Abstract
Neurotrophins provide trophic and tropic support for different neuronal subpopulations in the developing and adult nervous systems. Expression of the neurotrophins and their receptors can be altered in several different disease or injury states that impact upon the functions in the central and peripheral nervous systems. The intracellular signals used by the neurotrophins are triggered by ligand binding to the cell surface Trk and p75NTR receptors. In general, signals emanating from Trk receptors support survival, growth and synaptic strengthening, while those emanating from p75NTR induce apoptosis, attenuate growth and weaken synaptic signaling. Mature neurotrophins are the preferred ligand for Trk proteins while p75NTR binds preferentially to the proneurotrophins and serves as a signaling component of the receptor complex for growth inhibitory molecules of central nervous system myelin [ie, myelin‐associated glycoprotein (MAG), oligodendrocyte‐myelin glycoprotein (OMgP) and Nogo]. The functional antagonism between Trk and p75NTR signaling may significantly impact the pathogenesis of human neurodevelopmental and neurodegenerative diseases and further complicate therapeutic uses of exogenous neurotrophins. The potential for each is discussed in this review.
INTRODUCTION
Neurotrophins are a group of structurally related polypeptide growth factors that contribute to the development, maintenance and function of the peripheral and central nervous systems (PNS and CNS, respectively). These trophic factors have unique activities on target neurons and, more recently recognized, on other cell types in the nervous system and beyond that warrant consideration in the pathophysiology of diseases and injury responses in the human nervous system. The accompanying reviews in this mini‐symposium focus on distinct diseases or disease processes in which actions of the neurotrophins have been clearly implicated (16, 105, 155, 159). In most settings, a decrease or a presumed decrease in effective neurotrophin levels is detrimental. However, increases in neurotrophins can also alter cellular function in harmful ways. This bears particular consideration for inflammatory states, neoplasia and when considering therapeutic potentials for neurotrophins. There have been several recently published general and specialized reviews focusing on neurotrophins’ biological effects and signaling mechanisms (38, 153, 199, 201). Here, we will present mechanisms of actions of mammalian neurotrophins that raise unique considerations for neurotrophin pathophysiology with regard to human CNS and PNS diseases of pathological interest.
The biological activity of nerve growth factor (NGF), the first known neurotrophin, was initially identified in the 1950s from seminal transplantation experiments performed by Victor Hamburger and Rita Levi‐Montalcini (115). These and other experiments led to the “neurotrophic hypothesis” that developing neurons compete for limited quantities of neurotrophic factors that are secreted by their target tissues. The target‐derived trophic activity that the chick dorsal root ganglion (DRG) neurons competed for was coined “nerve growth factor” (41, 116). Isolation of NGF provided the necessary reagents and knowledge to fuel over three decades of fruitful research into the range of NGF’s biological activities and its mechanism of action (114). Shortly after the Nobel Prize was awarded to Levi‐Montalcini and Cohen in 1986 for the identification of NGF (and epidermal growth factor), it was recognized that NGF is only a single member of a family of neurotrophins. Indeed, when Liebrock et al isolated brain‐derived neurotrophic factor (BDNF), structural homologies between BDNF and NGF pointed to a family of related neurotrophic proteins (112).
With the advent of polymerase chain reaction (PCR), other members of the neurotrophin family were rapidly discovered, including neurotrophin‐3 (NT‐3), neurotrophin‐4 (NT‐4) and neurotrophin‐5 (NT‐5) (13, 49, 72, 79, 87, 88, 127, 156). NT‐4 and NT‐5 represent the Xenopus and mammalian orthologs of a single gene product and will be referred to as NT‐4/5 in the remainder of this text. Two additional neurotrophins have been identified in fish, neurotrophin‐6 (NT‐6) and neurotrophin‐7 (NT‐7) (139). The neurotrophins bind to members of a family of tyrosine kinase receptors, the tropomyosin‐related kinases (Trks) TrkA, TrkB and TrkC (81). Additionally, all members of the neurotrophin family bind to a second receptor protein, p75NTR, which was first identified as the NGF receptor (37).
SYNTHESIS AND ACTIVITIES OF NEUROTROPHINS
Neurotrophins are synthesized by a number of different cell types in the CNS and PNS. In general, the individual neurotrophins act upon different neuronal populations or subpopulations, but there can be overlaps in ligand responsiveness. Injury and other stimuli can also alter the repertoire of Trk receptor expression, changing a neuron’s ligand responsiveness. In the CNS, BDNF expression is overall more widespread than that of NGF, NT‐3 or NT‐4/5. Injury and neuronal activity can similarly alter the production of neurotrophins by target tissues and glial cells in the CNS and PNS (62, 131). The four mammalian neurotrophin genes encode prepropeptides, which are secreted as mature proteins. It has now become clear that the propeptides, at least NGF and BDNF, can be secreted and in some cases are processed extracellularly into mature proteins by activities of plasmin, metalloproteases or other as yet unidentified proteases (119). The mature neurotrophins are active as noncovalently linked homodimers. NGF, BDNF, NT‐3 and NT‐4/5 share approximately 50% identity (117). BDNF is secreted through both constitutive and regulated pathways (eg, activity‐dependent). While it has been suggested that the other neurotrophins only show constitutive secretion, activity‐dependent release of NGF and NT‐3 has been demonstrated in some systems (19, 29, 189).
The neurotrophins are essential for proper development of the embryonic nervous system through their ability to promote survival and stimulate neurite outgrowth from CNS and PNS neurons (117). After development, neurotrophins play critical roles in maintaining neuronal morphologies and functions as well as providing trophic and tropic activities in the neuronal responses to injury (15, 168). Although most experimental efforts have focused on activities of the mature neurotrophins, recent observations indicate that proneurotrophins are biologically active and actually counter the growth‐promoting and survival effects of the mature proteins (76).
NGF. In the PNS, NGF promotes survival and maturation of sympathetic and sensory neurons (117). Mice with the NGF gene “knocked out” (NGF−/−) exhibit severe loss of sympathetic and sensory neurons in the PNS (43). The small nociceptive neurons in the DRG, which mediate pain and temperature sensation, do not survive in animals treated with neutralizing NGF antibodies or in the NGF−/− mice (167). Clinically, trophic activity of NGF in the sensory nervous system, particularly the small nociceptive fibers, has been suggested to contribute to diabetic neuropathy (178), and mutations of the NGF receptor TrkA have been shown to cause congenital anhidrosis and pain insensitivity (82, 113).
The highest levels of mature NGF in the CNS are found in the hippocampus, which supplies trophic support for septal and basal forebrain cholinergic neurons through retrograde transport (97, 173). In these neurons, NGF regulates the expression of cholinergic markers (eg, choline acetyltransferase) needed for acetylcholine synthesis and neurotransmission (136). The atrophy of cholinergic neurons and memory dysfunction seen after axotomy of basal forebrain neurons (ie, with lesion of fornix) can be prevented by providing exogenous NGF to these neuronal cell bodies (57, 71, 99). Recent work from Salehi et al has also provided evidence for decreased retrograde transport of NGF from hippocampus to basal forebrain in the triosomy 16 mice that display an Alzheimer’s‐like phenotype (160). Although the NGF−/− mice die in the postnatal period (43), mice heterozygous for the NGF gene (NGF+/−) are viable, showing decreased cholinergic innervation of the hippocampus and deficits in memory acquisition (39). Beyond proving that NGF is needed throughout life, this haplotype insufficiency for NGF indicates that partial depletion of the ligand can alter neuronal function in vivo. These and other studies have provided support for the notion that exogenous NGF could be used as a treatment for Alzheimer’s disease. This issue is discussed in detail in the accompanying article by Blesch (16). Effects of NGF and other neurotrophins on myelination is discussed in the accompanying article by Rosenberg et al (155).
BDNF and NT‐4/5. In the PNS, BDNF is a trophic factor for sensory neurons of both neural crest (eg, DRG neurons that mediate mechano‐reception) and ectodermal placode origin (eg, nodose ganglion neurons that innervate viscera) (8). NT‐4/5 binds to the same TrkB receptor as BDNF, and, consequently, there is a significant overlap between the actions of these two factors in the PNS (81). In contrast to knockouts for the other neurotrophins or the cognate Trk receptors, NT‐4/5−/− mice show no obvious behavioral deficits and have normal life spans (42, 120). However, a closer inspection of NT‐4/5−/− animals has shown abnormalities in innervation of sympathetic ganglia, loss of vagal afferents to enteral mechanoreceptors and decreased tolerance to morphine (58, 154, 166).
By and large, the CNS sets BDNF and NT‐4/5 apart, as BDNF is more widely expressed in the CNS and shows regulated secretion in response to activity. BDNF knockout mice, both homozygotes and heterozygotes, show reduced long‐term potentiation (LTP) (102), the cellular correlate of long‐term memory, suggesting a role for BDNF and its TrkB receptor in synaptic plasticity. Exogenously applied BDNF has been shown to facilitate induction of LTP, particularly the transition to long‐lasting LTP that is protein synthesis‐dependent (103). These and other studies have led to the hypothesis that BDNF plays a critical role in the activity‐dependent neuroplasticity underlying learning and memory (124). Neurotrophins, including BDNF, enhance neurotransmission at the neuromuscular junction, and postsynaptic sources of BDNF and NT‐4/5 facilitate synaptogenesis in neuron/muscle cocultures (122, 171, 183, 190). Recent studies further implicate BDNF with the cognitive deficits associated with Alzheimer’s disease (48, 73). Trisomy 16 mice, which provide a mouse model of Down’s syndrome with overexpression of amyloid precursor protein, show abnormal BDNF signaling, and restoration of BDNF signaling by expression of full‐length TrkB prevents neuronal apoptosis in cortex (47) Together, these data suggest that BDNF plays a prominent role in both the development and function of the CNS. This is not to say that NT‐4/5 is without any CNS functions that are relevant to pathologies of the brain and spinal cord. Injection of NT‐4/5 into the marginal zone of embryonic rodent brain results in neuronal heterotopias through a TrkB‐dependent mechanism; surprisingly, NT‐4/5 shows more than 10‐fold greater propensity to induce heterotopias than does BDNF (21).
The propeptide sequence of endogenous BDNF sends this protein uniquely into a secretory pathway with activity‐dependent release of BDNF‐containing secretory vesicles (176). The propeptide regions of NGF, NT‐3 and NT‐4/5 are argued not to provide this level of directed secretion, although stimulus‐dependent release of NGF and NT‐3 has been demonstrated (19, 29, 78, 104, 189). Pro‐BDNF requires sorting by carboxy‐peptidase E to yield mature peptide in the regulated secretory pathway (123), while furin cleaves the proneurotrophins in the constitutive pathway [for reviews, see (119, 176)]. A single nucleotide polymorphism in the human BDNF gene has been shown to alter its regulated secretion such that BDNF with methionine rather than valine at residue 66 only shows constitutive release. Individuals carrying this polymorphism show evidence of altered memory acquisition (48). Activity‐dependent secretion of BDNF at the synapse has been demonstrated in several different neuronal systems, including cortical neurons and the neuromuscular junction [for a review, see (176)]. Locally released BDNF enhances synaptic efficacy both through effects locally at, or adjacent to, the synapse and through retrogradely signaling to the cell body (124). For example, BDNF has been shown to both regulate localized mRNA translation in dendrites and alter the delivery of mRNAs into dendrites (1, 177, 195). Localized effects of BDNF and the other neurotrophins are discussed in more detail in subsequent sections.
NT‐3. NT‐3 promotes survival and neurite outgrowth of the large‐diameter proprioceptive neurons of the DRG that innervate stretch and tension receptors in muscle and joints (50, 53). NT‐3 also has trophic actions on neurons of the nodose ganglia, sympathetic ganglia, Remak’s ganglia, ciliary ganglia, trigeminal mesencephalic nucleus and spiral ganglia (42, 117). As NT‐3 binds to all Trk receptors, some of the biological effects of NT‐3 can occur through binding to Trks other than TrkC. Indeed, sympathetic neurons of the superior cervical ganglion (SCG) require both NT‐3 and NGF for survival during development [for a review, see (67)]. NT‐3 supports survival and axonal outgrowth from SCG neurons as their axons are traversing toward their target of innervation; these SCG neurons switch from NT‐3 to NGF responsiveness upon reaching their targets of innervation (107). Interestingly, the effect of NT‐3 on sympathetic neurons is through TrkA rather than its preferred TrkC binding partner. Thus, the promiscuity of NT‐3 in its interactions with Trk family members has clear biological relevance during development. Beyond its effects on survival and neurite outgrowth, NT‐3 has been found to be important for keeping neural crest derivatives in a proliferative state during early development (52). NT‐3−/− mice die within a few weeks after birth, and show severe sensory and sympathetic neuron loss with abnormal limb movement (42). Crossing neurotrophin knockout animals with mice that have altered expression of Bcl‐2 family proteins, which affords neurotrophin‐independent neuronal survival during development, has led to the discovery of new activities for some neurotrophins. For NT‐3, NT‐3−/−Bax−/− mice show altered central connectivity of proprioceptive neurons in the spinal cord, indicating that NT‐3 plays a role in central patterning for proprioception, in addition to survival of proprioceptive DRG neurons (144).
Proneurotrophins. These precursors to mature neurotrophins were known to exist for quite some time, but biological activity of the proneurotrophins was only recently discovered (110). In general, the actions of the proneurotrophins are antagonistic to those of the mature neurotrophins [for a review, see (76)]. The proneurotrophins preferentially bind to p75NTR, but not all p75NTR expressing cells are sensitive to proneurotrophins; expression of the neurotensin receptor sortilin is apparently needed for proneurotrophins to induce biological effects (140). The binding characteristics of proneurotrophins to Trks has not been as extensively studied as those of the mature neurotrophins, and Fayard et al have reported that pro‐BDNF can bind to TrkB (54). Thus, the proneurotrophins may hold many surprises just as the p75NTR has over the years (see below). Nonetheless, currently available evidence indicates that the antagonistic effects of the proneurotrophins have pathophysiological relevance for human disease and injury. Axotomy through lesion of the internal capsule increases levels of pro‐NGF, resulting in apoptosis of corticospinal neurons through binding to p75NTR (74). Pro‐BDNF similarly induces neuronal apoptosis though the p75NTR/sortilin receptor complex (175). Actions of the proneurotrophins are not limited to neurons. Indeed, work from the Yoon group has shown that induction of pro‐NGF after spinal cord injury kills oligodendrocytes (11). Previous work indicated that mature NGF can induce apoptosis in cultured oligodendrocytes and transfection of TrkA prevented NGF from killing these glial cells (196). It should be noted that other studies have demonstrated p75NTR signaling in oligodendrocytes but did not report apoptotic effects of NGF or pro‐NGF (2, 109). Analyses of rodent and human tissues indicates that pro‐NGF, rather than the mature NGF peptide, is the predominant form in the brain, and pro‐NGF appears to be increased in Alzheimer’s disease as well as patients with mild cognitive impairment (51, 146). It will be interesting to see if the proneurotrophins play any role in the primary and metastatic CNS tumors that are discussed in the accompanying review by Krüttgen et al (105).
The antagonistic functions of mature neurotrophins and proneurotrophins extend beyond cell death. As noted above, mature BDNF plays a central role in synaptic strengthening for LTP (55, 102, 103, 145). Pro‐BDNF was recently shown to weaken synaptic strength, resulting in long‐term depression (LTD) (187). p75NTR also appears to play a role in dendritic growth as p75NTR−/− mice have increased dendritic spine complexity and overexpression of p75NTR decreases dendritic spine complexity (198). As discussed below, p75NTR has assumed a major role in the failed regeneration of CNS axons, suggesting that there could be an overlap between the mechanisms for axonal growth and spine morphogenesis. As Trk induces signals that generally counter p75NTR’s effects (182), activation of Trk may be one means to overcome the effects of proneurotrophins on synaptic activity. Extracellular cleavage of pro‐BDNF by activity‐dependent stimuli tips the balance toward the synaptic strengthening functions of neurotrophins (143).
INTRACELLULAR EFFECTORS FOR THE NEUROTROPHINS
Neurotrophins exert their biological actions by binding to two different classes of transmembrane receptors, the Trk family of receptors and p75NTR. Both receptors can trigger downstream signaling pathways to exert the biological effects of neurotrophins, proneurotrophins and other relevant ligands. One can broadly divide these pathways into Trk‐mediated signaling, which is generally growth‐promoting and prosurvival, and p75NTR‐mediated signaling, which is generally proapoptotic and growth‐inhibiting (Figure 1).
Figure 1.
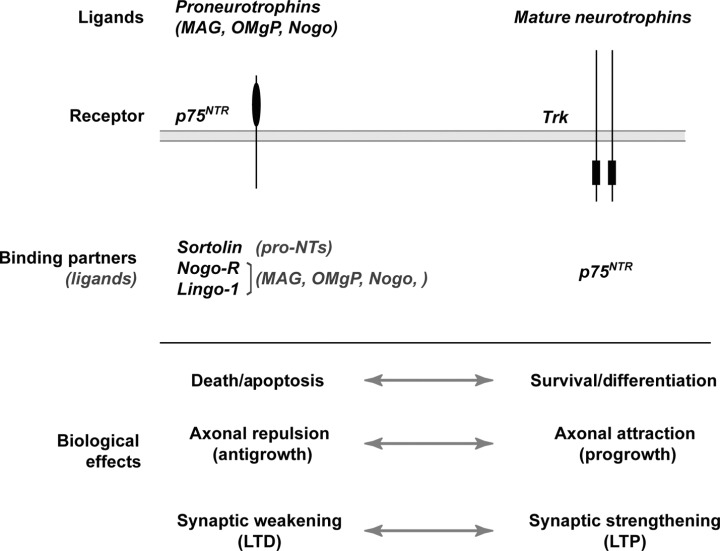
Antagonistic functions of the neurotrophin receptors, Trk and p75NTR. Summary of the directly antagonistic biological outcomes of ligand binding to Trk or p75NTR proteins along the neuronal cell surface is outlined. Mature neurotrophins are the preferred ligand for TrkA, TrkB and TrkC with p75NTR facilitating interactions with Trks (see text). Although all of the mature neurotrophins will also bind to p75NTR, proneurotrophins apparently use p75NTR as their preferred receptor but also require the presence of sortilin to generate an intracellular effect. The myelin inhibitor proteins, MAG, OMgP and Nogo, also signal through a multiprotein receptor complex consisting of Nogo‐R, Lingo‐1 and p75NTR. This ligand–receptor complex is best characterized for inducing axonal retraction or preventing axonal regrowth/regeneration in the CNS. LTD = long‐term depression; LTP = long‐term potentiation; MAG = myelin‐associated glycoprotein; NTR = neurotrophin receptor; NTs = neurotrophins; OMgP = oligodendrocyte‐myelin glycoprotein; Trk = tropomyosin‐related kinase.
The first member of the Trk family was discovered as an oncogene in which tropomyosin was fused to the kinase domain of TrkA (129). TrkA was later identified as an NGF receptor (94). The Trk proteins, TrkA, TrkB and TrkC, share the greatest degree of homology in their intracellular regions that possess tyrosine kinase activity. The extracellular regions that confer ligand‐binding specificity are the most variable (81). NGF preferentially binds to TrkA, BDNF and NT‐4/5 bind to TrkB, and NT‐3 binds to TrkC (18). As mentioned above, NT‐3 is promiscuous and can bind to TrkA and TrkB under some conditions. Expression of p75NTR appears to allow NT‐3 to discriminate its preferred TrkC from the other Trk receptors (135). These different Trk receptors are expressed in both primary neurons and neuronal cell lines and targeted mutation of trkA, trkB and trkC genes in mice disrupts neuronal development consistent with a loss of neurotrophin action (7).
Many of the signaling mechanisms activated by the Trks converge upon the nucleus to alter gene expression programs. Downstream signaling has been best characterized for TrkA, but signals activated by TrkB and TrkC appear generally similar (81). NGF binding to TrkA causes receptor dimerization leading to autophosphorylation of tyrosine residues within its kinase domain (27, 157). Subsequent phosphorylation of other tyrosine residues promote signaling by creating docking sites for proteins with Src homology 2 motifs (SH2) (121, 141, 170). Activation of the Ras/Rap–mitogen‐activated protein (MAP) kinase and phospholipase C γ (PLCγ) pathways can trigger changes in gene expression by direct or indirect activation of transcription factors. Downstream effectors for these two pathways include the extracellular signal‐regulated kinases 1 and 2 (Erk 1/2) and protein kinase C (PKC) isoforms, including the atypical PKCs (199). Trk‐dependent activation of phosphoinositoyl‐3 kinase (PI3K) can also regulate transcription through downstream regulation of Akt activity (93). However, this is more frequently considered a prosurvival pathway, with Akt preventing activation of proapoptotic members of the Bcl2 family (60). In addition to transcriptional regulation, both the Ras–MAP kinase and PI3K pathways can regulate activity of the translational machinery (66, 158). Likewise, each of the Trk‐activated pathways can have localized effects in the cytoplasm by regulating activity of proteins involved in synaptic function, cytoskeletal remodeling and bioenergetics (see below). Additionally, transcriptional regulation through Trk signaling can occur by epigenetic modifications of chromosome‐binding proteins, in addition to the classic transcription factor‐mediated events outlined above (34).
p75NTR, the first neurotrophin receptor to be identified (86, 151), is a transmembrane glycoprotein that shares a high degree of homology with members of the TNF receptor superfamily (76). Cysteine repeats in the extracellular domain confer relatively equal binding affinity to all members of the neurotrophin family (36). For mature neurotrophin interaction to the p75NTR, opposite cellular effects occur depending upon whether or not a cognate Trk receptor is present. For example, cells expressing both p75NTR and TrkA display a much higher binding affinity for NGF than under circumstances where either receptor is expressed alone (37). Ligand binding to p75NTR can potentiate TrkA autophosphorylation at subsaturating NGF concentrations and this appears to be dependent on the relative levels of p75NTR and TrkA (10, 28, 126, 180, 181). In stark contrast to these synergistic effects upon Trk signaling, p75NTR has also been shown to mediate neurotrophin‐induced cell death (31, 150). This is exemplified in circumstances in which p75NTR is expressed in the absence of an appropriate neurotrophin Trk receptor. For example, BDNF can promote apoptosis in primary sympathetic neurons that express p75NTR and TrkA but not TrkB (6), and endogenous NGF induces death of developing chick retinal neurons that express p75NTR and TrkB but not TrkA (59). NGF has been shown to promote cell death of mature oligodendrocytes, which express p75NTR but not TrkA (31), and introduction of TrkA negates the p75NTR‐dependent cell death seen with mature NGF (196). Thus, the survival vs. death decisions by neurotrophins rely critically upon matching the ligand with the appropriate Trk receptor.
The story of p75NTR has held many surprises since its first discovery as “the NGF receptor” and then the realization that it contained no clearly recognizable signal transducing domains. With the discovery of Trks, attention waned from the possibility that p75NTR could signal on its own. In addition to the effect of mature neurotrophins binding to p75NTR, proneurotrophins preferentially bind to p75NTR and CNS molecules that actively block axonal outgrowth use p75NTR as a signaling coreceptor. These ligands activate p75NTR signaling that in some cases is similar to those signals utilized by the related TNF receptors (98), while in other cases the signaling pathways appear unique to p75NTR, at least for the present time. Ligand binding to p75NTR can activate the c‐Jun N‐terminal kinase and NFκB transcription factor (14, 30, 31). Ligand‐dependent cleavage of p75NTR was very recently shown to provide a means for the intracellular domain (ICD) of p75NTR to signal (96). This solved a mystery of how expression of just the p75NTR ICD can induce cell death (128) and of the functional role of neurotrophin receptor interacting factor (NRIF), a zinc‐finger protein that was cloned as a p75NTR binding protein (32). Mature BDNF and pro‐BDNF can induce cleavage of p75NTR through γ‐secretase with release of p75NTR ICD, nuclear translocation of NRIF and apoptosis in sympathetic neurons (96). p75NTR has also provided some resolution to the mystery of how the myelin‐associated glycoprotein (MAG), oligodendrocyte‐myelin glycoprotein (OMgP) and Nogo, myelin proteins that inhibit axonal outgrowth in the CNS (56), can transduce intracellular signals. These myelin proteins bind to a multisubunit receptor complex that uses p75NTR as its signal transducer (132) [for a review, see (9)]. This receptor complex consists of the Nogo receptor (Nogo‐R), LINGO‐1 and p75NTR. The Rho‐guanosine diphosphate (GDP) dissociation inhibitor α (Rho‐GDIα) binds to the ICD of p75NTR upon MAG’s interaction with the cell surface and shifts activity of the small G‐protein Rho toward activation to favor F‐actin depolymerization (192). Mature neurotrophins binding to p75NTR have the opposite effect, shifting the balance of Rho activity toward inhibition (193). This could place p75NTR as a central determinant in the balance of axonal microfilament polymerization vs. depolymerization by intracellularly changing Rho activity based upon what ligands/receptor partners p75NTR is interacting with extracellularly (see Figure 2B).
Figure 2.
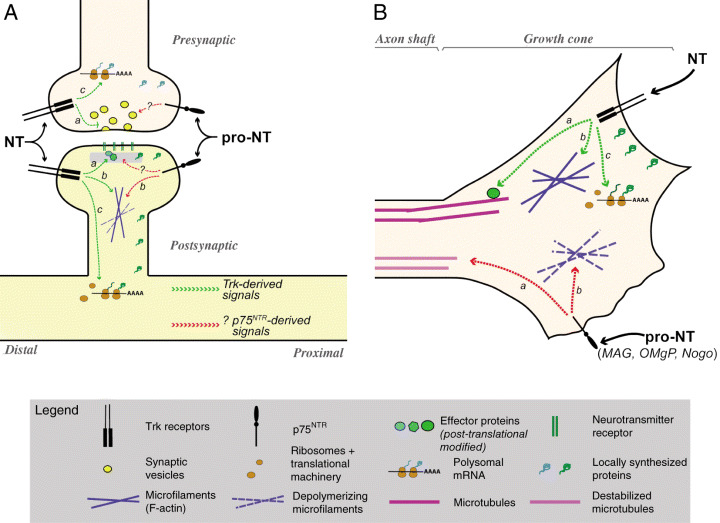
Local tropic and trophic effects of the neurotrophins. Schematic summarizing the effects of mature neurotrophins (NT) and proneurotrophins (pro‐NT) at the synapse (A) and growth cone (B) is shown. Anatomic designations are shown in gray italicized text. Symbol identities are designated in the legend. Green dashed lines illustrate intracellular effects of Trks and red dashed lines illustrate effects of p75NTR. A. Presynaptic effects of the NTs include facilitating release of neurotransmitters (a) and activating localized translation to generate new proteins (c). Postsynaptically, NTs can modify synaptic proteins through phosphorylation (a), antagonize effects of p75NTR to favor actin filament polymerization (b) and activate localized protein synthesis to generate new proteins (c). Although pro‐NTs have been shown to play a role in long‐term depression with synaptic weakening, the exact mechanism of this is not clear. In other systems, signaling through p75NTR has been shown to favor actin filament depolymerization (b). p75NTR may also signal directly to other pre‐ or postsynaptic components (?) to repress synaptic strength. Signals through Trks are retrogradely transmitted to the cell body, but it is not clear whether p75NTR generates any retrograde signals in dendrites or axons. Note that neither activity‐dependent nor neurotrophin‐dependent effects on neurotrophin release are represented in this schematic; refer to text for information on these stimuli for regulated release of neurotrophins. B. In growing axons, NTs can modify effector proteins to support axonal growth [eg, microtubule‐associated proteins, (a)], facilitate actin filament polymerization (b) and modulate localized protein synthesis (c). Signaling through p75NTR, particularly for the myelin inhibitor proteins (MAG, OMgP and Nogo), has been shown to favor actin filament depolymerization with growth cone collapse (b) and destabilization of microtubules (c). Antagonistic signals from p75NTR and Trks shown in this terminal axon would actively induce turning upward toward the NT source and away from sources of pro‐NT, MAG, OMgP and Nogo. MAG = myelin‐associated glycoprotein; NTR = neurotrophin receptor; OMgP = oligodendrocyte‐myelin glycoprotein; Trk = tropomyosin‐related kinase.
The intracellular signaling pathways that are activated by Trks and p75NTR are shared by many different ligand–receptor systems. The biological response to a specific ligand can be determined by the timing and magnitude of downstream signaling events as well as the combination of signals activated. For example, in the PC12 cell line, NGF induces neuronal differentiation while epidermal growth factor (EGF) is mitogenic. This distinction is determined by the kinetics of activation of the Erk 1/2 [see (105)]. EGF causes transient activation of Erk 1/2 through activation of the G‐protein Ras, while NGF activation of Erk 1/2 lasts for hours and uses both Ras and Rap (68, 92, 197). Despite the fall in Erk 1/2 activity after initial exposure to NGF, a continued basal level of Trk activity (and downstream signals) is required to drive transcription in cells that need neurotrophins for survival and phenotypic maintenance (33).
LOCAL TROPIC AND TROPHIC ACTIVITIES OF THE NEUROTROPHINS—CONSIDERATIONS FOR INJURY AND PLASTICITY
Mechanistically, the effects of the neurotrophins can be divided into local effects that occur adjacent to points of release of the ligands (eg, at the synapse or growth cone) and systemic or whole cell effects that are initiated by retrograde signaling to the cell body and nucleus. Retrograde signaling by internalized Trk receptors is particularly relevant for transcriptional responses to localized sources of neurotrophins. p75NTR is also internalized, but this process is much slower than that seen with the Trks and the full biological relevance of internalized p75NTR has yet to be demonstrated (20). Here, we will focus on recent advances in the localized responses to neurotrophins. We refer the reader to Zweifel et al for an excellent review on the topic of retrograde signaling (201). To exert their local effects, neurotrophins act upon existing macromolecules to alter protein function and cytoskeletal organization. Additionally, the neurotrophins can alter protein levels by directly modulating activity of the protein synthesis machinery that is concentrated near dendritic spines and within growth cones of axons (Figure 2). As discussed below, a surprising finding with translational regulation is the specificity that the protein synthesis machinery exhibits for individual mRNAs.
Local effects of neurotrophins in synaptic transmission and plasticity. Neurotrophin expression can be regulated by neuronal activity, and increase in neurotrophin levels has been shown to facilitate neurotransmission [for reviews, see (119, 124)]. Analyses of mice after kainic acid‐induced seizures suggested a local effect of neurotrophins with release of BDNF into the neuropil surrounding hippocampal dendrites (185). Using hippocampal slice preparations, Kang and Schuman showed that BDNF and NT‐3 can enhance synaptic efficacy through local effects at the synapse (89, 90, 91). Application of exogenous BDNF or NT‐3 to the developing neuromuscular junction also acutely enhances neurotransmitter release (122). For BDNF, this occurs through influx of calcium into the presynaptic cell and requires intracellular cyclic adenosine monophosphate (cAMP) (171). In contrast, NT‐3 induces release of intracellular calcium stores as well as activations of PI3K with resultant generation of free inositol triphosphate and calcium/calmodulin kinase (75, 194). Long‐term changes in synaptic neurotransmission at the neuromuscular junction require internalization of NT‐3 with activation of Akt, target of rapamycin and new protein synthesis (84). Localized activation of postsynaptic and presynaptic protein synthesis has been shown to modulate synaptic efficiency in several different neuronal systems [for recent reviews, see (148, 163, 184)]. In the neuromuscular junction, this BDNF‐triggered protein synthesis occurs directly within the axon terminus (200). Neurotrophins can directly regulate translation of specific mRNAs in dendrites whose products have been associated with learning and memory (1, 177, 195). With limited quantities of proteins derived from primary neuronal cultures and inefficiency of metabolic labeling approaches, the study of translational activation has not progressed at the same rates as analyses of changes in neuronal gene expression, where PCR can be used to amplify signals. Schratt et al addressed this technical limitation using a polysomal RNA fractionation approach to identify previously unrecognized mRNA targets for translational regulation by BDNF (162). Additional studies are needed to determine how many of these translationally regulated mRNAs will be relevant for localized effects of the neurotrophins. Although there has not been a direct link between neurotrophin‐regulated pre‐ or postsynaptic protein synthesis and human disease, brain tissue from Alzheimer’s disease show alterations in the translational machinery, suggesting that protein synthesis could be hindered in diseased neurons (45, 80). This, combined with altered neurotrophin levels, could compromise presynaptic and postsynaptic protein levels (and synaptic function) in neurodegenerative diseases.
Synaptic plasticity has been associated with growth of neuronal processes generating increased dendritic spine complexity. Interestingly, signaling through p75NTR−/− was recently shown to decrease dendritic spine complexity (198). It is not clear whether the ligand for this effect is mature or proneurotrophins; however, as noted above, proneurotrophins induce LTD that is generally associated with a decrease in synaptic strengthening (187). Despite this effect, pro‐BDNF can be converted to mature BDNF extracellularly through tissue plasminogen activator (tPA)/plasmin facilitating protein synthesis‐dependent late‐phase LTP in mouse hippocampus (143). Balance between Trk and p75NTR signaling could be a critical determinant of synapse structure, with Trk signaling favoring increased dendritic spine complexity and p75NTR favoring decreased dendritic spine complexity. In growing axons, activation of apoptotic pathways can rapidly trigger retraction of growth cones (26). Such local effects of apoptotic pathways could underlie the effect of p75NTR on dendritic spines. In cultures of basal forebrain neurons, the balance between apoptotic signaling through p75NTR and survival signaling through TrkA is determined by activity levels of PI3K and Ras/Rap–MAP kinase pathways (182).
Alterations in dendritic spine complexity have been demonstrated in several human neurodevelopmental disorders (4, 95, 152). Post‐mortem analyses of brains from patients with Rett syndrome, an X‐linked disorder that is most frequently caused by mutation of the methyl‐CpG binding protein (MeCP2) (3), show a reduction in dendritic arborization with decreased numbers of dendritic spines in frontal and motor cortex (4, 5, 12), and mice with targeted disruption of the Mecp2 gene display similar neuropathology (61). MeCP2 binds to methylated regions of chromosomes and functions as a transcriptional repressor by interacting with chromatin remodeling complexes including histone deacetylases (118, 138). MeCP2 was recently shown to differentially repress one of the BDNF gene promoters and neuronal activity triggers decrease in CpG island methylation with derepression of the BDNF gene (40, 130). Impaired regulation of BDNF expression may be critical in the pathogenesis of the neuronal lesion in Rett syndrome. The pathological effects of dysregulated BDNF expression in Rett syndrome could be further exacerbated by other neurotrophins or proneurotrophins acting through p75NTR to alter spine morphogenesis. Overexpression of a BDNF transgene was recently shown to improve survival and neurophysiological measures of neuronal dysfunction in the MeCP2 mutant mice that have provided a mouse model of Rett syndrome (35). Thus, activity‐dependent regulation of BDNF expression could indeed alter dendritic complexity in human neurodevelopmental disorders. Although not associated with neurotrophins, at least thus far, the increase in dendritic spine complexity seen in fragile X mental retardation is thought to be caused by loss of a translational suppressor that localizes to dendrites, the fragile X mental retardation protein (70). This suggests that a delicate balance in protein synthesis within dendrites is required for appropriate dendritic spine morphogenesis in human development.
Tropic effects of neurotrophins in development and regeneration. The interaction between Trk and p75NTR signaling also plays a role in axonal outgrowth during development and regeneration. Localized sources of neurotrophins serve as pathfinding cues, providing a tropic source for developing and regenerating axons through Trk signaling. In embryonic development, target tissues can serve as a localized source of neurotrophins. In injured peripheral nerves, increased synthesis and release of NGF by axotomized Schwann cells may play a role in guiding axons into degenerating distal stump of the nerve (77). Other neurotrophins and non‐neurotrophin trophic factors undoubtedly contribute to successful regeneration in the PNS (164, 191). Also, the potential role of proneurotrophins needs to be considered. In the CNS, myelin proteins and proteoglycans expressed within glial scars after injury actively prevent regeneration (56, 69). Although axonal regeneration is rarely successful in the CNS, neurotrophins have been used as one means to lure axons into the nonpermissive environment of the adult CNS (22, 101, 108). As indicated previously, signaling through p75NTR provides the CNS myelin inhibitory molecules with a mechanism to block axonal growth (9). This inhibition of growth appears to be a local effect on the growing axon, at least initially, rather than a systemic affect generated at the cell body.
In cultured neurons, the growth‐promoting or attractant effects of localized sources of neurotrophins induce turning of growth cones and sprouting along axon shafts (63). Both of these events require signaling to the axonal cytoskeleton, particularly the microfilaments that are required for growth cone motility (64). Work from several laboratories has pointed to a role for localized protein synthesis in pathfinding for developing axons (25, 134, 149, 188). As adult mammalian axons also have the capacity to locally synthesize proteins (179), this mechanism is also relevant for axonal regeneration. In retinal and motor neurons, application of BDNF to growth cones triggers intracellular signals that converge upon the protein synthesis machinery. Blocking BDNF’s activation of localized protein synthesis prevents axonal turning in response to this ligand (25, 134). Focal sources of neurotrophins can also modulate the levels of mRNAs in regenerating sensory axons that are needed for growth cone motility and responses to injury (147, 186). Some repellant cues for the growth cone similarly require localized protein synthesis; other repellant stimuli activate both protein synthesis and proteolysis, while some just trigger proteolysis in the growth cone (25). The proteolytic events are triggered by activation of apoptotic signaling in the growth cone, including activation of the stress‐activated kinase p38MAPK and caspase 3 (26). On the other hand, the turning response to attractant cues requires activation of Erk 1/2 and calcium signaling (26, 134). Thus, whether a stimulus invokes attraction or repulsion is likely determined by the balance of intracellular signals that are activated locally in the terminal axon. The recent history of what stimuli the growing axon has encountered also plays a role in determining its response to subsequent stimuli (44, 134, 149).
Activation of p75NTR by MAG, OMgP and Nogo inhibitors prevents axonal outgrowth. In cultured neurons, localized sources of MAG induce axonal retraction or turning (165). MAG induces intramembranous cleavage of p75NTR similar to the proneurotrophins activating Rho and downstream signals that can favor actin depolymerization (46). Furthermore, MAG blocks microtubule assembly through downstream activation of Rho kinase (133). Primary sensory neurons and cerebellar granular cells can be coaxed to extend axons across purified MAG and biochemical preparations of CNS myelin if they are first primed with neurotrophins (22, 165). Developing neurons see MAG as an attractant cue for growth because their levels of cAMP are higher than the cAMP levels of adult neurons (23). This elevation of cAMP results in the synthesis of polyamines through increased arginase levels and activation of the transcription factor cAMP‐response element‐binding protein (CREB) (24, 65). Activation of CREB is sufficient to overcome the effects of myelin inhibitors in adult neurons (65). Thus, stimulus history can alter how an axon interprets guidance cues that work through p75NTR, but this requires a transcriptional response in the cell body. Interestingly, the attractant responses of NGF, BDNF, NT‐4/5 and NT‐3 can be modified by intracellular levels of cyclic nucleotides. Decreases in cAMP for NGF, BDNF and NT‐4/5, and cGMP for NT‐3 convert these tropic factors from attractant to repulsant cues [for a review, see (169)]. In retrospect, this shift could indeed result from altering the balance of Trk to p75NTR signaling within the growth cone. Curiously, cAMP signaling also regulates the BDNF‐dependent formation of dendritic spines (85), pointing to overlapping mechanisms of axonal and dendritic growth.
Although the above mechanisms hold great potential for facilitating regenerative growth after spinal cord injury and stroke, there are not yet obvious examples of axonal pathfinding or repair deficits that have been shown to result directly from alterations in Trk or p75NTR signaling. However, searches for proteins that bind the p75NTR’s ICD have provided some insight into mechanisms underlying human neurodevelopmental disorders. Salehi et al initially showed that a novel melanoma‐associated antigen gene (MAGE) family member, termed NRAGE, binds to the juxtamembrane domain of p75NTR. NRAGE binding to p75NTR appears to block p75NTR–Trk interactions, favoring p75NTR‐dependent apoptosis (161). Other MAGE family members, including necdin, bind p75NTR and increase neuritogenesis through Trk‐independent means (174). Notably, four members of the MAGE family (Necdin, MAGE‐G1, MAGE‐L1 and MAGE‐H1) map to the imprinted region on chromosome 15q that is deleted in Prader–Willi and Angelman syndromes (17, 83, 100, 125, 172), and mice lacking the paternally expressed necdin gene show some characteristics of Prader–Willi syndrome, in conjunction with deficits in axonal growth, including altered fasciculation of processes in white matter tracts, pathfinding and target innervation (111, 142). Although the exact mechanism by which p75NTR/necdin interactions are connected to axonal outgrowth remain unclear, these findings certainly imply that altered p75NTR signaling can contribute to the axonal outgrowth abnormalities seen in human neurodevelopmental disorders. Necdin and MAGE‐G1 have been shown to repress transcriptional activity of the apoptosis‐promoting protein E2F1, but the data suggest that this is exclusive of p75NTR–MAGE protein interaction (106). Necdin interacts with other proteins in the cytoplasm that have been associated with cytoskeletal modulations of axonal outgrowth (111), which may provide a more direct link between p75NTR/necdin interaction and axonal growth. Regardless of exact mechanisms, the MAGE family’s interaction with p75NTR represents another point where proapoptotic and growth‐promoting activities of the neurotrophin receptors intersect.
CONCLUSIONS AND PERSPECTIVE
Survival‐ and growth‐promoting activities of the neurotrophins in the CNS and PNS have generated much interest in therapeutic applications for these growth factors in many different neurological diseases and injury states. Although systemic treatment with neurotrophins has generally proven ineffective, the accompanying reviews by Sahenk and Blesch emphasize high promise for the use of localized neurotrophin therapy for neuropathies and neurodegeneration (16, 159). However, there is still much to learn about the mechanisms of actions of the neurotrophins. In this short review, we have tried to emphasize the overlapping mechanisms that the neurotrophins use to support survival, growth and synaptic plasticity in different neuronal populations. Common intracellular mechanisms could prove beneficial in planning treatments with exogenous neurotrophins. For example, activity‐dependent increase in BDNF and NT‐3 expression in sensory neurons results in an enhanced regeneration capacity after PNS nerve injury by priming the neurons for increased growth (137). Thus, animals with decreased activity may have an overall poorer regenerative response to axotomy. It will be important to determine whether this observation can be extended to the CNS and if mechanisms of traumatic injury can be extrapolated to cellular injuries with neurodegeneration. Intracellular mechanisms triggered by the neurotrophins are able to overcome at least some components in the inhibitory environment of the CNS white matter (22). Overlapping intracellular mechanisms for the neurotrophins may also exacerbate the pathophysiology of disease states accompanied by decrease in endogenous neurotrophins. The secondary effects on endogenous neurotrophins also warrant some consideration in planning neurotrophin therapies as activity‐dependent release of endogenous neurotrophins can be regulated (29, 104, 189). This effectively provides a feed‐forward mechanism to facilitate neuronal survival, growth and plasticity. With the discovery of antagonistic effects of proneurotrophins and p75NTR signaling, consideration must also be given to how to overcome these “bad actors.”
ACKNOWLEDGMENTS
The authors thank Drs. Armin Blesch, Jonah Chen, Alex Kruttgen, and Zarife Sahenk for constructive criticisms on this manuscript. Work on related topics has been supported by R01‐HD037874 (NCS), R01‐NS041596 and R01‐NS049041 (JLT), and programmatic funds from the Nemours Foundation (NCS and JLT).
REFERENCES
- 1. Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM (2001) Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30:489–502. [DOI] [PubMed] [Google Scholar]
- 2. Althaus HH, Kloppner S (2006) Mature pig oligodendrocytes rapidly process human recombinant pro‐nerve growth factor and do not undergo cell death. J Neurochem 98:506–517. [DOI] [PubMed] [Google Scholar]
- 3. Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY (1999) Rett syndrome is caused by mutations in X‐linked MECP2, encoding methyl‐CpG‐binding protein 2. Nat Genet 23:185–188. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong DD (2005) Neuropathology of Rett syndrome. J Child Neurol 20:747–753. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong DD, Dunn K, Antalffy B (1998) Decreased dendritic branching in frontal, motor and limbic cortex in Rett syndrome compared with trisomy 21. J Neuropathol Exp Neurol 57:1013–1017. [DOI] [PubMed] [Google Scholar]
- 6. Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD (1998) The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol 140:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barbacid M (1995) Neurotrophic factors and their receptors. Curr Opin Cell Biol 7:148–155. [DOI] [PubMed] [Google Scholar]
- 8. Barde Y‐A (1989) Trophic factors and neuronal survival. Neuron 2:1525–1534. [DOI] [PubMed] [Google Scholar]
- 9. Barker PA (2004) p75NTR is positively promiscuous: novel partners and new insights. Neuron 42:529–533. [DOI] [PubMed] [Google Scholar]
- 10. Barker PA, Barbee G, Misko TP, Shooter EM (1994) The low affinity neurotrophin receptor, p75LNTR, is palmitoylated by thioester formation through cysteine 279. J Biol Chem 269:30645–30650. [PubMed] [Google Scholar]
- 11. Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO (2002) ProNGF induces p75‐mediated death of oligodendrocytes following spinal cord injury. Neuron 36:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC (2004) Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J Comp Neurol 480:281–298. [DOI] [PubMed] [Google Scholar]
- 13. Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A (1991) Neurotrophin‐5: a novel neurotrophic factor that activates trk and trkB. Neuron 7:857–866. [DOI] [PubMed] [Google Scholar]
- 14. Bhakar AL, Roux PP, Lachance C, Kryl D, Zeindler C, Barker PA (1999) The p75 neurotrophin receptor (p75NTR) alters tumor necrosis factor‐mediated NF‐kappaB activity under physiological conditions, but direct p75NTR‐mediated NF‐kappaB activation requires cell stress. J Biol Chem 274:21443–21449. [DOI] [PubMed] [Google Scholar]
- 15. Bibel M, Barde YA (2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14:2919–2937. [DOI] [PubMed] [Google Scholar]
- 16. Blesch A (2006) Neurotrophic factors in neurodegeneration. Brain Pathol 16:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boccaccio I, Glatt‐Deeley H, Watrin F, Roëckel N, Lalande M, Muscatelli F (1999) The human MAGEL2 gene and its mouse homologue are paternally expressed and mapped to the Prader‐Willi region. Hum Mol Genet 8:2497–2505. [DOI] [PubMed] [Google Scholar]
- 18. Bothwell M (1995) Functional interactions of neurotrophins and neurotrophin receptors. Ann Rev Neurosci 18:223–253. [DOI] [PubMed] [Google Scholar]
- 19. Brigadski T, Hartmann M, Lessmann V (2005) Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci 25:7601–7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M (2003) Ligand‐induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J Neurosci 23:3209–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brunstrom JE, Gray‐Swain MR, Osborne PA, Pearlman AL (1997) Neuronal heterotopias in the developing cerebral cortex produced by neurotrophin‐4. Neuron 18:505–517. [DOI] [PubMed] [Google Scholar]
- 22. Cai D, Shen Y, De Bellard M, Tang S, Filbin M (1999) Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP‐dependent mechanism. Neuron 22:89–101. [DOI] [PubMed] [Google Scholar]
- 23. Cai D, Qui J, Cai Z, McAtee M, Bregman B, Filbin M (2001) Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci 21:4731–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT (2002) Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro . Neuron 35:711–719. [DOI] [PubMed] [Google Scholar]
- 25. Campbell D, Holt C (2001) Chemotropic responses of reginal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32:1013–1016. [DOI] [PubMed] [Google Scholar]
- 26. Campbell DS, Holt CE (2003) Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 37:939–952. [DOI] [PubMed] [Google Scholar]
- 27. Canossa M, Rovelli G, Shooter EM (1996) Transphosphorylation of the neurotrophin Trk receptors. J Biol Chem 271:5812–5818. [DOI] [PubMed] [Google Scholar]
- 28. Canossa M, Twiss JL, Verity AN, Shooter EM (1996) p75(NGFR) and TrkA receptors collaborate to rapidly activate a p75(NGFR)‐associated protein kinase. EMBO J 15:3369–3376. [PMC free article] [PubMed] [Google Scholar]
- 29. Canossa M, Gartner A, Campana G, Inagaki N, Thoenen H (2001) Regulated secretion of neurotrophins by metabotropic glutamate group I (mGluRI) and Trk receptor activation is mediated via phospholipase C signalling pathways. EMBO J 20:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carter B, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm‐Matthaei R, Baeuerle P, Barde Y‐A (1996) Selective activation of NF‐kB by nerve growth factor through the neurotrophin receptor p75. Science 272:542–545. [DOI] [PubMed] [Google Scholar]
- 31. Casaccia‐Bonnefil P, Carter BD, Dobrowsky RT, Chao MV (1996) Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature 383:716–719. [DOI] [PubMed] [Google Scholar]
- 32. Casademunt E, Carter BD, Benzel I, Frade JM, Dechant G, Barde YA (1999) The zinc finger protein NRIF interacts with the neurotrophin receptor p75(NTR) and participates in programmed cell death. EMBO J 18:6050–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang JH, Mellon E, Schanen NC, Twiss JL (2003) Persistent TrkA activity is necessary to maintain transcription in neuronally differentiated PC12 cells. J Biol Chem 278:42877–42885. [DOI] [PubMed] [Google Scholar]
- 34. Chang JH, Van Niekerk E, Trepel JH, Schanen NC, Twiss JL (2006) PC12 cells regulate icer expression to differentially control CRE‐dependent transcription in response to NGF and cAMP. J Neurochem (in press). [DOI] [PubMed] [Google Scholar]
- 35. Chang Q, Khare G, Dani V, Nelson S, Jaenisch R (2006) The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 49:341–348. [DOI] [PubMed] [Google Scholar]
- 36. Chao M (1994) The p75 neurotrophin receptor. J Neurobiol 25:1373–1385. [DOI] [PubMed] [Google Scholar]
- 37. Chao M, Hempstead B (1995) p75 and Trk: a two‐receptor system. Trends Neurosci 18:321–326. [PubMed] [Google Scholar]
- 38. Chao MV, Rajagopal R, Lee FS (2006) Neurotrophin signalling in health and disease. Clin Sci (Lond) 110:167–173. [DOI] [PubMed] [Google Scholar]
- 39. Chen K, Nishimura M, Armanini M, Crowley C, Spencer S, Phillips H (1997) Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J Neurosci 17:7288–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME (2003) Depression of BDNF transcription involves calcium‐dependent phosphorylation of MeCP2. Science 302:885–889. [DOI] [PubMed] [Google Scholar]
- 41. Cohen S, Levi‐Montalcini R (1957) Purification and properties of a nerve growth‐promoting factor isolated from mouse sarcoma 180. Cancer Res 17:15–20. [PubMed] [Google Scholar]
- 42. Conover JC, Yancopoulos GD (1997) Neurotrophin regulation of the developing nervous system: analyses of knockout mice. Rev Neurosci 8:13–27. [DOI] [PubMed] [Google Scholar]
- 43. Crowley C, Spencer S, Nishimura M, Chen K, Pitts‐Meek S, Armanini M, Ling L, McMahon S, Shelton D, Levinson A, Phillips H (1994) Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76:1001–1011. [DOI] [PubMed] [Google Scholar]
- 44. Diefenbach TJ, Guthrie PB, Kater SB (2000) Stimulus history alters behavioral responses of neuronal growth cones. J Neurosci 20:1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ding Q, Markesbery WR, Chen Q, Li F, Keller JN (2005) Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci 25:9171–9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT (2005) MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron 46:849–855. [DOI] [PubMed] [Google Scholar]
- 47. Dorsey SG, Renn CL, Carim‐Todd L, Barrick CA, Bambrick L, Krueger BK, Ward CW, Tessarollo L (2006) In vivo restoration of physiological levels of truncated TrkB.T1 receptor rescues neuronal cell death in a trisomic mouse model. Neuron 51:21–28. [DOI] [PubMed] [Google Scholar]
- 48. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity‐dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269. [DOI] [PubMed] [Google Scholar]
- 49. Ernfors P, Ibanez CF, Ebendal T, Olson L, Persson H (1990) Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci USA 87:5454–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ernfors P, Lee KF, Kucera J, Jaenisch R (1994) Lack of neurotrophin‐3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77:503–512. [DOI] [PubMed] [Google Scholar]
- 51. Fahnestock M, Michalski B, Xu B, Coughlin MD (2001) The precursor pro‐nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci 18:210–220. [DOI] [PubMed] [Google Scholar]
- 52. Farinas I (1999) Neurotrophin actions during the development of the peripheral nervous system. Microsc Res Tech 45:233–242. [DOI] [PubMed] [Google Scholar]
- 53. Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF (1994) Severe sensory and sympathetic deficits in mice lacking neurotrophin‐3. Nature 369:658–661. [DOI] [PubMed] [Google Scholar]
- 54. Fayard B, Loeffler S, Weis J, Vogelin E, Kruttgen A (2005) The secreted brain‐derived neurotrophic factor precursor pro‐BDNF binds to TrkB and p75NTR but not to TrkA or TrkC. J Neurosci Res 80:18–28. [DOI] [PubMed] [Google Scholar]
- 55. Figurov A, Pozzo‐Miller LD, Olafsson P, Wang T, Lu B (1996) Regulation of synaptic responses to high‐frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381:706–709. [DOI] [PubMed] [Google Scholar]
- 56. Filbin MT (2003) Myelin‐associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci 4:703–713. [DOI] [PubMed] [Google Scholar]
- 57. Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH (1987) Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 329:65–68. [DOI] [PubMed] [Google Scholar]
- 58. Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL (2001) Neurotrophin‐4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short‐term satiety. J Neurosci 21:8602–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frade J, Rodriguez‐Tebar A, Barde Y‐A (1996) Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature 383:166–168. [DOI] [PubMed] [Google Scholar]
- 60. Franke TF, Kaplan DR, Cantley LC (1997) PI3K: downstream AKTion blocks apoptosis. Cell 88:435–437. [DOI] [PubMed] [Google Scholar]
- 61. Fukuda T, Itoh M, Ichikawa T, Washiyama K, Goto Y (2005) Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2‐deficient mice. J Neuropathol Exp Neurol 64:537–544. [DOI] [PubMed] [Google Scholar]
- 62. Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H (1993) Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 123:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gallo G, Letourneau P (1998) Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci 18:5403–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gallo G, Letourneau PC (2004) Regulation of growth cone actin filaments by guidance cues. J Neurobiol 58:92–102. [DOI] [PubMed] [Google Scholar]
- 65. Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT (2004) Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo . Neuron 44:609–621. [DOI] [PubMed] [Google Scholar]
- 66. Gingras AC, Raught B, Sonenberg N (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68:913–963. [DOI] [PubMed] [Google Scholar]
- 67. Glebova NO, Ginty DD (2005) Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28:191–222. [DOI] [PubMed] [Google Scholar]
- 68. Grewal SS, York RD, Stork PJ (1999) Extracellular‐signal‐regulated kinase signalling in neurons. Curr Opin Neurobiol 9:544–553. [DOI] [PubMed] [Google Scholar]
- 69. Grimpe B, Pressman Y, Lupa MD, Horn KP, Bunge MB, Silver J (2005) The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol Cell Neurosci 28:18–29. [DOI] [PubMed] [Google Scholar]
- 70. Grossman AW, Aldridge GM, Weiler IJ, Greenough WT (2006) Local protein synthesis and spine morphogenesis: fragile X syndrome and beyond. J Neurosci 26:7151–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gustilo MC, Markowska AL, Breckler SJ, Fleischman CA, Price DL, Koliatsos VE (1999) Evidence that nerve growth factor influences recent memory through structural changes in septohippocampal cholinergic neurons. J Comp Neurol 405:491–507. [DOI] [PubMed] [Google Scholar]
- 72. Hallbook F, Ibanez CF, Persson H (1991) Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron 6:845–858. [DOI] [PubMed] [Google Scholar]
- 73. Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR (2003) Brain‐derived neurotrophic factor val66met polymorphism affects human memory‐related hippocampal activity and predicts memory performance. J Neurosci 23:6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM (2004) Secreted proNGF is a pathophysiological death‐inducing ligand after adult CNS injury. Proc Natl Acad Sci USA 101:6226–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. He X, Yang F, Xie Z, Lu B (2000) Intracellular Ca(2+) and Ca(2+)/calmodulin‐dependent kinase II mediate acute potentiation of neurotransmitter release by neurotrophin‐3. J Cell Biol 149:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hempstead BL (2006) Dissecting the diverse actions of pro‐ and mature neurotrophins. Curr Alzheimer Res 3:19–24. [DOI] [PubMed] [Google Scholar]
- 77. Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H (1987) Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci USA 84:8735–8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Heymach JV Jr, Kruttgen A, Suter U, Shooter EM (1996) The regulated secretion and vectorial targeting of neurotrophins in neuroendocrine and epithelial cells. J Biol Chem 271:25430–25437. [DOI] [PubMed] [Google Scholar]
- 79. Hohn A, Leibrock J, Bailey K, Barde YA (1990) Identification and characterization of a novel member of the nerve growth factor/brain‐derived neurotrophic factor family. Nature 344:339–341. [DOI] [PubMed] [Google Scholar]
- 80. Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM, Hattier T, Harris PL, Siedlak SL, Fujioka H, Liu Q, Moreira PI, Miller FP, Nunomura A, Shimohama S, Perry G (2005) Ribosomal RNA in Alzheimer disease is oxidized by bound redox‐active iron. J Biol Chem 280:20978–20986. [DOI] [PubMed] [Google Scholar]
- 81. Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Indo Y (2002) Genetics of congenital insensitivity to pain with anhidrosis (CIPA) or hereditary sensory and autonomic neuropathy type IV. Clinical, biological and molecular aspects of mutations in TRKA(NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Clin Auton Res 12(Suppl.1):I20–I32. [DOI] [PubMed] [Google Scholar]
- 83. Jay P, Rougeulle C, Massacrier A, Moncla A, Mattei MG, Malzac P, Roeckel N, Taviaux S, Lefranc JL, Cau P, Berta P, Lalande M, Muscatelli F (1997) The human necdin gene, NDN, is maternally imprinted and located in the Prader‐Willi syndrome chromosomal region. Nat Genet 17:357–361. [DOI] [PubMed] [Google Scholar]
- 84. Je HS, Zhou J, Yang F, Lu B (2005) Distinct mechanisms for neurotrophin‐3‐induced acute and long‐term synaptic potentiation. J Neurosci 25:11719–11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ji Y, Pang PT, Feng L, Lu B (2005) Cyclic AMP controls BDNF‐induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci 8:164–172. [DOI] [PubMed] [Google Scholar]
- 86. Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M (1986) Expression and structure of the human NGF receptor. Cell 47:545–554. [DOI] [PubMed] [Google Scholar]
- 87. Jones KR, Reichardt LF (1990) Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci USA 87:8060–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kaisho Y, Yoshimura K, Nakahama K (1990) Cloning and expression of a cDNA encoding a novel human neurotrophic factor. FEBS Lett 266:187–191. [DOI] [PubMed] [Google Scholar]
- 89. Kang H, Schuman EM (1995) Long‐lasting neurotrophin‐induced enhancement of synaptic transmission in the adult hippocampus. Science 267:1658–1662. [DOI] [PubMed] [Google Scholar]
- 90. Kang H, Schuman EM (1995) Neurotrophin‐induced modulation of synaptic transmission in the adult hippocampus. J Physiol Paris 89:11–22. [DOI] [PubMed] [Google Scholar]
- 91. Kang H, Schuman EM (1996) A requirement of local protein synthesis in neurotrophin‐induced hippocampal synaptic plasticity. Science 273:1402–1405. [DOI] [PubMed] [Google Scholar]
- 92. Kao S, Jaiswal RK, Kolch W, Landreth GE (2001) Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem 276:18169–18177. [DOI] [PubMed] [Google Scholar]
- 93. Kaplan DR, Miller FD (2000) Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10:381–391. [DOI] [PubMed] [Google Scholar]
- 94. Kaplan DR, Hempstead BL, Martin‐Zanca D, Chao MV, Parada LF (1991) The trk proto‐oncogene product: a signal transducing receptor for nerve growth factor. Science 252:554–558. [DOI] [PubMed] [Google Scholar]
- 95. Kaufmann WE, MacDonald SM, Altamura CR (2000) Dendritic cytoskeletal protein expression in mental retardation: an immunohistochemical study of the neocortex in Rett syndrome. Cereb Cortex 10:992–1004. [DOI] [PubMed] [Google Scholar]
- 96. Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD (2006) Ligand‐dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron 50:219–232. [DOI] [PubMed] [Google Scholar]
- 97. Kew JN, Smith DW, Sofroniew MV (1996) Nerve growth factor withdrawal induces the apoptotic death of developing septal cholinergic neurons in vitro: protection by cyclic AMP and high potassium. Neuroscience 70:329–339. [DOI] [PubMed] [Google Scholar]
- 98. Khursigara G, Orlinick JR, Chao MV (1999) Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem 274:2597–2600. [DOI] [PubMed] [Google Scholar]
- 99. Klein RL, Hirko AC, Meyers CA, Grimes JR, Muzyczka N, Meyer EM (2000) NGF gene transfer to intrinsic basal forebrain neurons increases cholinergic cell size and protects from age‐related, spatial memory deficits in middle‐aged rats. Brain Res 875:144–151. [DOI] [PubMed] [Google Scholar]
- 100. Knoll JH, Nicholls RD, Magenis RE, Graham JM Jr, Lalande M, Latt SA (1989) Angelman and Prader‐Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet 32:285–290. [DOI] [PubMed] [Google Scholar]
- 101. Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W (1997) BDNF and NT‐4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP‐43 and Talpha1‐tubulin mRNA expression, and promote axonal regeneration. J Neurosci 17:9583–9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T (1995) Hippocampal long‐term potentiation is impaired in mice lacking brain‐derived neurotrophic factor. Proc Natl Acad Sci USA 92:8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Korte M, Kang H, Bonhoeffer T, Schuman E (1998) A role for BDNF in the late‐phase of hippocampal long‐term potentiation. Neuropharmacology 37:553–559. [DOI] [PubMed] [Google Scholar]
- 104. Kruttgen A, Moller JC, Heymach JV Jr, Shooter EM (1998) Neurotrophins induce release of neurotrophins by the regulated secretory pathway. Proc Natl Acad Sci USA 95:9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Krüttgen A, Schneider I, Weis J (2006) The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol 16:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kukowa K‐I, Taniura H, Yoshikawa K (2004) Necdin‐related MAGE proteins differentially interact with E2F1 transcription factor and the p75 neurotrophin receptor. J Biol Chem 279:1703–1712. [DOI] [PubMed] [Google Scholar]
- 107. Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD (2004) A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118:243–255. [DOI] [PubMed] [Google Scholar]
- 108. Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W (2002) Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci USA 99:3246–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ladiwala U, Lachance C, Simoneau SJ, Bhakar A, Barker PA, Antel JP (1998) p75 neurotrophin receptor expression on adult human oligodendrocytes: signaling without cell death in response to NGF. J Neurosci 18:1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294:1945–1948. [DOI] [PubMed] [Google Scholar]
- 111. Lee S, Walker CL, Karten B, Kuny SL, Tennese AA, O’Neill MA, Wevrick R (2005) Essential role for the Prader‐Willi syndrome protein necdin in axonal outgrowth. Hum Mol Genet 14:627–637. [DOI] [PubMed] [Google Scholar]
- 112. Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA (1989) Molecular cloning and expression of brain‐derived neurotrophic factor. Nature 341:149–152. [DOI] [PubMed] [Google Scholar]
- 113. Leinninger GM, Vincent AM, Feldman EL (2004) The role of growth factors in diabetic peripheral neuropathy. J Peripher Nerv Syst 9:26–53. [DOI] [PubMed] [Google Scholar]
- 114. Levi‐Montalcini R (1987) The nerve growth factor 35 years later. Science 237:1154–1162. [DOI] [PubMed] [Google Scholar]
- 115. Levi‐Montalcini R, Hamburger V (1951) Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool 116:321–361. [DOI] [PubMed] [Google Scholar]
- 116. Levi‐Montalcini R, Meyer H, Hamburger V (1954) In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res 14:49–57. [PubMed] [Google Scholar]
- 117. Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19:289–317. [DOI] [PubMed] [Google Scholar]
- 118. Lewis JD, Meehan RR, Henzel WJ, Maurer‐Fogy I, Jeppesen P, Klein F, Bird A (1992) Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69:905–914. [DOI] [PubMed] [Google Scholar]
- 119. Lim KC, Lim ST, Federoff HJ (2003) Neurotrophin secretory pathways and synaptic plasticity. Neurobiol Aging 24:1135–1145. [DOI] [PubMed] [Google Scholar]
- 120. Liu X, Ernfors P, Wu H, Jaenisch R (1995) Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature 375:238–241. [DOI] [PubMed] [Google Scholar]
- 121. Loeb DM, Stephens RM, Copeland T, Kaplan DR, Greene LA (1994) A Trk nerve growth factor (NGF) receptor point mutation affecting interaction with phospholipase C‐gamma 1 abolishes NGF‐promoted peripherin induction but not neurite outgrowth. J Biol Chem 269:8901–8910. [PubMed] [Google Scholar]
- 122. Lohof A, Ip N, Poo M (1993) Potentiation of developing neuromuscular synapses by the neurotrophins NT‐3 and BDNF. Nature 363:350–353. [DOI] [PubMed] [Google Scholar]
- 123. Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP (2005) Sorting and activity‐dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase e. Neuron 45:245–255. [DOI] [PubMed] [Google Scholar]
- 124. Lu B (2003) BDNF and activity‐dependent synaptic modulation. Learn Mem 10:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. MacDonald HR, Wevrick R (1997) The necdin gene is deleted in Prader‐Willi syndrome and is imprinted in human and mouse. Hum Mol Genet 6:1873–1878. [DOI] [PubMed] [Google Scholar]
- 126. Mahadeo D, Kaplan L, Chao MV, Hempstead BL (1994) High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi‐subunit polypeptide receptors. J Biol Chem 269:6884–6891. [PubMed] [Google Scholar]
- 127. Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD (1990) Neurotrophin‐3: a neurotrophic factor related to NGF and BDNF. Science 247:1446–1451. [DOI] [PubMed] [Google Scholar]
- 128. Majdan M, Lachance C, Gloster A, Aloyz R, Zeindler C, Bamji S, Bhakar A, Belliveau D, Fawcett J, Miller FD, Barker PA (1997) Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis. J Neurosci 17:6988–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Martin‐Zanca D, Hughes SH, Barbacid M (1986) A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 319:743–748. [DOI] [PubMed] [Google Scholar]
- 130. Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE (2003) DNA methylation‐related chromatin remodeling in activity‐dependent BDNF gene regulation. Science 302:890–893. [DOI] [PubMed] [Google Scholar]
- 131. Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H (1992) Enhanced synthesis of brain‐derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol 119:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB (2004) LINGO‐1 is a component of the Nogo‐66 receptor/p75 signaling complex. Nat Neurosci 7:221–228. [DOI] [PubMed] [Google Scholar]
- 133. Mimura F, Yamagishi S, Arimura N, Fujitani M, Kubo T, Kaibuchi K, Yamashita T (2006) Myelin‐associated glycoprotein inhibits microtubule assembly by a Rho‐kinase‐dependent mechanism. J Biol Chem 281:15970–15979. [DOI] [PubMed] [Google Scholar]
- 134. Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM (2002) Adaptation in the chemotactic guidance of nerve growth cones. Nature 417:411–418. [DOI] [PubMed] [Google Scholar]
- 135. Mischel PS, Smith SG, Vining ER, Valletta JS, Mobley WC, Reichardt LF (2001) The extracellular domain of p75NTR is necessary to inhibit neurotrophin‐3 signaling through TrkA. J Biol Chem 276:11294–11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mobley WC, Rutkowski JL, Tennekoon GI, Gemski J, Buchanan K, Johnston MV (1986) Nerve growth factor increases choline acetyltransferase activity in developing basal forebrain neurons. Brain Res 387:53–62. [DOI] [PubMed] [Google Scholar]
- 137. Molteni R, Zheng JQ, Ying Z, Gomez‐Pinilla F, Twiss JL (2004) Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci USA 101:8473–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Nan X, Campoy FJ, Bird A (1997) MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88:471–481. [DOI] [PubMed] [Google Scholar]
- 139. Nilsson AS, Fainzilber M, Falck P, Ibanez CF (1998) Neurotrophin‐7: a novel member of the neurotrophin family from the zebrafish. FEBS Lett 424:285–290. [DOI] [PubMed] [Google Scholar]
- 140. Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM (2004) Sortilin is essential for proNGF‐induced neuronal cell death. Nature 427:843–848. [DOI] [PubMed] [Google Scholar]
- 141. Ohmichi M, Decker SJ, Pang L, Saltiel AR (1991) Nerve growth factor binds to the 140 kd trk proto‐oncogene product and stimulates its association with the src homology domain of phospholipase C gamma 1. Biochem Biophys Res Commun 179:217–223. [DOI] [PubMed] [Google Scholar]
- 142. Pagliardina S, Ren J, Wevrick R, Greer JJ (2005) Developmental abnormalities of neuronal structure and functional in prenatal mice lacking the Prader‐Willi syndrome gene necdin. Am J Pathol 167:175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S, Teng KK, Yung WH, Hempstead BL, Lu B (2004) Cleavage of proBDNF by tPA/plasmin is essential for long‐term hippocampal plasticity. Science 306:487–491. [DOI] [PubMed] [Google Scholar]
- 144. Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD (2003) Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron 38:403–416. [DOI] [PubMed] [Google Scholar]
- 145. Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M (1992) Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT‐3 mRNAs. Neuron 9:1081–1088. [DOI] [PubMed] [Google Scholar]
- 146. Peng S, Wuu J, Mufson EJ, Fahnestock M (2004) Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol 63:641–649. [DOI] [PubMed] [Google Scholar]
- 147. Perlson E, Hanz S, Ben‐Yaakov K, Segal‐Ruder Y, Segar R, Fainzilber M (2005) Vimentin‐dependent spatial translocation of an activated MAP kinse in injured nerve. Neuron 45:715–726. [DOI] [PubMed] [Google Scholar]
- 148. Pfeiffer BE, Huber KM (2006) Current advances in local protein synthesis and synaptic plasticity. J Neurosci 26:7147–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Piper M, Salih S, Weinl C, Holt CE, Harris WA (2005) Endocytosis dependent desensitization and protein synthesis‐dependent resensitization in retional growth cone adaptation. Nat Neurosci 8:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE (1993) Induction of apoptosis by the low‐affinity NGF receptor. Science 261:345–348. [DOI] [PubMed] [Google Scholar]
- 151. Radeke M, Misko T, Hsu C, Herzenberg L, Shooter E (1987) Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature 325:593–597. [DOI] [PubMed] [Google Scholar]
- 152. Raymond GV, Bauman ML, Kemper TL (1996) Hippocampus in autism: a Golgi analysis. Acta Neuropathol (Berl) 91:117–119. [DOI] [PubMed] [Google Scholar]
- 153. Reichardt LF (2006) Neurotrophin‐regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361:1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Roosen A, Schober A, Strelau J, Bottner M, Faulhaber J, Bendner G, McIlwrath SL, Seller H, Ehmke H, Lewin GR, Unsicker K (2001) Lack of neurotrophin‐4 causes selective structural and chemical deficits in sympathetic ganglia and their preganglionic innervation. J Neurosci 21:3073–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rosenberg SS, Ng BK, Chen J (2006) The quest for remyelination: a new role for neurotrophins and their receptors. Brain Pathol 16:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rosenthal A, Goeddel DV, Nguyen T, Lewis M, Shih A, Laramee GR, Nikolics K, Winslow JW (1990) Primary structure and biological activity of a novel human neurotrophic factor. Neuron 4:767–773. [DOI] [PubMed] [Google Scholar]
- 157. Rovelli G, Heller RA, Canossa M, Shooter EM (1993) Chimeric tumor necrosis factor‐TrkA receptors reveal that ligand‐dependent activation of the TrkA tyrosine kinase is sufficient for differentiation and survival of PC12 cells. Proc Natl Acad Sci USA 90:8717–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ruggero D, Sonenberg N (2005) The Akt of translational control. Oncogene 24:7426–7434. [DOI] [PubMed] [Google Scholar]
- 159. Sahenk Z (2006) Neurotrophins and peripheral neuropathies. Brain Pathol 16:311–319 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto‐Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC (2006) Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51:29–42. [DOI] [PubMed] [Google Scholar]
- 161. Salehi AH, Roux PP, Kubu CJ, Zeindler C, Bhakar A, Tannis L‐L, Verdi JM, Barker PA (2000) NRAGE, a novel MAGE protein, interacts with the p75 neurotrophin receptor and facilitates nerve growth factor‐dependent apopotosis. Neuron 27:279–288. [DOI] [PubMed] [Google Scholar]
- 162. Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME (2004) BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin‐phosphatidylinositol 3‐kinase‐dependent pathway during neuronal development. J Neurosci 24:7366–7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Schuman EM, Dynes JL, Steward O (2006) Synaptic regulation of translation of dendritic mRNAs. J Neurosci 26:7143–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Sebert ME, Shooter EM (1993) Expression of mRNA for neurotrophic factors and their receptors in the rat dorsal root ganglion and sciatic nerve following nerve injury. J Neurosci Res 36:357–367. [DOI] [PubMed] [Google Scholar]
- 165. Shen YJ, DeBellard ME, Salzer JL, Roder J, Filbin MT (1998) Myelin‐associated glycoprotein in myelin and expressed by Schwann cells inhibits axonal regeneration and branching. Mol Cell Neurosci 12:79–91. [DOI] [PubMed] [Google Scholar]
- 166. Smith DJ, Leil TA, Liu X (2003) Neurotrophin‐4 is required for tolerance to morphine in the mouse. Neurosci Lett 340:103–106. [DOI] [PubMed] [Google Scholar]
- 167. Snider WD (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77:627–638. [DOI] [PubMed] [Google Scholar]
- 168. Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24:1217–1281. [DOI] [PubMed] [Google Scholar]
- 169. Song HJ, Poo MM (1999) Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol 9:355–363. [DOI] [PubMed] [Google Scholar]
- 170. Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR (1994) Trk receptors use redundant signal transduction pathways involving SHC and PLC‐gamma 1 to mediate NGF responses. Neuron 12:691–705. [DOI] [PubMed] [Google Scholar]
- 171. Stoop R, Poo MM (1996) Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain‐derived neurotrophic factor and ciliary neurotrophic factor. J Neurosci 16:3256–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sutcliffe JS, Han M, Christian SL, Ledbetter DH (1997) Neuronally‐expressed necdin gene: an imprinted candidate gene in Prader‐Willi syndrome [letter]. Lancet 350:1520–1521. [DOI] [PubMed] [Google Scholar]
- 173. Svendsen CN, Kew JN, Staley K, Sofroniew MV (1994) Death of developing septal cholinergic neurons following NGF withdrawal in vitro: protection by protein synthesis inhibition. J Neurosci 14:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tcherpakaov M, Bronfman FC, Conticello SG, Vaskovsky A, Levy Z, Niinobe M, Yoshikawa K, Arenas E, Fainzilber M (2002) The p75 neurotrophin receptor interacts with multiple MAGE proteins. J Biol Chem 277:49101–49104. [DOI] [PubMed] [Google Scholar]
- 175. Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL (2005) ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci 25:5455–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Thomas K, Davies A (2005) Neurotrophins: a ticket to ride for BDNF. Curr Biol 15:R262–R264. [DOI] [PubMed] [Google Scholar]
- 177. Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, Singer RH, Bassell GJ (2003) Activity‐dependent trafficking and dynamic localization of zipcode binding protein 1 and beta‐actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci 23:3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Tomlinson DR, Fernyhough P, Diemel LT (1996) Neurotrophins and peripheral neuropathy. Philos Trans R Soc Lond B Biol Sci 351:455–462. [DOI] [PubMed] [Google Scholar]
- 179. Twiss JL, Van Minnen J (2006) New insights into neuronal regeneration: the role of axonal protein synthesis in pathfinding and axonal extension. J Neurotrauma 23:295–308. [DOI] [PubMed] [Google Scholar]
- 180. Twiss JL, Wada HG, Fok KS, Chan SDH, Verity AN, Baxter GT, Shooter EM, Sussman HH (1998) Duration and magnitude of nerve growth factor signaling depend on the ratio of p75LNTR to TrkA. J Neurosci Res 51:442–453. [DOI] [PubMed] [Google Scholar]
- 181. Verdi J, Birren S, Inanez C, Persson H, Kaplan D, Benedetti M, Chao M, Anderson D (1994) p75LNGFR regulates Trk signal transduction and NGF‐induced neuronal differentiation in MAH cells. Neuron 12:733–745. [DOI] [PubMed] [Google Scholar]
- 182. Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ (2006) Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci 26:7756–7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wang T, Xie K, Lu B (1995) Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci 15:4796–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wells DG (2006) RNA‐binding proteins: a lesson in repression. J Neurosci 26:7135–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wetmore C, Olson L, Bean AJ (1994) Regulation of brain‐derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non‐NMDA type glutamate receptors. J Neurosci 14:1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Willis DE, Li KW, Zheng J‐Q, Smit AB, Kelly TK, Merianda TT, Sylvester J, Van Minnen J, Twiss JL (2005) Differential transport and local translation of cytoskeletal, injury‐response, and neurodegeneration protein mRNAs in axons. J Neurosci 25:778–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B (2005) Activation of p75NTR by proBDNF facilitates hippocampal long‐term depression. Nat Neurosci 8:1069–1077. [DOI] [PubMed] [Google Scholar]
- 188. Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR (2005) Local translation of RhoA regulates growth cone collapse. Nature 436:1020–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wu YJ, Kruttgen A, Moller JC, Shine D, Chan JR, Shooter EM, Cosgaya JM (2004) Nerve growth factor, brain‐derived neurotrophic factor, and neurotrophin‐3 are sorted to dense‐core vesicles and released via the regulated pathway in primary rat cortical neurons. J Neurosci Res 75:825–834. [DOI] [PubMed] [Google Scholar]
- 190. Xie K, Wang T, Olafsson P, Mizuno K, Lu B (1997) Activity‐dependent expression of NT‐3 in muscle cells in culture: implications in the development of neuromuscular junctions. J Neurosci 17:2947–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T (1996) Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT‐3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem Res 21:929–938. [DOI] [PubMed] [Google Scholar]
- 192. Yamashita T, Tohyama M (2003) The p75 receptor acts as a displacement factor that releases Rho from Rho‐GDI. Nat Neurosci 6:461–467. [DOI] [PubMed] [Google Scholar]
- 193. Yamashita T, Tucker KL, Barde YA (1999) Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 24:585–593. [DOI] [PubMed] [Google Scholar]
- 194. Yang F, He XP, Russell J, Lu B (2003) Ca2+ influx‐independent synaptic potentiation mediated by mitochondrial Na(+)‐Ca2+ exchanger and protein kinase C. J Cell Biol 163:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Yin Y, Edelman GM, Vanderklish PW (2002) The brain‐derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA 99:2368–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Yoon SO, Casaccia‐Bonnefil P, Carter B, Chao MV (1998) Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci 18:3273–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ (1998) Rap1 mediates sustained MAP kinase activation induced by nerve growth factor [see comments]. Nature 392:622–626. [DOI] [PubMed] [Google Scholar]
- 198. Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M (2005) The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci 25:9989–9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zampieri N, Chao MV (2006) Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans 34:607–611. [DOI] [PubMed] [Google Scholar]
- 200. Zhang X, Poo MM (2002) Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron 36:675–688. [DOI] [PubMed] [Google Scholar]
- 201. Zweifel LS, Kuruvilla R, Ginty DD (2005) Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci 6:615–625. [DOI] [PubMed] [Google Scholar]


