Abstract
Glucocorticoids applied prenatally alter birth weight and the maturation of the lungs. Moreover, glucocorticoids impair neuronal proliferation and differentiation in the hippocampal dentate gyrus. In the present study proliferation and neuronal differentiation in the dentate gyrus were studied in newborn common marmoset monkeys which were intrauterinely exposed to the synthetic glucocorticoid dexamethasone (DEX). Pregnant marmoset monkeys received DEX (5 mg/kg body weight) daily either during early (days 42–48) or late (days 90–96) pregnancy. In the hippocampi of newborn monkeys immunohistochemistry was performed with markers of proliferation (Ki‐67), apoptosis (in situ tailing) as well as early and late neuronal differentiation (calretinin and calbindin). Both after early and late intrauterine exposure to DEX, proliferation of dentate gyrus cells was significantly decreased (P < 0.05). The density of apoptotic neurons was not altered by DEX treatment. Quantification of calretinin‐ and calbindin‐immunoreactive neurons showed no significant differences between DEX‐exposed and control animals. In conclusion, the proliferation of putative precursor cells but not the differentiation into mature cells was impaired in the dentate gyrus of newborn marmosets exposed intrauterinely to DEX.
INTRODUCTION
Factors influencing fetal development such as hormones, nutrients and growth factors may also exert long‐lasting effects that persist to adolescence and adulthood 14, 36. In this context glucocorticoid hormones are of special interest because their corresponding receptors are found in many different cell types 3, 38, 45. Experimental studies in rats revealed that fetal exposure to glucocorticoid hormones may result in a disturbed hormone feed‐back mechanism of the hypothalamic‐pituitary‐adrenal (HPA) axis leading to higher endogenous glucocorticoid levels as well as alterations in glucose tolerance and blood pressure 5, 37, 53. Synthetic glucocorticoid hormones such as dexamethasone (DEX) can exert detrimental effects on neuronal tissue including loss of neurons, reduction of brain volume, increased apoptosis, altered neuronal structure and synapse formation 2, 27, 29. In rodents, overexposure to synthetic glucocorticoids can lead to alterations in adult behavior such as impaired coping in adverse situations (63). Studies of maternal or postnatal administration of DEX revealed a reduced body and brain weight (4), and alterations in the expression of transcription factors (57) and monoamine metabolism (43) in the brain. In accordance to these observations in rodent models, children at school age who had received early postnatal DEX for the prevention of chronic lung disease of prematurity suffered from impaired motor and cognitive function (69).
A brain area known to be very sensitive to circulating glucocorticoids is the hippocampal formation because of its high density of glucocorticoid receptors (GR). High abundance of these receptors was shown in rats 1, 41, 42, mice (58) and in non‐human primates. Within the hippocampal formation of common marmoset monkeys, in situ hybridization of GR mRNA revealed high expression levels in the granule cell layer of the dentate gyrus (47). In contrast, in the rhesus monkey the GR is only weakly expressed in the dentate gyrus but more abundant in the hypothalamus, cerebellum and cerebral cortex (51). In humans, GR are highly expressed in the dentate gyrus, CA3 and CA4 (54).
In postnatal neurogenesis, precursor cells proliferate within the subgranular layer of the dentate gyrus. Many of these cells die shortly after division but a significant subpopulation of these cells survive and partly differentiate into neurons (31). These neurons are believed to be integrated into the neuronal network by extending their dendrites to mature pyramidal neurons in CA3 through the mossy fiber pathway 28, 40. In the adult brain, proliferation of neuronal precursor cells is increased by various stimuli such as physical exercise (46), enriched environment 9, 33 or antidepressant drugs (67). Glucocorticoids (70) as well as stress (24) decrease neuronal cell proliferation probably through an N‐methyl‐D‐aspartate (NMDA)‐receptor‐mediated mechanism 10, 11, 22. Conversely, mineralocorticoids stimulate neuronal proliferation within the hippocampal granule cell layer (17).
Synthetic steroid hormones such as DEX, prednisolone or prednisone are widely used for the treatment of optic neuritis and multiple sclerosis during pregnancy and for the prevention of the neonatal respiratory distress syndrome prenatally or immediately postnatally 16, 25, 26, 39. To our knowledge there are no consistent experimental studies concerning the effects of prenatal glucocorticoid exposure on neuronal development in newborn primates. Most of the studies investigating the influence of prenatal glucocorticoids were performed with rodents, mainly rats 2, 3, 36, 63. Because of the physiological differences between rodents and humans these results can only partly be transferred to man. The continuous use of glucocorticoids during pregnancy in clinical medicine illustrates the necessity of a study in primates on the impact of glucocorticoids on neuronal proliferation and death in the hippocampal formation. Therefore, the present study was carried out with the common marmoset monkey. In comparison with old world monkeys common marmoset monkeys carry twins and a high percentage of triplets which cannot be brought up by their mothers. Moreover, compared with the gestation and pregnancy in old world monkeys such as rhesus and baboon, in new world monkeys such as common marmosets the speed of development during organogenesis is almost identical with that in man (44). DEX as a specific GR agonist, instead of the non‐selective compounds prednisolone or prednisone was used in order to study the stimulation of the GR only. To be able to distinguish whether there are divergent effects of DEX during different stages of pregnancy, female marmosets were exposed to DEX either in the phase of organogenesis or at a time point where organogenesis was already terminated. As organogenesis in the common marmoset monkey approximately occurs from day 46 to day 61 of gestation (44), time points of DEX exposure were set during organogenesis from day 42 to day 46 and later during pregnancy from day 90 to day 96.
MATERIAL AND METHODS
Animals and experimental groups. Adult common marmoset monkeys (Callithrix jacchus) were used for this experiment. The animals were obtained from the breeding colony at the German Primate Center in Göttingen, Germany, and housed in pairs on a regular day/night cycle (lights on from 07:00 h to 19:00 h) at 26°C, 55% relative humidity, with free access to food and water. Animal experiments were conducted in accordance with the European Communities Council Directive of November 24, 1986 (86/EEC), and were approved by the Government of Lower Saxony, Germany. We used the minimum number of animals required to obtain consistent data. This study was performed within the EC‐funded project EUPEAH.
Pregnant marmosets were treated orally with 5 mg DEX/kg body weight daily either during early pregnancy from day 42 to day 48 or during late pregnancy from day 90 to day 96 post conception, respectively. The average expected full‐term gestation for a marmoset is 145 days. For this purpose DEX tablets (Dexamethasone 0.5, 1.5 or 4 mg Jenapharm®, Jena, Germany) were split and dosed according to the body weight of the individual animal. Each dose was dissolved in 0.4 mL of tap water and mixed with 1.6 mL Nutri‐Cal (Albrecht, Aulendorf, Germany) in a syringe. This mixture was taken voluntarily, control animals received vehicle only either during early or late pregnancy.
While common marmoset monkey pairs naturally can bring up only two offspring, more than 50% of the breeding females deliver more than two newborns in captivity. Natural rearing of triplet litters by the parents is generally not possible and without intervention the weakest young will die some days after birth (65). In most breeding facilities surplus newborn marmoset monkeys are killed for reasons of animal protection because hand‐reared animals often are not normally socialized and suffer from metabolic diseases in adulthood as it has been demonstrated for other species (62). Therefore, in triplet litters one animal was killed within 2 days after birth. Only such third offspring were included in the study and each animal was from a different mother. The early DEX‐exposed group comprised nine females and two males (n = 11); the late DEX‐exposed group comprised six females and one male (n = 7) and the control group consisted of eight females and one male (n = 9) (five animals received vehicle from day 42 to day 48, four received vehicle from day 90 to day 96). These animals were killed in general anesthesia with an overdose of xylazine (Rompun®, Bayer, Leverkusen, Germany) and ketamine (Ketavet®, Pharmacia & Upjohn, Erlangen, Germany) after birth. The brain was removed and the dorsal part of the left hemisphere was immersion fixed in 4% paraformaldehyde.
Immunohistochemistry. Deparaffinized and hydrated brain tissue was cut into 1‐µm‐thick sections. Sections were then pretreated with microwaving for 3 × 5 minutes in citric acid buffer, 10 mmol/L, pH 6.0. After blocking with 10% fetal calf serum/phosphate‐buffered saline (FCS/PBS) for 30 minutes, primary antibodies were applied at the concentrations indicated and allowed to bind overnight at 4°C (calretinin) or for 90 minutes at room temperature (Ki‐67 and calbindin).
Ki‐67 was detected by binding of mouse monoclonal anti‐Ki‐67 (1:100, Dako, Kopenhagen, Denmark). Calretinin and calbindin were visualized by binding of rabbit polyclonal anti‐calretinin (1:200, Swant, Bellinzona, Switzerland) and mouse monoclonal anti‐calbindin‐D‐28k (1:150, Sigma‐Aldrich, St. Louis, MO, USA) antibodies. For light microscopy, sections were incubated with appropriate biotinylated secondary antibodies (Amersham, Buckinghamshire, UK) followed by treatment with avidinperoxidase (Sigma‐Aldrich, St. Louis, MO, USA). DAB was used as the chromogenic substrate (Roche, Mannheim, Germany) for Ki‐67. For calretinin and calbindin the color reaction was developed with Newfuchsin (Dako, Kopenhagen, Denmark). All slices were counterstained with hemalum (Merck, Darmstadt, Germany). Control sections were incubated with isotype control antibodies or without primary antibody.
In situ tailing. Deparaffinized 1‐µm sections were incubated with 100 µg/mL proteinase K (Sigma‐Aldrich, St. Louis, MO, USA) for 15 minutes at 37°C. Then they were treated for 1 h at 37°C with a mixture of 5 × tailing buffer, 1‐µL digoxigenin DNA labeling mix, 2‐µL cobalt chloride, 12.5 U terminal transferase and distilled water to a final volume of 50 µL. Sections were incubated with 10% FCS for 20 minutes and treated with anti‐digoxigenin antibody (1:250, Roche, Mannheim, Germany) for 90 minutes at room temperature. The color reaction was developed with 4‐nitroblue‐tetrazolium‐chloride/5‐bromine‐4‐chloride‐3‐indolyl‐phosphate (NBT/BCIP), and sections were counterstained with red‐aluminum hydroxide (Roche, Mannheim, Germany) 19, 21, 71.
Double‐labeling of Ki‐67 and glial fibrillary acidic protein (GFAP). Double‐labeling with Ki‐67 and the specific glial marker GFAP was performed to exclude that the proliferation observed was represented by cells of glial origin only. For fluorescence double‐labeling, sections were incubated for 90 minutes at room temperature with rabbit anti‐GFAP (Dako, Kopenhagen, Denmark) followed by 1:200 biotinylated secondary antibody and for 1 h with 1:200 Cy‐2‐streptavidin (Jackson Immunoresearch, West Grove, PA, USA) and then incubated for 90 minutes with mouse monoclonal anti‐Ki‐67. Finally, slices were incubated with 1:200 goat‐anti‐mouse Cy3 (Jackson Immunoresearch, West Grove, PA, USA) for 1 h.
Thickness of the dentate granule cell layer. The thickness of the dentate granule cell layer was evaluated with a 10× objective and an imaging system (BX51; Olympus, Hamburg, Germany; software AnalySIS® 3.2; Soft Imaging System GmbH, Münster, Germany). Two straight lines along the borders of the upper and the lower blade were drawn at the curvature of the dentate gyrus, and 300 µm from the intersection of the straight lines the thickness of the upper and lower blade of the dentate gyrus was measured (Figure 1).
Figure 1.
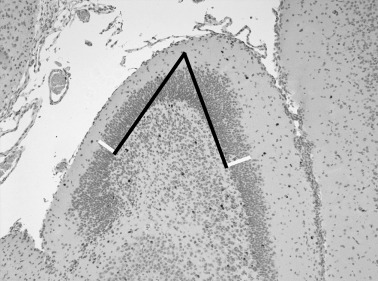
Determination of the thickness of the granule cell layer of the dentate gyrus: two straight lines (black) were drawn at the curvature of the dentate gyrus along the borders of the upper and lower blade of the granule cell layer. Three hundred micrometers from the intersection of the straight lines in a 90° angle the thickness of the upper and lower blade was measured (white lines).
Quantification of immunoreactive cells. The marmoset brains were cut in regular intervals of 5 µm in posterior–anterior direction. Quantification of immunoreactive cells was performed in brain sections containing the hippocampal formation and the pulvinar medialis beginning 500 µm behind the first slice with cells of the fascia dentata. The coronal level coordinates used for quantification ranged from anterior–posterior 4.5 to 6.5 mm, the whole dentate gyrus extending from anterior–posterior 0.5 to 7.5 mm.
All slices were randomized and evaluated in a blinded fashion. The sections were examined using a 40× objective and all immunoreactive cells in the region of interest were quantified. Furthermore, the total number of neurons in the dentate gyrus was counted. Only cells within the granule cell layer and on the border of the subgranular zone were considered for analysis. The area of the granule cell layer in the dentate gyrus was measured by using an imaging system (BX51; software AnalySIS® 3.2). The density of immunolabeled cells was expressed as the number of marked cells per mm2 of the area measured and per total number of cells in the dentate gyrus. The quotient of total cell number divided by the area measured was also calculated.
The marmoset brains were included in the EUPEAH brain bank; the tissue block containing the dorsal part of the left hippocampal formation was fixed in 4% paraformaldehyde and embedded in paraffin. Therefore only a part of the hippocampal formation was available for this investigation and it was impossible to determine the hippocampal volume. Quantification of cells by means of stereology was limited by the fixation of the tissue with paraformaldehyde. Paraformaldehyde‐fixed and paraffin‐embedded tissue—as used in the present study—cannot be cut into 20–40‐µm‐thick slices necessary for stereology.
Statistical analysis. Values are expressed as mean ± standard deviation. Data were compared by the two‐tailed parametric one‐way analysis of variance (ANOVA) followed by Dunnett’s post hoc test, and P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA, USA).
RESULTS
Birth weights. The birth weights of the early and late DEX‐exposed newborn marmosets were not significantly different compared with control animals (P > 0.05; Table 1). The birth weights of the littermates, which were brought up by their mothers, were not significantly different from marmosets which had to be killed immediately after birth (P > 0.05 surviving vs. sacrificed offspring; Table 1).
Table 1.
Birth weights of controls, early and late dexamethasone‐exposed newborn marmosets in comparison with birth weights of the littermates (mean ± standard deviation; P > 0.05).
| Control | Early treatment | Late treatment | |
|---|---|---|---|
| Newborn | 29.7 ± 3.3 g | 30.6 ± 2.7 g | 29.5 ± 3.2 g |
| Littermates | 30.2 ± 2.6 g | 30.6 ± 2.8 g | 32.2 ± 4.4 g |
Suppression of urinary cortisol‐excretion after DEX exposure. Compared with old world monkeys common marmoset monkeys exhibit up to 25 times higher glucocorticoid concentrations in blood plasma, that is, 2000–3900 ng/mL, with cortisol being the main component 64, 68. Plasma cortisol concentrations in common marmoset monkeys were suppressed to about 250 ng/mL after a single intramuscular injection of 5 mg/kg DEX (50). In the experiments reported here, in the pregnant mothers after normalization for creatinine concentration the same magnitude of suppression of urinary cortisol excretion was observed at the third and the fifth days of DEX treatment.
Thickness of the dentate gyrus. Analysis of thickness of the dentate granule cell layer did not reveal differences between control, early and late DEX‐exposed animals (P > 0.05; Table 2).
Table 2.
Thickness of the dentate granule cell layer of controls, early and late dexamethasone‐exposed newborn marmosets (mean ± standard deviation; P > 0.05).
| Control | Early treatment | Late treatment |
|---|---|---|
| 79.01 ± 15.8 µm | 83.21 ± 6.8 µm | 75.35 ± 7.9 µm |
Decreased proliferation after exposure to DEX. The proliferation of putative neuronal precursor cells in the dentate gyrus was determined by using Ki‐67. This marker labels a specific nuclear antigen which is present in all proliferating cells during cell cycle G1, S, G2 and mitosis but is absent in the G0 phase (20). The density of Ki‐67‐immunoreactive cells showed no significant difference between control animals that received vehicle early or late during pregnancy. Control animals were therefore united to one control group. The density of proliferating cells in the granule cell layer of the dentate gyrus was significantly decreased after early exposure to DEX by 37% relative to untreated control animals (P < 0.05; Table 3). Similarly, after exposure to DEX during late pregnancy the proliferation rate of dentate gyrus cells was significantly decreased in comparison to controls by 44% (P < 0.05; Table 3; 2, 3). Determination of Ki‐67‐immunoreactive cells per total number of neurons of the dentate gyrus confirmed the decreased proliferation rate after exposure to DEX (Table 3).
Table 3.
Number of proliferating cells/mm2 and number of proliferating cells/total number of neurons (Ki‐67‐immunoreactive cells) in the granular cell layer of the dentate gyrus in controls, early and late dexamethasone‐exposed newborn marmosets (mean ± standard deviation; P < 0.05 control vs. early/late treatment*).
| Ki‐67 | Control | Early treatment | Late treatment |
|---|---|---|---|
| /mm2 | 164.1 ± 72.0 | 103.0 ± 56.5* | 91.1 ± 33.1* |
| /total cell number | 0.0137 ± 0.007 | 0.0074 ± 0.004* | 0.0068 ± 0.003* |
Figure 2.
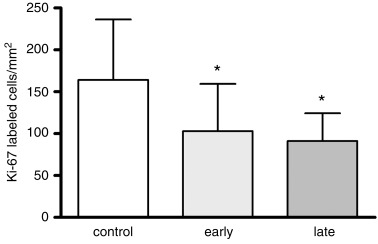
Density of Ki‐67‐immunoreactive cells in the dentate gyrus of untreated and early and late dexamethasone‐exposed newborn marmosets. Cell proliferation was significantly decreased both after early and late intrauterine exposure to dexamethasone. Results are presented as means ± standard deviation; *P < 0.05.
Figure 3.
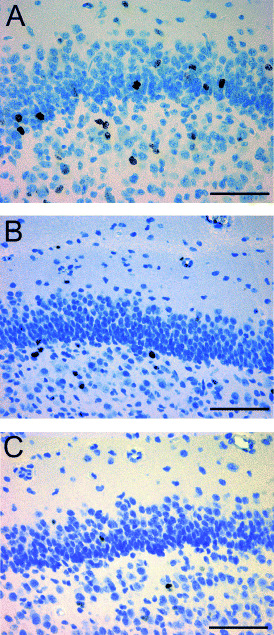
Ki‐67‐immunoreactive cells in a newborn control monkey (A) and marmosets with dexamethasone treatment during early (B) and late pregnancy (C) showing a decreased density of proliferating cells in the dentate gyrus (scale bar = 50 µm).
The quotient of number of neurons per mm2 dentate gyrus showed no difference between all groups. The means ± standard deviations of total number of neurons per mm2 dentate gyrus for controls, early and late treatment were 14 161 ± 7120/mm2 vs. 14 201 ± 3296/mm2 vs. 11 730 ± 4928/mm2 (P > 0.05).
Furthermore, there was no difference in the density of hippocampal pyramidal neurons or any neuronal degeneration observed among the investigated groups in the CA1‐4 region. In particular, in DEX‐exposed animals no morphological abnormalities of pyramidal neurons were observed. Rarely, Ki‐67‐immunoreactive cells were seen in the hippocampal pyramidal layer.
Density of apoptotic neurons. Apoptotic neurons were detected by in‐situ tailing of fragmented DNA and by morphological criteria 19, 21. The density of apoptotic neurons/mm2 and per total number of neurons in the dentate gyrus in early and late DEX‐exposed and control animals revealed no major difference between these groups (P > 0.05; Table 4; Figure 4A,B).
Table 4.
Number of apoptotic cells/mm2 and number of apoptotic cells/total number of neurons (in situ tailing, IST) in the granular cell layer of the dentate gyrus in controls, early and late dexamethasone‐exposed newborn marmosets (mean ± standard deviation; P > 0.05 control vs. early/late treatment).
| IST | Control | Early treatment | Late treatment |
|---|---|---|---|
| /mm2 | 40.5 ± 21.6 | 36.2 ± 19.2 | 30.0 ± 9.2 |
| /total cell number | 0.0022 ± 0.001 | 0.0036 ± 0.002 | 0.0021 ± 0.001 |
Figure 4.
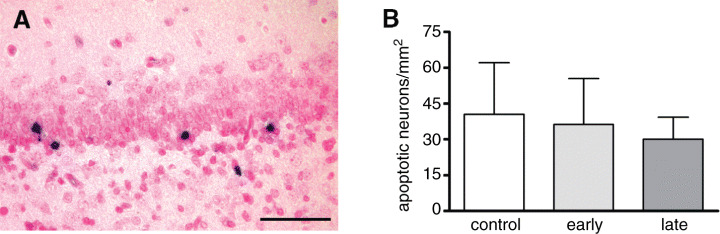
A. Apoptotic neurons in the subgranular cell layer of the dentate gyrus (scale bar = 50 µm). B. Density of apoptotic cells in the dentate gyrus of marmosets in controls and after early and late intrauterine exposure to dexamethasone (mean ± standard deviation; P > 0.05).
Detection of calretinin and calbindin in the dentate gyrus. Calretinin and calbindin are both calcium‐binding proteins and markers for postmitotic granule cells. While calretinin is expressed only transiently in early postmitotic and still immature granule cells, calbindin labels more differentiated granule cells. For how long calbindin is expressed, is not known yet 8, 32. Calretinin‐immunoreactive cells were most abundant in the subgranular layer of the dentate gyrus and to a smaller extent in the area CA1/2 of the hippocampus. The density of cells expressing calretinin in the subgranular layer of the dentate gyrus was not significantly different between control marmosets and DEX‐exposed animals (P > 0.05; Table 5; Figure 5A,B).
Table 5.
Number of calretinin‐ and calbindin‐immunoreactive cells/mm2 and number/total number of neurons in the granular cell layer of the dentate gyrus in controls, early and late dexamethasone‐exposed newborn marmosets (mean ± standard deviation; P > 0.05).
| Control | Early treatment | Late treatment | |
|---|---|---|---|
| Calretinin | |||
| /mm2 | 137.4 ± 75.7 | 133.3 ± 84.4 | 125.3 ± 62.2 |
| /total cell number | 0.05 ± 0.018 | 0.057 ± 0.043 | 0.054 ± 0.043 |
| Calbindin | |||
| /mm2 | 68.2 ± 20.1 | 69.0 ± 26.5 | 50.2 ± 24.8 |
| /total cell number | 0.029 ± 0.01 | 0.024 ± 0.01 | 0.024 ± 0.02 |
Figure 5.
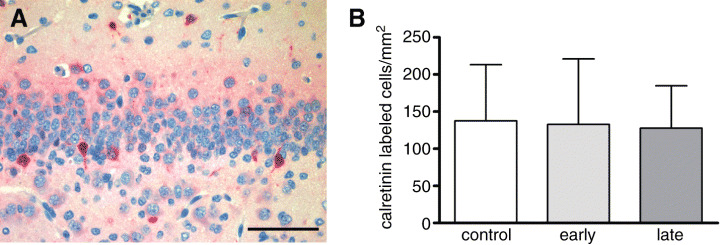
A. Cells expressing the neuronal marker calretinin in the subgranular layer of the dentate gyrus. B. Comparison of calretinin‐immunoreactive cells/mm2 in the dentate gyrus of control marmosets and after early and late exposure to prenatal dexamethasone (means ± standard deviation; P > 0.05).
Calbindin was predominantly seen in the subgranular layer of the dentate gyrus and in the area CA1/2 of the hippocampus. Comparison of the density of calbindin‐immunoreactive neurons in the dentate gyrus of DEX‐exposed and control animals revealed a similar density of cells in all groups investigated (P > 0.05; Table 5; Figure 6A,B).
Figure 6.
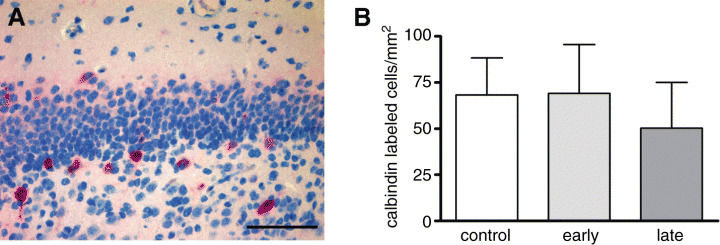
A. Cells expressing the neuronal marker calbindin which represents more differentiated granule cells in the subgranular cell layer of the dentate gyrus (scale bar = 50 µm). B. No significant changes in the density of calbindin‐immunoreactive cells were observed between controls and dexamethasone‐treated animals.
In all three groups investigated, the density of calretinin‐immunoreactive neurons of the dentate gyrus was higher than the density of calbindin‐immunoreactive cells. This is probably due to the newborn state of the brain where a large fraction of neurons may not be finally differentiated.
Double‐labeling of Ki‐67 and GFAP. Double‐labeling of Ki‐67 and GFAP was observed only seldom while the majority of Ki‐67‐immunoreactive cells did not coexpress GFAP (Figure 7A–C).
Figure 7.
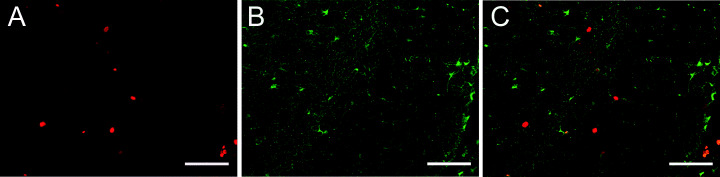
Detection of Ki‐67 (red) (A) and glial fibrillary acidic protein (GFAP) (green) (B) by double‐label fluorescent immunocytochemistry in the dentate gyrus. The merge of both markers is illustrated in C. The majority of Ki‐67‐immunoreactive cells did not colocalize with GFAP, indicating that the newly divided cells in the subgranular layer of the dentate gyrus did not express the glial marker. However, very few double‐labeled cells were detected (scale bar = 50 µm).
DISCUSSION
There is accumulating evidence that excessive fetal exposure to glucocorticoid hormones not only influences prenatal development but can also be the origin of diseases in adulthood such as high blood pressure and deranged glucose tolerance 5, 14, 36, 37, 53. Prenatal glucocorticoids exert their actions during the so‐called phase of “early life programming” where the prenatal environment including hormones, growth factors, nourishment, drugs etc. has effects that may persist throughout life if induced in a vulnerable phase during development or may even cause intergenerational effects (15).
Glucocorticoids can induce neuronal apoptosis 27, 29 and cause alterations in gene expression of transcription factors (57) and protein synthesis in monoamine metabolism of the nervous system (43). Exposure of pregnant rhesus monkeys to adrenocorticotropic hormone (ACTH) for 14 consecutive days during mid‐pregnancy impaired the newborn’s motor coordination (49). As the hippocampal formation is most sensitive to the effects of glucocorticoids and also a region where neurogenesis in adults takes place, research has emphasized the impact of these hormones on neuronal proliferation in the hippocampal formation. The administration of high doses of prenatal DEX to fetal rhesus monkeys (59) or recurrent stressing of the pregnant mother (13) resulted in a modestly decreased hippocampal volume of the neonatal monkey. Similarly, administration of intramuscular DEX to pregnant rhesus monkeys one month before term resulted in a reduced hippocampal volume of the offspring (60).
In our study, prenatal treatment of the common marmoset monkey with 5 mg/kg body weight DEX for 7 days during early and late pregnancy led to a distinct decrease in dentate granule cell proliferation by 37% and 44%, respectively after birth. Interestingly, the degree of the impairment of proliferation was independent of the chosen time point during pregnancy although there are differences in neuronal development at the two selected periods chosen for gestational manipulation. Moreover, DEX exerted a long‐lasting effect that was still detectable many weeks after administration. In this study we measured the direct effect of intrauterine exposure to DEX, because the offspring were killed within 2 days after birth, and the proliferation of dentate gyrus cells was not influenced by effects like postpartal maternal behavior, rearing conditions or carry‐over effects of influencing factors by breast milk. Our observations compare well with work showing a reduced number of BrdU‐positive cells in the dentate gyrus of juvenile rhesus monkeys after prenatal stress during either early or late pregnancy (13).
Although there are studies addressing the effects of synthetic steroids on the hippocampal volume 13, 59, less is known about the impact of prenatal glucocorticoids on neuronal differentiation. In adult neurogenesis, new cells are generated within the subgranular layer of the dentate gyrus. Many of these cells die shortly after division but a part of these cells do survive and differentiate into functionally significant neurons which are believed to be integrated into the neuronal network 7, 31. Which role glucocorticoids play in neuronal differentiation is not completely understood yet. A recent study in rats revealed that corticosterone influenced the survival of precursor cells in the adult hippocampus depending on the pre‐ or postmitotic stage of the cells (66). Dehydroepiandrosterone (DHEA) prevented the inhibitory effects of high doses of glucocorticoids on precursor cell proliferation and on the survival of newly formed cells in the dentate gyrus (30). In a recent study in mice approximately 50% of the dividing precursor cells expressed GR whereas no expression of mineralocorticoid receptors was detected early after division (18). In contrast to these observations in mice, no expression of GR was found in [3H]thymidine‐labeled cells early after division in the dentate gyrus of adult rats (12). In rodents, gene expression levels of steroid hormone receptors change with age. In rat and mouse low levels of GR were detected at birth with an increase during adulthood 48, 52. Contrarily, no major changes in GR mRNA was observed from neonatal development to adulthood in common marmoset monkeys (47). With respect to the expression of GR in precursor cells, the effects of steroid hormones could be either due to direct receptor binding or being mediated indirectly by surrounding cells such as glia. The effect of DEX might in part be induced by alterations in gene expression and protein synthesis of cell cycle mediators, transcription factors, growth factors or neurotrophins. More studies with respect to the underlying mechanisms involved in steroid effects will support our understanding of the complex interactions in the development and maintenance of the cellular homeostasis in the hippocampal formation.
We investigated the impact of prenatal DEX administration on early and late neuronal differentiation by evaluating the expression pattern of calretinin and calbindin in the dentate gyrus. Calretinin is transiently expressed in immature granule cells which represent the early neuronal development. Later during differentiation the expression is switched from calretinin to calbindin, a calcium‐binding protein characterizing mature granule cells (32). In the common marmoset, no difference in the density of calretinin‐ or calbindin‐immunoreactive neurons was observed between DEX‐exposed and control animals. Taken together, the proliferation of putative precursor cells was reduced while the neuronal differentiation as marked by calretinin and calbindin expression was unchanged.
Additionally, the density of neurons labeled by in situ tailing was similar between the investigated groups and showed that DEX did not result in a long‐term increase of neuronal apoptosis in the hippocampal formation. Similar to these results in rats, treatment with DEX intraperitoneally caused a reduced proliferation rate in the hippocampal formation while no change in neuronal apoptosis or differentiation was observed (34). A reduced number of neurons and degeneration of neuronal perikarya and dendrites of granule and pyramidal neurons were detected in the hippocampal formation of fetal rhesus monkeys after intrauterine exposure to a single dose of 0.5–10 mg/kg body weight DEX 3 days before delivery (60). This is in contrast to our findings in newborn marmosets where no degeneration of neurons or dendrites in the hippocampal formation was detected. Similar to our observations, elevated glucocorticoid levels caused by stress did not affect the number of neurons in the tree shrew hippocampus (61).
DEX treatment in pregnancy impaired putative precursor cell proliferation in offspring without change of apoptotic cell death or neuronal differentiation as indicated by expression of calretinin and calbindin. Consequently, a reduction of proliferation should have resulted in a decreased volume of the dentate granule cell layer and the hippocampus, respectively. As only the dorsal part of the left hippocampal formation was available in this study it was impossible to evaluate the overall size of the hippocampus. To get indirect information about the size of the dentate gyrus, the thickness of the dentate granule cell layer was measured in coronal slices showing no significant differences between all three groups. This indirect indicator of hippocampal volume, however, might have missed moderate changes. Furthermore, investigation of the coronal plane cannot detect changes in the anterior–posterior plane of the hippocampal formation. Therefore, immunohistochemical quantification demonstrating an impaired proliferation rate without changes of neuronal differentiation marked by calbindin and calretinin and apoptotic cell death suggests a decrease of the hippocampal volume which was probably not detected by the measurement of the thickness of the dentate granule cell layer in coronal slices.
DEX unfolds a direct effect on proliferation independent of the time period of administration during gestation. Whether these effects are long‐lasting and persist until adolescence and adulthood remains to be further investigated. The fetal development of the hippocampal dentate gyrus of monkeys and humans during pregnancy is rather similar (6). At term, the majority of the granule cells within the dentate gyrus have been formed in humans and monkeys. Nevertheless, the proliferation and differentiation of granule cells continues well into the postnatal period 23, 35, 55, 56. As the human brain reaches its final form after birth, there might be capacity for compensating prenatally acquired deficits. We showed that the effect of intrauterine DEX exposure on the proliferation of putative neuronal precursor cells is still detectable after birth. The question of whether prenatal glucocorticoid exposure leads to effects on neuronal proliferation and differentiation still detectable in adulthood will be answered with the siblings of the common marmoset monkeys studied by us, which will be investigated in a subsequent study 2.5 years after birth within the EUPEAH project.
In conclusion, intrauterine exposure to DEX both during early and late pregnancy led to a decreased density of proliferating dentate gyrus cells. In contrast, no influence on the rate of neuronal apoptosis was observed. Additionally, no major difference in the density of cells expressing early and late neuronal markers was detected. Taken together, these results suggest that the proliferation of dentate granule cells but not the differentiation into dentate gyrus neuronal cells is impaired by intrauterine exposure to DEX in newborn common marmoset monkeys.
ACKNOWLEDGMENTS
This work was supported by the European Commission grant QLRT‐2001‐02758 (EUPEAH) and by the DFG Research Center Molecular Physiology of the Brain (CMPB). The authors thank Stephanie Bunkowski for excellent technical assistance.
REFERENCES
- 1. Ahima R, Harlan RE (1991) Differential corticosteroid regulation of type II glucocorticoid receptor‐like immunoreactivity in the rat central nervous system: topography and implications. Endocrinology 129:226–236. [DOI] [PubMed] [Google Scholar]
- 2. Antonow‐Schlorke I, Schwab M, Li C, Nathanielsz PW (2003) Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol 547:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnes PJ (2004) Distribution of receptor targets in the lung. Proc Am Thorac Soc 1:345–351. [DOI] [PubMed] [Google Scholar]
- 4. Baud O, Verney C, Evrard P, Gressens P (2005) Injectable dexamethasone administration enhances cortical GABAergic neuronal differentiation in a novel model of postnatal steroid therapy in mice. Pediatr Res 57:149–56. [DOI] [PubMed] [Google Scholar]
- 5. Benediktsson R, Lindsay R, Noble J, Seckl JR, Edwards CR (1993) Glucocorticoid exposure in utero: a new model for adult hypertension. Lancet 341:339–341. [DOI] [PubMed] [Google Scholar]
- 6. Berger B, Alvarez C, Goldmann‐Rakic PS (1993) Neurochemical development of the hippocampal region in the fetal rhesus monkey. I. Early appearance of pepties, calcium‐binding protines, DARPP‐32, and monoamine innervation in the entorhinal cortex during the first half of gestation (E47‐90). Hippocampus 3:279–305. [DOI] [PubMed] [Google Scholar]
- 7. Biebl M, Cooper CM, Winkler J, Kuhn HG (2000) Analysis of neurogenesis and programmed cell death reveals a self‐renewing capacity in the adult rat brain. Neurosci Lett 291:17–20. [DOI] [PubMed] [Google Scholar]
- 8. Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick‐Sander A, Von Der Behrens W, Kempermann G (2003) Transient calretinin‐expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci 24:603–13. [DOI] [PubMed] [Google Scholar]
- 9. Brown J, Cooper‐Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG (2003) Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci 17:2042–2046. [DOI] [PubMed] [Google Scholar]
- 10. Cameron HA, McEwen BS, Gould E (1995) Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci 15:4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cameron HA, Tanapat P, Gould E (1998) Adrenal steroids and N‐methyl‐D‐aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats trough a common pathway. Neuroscience 82:349–354. [DOI] [PubMed] [Google Scholar]
- 12. Cameron HA, Woolley CS, Gould E (1993) Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res 611:342–346. [DOI] [PubMed] [Google Scholar]
- 13. Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E (2003) Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 54:1025–1034. [DOI] [PubMed] [Google Scholar]
- 14. Doyle LW, Ford GW, Davis NM, Callanan C (2000) Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci 98:137–142. [PubMed] [Google Scholar]
- 15. Drake AJ, Walker BR, Seckl JR (2005) Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol 288:R34–R38. [DOI] [PubMed] [Google Scholar]
- 16. Ferrero S, Pretta S, Ragni N (2004) Multiple sclerosis: management issues during pregnancy. Eur J Obstet Gynecol Reprod Biol 115:3–9. [DOI] [PubMed] [Google Scholar]
- 17. Fischer AK, Von Rosenstiel P, Fuchs E, Goula D, Almeida OF, Czéh B (2002) The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat. Brain Res 947:290–293. [DOI] [PubMed] [Google Scholar]
- 18. Garcia A, Steiner B, Kronenberg G, Bick‐Sander A, Kempermann G (2004) Age‐dependent expression of glucocorticoid‐ and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell 3:363–371. [DOI] [PubMed] [Google Scholar]
- 19. Gerber J, Raivich G, Wellmer A, Noeske C, Kunst T, Werner A, Bruck W, Nau R (2001) A mouse model of Streptococcus pneumoniae meningitis mimicking several features of human disease. Acta Neuropathol 101:499–508. [DOI] [PubMed] [Google Scholar]
- 20. Gerdes J (1990) Ki‐67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin Cancer Biol 1:199–206. [PubMed] [Google Scholar]
- 21. Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H (1994) Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 71:219–225. [PubMed] [Google Scholar]
- 22. Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17:2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E (1999) Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA 96:5263–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E (1998) Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 95:3168–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grier DG, Halliday HL (2004) Effects of glucocorticoids on fetal and neonatal lung development. Treat Respir Med 3:295–306. [DOI] [PubMed] [Google Scholar]
- 26. Gross SJ, Anbar RD, Mettelman BB (2005) Follow‐up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics 115:681–687. [DOI] [PubMed] [Google Scholar]
- 27. Hassan AH, Von Rosenstiel P, Patchev VK, Holsboer F, Almeida OF (1996) Exacerbation of apoptosis in the dentate gyrus of the aged rat by dexamethasone and the protective role of corticosterone. Exp Neurol 140:43–52. [DOI] [PubMed] [Google Scholar]
- 28. Hastings NB, Gould E (1999) Rapid extension of axons into the CA3 region by adult‐generated granule cells. J Comp Neurol 413:146–154. [DOI] [PubMed] [Google Scholar]
- 29. Haynes LE, Griffiths MR, Hyde RE, Barber DJ, Mitchell IJ (2001) Dexamethasone induces limited apoptosis and extensive sublethal damage to specific subregions of the striatum and hippocampus: implications for mood disorders. Neuroscience 104:57–69. [DOI] [PubMed] [Google Scholar]
- 30. Karishma KK, Herbert J (2002) Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone‐induced suppression. Eur J Neurosci 16:445–453. [DOI] [PubMed] [Google Scholar]
- 31. Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH (2003) Early determination and long‐term persistence of adult‐generated new neurons in the hippocampus of mice. Development 130:391–399. [DOI] [PubMed] [Google Scholar]
- 32. Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452. [DOI] [PubMed] [Google Scholar]
- 33. Kempermann G, Kuhn HG, Gage FH (1997) More hippocampal neurons in adult mice living in an enriched environment. Nature 386:493–495. [DOI] [PubMed] [Google Scholar]
- 34. Kim JB, Ju JY, Kim JH, Kim TY, Yang BH, Lee YS, Son H (2004) Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro . Brain Res 1027:1–10. [DOI] [PubMed] [Google Scholar]
- 35. Kornack DR, Rakic P (1999) Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA 96:5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lesage J, Del‐Favero F, Leonhardt M, Louvart H, Maccari S, Vieau D, Darnaudery M (2004) Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behavior disturbances in the aged rat. J Endocrinol 181:291–296. [DOI] [PubMed] [Google Scholar]
- 37. Levitt N, Lindsay RS, Holmes MC, Seckl JR (1996) Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 64:412–418. [DOI] [PubMed] [Google Scholar]
- 38. Liesegang P, Romalo G, Sudmann M, Wolf L, Schweikert HU (1994) Human osteoblast‐like cells contain specific, saturable, high‐affinity glucocorticoid, androgen, estrogen, and 1‐alpha, 25‐dihydroxycholecalciferol receptors. J Androl 15:194–199. [PubMed] [Google Scholar]
- 39. Lin YJ, Lin CH, Wu JM, Tsai WH, Yeh TF (2005) The effects of early postnatal dexamethasone therapy on pulmonary outcome in premature infants with respiratory distress syndrome: a two‐year follow‐up study. Acta Paediatr 94:310–306. [DOI] [PubMed] [Google Scholar]
- 40. Markakis EA, Gage FH (1999) Adult‐generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol 406:449–460. [PubMed] [Google Scholar]
- 41. Meijer OC, De Kloet ER (1998) Corticosterone and serotonergic neurotransmission in the hippocampus: functional implications of central corticosteroid receptor diversity. Crit Rev Neurobiol 12:1–20. [PubMed] [Google Scholar]
- 42. Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M (1996) Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res 26:235–269. [DOI] [PubMed] [Google Scholar]
- 43. Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, Takahashi K (1997) Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am J Physiol 273(5 Pt 2):R1669–R1675. [DOI] [PubMed] [Google Scholar]
- 44. Neubert D, Heger W, Merker HJ, Sames K, Meister R (1988) Embryotoxic effects of thalidomide derivates in the non‐human primate Callithrix jacchus. Arch Toxicol 61:180–191. [DOI] [PubMed] [Google Scholar]
- 45. Oakley RH, Webster JC, Sar M, Parker CR Jr, Cidlowski JA (1997) Expression and subcellular distribution of the beta‐isoform of the human glucocorticoid receptor. Endocrinology 138:5028–5038. [DOI] [PubMed] [Google Scholar]
- 46. Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. [DOI] [PubMed] [Google Scholar]
- 47. Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen‐Relo A (2005) Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur J Neurosci 21:1521–1535. [DOI] [PubMed] [Google Scholar]
- 48. Rosenfeld P, Sutanto W, Levine S, De Kloet ER (1988) Ontogeny of type I and type II corticosteroid receptors in the rat hippocampus. Brain Res 470:113–118. [DOI] [PubMed] [Google Scholar]
- 49. Roughton EC, Schneider ML, Bromley LJ, Coe CL (1998) Maternal endocrine activation during pregnancy alters neurobehavioural state in primate infants. Am J Occup Ther 52:90–98. [DOI] [PubMed] [Google Scholar]
- 50. Saltzman W, Prudom SL, Schultz‐Darken NJ, Abbott DH (2000) Reduced adrenocortical responsiveness to adrenocorticotropic hormone (ACTH) in socially subordinate female marmoset monkeys. Psychoneuroendocrinology 25:463–477. [DOI] [PubMed] [Google Scholar]
- 51. Sanchez MM, Young LJ, Plotsky PM, Insel TR (2000) Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci 20:4657–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmidt M, Enthoven L, Van Der Mark M, Levine S, De Kloet ER, Oitzl MS (2003) The postnatal development of the hypothalamic‐pituitary‐adrenal axis in the mouse. Int J Dev Neurosci 21:125–132. [DOI] [PubMed] [Google Scholar]
- 53. Seckl J (2004) Prenatal glucocorticoids and long‐term programming. Eur J Endocrinol 151(Suppl. 3):U49–U62. [DOI] [PubMed] [Google Scholar]
- 54. Seckl JR, Dickson KL, Yates C, Fink G (1991) Distribution of glucocorticoid and mineralocorticoid receptor mRNA expression in human postmortem hippocampus. Brain Res 561:332–337. [DOI] [PubMed] [Google Scholar]
- 55. Seress L (1992) Morphological variability and developmental aspects of monkey and human granule cells: differences between the rodent and primate dentate gyrus. Epilepsy Res Suppl 7:3–28. [PubMed] [Google Scholar]
- 56. Seress L, Abraham H, Tornoczky T, Kosztolanyi G (2001) Cell formation in the human hippocampal formation from mid‐gestation to the late postnatal period. Neuroscience 105:831–843. [DOI] [PubMed] [Google Scholar]
- 57. Slotkin TA, Zhang J, McCook EC, Seidler FJ (1998) Glucocorticoid administration alters nuclear transcription factors in fetal rat brain: implications for the use of antenatal steroids. Brain Res Dev Brain Res 111:11–24. [DOI] [PubMed] [Google Scholar]
- 58. Speirs HJ, Seckl JR, Brown RW (2004) Ontogeny of glucocorticoid receptor and 11beta‐hydroxysteroid dehydrogenase type‐1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol 181:105–116. [DOI] [PubMed] [Google Scholar]
- 59. Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J (1994) Neurotoxicity of glucocorticoids in the primate brain. Horm Behav 28:336–348. [DOI] [PubMed] [Google Scholar]
- 60. Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM (1990) Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. Brain Res Dev Brain Res 53:157–167. [DOI] [PubMed] [Google Scholar]
- 61. Vollmann‐Honsdorf GK, Flügge G, Fuchs E (1997) Chronic psychosocial stress does not affect the number of pyramidal neurons in tree shrew hippocampus. Neurosci Lett 233:121–124. [DOI] [PubMed] [Google Scholar]
- 62. Waterland RA, Jirtle RL (2004) Early nutrition, epignetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 20:63–68. [DOI] [PubMed] [Google Scholar]
- 63. Welberg LA, Seckl JR, Holmes MC (2001) Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin‐releasing hormone: possible implications for behavior. Neuroscience 104:71–79. [DOI] [PubMed] [Google Scholar]
- 64. Whitehouse BJ, Abayasekara DRE (2000) Adrenocortical function in a new world primate, the marmoset monkey, Callithrix jacchus. Gen Comp Endocrinol 120:2–7. [DOI] [PubMed] [Google Scholar]
- 65. Windle CP, Baker HF, Ridley RM, Oerke AK, Martin RD (1999) Unrearable litters and prenatal reduction of litter size in the common marmoset (Callithrix jacchus). J Med Primatol 28:73–73. [DOI] [PubMed] [Google Scholar]
- 66. Wong EY, Herbert J (2004) The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci 20:2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu H, Steven Richardson J, Li XM (2003) Dose‐related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl‐2 and Cu/Zn‐SOD in rat hippocampus. Neuropsychopharmacology 28:53–62. [DOI] [PubMed] [Google Scholar]
- 68. Yamamoto S, Utsu S, Tanioka Y, Ohsawa N (1977) Extremely high levels of corticosteroids and low levels of corticosteroid binding macromolecule in plasma of marmoset monkeys. Acta Endocrinol 85:398–405. [DOI] [PubMed] [Google Scholar]
- 69. Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH (2004) Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med 350:1304–1313. [DOI] [PubMed] [Google Scholar]
- 70. Yu IT, Lee SH, Lee YS, Son H (2004) Differential effects of corticosterone and dexamethasone on hippocampal neurogenesis in vitro . Biochem Biophys Res Commun 317:484–490. [DOI] [PubMed] [Google Scholar]
- 71. Zysk G, Brück W, Gerber J, Brück Y, Prange HW, Nau R (1996) Anti‐inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol 55:722–728. [DOI] [PubMed] [Google Scholar]


