Abstract
Stem cell biology has provided constant alteration if not reversal of dogma related to the understanding of the behaviors of primitive and dynamic cells. This review summarizes recent findings on dynamic changes of phenotype that accompany the in vitro growth and differentiation of not only stem and progenitor cells, but also differentiated cells derived from a variety of normal and pathological tissues. As there are examples of apparent dedifferentiation and transdifferentiation of neural cells that appear to be terminally differentiated, there is a need to reconsider elements of cellular fate choice that have relevance to neurooncology and neural repair. Recent findings of dynamic behaviors and mixed phenotype of both normal and cancer stem cells suggest that some of the diverse lineage attributes of different solid tumors may owe their existence to dynamic cellular phenotypy gone awry.
INTRODUCTION
It has long been appreciated, as one of the major tenets of cell biology, that eukaryotic cells evolve from primitive precursor cells during embryogenesis and contribute to tissue histogenesis via precise rules for cellular proliferation, fate choice, commitment, and differentiation. Recent studies in the field of stem cell biology, however, have begun to raise issues as to whether or not it is possible for cells to change their fate rather abruptly and dramatically, without oncogenic transformation and even when they may be in a state of committed differentiation. As stem and progenitor cells (“stem/progenitor” when stemness or degree of stemness is uncertain or questioned; the term “precursor” is even more indifferent) have the profound ability to undergo impressive phenotypic changes during their course of differentiation from a primitive precursor to a fully differentiated tissue (somatic) cell, a concept of dynamic cellular differentiation that could contribute to “phenotypic fluidity” has not been fully considered—until recently—because intermediate forms of mammalian neural cells now have been observed (38). In fact, surprising new evidence suggests that adult rodent and human neural cells can be isolated from the brain and observed to undergo forwards (58) and backwards (38) differentiation programs that even generate cells at certain times during this differentiation cascade that adopt the fate of both a neuron and an astrocyte, or “asteron”(38).
Stem cell biology and regenerative medicine has evolved as a field over the last decade; both the scientific and lay communities have great expectations of it for translating the fruits of this highly technological research to biomedical applications, including new therapeutics for most if perhaps not all human diseases. As such, the rapid advances in our understanding of very potent cells in embryonic, fetal, and adult tissues and organisms have also contributed to numerous reversals of dogma as well as contributions that have evoked a significant amount of question and controversy. Stem cell biology owes its origins to the pioneering work of the bone marrow hematopoiesis field that embarked on elucidating the importance of clonogenic precursor cells which are vulnerable and at‐risk to environmental, eg, ionizing radiation (45) insults that prior to the 1940s and 1950s were not an issue for maintaining homeostasis and survival of the human species.
EMBRYONIC, FETAL, AND ADULT STEM CELL BIOLOGY—A VERY BRIEF SYNOPSIS
Embryonic stem cells. Embryonic stem (ES) cells, isolated from the inner cell mass of blastocysts, present a profound potential for combining cell and gene therapy to eventually attack many if not all diseases that impact the human condition. Advantages of ES cells as a donor source include their pluripotency, their potential for virtually unlimited proliferation, their amenability to genetic modification, and the possibility to differentiate them into homogeneous and in particular neural (eg, 12, 47) and nonneural cell populations. ES cell‐derived neural precursors (ESNPs) have been efficiently derived from both rodent and human ES cells (65); following their intracerebral transplantation ESNPs integrate widely throughout the central nervous system (CNS) and differentiate into neurons, astrocytes, and oligodendrocytes 13, 73. Many studies support the notion that primary and immortalized CNS stem cells as well as ES cell‐derived neural cells can contribute to behavioral improvement when grafted into rodent models of neurodegenerative disease or traumatic CNS lesions (see 42 for review). ES cells have been touted and generally accepted to be the most plastic and potent source of large numbers of precursor cells for therapeutic transplantation, yet once they have committed to a tissue type (eg, Neural Restricted or Glial Restricted Precursors, NRPs and GRPs, eg, see 4, 57), there is no evidence for their ability to switch their fate choice; their phenotype is therefore only dynamic until they choose their final fate, at least as studied to date. Nonetheless, their ability to generate cell lineage diversity is their hallmark, and also the basis for their contribution to hyperplasia, ie, teratomas, that exhibit extremely diverse cellular fate choices. These hyperplasias have been reported after ES cell transplantation in rodent models of Parkinson’s Disease following their grafting into the dopamine‐depleted neostriatum (6).
Fetal neural stem cells. There are stem and progenitor cells in fetal tissues that are generally presumed to be more plastic than their counterparts in adult tissues, and also less potent than ES cells. Cord blood stem cells are somewhat intermediary in their nature and ability to differentiate into a variety of tissues and potentially provide a valuable source of replacement cells for tissues at risk following injury or disease. These cells may have potential to differentiate into both hematopoietic and nonhematopoietic cells and they “. . . may exist in a continuum with continually and reversibly changing phenotype. . . .”(52), a notion that is particularly relevant to “phenotypic fluidity” of stem/progenitor cells in general to be discussed below. Fetal neural stem cells have not been reported to participate in rapid or unique phenotypic transformations, and cord blood stem cells are presumed to exhibit the same type of differentiation potential and plasticity, certainly if not more, as that seen with adult bone marrow‐derived hematopoietic stem cells (HSCs, see below). Recent reviews by Lindvall and Bjorklund (42) and Reier (53) discuss the application of fetal neural stem cell biology to human transplantation for Parkinson’s Disease and spinal cord injury. In short, fetal human neural stem cell trials have yielded positive safety and to some degree proof of principle, but more studies are needed both in animal models and human diseases before we pronounce these cells as valuable or insignificant therapeutic reagents. As nicely stated by Lindvall and Bjorklund (42), regarding human fetal neural stem cell shortcomings, they suffer from, “. . . 1) lack of sufficient amounts of tissue for transplantation in a large number of patients, 2) variability of functional outcome with some patients showing major improvement and others modest if any clinical benefit, and 3) occurrence of troublesome dyskinesias in a significant proportion of patients after transplantation. . . .” Technological issues surrounding the preparation and use of fetal human neural cells in Parkinson’s and spinal cord injury transplants are worthy of more attention. With proper attention, fetal stem/progenitor cells may be amenable to coaxing into a variety of specified and desired phenotypes that could survive transplantation and contribute to circuitry protection and replacement in neurological disorders.
STEM/PROGENITOR CELLS FROM THE ADULT CNS
Stem/progenitor populations from the postnatal and adult mammalian CNS have been characterized using a variety of in vitro protocols that expand, enrich, and differentiate them, leading to a better understanding of the nature of these cells for eventual use in cell‐replacement therapies. The periventricular subventricular zone (SVZ) and rostral migratory stream (RMS) of the forebrain is a neuropoietic niche that represents the vestigial embryonic germinal zone that builds the telencephalon, and they display a high level of constitutive proliferation with presumably the greatest density of putative neural stem cells in the adult brain. Work from our laboratory (40; Figure 1) has shown that a cell exhibiting characteristics of an astrocyte, from the developing brain until the end of the second postnatal week, and from the SVZ throughout life, is a multipotent stem cell (that we refer to as a multipotent astrocytic stem cell, “MASC”) that in essence transdifferentiates into a clonogenic cell in vitro which can give rise to neuropsheres containing both glia and neurons. The MASC has been lineage‐associated to a variety of progenitor cells that owe their nature and behaviors to the most primitive radial neuroepithelial precursor cells that generate the CNS during embryonic histogenesis (62), and which inhabit the entire neuraxis until the end of the second postnatal week in the mouse. The multipotent MASC persists throughout life in the SVZ and other astrocytic cells throughout the neuraxis may be amenable to dedifferentiation in vitro, or possibly attempted dedifferentiation in vivo following injury, disease, or proper exogenous growth factor exposure. Figure 1E shows dividing glial fibrillary acidic protein (GFAP+) reactive astrocytes in the injured cerebral cortex that may not only be contributing to the astroglial scar, but also attempting dedifferentiation to again become multipotent (eg, upregulating nestin and proliferating), but failing at this despite exposure to significant levels of blood‐borne and CNS‐derived growth factors following such penetrating injuries and disruption of the vasculature (see 62 for review).
Figure 1.
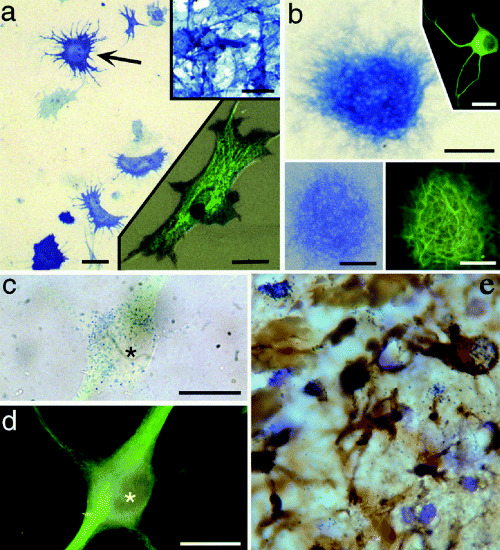
Astrocyte cultures can give rise to neurons and neurospheres; an example of transdifferentiation potential. Alkaline phosphatase (AP) enzyme histochemistry and immunocytochemistry of cells and neurospheres derived from Gtv‐a transgenic mouse astrocytes infected with RCAS–AP, showing cells expressing both AP and neuronal phenotype markers [Gtv‐a, a mouse strain that contains the tv‐a gene encoding the TVA receptor for avian leukosisvirus under control of the glial fibrillary acidic protein (GFAP) promoter, which allows for the selective infection of GFAP‐expressing astrocytes with the avian leukosisvirus (RCAS)]. A. P2 astrocyte monolayer after infection with the avian leukosisvirus expressing the AP reporter gene, showing infected astrocytes (eg, arrow). Upper right inset shows AP histochemistry of the DF1 chicken embryo fibroblast line engineered to produce the RCAS–AP leukosisvirus. Lower right inset shows a single infected astrocyte with both AP histochemical labeling (blue‐black punctae) and GFAP immunofluorescence (green, FITC). Scale bars = 25 µm in (A), 30 µm in both insets. B. A neurosphere derived from a Gtv‐a astrocyte monolayer. AP histochemistry reveals cells of this neurosphere expressing the RCAS–AP gene, thus indicating derivation of the clone from a single, infected astrocyte. Upper right inset shows an example of a neuron derived from such a neurosphere, immunofluorescence for β‐III tubulin (green, FITC). Lower pair of insets show the same neurosphere expressing AP (left) and MAP2 (right), revealing numerous MAP2‐positive processes emanating from an RCAS‐infected neurosphere. Scale bars = 100 µm in (B), 50 µm in the lower insets, and 40 µm in the upper inset. C and D. A single RCAS–AP‐infected neuron that has migrated away from its neurosphere and differentiated. Brightfield labeling of AP reaction product (blue dots) in this neuron (C), colocalized with β‐III tubulin immunofluorescence (FITC green) shown in (D). Asterisk marks the nucleus of this cell in each figure. Scale bars in (C) and (D) =10 µm. E. Following penetrating injuries to the adult brain, reactive astrocytes express GFAP and take up tritiated thymidine that help constitute the glial scar and possibly indicate an attempt to dedifferentiate and possibly become neurogenic. Reactive astrocytes are up to 20 µm in diameter. [A–D from 40; copyright PNAS 2000, with permission.]
We have recently modified a monolayer culture system similar to that described by Song, Gage and collaborators (61) that affords what appears to be a complete recapitulation of SVZ neuropoiesis in vitro, resulting in the generation of very large numbers of neuroblasts rather rapidly from a nestin+/A2B5+/GFAPlow/PSA‐NCAM‐/Dlx2‐ primed or induced precursor cell; functional GABAergic interneurons are generated as produced in the SVZ–RMS in vivo (58; Figure 2). These cells and presumed MASCs are acquiescent to extremely dynamic phenotypic alterations (see section below, “Phenotypic Fluidity and the Generation of Intermediate Cellular Forms in Normal Brain Cell Cultures”) that can lead to very unique hybrid cell types.
Figure 2.
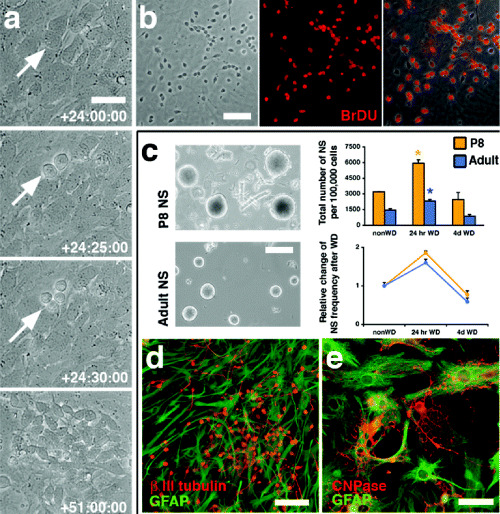
Induction of neurogenesis in postnatal mouse astrocyte cultures shows a rapid phenotypic change in a primitive astrocytic stem/progenitor cell such that it can give rise to large numbers of neuroblasts in 27 h. A mitotically active, multipotent cell emerges transiently during early stages of in vitro neuropoiesis. A. Time‐lapse microscopy of growth factor‐withdrawn subventricular zone (SVZ) cultures reveals a transient period, characterized by rapid cell divisions, leading to the initial appearance of neuroblasts within 27 h. B. BrdUrd applied at 48–72 h after the initiation of differentiation labels 95% of all generated neuroblasts. C. Clonal neurospheres were derived from cultured P8 and adult SVZ cells (left for morphological comparison). Total numbers and relative frequencies (right) of neurospheres (NS) generated from 100 000 cells per condition increase significantly but transiently at 24 h after growth factor withdrawal (WD) (*P < 0.01 for adult and P8 compared with non‐WD and 4‐d WD). D and E. Primary and secondary P8 and adult (shown here) neurospheres yield neurons (D) and glia (E). Scale bars (in µm): A, 15; B, 60; C, 200; D and E, 20. [From 58; copyright PNAS 2005, with permission.]
Findings that both adult mouse and human neural stem/progenitor cells can survive in tissue with rather protracted post‐mortem intervals 39, 51 suggests that the cadaveric human brain is a valuable resource of such cells that could be manipulated for therapeutic applications in diseases. A complete understanding of clonogenic, highly expandable stem/progenitor cells from the adult brain could lead to exploitation of the plasticity of these cells, if they exhibit any of the repopulation attributes of their embryonic counterparts, for cell replacement protocols for a variety of neurological disorders. One could even propose that translating insights from ES cell research to adult stem cell biology could lead to greater control and manipulation of these cells for desired fate and differentiation leading to their functional integration within compromised adult CNS circuitries.
BONE MARROW‐DERIVED NEURAL CELLS
In addition to neuropoietic adult stem/progenitor sources in brain, the hematopoietic system should be another valuable source of multipotent cells that might be able to be coaxed into neuronal and glial differentiation. HSCs may retain their pluripotent differentiation capacity throughout our lifespan, and their homeostasis is maintained by a consistent and orderly regulated developmental cascade and hierarchy. Bone marrow‐derived HSCs can differentiate through a series of multipotent and unipotent progenitor cells to form all blood cell types, including lymphocytes, granulocytes, monocytes, erythrocytes and megakaryocytes. The precise signal transduction pathways that control hematopoiesis are made up of several interconnected mechanisms, with one level of regulation occurring at the transcriptional control of hematopoiesis‐specific gene expression; a second mechanism involves the interaction of hematopoietic growth factors with particular cell‐surface receptors. Cell–cell and cell–substrate signaling mediated by adhesion and extracellular matrix (ECM) molecules (72) also plays an important role in stem cell–stromal cell interactions that regulate hematopoiesis. The stromal cell and its role in both supporting hematopoiesis and exhibiting multipotency (60) may be quite similar to the MASC and the reactive astrocyte described above, that express similar batteries of ECM and can contribute to multipotency and attempted neuropoiesis under particular growth conditions.
In vitro studies indicate that conditions can be found that coax hematopoietic cells into expressing certain neural characteristics. For example, Sanchez‐Ramos and collaborators (56), and Black and collaborators (69) showed that bone marrow stromal cells in culture express neural markers of astrocytes and neurons when exposed to particular growth conditions and factors. It also has been shown that bone marrow stromal cells injected into the rodent brain, in pioneering studies by the Prockop group, could adopt neural fates as evidenced by their expression of the intermediate filament protein of astrocytes, GFAP (3). We have also recently reported (19) that bone marrow‐derived cells can express such neural antigens in culture (Figure 3) even prior to the implementation of neutralization protocols. Furthermore, as shown for these cells following grafting to the developing rodent brain, where they appear to migrate on radial glia to distribute within developing telencephalic structures (48), when these cells are grafted into the adult rodent brain with access to the SVZ/RMS neurogenic niche they do migrate and assume some phenotypic characteristics of immature neurons (19). There is a growing opinion in the field that bone marrow and differentiated cells can adopt morphologies of cells from a variety of tissues, undergo rapid shape changes under particular growth conditions in vitro, and also express phenotypic markers of neural and other cell types 5, 22, but that this may not constitute a true and complete “transdifferentiation.” They potentially still retain gene expression profiles associated with their original ancestral (eg, mesodermal) cell (5). Even within an ectopic tissue location following grafting (19) or homing, despite the strong induction of host tissue phenotypy conferred upon them from their new location, they still possess cellular phenotypic characteristics associated with their original “home.” There are examples, however, even following human transplantation (see review of 18, below) of what appears to be a complete transdifferentiation of bone marrow‐derived cells in the human brain; this is a controversial issue that still elicits strong debate and even refutation 44, 67 despite applications of careful phenotypic criteria. Recent insights into potential neural crest origins of multipotent cells in presumed nonneural epithelium (66) suggest that it is important to understand the embryology of the cells and tissues in question before assigning true transdifferentiation potential.
Figure 3.
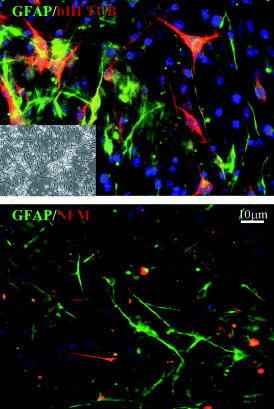
Bone marrow‐derived cell (BMDC) clones exhibit multipotency by generating progeny of different lineages through symmetric and asymmetric division. Inset in the top figure shows a phase image of the starting BMDC population. Both top and bottom panels show double immunolabeling of BMDCs treated with dbcAMP (dibutyryl cyclic AMP)/IBMX (isobutylmethylxanthine) using antibodies against neuronal and astrocyte‐specific proteins. Glial fibrillary acidic protein (GFAP) is green; βIII tubulin and NFM are red; blue is DAPI counterstain. [From 18; copyright Stem Cells 2005, with permission.]
HETEROGENEITY OF STEM CELLS, EVEN IN THE SAME ADULT TISSUE, CAN LEAD TO CELL LINEAGE DIVERSITY
There are more studies beginning to acknowledge the possibility that more than one type of multipotent cell resides within a single neurogenic niche (eg, the “B” and “C” cells, 20). Certainly within bone marrow it has been assumed that the existence of multipotential adult progenitor cells (MAPCs) (33) and other multipotent cells exemplify such heterogeneity. Current in vitro models of neurogenesis suggest that neurogenesis is driven by multipotent neural stem cells that can self‐renew and progressively generate more developmentally restricted but still multipotent progenitors and ultimately more developmentally committed progeny. We previously tested the hypothesis that a subset of parental cells that are clonogenic in an in vitro system, give rise to neurons and glia that could be retrospectively subdivided into distinct subsets of cells with different developmental commitment based on distinct molecular phenotypes of their clonal progeny (63). This study revealed temporal and sequential order in the expression of genes involved in neural stem cell growth and differentiation; a resulting model was generated to elucidate the molecular phenotype of any clonogenic stem/progenitor cell population, eg, those involved in developmental and persistent neurogenesis. Findings of clonal heterogeneity from single neurosphere expression profilings (63) suggested that the size of a clone might reflect the responsiveness to growth factors and the proliferation/differentiation status of the parental clone‐forming stem/progenitor cell; however, recent work from Reynolds and collaborators (55) have advised caution in inferring a state of stemness based on insights from conventional neurosphere versus colony‐forming‐assay approaches. Regionally distinct CNS stem/progenitor cells may also contribute to heterogeneity, with multipotent cells present in even mature areas of the CNS, supporting a notion of a variety of clonogenic cells that possess distinct developmental and environmental histories.
The data we presented in our neurosphere gene expression and temporal profiling study (63) revealed that clonal neurospheres derived from the human brain, which were initially arranged within a panel according to their size (from small to large), did display different combinations of transcripts that overall contributed to distinct molecular phenotypy, and reflecting distinct developmental potential of clone‐forming cells from the developing and adult human brain. Heterogeneity of stem and progenitor cells in the neurogenic SVZ and hippocampus is certainly a reasonable possibility, even though we do not know if it is true heterogeneity or variance in state of proliferative potential or differentiation related to what state (eg, cell cycle) the isolated clonogenic cells were in when subjected to proliferative or differentiating culture conditions. All evidence points to the existence of different types of stem and progenitor cells in both the developing and adult brain, and they seem to be amenable to changes in growth conditions that generate lineage diversity of their progeny. This could also lead to lineage diversity following oncogenic transformation that ultimately generates the varieties of cell types found in tumors, including those seen in gliomas (see below).
Growth conditions may also be able to induce unexpected events of a terminally differentiated cell in culture. Growing embryonic and adult rodent neural cells in Neurobasal/B27 medium with FGF‐2 yielded large numbers of cells with neuronal characteristics, including neurofilament, tau, and MAP2 expressions, glutamatergic transmitter phenotype, and the ability to generate action potentials; apparently differentiated neurons were also shown to be able to take up BrdU and divide after 3 days in culture (10). The appearance of nestin expression in these cells under these culture conditions suggested the possibility of dedifferentiation to a precursor state that could possibly be exploited to regenerate significant numbers of neurons from cells with terminally differentiated phenotypy. Dedifferentiation has been described for ES cells giving rise to primordial germ cells in culture. As described by Hubner and colleagues, “. . . Cultured embryonic stem cells are generally considered pluripotent rather than totipotent because of the failure to detect germline cells under differentiating conditions . . . mouse embryonic stem cells in culture can develop into oogonia that enter meiosis, recruit adjacent cells to form follicle‐like structures, and later develop into blastocysts (31).” It is interesting to consider that ES cell generation of oocytes and sperm (34) represents a recapitulation of embryonic development in vitro that has attributes of both differentiation and dedifferentiation of germ cells, depending on perspective and developmental biology that hitherto was assumed to have cellular differentiation proceed in primarily one direction.
AMBIGUOUS PHENOTYPY IN THE “TRANSDIFFERENTIATION” OF MESENCHYMAL CELLS TO NEURAL PROGENITOR CELLS—CELLULAR FUSION VERSUS PLASTICITY
A large number of papers have appeared over the last several years touting an ability of stem/progenitor cells from bone marrow to differentiate from one germ layer to another, eg, mesoderm to ectoderm, with the apparent differentiation of mesenchymal stem cells into glial and neuronal lineages as reviewed above. A very recent study from the Gage group has even presented compelling evidence that neural stem cells can give rise to endothelial cells and vascular outgrowths in the brain (70). This is in keeping with ectoderm and mesoderm fate switching from stem/progenitor cells in bone marrow and muscle 7, 25, 28. Cell fusion‐independent homing of a putative hemangioblast from bone marrow to retinal neovascular beds in an animal model of diabetic retinopathy shows the potential clinical significance of stem cell transdifferentiation, where attempted wound healing and tissue regeneration under control of the potent SDF‐1/CXCR 4 cytokine axis (14) can lead to novel forms of tissue growth. Insights gained from studying stem cell behaviors and biogenic factors that control their homing, proliferation, migration, fate choice and differentiation should be applied to new therapeutic protocols that manipulate these behaviors and factors.
Looking at post‐mortem brain specimens containing the hippocampus from female patients receiving bone marrow transplants from male siblings, we found significant numbers of beta III tubulin/neurofilament+ neurons with XY FISH+ chromosome labeling in the hippocampus of the longest surviving transplant patients, suggesting transdifferentiation of bone marrow‐derived cells into neurons without evidence of cell fusion (aneuploidy) (18; Figure 4). We do know that neural cells are capable of fusing in culture (16; Figure 4) suggesting that we must be cautious in pronouncing cellular plasticity and transdifferentiation with a need for proper tissue and cell ploidy and phenotypic analyses. As recently reviewed (62), findings regarding the transdifferentiation potential of stem cells from different tissues, including blood‐to‐brain, require extensive attention not only to differentiation potential and fate control, but also to the ways in which we assess a cell’s so‐called conversion. The potential but not propensity of primitive, undifferentiated cells to fuse with other more differentiated cells has now been seen and addressed in numerous in vitro and in vivo studies 2, 64, 68, 71. It is necessary to monitor the ploidy of all putatively transdifferentiated, dedifferentiated, or incorporated (following transplantation) cells. Studies by Brazelton et al (9) showed the expression of neuronal markers following repopulation attempts of bone marrow‐derived HSCs in the brain, but their morphology did not easily conform to that of differentiated neurons in spite of their expression of so‐called mature neuronal immunomarkers. Electrophysiological profiling of a differentiated and integrated new “neuron” helps the cause, but there is also the existence of ambiguous somatodendritic morphologies in cultured blood‐to‐brain cell examples. Even though the study of Cogle et al (18) supports an apparent uncanny potential of bone marrow‐derived cells in humans to contribute to mature neuronal and glial cells in the brains of human patients undergoing transplantation for cancer, the nature of this homing, fate switch, and apparent terminal transdifferentiation must be studied in more detail with an eye toward resolving the “rare event” issue raised by Wagers et al (67) versus what normal or abnormal (eg, radiation and chemotherapy that compromised the blood brain barrier as well as potentially CNS circuitry) physiological conditions beckoned these cells from bone marrow to brain and allowed them to apparently integrate within established CNS circuitries; this certainly has a tremendous amount of significance toward the understanding and development of autologous repair approaches for many debilitating diseases. Specialized growth conditions in vitro that could be recreated in vivo following disease‐ and treatment‐related insults might yield conversion of a cell’s phenotypic attributes to exhibit transdifferentiated, eg, neuronal or glial phenotype. Growth factors, growth constraint conditions and unusual cell–cell interactions might also lead a cell to express ectopic antigens, thus any cell may look like any other cell in vitro, but it may not have completed a normal differentiation program for that cell, hence falling somewhere in‐between on a cellular differentiation continuum. Placing cells in neutralizing conditions, such as in vivo transplantation into neuropoietic brain marrow 62, 74, 75, may lead to a more favorable environment in which to coax cells into completing ectopic differentiation programs. Recent studies by Weissman and collaborators (67) relied solely on systemic introductions of nonneural (eg, bone marrow‐derived hematopoeitic precursors) cells into stem cell‐depleted mice, some of which had brain injuries that should have been able to induce neural repopulation events if, in fact, transdifferentiation of blood cells to brain is possible. The results were unimpressive, and did not meet a standard exhibited by, eg, Verfaillie’s adult mesenchymal stem cells that not only appear to transdifferentiate, but also contribute to an impressive whole animal chimerism following their introduction into blastocyts (33). The most stringent test of stemness is the ability of a single cell to repopulate following a serial transplantation paradigm 28, 35, 62.
Figure 4.
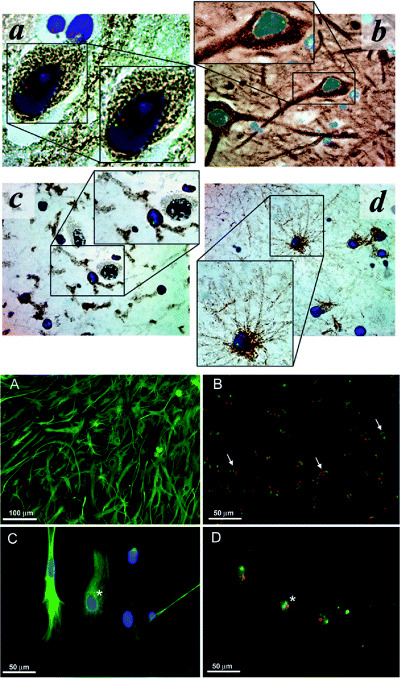
Transdifferentiation potential, without cell fusion, of human bone marrow‐derived cells following transplantation in human leukemia patients; example of limited cell fusion potential in mouse neural stem/progenitor cell cultures. Top figure: Multilineage CNS engraftment in human bone marrow hematopoeitic stem cell (HSC) transplant patients. Representative micrographs of hippocampus from female recipients of HSC transplants from sibling male donors. Cells were stained for Y‐chromosome (green), X‐chromosome (red), nuclei (DAPI, blue), and differentiation markers (DAB, brown) for neurons (panel A, anti‐ß‐III tubulin, ×160 magnification; panel B, antineurofilament, ×100 magnification), microglia (panel C, anti‐CD45, ×100 magnification), and astrocytes [panel D, anti‐glial fibrillary acidic protein (GFAP), ×100 magnification]. No cell fusion is observed in these engrafted hippocampal cells, revealed by normal diploid XY chromosomal staining patterns. [From 18; copyright Lancet, 2002, with permission.] Bottom figure: Astrocyte monolayers from mouse, however, do reveal evidence of stem/progenitor cell fusion, as also seen following induction of neurospheres in these cultures. These cultures contain cells with polyploid sex chromosomes. In (A), an astrocyte monolayer derived from the subventricular zone (SVZ) shown to consist mostly of cells immunopositive for GFAP (green). In (B), chromosome painting specific for the mouse X‐chromosome (green) and Y‐chromosome (red) reveals cells with abnormal chromosome counts (arrows) within the astrocyte monolayer culture. In (C), this high magnification photomicrograph shows a group of cells immunopositive for GFAP (green) before chromosome painting. In (D), the same group of cells as seen in (C) are shown after chromosome painting. Asterisks indicate corresponding cell that is immunolabeled for GFAP (C) and contains three sex chromosomes (D). [From 16; copyright Exp Neurol 2006, with permission.]
PHENOTYPIC FLUIDITY AND THE GENERATION OF INTERMEDIATE CELLULAR FORMS IN NORMAL BRAIN CELL CULTURES
In a recent study, we analyzed the fate of cells derived from differentiating primary neurospheres and showed that unambiguously identified neurons can undergo a phenotypic shift into cells with astrocyte characteristics by transitioning through an “asteron” (neuron/astrocyte hybrid) morphotype. Asterons are an apparent transient population under our culture conditions of growing stem/progenitor cells in unique monolayers derived from the SVZ or postnatal cerebellum. They coexpress a variety of neuron and astrocyte proteins and genes (Figure 5), exhibit membrane biophysical and physiological properties that appear to combine properties of neurons and astrocytes, and do not result from neuron/astrocyte fusion (38; see 16, and Figure 5 to show that astrocytes can be involved in stem cell fusion in vitro, as “rare events,” but not in the case of asteron generation). Under particular culture conditions neurons can alter their phenotype to assume characteristics associated with astrocytes. This transition seems to result from transdifferentiation because even though asterons can take up BrdU, their proliferative and differentiation behaviors in asteron cultures does not support the notion of cell division or cell death involved in the appearance of mixed phenotype under our in vitro conditions (38). This is also supported by recent reports describing the in vitro presence of astrocyte/oligodendrocyte “hybrids”(46), as well as neurons with some astrocyte characteristics in cultured cerebellar dissociates (50).
Figure 5.
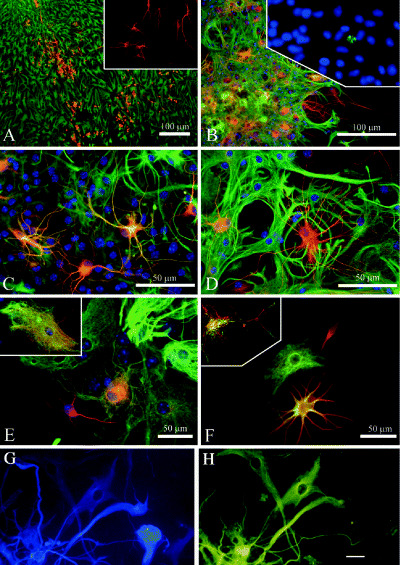
Examples of phenotypic fluidity in normal postnatal mouse astrocyte monolayer and neurosphere cultures, and in hybrid cells from adult human brain gliomas. Time‐dependent neuron/astrocyte hybrids in differentiating cerebellar spheres. A, and inset. Thirty‐six hours after attachment, spheres consist of separate populations of neurons (labeled for β‐III tubulin, red) and astrocytes [labeled for glial fibrillary acidic protein (GFAP), green]. B. Three days after attachment many yellow cells are seen which coexpress β‐III tubulin (red) and GFAP (green); inset in (B) caspase 3 immunolabeling (green) within a differentiating sphere at 72 h after attachment shows the fragmented nucleus of an apoptotic cell. Such labeling was rare, and most nuclei remained intact for extended in vitro periods. C and D. Higher magnification of neuron/astrocyte hybrids at 1 week post attachment show some cells expressing β‐III tubulin (red) and GFAP (green). Notice the hybrid morphology of thin, finely branched processes combined with stellate somata. Arrow in (D) indicates a coexpressing cell with two nuclei. E and F.β‐III tubulin and GFAP labeling reveals neuronal (red), astrocytic (green) and hybrid (yellow) phenotypes in adjacent cells. Inset in (E) is a high magnification of a cell with astrocytic morphology, showing β‐III tubulin (red) and GFAP (green) labeling of separate intracellular elements. Inset in (F) is a confocal micrograph of a single hybrid cell immunolabeled for β‐III tubulin (red) and GFAP (green) showing colocalization of these markers. G and H. Glioblastoma contain cells that under our culture conditions give rise to βIII tubulin+ neuron‐like cells (FITC), and cells that express the astroglial marker GFAP (blue, AMCA). In (G) there is a cell that is not labeled for GFAP, but is immunopositive for β‐III tubulin. Conversely, there are cells that are immunolabeled for GFAP, but immunonegative for β‐III tubulin, and a cell that is labeled with both immunomarkers (a so‐called “asteron”). Scale bars are 10 µm (G and H) and 100 µm (A–E). [From 38; copyright J Comp Neurol 2005, and 32; copyright Glia, 2002, with permission]
The appearance of asterons in our monolayer culture system and the retrieval of floating neurospheres for multipotency and neural cell differentiation analysis led to the discovery of novel cellular states during the differentiation of neural stem/progenitor cells, whereby attributes of both neuronal and astroglial phenotype could be present in the same cell. Thus, such “phenotypic fluidity” appears to be a tissue culture‐generated phenomenon, characterized using a variety of molecular and physiological approaches, and support a notion that, not only do astrocyte‐like stem cells have the ability to generate neurons 21, 40, but newly‐generated neurons can assume or revert to an astrocytic phenotype most likely as a default under the influence of stressful, eg, in vitro conditions that may not present the required neuron‐supporting growth factor environment. Even if this is some type of “tissue culture artifact,” it might be a useful one because the transformation does occur in our culture studies and suggests a cell’s ability to dramatically alter its phenotype. Exploiting dedifferentiation and transdifferentiation potential of committed neural cells provides insights into the nature of stem, progenitor, and differentiated cellular plasticity, and ultimately may lead to new cellular therapeutic approaches. The apparent resiliency of glial cells derived from stem cells in neural grafting studies might also be explained by a posttransplantation transition of neurons into astrocytes. Neuron to astrocyte neuron‐to‐astrocyte, in addition to astrocyte to neuron astrocyte‐to‐neuron 21, 26, 40 transdifferentiation could have significant ramifications toward understanding neural fate choice decisions, and a new thinking regarding the propensity for glial cell “survival” seen in neural grafting studies (15). The bidirectional differentiation potential of neural stem/progenitor cells also speaks to a continuum of cell fate potential of a very complex cell type, a brain cell, where all stops and checkpoints in a differentiation cascade now seem amenable to manipulation. Such a challenge of dogma may also provide insights into so‐called transformation of primitive cells in cancers that lead to unique cellular phenotypy as well as complex cell lineage diversity as seen in solid tumors including gliomas (see below).
CELLULAR PLASTICITY AND DIVERSITY FOLLOWING ONCOGENIC TRANSFORMATION
As recent studies have shown that mesenchymal (33) as well as epithelial stem/progenitor cells can contribute to many aspects of embryogenesis including the generation of tissues and embryos, such cellular plasticity must also be considered in light of oncogenic transformation. Likewise, recent work from the Curran group showing that cancer stem/progenitor cells from medulloblastoma can also be used to generate viable embryos (41) adds credence to a notion of plasticity that persists following oncogenic transformation, which might be controllable for novel cell and chemotherapeutic approaches for cancers.
The cancer stem cell hypothesis has now been addressed and re‐addressed, since Bonnet and Dick (8) and the position paper by Reya et al (54). Several groups have now shown that a variety of solid tumors possess cells with stem cell‐like activity. Gliomas were first shown by our group to contain a stem‐like cell population within cortical glioblastomas and anaplastic astrocytomas (32), and the Kornblum, Vescovi and Dirks groups 24, 29, 59 also showed that pediatric and adult brain tumors contain clonogenic cells that can reconstitute the tumor both in vitro and in vivo in rodent transplants. Our recent studies have looked at osteo‐ and chondrosarcomas that also contain clonogenic cells which form “sarcospheres” in semisolid media (27). Bone sarcomas are part of a group of mesenchymal malignancies that exhibit clinical, cell, molecular, and histological heterogeneity. Stem‐like cells also have been implicated in the pathogenesis of leukemia, breast and other tumors, and it is now worth considering that the cancer stem cell theory is supported by the existence of a small subpopulation of cells within solid tumors that have the ability, as do normal stem cells, to self‐renew (1). Tumor‐initiating cancer stem‐like cells would have to divide asymmetrically, produce an identical copy of themselves and more differentiated daughter cells that go on to generate the majority of the tumor bulk. Such rare stem‐like cells could initiate and maintain the growth of the tumor as well as potential local and distant recurrence in bone sarcoma, glioma and other types of cancers.
Our recent study of bone sarcoma stem‐like cells (27; Figure 6) included a hypothesis that the heterogeneity and the relative resistance to chemotherapy of these tumors might be associated with the presence of tumor stem‐like cells as observed in our glioma studies, in which we observed clonogenic sphere‐forming cells under particular in vitro growth conditions. The osteosarcoma‐ and chondrosarcoma‐initiating cells appear to have “dedifferentiated” to a level in vitro and possibly in vivo where they express genes normally only associated with ES cells—Oct3/4 and Nanog. Our culture conditions 32, 36, 37 pose a unique set of stressful growth conditions that either dedifferentiate cells or induce a selection for the most primitive clonogenic cells; the most differentiated cells that are unable to survive serum and anchorage withdrawal presumably die under these conditions. Dissociated normal and cancerous tissue in suspension, using semisolid media without serum, thus selects for primitive clonogenic cells that are then able to be expanded and give rise to different classes of cells, suggesting a stem/progenitor cell population within the primary dissociate and thus the tumor itself 32, 36. In these studies of glioblastoma and anaplastic astrocytoma, clonogenic cells from gliomas that manifested some degree of stemness in culture were shown to be neural cancer stem‐like cells based on the expression of phenotypic markers of neuronal and astroglial differentiation (and see Figure 5). Cell surface marker expression, fluorescence‐activated cell sorting (FACS) isolation and cloning, and reestablishment of tumorigenicity following xeno‐grafting was also described for both adult and pediatric brain tumors 24, 29, 59. Even with a prospective in addition to a retrospective identification of tumor stem cells using such isolation and culture enrichment approaches and potential biomarker applications, genetic and molecular mechanisms underlying and controlling their self‐renewal and differentiation are still unfortunately poorly understood.
Figure 6.
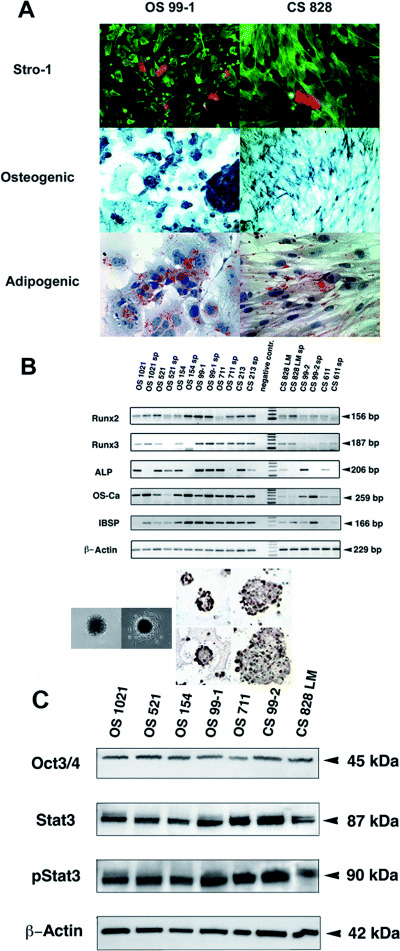
Examples of transformed cellular transdifferentiation potential from human bone tumors. Mesenchymal lineage and differentiation. A. Demonstration in osteosarcoma (OS 99‐1) and chondrosarcoma (CS 828) cells of a subpopulation of Stro‐1 positive cells. Activation of osteogenic and adipogenic programs of differentiation in bone sarcoma cultures grown in specific differentiation media. Osteogenic lineage is indicated by expression of alkaline phosphatase and adipogenic by accumulation of lipid vacuoles stained with Oil‐red‐O. B. Preservation of multiple osteo/chondral mesenchymal lineage markers (Runx2, Runx3, alkaline phosphatase, osteocalcin, and bone‐sialo protein in both sarcospheres and adherent cultures demonstrated by RT‐PCR. C. Western blot analysis of lysates from representative bone sarcoma cell cultures for the protein expression of Oct 3/4, STAT3, and activated (phosphorylated, p) STAT3. β‐Actin was used as a positive control for loading, membrane transfer, and immunoblotting (all cultures showed positive staining of protein bands of appropriate sizes, as indicated). The inset between (B) and (C) shows floating, attached sarcospheres, and following attachment their immunostaining for the pluripotency‐associated markers Oct3/4 and Nanog. [From 27; copyright Neoplasia 2005, with permission.]
As primitive neuroectodermal tumors, such as medulloblastoma, can be coaxed into a unique differentiation cascade that ultimately leads to the apparent generation of a viable embryo (41), it seems reasonable to assume that cancer stem cells in vitro should also be able to transdifferentiate and give rise to “normal” and heterologous cells and tissue types. Figure 6 is from our recent study of chondrosarcoma and osteosarcoma stem‐like cells (27) showing the ability to coax these cells into potentially normal bone and fat cells that possess all of the appropriate molecular markers of these tissues. Such a finding also provides a significant application of transdifferentiation principles to future treatments of mesenchymal stem cell‐derived tumors that exploits cellular rehabilitation in addition to chemotherapeutic cancer cell destruction.
CONCLUSIONS
In order to apply knowledge gained from differentiation, dedifferentiation and transdifferentiation of clonogenic stem/progenitor cells in culture to regenerative medicine, eg, restorative neuroscience, it seems necessary that we understand how to manipulate and insinuate new cells in the established and compromised circuitries within normal and diseased CNS. Brain reorganization following injury or disease may actually include “reactive” neurogenesis, because we have shown that a type of astrocytic cell, the MASC (40)—the same cell Alvarez‐Buylla and colleagues categorized as the “B” cell (21)—is the neurogenic cell during development (58) that may also attempt to dedifferentiate following CNS injury (62). The astroglial scar and its cadre of ECM‐expressing astrocytic cells may actually represent an attempt by reactive astrocytes to become neurogenic again to replace lost neurons, rather than just create a neurite‐growth inhibitory environment as mostly believed by the neuroregeneration field (for review, see 11, 23). It is postulated that MASCs and ESNPs have many attributes in common with neurogenic radial neuroepithelial cells (49) found throughout the neuraxis during CNS development. MASCs constitute a unique neurogenic niche in the adult CNS, but injuries and disease may induce what appear to be fully differentiated astrocytes to assume a neurogenic role whereby they upregulate their expressions of developmentally regulated proteins and attempt to recapitulate neurogenic programs. Neurogenic astrocyte‐like cells change their biochemistry throughout the neuraxis at the end of the second postnatal week in mice, and then assume a more differentiated phenotype where their activities are associated with standard operating procedures of a normal CNS. In vitro conditions can be created that restore neurogenic programs of these cells; and a goal of future studies is to resolve the precise molecular cascades responsible for lifelong plasticity of CNS cells. Unlocking multipotency of apparently differentiated mammalian CNS astrocytic cells, and further revealing their “phenotypic fluidity”(38), should contribute important information to the arsenal of known and unknown molecular messengers that could eventually direct reactive neurogenesis for lifelong neural repair.
As stem/progenitor and even differentiated cells seem to be able to change their nature rather abruptly and quickly in culture, there must be molecular interactions that can be tapped to guide dynamic alterations in phenotype for controlled fate choice and differentiation leading to desired cellular regeneration following cell loss that accompanies injury and disease. Likewise, tapping such controlled differentiation can have ramifications toward manipulating out of control growth of aberrant cell populations that is a hallmark of tumorigenesis. Recent reports have indicated that posttranscriptional regulators, including microRNAs (17) as well as the induction of neurogenic transcription factors (eg, NeuroD; 30) affect cell type‐specific gene expression, stem cell renewal, fate choice decisions and differentiation of neural precursors. Such genetic and epigenetic events are certainly involved in the rapid changes that occur in culture, whereby cells appear to be able to change their fate within surprisingly short periods of time, with apparent trans‐ and dedifferentiation potential that does support a notion of phenotypic fluidity. Within a continuum of cellular differentiation from the most primitive cells, eg, ES and germ cells, the most differentiated cells being terminally differentiated neurons or glia, the examples of unusual phenotypy reviewed here suggest we have yet to understand how cells decide to expand, choose their fate, or differentiate. As pointed out by Doetsch and colleagues in a recent review of roles for microRNAs in stem cell biology, “. . . The intrinsic state of stem cells depends on their spatial and temporal history and affects their responsiveness to extrinsic signals from the microenvironment. Stem cell self‐renewal and differentiation along neuronal and glial lineages are defined by the dynamic interplay between transcription, epigenetic control, and posttranscriptional regulators . . .”(17). This dynamic interplay may underlie some of the unexpected dedifferentiation and transdifferentiation events of astrocytes and neurons under particular culture conditions that may mimic aspects of the molecular milieu of developing tissue environments during embryonic and fetal development, as well as during adult neuropoiesis. There is still a lack of evidence for the existence of intermediate phenotypes such as asterons in vivo, but we cannot rule them out. They have been observed in solid tumor specimens where glioma cells express neuronal markers including beta III tubulin (43) that may relate to their dynamic organization of the microtubule cytoskeleton associated with cancer and cancer cells in vitro, where again asterons have been observed (32; Figure 5). It is hoped that an understanding of unusual cellular phenotypy, including the generation of intermediate and hybrid cellular forms, will increase our understanding of stem, progenitor, and differentiated cell plasticity that could be translated to regenerative medicine and new therapies for human diseases.
ACKNOWLEDGMENTS
The author was supported by grants from the NIH (NINDS NS 37556; NHLBI HL 70143), and the McKnight Brain Research Foundation.
REFERENCES
- 1. Al‐Hajj M, Clarke MF (2004) Self‐renewal and solid tumor stem cells. Oncogene 23:7274–7282. [DOI] [PubMed] [Google Scholar]
- 2. Alvarez‐Dolado M, Pardal R, Garcia‐Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez‐Buylla A (2003) Fusion of bone‐marrow‐derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425:968–973. [DOI] [PubMed] [Google Scholar]
- 3. Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ (1998) Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA 95:3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benninger F, Beck H, Wernig M, Tucker KL, Brustle O, Scheffler B (2003) Functional integration of embryonic stem cell‐derived neurons in hippocampal slice cultures. J Neurosci 23:7075–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertani N, Malatesta P, Volpi G, Sonego P, Perris R (2005) Neurogenic potential of human mesenchymal stem cells revisited: analysis by immunostaining, time‐lapse video and microarray. J Cell Sci 118:3925–3936. [DOI] [PubMed] [Google Scholar]
- 6. Bjorklund LM, Sanchez‐Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O (2002) Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA 99:2344–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL (1999) Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo . Science 283:534–537. [DOI] [PubMed] [Google Scholar]
- 8. Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730–737. [DOI] [PubMed] [Google Scholar]
- 9. Brazelton TR, Rossi FM, Keshet GI, Blau HM (2000) From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290:1775–1779. [DOI] [PubMed] [Google Scholar]
- 10. Brewer GJ (1999) Regeneration and proliferation of embryonic and adult rat hippocampal neurons in culture. Exp Neurol 159:237–247. [DOI] [PubMed] [Google Scholar]
- 11. Brodkey JA, Gates MA, Laywell ED, Steindler DA (1993) The complex nature of interactive neuroregeneration‐related molecules. Exp Neurol 123:251–270. [DOI] [PubMed] [Google Scholar]
- 12. Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD (1999) Embryonic stem cell‐derived glial precursors: a source of myelinating transplants [see comments]. Science 285:754–756. [DOI] [PubMed] [Google Scholar]
- 13. Brustle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RD (1997) In vitro‐generated neural precursors participate in mammalian brain development. Proc Natl Acad Sci USA 94:14809–14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW (2005) SDF‐1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 115:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR (2001) Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol 167:48–58. [DOI] [PubMed] [Google Scholar]
- 16. Chen KA, Laywell ED, Marshall G, Walton N, Zheng T, Steindler DA (2006) Fusion of neural stem cells in culture. Exp Neurol 198:129–135. [DOI] [PubMed] [Google Scholar]
- 17. Cheng LC, Tavazoie M, Doetsch F (2005) Stem cells: from epigenetics to microRNAs. Neuron 46:363–367. [DOI] [PubMed] [Google Scholar]
- 18. Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, Scott EW (2004) Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet 363:1432–1437. [DOI] [PubMed] [Google Scholar]
- 19. Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED (2005) Mesenchymal stem cells spontaneously express neural proteins in culture, and are neurogenic after transplantation. Stem Cells 24:1054–1064. [DOI] [PubMed] [Google Scholar]
- 20. Doetsch F (2003) The glial identity of neural stem cells. Nat Neurosci 6:1127–1134. [DOI] [PubMed] [Google Scholar]
- 21. Doetsch F, Caille I, Lim DA, Garcia‐Verdugo JM, Alvarez‐Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97:703–716. [DOI] [PubMed] [Google Scholar]
- 22. Dravida S, Pal R, Khanna A, Tipnis SP, Ravindran G, Khan F (2005) The transdifferentiation potential of limbal fibroblast‐like cells. Brain Res Dev Brain Res 160:239–251. [DOI] [PubMed] [Google Scholar]
- 23. Faissner A, Steindler D (1995) Boundaries and inhibitory molecules in developing neural tissues. Glia 13:233–254. [DOI] [PubMed] [Google Scholar]
- 24. Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A (2004) Isolation and characterization of tumorigenic, stem‐like neural precursors from human glioblastoma. Cancer Res 64:7011–7021. [DOI] [PubMed] [Google Scholar]
- 25. Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, Mora M, De Angelis MG, Fiocco R, Cossu G, Vescori AL (2000) Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci 3:986–991. [DOI] [PubMed] [Google Scholar]
- 26. Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV (2004) GFAP‐expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 7:1233–1241. [DOI] [PubMed] [Google Scholar]
- 27. Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN, Steindler DA (2005) Stem‐like cells in bone sarcomas: implications for tumorigenesis. Neoplasia 7:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB et al (2002) Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 8:607–612. [DOI] [PubMed] [Google Scholar]
- 29. Hemmati HD, Nakano I, Lazareff JA, Masterman‐Smith M, Geschwind DH, Bronner‐Fraser M, Kornblum HI (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 100:15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH (2004) Histone deacetylase inhibition‐mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA 101:16659–16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF 3rd, Boiani M, Scholer HR (2003) Derivation of oocytes from mouse embryonic stem cells. Science 300:1251–1256. [DOI] [PubMed] [Google Scholar]
- 32. Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA (2002) Human cortical glial tumors contain neural stem‐like cells expressing astroglial and neuronal markers in vitro. Glia 39:193–206. [DOI] [PubMed] [Google Scholar]
- 33. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz‐Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M et al (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49. [DOI] [PubMed] [Google Scholar]
- 34. Kehler J, Hubner K, Garrett S, Scholer HR (2005) Generating oocytes and sperm from embryonic stem cells. Semin Reprod Med 23:222–233. [DOI] [PubMed] [Google Scholar]
- 35. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ (2001) Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell 105:369–377. [DOI] [PubMed] [Google Scholar]
- 36. Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA (1999) Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol 156:333–344. [DOI] [PubMed] [Google Scholar]
- 37. Kukekov VG, Laywell ED, Thomas LB, Steindler DA (1997) A nestin‐negative precursor cell from the adult mouse brain gives rise to neurons and glia. Glia 21:399–407. [DOI] [PubMed] [Google Scholar]
- 38. Laywell ED, Kearns SM, Zheng T, Chen KA, Deng J, Chen HX, Roper SN, Steindler DA (2005) Neuron‐to‐astrocyte transition: phenotypic fluidity and the formation of hybrid asterons in differentiating neurospheres. J Comp Neurol 493:321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laywell ED, Kukekov VG, Steindler DA (1999) Multipotent neurospheres can be derived from forebrain subependymal zone and spinal cord of adult mice after protracted postmortem intervals. Exp Neurol 156:430–433. [DOI] [PubMed] [Google Scholar]
- 40. Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA (2000) Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA 97:13883–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li L, Connelly MC, Wetmore C, Curran T, Morgan JI (2003) Mouse embryos cloned from brain tumors. Cancer Res 63:2733–2736. [PubMed] [Google Scholar]
- 42. Lindvall O, Bjorklund A (2004) Cell therapy in Parkinson’s disease. NeuroRx 1:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martinez‐Diaz H, Kleinschmidt‐DeMasters BK, Powell SZ, Yachnis AT (2003) Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for class III beta‐tubulin. Arch Pathol Lab Med 127:1187–1191. [DOI] [PubMed] [Google Scholar]
- 44. Massengale M, Wagers AJ, Vogel H, Weissman IL (2005) Hematopoietic cells maintain hematopoietic fates upon entering the brain. J Exp Med 201:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCulloch EA, Till JE (1960) The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res 13:115–125. [PubMed] [Google Scholar]
- 46. Milosevic A, Goldman JE (2002) Progenitors in the postnatal cerebellar white matter are antigenically heterogeneous. J Comp Neurol 452:192–203. [DOI] [PubMed] [Google Scholar]
- 47. Mujtaba T, Rao MS (2002) Isolation of lineage‐restricted neural precursors from cultured ES cells. Methods Mol Biol 185:189–204. [DOI] [PubMed] [Google Scholar]
- 48. Munoz‐Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB (2004) Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long‐term survival. J Neurosci 24:4585–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR (2002) Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci 22:3161–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okano‐Uchida T, Himi T, Komiya Y, Ishizaki Y (2004) Cerebellar granule cell precursors can differentiate into astroglial cells. Proc Natl Acad Sci USA 101:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH (2001) Cell culture. Progenitor cells from human brain after death. Nature 411:42–43. [DOI] [PubMed] [Google Scholar]
- 52. Quesenberry PJ, Dooner G, Colvin G, Abedi M (2005) Stem cell biology and the plasticity polemic. Exp Hematol 33:389–394. [DOI] [PubMed] [Google Scholar]
- 53. Reier PJ (2004) Cellular transplantation strategies for spinal cord injury and translational neurobiology. NeuroRx 1:424–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111. [DOI] [PubMed] [Google Scholar]
- 55. Reynolds BA, Rietze RL (2005) Neural stem cells and neurospheres—re‐evaluating the relationship. Nat Methods 2:333–336. [DOI] [PubMed] [Google Scholar]
- 56. Sanchez‐Ramos J, Song S, Cardozo‐Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sandberg PR (2000) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164:247–256. [DOI] [PubMed] [Google Scholar]
- 57. Scheffler B, Schmandt T, Schroder W, Steinfarz B, Husseini L, Wellmer J, Seifert G, Karram K, Beck H, Blumcke I, Wiestler OD, Steinhauser C, Brüstle O (2003) Functional network integration of embryonic stem cell‐derived astrocytes in hippocampal slice cultures. Development 130:5533–5541. [DOI] [PubMed] [Google Scholar]
- 58. Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA (2005) Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci USA 102:9353–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401. [DOI] [PubMed] [Google Scholar]
- 60. Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ (2004) Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells 22:823–831. [DOI] [PubMed] [Google Scholar]
- 61. Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44. [DOI] [PubMed] [Google Scholar]
- 62. Steindler DA, Laywell ED (2003) Astrocytes as stem cells: nomenclature, phenotype, and translation. Glia 43:62–69. [DOI] [PubMed] [Google Scholar]
- 63. Suslov ON, Kukekov VG, Ignatova TN, Steindler DA (2002) Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci USA 99:14506–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416:542–545. [DOI] [PubMed] [Google Scholar]
- 65. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts [see comments] [published erratum appears in Science 1998 Dec 4;282(5395): 1827]. Science 282:1145–1147. [DOI] [PubMed] [Google Scholar]
- 66. Toma JG, Akhavan M, Fernandes KJ, Barnabe‐Heider F, Sadikot A, Kaplan DR, Miller FD (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–784. [DOI] [PubMed] [Google Scholar]
- 67. Wagers AJ, Sherwood RI, Christensen JL, Weissman IL (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297:2256–2259. [DOI] [PubMed] [Google Scholar]
- 68. Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al‐Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M (2003) Cell fusion is the principal source of bone‐marrow‐derived hepatocytes. Nature 422:897–901. [DOI] [PubMed] [Google Scholar]
- 69. Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364–370. [DOI] [PubMed] [Google Scholar]
- 70. Wurmser AE, Nakashima K, Summers RG, Toni N, D’Amour KA, Lie DC, Gage FH (2004) Cell fusion‐independent differentiation of neural stem cells to the endothelial lineage. Nature 430:350–356. [DOI] [PubMed] [Google Scholar]
- 71. Ying QL, Nichols J, Evans EP, Smith AG (2002) Changing potency by spontaneous fusion. Nature 416:545–548. [DOI] [PubMed] [Google Scholar]
- 72. Yoder MC, Williams DA (1995) Matrix molecule interactions with hematopoietic stem cells. Exp Hematol 23:961–967. [PubMed] [Google Scholar]
- 73. Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA (2001) In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19:1129–1133. [DOI] [PubMed] [Google Scholar]
- 74. Zheng T, Steindler DA, Laywell ED (2002) Transplantation of an indigenous neural stem cell population leading to hyperplasia and atypical integration. Cloning Stem Cells 4:3–8. [DOI] [PubMed] [Google Scholar]
- 75. Zigova T, Betarbet R, Soteres BJ, Brock S, Bakay RA, Luskin MB (1996) A comparison of the patterns of migration and the destinations of homotopically transplanted neonatal subventricular zone cells and heterotopically transplanted telencephalic ventricular zone cells. Dev Biol 173:459–474. [DOI] [PubMed] [Google Scholar]


