Abstract
Although neurotrophins of the nerve growth factor (NGF) family are best known for their neurite outgrowth‐inducing and survival‐promoting effects on neuronal cells, these are actually pleiotropic growth factors acting physiologically on many different cell types of our body. As for many other growth factors, dysregulation of neurotrophin signal transduction is found in a number of tumors where they can accompany or contribute to malignant transformation. Interestingly, tropomyosin‐related kinase (Trk) receptor activation can either support or suppress tumor growth, depending on the tumor type. These same divergent responses have been observed with neurotrophins binding to the p75NTR neurotrophin receptor on tumor cells. This article summarizes the current knowledge on the role of neurotrophins and their receptors in malignancies, with special focus on tumors of neuropathological interest.
TWO TYPES OF RECEPTORS, MANY DIFFERENT SIGNALING OUTCOMES
Nerve growth factor (NGF), brain‐derived neurotrophic factor (BDNF), neurotrophin‐3 (NT‐3) and neurotrophin‐4/5 (NT‐4/5) comprise the mammalian neurotrophins, a family of structurally related growth factors (GFs) that play a crucial role in the survival, differentiation, neurite outgrowth and maintenance of specific neuronal populations in the nervous system (4, 79). The effects of neurotrophins are mediated by two classes of cell‐surface receptors, a family of receptor tyrosine kinases (RTKs) called Trks (TrkA, TrkB and TrkC) as well as the still enigmatic p75NTR receptor, the first cloned member of the tumor necrosis factor receptor (TNFR) family (5). Whereas p75NTR binds all neurotrophins with lower affinity and specificity, Trk receptors bind neurotrophins with higher affinity and specificity. TrkA binds NGF and NT‐3, TrkB binds BDNF, NT‐3, NT‐4/5, and TrkC only binds NT‐3 (74).
Although classically known for their effects on neurons, neurotrophins are multifunctional GFs and exert numerous effects on non‐neuronal cells, including, but not limited to, differentiation of B‐lymphocytes (58), histamine release from mast cells (56), formation of intramyocardial blood vessels (88), hair follicle development (81), inhibition of myogenic differentiation in skeletal muscle satellite cells (53), migration of Schwann cells (2) and growth of follicles in the ovaries (59).
Under physiological circumstances, GF receptor activation needs to be limited in duration and amplitude. To this end, GF signaling is subjected to multiple layers of control, ensuring that ligand‐stimulated activated GF receptors are fine‐tuned and eventually down‐regulated. Negative regulation of GF signaling occurs via diverse mechanisms, including: (i) removal of ligand from the extracellular space by endocytosis; (ii) downregulation of surface receptors by shedding or endocytosis; (iii) dephosphorylation by phosphatases; and (iv) endosomal trafficking to lysosomes followed by proteolytic degradation.
A hallmark of cancer cells is the acquired self‐sufficiency of GF signals (23). The purpose of GFs in normal cell–cell communication, exogenous instructions on how to behave in order to serve the organism, can be perverted in tumors as their cells develop autocrine loops by coexpression of GFs and their receptors. Alternatively, malignant cells might express altered GF receptors that behave in a constitutively active way, thus escaping control by exogenous or endogenous down‐regulation mechanisms.
Like many other GFs, NGF signal transduction may be “hijacked” by malignant cells to use for their own advantage. Although neurotrophins are well known for their role in neuronal development and physiology, the relevance of neurotrophins to the important field of tumor biology is, unfortunately, not sufficiently appreciated by most neuroscientists. This is surprising, given that NGF was originally purified from a sarcoma (77), that the most popular bioassay for NGF is performed using a pheochromocytoma cell line (rat PC12 cells) (21), that TrkA was discovered in a human colon carcinoma biopsy (44) and that p75NTR was purified from a human melanoma cell line (42).
Interestingly, a given RTK might signal proliferation or differentiation, depending on the cell type. Moreover, two different RTKs can signal differentiation or proliferation within the same cell. Accordingly, neurotrophins can trigger distinct (sometimes opposing) biological outcomes, such as life vs. death, and proliferation vs. differentiation (27). The decision of a cell between proliferation and differentiation is the most dramatic example of RTK signaling, and highly relevant to the oncology field. The PC12 pheochromocytoma cell line, established by Greene and Tischler (21), is a suitable model to study the signaling pathways that underlie RTK‐mediated differentiation vs. proliferation. These cells express both the epidermal growth factor receptor (EGFR) and TrkA RTKs, and respond to NGF with differentiation and to EGF with proliferation. What are the pathways specific to proliferation or differentiation? In PC12 cells, the duration of mitogen‐activated protein kinase signaling is of high importance, because long‐term extracellular signal‐regulated kinases 1 and 2 (ERK1/2) activation (by NGF/TrkA) triggers differentiation whereas short‐term activation (by EGF/EGFR) triggers proliferation. NGF‐dependent long‐term ERK1/2 activation and differentiation depends on a signaling pathway containing the proteins Crk, FRS‐2 and Rap1 (30, 48, 91). Accordingly, phosphorylation of FRS‐2 and prolonged activation of ERK1/2 correlates with neurotrophin‐dependent cell cycle arrest and differentiation. Thus, the context and specific downstream complexes determine the downstream effect of activated RTKs.
Rap1 is of special interest because it is activated in endosomes and thus leads to another emerging aspect controlling RTK signaling, the subcellular localization of activated RTKs (20, 26). Recent research on the EGFR and Trk receptors has shown that activated receptors along the cell surface are internalized into endosomes where they continue to signal intracellularly. Internalization and endosomal sorting also determines the destruction RTKs by trafficking to lysosomes. Internalized receptors are moved to early endosomes. In these organelles, RTKs are sorted to two different trafficking pathways: they either return to the cell surface via recycling endosomes or move into late endosomes (also called multivesicular bodies) and eventually lysosomes, where they undergo proteolysis (80). The sorting decision in early endosomes controls RTK responsiveness by controlling the half‐life and surface levels of RTKs. Most emphasis for neurotrophin‐mediated Trk signaling has focused on the issue of retrograde signaling from distal neuronal processes (95). The regulation of Trk trafficking is also of high importance for tumor cell biology (3). Rab GTPases, which are attached to endosomal membranes via lipid anchors, are key regulators of endosome trafficking and should therefore be of interest to oncologists (6). Accordingly, our group recently revealed the importance of Rab7 activity in controlling the endosomal transport and signaling of TrkA in PC12 pheochromocytoma cells: inhibiting Rab7 function potentiated the differentiation response to NGF (72). Supporting an important role of Rab7 and endosomal trafficking in tumor cells, Rab7 was recently found as one of the major targets of farnesyl‐transferase inhibitors, an emerging class of anticancer drugs (36).
Dysregulation of RTK signaling can occur by means other than altered trafficking. Classically, tumors synthesize altered receptor proteins with enhanced activity. Structural alterations of RTKs may occur by point mutations, deletions, chromosomal rearrangements and alternative RNA splicing (23). Regarding Trk receptors, all of these scenarios have been observed in tumors (9). Thus far, not much is known regarding tumor‐specific dysregulation of p75NTR caused by such structural alterations, and as opposed to Trk receptors, the mechanisms of p75NTR effects are still largely unknown. As the first cloned member of the TNFR family (8, 65), the p75NTR pan‐neurotrophin receptor harbors a type II death domain within its intracellular domain (ICD). However, domain‐swapping experiments between p75NTR and the prototypical death receptor Fas revealed that p75NTR is not an efficient death inducer (34). Although the role of p75NTR as a physiological proapoptotic receptor in vivo is a matter of discussion, it has often been observed that p75NTR triggers death in cells that are subjected to stress or overexpress p75NTR (69). In vivo experiments showed that transgenic mice overexpressing p75NTR in the brain are viable, lacking any apparent neuropathological alterations such as neurodegeneration or tumors (60), whereas mice overexpressing the ICD of p75NTR showed increased neuronal death (41). Loss‐of‐function experiments using two different strains of p75NTR knockout mice led to complicated results (38, 61, 86), some of which support a proapoptotic role for p75NTR. However, the phenotype of p75NTR knockout mice also clearly showed that p75NTR promotes survival of sensory neurons and sympathetic neurons (39). High expression levels of p75NTR have been noted in many tumor cell types (see below), and functional experiments in vitro have found evidence for a tumor‐supportive, as well as tumor‐suppressive, function of p75NTR, depending on the tumor type. Unfortunately, there is currently no unifying model on the p75NTR downstream signaling pathways that integrates all of the numerous p75NTR ICD interacting proteins identified by yeast‐two‐hybrid technology (19).
NEUROTROPHINS AND TUMORS OF THE NERVOUS SYSTEM
Many studies have examined expression of neurotrophins and their receptors in tumor‐derived biopsies, and some striking correlations between their expression in tumors and classification/prognosis have been revealed. As previously discussed, expression or activity of a GF receptor per se is not a sign of malignancy; rather the signaling context (eg, FRS‐2 phosphorylation or Rap1 activation in PC12 cells) determines proliferation vs. differentiation in response to RTK activation. Thus, positive immunostaining for an RTK (such as neurotrophin receptors) in a large percentage of biopsies from a certain tumor does not prove a causal relationship between RTK activity and tumor progression. However, functional experiments from cell cultures or animals often provided compelling evidence for a causal role of neurotrophins in certain neoplasias. These results should be relevant to patients, as some neurotrophins (eg, BDNF) are highly expressed in the adult brain (40) and also reach considerable concentrations in adult human serum (ie, 15 ng/mL for BDNF) (68). In the following paragraphs, we will review important examples of tumors where neurotrophins have been shown to play a significant role.
Neuroblastoma. Neuroblastoma is one of the most common malignant childhood tumors and is characterized by a high rate of spontaneous remissions (25). Neuroblastomas develop from neural crest‐derived sympathetic precursor cells, well known to be responsive to neurotrophins (85). Consistent with this notion, it was revealed that neurotrophins play a major role in the development and progression of neuroblastomas. The groups of Nakagawara and Brodeur first established that expression of full‐length TrkA and TrkC correlates with favorable prognosis, while expression of full‐length TrkB is associated with unfavorable, more aggressive, N‐myc‐amplified neuroblastoma (54, 92). Moreover, truncated TrkB lacking a kinase domain was preferentially expressed in differentiated tumors (ganglioneuromas and ganglioneuroblastomas), while full‐length TrkB is expressed almost exclusively in immature neuroblastomas with N‐myc amplification (55). These authors also found coexpression of BDNF with its TrkB receptor and concluded that BDNF promotes cellular survival in an autocrine or paracrine manner in TrkB‐expressing human neuroblastomas. Matsumoto et al came to similar conclusions by showing that a large number of prognostically poor neuroblastomas constitutively express BDNF and variably coexpress TrkB, whereas high levels of TrkA predict a better prognosis (45). It is interesting to note that adult sensory neurons physiologically require TrkB/BDNF autocrine loops for survival (1).
As one study using a series of primary neuroblastoma biopsies demonstrated that TrkA expression was actually not necessarily associated with TrkA activation (29), it was important to perform functional studies on neuroblastoma cell lines stimulated with neurotrophins to elucidate the effects of neurotrophins in neuroblastoma cells. Some of these studies showed that TrkA triggers proapoptotic signaling pathways (37), whereas others found that TrkA triggers neuronal differentiation (46, 54). In both cases, TrkA signaling would be beneficial for patients.
When similar in vitro assays were performed with TrkB‐expressing neuroblastoma cell lines, it was found that TrkB triggers prosurvival pathways, which is compatible with the association of TrkB expression and a poor prognosis for patients (45, 73). However, it was also reported that activation of TrkB stimulates differentiation and neurite outgrowth in neuroblastoma cell lines or primary isolates (31, 82). These results exemplify that “all or nothing results” are rarely seen in tumors, given the heterogeneity among primary isolates and possibly even between different areas within one tumor. Moreover, these findings might also indicate that the role of Trk receptors in neuroblastomas is more complex than previously thought. Indeed, most recently, Tacconelli et al revised and extended the previous hypothesis (TrkA = good prognosis) by the discovery of a novel TrkA splice variant, coined TrkAIII. TrkAIII lacks exons 6, 7 and 9 (amino acids 192–284) within its extracellular domain, and cannot bind to NGF. This TrkA variant acts as a ligand‐independent, constitutively active RTK, analogous to many oncogenes (83). Interestingly, TrkAIII expression is restricted to undifferentiated neuronal progenitors, neuroblastomas (24 primary isolates were examined) and a subset of neural crest‐derived tumors. Importantly, the expression of this new TrkA splice variant was found to be induced by hypoxia, a condition often encountered within tumors. Although these findings need to be extended to larger series of clinical samples, this novel TrkA splice variant adds an additional layer of complexity to our view on the action of neurotrophins in neuroblastoma. It will be interesting to determine if other Trk receptors have novel neuroblastoma‐specific splice variants. Moreover, the TrkAIII‐specific epitope within the extracellular domain created by splicing in tumors might turn out to be an attractive target for therapeutic monoclonal antibodies, in a similar way to promising monoclonal antibodies against the vIII splice variant of EGFR, which has been detected in some gliomas (49).
Medulloblastoma. Medulloblastomas originate from cerebellar granule cells and represent the most common central nervous system malignancy in children. Neurotrophins play an important role in the normal development of cerebellar neurons. Accordingly, two groups (22, 75) noticed a correlation between TrkC expression and prognosis of medulloblastoma patients, with TrkC expression representing the most powerful predictor of clinical outcome. It was reported that patients whose tumors had little or no TrkC showed an approximately fivefold greater risk of death than children with tumors expressing high levels of TrkC. In a cohort consisting of 81 medulloblastoma [considered infratentorial primitive neuroectodermal tumors (PNETs)] and six supratentorial PNETs, 5‐year survival was 89% for patients with tumors expressing high levels of TrkC and 47% for patients with tumors that expressed little/no levels of TrkC (22). Accordingly, analysis of biopsies showed that TrkC expression in individual tumor cells is strongly correlated with apoptosis. Moreover, functional analyses of medulloblastoma cell lines found that the TrkC ligand NT‐3 induces apoptosis via activation of TrkC in vitro and overexpression of TrkC inhibits the growth of intracerebral xenografts of TrkC‐transfected medulloblastoma cells in nude mice (32). Examination of the responsible downstream pathways revealed that the immediate early genes c‐jun and c‐fos play major roles in this proapoptotic Trk signaling pathway. Thus, the ability of NT‐3/TrkC to induce apoptosis in medulloblastoma is reminiscent of the ability of NGF/TrkA (full‐length isoform) to trigger apoptosis in neuroblastoma cells, even though the downstream signaling pathways are different.
Taken together, these findings provide compelling evidence for TrkC being a useful prognostic marker in medulloblastoma patients, and recently large‐scale gene array expression analyses confirmed the correlation of TrkC expression with positive prognosis (64). Also, other neurotrophins and Trks have been detected in medulloblastomas (15), but no predictive value has been established. Buhren et al reported that p75NTR expression is characteristic of a subvariant of medulloblastoma, the desmoplastic/nodular medulloblastoma, and that p75NTR is predominantly found in proliferative areas (7). The functional role of p75NTR and its ligands in desmoplastic medulloblastoma is so far not known.
Glioma. Gliomas are the most common nervous system tumor in adults, with glioblastomas, astrocytomas and oligodendrogliomas representing the most frequent subtypes (66). Wang et al used immunohistochemistry to examine the expression of Trk receptors in a cohort of glioma cases. Interestingly, they found that TrkA, TrkB and TrkC immunoreactivity was only found in astrocytomas but not in oligodendrogliomas (90). Strikingly, Trk expression appeared to be limited to the astrocytic component in mixed gliomas (oligoastrocytomas) (see Figure 1). Moreover, Trk staining was also observed in reactive astrocytes surrounding tumors.
Figure 1.
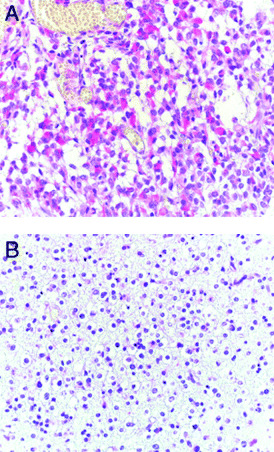
A. Astrocytic component in a mixed oligoastrocytoma showing distinct cytoplasmic TrkC immunoreactivity (red). Paraffin section, hematoxilin counterstain; ×350. B. The oligodendroglial component of the same tumor lacks TrkC immunoreactivity. Paraffin section, hematoxilin counterstain; ×350.
Is there a correlation between Trk expression and histological grade of gliomas? Whereas one group reported no such correlation in their biopsies (90), another group found an interesting pattern, in that high expression of Trks was found in low‐grade astrocytoma (I and II), whereas no expression was found in high‐grade gliomas (giant cell glioblastoma and glioblastoma multiforme) (87). This indicates that TrkA and TrkB may play a role in the early stage tumor pathophysiology. Some human glioblastoma cell lines (U251, U87 and U373) are actually responsive to NGF, with NGF exerting a mitogenic effect via TrkA receptors (78). However, it has been reported that Trk receptor expression is rapidly lost when cell lines are being established from primary astrocytoma, preventing the examination of the signaling pathways of endogenous Trk receptors in these astrocytoma cells (90). Taken together, these results leave the question of any functional role of Trk receptors in astrocytoma development or progression unanswered.
NEUROTROPHINS AND TUMORS OUTSIDE THE NERVOUS SYSTEM
There are many reports on neurotrophins in neoplasms unrelated to the nervous system (18), such as myeloma (62), acute myeloid leukemia (16), fibrosarcoma (33), hepatocellular carcinoma (93), pancreatic cancer (57), lung cancer (67) and thyroid papillary carcincoma (47). Because of space limitations, we will discuss only a few selected examples.
Prostate cancer. The normal prostate is one of the most abundant sources of NGF outside the nervous system. Smooth muscle stromal cells express NGF and BDNF but lack any NGF or BDNF receptors. In contrast, epithelial cells express both NGF receptors (p75NTR and TrkA) and both BDNF receptors (TrkB and p75NTR) (10). This suggests a molecular choreography in which stromal cell‐derived NGF and BDNF may interact via paracrine mechanisms with TrkA and TrkB receptors on the adjacent epithelial cells. In striking contrast, malignant prostate epithelial cells not only express NGF and BDNF but also coexpress the corresponding TrkB and TrkC receptors, but apparently lose expression of p75NTR (52). This fascinating finding suggests the development of autocrine loops during progression to malignancy (71). Interestingly, migration of malignant cells frequently occurs along nerves within the prostate (10), which may provide a rich source of neurotrophins, acting as chemoattractive guidance clues for tumor cell migration.
Expression of p75NTR is lost during tumor progression, possibly indicating a role for p75NTR as a tumor suppressor in the prostate gland. This hypothesis is indeed supported by functional data (35). Expression of p75NTR increased quiescence in a series of prostate tumor cells in vitro in a dose‐dependent fashion. When this same series of tumor cells were injected into the flanks of immunosupressed mice, the growth of the resulting tumors was diminished in direct proportion to p75NTR expression levels. In addition, the increase in p75NTR expression correlated with a dose‐dependent increase in tumor cell apoptosis.
In contrast to p75NTR, the Trk receptors appear to be involved in malignant progression as their levels appear to increase with increasing malignancy of prostate cancer. Rodent models have shown that inhibition of autocrine Trk signaling via the small molecule Trk inhibitor CEP‐751, developed by Cephalon Inc, induces the apoptotic death of malignant prostate cells (50). Also, neutralizing antineurotrophin antibodies were effective for inhibiting the growth of prostate tumor xenografts in nude mice (51).
Breast cancer. High expression of NGF was detected in breast cancer cell lines in vitro as well as in tumor biopsies (13). In contrast, NGF production could not be detected in normal mammary gland epithelial cells. NGF receptor expression was examined in a series of 363 biopsies, and expression of TrkA positively correlated with the histological grade. On the other hand, expression of p75NTR was linked to tumor type, with low levels of p75NTR expression more frequently in ductular‐type tumors (11). Rather unexpectedly, high levels of TrkA expression correlate with a better overall survival. Potentially, TrkA changes the signaling pathway from one that initiates proliferation to one that promotes differentiation. Alternatively, differential expression of different TrkA isoforms might contribute to this unexpected observation.
Functional experiments demonstrated that the survival and growth of breast cancer cells in vitro was strongly inhibited by either neutralizing anti‐NGF antibodies or pharmacological inhibitors of TrkA, indicating an NGF autocrine loop for breast cancer cells (12). Moreover, TrkA collaborates with human epidermal growth factor receptor 2 (HER2) in promoting the growth of breast cancer cells (84).
Melanoma. Melanomas are neural crest‐derived neoplasms and considered among the most aggressive tumors known in humans. Morphologically, melanomas can be subdivided in several subtypes, such as epithelioid, pleomorphic spindle cell and desmoplastic melanoma. Iwamoto et al found that there was no detectable p75NTR immunostaining in melanocytes of the normal epidermis, whereas 13 of 14 benign nevi showed detectable p75NTR. This was primarily within the spindled nevocytic structures within the dermis (28). Interestingly, p75NTR is highly expressed in desmoplastic melanomas and spindle cell melanomas and weakly expressed in the epithelioid melanomas. Is there a functional role of p75NTR signaling in melanomas? As no TrkA expression is found in melanoma cells, responsiveness to NGF should be solely mediated by p75NTR. Marchetti et al performed in vitro experiments using melanoma cell lines to address the role of neurotrophins in these cells and found that NGF/p75NTR signaling promotes the survival of melanoma cells (43). These authors also found TrkC expression in these cells. Interestingly, they observed the presence of NGF and NT‐3 in tumor‐adjacent tissues at the invasive front of melanoma brain metastases, which might indicate a paracrine activation of p75NTR and TrkC on melanoma cells by NGF and NT‐3 produced by nearby glial cells. Besides promoting melanoma cell survival, neurotrophins also induce the expression of heparanase by melanoma cells, an enzyme important for local invasion and metastasis by cleaving heparan sulfate chains of proteoglycans and thus modifying the extracellular matrix of tumor cells (89). Moreover, Shonukan et al established that neurotrophins are chemotactic for melanoma cells, triggered by p75NTR‐mediated dephosphorylation of the actin‐bundling protein fascin (76).
CONCLUSIONS AND CONSIDERATIONS FOR THE FUTURE
Neurotrophin receptors are expressed in many important human cancers, and in some cases there is compelling evidence that neurotrophins play a major role in tumor cell biology. Neurotrophins can either support (eg, p75NTR in melanoma; p75NTR and TrkA in breast cancer) or suppress (eg, p75NTR in prostate cancer and TrkC in medulloblastoma) tumor growth, depending on the tumor type. Understanding the receptor signal transduction context that leads to opposing outcomes in different tumors continues to be a challenge. For potential treatment options, inhibition of neurotrophin signaling can be beneficial in some cases and detrimental in other cases. Fortunately, neurotrophin signaling blocking reagents such as neutralizing monoclonal antibodies (24), receptor extracellular domain‐Fc fusion proteins (17) and low molecular weight chemical inhibitors (70) are already available and might be part of the oncologist’s therapeutic repertoire in the future. On the other hand, it will be necessary to stimulate neurotrophin signaling in certain other tumor patients. To this end, recombinant neurotrophins, agonistic peptides (94) and low molecular weight compounds (63) might be applied to patients some day.
The potential for novel, tumor‐specific splice variants of Trks and p75NTR is of special interest to tumor pathophysiology. By analogy to the rewarding work performed on tumor‐specific splice variants of EGFR carrying neo‐epitopes within their extracellular domain (such as the EGFRvIII expressed in gliomas), this line of research might yield novel avenues to attack tumors with tumor splice variant‐specific monoclonal antibodies, which will be useful not only for therapeutic but also diagnostic purposes. In addition to the emerging importance of novel neurotrophin receptor splice variants, the recent finding of TrkB as a key player in tumor cell survival after detachment from the extracellular matrix (a process called “anoikis”) will be of major importance (14). Understanding these mechanisms and learning to inhibit anoikis would benefit essentially all patients threatened with those tumors known to metastasize.
ACKNOWLEDGMENTS
We would like to acknowledge the financial support of the START program of the medical faculty of the RWTH Aachen, the Bundesministerium für Bildung und Forschung (BMBF), the European Union (EURON MC‐EST), the Swiss National Science Foundation, the Bernese Cancer League, the Ehmann Foundation (Savognin) and the Stiftung zur Krebsbekämpfung (Zürich).
REFERENCES
- 1. Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM (1995) A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 374:450–453. [DOI] [PubMed] [Google Scholar]
- 2. Anton ES, Weskamp G, Reichardt LF, Matthew WD (1994) Nerve growth factor and its low‐affinity receptor promote Schwann cell migration. Proc Natl Acad Sci USA 91:2795–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bache KG, Slagsvold T, Stenmark H (2004) Defective downregulation of receptor tyrosine kinases in cancer. EMBO J 23:2707–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bibel M, Barde YA (2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14:2919–2937. [DOI] [PubMed] [Google Scholar]
- 5. Bothwell M (1995) Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci 18:223–253. [DOI] [PubMed] [Google Scholar]
- 6. Bucci C, Chiariello M (2006) Signal transduction grabs attention. Cell Signal 18:1–8. [DOI] [PubMed] [Google Scholar]
- 7. Buhren J, Christoph AH, Buslei R, Albrecht S, Wiestler OD, Pietsch T (2000) Expression of the neurotrophin receptor p75NTR in medulloblastomas is correlated with distinct histological and clinical features: evidence for a medulloblastoma subtype derived from the external granule cell layer. J Neuropathol Exp Neurol 59:229–240. [DOI] [PubMed] [Google Scholar]
- 8. Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR, Sehgal A (1986) Gene transfer and molecular cloning of the human NGF receptor. Science 232:518–521. [DOI] [PubMed] [Google Scholar]
- 9. Coulier F, Kumar R, Ernst M, Klein R, Martin‐Zanca D, Barbacid M (1990) Human trk oncogenes activated by point mutation, in‐frame deletion, and duplication of the tyrosine kinase domain. Mol Cell Biol 10:4202–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dalal R, Djakiew D (1997) Molecular characterization of neurotrophin expression and the corresponding tropomyosin receptor kinases (trks) in epithelial and stromal cells of the human prostate. Mol Cell Endocrinol 134:15–22. [DOI] [PubMed] [Google Scholar]
- 11. Descamps S, Pawlowski V, Revillion F, Hornez L, Hebbar M, Boilly B, Hondermarck H, Peyrat JP (2001) Expression of nerve growth factor receptors and their prognostic value in human breast cancer. Cancer Res 61:4337–4340. [PubMed] [Google Scholar]
- 12. Dolle L, El Yazidi‐Belkoura I, Adriaenssens E, Nurcombe V, Hondermarck H (2003) Nerve growth factor overexpression and autocrine loop in breast cancer cells. Oncogene 22:5592–5601. [DOI] [PubMed] [Google Scholar]
- 13. Dolle L, Adriaenssens E, El Yazidi‐Belkoura I, Le Bourhis X, Nurcombe V, Hondermarck H (2004) Nerve growth factor receptors and signaling in breast cancer. Curr Cancer Drug Targets 4:463–470. [DOI] [PubMed] [Google Scholar]
- 14. Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS (2004) Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 430:1034–1039. [DOI] [PubMed] [Google Scholar]
- 15. Eberhart CG, Kaufman WE, Tihan T, Burger PC (2001) Apoptosis, neuronal maturation, and neurotrophin expression within medulloblastoma nodules. J Neuropathol Exp Neurol 60:462–9. [DOI] [PubMed] [Google Scholar]
- 16. Eguchi M, Eguchi‐Ishimae M, Tojo A, Morishita K, Suzuki K, Sato Y, Kudoh S, Tanaka K, Setoyama M, Nagamura F, Asano S, Kamada N (1999) Fusion of ETV6 to neurotrophin‐3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25). Blood 93:1355–1363. [PubMed] [Google Scholar]
- 17. Evangelopoulos ME, Weis J, Kruttgen A (2004) Neurotrophin effects on neuroblastoma cells: correlation with trk and p75NTR expression and influence of Trk receptor bodies. J Neurooncol 66:101–110. [DOI] [PubMed] [Google Scholar]
- 18. Fanburg‐Smith JC, Miettinen M (2001) Low‐affinity nerve growth factor receptor (p75) in dermatofibrosarcoma protuberans and other nonneural tumors: a study of 1150 tumors and fetal and adult normal tissues. Hum Pathol 32:976–983. [DOI] [PubMed] [Google Scholar]
- 19. Gentry JJ, Barker PA, Carter BD (2004) The p75 neurotrophin receptor: multiple interactors and numerous functions. Prog Brain Res 146:25–39. [DOI] [PubMed] [Google Scholar]
- 20. Ginty DD, Segal RA (2002) Retrograde neurotrophin signaling: trk‐ing along the axon. Curr Opin Neurobiol 12:268–274. [DOI] [PubMed] [Google Scholar]
- 21. Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73:2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grotzer MA, Janss AJ, Fung K, Biegel JA, Sutton LN, Rorke LB, Zhao H, Cnaan A, Phillips PC, Lee VM, Trojanowski JQ (2000) TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol 18:1027–1035. [DOI] [PubMed] [Google Scholar]
- 23. Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. [DOI] [PubMed] [Google Scholar]
- 24. Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, Davies AM (2006) Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci 27:85–91. [DOI] [PubMed] [Google Scholar]
- 25. Hoehner JC, Hedborg F, Eriksson L, Sandstedt B, Grimelius L, Olsen L, Pahlman S (1998) Developmental gene expression of sympathetic nervous system tumors reflects their histogenesis. Lab Invest 78:29–45. [PubMed] [Google Scholar]
- 26. Howe CL, Mobley WC (2005) Long‐distance retrograde neurotrophic signaling. Curr Opin Neurobiol 15:40–48. [DOI] [PubMed] [Google Scholar]
- 27. Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwamoto S, Burrows RC, Agoff SN, Piepkorn M, Bothwell M, Schmidt R (2001) The p75 neurotrophin receptor, relative to other Schwann cell and melanoma markers, is abundantly expressed in spindled melanomas. Am J Dermatopathol 23:288–294. [DOI] [PubMed] [Google Scholar]
- 29. Iwata H, Ito T, Mutoh T, Ishiguro Y, Xiao H, Hamaguchi M (1994) Abundant but inactive‐state gp140proto‐trk is expressed in neuroblastomas of patients with good prognosis. Jpn J Cancer Res 85:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kao S, Jaiswal RK, Kolch W, Landreth GE (2001) Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem 276:18169–18177. [DOI] [PubMed] [Google Scholar]
- 31. Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ (1993) Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Eukaryotic Signal Transduction Group. Neuron 11:321–331. [DOI] [PubMed] [Google Scholar]
- 32. Kim JY, Sutton ME, Lu DJ, Cho TA, Goumnerova LC, Goritchenko L, Kaufman JR, Lam KK, Billet AL, Tarbell NJ, Wu J, Allen JC, Stiles CD, Segal RA, Pomeroy SL (1999) Activation of neurotrophin‐3 receptor TrkC induces apoptosis in medulloblastomas. Cancer Res 59:711–719. [PubMed] [Google Scholar]
- 33. Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH (1998) A novel ETV6‐NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet 18:184–187. [DOI] [PubMed] [Google Scholar]
- 34. Kong H, Kim AH, Orlinick JR, Chao MV (1999) A comparison of the cytoplasmic domains of the Fas receptor and the p75 neurotrophin receptor. Cell Death Differ 6:1133–1142. [DOI] [PubMed] [Google Scholar]
- 35. Krygier S, Djakiew D (2002) Neurotrophin receptor p75(NTR) suppresses growth and nerve growth factor‐mediated metastasis of human prostate cancer cells. Int J Cancer 98:1–7. [DOI] [PubMed] [Google Scholar]
- 36. Lackner MR, Kindt RM, Carroll PM, Brown K, Cancilla MR, Chen C, De Silva H, Franke Y, Guan B, Heuer T, Hung T, Keegan K, Lee JM, Manne V, O’Brien C, Parry D, Perez‐Villar JJ, Reddy RK, Xiao H, Zhan H, Cockett M, Plowman G, Fitzgerald K, Costa M, Ross‐Macdonald P (2005) Chemical genetics identifies Rab geranylgeranyl transferase as an apoptotic target of farnesyl transferase inhibitors. Cancer Cell 7:325–336. [DOI] [PubMed] [Google Scholar]
- 37. Lavoie JF, Lesauteur L, Kohn J, Wong J, Furtoss O, Thiele CJ, Miller FD, Kaplan DR (2005) TrkA induces apoptosis of neuroblastoma cells and does so via a p53‐dependent mechanism. J Biol Chem 280:29199–29207. [DOI] [PubMed] [Google Scholar]
- 38. Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R (1992) Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69:737–749. [DOI] [PubMed] [Google Scholar]
- 39. Lee KF, Davies AM, Jaenisch R (1994) p75‐deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development 120:1027–1033. [DOI] [PubMed] [Google Scholar]
- 40. Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD (1990) NT‐3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron 5:501–509. [DOI] [PubMed] [Google Scholar]
- 41. Majdan M, Lachance C, Gloster A, Aloyz R, Zeindler C, Bamji S, Bhakar A, Belliveau D, Fawcett J, Miller FD, Barker PA (1997) Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis. J Neurosci 17:6988–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marano N, Dietzschold B, Earley JJ Jr, Schatteman G, Thompson S, Grob P, Ross AH, Bothwell M, Atkinson BF, Koprowski H (1987) Purification and amino terminal sequencing of human melanoma nerve growth factor receptor. J Neurochem 48:225–232. [DOI] [PubMed] [Google Scholar]
- 43. Marchetti D, Denkins Y, Reiland J, Greiter‐Wilke A, Galjour J, Murry B, Blust J, Roy M (2003) Brain‐metastatic melanoma: a neurotrophic perspective. Pathol Oncol Res 9:147–158. [DOI] [PubMed] [Google Scholar]
- 44. Martin‐Zanca D, Hughes SH, Barbacid M (1986) A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 319:743–748. [DOI] [PubMed] [Google Scholar]
- 45. Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ (1995) Expression of brain‐derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res 55:1798–1806. [PubMed] [Google Scholar]
- 46. Matsushima H, Bogenmann E (1990) Nerve growth factor (NGF) induces neuronal differentiation in neuroblastoma cells transfected with the NGF receptor cDNA. Mol Cell Biol 10:5015–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD (1999) Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA 96:4540–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meakin SO, MacDonald JI, Gryz EA, Kubu CJ, Verdi JM (1999) The signaling adapter FRS‐2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem 274:9861–9870. [DOI] [PubMed] [Google Scholar]
- 49. Mellinghoff IK, Wang MY, Vivanco I, Haas‐Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, Riggs BL, Horvath S, Liau LM, Cavenee WK, Rao PN, Beroukhim R, Peck TC, Lee JC, Sellers WR, Stokoe D, Prados M, Cloughesy TF, Sawyers CL, Mischel PS (2005) Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 353:2012–2024. [DOI] [PubMed] [Google Scholar]
- 50. Miknyoczki SJ, Chang H, Klein‐Szanto A, Dionne CA, Ruggeri BA (1999) The Trk tyrosine kinase inhibitor CEP‐701 (KT‐5555) exhibits significant antitumor efficacy in preclinical xenograft models of human pancreatic ductal adenocarcinoma. Clin Cancer Res 5:2205–2212. [PubMed] [Google Scholar]
- 51. Miknyoczki SJ, Wan W, Chang H, Dobrzanski P, Ruggeri BA, Dionne CA, Buchkovich K (2002) The neurotrophin‐trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma xenografts in nude mice. Clin Cancer Res 8:1924–1931. [PubMed] [Google Scholar]
- 52. Montano X, Djamgoz MB (2004) Epidermal growth factor, neurotrophins and the metastatic cascade in prostate cancer. FEBS Lett 571:1–8. [DOI] [PubMed] [Google Scholar]
- 53. Mousavi K, Jasmin BJ (2006) BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci 26:5739–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nakagawara A, Arima‐Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM (1993) Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 328:847–854. [DOI] [PubMed] [Google Scholar]
- 55. Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM (1994) Expression and function of TRK‐B and BDNF in human neuroblastomas. Mol Cell Biol 14:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nassenstein C, Braun A, Nockher WA, Renz H (2005) Neurotrophin effects on eosinophils in allergic inflammation. Curr Allergy Asthma Rep 5:204–211. [DOI] [PubMed] [Google Scholar]
- 57. Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ (2004) Nerve growth factor stimulates MMP‐2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis 21:285–292. [DOI] [PubMed] [Google Scholar]
- 58. Otten U, Ehrhard P, Peck R (1989) Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci USA 86:10059–10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paredes A, Romero C, Dissen GA, DeChiara TM, Reichardt L, Cornea A, Ojeda SR, Xu B (2004) TrkB receptors are required for follicular growth and oocyte survival in the mammalian ovary. Dev Biol 267:430–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patil N, Lacy E, Chao MV (1990) Specific neuronal expression of human NGF receptors in the basal forebrain and cerebellum of transgenic mice. Neuron 4:437–447. [DOI] [PubMed] [Google Scholar]
- 61. Paul CE, Vereker E, Dickson KM, Barker PA (2004) A pro‐apoptotic fragment of the p75 neurotrophin receptor is expressed in p75NTRExonIV null mice. J Neurosci 24:1917–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL (2005) A neurotrophin axis in myeloma: trkB and BDNF promote tumor‐cell survival. Blood 105:4429–4436. [DOI] [PubMed] [Google Scholar]
- 63. Pollack S, Young L, Bilsland J, Wilkie N, Ellis S, Hefti F, Broughton H, Harper S (1999) The staurosporine‐like compound L‐753,000 (NB‐506) potentiates the neurotrophic effects of neurotrophin‐3 by acting selectively at the TrkA receptor. Mol Pharmacol 56:185–195. [DOI] [PubMed] [Google Scholar]
- 64. Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, Allen JC, Zagzag D, Olson JM, Curran T, Wetmore C, Biegel JA, Poggio T, Mukherjee S, Rifkin R, Califano A, Stolovitzky G, Louis DN, Mesirov JP, Lander ES, Golub TR (2002) Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415:436–442. [DOI] [PubMed] [Google Scholar]
- 65. Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM (1987) Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature 325:593–597. [DOI] [PubMed] [Google Scholar]
- 66. Reifenberger G, Collins VP (2004) Pathology and molecular genetics of astrocytic gliomas. J Mol Med 82:656–670. [DOI] [PubMed] [Google Scholar]
- 67. Ricci A, Greco S, Mariotta S, Felici L, Bronzetti E, Cavazzana A, Cardillo G, Amenta F, Bisetti A, Barbolini G (2001) Neurotrophins and neurotrophin receptors in human lung cancer. Am J Respir Cell Mol Biol 25:439–446. [DOI] [PubMed] [Google Scholar]
- 68. Rosenfeld RD, Zeni L, Haniu M, Talvenheimo J, Radka SF, Bennett L, Miller JA, Welcher AA (1995) Purification and identification of brain‐derived neurotrophic factor from human serum. Protein Expr Purif 6:465–471. [DOI] [PubMed] [Google Scholar]
- 69. Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67:203–233. [DOI] [PubMed] [Google Scholar]
- 70. Ruggeri BA, Miknyoczki SJ, Singh J, Hudkins RL (1999) Role of neurotrophin‐trk interactions in oncology: the anti‐tumor efficacy of potent and selective trk tyrosine kinase inhibitors in pre‐clinical tumor models. Curr Med Chem 6:845–857. [PubMed] [Google Scholar]
- 71. Satoh F, Mimata H, Nomura T, Fujita Y, Shin T, Sakamoto S, Hamada Y, Nomura Y (2001) Autocrine expression of neurotrophins and their receptors in prostate cancer. Int J Urol 8:S28–S34. [DOI] [PubMed] [Google Scholar]
- 72. Saxena S, Bucci C, Weis J, Kruttgen A (2005) The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci 25:10930–10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schulte JH, Schramm A, Klein‐Hitpass L, Klenk M, Wessels H, Hauffa BP, Eils J, Eils R, Brodeur GM, Schweigerer L, Havers W, Eggert A (2005) Microarray analysis reveals differential gene expression patterns and regulation of single target genes contributing to the opposing phenotype of TrkA‐ and TrkB‐expressing neuroblastomas. Oncogene 24:165–177. [DOI] [PubMed] [Google Scholar]
- 74. Segal RA, Greenberg ME (1996) Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19:463–489. [DOI] [PubMed] [Google Scholar]
- 75. Segal RA, Goumnerova LC, Kwon YK, Stiles CD, Pomeroy SL (1994) Expression of the neurotrophin receptor TrkC is linked to a favorable outcome in medulloblastoma. Proc Natl Acad Sci USA 91:12867–12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shonukan O, Bagayogo I, McCrea P, Chao M, Hempstead B (2003) Neurotrophin‐induced melanoma cell migration is mediated through the actin‐bundling protein fascin. Oncogene 22:3616–23. [DOI] [PubMed] [Google Scholar]
- 77. Shooter EM (2001) Early days of the nerve growth factor proteins. Annu Rev Neurosci 24:601–629. [DOI] [PubMed] [Google Scholar]
- 78. Singer HS, Hansen B, Martinie D, Karp CL (1999) Mitogenesis in glioblastoma multiforme cell lines: a role for NGF and its TrkA receptors. J Neurooncol 45:1–8. [DOI] [PubMed] [Google Scholar]
- 79. Snider WD (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77:627–638. [DOI] [PubMed] [Google Scholar]
- 80. Sorkin A, Von Zastrow M (2002) Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol 3:600–614. [DOI] [PubMed] [Google Scholar]
- 81. Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M (1998) Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci 18:7040–7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sugimoto T, Kuroda H, Horii Y, Moritake H, Tanaka T, Hattori S (2001) Signal transduction pathways through TRK‐A and TRK‐B receptors in human neuroblastoma cells. Jpn J Cancer Res 92:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tacconelli A, Farina AR, Cappabianca L, Desantis G, Tessitore A, Vetuschi A, Sferra R, Rucci N, Argenti B, Screpanti I, Gulino A, Mackay AR (2004) TrkA alternative splicing: a regulated tumor‐promoting switch in human neuroblastoma. Cancer Cell 6:347–360. [DOI] [PubMed] [Google Scholar]
- 84. Tagliabue E, Castiglioni F, Ghirelli C, Modugno M, Asnaghi L, Somenzi G, Melani C, Menard S (2000) Nerve growth factor cooperates with p185(HER2) in activating growth of human breast carcinoma cells. J Biol Chem 275:5388–5394. [DOI] [PubMed] [Google Scholar]
- 85. Verdi JM, Anderson DJ (1994) Neurotrophins regulate sequential changes in neurotrophin receptor expression by sympathetic neuroblasts. Neuron 13:1359–1372. [DOI] [PubMed] [Google Scholar]
- 86. Von Schack D, Casademunt E, Schweigreiter R, Meyer M, Bibel M, Dechant G (2001) Complete ablation of the neurotrophin receptor p75NTR causes defects both in the nervous and the vascular system. Nat Neurosci 4:977–978. [DOI] [PubMed] [Google Scholar]
- 87. Wadhwa S, Nag TC, Jindal A, Kushwaha R, Mahapatra AK, Sarkar C (2003) Expression of the neurotrophin receptors Trk a and Trk B in adult human astrocytoma and glioblastoma. J Biosci 28:181–188. [DOI] [PubMed] [Google Scholar]
- 88. Wagner N, Wagner KD, Theres H, Englert C, Schedl A, Scholz H (2005) Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes Dev 19:2631–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Walch ET, Albino AP, Marchetti D (1999) Correlation of overexpression of the low‐affinity p75 neurotrophin receptor with augmented invasion and heparanase production in human malignant melanoma cells. Int J Cancer 82:112–120. [DOI] [PubMed] [Google Scholar]
- 90. Wang Y, Hagel C, Hamel W, Muller S, Kluwe L, Westphal M (1998) Trk A, B, and C are commonly expressed in human astrocytes and astrocytic gliomas but not by human oligodendrocytes and oligodendroglioma. Acta Neuropathol (Berl) 96:357–364. [DOI] [PubMed] [Google Scholar]
- 91. Wu C, Lai CF, Mobley WC (2001) Nerve growth factor activates persistent Rap1 signaling in endosomes. J Neurosci 21:5406–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yamashiro DJ, Nakagawara A, Ikegaki N, Liu XG, Brodeur GM (1996) Expression of TrkC in favorable human neuroblastomas. Oncogene 12:37–41. [PubMed] [Google Scholar]
- 93. Yang ZF, Ho DW, Lam CT, Luk JM, Lum CT, Yu WC, Poon RT, Fan ST (2005) Identification of brain‐derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res 65:219–225. [PubMed] [Google Scholar]
- 94. Zaccaro MC, Lee HB, Pattarawarapan M, Xia Z, Caron A, L’Heureux PJ, Bengio Y, Burgess K, Saragovi HU (2005) Selective small molecule peptidomimetic ligands of TrkC and TrkA receptors afford discrete or complete neurotrophic activities. Chem Biol 12:1015–1028. [DOI] [PubMed] [Google Scholar]
- 95. Zweifel LS, Kuruvilla R, Ginty DD (2005) Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci 6:615–625. [DOI] [PubMed] [Google Scholar]


