Abstract
Purpose
Three related male English Cocker Spaniels (ECS) were reported to be congenitally blind. Examination of one of these revealed complete retinal detachment. A presumptive diagnosis of retinal dysplasia (RD) was provided and pedigree analysis was suggestive of an X-linked mode of inheritance. We sought to investigate the genetic basis of RD in this family of ECS.
Methods
Following whole genome sequencing (WGS) of the one remaining male RD-affected ECS, two distinct investigative approaches were employed: a candidate gene approach and a whole genome approach. In the candidate gene approach, COL9A2, COL9A3, NHEJ1, RS1 and NDP genes were investigated based on their known associations with RD and retinal detachment in dogs and humans. In the whole genome approach, affected WGS was compared with 814 unaffected canids to identify candidate variants, which were filtered based on appropriate segregation and predicted pathogenic effects followed by subsequent investigation of gene function. Candidate variants were tested for appropriate segregation in the ECS family and association with disease was assessed using samples from a total of 180 ECS.
Results
The same variant in NDP (c.653_654insC, p.Met114Hisfs*16) that was predicted to result in 15 aberrant amino acids before a premature stop in norrin protein, was identified independently by both approaches and was shown to segregate appropriately within the ECS family. Association of this variant with X-linked RD was significant (P = 0.0056).
Conclusions
For the first time, we report a variant associated with canine X-linked RD. NDP variants are already known to cause X-linked RD, along with other abnormalities, in human Norrie disease. Thus, the dog may serve as a useful large animal model for research.
Introduction
Retinal dysplasia (RD) is a developmental disorder that is defined as abnormal differentiation of the retina with proliferation of one or more of its components [1]. It has been described in multiple dog breeds and can be subdivided into focal, multifocal, geographic and total retinal dysplasia types [2]. RD can present as a nonsyndromic form such as that described in the Bedlington and Sealyham Terriers or it can present as a syndromic RD such as in oculoskeletal dysplasia described in the Labrador Retriever, Samoyed and Northern Inuit dog [3–7]. Genetic variants associated with oculoskeletal dysplasia have been identified in all of these breeds [7, 8]. Total RD presents clinically as total retinal detachment. This can be associated with non-attachment due to failure of contact between the neurosensory retina and retinal pigment epithelium during embryogenesis or due to complete detachment of the retina. It may also be seen in conjunction with microphthalmos, vitreal dysplasia, leukocoria and a rotary-searching nystagmus [2].
Retinal detachment has also been reported as a clinical feature in Collie Eye Anomaly (CEA) in multiple breeds [9]. CEA is characterized by regional hypoplasia of the choroid. In severe cases and especially those with colobomas, affected dogs can develop retinal detachments, intraocular haemorrhage and subsequent blindness [9, 10]. The genetic variant for CEA has been identified in non-homologous end joining factor 1 (NHEJ1) [11]. The mode of inheritance of total RD and CEA has been reported to be autosomal recessive in all breeds [3, 4, 7, 8, 10]. Although other retinal pathologies have been found to be X-linked in dogs, such as XLPRA in the Siberian Husky as well as other breeds, to the author’s knowledge an X-linked RD has not been reported before [12].
Many genetic disorders that affect humans have an equivalent disease that is recognised in the dog [13]. Retinal diseases that can result in blindness in people that are known to be X-linked include retinoschisis and NDP (norrin cystine knot growth factor)-related retinopathies [14, 15]. Retinoschisis can present in infants as young as two months of age but more commonly presents in males at five years and upwards with macular dysfunction. In more severe forms of the disease retinal detachment can occur with a variable reported incidence of 16.6–30% [14, 16, 17]. Variants in XLRS1 (X-linked (juvenile) retinoschisis 1) have previously been associated with retinoschisis [18–20].
NDP-related retinopathies are characterised by a spectrum of fibrous and vascular changes of the retina at birth that progress and cause varying degrees of visual impairment in males. The most severe phenotype described is Norrie disease that presents clinically with a grey-yellow fibrovascular mass (pseudoglioma) secondary to retinal vascular dysgenesis and retinal detachment with congenital blindness [21]. Cognitive impairment and behavioural disturbances occur in approximately 33% and 45% of people with the disease respectively. Progressive sensorineural hearing loss usually develops, with one study revealing 85–90% of patients experiencing the onset of hearing loss by their mid-20s and cryptorchidism has also been rarely reported in cases of Norrie disease [22–24]. Associated genetic variants have been identified within NDP, which encodes a small, presumably secreted protein (norrin), with a cysteine-knot motif [25]. Greater than 100 different disease-causing NDP variants have been identified in humans to date [26]. In mice, norrin has been shown to play important roles in angiogenesis, not only in development of the eye but also the ear, brain, and female reproductive system [27].
Here we describe a novel form of RD associated with a variant in NDP in the English Cocker Spaniel (ECS), a small dog of the gundog breed, first recognised as a separate variety in 1893 [28]. In a family of ECS where three of the males were reported to be congenitally blind and pedigree analysis was suggestive of an X-linked mode of inheritance, we sought to investigate the genetic basis of the RD. Using two distinctive investigative approaches; a candidate gene approach and a whole genome sequencing (WGS) approach, we identified a provocative candidate variant. To the best of our knowledge, this is the first description of canine X-linked RD and the first time a variant in NDP has been associated with any disease in the dog. These findings also reveal the dog as a potential large animal model for studying NDP-related retinopathies in humans.
Materials and methods
Ethics statement
All dogs used for this study were privately owned pet dogs that were examined at the owners’ request following informed and written owner consent. Buccal mucosal swab sampling was performed following written owner consent and no in vivo experiments were undertaken. All clinical examinations were conducted as part of routine ophthalmic examination and not specifically for research purposes. All sample collection was approved by the Animal Health Trust Ethics Committee (04–2018).
Animals used
In this study a total of 1260 samples of 178 breeds were used. This consisted of DNA samples from one affected ECS, nine related ECS, 170 unaffected ECS, 264 unaffected dogs, WGS data from an additional 814 canids and clinical information only from two more affected ECS. Affected dogs were defined as those with RD in the form of retinal detachment. Twelve related ECS from two litters (three affected and nine unaffected) were included in the study. DNA was collected from ten of the twelve dogs, including one affected. The remaining two affected were deceased and were therefore unavailable for DNA collection.
DNA samples from an additional 170 unaffected ECS and from 264 unaffected dogs of 93 other breeds that were previously collected for unrelated research projects were used in the candidate variant genotyping.
WGS data from 814 canids were used as ‘controls’ in the whole genome approach. These comprised two distinct groups: WGS data from 198 unaffected dogs (comprising of 98 breeds and multiple crossbreeds) previously sequenced by the authors for other studies, and WGS from 616 canids of unknown phenotypes (comprising 122 dog breeds, multiple crossbreeds, and eight wolves, of unknown clinical status) derived from the Dog Biomedical Variant Database Consortium (DBVDC) [29].
Clinical investigation
Ocular examinations were performed on all twelve related ECS, between 2010–2018, at the Animal Health Trust (AHT) veterinary hospital’s ophthalmology department, by veterinary ophthalmologists, due to owners’ concerns of visual impairment in three of the males. The original two affected males were examined at 6 weeks old in 2010, whilst the affected case was examined at 6weeks, 12 weeks and 12 months old in 2017–18. Five generation pedigrees were obtained for all related ECS (Fig 1). Ocular ultrasonography was performed in the three affected males. The sire of the second litter had been examined for hereditary eye diseases as part of the British Veterinary Associated/Kennel Club/International Sheep Dog Society Eye Scheme.
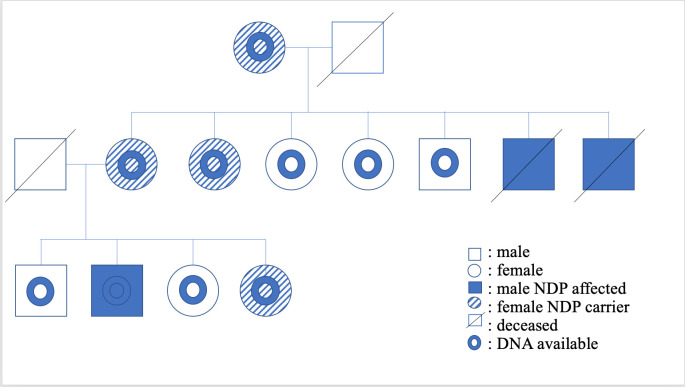
Fig 1. Pedigree of RD affected and related ECS.
RD-affected and obligate carriers (sires and dams of the RD-affected ECS), and the dog(s) from which DNA was available are indicated in the legend. Segregation is consistent with an X-linked autosomal recessive mode of inheritance.
Ocular examination
All ophthalmic examinations performed in the study included: vision assessment via maze testing and visual responses; rebound tonometry (TonoVetTM iCare, FI-01510 Vantaa, Finland); slit-lamp biomicroscopy (Kowa SL-17, Torrance, California, USA); and direct and indirect ophthalmoscopy (Welch Allyn Ltd, Ashby de la Zouch, Leicestershire, UK and Keeler Professional, Windsor, Surrey, UK) after dilation with 1% tropicamide (Minims, Bausch & Lomb, Kingston-upon-Thames, Surrey, UK). B-mode ocular ultrasound was performed on the three affected ECS (Easoate MyLabONE, 10-18MHz 30mm linear probe, Imotek, Somersham, Cambs, UK). Topical anaesthetic 0.5% proxymetacaine (Minims, Bausch & Lomb, Kingston-upon-Thames, Surrey, UK) was instilled in the eyes prior to ocular ultrasonography. Carbomer gel (Viscotears, Bausch & Lomb, Kingston-upon-Thames, Surrey, UK) was used as a coupling medium for ocular ultrasonography, and the probe was placed in a horizontal position in contact with the central cornea and axial eye length was measured from the corneal endothelium to the internal sclera. Fundus photography was performed in four ECS (RetCam Shuttle, Clarity, Macclesfield, Cheshire, UK).
DNA extraction
DNA was extracted from the buccal mucosal swabs using the QIAamp DNA Blood Mini Kit (Qiagen, Manchester, UK) according to the manufacturer’s instructions. DNA concentration and purity were determined by using the NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Loughborough, UK) and/or the Qubit fluorometer with the Qubit dsDNA broad range (BR) Assay Kit (Invitrogen, Loughborough, UK). Initial primers for the candidate gene approach were designed using Primer3 and purchased from Integrated DNA Technologies (IDT, Leuven, Belgium) [30].
Whole genome sequencing
WGS of DNA from one RD-affected ECS was outsourced to Edinburgh Genomics, UK. Illumina sequencing of a TruSeq Nano library on a HiSeq X sequencing platform and paired-end sequencing generated with read lengths of 150 base pairs and approximately 30x coverage of the dog genome. Reads were then aligned to the canine reference genome (CanFam3.1) using the Burrows-Wheeler Aligner and variant calls were made using GATK (HaplotypeCaller) [31, 32]. The WGS was also visualised and manually interrogated using Integrative Genomics Viewer (IGV) [33].WGS data from this dog is available from the European Nucleotide Archive (ENA, Accession number: PRJEB39869).
Candidate gene approach
Five candidate genes that have been associated with retinal detachment in dogs or X-linked retinal detachments in humans were selected (NHEJ1, COL9A3, COL9A2, RS1 and NDP) [7, 8, 11, 18, 34]. Using IGV, the exons and introns of each candidate gene and 5000bp of flanking DNA were examined for single nucleotide variants (SNVs), insertions and deletions using WGS from an unaffected American cocker spaniel and the CanFam3.1 for reference and comparison. The potential variants were filtered by consideration of their genomic context (i.e. if they are within coding or exonic sequence, or are splice site variants), their presence in the control genomes, their location with respect to repetitive regions and their mapping quality score. Ensembl genome browser, PolyPhen and Provean were used to further analyse potential causal variants to predict pathogenicity [35–37]. PredictProtein and Swiss-Model were used to assess structural differences between the normal and altered NDP protein [38, 39].
Whole genome approach
WGS from 198 dogs were processed through the same analysis pipeline as the ECS case. Genomic Variant Call Format (VCF) files from 199 canine WGS (198 “controls” and one ECS case) were combined using HaplotypeCaller into a multi-sample VCF file. For cross-genome analysis, Variant Effect Predictor (VEP) was run on the multi-sample file. Two greyhounds were affected with a similar phenotype and were therefore omitted from the multi-sample file. Variants predicted to result in premature start/stop codons, splicing variants, nonsense variants, coding insertions or deletions, or missense variants predicted by Polyphen and Provean to be pathogenic were retained for further investigation [35, 37]. The remaining variants were then filtered based on appropriate segregation (homozygous for the risk-variant in the case and homozygous wild type or heterozygous for the risk variant in the controls). The DBVDC genome dataset containing 616 additional canids was used for additional variant filtering based on appropriate segregation. Pubmed, OMIM, Retnet and Varelect were used to investigate gene function, expression and phenotype of remaining variants [40–42].
Candidate variant genotyping
Candidate variants that remained after filtering were investigated further by Sanger sequencing of the 10 related ECS (1 affected and 9 unaffected ECS) and appropriate segregation for an autosomal recessive or X-linked mode of inheritance was assessed (S1 Appendix).
The remaining candidate variant in NDP was genotyped in 170 ECS and 264 dogs of 93 breeds, using allele specific probes in an allelic discrimination assay. Custom primers designed to be 135 base pairs in length (forward: 5’-GGAGAGGATGTACCGGTAGGT; reverse: ACTTCGGCTGCGCTGTT) and allele-specific probes (variant: FAM-CCTCATGCCCCCCCG; reference: VIC-AGCCTCATGCCCCCCG) (Thermo Fisher Scientific, Loughborough, UK) were used. Reactions were carried out in 8 μL volumes including 4 μL LUNA Universal qPCR Master Mix (New England Biolabs), 1.4 μL MQ, 0.4 μL DMSO, 0.2 μL of primers and 2 μL genomic DNA. Cycling parameters were 25°C for 30 seconds, 95°C for 3 minutes, 40 cycles of 95°C for 3 seconds and 60°C for 10 seconds, and finally 25°C for 30 seconds.
Statistics
Using Microsoft Excel, a Fisher’s exact test using a 2 x 2 contingency table was used to assess significance of association of the candidate variant with RD using the one affected and 170 unaffected ECS. Statistical significance was set at P ≤ 0.05.
Results
Clinical findings
The related ECS were all purebred dogs that resided in the UK. A pedigree was established of those related ECS (Fig 1). In the original litter examined (seven puppies), two males were found to have behavioural signs suggestive of severe visual deficits (determined by observing their movements in unfamiliar surroundings), absent pupillary light reflexes, wandering nystagmus, darkened irises, and extensive posterior segment haemorrhage. Ocular ultrasonography revealed total retinal detachment and increased echogenicity within posterior segment in all eyes. The globe diameters (anterior to posterior) were comparable with their normal litter mates at 16mm in all dogs except one affected dog, whose globes were 15mm OU. Both males were euthanised, one dog when less than one year old due to behavioural abnormalities and blindness, the other at six years old due to suspected glaucoma and aggression. One male was found to be bilaterally cryptorchid on clinical examination.
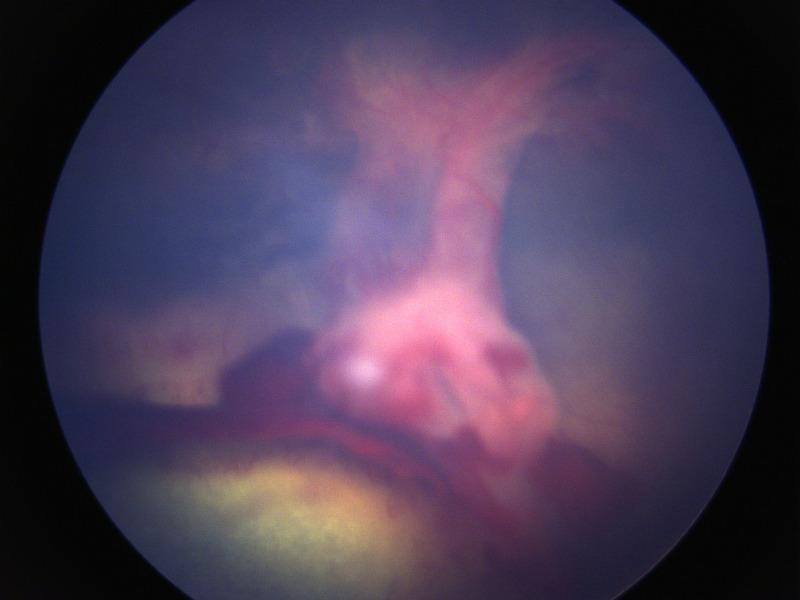
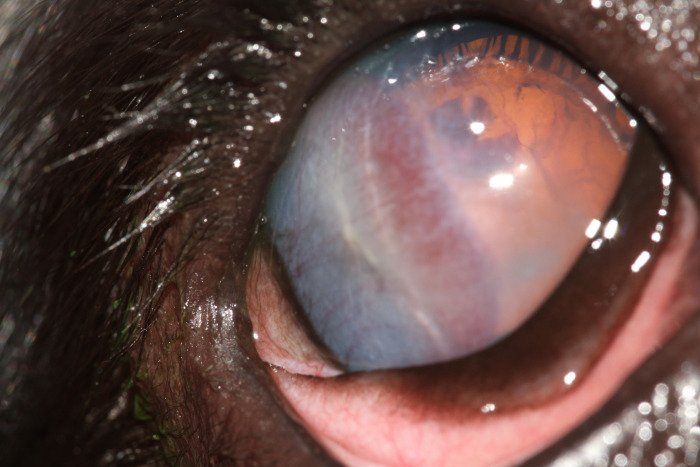
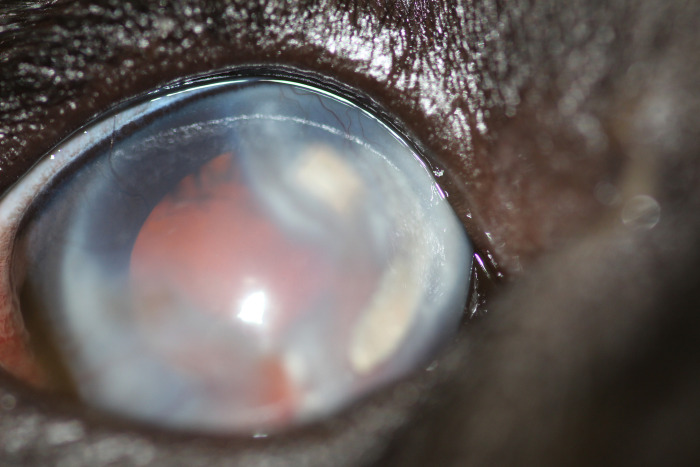
The sire of the second litter had no abnormalities detected on ocular examination. In the second litter examined (four puppies), one male displayed behavioural signs suggestive of severe visual deficits (determined by observing his movements in unfamiliar surroundings), and was found to have absent dazzle and pupillary light reflexes, a wandering nystagmus, corneal endothelial opacities ventrally in the right eye, darkened irises, hyphema of the right eye and extensive posterior segment haemorrhage in both eyes, with the retina in the left eye appearing detached and as a fibrovascular retrolental mass (Figs 2 and 3). Ocular ultrasonography revealed total retinal detachment and increased echogenicity within the posterior segment in both eyes. The left globe appeared microphthalmic with an anterior-posterior diameter of 16.42mm versus 21.88mm in the right eye. The affected male was subsequently re-examined, the left eye appeared relatively unchanged whereas the corneal pathology had advanced to diffuse corneal degeneration with neovascularisation and dense crystalline stromal deposits in the right eye (Fig 4). Microphakia was evident along with incipient nuclear cataract being present (Fig 5). On clinical examination the dog was found to be cryptorchid at 10 months old.
Fig 2. Ocular defects associated with affected ECS.
Showing RD-affected male with hyphema of the right eye.
Fig 3. Ocular defects associated with affected ECS.
Showing the retinal detachment as a fibrovascular mass.
Fig 4. Ocular defects associated with affected ECS.
Showing corneal and lenticular abnormalities.
Fig 5. Ocular defects associated with affected ECS.
Showing corneal and lenticular abnormalities.
Identification of candidate variants by a candidate gene approach
Manual visual inspection of the DNA sequence of the five candidate genes, comparing the affected ECS with 1 unaffected dog and the CanFam3.1 reference using IGV, identified 196 variants that were initially narrowed down to 12 potential variants by the manual interrogation described above. This was then further narrowed down on the basis of predicted pathogenicity (using Provean and Polyphen-2) to two potential variants. The first was a missense variant in exon seven in COL9A3 (c.1240G>C, p.Asp414His), predicted to be “probably damaging” by PolyPhen and “neutral” by Provean. The second was a frameshift variant in NDP (c.338_339insC), that was predicted to result in 15 aberrant amino acids before a premature stop in norrin protein (p.Met114Hisfs*16), resulting in a loss of the last five amino acids. Each of the aberrant amino acids was independently assessed for pathogenicity using PolyPhen-2 and Provean (Table): four changes were predicted to be “Probably Damaging” by PolyPhen-2 and nine changes were predicted to be “Deleterious” by Provean (Table 1). The NDP protein is made up of 133 amino acids; therefore, 15.0% of the protein is predicted to be either changed or lost. In addition, significant structural differences between the wildtype and altered proteins are predicted (Fig 6).
Table 1. Predicted pathogenicity.
| Amino acid change | PolyPhen Result | PolyPhen Score | Provean Result | Provean Score (Threshold <-2.5) |
|---|---|---|---|---|
| M114H | Possibly Damaging | 0.566 | Neutral | -0.102 |
| R115E | Possibly Damaging | 0.462 | Neutral | -0.647 |
| L116A | Possibly Damaging | 0.816 | Deleterious | -3.109 |
| T117H | Probably Damaging | 0.974 | Deleterious | -3.353 |
| A118R | Probably Damaging | 0.958 | Neutral | -1.118 |
| T119H | Probably Damaging | 0.974 | Neutral | -1.647 |
| Y120L | Possibly Damaging | 0.816 | Deleterious | -8 |
| R121P | Possibly Damaging | 0.827 | Deleterious | -4.941 |
| Y122V | Possibly Damaging | 0.816 | Deleterious | -6.235 |
| I123H | Possibly Damaging | 0.944 | Deleterious | -6.882 |
| L124P | Probably Damaging | 0.974 | Neutral | -1.588 |
| S125L | Possibly Damaging | 0.633 | Deleterious | -3.176 |
| C126L | Possibly Damaging | 0.462 | Deleterious | -10 |
| H127S | Possibly Damaging | 0.462 | Neutral | 2.255 |
| C128L | Possibly Damaging | 0.462 | Deleterious | -7.118 |
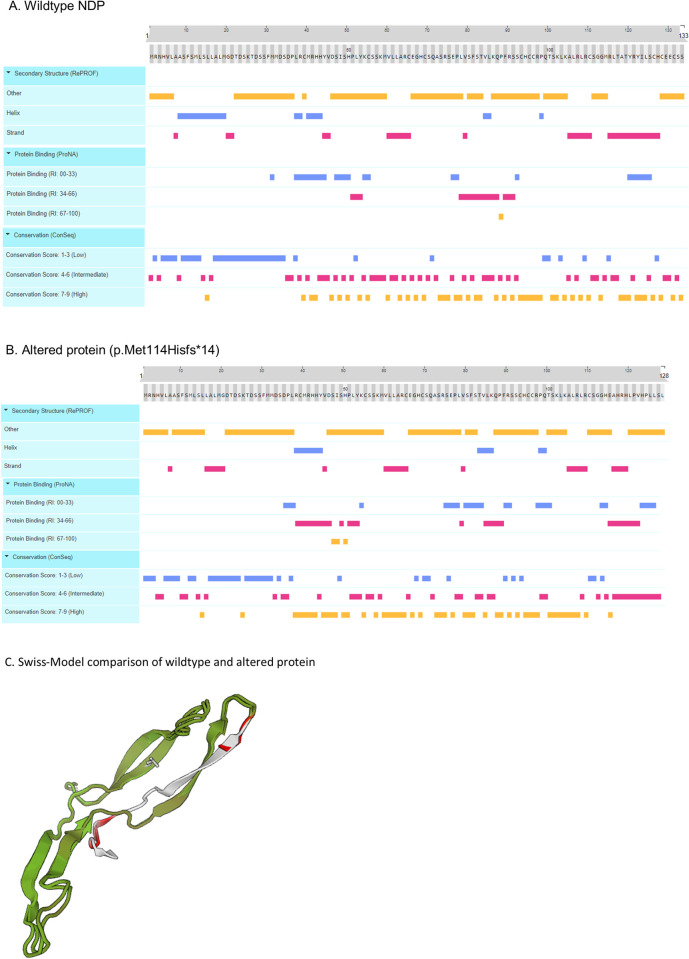
Fig 6.
Comparison of the wildtype (A) and altered protein (B). Using PredictProtein reveal that the two proteins are expected to differ throughout with regards to secondary structure, protein binding and conservation. In addition, modelling of both proteins using Swiss-Model (C) reveal that in the altered molecule, the 15 amino acids at the C-terminus, involved in the shifted reading frame, do not align with the N-terminus (white and red in C) of the wildtype protein at all, and also do not align with any other known protein structures.
Identification of candidate variants by a whole genome sequencing approach
WGS data from the RD-affected ECS was compared to those from 198 unaffected control dogs of 98 other breeds. There was a total of 27,874,423 variants in at least one of these genomes. These variants were first filtered based on predicted effect on a protein using the Ensembl Variant Effect Predictor (VEP) [43, 44], resulting in 530 variants. These variants were further filtered based on appropriate segregation for an autosomal recessive mode of inheritance which resulted in 23 remaining variants (Table 2).
Table 2. 23 remaining variants.
| Chromosome | Genomic coordinates | Reference allele | Alternate allele | Consequence | Gene Symbol | Gene Full Name | Human Orthologue | Present in DBVDC | Base change | Amino acid change | CDS Position | Protein Position |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 33957623 | A | C | missense_variant | DACT1 | Dishevelled Binding Antagonist of Beta Catenin 1 | DACT1 | Yes | gAg/gCg | E/A | 1700 | 567 |
| 25 | 35087169 | C | G | missense_variant | BMP1 | Bone Morphogenetic Protein 1 | BMP1 | Yes | gaG/gaC | E/D | 114 | 38 |
| 26 | 9447613 | TA | T,TTCTTCTGGTAAA,TTCTTCTGGTAA | frameshift_variant | ENSCAFG00000008764 | None | Yes | Atc/tc | I/X | 1549 | 517 | |
| 38 | 21369593 | G | GT | frameshift_variant | KLHDC9 | kelch domain containing 9 | KLHDC9 | No | -/A | -/X | 735–736 | 245–246 |
| 31 | 39447278 | G | A | missense_variant | COL6A2 | Collagen Type IX Alpha 2 Chain | COL6A2 | Yes | aGa/aAt | S/N | 2495 | 832 |
| 17 | 20301410 | G | GC | frameshift_variant | GAREM2 | Grb2-associated regulator of Erk/MAPK1 | GAREM2 | No | ggg/ggCg | G/GX | 620–621 | 207 |
| 18 | 32393127 | CGCCGCCTCCAGCCACGCGGCCGCCTGCGCCTCCGAGAAGCCGCGGGGCACCCGCTCCTCCAG | C | frameshift_variant | FJX1 | Four Jointed Box Kinase 1 | FJX1 | No | CTGGAGGAGCGGGTGCCCCGCGGCTTCTCGGAGGCGCAGGCGGCCGCGTGGCTGGAGGCGGCg/g | LEERVPRGFSEAQAAAWLEAA/X | 199–260 | 67–87 |
| 6 | 8811462 | ATCTGCTCGTATCTGGAGATGCTGTTAGAGTGCTGG | *,GTCTGCTCGTATCTGGAGATGCTGTTAGAGTGCTGG | missense_variant | ENSCAFG00000014044 | None | Yes | aCCAGCACTCTAACAGCATCTCCAGATACGAGCAGATcc/aCCAGCACTCTAACAGCATCTCCAGATACGAGCAGACcc | TSTLTASPDTSRS/TSTLTASPDTSRP | 7454–7489 | 2485–2497 | |
| 12 | 52207701 | GAAAGAAAGAAAGAAAA | *,GAAAGAAGAAAAAGAAAGAAAGAAAA,G,GAAAAGAAAGAAAGAAAA,GAAGAAAGAAAGAAAA | inframe_insertion+frameshift_variant | ENSCAFG00000030881+ENSCAFG00000030881 | None | Yes | tTTTTCTTTCTTTCTTTc/tTTTTCTTTCTTTCTTTTTCTTCTTTc tTTTTCTTTCTTTCTTTc/tc | FFFLSF/FFFLSFSSF FFFLSF/X | 203–218+203–218 | 68–73+68–73 | |
| 1 | 77496026 | CTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCT | CTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCT,C,*,CTTTTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCT | splice_acceptor_variant&intron_variant&non_coding_transcript_variant | ENSCAFG00000034426 | None | Yes | |||||
| 6 | 55325921 | AAG | *,A | splice_acceptor_variant&intron_variant | ENSCAFG00000032258 | None | Yes | |||||
| 6 | 54025399 | G | GAAA,GA,A,*,GAAAA,GAAAGAAAA | inframe_insertion+frameshift_variant+missense_variant | ENSCAFG00000029585+ENSCAFG00000029585+ENSCAFG00000029585 | None | Yes | aGg/aGAAAg aGg/aGAg aGg/aAg | R/RK R/RX R/K | 77+77+77 | 26+26+26 | |
| 16 | 12395809 | A | G,* | missense_variant | ENSCAFG00000031012 | None | Yes | aAa/aGa | K/R | 422 | 141 | |
| 1 | 77496030 | CTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCT | C,*,CTTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCT,CTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCT,CTCTTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTCTTTCTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCTTTCT | splice_acceptor_variant&intron_variant&non_coding_transcript_variant | ENSCAFG00000034426 | None | Yes | |||||
| 11 | 54593727 | C | G | missense_variant | ENSCAFG00000031828 | None | No | Gac/Cac | D/H | 148 | 50 | |
| 21 | 4789963 | T | G | missense_variant | CCDC82 | Coiled-coil Domain Containing 82 | CCDC82 | Yes | gaT/gaG | D/E | 171 | 57 |
| 25 | 44173062 | G | A | missense_variant | CHRND | Cholinergic Receptor Nicotinic Delta Subunit | CHRND | No | cGc/cAc | R/H | 1400 | 467 |
| 34 | 19179708 | G | A | missense_variant | TBCCD1 | TBCC Domain Containing 1 | TBCCD1 | No | aCg/aTg | T/M | 167 | 56 |
| 39 | 1615231 | G | A | missense_variant&splice_region_variant | ARSF | Arylsulfatase F | ARSF | No | aGg/aAg | R/K | 161 | 54 |
| 39 | 37950668 | G | GC | frameshift_variant | NDP | Norrin Cystine Knot Growth Factor | NDP | No | ggc/ggGc | G/GX | 338–339 | 113 |
| 6 | 55887792 | C | G | missense_variant | CCDC18 | Coiled-Coil Domain Containing 18 | CCDC18 | Yes | atG/atC | M/I | 1230 | 410 |
| 7 | 9830091 | C | A | missense_variant | TRAF5 | TNF Receptor Associated Factor 5 | TRAF5 | No | Caa/Aaa | Q/K | 1003 | 335 |
| 9 | 59422620 | G | A | missense_variant | DENND1A | DENN Domain Containing 1A | DENND1A | No | cGc/cAc | R/H | 2279 | 760 |
Two remaining variants FJX1 and NDP involved in retinal development highlighted in blue.
Of the 23 variants only 14 were within known genes that had human orthologues. Five of these variants were found in the additional 648 canid genomes within the DBVDC and were excluded from further investigation. This resulted in 9 variants in 9 individual genes. Gene function was investigated using Pubmed, OMIM, Retnet and Varelect. Only two of these genes were known to be involved in retinal development: NDP and FJX1 (four jointed box kinase 1). The variant in NDP was identical to that discovered by the candidate gene approach (c.338_339insC). The variant in FJX1 was a frameshift variant (c.199_260del).
Genotyping the candidate variants
Sanger sequencing was used to genotype the nine related ECS and the one RD-affected dog for which DNA was available. The RD-affected dog was homozygous for two of the variant alleles (NDP, COL9A3) but was homozygous for the wildtype FJX1 allele with sanger sequencing. Manual inspection of the FJX1 variant region in the affected WGS revealed highly repetitive sequence and reads with poor alignment with CanFam3.1, and the calling of this as a variant is therefore, a false positive. Three unaffected ECS were also homozygous for the COL9A3 variant, whereas all nine unaffected dogs were either heterozygous or homozygous for the wildtype NDP allele and all nine unaffected dogs were homozygous wild type for FJX1. Therefore, only the NDP variant segregated appropriately for an autosomal recessive or X-linked mode of inheritance among the nine related ECS (Fig 6). An allelic discrimination assay was then performed in the additional 170 unaffected ECS and 264 unaffected dogs of 93 other breeds. All these dogs were homozygous for the wild type allele. Association of the NDP variant with RD was significant (P = 0.0056).
Discussion
In this study, two different approaches, candidate-gene and whole genome, were used to identify a single exonic insertion variant in NDP which was shown to be associated with X-linked RD in the ECS. This variant is predicted to result in a 15 residue shift in the reading frame, a premature stop codon and norrin protein truncated by five residues, out of a normal length of 133 amino acids. While structural variants near exons would have been detected in the candidate gene approach, our current whole genome analysis pipeline does not detect structural variants. It is, therefore, worth noting that the presence of structural variation causing disease in this study cannot be fully excluded. In addition, the canine genome is not well annotated with regulatory regions and lncRNAs, and these were therefore not investigated and cannot be excluded. Indeed, a lncRNA (NDP-AS1) on the reverse strand is annotated on the human genome build GRCh38.p13), but it is unclear if the canine genome shares this feature, and if it does whether the variant described here affects the NDP protein, the lncRNA or both. It is also worth noting linkage disequilibrium (LD) was not investigated due the small number of WGS available for ECS in this study (four in total including the case), which was considered too few samples for an accurate estimate of LD in the breed in general and especially in the vicinity of the variant of interest by the authors.
This is the first time a variant in NDP has been described in the dog and the first time X-linked RD has been reported in the dog. The results suggest that this variant is likely to be private to this family of ECS as it was not identified in the additional 170 ECS and the 1078 other non-ECS canids. The pedigree analysis was helpful in determining it was most likely a recessive condition as only a small proportion of the progeny was affected and, because it was only observed in male progeny, a X-linked mode of inheritance was also hypothesized. This narrowed down the potential candidate genes to be investigated. Genomic material was only available from one affected as the other two affected were deceased. The low number of affected animals is a limiting factor for this study. Ocular tissue from RD-affected ECS would also be helpful for assessing changes in the mRNA and protein expression within the retina of these dogs in comparison to age matched control dog retinas. Further work is required to prove this is the causal variants of RD in the ECS, although the predicted effect on the protein is supportive of this hypothesis.
WGS is a very useful and cost-effective method of identifying variants, when faced with a small number of affected cases. Unlike genome wide association studies, a small number, or even a single case may be sufficient to identify underlying variant as in this study. If successful, WGS can help identify risk variants early on in the disease emergence process, hence eliminating it before the disease becomes widespread within the population, which is especially important in dogs due to high levels of inbreeding, popular sire effects and relatively fast turnover of progeny [45, 46]. A limitation to using WGS as with other sequencing techniques, is the inherent difficulty in the detection of structural variants, for example, transposons, inversions, or large insertions and deletions which our current WGS pipeline cannot identify. Genome-wide association studies can be useful in identifying the approximate location of the disease variant even when there is a large structural variant, so its use in combination with WGS could help mitigate this limitation. Unfortunately, due to our low case numbers, this was not possible in this study. It is therefore worth noting that the presence of structural variation causing disease cannot be fully excluded.
The candidate gene approach was considered appropriate for this study because similar phenotypes had been reported in humans and dogs which have been shown to be associated with a limited number of genes. This approach is useful when trying to identify the genetic basis of a disease thought to be of simple inheritance using only a small number of cases [47]. It requires existing knowledge about the known or presumed biology of the phenotype under investigation, either in other breeds of dog or in other species, which was available in this instance [48]. This method is however biased and limited, as it only includes genes known to be associated with the relevant phenotype, meaning variants in novel genes not previously associated with the phenotype will go undetected [49]. A whole genome approach was also used, which does not require prior knowledge of gene candidacy and thus is considered a less biased approach compared to candidate gene analysis [50].
NDP encodes for norrin, a cysteine knot growth factor, that is required for angiogenesis in the eye, ear, brain and female reproductive system [47]. Norrin is an atypical Wnt (wingless-related integration site) ligand, that has binding sites for both Fz4 (Frizzled 4) and LRP5/6 (low-density lipoprotein receptor-related protein) which activates β-catenin signalling, that plays a central role in retinal vascularisation [47, 51]. Its structure is a semi-circular shaped homodimer linked through three intermolecular disulphide bonds. Disease-causing variants will affect the protein folding and stabilisation of the norrin structure and hence affect protein function [52].
Over 100 disease-causing NDP variants have been reported in humans [26]. Analysis of gnomAD data within the equivalent human C-terminal region affected by the frameshift and truncation (X:43809045–43809107, based on genome build GRCh37/hg19) revealed eight variants. Of these, three are missense, five are synonymous and none are truncating. However, analysis of the ClinVar data in gnomAD revealed five additional “likely pathogenic” variants, including three missense and two nonsense/stop gained variants [53]. These variants can result in inherited retinopathies including Norrie disease, X-linked familial exudative vitreoretinopathy, retinopathy of prematurity and Coat’s disease [54]. Retinal detachment has been reported in all of these conditions to varying degrees at different clinical stages [55–57]. Retinal detachment in Norrie disease has even been reported in utero during the third trimester in a known carrier of the condition [58]. The affected ECS males were examined at 6 weeks old, although blindness was suspected earlier than this. Puppies open their eyes at approximately two weeks of age [59]. In the affected ECS, retinal detachment was suspected to be congenital, but this cannot be confirmed.
Histopathological characterisation is usually not possible in humans with NDP-related retinopathies. Therefore Ndph (Norrie disease homologue)-knockout mice have been used as a small animal model to study early histopathological changes [60]. In mice with targeted inactivation of Ndp, histological data suggest retrolental structures and a disorganized ganglion cell layer as primary pathogenic events [61]. Histopathology of the retina and posterior segment of an RD-affected ECS would be useful to better identify the phenotypic characteristics of this type of RD in the dog. No ocular tissue was available from the affected dog in this study, so the pathogenesis can only be hypothesised based on the clinical findings observed. Although there were subtle ophthalmic differences in the three affected dogs, the overall ocular changes were similar, as they all shared the congenital blindness, extensive posterior segment haemorrhage and the retinal detachment. The congenital retinal detachments in the form of fibrovascular retrolental masses and extensive vitreal haemorrhage, is more similar to that described in clinical cases of human Norrie disease than the other inherited retinopathies associated with NDP variants [62, 63].
Cognitive impairment, behavioural disturbances and progressive sensorineural hearing loss have been described in humans with Norrie disease. The two affected males of the initial litter of ECS were euthanised early in life due to behavioural abnormalities that were thought to be related to their lack of vision. The affected male in the second litter was not reported to show behavioural abnormalities. No males were reported to have any hearing impairment, although brainstem auditory evoked response testing was not performed to confirm this, in the affected ECS to confirm this. Hearing loss is reported to develop in humans in their early twenties so it is possible that the affected males had not yet reached an appropriate age for this to have developed and the one surviving affected male could develop hearing loss as he continues to mature [22].
This is the first time a variant in NDP has been associated with a retinal disease in the dog and also the first X-linked RD to be reported in the dog. Therefore, this study also suggests the dog may serve as a useful model for understanding human NDP-related retinopathies and the development of gene and other therapies.
Supporting information
(DOCX)
Acknowledgments
The authors are grateful to owners of ECS for their support and enthusiasm. We would like to sincerely thank Edinburgh Genomics (University of Edinburgh) for the generation of WGS data. We thank the Dog Biomedical Variant Database Consortium (Gus Aguirre, Catherine André, Danika Bannasch, Doreen Becker, Brian Davis, Cord Drögemüller, Kari Ekenstedt, Kiterie Faller, Oliver Forman, Steve Friedenberg, Eva Furrow, Urs Giger, Christophe Hitte, Marjo Hytönen, Vidhya Jagannathan, Tosso Leeb, Hannes Lohi, Cathryn Mellersh, Jim Mickelson, Leonardo Murgiano, Anita Oberbauer, Sheila Schmutz, Jeffrey Schoenebeck, Kim Summers, Frank van Steenbeek, Claire Wade) and Prof Natasha Olby (North Carolina State University) for sharing WGS data from control dogs. We also acknowledge all canine researchers who deposited dog WGS data into public databases.
Data Availability
The whole genome sequencing data, has been uploaded to the European Nucleotide Archive (ENA, Accession number: PRJEB39869).
Funding Statement
The work was supported by the Animal Health Trust, the Kennel Club Charitable Trust and the British Association of Veterinary Ophthalmologists (BrAVO). Two of the funders provided support in the form of salaries for authors HJ and LF (Animal Health Trust) and LMB, HW, and CM (Kennel Club Charitable Trust), but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. Three of the authors (HJ, LF and JO) are currently employed by a commercial company, Dick White Referrals, but this affiliation did not play a role in the study as the authors were based at the Animal Health Trust at the time of their contribution to this study. Dick White Referrals are not a funder of this study and had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The BrAVO Research grant awarded to the main author, HJ, for the whole genome analysis of the affected case was for solely this and the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. https://www.bravo.org.uk
References
- 1.Iwabe S, Dufour VL, Guzmán JM, Holle DM, Cohen JA, Beltran WA, et al. Focal/multifocal and geographic retinal dysplasia in the dog—In vivo retinal microanatomy analyses. Veterinary Ophthalmology. 2020. March;23(2):292–304. 10.1111/vop.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narfström K and Petersen-Jones S. Diseases of the canine ocular findus. In: Gelatt KN, Gilger BC, Kern TJ. Veterinary Ophthalmology. John Wiley & Sons; 2012. Pp. 1316–1320. [Google Scholar]
- 3.Rubin LF. Heredity of retinal dysplasia in Bedlington terriers. Journal of the American Veterinary Medical Association. 1968. January 1;152(3):260. [Google Scholar]
- 4.Ashton N, Barnett KC, Sachs DD. Retinal dysplasia in the Sealyham terrier. The Journal of Pathology and Bacteriology. 1968. October;96(2):269–72. 10.1002/path.1700960203 [DOI] [PubMed] [Google Scholar]
- 5.Carrig CB, MacMillan A, Brundage S, Pool RR, Morgan JP. Retinal dysplasia associated with skeletal abnormalities in Labrador Retrievers. Journal of the American Veterinary Medical Association. 1977. January;170(1):49–57. [PubMed] [Google Scholar]
- 6.Meyers VN, Jezyk PF, Aguirre GD, Patterson DF, Short-Limbed dwarfism and ocular defects in the Samoyed dog. J Am Vet Med Assoc. 1983; 183(9):975–9 Epub 1983/11/01. [PubMed] [Google Scholar]
- 7.Stavinohova R, Hartley C, Burmeister LM, Ricketts SL, Pettitt L, Pont RT, et al. Clinical, histopathological and genetic characterisation of oculoskeletal dysplasia in the Northern Inuit Dog. PLOS ONE. 2019;14(8). 10.1371/journal.pone.0220761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein O, Guyon R, Kukekova A, Kuznetsova TN, Pearce-Kelling SE, Johnson J, et al. COL9A2 and COL9A3 mutations in canine autosomal recessive oculoskeletal dysplasia. Mammalian Genome. 2010. August 1;21(7–8):398–408. 10.1007/s00335-010-9276-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett KC. Collie eye anomaly (CEA). Journal of Small Animal Practice. 1979. September;20(9):537–42. [DOI] [PubMed] [Google Scholar]
- 10.Lowe JK, Kukekova AV, Kirkness EF, Langlois MC, Aguirre GD, Acland GM, et al. Linkage mapping of the primary disease locus for collie eye anomaly. Genomics. 2003. July 1;82(1):86–95. 10.1016/s0888-7543(03)00078-8 [DOI] [PubMed] [Google Scholar]
- 11.Parker HG, Kukekova AV, Akey DT, Goldstein O, Kirkness EF, Baysac KC, et al. Breed relationships facilitate fine-mapping studies: a 7.8-kb deletion cosegregates with Collie eye anomaly across multiple dog breeds. Genome research. 2007. November 1;17(11):1562–71. 10.1101/gr.6772807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kropatsch R, Akkad DA, Frank M, Rosenhagen C, Altmüller J, Nürnberg P, et al. A large deletion in RPGR causes XLPRA in Weimaraner dogs. Canine Genetics and Epidemiology. 2016. December 1;3(1):7. 10.1186/s40575-016-0037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurda BL, Bradbury AM, Vite CH. Focus: Comparative Medicine: Canine and feline models of human genetic diseases and their contributions to advancing clinical therapies. The Yale journal of Biology and Medicine. 2017. September;90(3):417. [PMC free article] [PubMed] [Google Scholar]
- 14.George ND, Yates JR, Moore AT. X linked retinoschisis. The British Journal of Ophthalmology. 1995. July;79(7):697. 10.1136/bjo.79.7.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warburg M. Norrie’s disease. A congenital progressive oculo-acoustico-cerebral degeneration. Acta Ophthalmol (Copenh). 1966;89:1–47. [PubMed] [Google Scholar]
- 16.Neriyanuri S, Dhandayuthapani S, Arunachalam JP, Raman R. Phenotypic characterization of X-linked retinoschisis: Clinical, electroretinography, and optical coherence tomography variables. Indian Journal of Ophthalmology. 2016. July;64(7):513. 10.4103/0301-4738.190140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pimenides D, George ND, Yates JR, Bradshaw K, Roberts SA, Moore AT, et al. X-linked retinoschisis: clinical phenotype and RS1 genotype in 86 UK patients. Journal of Medical Genetics. 2005. June 1;42(6):e35-. 10.1136/jmg.2004.029769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauer CG, Gehrig A, Warneke-Wittstock R, Marquardt A, Ewing CC, Gibson A, et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nature Genetics. 1997. October;17(2):164. 10.1038/ng1097-164 [DOI] [PubMed] [Google Scholar]
- 19.Retinoschisis Consortium. Functional implications of the spectrum of mutations found in 234 cases with X-linked juvenile retinoschisis (XLRS). Human Molecular Genetics. 1998. July 1;7(7):1185–92. 10.1093/hmg/7.7.1185 [DOI] [PubMed] [Google Scholar]
- 20.Yi J, Li S, Jia X, Xiao X, Wang P, Guo X, et al. Novel RS1 mutations associated with X-linked juvenile retinoschisis. International Journal of Molecular Medicine. 2012. April 1;29(4):644–8. 10.3892/ijmm.2012.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sims KB. NDP-related retinopathies. InGeneReviews®[Internet] 2014 Sep 18. University of Washington, Seattle.
- 22.Smith SE, Mullen TE, Graham D, Sims KB, Rehm HL. Norrie disease: extraocular clinical manifestations in 56 patients. American Journal of Medical Genetics Part A. 2012. August;158(8):1909–17 10.1002/ajmg.a.35469 [DOI] [PubMed] [Google Scholar]
- 23.Moreira-Filho CA, Neustein I. A presumptive new variant of Norrie’s disease. Journal of Medical Genetics. 1979. April 1;16(2):125–8. 10.1136/jmg.16.2.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gal A, Wieringa B, Smeets DF, Bleeker-Wagemakers L, Ropers HH. Submicroscopic interstitial deletion of the X chromosome explains a complex genetic syndrome dominated by Norrie disease. Cytogenetic and Genome Research. 1986;42(4):219–24. 10.1159/000132282 [DOI] [PubMed] [Google Scholar]
- 25.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004. March 19;116(6):883–95. 10.1016/s0092-8674(04)00216-8 [DOI] [PubMed] [Google Scholar]
- 26.Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends in Molecular Medicine. 2010. September 1;16(9):417–25. 10.1016/j.molmed.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehm HL, Zhang DS, Brown MC, Burgess B, Halpin C, Berger W, et al. Vascular defects and sensorineural deafness in a mouse model of Norrie disease. Journal of Neuroscience. 2002. June 1;22(11):4286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunliffe J. The encyclopedia of dog breeds, 1st ed, Bath: Parragon; 2000 [Google Scholar]
- 29.Jagannathan V, Drögemüller C, Leeb T. Dog Biomedical Variant Database Consortium (DBVDC). A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and 8 wolves. Anim. Genet. 2019;50:695–704. 10.1111/age.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000; 132:365–86. 10.1385/1-59259-192-2:365 [DOI] [PubMed] [Google Scholar]
- 31.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009; 25(14);1754–60. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit;a MapReduce framework for analysing next-generation DNA sequencing data. Genome Res. 2010; 20(9):1297–303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nature biotechnology. 2011. January 10;29(1):24. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger W, Meindl A, Van de Pol TJ, Cremers FP, Ropers HH, Döerner C, et al. Isolation of a candidate gene for Norrie disease by positional cloning. Nature genetics. 1992. June;1(3):199. 10.1038/ng0692-199 [DOI] [PubMed] [Google Scholar]
- 35.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;Chapter 7:Unit7 20. 10.1002/0471142905.hg0720s76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, et al. Ensembl 2018. Nucleic Acids Research. 2017. November 16;46(D1):D754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi Y, Chan AP. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015. April 6;31(16):2745–7. 10.1093/bioinformatics/btv195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yachdav G, Kloppmann E, Kajan L, Hecht M, Goldberg T, Hamp T, et al. PredictProtein—an open resource for online prediction of protein structural and functional features. Nucleic Acids Res. 2014. July;42(Web Server issue):W337–43. 10.1093/nar/gku366 Epub 2014 May 5. ; PMCID: PMC4086098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterhouse Andrew, Bertoni Martino, Bienert Stefan, Studer Gabriel, Tauriello Gerardo, Gumienny Rafal, et al. SWISS-MODEL: homology modelling of protein structures and complexes, Nucleic Acids Research, Volume 46, Issue W1, 2 July 2018, Pages W296–W303 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amberger J, Bocchini CA, Scott AF, Hamosh A. McKusick’s online Mendelian inheritance in man (OMIM®). Nucleic Acids Research. 2008. October 7;37(suppl_1):D793–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stelzer G, Plaschkes I, Oz-Levi D, Alkelai A, Olender T, Zimmerman S, et al. VarElect: the phenotype-based variation prioritizer of the GeneCards Suite. BMC Genomics. 2016. June;17(2):444. 10.1186/s12864-016-2722-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daiger SP, Sullivan LS, Bowne SJ, Rossiter BJ. RetNet: Retinal Information Network. Na+ Ca2. 2013. June;5. [Google Scholar]
- 43.Cunningham F, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2015. Nucleic Acids Research. 2015. January 28;43(D1):D662–9. 10.1093/nar/gku1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yates A, Akanni W, Amode MR, Barrell D, Billis K, Carvalho-Silva D, et al. Ensembl 2016. Nucleic Acids Research. 2016. January 4;44(D1):D710–6. 10.1093/nar/gkv1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitti RJ, Oliver JA, Schofield EC, Bauer A, Kaukonen M, Forman OP, et al. Whole genome sequencing of Giant Schnauzer Dogs with progressive retinal atrophy establishes NECAP1 as a novel candidate gene for retinal degeneration. Genes. 2019. May;10(5):385. 10.3390/genes10050385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broeckx BJ. The dog 2.0: Lessons learned from the past. Theriogenology. 2020. January 21. 10.1016/j.theriogenology.2020.01.043 [DOI] [PubMed] [Google Scholar]
- 47.Zhu M, Zhao S. Candidate gene identification approach: progress and challenges. International Journal of Biological Sciences. 2007;3(7):420. 10.7150/ijbs.3.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaid DJ, Sommer SS. Genotype relative risks: methods for design and analysis of candidate-gene association studies. American Journal of Human Genetics. 1993. November;53(5):1114. [PMC free article] [PubMed] [Google Scholar]
- 49.Tabor HK, Risch NJ, Myers RM. Candidate-gene approaches for studying complex genetic traits: practical considerations. Nature Reviews Genetics. 2002. May;3(5):391–7. 10.1038/nrg796 [DOI] [PubMed] [Google Scholar]
- 50.Van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends in Genetics. 2014. September 1;30(9):418–26. 10.1016/j.tig.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 51.Ohlmann A, Merkl R, Tamm ER. Focus on molecules: norrin. Experimental Eye Research. 2012. September 1;102:109–10. 10.1016/j.exer.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 52.Ke J, Harikumar KG, Erice C, Chen C, Gu X, Wang L, et al. Structure and function of Norrin in assembly and activation of a Frizzled 4–Lrp5/6 complex. Genes & Development. 2013. November 1;27(21):2305–19. 10.1101/gad.228544.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karczewski K.J., Francioli L.C., Tiao G. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020). https://doi-org.ezp.lib.cam.ac.uk/10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickinson JL, Sale MM, Passmore A, FitzGerald LM, Wheatley CM, Burdon KP, et al. Mutations in the NDP gene: contribution to Norrie disease, familial exudative vitreoretinopathy and retinopathy of prematurity. Clinical & Experimental Ophthalmology. 2006. September;34(7):682–8. 10.1111/j.1442-9071.2006.01314.x [DOI] [PubMed] [Google Scholar]
- 55.Vallat M, Mathon C, Vancoppenolle F, Detre J, Fritsch D, Clavel D, et al. Retinopathy of prematurity-retinal-detachment in late stages. Journal Fransais D Ophthalmologie. 1986. January 1;9(8–9):573–82. [PubMed] [Google Scholar]
- 56.Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye. 2015. January;29(1):1. 10.1038/eye.2014.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture. American Journal of Ophthalmology. 2001. May 1;131(5):561–71. 10.1016/s0002-9394(00)00883-7 [DOI] [PubMed] [Google Scholar]
- 58.Redmond RM, Vaughan JI, Jay M, Jay B. In-utero diagnosis of Norrie disease by ultrasonography. Ophthalmic Paediatrics and Genetics. 1993. January 1;14(1):1–3. 10.3109/13816819309087615 [DOI] [PubMed] [Google Scholar]
- 59.Scott JP. Critical periods in the development of social behavior in puppies. Psychosomatic Medicine. 1958. January 1;20(1):42–54. 10.1097/00006842-195801000-00005 [DOI] [PubMed] [Google Scholar]
- 60.Luhmann UF, Lin J, Lammel S, Feil S, Hammes HP, Berger W. Differential expression of angiogenic factors during postnatal retinal vascular development in Norrie disease mice. Investigative Ophthalmology & Visual Science. 2004. May 1;45(13):496-. [Google Scholar]
- 61.Berger W, van de Pol D, Bächner D, Oerlemans F, Winkens H, Hameister H, et al. An animal model for Norrie disease (ND): gene targeting of the mouse ND gene. Human Molecular Genetics. 1996. January 1;5(1):51–9. 10.1093/hmg/5.1.51 [DOI] [PubMed] [Google Scholar]
- 62.Pelcastre EL, Villanueva‐Mendoza C, Zenteno JC. Novel and recurrent NDP gene mutations in familial cases of Norrie disease and X‐linked exudative vitreoretinopathy. Clinical & Experimental Ophthalmology. 2010. May;38(4):367–74. 10.1111/j.1442-9071.2010.02245.x [DOI] [PubMed] [Google Scholar]
- 63.Meindl A, Lorenz B, Achatz H, Hellebrand H, Schmitz-Valckenberg P, Meitinger T. Missense mutations in the NDP gene in patients with a less severe course of Norrie disease. Human Molecular Genetics. 1995. March 1;4(3):489–90. 10.1093/hmg/4.3.489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The whole genome sequencing data, has been uploaded to the European Nucleotide Archive (ENA, Accession number: PRJEB39869).