Abstract
The purpose of this systematic review is to synthesize the study design features as well as the attributes and outcomes of technology-based health interventions targeting chronically ill adults and their family caregivers. Twenty papers representing 19 studies met the inclusion criteria. Various theoretical foundations or approaches guided the interventions in 11 studies. Interventions either aimed to support patient self-management and improve patient outcomes or enhance shared illness management and improve patient and caregiver outcomes. The interventions included educational, behavioral, and support components and were delivered using various technologies ranging from text messaging to using the Internet. Overall, patients and caregivers expressed improvements in self-management outcomes (or support) and quality of life. Interventions with a dyadic focus reported on interpersonal outcomes, with improvements noted mostly in patients. This review captures an emerging area of science, and findings should be interpreted in light of the methodological limitations of the included studies.
Keywords: self-management, technology-based, intervention, family caregiver, dyad
Chronic conditions are a significant public health concern. In the United States, 6 in 10 adults are living with at least one chronic condition, and 4 in 10 have more than one (Buttorff et al., 2017). The proportion of people with chronic conditions is gradually increasing, and the number of Americans with multiple chronic conditions is predicted to increase by 37% between 2000 and 2030 (Anderson, 2010). Chronic conditions are physical or mental health conditions characterized by a long duration (lasting more than one year), functional limitations, and the need for ongoing monitoring and treatment (Hwang et al., 2001). People with chronic conditions have poor functional status and quality of life (QOL) and often higher utilization of health care resources (Jindai, et al., 2016; Lehnert et al., 2011). Chronic disease management involves a complex self-care regimen that is further complicated for people living with more than one chronic disease (Liddy et al., 2014). In light of the escalating prevalence of adults living with chronic conditions and the impact of these conditions on the person and the health care system, it is important to find new models that support self-care practices.
In the conventional patient-centric paradigm, people with chronic illness often seek support from their close family members, friends, and unpaid persons who provide instrumental assistance and emotional support at different stages of the disease trajectory, which is referred to as family caregiver (Buck et al., 2015). On the other hand, family caregivers report high levels of stress and reduced QOL (Bom et al., 2018; Wolff et al., 2016). Dyadic illness management is emerging as a novel behavioral paradigm that focuses on partnerships between patients and family caregivers to manage health and illness for both members of the dyad (Lyons & Lee, 2018). Therefore, dyadic interventions may be beneficial to both members of the dyad by contributing to better QOL and well-being for patients and caregivers. Dyadic health interventions are delivered to both patients and their family caregivers with expectations that both members of the dyad are collaboratively engaging in healthy behaviors and disease management. The existing literature highlights the importance of addressing psychosocial and relational factors for health promotion and disease management in a dyadic context. The psychological well-being of patients and family caregivers influences self-management outcomes (Bidwell et al., 2017; Chung et al., 2009). Additionally, relationship quality is associated with varying degrees of engagement in disease management for both partners and influences patient and caregiver outcomes (Bidwell et al., 2015; Hooker et al., 2018; Park & Schumacher, 2014). Therefore, dyadic health interventions are aimed at collaboratively managing chronic illness and improving psychosocial, relational, and physical health outcomes for patients and caregivers.
Traditionally, behavioral interventions have consisted of face-to-face contact, with participants interacting with a moderator in order to reexamine their beliefs, enhance their knowledge, and learn new skills to maintain a healthy lifestyle or manage their conditions. More recently, there has been an increased interest in technology-based interventions as an alternate paradigm for intervention delivery (Murray, 2012). Depending on the type of technology used, researchers are able to provide participants with the flexibility of completing the intervention at their own pace and convenience. Moreover, researchers can overcome access barriers and reach more people across geographic boundaries, specifically those with limited transportation and those living in rural areas.
To date, the majority of technology-based interventions have targeted individual people to promote behavior change, support chronic disease management, and enhance coping strategies. Researchers have predominately focused on either patients or their family caregivers, but more recently, there is an emerging interest in engaging both care partners in managing health and illness. Recent systematic reviews reported on dyadic interventions or programs focused on specific health behaviors (e.g., physical activity, [Carr et al., 2019]) or specific disease populations (e.g., individuals with cancer, [Hu et al., 2019]; traumatic brain injury [Kreitzer et al., 2018]; or heart failure [Buck et al., 2018]). However, no systematic assessment of technology-based dyadic interventions has been conducted across disease populations. Therefore, understanding how researchers are using technological advances in the context of interventions that target both patients and their family caregivers is warranted.
Purpose
The purpose of this systematic review is threefold: (a) describe the characteristics of studies that evaluated technology-based health interventions targeting chronically ill adults and their family caregivers; (b) identify the theoretical foundations and design elements of technology-based interventions; and (c) summarize the impact of technology-based intervention among the chronically ill and their family caregivers.
Methods
Design
This is a systematic review that is guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework. For this study, we defined technology-based dyadic health interventions as any intervention that targeted chronically ill adults and their family caregivers; encompassed cognitive-behavioral, psychoeducational, peer support, or coaching strategies; and was delivered via computer or mobile technology to improve patient or family caregiver outcomes.
Study Eligibility
Studies that met the following eligibility criteria were included: (1) consisted of a sample of chronically ill adults (18 years of age or older) and their family caregivers, (2) had a quasi-experimental or experimental design, which included feasibility or pilot studies, (3) exposed participants to a technology-based health intervention, and (4) reported health-related outcomes for the chronically ill or their family caregivers. Given the rapidly growing nature of the body of literature focused on technology-based health interventions, we included pilot studies that assessed feasibility, acceptability, and efficacy to capture emerging dyadic interventional studies and forecast future directions in this line of inquiry. Studies were excluded if they: (1) were not conceptualized to influence chronic disease management, (2) were delivered via telephone calls, such as the delivery of motivational interviewing over the telephone, and did not combine this strategy with other forms of technology, (3) were not available in English or in full-text.
Search Strategy
The search strategy included the abstraction of studies from PubMed, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and PsycINFO. Three groups of keywords and MeSH terms were developed to search for the following concepts: dyad (involving a patient and a family caregiver), technology, and chronic disease management (Table 1). Additional papers were identified by hand searching the reference list of all eligible studies and by tracking citations of the eligible studies in Google Scholar. Authors of published study protocols were contacted to inquire whether they completed their study and had any upcoming paper focused on the primary findings. We concluded our search of the studies in February 2019.
Table 1:
Exemplar Search Terms
| Concept | Search term |
|---|---|
| Dyad | Patient, care receiver, family, caregiver, carer, care taker, care partner, family caregiver, informal caregiver, couple, spouse, husband, wife, adult child, son, daughter, dyad, dyadic, interpersonal relations, dyadic relationship |
| Technology | Technology, internet, eHealth, electronic health, mHealth, mobile health, web-based, website, online, video conferenc*, text messag*, SMS messag* or SMS text*, mobile phone, cell phone, email, electronic mail, computer, DVD, CD, VCD or video CD, telemedicine, educational technology, audiovisual aids |
| Chronic disease management | Self-care, self-management, disease management, symptom management, palliative care, behavior change, behavior modification, health behavior, shared management, shared care |
Note: Search terms were used as keywords in the title and abstract and as MeSH terms when available.
Truncation was used with some search terms to capture various word endings and spellings.
Study Selection
The first author completed the initial search of the databases as well as the first-level screening of all titles and abstracts after eliminating duplicates. Studies were retained to the second level of screening in cases of uncertainty, and their full-text papers were independently assessed for eligibility by two authors. The first two authors discussed their decisions and reached consensus regarding the final decision. Any disagreement over study eligibility was resolved through discussion with the senior author.
Data Extraction
Each study that met the inclusion criteria underwent data extraction using a predesigned data matrix. Extracted information included study design; study population and participant characteristics; details of the intervention, including theoretical underpinning, intervention components, and type of technology used; control group description; outcomes and times of measurement; and indicators of participant acceptability. We discussed the extracted data and identified shared and unique features in the reviewed interventions and the technologies used to deliver those interventions. We synthesized the results using a narrative summary approach.
Risk of Bias Assessment
Two authors independently evaluated the risk of bias of each included study against the following criteria: random sequence generation; allocation concealment; blinding of participants, personnel, and outcomes; incomplete outcome data; and selective outcome reporting, as recommended by The Cochrane Collaboration (Higgins et al., 2011). The following judgments were used: low risk, high risk, or unclear risk. Two authors compared their ratings and resolved disagreement by consensus, and if necessary, the third author was consulted to resolve disagreements. The risk of bias was not assessed for pilot studies that only included acceptability measures. No papers were excluded based on the risk of bias assessments because the majority of papers reported on pilot and quasi-experimental studies and we wanted to capture the current state of the science of this emerging area.
Results
Search Results
The primary search of the databases yielded 407 papers extracted from PubMed (n = 379) and CINAHL and PsycINFO (n = 28). Additional papers (n = 10) were retrieved through citation tracking and hand searching of the reference lists of the relevant papers. After removing duplicates, 397 records were screened by reviewing titles and abstracts. Of these, 356 records were excluded, leaving the remaining 41 papers for a full-text eligibility assessment. Twenty papers representing 19 unique intervention studies met the search criteria and were included in this review. Papers were excluded because they reported on intervention development or protocols (n = 8), the intervention targeted either patients (n = 1) or caregivers (n = 6), the intervention did not address chronic illness management (n = 5), and the patient population included children (n = 1). Figure 1 illustrates the results of the search strategy.
Figure 1:
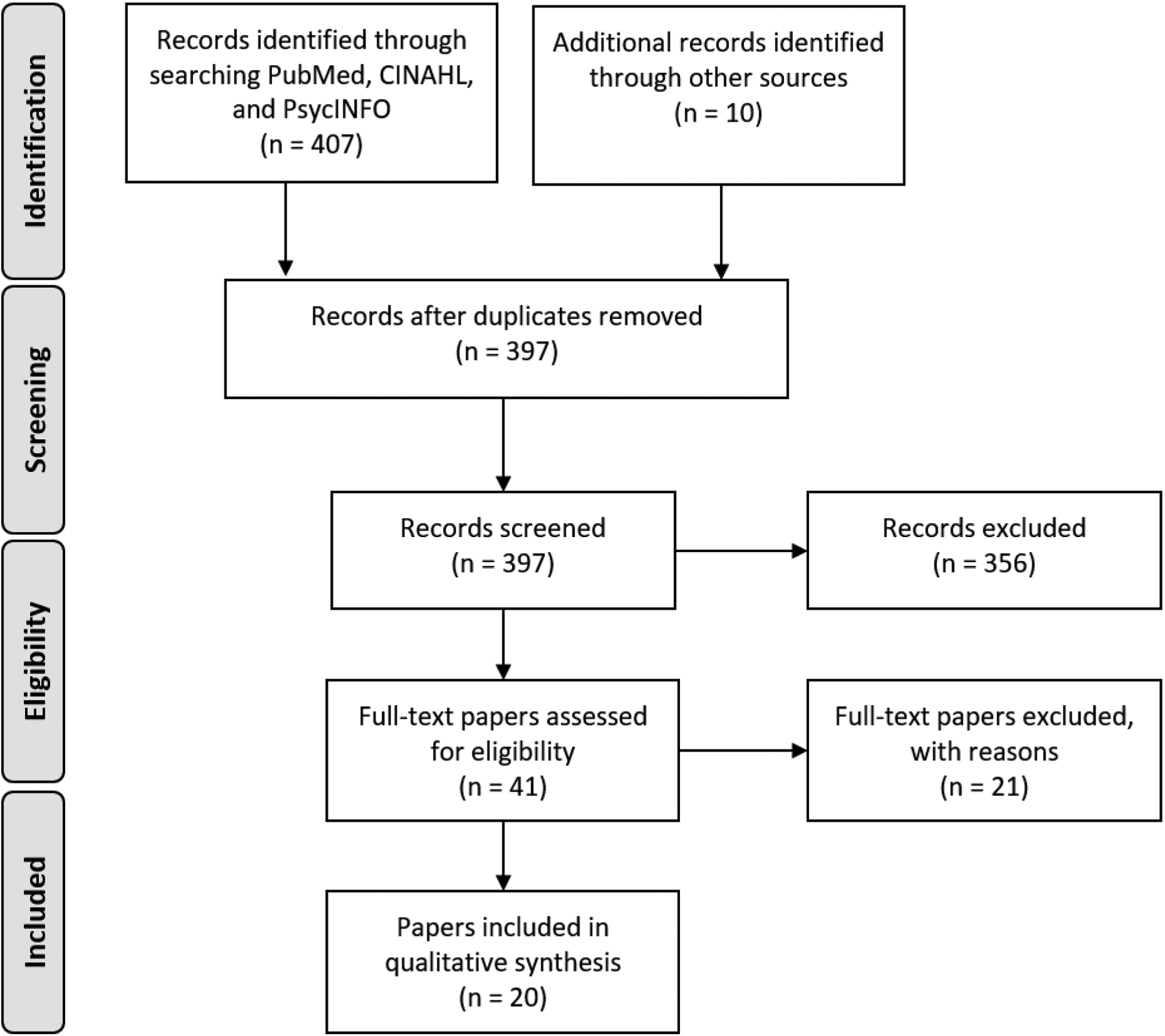
PRISMA Flow Chart
Study Characteristics
Design.
Nine studies were randomized controlled trials (RCTs), with a third of them being pilot trials that focused on assessing acceptability and feasibility, and in some instances, establishing preliminary efficacy of the interventions. The remaining 10 studies had a quasi-experimental design (specifically pretest-posttest and patient preference clinical trial designs) and were largely (8 out of 10) pilot studies. Randomized trial studies were heterogeneous with respect to the types of control groups used. These studies were designed using standard usual care (n = 2), wait list (n = 2), attention control (n = 3), and comparison of other unique conditions (n = 2). Characteristics of the selected studies are presented in Table 2.
Table 2:
Study Characteristics
| Authors (Country) | Design and Follow-up Period | Control group | Sample | Recruitment site |
|---|---|---|---|---|
| (Agren et al., 2012) (Sweden) | Randomized controlled design with 3 and 12 months follow-up | Usual care in hospital and outpatient education and psychosocial support, but not systematically involving the partner | N = 155 patients with heart failure and their coresiding partners |
|
| Patient age: 71.2 ± 11.4 | ||||
| Patient gender: 75.5% male | ||||
| Caregiver age: 68.6 ± 10.9 | ||||
| Caregiver gender: 24.5% male | ||||
| (Aikens, Trivedi, Aron, & Piette, 2015) (USA) | Quasi-experimental patient preference trial | N = 303 patients with type 2 diabetes; 118 patients participated with a noncoresiding caregiver (family member or friend) |
|
|
| Patient age: 66.6 ± 9.8 | ||||
| Patient gender: 97% male | ||||
| Caregiver age: No data | ||||
| Caregiver gender: No data | ||||
| (Aikens, Trivedi, Heapy et al., 2015) (USA) | Quasi-experimental patient preference trial | N = 221 patients with an active depression diagnosis; 135 patients participated with a caregiver (family member or friend) |
|
|
| Patient age: 51.4 ± 12.7 | ||||
| Patient gender: 21.4% male | ||||
| Caregiver age: No data | ||||
| Caregiver gender: No data | ||||
| (Badger et al., 2013) (USA) | Randomized trial with 8- and 16-week follow-up | Three-group design comparing telephone health education, telephone interpersonal counseling, and videophone interpersonal counseling | N = 49 women with breast cancer and their partners (42.9% spouses or significant others, 18.4% daughters 12.2% siblings, 10.2% mothers, 16.3% friends). |
|
| Patient age: 53.1 ± 12.2 | ||||
| Patient gender: 0% male | ||||
| Caregiver age: 51.6 ± 13.9 | ||||
| Caregiver gender: 42.9% male | ||||
| Single-blind randomized controlled trial with 2-week follow-up | Attention control: individuals were not instructed to set behavioral intentions but received 1 text message every weekday over 2 weeks reminding them to fill in the diary | N = 121 overweight or obese heterosexual couples |
|
|
| Target person age: 46.1 ± 13.6 | ||||
| Target person gender: 48.8 % male | ||||
| Partner age: No data | ||||
| Partner gender: 51.2 % male | ||||
| (Dew et al., 2004) (USA) | Quasi-experimental trial with historic comparison group | N = 64 heart recipients; 59 participated with their family caregivers (75% spouses) |
|
|
| Patient age: 53.1% ≤ 55 years | ||||
| Patient gender: 75% male | ||||
| Caregiver age: 58.3% ≤ 55 years | ||||
| Caregiver gender: 15% male | ||||
| (Fergus et al., 2014) (Canada) | Pilot testing; feasibility and acceptability | N = 10 women with breast cancer and their male partners |
|
|
| Patient age: 33.6 ± 4.5 | ||||
| Patient gender: 0% male | ||||
| Caregiver age: 34.9 ± 4.3 | ||||
| Caregiver gender: 100% male | ||||
| (Mayberry et al., 2016) (USA) | Pilot testing; feasibility and acceptability | N = 19 patients with type 2 diabetes; 7 patients participated with a caregiver (six spouses/significant others and one son) |
|
|
| Patient age: 51.7 ± 10.2 | ||||
| Patient gender: 47% male | ||||
| Caregiver age: No data | ||||
| Caregiver gender: No data | ||||
| (Northouse et al., 2014) (USA) | Preintervention and postintervention feasibility study with 2-week follow-up | N = 38 patients with cancer (lung, breast, colorectal, prostate) and their family caregivers (68% spouses, 32% non-spouses) |
|
|
| Patient age: 54.8 ± 12.6 | ||||
| Patient gender: 42% male | ||||
| Caregiver age: 50.6 ± 14.7 | ||||
| Caregiver gender: 39% male | ||||
| (Pfeiffer et al., 2017) (USA) | Pilot testing; pretest-posttest design with 3- and 6-month follow-up | N = 48 patients with depression discharged from inpatient psychiatric units; 19 patients participated with their family member/friend and 29 patients chose to work with a trained peer specialist |
|
|
| Patient age: 50.1 ± 14.7 | ||||
| Patient gender: 75% male | ||||
| Caregiver age: No data | ||||
| Caregiver gender: No data | ||||
| (Piette et al., 2016) (Bolivia) | Randomized trial over a 4-month period | Attention control: patients received weekly self-monitoring and self-management support calls for 4 months | N = 72 patients with diabetes and/or hypertension; 45 patients participated with their family caregivers (65.3% adult children, 20.8% spouses, 13.9% friends and siblings) |
|
| Patient age: 62.5% ≥ 60 years | ||||
| Patient gender: 37.5% male | ||||
| Caregiver age: 42.2 ± 14.9 | ||||
| Caregiver gender: 34.2% male | ||||
| (Piette, Striplin, Marinec, Chen, & Aikens, 2015) (USA) | Randomized comparative effectiveness trial with 6- and 12-month follow-up | Attention control: besides educational booklets for patients and caregivers, patients received weekly self-monitoring and self-management support calls for 12 months | N = 369 patients with heart failure and their noncoresiding family caregivers (41.1% daughter/daughter-in-law, 19.7% son/son-in-law, 9.5% sisters/sisters-in-law, and 29.7% included other family members and friends) |
|
| Patient age: 67.9 ± 10.2 | ||||
| Patient gender: 99.2% male | ||||
| Caregiver age: 47.1 ± 13.2 | ||||
| Caregiver gender: 34.9% male | ||||
| (Piette, Striplin, Marinec, Chen, Trivedi, et al., 2015) (USA) | N = 331 patients with heart failure and their noncoresiding informal caregivers (41.1% daughter/daughter-in-law, 20.2% son/son-in-law, 9.1% sisters/sisters-in-law, and 29.3% included other family members and friends) | |||
| Patient age: 67.8 ± 10.2 | ||||
| Patient gender: 99.4% male | ||||
| Caregiver age: 46.7 ± 13.2 | ||||
| Caregiver gender: 35% male | ||||
| (Piette et al., 2013) (USA) | Feasibility study | N = 387 patients with an active depression diagnosis or an antidepressant prescription; 227 patients participated with a family caregiver (family member or friend) |
|
|
| Patient age: 59% ≤ 55 years | ||||
| Patient gender: 20% male | ||||
| Caregiver age: No data | ||||
| Caregiver gender: No data | ||||
| (Piette et al., 2008) (USA) | Feasibility study | N = 52 patients with heart failure and their non-coresiding family caregivers (40% daughter, 35% son, 25% other relative, 10% friend). |
|
|
| Patient age: 65.9 ± 10.8 | ||||
| Patient gender: 89% male | ||||
| Caregiver age: 42.3 ± 9.8 | ||||
| Caregiver gender: 42% male | ||||
| (Porter et al., 2018) (USA) | Randomized pilot trial | Wait list control | N = 20 cancer survivors (25% prostate cancer, 75% breast cancer) and their coresiding partners (2 couples were same sex) |
|
| Patient age: 63.0 ± 8.9 | ||||
| Patient gender: 30% male | ||||
| Caregiver age: 62.8 ± 7.7 | ||||
| Caregiver gender: 65% male | ||||
| (Porter et al., 2017) (USA) | Randomized pilot trial with 2–3 weeks follow-up | Educational comparison condition: six 60-min sessions via videoconference about healthy lifestyle information relevant to cancer | N = 32 patients with advanced GI cancer (stage 3 or 4) and their spouses or intimate partners who reported difficulties communicating about cancer-related concerns |
|
| Patient age: 54.7 ± 10.4 | ||||
| Patient gender: 68.8% male | ||||
| Caregiver age: 52.3 ± 10.1 | ||||
| Caregiver gender: 31.2% male | ||||
| (Schover et al., 2012) (USA) | Randomized trial with 3-month, 6-month, and 12-month follow-up | Three-group design comparing a 3-month wait list control, face-to-face intervention, and Internet-based intervention | N = 186 men with prostate cancer and their coresiding partners (96.7% spouses) |
|
| Patient age: 64.0 ± 7.7 | ||||
| Patient gender: 100% male | ||||
| Caregiver age: No data | ||||
| Caregiver gender: 0% male | ||||
| (Song et al., 2015) (USA) | Preintervention and postintervention feasibility study | N = 22 men with prostate cancer and their partners (25 couples completed the intervention but only 22 were included in the analysis) |
|
|
| Patient age: 62.95 ± 8.22 | ||||
| Patient gender: 100% male | ||||
| Caregiver age: 59.32 ± 10.67 | ||||
| Caregiver gender: 0% male | ||||
| (Srisuk et al., 2017) (Thailand) | Randomized controlled trial with 3- and 6-month follow-up | Usual care from the hospital and equivalent contact with research team about general health topics | N = 100 patients with heart failure and their coresiding family caregivers (27% spouse, 39% child, 26% sibling, 8% parent) |
|
| Patient age: 62 ± 16.2 | ||||
| Patient gender: 47% male | ||||
| Caregiver age: 41 ± 10.7 | ||||
| Caregiver gender: 27% male |
Nine of the 19 included studies had an RCT design and minimized selection bias. However, authors of only five studies provided a description of the allocation concealment process. Blinding of participants was not feasible for most study conditions, and in only one study participants were blinded (Berli et al., 2016). Blinding of outcome assessment was achieved in two studies where the outcome (physical activity) was measured using accelerometers (Berli et al., 2016) or the data collector was blinded to group assignment (Srisuk et al., 2017). Therefore, many studies had high or unclear risk of bias and the results should be interpreted with caution. Risk of bias assessment is presented in Table 3.
Table 3:
Risk of Bias Assessment Based on the Cochrane Collaboration’s Tool
| Authors | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) |
|---|---|---|---|---|---|---|
| (Agren et al., 2012) | Low risk | Unclear risk | High risk | High risk | Unclear risk | Low risk |
| (Aikens, Trivedi, Aron et al., 2015) | High risk | High risk | High risk | High risk | Unclear risk* | Low risk |
| (Aikens, Trivedi, Heapy et al., 2015) | High risk | High risk | High risk | High risk | Low risk* | Low risk |
| (Badger et al., 2013) | Low risk | Unclear risk | High risk | High risk | Low risk | Low risk |
| (Berli et al., 2016) | Low risk | Low risk | Low risk | Low risk | Low risk* | Low risk |
| (Dew et al., 2004) | High risk | High risk | High risk | High risk | Unclear risk | Low risk |
| (Northouse et al., 2014) | High risk | High risk | High risk | High risk | Low risk* | Low risk |
| (Pfeiffer et al., 2017) | High risk | High risk | High risk | High risk | Unclear risk | Low risk |
| (Piette et al., 2016) | Low risk | Unclear risk | High risk | High risk | Low risk* | Low risk |
| (Piette et al., 2015 and Piette, Striplin, Marinec, Chen, & Aikens, 2015) | Low risk | Low risk | High risk | High risk | Low risk | Low risk |
| (Porter et al., 2018) | Low risk | Low risk | High risk | High risk | Low risk* | Low risk |
| (Porter et al., 2017) | Low risk | Low risk | High risk | High risk | Low risk* | Low risk |
| (Schover et al., 2012) | Low risk | Unclear risk | High risk | High risk | Low risk | Low risk |
| (Song et al., 2015) | High risk | High risk | High risk | High risk | Low risk | Low risk |
| (Srisuk et al., 2017) | Low risk | Low risk | High risk | Low risk | Unclear risk | Low risk |
Note:
Risk of bias assessment for short-term outcomes only; no long-term outcomes available.
Risk of bias was not assessed for four studies that only included acceptability measures (Fergus et al., 2014; Mayberry et al., 2016; Piette et al., 2008; Piette et al., 2013).
Target population.
All studies used convenient sampling strategies to identify the target populations of interest. Sample sizes ranged from 10 (Fergus et al., 2014) to 369 (Piette et al., 2015) dyads. For papers that included the average age of study participants, the average age of patients across studies (n = 17) ranged from 33.6 to 71.2 years, whereas caregivers’ average age ranged from 34.9 to 68.6 years (n = 12). The proportion of family caregivers was predominantly female, but varied between patient groups. The studies that enrolled partners and focused on sex-linked cancers (n = 4) included either male- or female-predominant caregivers. Otherwise, the proportion of female caregivers ranged from 49% to 85%. Seven of the 19 studies included only spouse/partner dyads, and the other 12 studies included mixed dyads, primarily other family member or friend dyads. Three studies specifically enrolled caregivers who were noncoresiding family members or friends.
The samples included patients with a variety of chronic conditions. The interventions targeted patients with cancer (primarily breast and prostate cancer; n = 7), cardiovascular disease (n = 6) with a focus on heart failure, depression (n = 3), diabetes (n = 3), and obesity (n = 1). In one study, patient participants had a diabetes and/or hypertension diagnosis (Piette et al., 2016). One of the interventions targeting cancer dyads included mixed types of cancer (e.g., lung, colorectal, breast, prostate [Northouse et al., 2014]), and another intervention focused on breast and prostate cancer survivors (Porter et al., 2018).
Recruitment setting.
The interventions were tested in multiple countries, including the United States, Bolivia, Canada, Sweden, and Thailand. Most participants were recruited from the community and from outpatient clinics, including hospital-affiliated or community-based primary care clinics, oncology clinics at comprehensive cancer centers, and cardiology clinics. In one study, participants were identified by screening the electronic medical records for all admissions to an inpatient psychiatry unit (Pfeiffer et al., 2017). Four of the included studies recruited from a U.S. Veterans Administration clinic or hospital. In three studies, different sites were used and participants were recruited from the community and outpatient clinics (Badger et al., 2013; Fergus et al., 2014; Schover et al., 2012).
Intervention Characteristics
Conceptually, the interventions had either a patient or a dyadic focus. Patient-focused interventions targeted both patients and family caregivers but were primarily aimed at improving patient health outcomes through self-management support. Interventions focusing on the dyad consisted of interpersonal skills building and strategies for shared illness management and were aimed at improving outcomes for both members of the dyad. Characteristics of the interventions are described in the subsequent four sections and presented in Table 4.
Table 4:
Intervention Characteristics
| Authors | Intervention Focus | Theoretical Underpinnings and Guiding Principles | Intervention Components | Intervention Dosage | Type of Technology |
|---|---|---|---|---|---|
| (Agren et al., 2012) | Dyadic |
|
|
Three 60-minute sessions conducted 2, 6, and 12 weeks after hospital discharge | Computer-based CD-ROM educational program |
| (Aikens, Trivedi, Aron et al., 2015) | Patient | No information |
|
3 or 6 months of weekly calls and email reports (mid-project positive findings supported the extension of the intervention duration to 6 months) | IVR calls, email reports, DVD-based training |
| (Aikens, Trivedi, Heapy et al., 2015) | Patient | No information |
|
6 months of weekly calls and email reports | IVR calls and email reports |
| (Badger et al., 2013) | Dyadic | No information |
|
|
Videoconference (videophone or laptop computer to use Skype) |
| (Berli et al., 2016) | Patient |
|
Individuals were assigned to one of two intervention groups:
|
One text message every weekday over 2 weeks (total of 10 messages) | Text messages |
| (Dew et al., 2004) | Patient |
|
|
Weekly access to website for 4 months | Website |
| (Fergus et al., 2014) | Dyadic |
|
|
6 sessions over 8 weeks and weekly interaction with the online facilitator | Website |
| (Mayberry et al., 2016) | Patient |
|
|
One phone coaching session with the patient followed by two weeks of text message support (4 text messages per week for the patient and 3 text messages per week for the caregiver, if enrolled) | Text messages |
| (Northouse et al., 2014) | Dyadic |
|
|
3 sessions over a 6-week period | Website and email review of each session |
| (Pfeiffer et al., 2017) | Patient | No information |
|
|
IVR calls and email reports |
| (Piette et al., 2016) | Patient | No information |
|
Up to 4 months of weekly calls | IVR calls |
| (Piette et al., 2015; Piette, Striplin, Marinec, Chen, & Aikens, 2015) | Patient |
|
|
12 months of weekly calls and email reports | IVR calls and email reports |
| (Piette et al., 2013) | Patient | No information |
|
6–15 weeks (12 weeks on average) of IVR calls and email reports | IVR calls and email reports |
| (Piette et al., 2008) | Patient | No information |
|
Weekly IVR calls and email reports for the first 6 weeks, after which patients can reduce the frequency (unless they have increased depressive symptoms) | IVR calls and email reports |
| (Porter et al., 2018) | Dyadic |
|
|
Four 60-min sessions (three weekly sessions with a booster session occurring one month later) | Videoconference |
| (Porter et al., 2017) | Dyadic | No information |
|
Six 60-min weekly sessions | Videoconference |
| (Schover et al., 2012) | Dyadic |
|
|
|
Website and email communication with facilitator |
| (Song et al., 2015) | Dyadic |
|
|
|
Website |
| (Srisuk et al., 2017) | Dyadic |
|
|
|
DVD |
Theoretical foundation.
Eleven studies had a theoretical underpinning or guiding framework for the interventions and the description of the theoretical foundation varied in length and depth across studies. In five studies, the authors referred to one theoretical or practical model that informed the development of the intervention program. The following theories and frameworks were noted: dyadic action control, self-regulation theory, stress and coping theory, and a sensate focus framework. In the remaining six studies, the authors used a combination of theories and integrated approaches from multiple frameworks to develop the components of the intervention. For example, authors of two studies (Fergus et al., 2014; Porter et al., 2018) integrated concepts from multiple dyadic coping models. Approaches that were often used and may have been theory-informed included adult learning principles, and general behavior change techniques and coping strategies such as goal-setting, problem-solving, self-monitoring of behavior, and cognitive behavioral therapy.
Intervention components.
The interventions included educational, behavioral, and support components. All of the interventions were multicomponent and twelve studies included all three types of components. Educational components consisted of informational sessions about various topics such as disease processes, self-management skills, relationship building, and communication strategies. In some cases, education was tailored to participants’ characteristics (Northouse et al., 2014) or needs (such as in the interventions using Interactive Voice Response [IVR] calls). Educational components were often coupled with activities or experiential exercises for participants to develop their self-management and interpersonal skills.
Behavioral components included self-monitoring and goal-setting strategies. Nine studies had a self-monitoring component that consisted of receiving prompts to evaluate one’s own health behaviors on a regular basis. The prompts included receiving automated phone calls, text message reminders, or diaries to track health behaviors. Goal setting was central to three studies (Berli et al., 2016; Mayberry et al., 2016; Porter et al., 2018). Patients set behavioral goals in the presence of their informal caregivers and received regular reminders from their caregivers to reach their goals.
Lastly, support components consisted of providing direct support to participants during the intervention or guiding participants to support their care partners. Examples of support components included phone coaching (Mayberry et al., 2016), counseling sessions (Badger et al., 2013; Schover et al., 2012; Srisuk et al., 2017), and suggestions to support others (in most of the remaining studies).
Intervention dosage.
There was great variability in the intervention dosage across the 19 included studies. Some interventions (n = 9) adopted a more intensive approach by having frequent doses of short duration (e.g., weekly phone calls and e-mail reports, daily text messages), while others (n = 10) consisted of less frequent contact, but longer sessions. Sessions were either self-directed or facilitated by a trained professional. Four out of the 10 interventions that used sessions included self-directed modules that ranged from 2 to 7 in number. For the remaining 6 (out of 10) interventions, the number of professionally facilitated sessions ranged from 1 to 8, with sessions lasting between 30 and 90 minutes, and were often delivered weekly. In two papers, authors mentioned booster doses that were delivered at least a month after the main intervention components were completed (Porter et al., 2018; Schover et al., 2012).
Type of technology.
Several types of computer or mobile technology with varying levels of sophistication supported intervention delivery, highlighting the continuum of technology complexity. The range of technologies included using a computer or mobile phone to access a website or program, participate in a videoconference or an IVR call, receive text messages or e-mail messages, or play a video. Eight interventions were Internet-based and used either a website (n = 5) or videoconferencing (n = 3) as a delivery platform. Two of the interventions delivered via website also had a professional facilitator who regularly communicated with participants using e-mail (Schover et al., 2012) or a discussion board (Fergus et al., 2014). Seven studies tested one intervention program that was adapted to multiple disease populations. This intervention consisted of IVR calls following a tree-structured algorithm and tailored feedback to patients as well as e-mail messages to caregivers. Lastly, two interventions used a computer-based program or video, and the remaining two used personalized text messaging.
The type of technology used was associated with intervention intensity and exposure (i.e., the frequency and length of contact with participants). For instance, interventions that relied on IVR calls or text messages consisted of frequent contacts of short duration, whereas web-based interventions included less frequent sessions of longer duration.
Intervention Outcomes
Given the heterogeneity of the included studies and the exploratory nature of some of them, the reporting of outcomes varied from specifying point estimates, confidence intervals, and levels of significance to calculating effect sizes. Our synthesis of the measured outcomes resulted in five categories for patient outcomes, three categories for caregiver outcomes, and a category of interpersonal outcomes that were assessed for both patients and caregivers using the same measures. Details about intervention outcomes are presented in Table 5 and summarized below. Four studies included measures of feasibility and acceptability, without any outcome assessments. Therefore, authors of these studies explored participants’ experiences with the intervention and reported on the acceptability of their interventions.
Table 5:
Summary of Intervention Outcomes
| Patient outcomes | Caregiver outcomes | |||
|---|---|---|---|---|
| Significant* | Nonsignificant | Significant* | Nonsignificant | |
| Self-management behaviors | Medication adherence (Aikens, Trivedi, Aron, et al., 2015; Aikens, Trivedi, Heapy, et al., 2015; Piette et al., 2015); physical activity (Berli et al., 2016; Porter et al., 2018); medical compliance: medication adherence, clinic appointments, blood tests, physical activity, diet adherence (Dew et al., 2004); heart failure self-care (Srisuk et al., 2017) | Heart failure self-care (Agren et al., 2012; Piette et al., 2015) | Physical activity (Porter et al., 2018) | |
| Self-management processes | Perceived control at 3 months (Agren et al., 2012); self-efficacy to manage symptoms (Porter et al., 2017); partner support (Porter et al., 2018); heart failure self-efficacy and heart failure knowledge (Srisuk et al., 2017) | Perceived control at 12 months (Agren et al., 2012); cancer self-efficacy and social support (Northouse et al., 2014); Social support (Pfeiffer et al., 2017) | Cancer self-efficacy and social support (Northouse et al., 2014); care partners’ self-management support (Piette, Striplin, Marinec, Chen, & Aikens, 2015; Porter et al., 2018); self-efficacy to manage symptoms (Porter et al., 2017); heart failure knowledge at 3 and 6 months, and perceived control at 3 months only (Srisuk et al., 2017) | Perceived control (Agren et al., 2012) |
| Physical symptoms | Shortness of breath and clinically significant weight gain (Piette et al., 2015); symptom distress related to prostate cancer (bowel dysfunction, urinary irritability), and general symptoms (fatigue, pain, sleep disturbance) (Song et al., 2015) | General symptom distress (12 cancer-related symptoms) (Badger et al., 2013); erectile dysfunction (Schover et al., 2012) | Physical symptom (Badger et al., 2013); improvement in partners’ perception of patients’ prostate cancer’s symptoms as problems and small improvement of general symptoms (fatigue, pain, sleep disturbance) (Song et al., 2015) | Female sexual function (Schover et al., 2012) |
| Psychological symptoms | Depression remission (Aikens, Trivedi, Heapy et al., 2015); depression and anxiety (Dew et al., 2004); emotional distress (Northouse et al., 2014); depression and anxiety (Pfeiffer et al., 2017); cancer-related distress and psychological growth (Porter et al., 2017) | Depression (Agren et al., 2012); depression (Badger et al., 2013); emotional distress (Schover et al., 2012) | Distress level, anxiety symptoms, and depression symptoms (Dew et al., 2004); emotional distress (Northouse et al., 2014); depressive symptoms and caregiver strain at 6 and 12 months (Piette, Striplin, Marinec, Chen, & Aikens, 2015) | Symptoms of depression and caregiver burden (Agren et al., 2012); depression (Badger et al., 2013) |
| Quality of life | Social well-being (Badger et al., 2013); QOL in social functioning (Dew et al., 2004); overall (total) QOL, physical QOL, functional QOL (Northouse et al., 2014); general health status (Piette et al., 2016); physical QOL (Porter et al., 2018); overall, physical, and social QOL (Song et al., 2015); emotional dimension of HF-specific QOL at 3 and 6 months (Srisuk et al., 2017) | Spiritual well-being (Badger et al., 2013); physical and mental components of QOL (Agren et al., 2012); HF-specific QOL (Piette et al., 2015) | Social well-being (Badger et al., 2013); QOL in social functioning (Dew et al., 2004); overall (total) QOL, physical QOL, functional QOL (Northouse et al., 2014); social QOL (Song et al., 2015) | Physical and mental components of QOL (Agren et al., 2012; Srisuk et al., 2017) |
| Interpersonal outcomes | Communication (Piette et al., 2015; Porter et al., 2017); relationship satisfaction and intimacy (Porter et al., 2017) | Communication (Northouse et al., 2014; Song et al., 2015); relationship satisfaction (Schover et al., 2012; Song et al., 2015); sexual satisfaction (Schover et al., 2012) | Relationship satisfaction (Porter et al., 2017) | Communication (Northouse et al., 2014; Porter et al., 2017; Song et al., 2015); intimacy (Porter et al., 2017); relationship satisfaction (Schover et al., 2012; Song et al., 2015); sexual satisfaction (Schover et al., 2012) |
Note:
Significant findings included significant group × time differences for RCTs or significant changes over time for pre-post designs.
No outcomes reported in four studies (Fergus et al., 2014; Mayberry et al., 2016; Piette et al., 2008; Piette et al., 2013).
Patient outcomes.
Fifteen of the included studies assessed patient outcomes. The majority of them (14 out of 15) examined self-management behaviors (i.e., medication adherence, physical activity, and heart failure self-care) or self-management processes (i.e., self-efficacy, social support, knowledge, and perceived control). Eight studies reported on self-management behaviors. Overall, patients who received the intervention were more likely to have improved health behaviors compared to those in the comparison group (n = 7 studies). Authors of two studies (Agren et al., 2012; Piette et al., 2015) reported nonsignificant differences in heart failure self-care behaviors between the intervention and control groups using disease-specific measures of self-care. The studies with positive self-management behavior outcomes tested interventions with a behavioral component. On the other hand, six studies assessed the processes that are known to support self-management behaviors and found mixed results. While two studies reported nonsignificant improvements in social support and self-efficacy (Northouse et al., 2014; Pfeiffer et al., 2017), each of the remaining four studies found statistically significant differences or a tendency for improvement in at least one of the self-management processes, favoring the intervention.
Nine out of the 15 studies reported on QOL outcomes using generic or disease-specific measures. Authors examined multiple dimensions of QOL, including functional or physical, social, and emotional QOL. Overall, most studies (7 out of 9) found significant improvements or a tendency for improvement (in the case of exploratory studies) in QOL. Two authors reported nonsignificant differences in QOL between the compared groups and their interventions targeted adults with heart failure and their family caregivers (Agren et al., 2012; Piette et al., 2015).
Eight out of 15 studies assessed patient psychological symptoms, including depression, anxiety, and emotional distress. Three of them were RCTs and reported nonsignificant differences between groups. The remaining five were quasi-experimental studies and had a significant decline in psychological symptoms over time. Specifically, one study targeted patients with depression (Aikens, Trivedi, Heapy, et al., 2015) and concluded that those assigned to the intervention group were more likely to achieve depression remission compared to those in the comparison group. Lastly, only four studies assessed physical symptoms, and two of them found significant differences or a tendency for improvement in favor of the intervention (Piette et al., 2015; Song et al., 2015).
Caregiver outcomes.
Ten of the included studies assessed caregiver outcomes, and most of them (n = 8) had a dyadic focus. Six studies assessed processes that influence self-management behaviors, with self-efficacy and self-management support being the most common ones. Two studies reported improvements in caregiver self-efficacy over time (Northouse et al., 2014; Porter et al., 2017). Three (out of six) studies assessed support, and one of them (Northouse et al., 2014) reported a tendency for improvement in the support that caregivers received from the patient. The other two studies (Piette et al., 2015; Porter et al., 2018) found an improvement in caregivers’ self-management support, favoring the intervention. Perceived control was assessed in two out of the six studies, but only one study (Srisuk et al., 2017) reported significant differences between the groups, in favor of the intervention. Another set of six studies evaluated the QOL as an outcome for caregivers, and four of them reported statistically significant improvement or a tendency for improvement over time. Specifically, the social dimension of QOL improved in three studies. The two interventions associated with no improvement in caregiver QOL were dyadic in nature, targeted caregivers of adults with heart failure, used a DVD- or computer-based program, and assessed QOL using a generic measure (Agren et al., 2012; Srisuk et al., 2017). Lastly, psychological symptoms were assessed in five other studies. Three of them found statistically significant improvement in depressive symptoms or distress. Only two studies examined caregiver burden, and reported conflicting findings (Agren et al., 2012; Piette, Striplin, Marinec, Chen, & Aikens, 2015).
Interpersonal outcomes.
Five studies examined interpersonal outcomes (i.e., communication and relationship satisfaction) and four of them had a dyadic focus. In the study that focused on patients, the authors assessed communication from the patient’s perspective only and reported more active and positive communication over time in favor of the intervention (Piette et al., 2015). The studies with a dyadic focus had mixed findings. Two exploratory studies (Northouse et al., 2014; Song et al., 2015) and one RCT (Schover et al., 2012) did not find improvement over time in any of the interpersonal outcomes. The remaining study reported improvements in communication and relationship satisfaction mostly favoring patients in the intervention groups (Porter et al., 2017).
Participant experiences and perceived acceptability.
Out of the 19 studies, 11 included a description of participants’ experiences with the programs. Overall, most participants had high satisfaction ratings and reported that the interventions facilitated meaningful conversations and strengthened their relationship with their care partners. Authors reported the challenges and advantages related to the use of technology. A few participants faced connectivity problems during the videoconference and preferred the telephone as a more reliable tool for communication (Badger et al., 2013). Others disliked the impersonality of the computer when accessing the web-based intervention (Fergus et al., 2014). On the other hand, participants appreciated the flexibility and convenience of using the Internet to access the program (Porter et al., 2017; Song et al., 2015). Participants also expressed their satisfaction with tailoring the program to their own needs (Song et al., 2015) and receiving ongoing support via text reminders, automated phone calls, and e-mail reports (Mayberry et al., 2016; Piette et al., 2008). Besides the comments related to the use of technology, some participants criticized the time commitment associated with study participation (Fergus et al., 2014), while others appreciated the brevity of the web-based sessions (Porter et al., 2018).
Discussion
In this systematic review, we examined and reported specific characteristics and outcomes of technology-based intervention studies targeting chronically ill adults and their family caregivers. We identified 20 papers representing 19 unique studies. Four primary findings were observed. First, the RCTs included in this review had various types of control conditions. Second, more than half of the included studies had a theoretical foundation or approach that guided the intervention, but most of them lacked a precise reporting of how theory informed the intervention components. Third, participants reported diverse experiences interacting with technology. Lastly, the interventions had either a patient or a dyadic focus, which influenced the topics of the educational sessions and the type of outcomes assessed.
The RCTs included in this systematic review had a complex structure with respect to the types of control groups employed in the studies. In most instances, participants randomized to the comparison group received a treatment or a greater level of attention besides usual care. In an RCT, the efficacy of an intervention is judged relatively to the control condition. Therefore, the selection of control conditions is as important as the development of the intervention components. While studies that include control groups other than usual care and no treatment may be seen as more rigorous (or more credible) and may have lower rates of attrition, they are not always needed, especially if the intervention is in its early stages of testing (Mohr et al., 2009). Baskin and colleagues (2003) argued that if a study is primarily focused on determining the efficacy of an intervention rather than theoretically disentangling its potent components, then usual care or no treatment are appropriate control conditions. Therefore, authors are encouraged to carefully select the control condition based on the purpose of their studies, especially if the effect of an intervention on the outcome of interest is unknown.
Most authors referred to a theoretical foundation or approach that guided the intervention but few authors explicitly mapped the theoretical underpinnings to the intervention components. Our finding is consistent with the concerns raised by others about the lack of explanation of how theory is used as a basis for behavioral interventions (Michie & Prestwich, 2010). Existing reporting guidelines, such as the CONSORT checklist, do not address the theoretical or conceptual basis of the study or intervention. Therefore, it is at the authors’ discretion to provide information about their guiding framework. Moreover, other parameters such as journals’ submission guidelines may limit the authors’ description of theoretical foundations. Michie and Prestwich (2010) proposed a theory coding scheme as a guide to assess how behavioral interventions are informed by theory and how findings can subsequently substantiate and extend theory. Precise and rigorous reporting of theory-based interventions is important to understand behavior change mechanisms in order to build on the existing literature and develop targeted and more effective interventions. Future research would benefit from taxonomy mapping similar to the work that has been done by Michie and Prestwich (2010) to help better classify and compare intervention effects across studies.
While most participants were satisfied with the use of technology, some faced technical challenges and others had concerns about the impersonality of the technology. These diverse experiences may be related to how people adopt a new technology, which varies based on age, household income, educational attainment, and physical disability (M. Anderson & Perrin, 2017). Moreover, perceived value, confidence in ability to learn the technology, and the perceived impact on QOL influence technology adoption in older adults (Berkowsky et al., 2018). Some of the studies included in this systematic review did not exclusively rely on technology to deliver the intervention and included other types of contact with participants such as involving a professional facilitator. Similar strategies can mitigate the negative experiences that some participants may face while interacting with technology by providing additional opportunities for participants to improve their confidence in their ability to use the technology and appreciate its value. Researchers are encouraged to choose the mode of intervention delivery and the type of technology that best meet the needs of their target population while offering continuous support on the use of the technology.
All studies tested interventions that required both patients and family caregivers to be engaged with the programs but varied in their goals to improve patient and caregiver outcomes. This finding highlights the different paradigms in chronic disease management. Existing models have traditionally focused on providing patients with adequate support to improve their outcomes (Ryan & Sawin, 2009). Current dyadic illness management models highlight the partnership between patients and family caregivers and the interdependence of their health and well-being (Lyons & Lee, 2018). The studies included in this review that reported interpersonal outcomes did not have promising results, and the results were mixed for each member of the dyad. Overall, patients seemed to benefit more than their caregivers from the interventions. Most of these studies often lacked a description of the level of participant engagement with the intervention. The reader would assume that patients and caregivers completed the sessions and activities together unless otherwise specified. One potential explanation is that interventions may have a differential effect on patients and caregivers based on the dyad’s care type (Buck et al., 2019). Future research is needed to evaluate why patients and caregivers may respond differently to an intervention that equally targets both of their needs.
Lastly, findings of this systematic review also have practice implications. Clinicians are encouraged to evaluate if one member of the dyad is primarily responsible for specific aspects of illness management and assess relational factors that might influence how patients and caregivers work on managing chronic illness together.
Our review has several limitations. First, more than half of the studies included in this review were exploratory in nature, which highlights the developing nature of this area of science. Moreover, many studies had high or unclear risk of bias. Therefore, the findings should be interpreted in light of these design limitations. Second, although we consulted with a medical librarian while building the search strategy and perusing the databases, there is possibility that we may have missed including other relevant studies. Lastly, given the heterogeneity of the studies included in this review, we were not able to draw definitive and clear conclusions with respect to the linkage between intervention characteristics (e.g., components, dosage, and type of technology used) and outcomes of patients and their family caregivers. We used a narrative synthesis approach, which only allowed us to report statistically significant findings as indicated by the study investigators. Our review included several exploratory studies and the results should be interpreted with caution due to concerns for inadequate power. Despite these limitations, this systematic review highlights the current state of the science and the gaps in the literature related to the use of technology to deliver health interventions targeting chronically ill adults and their family caregivers. We synthesized the key components and approaches that can be used by researchers in the future to influence behavior change and illness management in a dyadic context and advance the science of technology-based dyadic interventions.
Technology-based interventions targeting chronically ill adults and their caregivers constitute an emerging body of literature. Overall, patient and caregiver participants expressed improvements in self-management outcomes (or support) and QOL. However, there was limited evidence to support improvements in psychological outcomes. Interventions with a dyadic focus reported on interpersonal outcomes, with improvements noted mostly in patients. Most of the included studies had some methodological limitations that might have influenced the findings. Future research should test technology-based interventions in larger samples using robust study designs. Moreover, researchers need to further explore why patients and caregivers may respond differently to dyadic health interventions and how to better engage both members of the dyad to concurrently target both of their needs and improve their well-being.
Funding Sources:
Dr. Irani’s postdoctoral training is supported by the National Institute of Nursing Research of the National Institutes of Health (T32NR015433: Multiple Chronic Conditions, Interdisciplinary Nurse Scientist Training; Principal Investigator, Dr. Shirley M. Moore).
Footnotes
The authors have no conflicts of interest to disclose.
References
- Agren S, Evangelista LS, Hjelm C, & Stromberg A (2012). Dyads affected by chronic heart failure: A randomized study evaluating effects of education and psychosocial support to patients with heart failure and their partners. Journal of Cardiac Failure, 18(5), 359–366. 10.1016/j.cardfail.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikens JE, Trivedi R, Aron DC, & Piette JD (2015). Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. Journal of General Internal Medicine, 30(3), 319–326. 10.1007/s11606-014-3101-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikens JE, Trivedi R, Heapy A, Pfeiffer PN, & Piette JD (2015). Potential impact of incorporating a patient-selected support person into mHealth for depression. Journal of General Internal Medicine, 30(6), 797–803. 10.1007/s11606-015-3208-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GF (2010). Chronic care: Making the case for ongoing care. Robert Wood Johnson Foundation. [Google Scholar]
- Anderson M, & Perrin A (2017). Tech adoption climbs among older adults. Pew research center. https://www.pewresearch.org/internet/2017/05/17/tech-adoption-climbs-among-older-adults/ [Google Scholar]
- Badger T, Segrin C, Pasvogel A, & Lopez AM (2013). The effect of psychosocial interventions delivered by telephone and videophone on quality of life in early-stage breast cancer survivors and their supportive partners. Journal of Telemedicine and Telecare, 19(5), 260–265. 10.1177/1357633X13492289 [DOI] [PubMed] [Google Scholar]
- Baskin TW, Tierney SC, Minami T, & Wampold BE (2003). Establishing specificity in psychotherapy: A meta-analysis of structural equivalence of placebo controls. Journal of Consulting and Clinical Psychology, 71(6), 973–979. 10.1037/0022-006X.71.6.973 [DOI] [PubMed] [Google Scholar]
- Berkowsky RW, Sharit J, & Czaja SJ (2018). Factors predicting decisions about technology adoption among older adults. Innovation in Aging. Advance online publication. 10.1093/geroni/igy002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berli C, Stadler G, Inauen J, & Scholz U (2016). Action control in dyads: A randomized controlled trial to promote physical activity in everyday life. Social Science & Medicine (1982), 163, 89–97. 10.1016/j.socscimed.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Bidwell JT, Lyons KS, & Lee CS (2017). Caregiver well-being and patient outcomes in heart failure: A meta-analysis. The Journal of Cardiovascular Nursing, 32(4), 372–382. 10.1097/JCN.0000000000000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell JT, Vellone E, Lyons KS, D’Agostino F, Riegel B, Juarez-Vela R, … Lee CS (2015). Determinants of heart failure self-care maintenance and management in patients and caregivers: A dyadic analysis. Research in Nursing & Health, 38(5), 392–402. 10.1002/nur.21675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bom JJ, Bakx PP, Schut EF, & van Doorslaer EE (2018). The impact of informal caregiving for older adults on the health of various types of caregivers. The Gerontologist, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck HG, Harkness K, Wion R, Carroll SL, Cosman T, Kaasalainen S, … Arthur HM (2015). Caregivers’ contributions to heart failure self-care: A systematic review. European Journal of Cardiovascular Nursing, 14(1), 79–89. 10.1177/1474515113518434 [DOI] [PubMed] [Google Scholar]
- Buck HG, Hupcey J, Juarez-Vela R, Vellone E, & Riegel B (2019). Heart failure care dyadic typology: Initial conceptualization, advances in thinking, and future directions of a clinically relevant classification system. Journal of Cardiovascular Nursing, 34(2), 159–165. 10.1097/JCN.0000000000000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck HG, Stromberg A, Chung ML, Donovan KA, Harkness K, Howard AM, … Evangelista LS (2018). A systematic review of heart failure dyadic self-care interventions focusing on intervention components, contexts, and outcomes. International Journal of Nursing Studies, 77, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttorff C, Ruder T, & Bauman M (2017). Multiple chronic conditions in the United States. RAND Corporation. 10.7249/TL221 [DOI] [Google Scholar]
- Carr RM, Prestwich A, Kwasnicka D, Thogersen-Ntoumani C, Gucciardi DF, Quested E, … Ntoumanis N (2019). Dyadic interventions to promote physical activity and reduce sedentary behaviour: Systematic review and meta-analysis. Health Psychology Review, 13(1), 91–109. 10.1080/17437199.2018.1532312 [DOI] [PubMed] [Google Scholar]
- Chung ML, Moser DK, Lennie TA, & Rayens MK (2009). The effects of depressive symptoms and anxiety on quality of life in patients with heart failure and their spouses: Testing dyadic dynamics using actor-partner interdependence model. Journal of Psychosomatic Research, 67(1), 29–35. 10.1016/j.jpsychores.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew MA, Goycoolea JM, Harris RC, Lee A, Zomak R, Dunbar-Jacob J, … Kormos RL (2004). An internet-based intervention to improve psychosocial outcomes in heart transplant recipients and family caregivers: Development and evaluation. The Journal of Heart and Lung Transplantation, 23(6), 745–758. [DOI] [PubMed] [Google Scholar]
- Fergus KD, McLeod D, Carter W, Warner E, Gardner SL, Granek L, & Cullen KI (2014). Development and pilot testing of an online intervention to support young couples’ coping and adjustment to breast cancer. European Journal of Cancer Care, 23(4), 481–492. 10.1111/ecc.12162 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, … Cochrane Statistical Methods Group. (2011). The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.), 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker SA, Schmiege SJ, Trivedi RB, Amoyal NR, & Bekelman DB (2018). Mutuality and heart failure self-care in patients and their informal caregivers. European Journal of Cardiovascular Nursing, 17(2), 102–113. 10.1177/1474515117730184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liu T, & Li F (2019). Association between dyadic interventions and outcomes in cancer patients: A meta-analysis. Supportive Care in Cancer, 27(3), 745–761. 10.1007/s00520-018-4556-8 [DOI] [PubMed] [Google Scholar]
- Hwang W, Weller W, Ireys H, & Anderson G (2001). Out-of-pocket medical spending for care of chronic conditions. Health Affairs, 20(6), 267–278. [DOI] [PubMed] [Google Scholar]
- Jindai K, Nielson CM, Vorderstrasse BA, & Quinones AR (2016). Multimorbidity and functional limitations among adults 65 or older, NHANES 2005–2012. Preventing Chronic Disease, 13, E151. 10.5888/pcd13.160174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer N, Kurowski BG, & Bakas T (2018). Systematic review of caregiver and dyad interventions after adult traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 99(11), 2342–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnert T, Heider D, Leicht H, Heinrich S, Corrieri S, Luppa M, … König H (2011). Review: Health care utilization and costs of elderly persons with multiple chronic conditions. Medical Care Research and Review, 68(4), 387–420. [DOI] [PubMed] [Google Scholar]
- Liddy C, Blazkho V, & Mill K (2014). Challenges of self-management when living with multiple chronic conditions: Systematic review of the qualitative literature. Canadian Family Physician, 60(12), 1123–1133. [PMC free article] [PubMed] [Google Scholar]
- Lyons KS, & Lee CS (2018). The theory of dyadic illness management. Journal of Family Nursing, 24(1), 8–28. 10.1177/1074840717745669 [DOI] [PubMed] [Google Scholar]
- Mayberry LS, Berg CA, Harper KJ, & Osborn CY (2016). The design, usability, and feasibility of a family-focused diabetes self-care support mHealth intervention for diverse, low-income adults with type 2 diabetes. Journal of Diabetes Research, 2016, 7586385. 10.1155/2016/7586385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S, & Prestwich A (2010). Are interventions theory-based? development of a theory coding scheme. Health Psychology, 29(1), 1–8. 10.1037/a0016939 [DOI] [PubMed] [Google Scholar]
- Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, … Kaplan R (2009). The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychotherapy and Psychosomatics, 78(5), 275–284. 10.1159/000228248 [DOI] [PubMed] [Google Scholar]
- Murray E (2012). Web-based interventions for behavior change and self-management: Potential, pitfalls, and progress. Medicine 2.0, 1(2), e3. 10.2196/med20.1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northouse L, Schafenacker A, Barr KL, Katapodi M, Yoon H, Brittain K, … An L (2014). A tailored web-based psychoeducational intervention for cancer patients and their family caregivers. Cancer Nursing, 37(5), 321–330. 10.1097/NCC.0000000000000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EO, & Schumacher KL (2014). The state of the science of family caregiver-care receiver mutuality: A systematic review. Nursing Inquiry, 21(2), 140–152. 10.1111/nin.12032 [DOI] [PubMed] [Google Scholar]
- Pfeiffer PN, Valenstein M, Ganoczy D, Henry J, Dobscha SK, & Piette JD (2017). Pilot study of enhanced social support with automated telephone monitoring after psychiatric hospitalization for depression. Social Psychiatry and Psychiatric Epidemiology, 52(2), 183–191. 10.1007/s00127-016-1288-2 [DOI] [PubMed] [Google Scholar]
- Piette JD, Aikens JE, Trivedi R, Parrish D, Standiford C, Marinec NS, … Bernstein SJ (2013). Depression self-management assistance using automated telephonic assessments and social support. The American Journal of Managed Care, 19(11), 892–900. https://doi.org/85248 [PubMed] [Google Scholar]
- Piette JD, Gregor MA, Share D, Heisler M, Bernstein SJ, Koelling T, & Chan P (2008). Improving heart failure self-management support by actively engaging out-of-home caregivers: Results of a feasibility study. Congestive Heart Failure (Greenwich, Conn.), 14(1), 12–18. [DOI] [PubMed] [Google Scholar]
- Piette JD, Marinec N, Janda K, Morgan E, Schantz K, Yujra AC, … Aikens JE (2016). Structured caregiver feedback enhances engagement and impact of mobile health support: A randomized trial in a lower-middle-income country. Telemedicine Journal and e-Health, 22(4), 261–268. 10.1089/tmj.2015.0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette JD, Striplin D, Marinec N, Chen J, & Aikens JE (2015). A randomized trial of mobile health support for heart failure patients and their informal caregivers: Impacts on caregiver-reported outcomes. Medical Care, 53(8), 692–699. 10.1097/MLR.0000000000000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette JD, Striplin D, Marinec N, Chen J, Trivedi RB, Aron DC, … Aikens JE (2015). A mobile health intervention supporting heart failure patients and their informal caregivers: A randomized comparative effectiveness trial. Journal of Medical Internet Research, 17(6), e142. 10.2196/jmir.4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter LS, Gao X, Lyna P, Kraus W, Olsen M, Patterson E, … Pollak KI (2018). Pilot randomized trial of a couple-based physical activity videoconference intervention for sedentary cancer survivors. Health Psychology, 37(9), 861–865. 10.1037/hea0000608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter LS, Keefe FJ, Baucom DH, Olsen M, Zafar SY, & Uronis H (2017). A randomized pilot trial of a videoconference couples communication intervention for advanced GI cancer. Psycho-Oncology, 26(7), 1027–1035. 10.1002/pon.4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, & Sawin KJ (2009). The individual and family self-management theory: Background and perspectives on context, process, and outcomes. Nursing Outlook, 57(4), 217–225.e6. 10.1016/j.outlook.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schover LR, Canada AL, Yuan Y, Sui D, Neese L, Jenkins R, & Rhodes MM (2012). A randomized trial of internet-based versus traditional sexual counseling for couples after localized prostate cancer treatment. Cancer, 118(2), 500–509. 10.1002/cncr.26308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Rini C, Deal AM, Nielsen ME, Chang H, Kinneer P, … Palmer MH (2015). Improving couples’ quality of life through a web-based prostate cancer education intervention. Oncology Nursing Forum, 42(2), 183–192. 10.1188/15.ONF.183-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisuk N, Cameron J, Ski CF, & Thompson DR (2017). Randomized controlled trial of family-based education for patients with heart failure and their carers. Journal of Advanced Nursing, 73(4), 857–870. 10.1111/jan.13192 [DOI] [PubMed] [Google Scholar]
- Wolff JL, Spillman BC, Freedman VA, & Kasper JD (2016). A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Internal Medicine, 176(3), 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]


