Abstract
Antibodies possess properties that make them valuable as therapeutics, diagnostics, and basic research tools. However, antibody chemical reactivity and covalent antigen binding are constrained, or even prevented, by the narrow range of chemistries encoded in canonical amino acids. In this work, we investigate strategies for leveraging an expanded range of chemical functionality using yeast displayed antibodies containing noncanonical amino acids (ncAAs) in or near antibody complementarity determining regions (CDRs). To enable systematic characterization of the effects of ncAA incorporation on antibody function, we first investigated whether diversification of a single antibody loop would support isolation of binding clones against immunoglobulins from three species. We constructed and screened a billion-member library containing canonical amino acid diversity and loop length diversity only within the 3rd complementarity determining region of the heavy chain (CDR-H3). Isolated clones exhibited moderate affinities (double- to triple-digit nanomolar affinities) and in several cases, single-species specificity, confirming that antibody specificity can be mediated by a single CDR. This constrained diversity enabled utilization of additional CDRs for the installation of chemically reactive and photo-crosslinkable ncAAs. Binding studies of ncAA-substituted antibodies revealed that ncAA incorporation is reasonably well tolerated, with observed changes in affinity occurring as a function of ncAA side chain identity, substitution site, and the ncAA incorporation machinery used. Multiple azide-containing ncAAs supported copper-catalyzed azide-alkyne cycloaddition (CuAAC) and strain-promoted azide-alkyne cycloaddition (SPAAC) without abrogation of binding function. Similarly, several alkyne substitutions facilitated CuAAC without apparen disruption of binding. Finally, antibodies substituted with a photo-crosslinkable ncAA were evaluated for ultraviolet-mediated crosslinking on the yeast surface. Competition-based assays revealed position-dependent covalent linkages, strongly suggesting successful crosslinking. Key findings regarding CuAAC reactions and photo-crosslinking on the yeast surface were confirmed using soluble forms of ncAA-substituted clones. The consistency of findings on the yeast surface and in solution suggest that chemical diversification can be incorporated into yeast display screening approaches. Taken together, our results highlight the power of integrating the use of yeast display and ncAAs in search of proteins with “chemically augmented” binding functions. This includes strategies for systematically introducing small molecule functionality within binding protein structures and evaluating protein-based covalent target binding. The efficient preparation and chemical diversification of antibodies on the yeast surface opens up new possibilities for discovering “drug-like” protein leads in high throughput.
Keywords: yeast display, synthetic antibodies, noncanonical amino acid, amber suppression, click chemistry, photo-crosslinking
Graphical Abstract

INTRODUCTION
Antibodies exhibit a versatile range of molecular recognition properties that make them cornerstones of therapeutics, diagnostics, and basic research tools.1–3 The rise of display technologies such as yeast and phage display has greatly expanded the range of methods for antibody discovery, characterization, and engineering.3–5 In addition to facilitating new approaches to mining antibody repertoires from immunologically derived sources, display technologies have resulted in the establishment of powerful, laboratory designed synthetic antibody libraries.6, 7 Specifically, constraining antibody repertoires with synthetic approaches has led to important insights into how antibodies recognize target antigens.3, 6–11 For example, researchers have found that restricting amino acid diversification to a single CDR,8 or limiting the diversification of antibody complementarity determining regions (CDRs) to as few as two amino acids11, 12 results in antibody repertoires capable of yielding antibodies with moderate target affinities (typically double-digit nanomolar to micromolar). Discoveries of functional antibodies from these studies and others indicate that antibody variable domains tolerate amino acid substitution patterns far beyond those employed by mammalian immune systems. These findings raise the following question: how extensively can antibody functions can be altered using chemical groups that are not genetically encoded in natural repertoires?
Several strategies for expanding the chemistries within antibodies are feasible,13, 14 including posttranslational modification of antibody structures containing canonical amino acids15–17 and the introduction of genetically encoded noncanonical amino acids (ncAAs).13, 18 Numerous approaches to antibody chemical diversification have been explored extensively within the context of antibody-drug conjugates (ADCs).19–21 However, most ADC development strategies focus on modification sites located in antibody constant regions that have little or no effect on antigen binding;21–24 the result is modular addition of new chemistries that leave antibody binding function unchanged. In contrast, only sparse studies exist that examine the effects of adding chemical functionality near antibody CDRs. Multiple groups have reported studies in which introducing ncAAs in antibody CDRs leads to altered antibody binding functions. Tirrell and coworkers observed that replacing the methionines of anti-digoxin antibody variants with the azide-containing ncAA azidohomoalanine unexpectedly results in clones exhibiting increased digoxin binding affinity.25 Sakamoto and coworkers recently reported improved antigen affinities in two antibodies upon replacing tyrosines with halogenated analogs.18 Multiple groups have integrated ncAAs containing side chains known to interact with target antigens into antibodies for the purpose of phage display screening (Schultz, Smider, and coworkers)26, 27 and systematic biochemical characterizations (Chang Liu and coworkers)28 to identify antibody constructs with improved binding properties. Several strategies for altering antibody binding functions with an expanded chemical repertoire have used antibodies containing only canonical amino acids as starting points. Barbas, Lerner, and coworkers described the use of catalytic antibodies to present peptide binders within antibody variable domains, effectively replacing antibody CDRs with peptide-mediated antigen recognition.29, 30 Multiple groups have leveraged thiol-mediated chemical conjugation strategies to present small molecules within antibody variable domains. For example, Winter and coworkers reported a phage display antibody library encoding a cysteine in order to introduce fluorescent dyes; library screening against a model antigen led to a clone exhibiting both dye-dependent binding and changes in fluorescence properties upon antigen binding.31 Finally, Wang, Miranda, and coworkers reported the design and construction of bivalent small molecule-antibody “hybrids” based on a crystal structure of a dipeptidyl peptidase IV (DPPIV)-antibody complex.32 Introduction of analogs of a known small molecule inhibitor of DPPIV into the noninhibitory antibody led to hybrid inhibitors with enhanced IC50 values in comparison to controls. Taken together, these studies indicate that there are several promising routes to chemically diversify antibody variable domains. Some of these studies also provide powerful examples of modulating antibody binding function with additional chemical groups. While promising, these studies also highlight opportunities to further generalize and expand approaches to the presentation of chemical groups within antibody structures.
In this work, we sought to establish strategies for generating simple synthetic antibodies and integrating chemical groups into the CDRs of the resulting clones. To facilitate efficient synthetic antibody discovery and derivatization, we utilized the combination of yeast display and noncanonical amino acid (ncAA) incorporation.33–35 We first developed an extremely simple, yeast-displayed synthetic single-chain variable fragment (scFv) library with amino acid and loop length diversity encoded solely in CDR-H3.8, 36 Construction and screening of a billion-member yeast display library enabled the isolation of antibodies that exhibit specificity towards only one of three structurally similar antigens (donkey, bovine, or rabbit immunoglobulins). These findings demonstrate that even conventional amino acid diversity within a single CDR is sufficient to isolate specific antibodies on the yeast surface, in line with synthetic antibody libraries prepared in other display formats.8, 11, 12 Limiting library diversification, and presumably antigen binding in isolated clones, to CDR-H3 enabled us to incorporate ncAAs within additional CDRs of multiple clones and evaluate the effects of these insertions on scFv function. We observed retention of antigen binding after substitutions with multiple ncAAs containing azide or alkyne side chains at three different CDR substitution positions in several clones. However, we also noted changes in apparent binding affinities that depended on the substitution site, specific ncAA substitution utilized, and clone identity. For clones that retained binding function, all of the substitutions that we investigated supported copper-catalyzed azide-alkyne cycloadditions (CuAAC; azides and alkynes) and strain-promoted azide-alkyne cycloadditions (SPAAC; azides) “click” chemistries. Surprisingly, numerous ncAA-substituted clones supported efficient chemical reactions while also retaining binding function following modification, indicating that even reactions resulting in the introduction of large substituents can be accommodated in these clones. Finally, we evaluated whether clones containing the photo-crosslinkable ncAA p-azido-L-phenylalanine (AzF) would support crosslinking on the yeast surface. In two different clones, we observed covalent crosslinking with efficiencies varying as a function of the position of the ncAA substitution site. These key observations were further confirmed by conducting crosslinking experiments with soluble forms of one series of ncAA-substituted scFvs. Overall, our work demonstrates the feasibility of introducing ncAAs within several CDRs of simple synthetic antibodies, frequently without abrogating binding function. In addition, these simple, ncAA-substituted antibodies exhibit chemical reactivity and photoreactivity properties that cannot be accessed with canonical amino acids alone. Our findings expand the range of engineering strategies that can be used to present additional chemistries within antibodies (and other binding proteins), providing opportunities for tailoring and expanding the functions of these proteins for use in basic research and therapeutic discovery.
RESULTS AND DISCUSSION
Library design, construction and characterization.
We first constructed an scFv library in yeast display format in which we employed a single antibody framework and introduced amino acid diversity within a single CDR (Figure 1). Similar to previously described synthetic antibody libraries, we utilized framework regions found in the known therapeutic antibody trastuzumab (Herceptin). These regions are derived from germlines known to tolerate a wide range of CDR diversification schemes.8, 37, 38 The light chain was based on IGKV1–39 V domain and JK4 Kappa joining segment. The heavy chain was based on the IGHV3–23 V domain and JH4 joining segment. Amino acid sequences in the light chain CDRs (L1–L3) and heavy chain CDRs H1 and H2 are identical to those found in the Immunogenetics database entries (http://www.imgt.org/; see also Materials and Methods). To leave the majority of the scFv available for exploring different sites for ncAA incorporation, genetic diversity was limited to the CDR-H3 loop (Figure 1A). Amino acid diversity in CDR-H3 was designed to mimic the amino acid frequencies observed in natural human antibodies using degenerate “XYZ” codons with customized oligonucleotides containing the frequencies of nucleotide bases.39 Clones in the library contain a stretch of 5–13 amino acids encoded by “XYZ” codons, followed by one position encoding either A or G and one position encoding F/I/L/M for total CDR-H3 loop length variations between 9 and 17 codons (Figure 1A; CDR-H3 ranges between positions 98–105 in the Kabat numbering scheme used here40). Including the A/G and F/I/L/M randomizations at the end of CDR-H3 enables CDR-H3 to assume multiple potential conformations as described previously by Sidhu and coworkers.11 In all, this CDR-H3 diversification scheme allows 48 different possible sequences in each “XYZ” codon, two sequences within the A/G codons, and six sequences within the F/I/L/M codons. The combination of the Herceptin-derived antibody scaffold and CDR-H3 randomization results in a library of synthetic antibody clones that possess key therapeutically relevant antibody properties.
Figure 1.

CDR-H3 library design and characterization. A) Antibody framework and CDR-H3 diversification scheme. Antibody structure generated using PyMOL Version 2.3.4 with PDB ID 1FVC.41 B) Predicted and observed frequencies of amino acid distribution in the “XYZ” diversified region of CDR-H3. C) Predicted and observed frequencies of amino acid distribution in the A/G and F/I/L/M positions in CDR-H3.
We prepared the CDR-H3 library as a series of sublibraries, each comprising a separate loop length of CDR-H3 within the range of 9 to 17 amino acids (Figure 1A) using homologous recombination yeast display library preparation.42, 43 The theoretical diversity of the sublibraries range between 3.06 × 109 (loop length 9) and 8.62 × 1022 (loop length 17) for a total possible diversity of 8.80 × 1022 (Table 1). Due to library size restrictions in yeast display systems, we aimed for a total of approximately 1 × 109 transformants, distributed equally into each of the nine sublibraries. The actual number of transformants we obtained, which totaled to 1.36 × 109 for the entire library, was determined by plating samples of transformed cells from each sublibrary (Table 1). The observed percentage of truncated clones was determined experimentally by evaluating the fraction of clones lacking a C-terminal c-Myc tag using flow cytometry (Supplementary Figure S1). As expected, the number of observed truncated clones increased with the number of “XYZ” codons since each additional “XYZ” codon introduces an additional possibility of a stop codon occurring in the diversified CDR-H3 loop that would truncate the scFv. The data also indicates that the observed percentage of truncated clones determined via flow cytometry was higher than the expected percentage of truncated clones in each sublibrary (Supplementary Figure S1B). We attribute this observation to the fact that the expected truncation frequency only takes into account the probability of a stop codon occurring in the “XYZ” codon and does not predict insertions or deletions that could occur throughout the scFv leading to a truncation. In sequencing analysis of 95 clones (see also below), we observed that 4 clones contained a frameshift mutation in the scFv outside CDR-H3 (Supplementary Table S4). Based on our flow cytometry analysis of a 22.4% truncation rate within the full library, we estimate that approximately 1.1 × 109 full-length transformants are present in the full CDR-H3 library (Table 1).
Table 1.
Characterization of CDR-H3 library. Theoretical Diversity and Predicted Truncated Clones were calculated for each sublibrary based on library design. Number of Transformants was determined by colony counting, and Observed Truncated Clones was determined by flow cytometry (see Materials and Methods for details).
| Sublibrary | Theoretical Diversity | Number of Transformants | Predicted Truncated Clones (%) | Observed Truncated Clones (%) |
|---|---|---|---|---|
| CDR9 | 3.06 × 109 | 1.20 × 108 | 9.60 | 18.2 |
| CDR10 | 1.47 × 1011 | 1.37 × 108 | 11.4 | 24.2 |
| CDR11 | 7.04 × 1012 | 1.52 × 108 | 13.2 | 20.4 |
| CDR12 | 3.38 × 1014 | 1.50 × 108 | 14.9 | 25.0 |
| CDR13 | 1.62 × 1016 | 1.80 × 108 | 16.6 | 24.6 |
| CDR14 | 7.79 × 1017 | 1.52 × 108 | 18.3 | 26.8 |
| CDR15 | 3.74 × 1019 | 1.22 × 108 | 19.9 | 30.3 |
| CDR16 | 1.80 × 1021 | 2.30 × 108 | 21.5 | 29.4 |
| CDR17 | 8.62 × 1022 | 1.20 × 108 | 23.1 | 30.8 |
| Full Library | 8.80 × 1022 | 1.36 × 109 | – | 22.4 |
We validated the CDR-H3 library design by sequencing a sample of 8–12 clones from each sublibrary, for a total of 95 clones. Each of these clones had a unique CDR-H3 sequence, indicating that the library contains many unique transformants (Supplementary Table S4). The distribution of amino acids in the designed “XYZ”, A/G, and F/I/L/M codons was analyzed using the sequencing data from clones containing no frameshift mutations. The “XYZ” codon was designed based on Woldring et al39 with the predicted amino acid frequencies shown in Figure 1A and nucleotide frequencies shown in Supplementary Figure S2. Observed frequencies of each amino acid and nucleotide were found to be similar to the predicted frequencies. The most notable differences between designed and observed amino acid frequencies at positions encoded by “XYZ” codons were with respect to the observed frequencies of histidine, asparagine, threonine, and serine (Figure 1B). We also investigated the distribution of amino acids at positions encoded by the A/G and F/I/L/M codons and observed some differences between predicted and observed frequencies (Figure 1C). While amino acids found at A/G and F/I/L/M positions deviated further from their predicted frequencies compared to the amino acid deviations found at “XYZ” codons, this could be due to the smaller number of A/G and F/I/L/M codons included sequenced (one per library clone compared to 5–13 per library clone for “XYZ” codons). Although deep sequencing characterizations could potentially provide a more in-depth analysis of how the constructed library compares to the intended design, the Sanger sequencing approach used here44 was sufficient to verify that key elements of the design were present in the assembled library. Overall, our sequencing and flow cytometry data confirmed that the library contained the diversity that was expected based on design.
Library screening and isolation of binders.
Once we confirmed that the constructed library consisted of clones consistent with the design, we performed a series of proof-of-principle enrichments45–47 to evaluate the performance of the library. Specifically, we enriched against immunoglobulins (IgGs) from donkey, bovine, and rabbit in order to determine whether this library contained binding clones capable of binding to and discriminating between these three antigens (Figure 2 and Supplementary Figure S3). IgGs are well-behaved, commercially available model targets commonly used to validate antibody libraries. In addition, the structural similarity of these proteins allows for evaluation of clone specificity. The depicted results come from enrichments consisting of magnetic bead sorting for four rounds and then a single round of fluorescence-activated cell sorting (FACS). Each round of magnetic bead sorting started with depletions against streptavidin-coated Dynabeads, beads precomplexed with biotin, and beads precomplexed with the murine IgG TA99 (see Materials and Methods for details). Then, the remaining cells were subjected to enrichments with target antigen-coated beads, grown to saturation, and analyzed for binding to the IgG by flow cytometry; progress in the enrichments was confirmed by this analysis after each round (Figure 2 and Supplementary Figure S3). After four rounds of bead sorting and one round of FACS, the majority of cells in the enriched populations exhibited binding to the target IgG in flow cytometry experiments (Figure 2 and Supplementary Figure S3). We then isolated and sequenced individual clones from the enriched populations targeting donkey IgG, bovine IgG, and rabbit IgG. From this sequencing, we identified several unique clones that appeared to be able to bind to their respective target antigens as evaluated by flow cytometry. Since antigen sorts were performed with biotinylated IgGs, competition studies were performed to identify clones binding to epitopes present on the native antigen while eliminating clones that bind preferentially to the biotinylated form of the antigen (Supplementary Figures S4 and S5). Our competition data indicate that we identified both clones that bind to the authentic antigens and clones only capable of binding to biotinylated forms of the antigens. While the identification of clones that bind to biotinylated forms of antigens indicates that our library contains clones binding to multiple epitopes, the majority of these clones were not considered further after the competition experiments. The CDR-H3 sequences of clones capable of binding to the unmodified forms of the IgGs are listed in Table 2. Together, the screening results demonstrate the isolation of unique protein binders to donkey, bovine and rabbit IgG using the CDR-H3 library, despite limiting amino acid diversity to just the CDR-H3 region.
Figure 2.
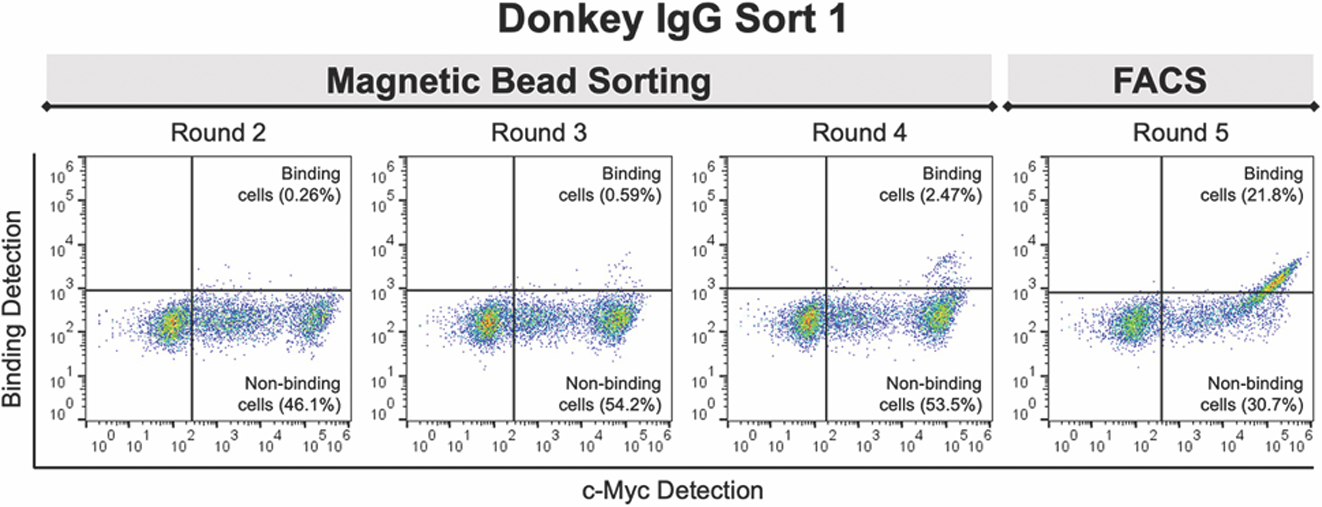
CDR-H3 library sorting against biotinylated donkey IgG. Binders were isolated using four rounds of magnetic bead sorting followed by one round of fluorescence-activated cell sorting (FACS). During FACS, cells were treated with 50 nM biotinylated IgG and the population sorted for cells exhibit both full-length display (c-Myc positive) and donkey IgG binding.
Table 2.
Properties of clones isolated from CDR-H3 library. Each scFv was isolated by sorting against the target antigen, and binding affinity against biotinylated forms of these IgGs was determined from binding titrations using a saturation binding model on GraphPad Prism Version 8.3.0 (see Materials and Methods for details).
| Clone | CDR-H3 Sequence | CDR-H3 Loop Length | Target Antigen | KD (95% CI), nM |
|---|---|---|---|---|
| Donkey1.1 | KYDKTHHNPDYALD | 14 | Donkey IgG | 8.75 (6.65–11.50) |
| Bovine2.1 | KHYDPYDDYNFYFGFD | 16 | Bovine IgG | 746 (620–915) |
| Bovine2.2 | KTYYDNSYAYTALD | 14 | 46.1 (43.1–49.3) | |
| Bovine2.3 | KSSIYDGYMYGLD | 13 | 38.4 (33.4–44.2) | |
| Rabbit1.1 | KYNYHHPFYSYDAFD | 15 | Rabbit IgG | 131 (121–142) |
Characterization of isolated binders.
In order to fully understand the properties of the scFvs isolated from this minimalist library, clones capable of binding to their target animal IgGs (donkey, bovine or rabbit) were further investigated for binding specificity as well as binding affinity (Figure 3 and Table 2). To determine the specificity of the isolated antibody fragments, we used a series of clones capable of binding to nonbiotinylated antigens as described above (Supplementary Figures S4 and S5): two donkey IgG-binding scFvs (Donkey1.1 and 1.2), three bovine IgG-binding scFvs (Bovine2.1, 2.2, and 2.3) and one rabbit IgG-binding scFv (Rabbit1.1). Since biotinylated forms of the IgGs were used for this assay, we also included one scFv that exhibited some amount of binding against only biotinylated form of donkey IgG (Donkey1.3) as a control for crossreactivity. ScFvs were displayed on the yeast surface and treated with IgGs to evaluate binding specificity. Figure 3A and Supplementary Figure S6 depict the results of flow cytometry experiments in which the set of scFvs was tested for binding against each of the three animal IgGs. At a fixed concentration of 200 nM IgG, assays with both Donkey1.2 and Donkey1.3 result in binding signal when any of the three IgGs are used as the antigen. This is as expected for Donkey1.3 based on its preference for biotinylated antigen (Supplementary Figures S4 and S5). Donkey1.2 appears to exhibit crossreactivity against multiple IgGs. While the highest levels of IgG detection occur against donkey IgG, there is also measurable detection of bovine and rabbit IgG binding. In contrast, Donkey1.1, all three bovine IgG binders, and Rabbit1.1, appear to exhibit specificity to their target antigens based on the flow cytometry evaluations (Figure 3A and Supplementary Figure S6); for all of these clones, binding signal is detected only against the cognate antigen. These experiments demonstrate that a single CDR loop is sufficient to yield binding proteins that discriminate between closely related antigens.
Figure 3.

Binding characterizations of wild-type scFvs isolated from the library. A) Analysis of antigen specificity via crossreactivity assays. B) Binding titrations for the best performing wild-type clones. ScFvs were treated with different concentrations of biotinylated IgG (starting from 1 μM with subsequent 4-fold dilutions) and labeled to detect biotinylated antigens. All characterizations were performed in technical triplicate, with error bars representing standard error (see Materials and Methods for details regarding normalization and analysis).
We also estimated the apparent affinities of the five antibody fragments exhibiting antigen specificity on the yeast surface (Table 2 and Supplementary Figure S7; due to the off-target binding observed for Donkey1.2 and Donkey1.3, these scFvs were not considered further). Three of these scFvs (Donkey1.1, Bovine2.2, and Bovine2.3) exhibit relatively strong affinities, with Donkey1.1 possessing an apparent KD of 8.75 nM (95% confidence interval: 6.65–11.50 nM) and two of the bovine IgG clones possessing KDs in the doubt digit nanomolar range (Table 2). The other clones characterized here exhibited more modest affinities, although even for a comparatively weak binder such as Bovine2.1 (KD = 746 nM, 95% confidence interval 620–915 nM), binding is readily detectable in flow cytometry-based assays. The affinities of clones isolated from this single-CDR antibody library are consistent with or stronger than the affinities of clones isolated from other libraries in which amino acid diversity is restricted to a single loop,8 confirming that a single loop is sufficient to support nanomolar binding affinities. With these simple, but specific, binding clones in hand, we investigated strategies for adding functionality to these antibodies via ncAA substitution and subsequent modifications based on the distinct side chain chemistries.
Effects of noncanonical amino acid incorporation on binding affinity and protein display.
In order to utilize the enhanced chemical diversity accessible through ncAAs, we sought to incorporate ncAAs without disrupting antibody function in the process. As such, we first investigated the effects of ncAA incorporation on antigen binding and full-length scFv display to determine the feasibility of this strategy. We made substitutions at multiple positions within scFv variable domains using stop codon suppression to genetically encode ncAAs in response to the amber (TAG) stop codon.34, 48, 49 Plasmids encoding the donkey IgG binder Donkey1.1, bovine IgG binders Bovine2.1 and Bovine2.2, and the rabbit IgG binder Rabbit1.1 were mutated to contain a TAG codon at one of three scFv sites: position 93 in the light chain (located in CDR-L1; originally serine (TCT)), position 31 in the heavy chain (located within CDR-H1; originally serine (AGC)) or position 54 in the heavy chain (located within CDR-H2; originally serine (TCT)). These positions will be referred to as L93, H31 and H54, respectively, and resulting mutants will be referred to as the L93TAG, H31TAG and H54TAG variants of the parent scFvs. These positions were chosen based on their locations outside of CDR-H3 but within other CDRs that can be involved in antigen binding,50 and based on the predicted surface accessibility of native residues using GetArea calculations in several antibody fragment structures (PDB 3AUV,51 2KH2,52 3K2U,53 and 5JYL54). NcAAs were incorporated using constitutively expressed orthogonal translation systems (OTSs; Figure 4A), which in this work are previously described aminoacyl-tRNA synthetase/tRNA (aaRS/tRNA) pairs.33–35 The ncAAs used in this work are p-azido-L-phenylalanine (AzF), p-azidomethyl-L-phenylalanine (AzMF), p-propargyloxyphenylalanine (OPG) and H-L-Lys(EO-N3)-OH (LysN3) (Figure 4B). These ncAAs were incorporated using aaRSs derived from either the E. coli tyrosyl-tRNA synthetase or the E. coli leucyl-tRNA synthetase known as AcFRS (AzF and OPG), LeuOmeRS (OPG and AzMF), or LeuRS BH5 T252A (LysN3). These aaRSs were paired with corresponding suppressor tRNACUATyr or tRNACUALeu 33, 34, 55, 56 (note: aaRS/tRNA pairs will be referred to by specifying only the aaRS for the remainder of this manuscript). Yeast cotransformed with TAG-containing scFv constructs and stop codon suppression machinery were induced in the presence of ncAAs to display substituted clones on the yeast surface, treated with a 200 nM concentration of the respective target IgG, and analyzed for display levels and antigen binding detection via flow cytometry.
Figure 4.

Evaluation of noncanonical amino acid (ncAA) substitution effects on antigen binding and full-length display. A) NcAA incorporation strategy using amber suppression machinery. B) NcAAs used in this study: p-azido-L-phenylalanine (AzF), p-azidomethyl-L-phenylalanine (AzMF), p-propargyloxyphenylalanine (OPG) and H-L-Lys(EO-N3)-OH (LysN3). C) Binding analysis of Donkey1.1 (left) and Bovine2.2 (right) clones substituted with ncAAs using 200 nM biotinylated donkey or bovine IgG, respectively. D) Full-length display detection of Donkey1.1 (left) and Bovine2.2 (right) clones via c-Myc epitope tag labeling. For data shown in both panels C and D, values here are background-subtracted median fluorescence intensity (MFI) values from experiments performed in technical triplicate (see Materials and Methods for details). E) Relative readthrough efficiency (RRE) and maximum misincorporation frequencies (MMF) of ncAA substitutions in Donkey1.1 L93TAG and Bovine2.2 L93TAG. AzF and OPG were inserted using the AcFRS and LeuOmeRS aaRSs, respectively. See Materials and Methods for calculation details. ND, not determined.
NcAA substitutions resulted in varying effects on antigen binding for the four clones analyzed here (Figure 4C and Supplementary Figures S8–S10). Three out of the four clones (Donkey1.1, Bovine2.1, and Bovine2.2) retained binding function after almost every ncAA substitution at position L93. This tolerance for substitutions at L93 is in line with the work of Jespers et al in which cysteine introduced at L93 of a phage displayed antibody library was found to be well-tolerated.31 Three out of four clones (Donkey1.1, Bovine2.2, and Rabbit1.1) retained binding function after ncAA substitution at H54, while substitutions at position H31 tended to result in lower antigen binding levels. Two clones exhibited broad tolerance of ncAA substitutions: in Donkey1.1 and Bovine2.2, almost every combination of aaRS/tRNA, ncAA, and substitution site tested resulted in detection of donkey IgG and bovine IgG binding, respectively (Figure 4C and Supplementary Figure S8). With the exception of LysN3 substitution at position H31 of Donkey1.1, all flow cytometry dot plots clearly indicate a population of cells binding the target antigen (Supplementary Figure S8). Several substitutions in Donkey1.1 and Bovine2.2 resulted in moderate to high levels of antigen detection at 200 nM concentration, with the highest antigen detection being OPG incorporation with LeuOmeRS in Donkey1.1 (antigen detection levels of approximately 50% of wild-type). On the other hand, ncAA substitutions at position H31 led to antigen binding levels of less than 10% of wild-type levels for all ncAAs tested in Donkey1.1 and Bovine2.2. Two clones exhibited poor tolerance for ncAA substitutions: ncAA substitutions in clones Bovine2.1 and Rabbit1.1 resulted in greatly diminished or undetectable antigen binding for all substitutions investigated (Supplementary Figures S8–S10). These results are particularly surprising for Rabbit1.1, as the parent clone exhibits moderate affinity towards rabbit IgG (KD = 131 nM, 95% confidence interval 121–142 nM). On the other hand, the loss of antigen detection in the case of ncAA-substituted Bovine2.1 clones is consistent with the weak affinity of the parent clone (KD = 746 nM, 95% confidence interval 620–915 nM).
Differences in antigen binding following ncAA substitution indicate that these four clones exhibit a range of tolerances for ncAA substitutions in positions located outside of CDR-H3. To address one potential reason for these variable tolerances, we examined how ncAA incorporation efficiency affects antigen binding. All ncAA substitutions investigated decreased c-Myc detection levels; detected display levels range from approximately 10% to 90% of parent clone display levels (Figure 4D and Supplementary Figure S9). Decreases in display are expected based on known ncAA incorporation efficiencies of the orthogonal translation systems (OTSs) used here.34 Since stop codon context can have large effects on ncAA incorporation efficiency and fidelity,33–35, 57 we determined relative readthrough efficiency (RRE) and maximum misincorporation frequency (MMF) for selected clones at the L93 site (Figure 4E; RRE and MMF are measures of ncAA incorporation efficiency and fidelity, respectively). For the variants Donkey1.1 L93TAG and Bovine2.2 L93TAG prepared with the aaRS/tRNA + ncAA combinations of AcFRS + AzF and LeuOmeRS + OPG, we determined RRE to be in the range of 0.47–0.77 (Figure 4E and Supplementary Table S10). These values indicate that ncAA insertions in these clones at position L93 are moderately efficient, possessing increased RRE values compared to the values obtained with the same OTS + ncAA combinations with our previously described yeast display reporter system.34 Measurements of MMF resulted in almost undetectable levels of misincorporation of canonical amino acids, confirming the apparent fidelity of these incorporation events. Thus, while inserting ncAAs in these displayed clones is a relatively efficient process, some loss of display levels is expected based on diminished ncAA incorporation efficiency alone.
We surveyed changes in display levels in Donkey1.1 and Bovine2.2 and compared these changes to changes in antigen binding levels. We focused on two key sets of comparisons for a number of aaRS/tRNA + ncAA + insertion site combinations. The data shown in Supplementary Figure S10A and S10B directly compares antigen binding and display levels observed for OPG-containing clones prepared with each of two aaRS/tRNA pairs (AcFRS and LeuOmeRS). As expected, clones prepared with LeuOmeRS exhibit higher antigen binding and display levels than clones prepared with AcFRS, confirming the role of display level in dictating antigen binding. However, we note that the decreases in binding levels observed are greater than corresponding decreases in display levels upon ncAA substitution. This trend also generally holds when comparing display levels and antigen binding levels in AzMF- and OPG-substituted clones prepared using the same LeuOmeRS (Supplementary Figure S10C and S10D). These variants exhibit display levels within 10–20 percent of one another, but differences in antigen binding between the two variants approach as high as 50% in some cases. A possible explanation for differences in display levels versus antigen binding levels is that the bulky substitutions made in this work (all replacing serines with ncAAs) interfere with proper antibody folding, reducing antigen affinity beyond what can be explained by changes in display levels. While the exact mechanisms mediating these effects will require further study, findings presented here clearly show that changes in display levels upon ncAA substitution directly account for only a portion of changes in antigen binding levels.
Based on the observation that antigen binding levels in ncAA-substituted clones tend to decrease more substantially than corresponding display levels, we investigated the possibility that ncAA substitutions decrease apparent binding affinities. To address this question, we used yeast-based titrations to evaluate potential changes in antigen binding affinity following ncAA substitutions in Donkey1.1 and Bovine2.2 (Supplementary Figure S12). Table 3 reports the KD values of ncAA-substituted scFvs prepared with two aaRS/tRNA + ncAA combinations (AcFRS + AzF and LeuOmeRS + OPG) on the yeast surface and the fold change of the KDs from the corresponding observed wild-type KDs. In general, OPG-containing clones exhibited relatively strong apparent affinities, with substitutions at L93 and H54 increasing apparent KDs by 3.9- to 6.4-fold compared to WT. In contrast, AzF substitutions at these positions were more poorly tolerated, resulting in increases in apparent KDs ranging from 7.2- to 24.4-fold. NcAA substitutions at position H31 of both Donkey1.1 and Bovine2.2 were not well-tolerated, resulting in increases in apparent KDs ranging from 11.9-fold to 64.1-fold. Given that some of the resulting KDs for these clones are well above 200 nM, this helps to explain the low levels of antigen detection observed in comparison to changes in clone display levels (Figure 4C and 4D). We further confirmed this observation by noting that antigen binding levels of substituted clones increase when treated with 1 μM antigen during titration experiments (Supplementary Figure S13). These data suggest that ncAA substitutions, although located outside of the primary specificity-determining CDR-H3 loop, can weaken apparent clone affinities.
Table 3.
Binding affinities of AzF- and OPG-containing variants of Donkey1.1 and Bovine2.2 clones. Affinities were determined from binding titrations with biotinylated IgGs using a saturation binding model on GraphPad Prism Version 8.3.0. Fold change from WT indicates the effect of ncAA incorporation on binding interactions between the scFv and IgGs (see Materials and Methods for details).
| Clone | aaRS/ncAA | Target Antigen | KD (95% CI), nM | Fold Change from WT |
|---|---|---|---|---|
| Donkey1.1 L93TAG | AcFRS/AzF | Donkey IgG | 125 (99–158) | 14.3 |
| LeuOmeRS/OPG | 45.0 (29.2–68.5) | 5.10 | ||
| Donkey1.1 H31TAG | AcFRS/AzF | 561 (517–610) | 64.1 | |
| LeuOmeRS/OPG | 365 (335–399) | 41.7 | ||
| Donkey1.1 H54TAG | AcFRS/AzF | 213 (173–263) | 24.4 | |
| LeuOmeRS/OPG | 55.8 (38.8–79.8) | 6.40 | ||
| Bovine2.2 L93TAG | AcFRS/AzF | Bovine IgG | 332 (273–406) | 7.20 |
| LeuOmeRS/OPG | 179 (155–208) | 3.90 | ||
| Bovine2.2 H31TAG | AcFRS/AzF | 546 (474–633) | 11.9 | |
| LeuOmeRS/OPG | 601 (515–708) | 13.0 | ||
| Bovine2.2 H54TAG | AcFRS/AzF | 380 (310–471) | 8.30 | |
| LeuOmeRS/OPG | 183 (132–254) | 4.00 |
Overall, these substitution studies demonstrate that some synthetic antibodies tolerate ncAA substitution at multiple sites. Our data also indicate that the degree to which clones tolerate ncAA incorporation depends on the specific clone, substitution site, ncAA, and even the OTS-dependent display level of ncAA-containing clones. While further studies will be required to fully understand the molecular underpinnings of some of our observations, the ncAA substitution tolerances of Donkey1.1 and Bovine2.2 enabled us to examine how to exploit the unique chemical properties of the resulting clones in the following sections.
Click chemistry and its effects on binding.
Genetically encoding azides or alkynes within antibodies enables the use of bioorthogonal chemistries to create well-defined conjugates,58, 59 including conjugates on the yeast surface.25, 33, 34 Here, we investigated modifications to Donkey1.1 and Bovine2.2 clones containing ncAAs at positions L93 and H54 to determine clone reactivity and investigate whether modifications disrupt antigen binding (Figure 5). While most investigations of antibody conjugation strategies focus on sites located distal to antibody CDRs, the L93 and H54 sites used in this work are located directly within CDR-L3 and CDR-H2, respectively; little is known about the effects of conjugations at these sites on antibody function. To evaluate reactivity and binding, we used copper-catalyzed azide-alkyne cycloaddition (CuAAC) and strain-promoted azide-alkyne cycloaddition (SPAAC) reactions (Figure 5A).60, 61 Biotin-alkyne and biotin-azide probes were used to evaluate CuAAC reactions with clones containing azide- and alkyne-containing ncAAs, respectively; biotin-dibenzocyclooctyne (DBCO) was used to evaluate SPAAC reactions. For all reactions reported here, we used the same set of empirically determined conditions for CuAAC and SPAAC reactions (Supplementary Figures S15 and S16). For CuAAC, 100 μM probe concentration led to maximum biotinylation within 15 minutes without detectable nonspecificity for a previously described model reporter protein containing AzF;34 we used these conditions for all CuAAC reactions (Supplementary Figure S15). For SPAAC, reactions with 10 μM biotin-DBCO at 4 °C suppressed nonspecific reactions, but we limited reaction times with biotin-DBCO to 2 hours based on the observation of biotinylation of WT Bovine2.2 beginning at 2 hours (Supplementary Figure S16).
Figure 5.

Click chemistry reactions and binding analysis of clones following reactions. A) Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and strain-promoted azide-alkyne cycloaddition (SPAAC) reaction schemes to modify yeast-displayed scFvs. B) Evaluation of CuAAC and SPAAC reactions using reactive biotin probes. All samples were subjected to CuAAC or SPAAC conditions, with DMSO controls omitting reactive alkyne probes. C) Binding with 200 nM donkey IgG after subjecting samples to CuAAC and SPAAC reactions. For data shown in both panels B and C, values here are background-subtracted median fluorescence intensity (MFI) values from experiments performed in technical triplicate (see Materials and Methods for details).
Every combination of aaRS, ncAA, substitution site, and clone resulted in substantial biotin detection for CuAAC and SPAAC, while all control reactions led to minimal or undetectable labeling (Figure 5 and Supplementary Figures S17–S21). For the combinations tested here, CuAAC reactions resulted in higher relative biotin detection levels than the corresponding SPAAC reactions (Figure 5B), which we attribute primarily to using SPAAC conditions that limit off-target reactions rather than maximizing yield. For Donkey1.1, scFvs containing azides (AzF, AzMF and LysN3) subjected to CuAAC all resulted in similar levels of biotin detection, whereas for Bovine2.2 clones substituted with AzMF exhibit higher levels of biotin detection than clones containing AzF or LysN3 (Supplementary Figure S21). Some of these differences are likely the result of changes in the levels of full-length constructs that depend on the specific suppression machinery used.34 As observed in Figure 4, LeuOmeRS (incorporating AzMF and OPG) appears to support high levels of full-length display, followed by moderate levels of display for AcFRS (incorporating AzF), and low levels of display with LeuRS BH5 T252A (incorporating LysN3). CuAAC reactions with OPG-substituted clones resulted in higher biotin detection levels than those observed with antibodies containing any of the azide groups considered here. Previous work has indicated that even subtle changes to reactants used in CuAAC can strongly influence reaction kinetics and product yield,62, 63 consistent with our observations that changes in ncAA side chain affect relative reaction levels under a fixed set of reaction conditions.
Following the click chemistry reactions, we evaluated whether the reactions affected the binding levels of displayed clones. We hypothesized that since our clones were engineered to contain only variable CDR-H3 loops, installation of chemical groups in other CDRs should be well-tolerated. Following CuAAC or SPAAC reactions, yeast displaying scFvs were treated with 200 nM target IgG (donkey IgG for Donkey1.1 and bovine IgG for Bovine2.2, respectively) and analyzed for antigen binding. For these experiments, antigen binding was detected by using anti-donkey or anti-bovine secondary antibodies to detect unmodified donkey or bovine IgG, respectively (since the click chemistry reactions result in the installation of a biotin moiety). Figure 5C and Supplementary Figure S21 compare the binding levels between biotin-clicked, DMSO-clicked and non-clicked (control) samples. Based on the amount of IgG detected, neither installation of the biotin probes nor the CuAAC and SPAAC reaction conditions appear to affect binding levels for most of the clones analyzed (for the OPG-containing Bovine2.2 H54TAG clone we did observe decreased binding after CuAAC and SPAAC reactions (Supplementary Figure S21), indicating that the addition of external chemical groups does affect binding levels in this case). It is especially noteworthy that even samples treated with the bulky DBCO reagent retain similar levels of antigen binding to untreated samples and to samples subjected to CuAAC. Although titration experiments would be needed to reveal whether or not these modifications have a measurable impact on binding affinities, the findings presented here demonstrate that conjugations at the L93 and H54 positions are well-tolerated. This is consistent with the hypothesis that interactions with CDR-H3 are a key element driving antigen binding. Overall, these studies demonstrate that scFvs containing ncAA substitutions positioned within CDRs can be chemically addressed using CuAAC and SPAAC on the yeast surface, and that the resulting conjugates retain binding function even upon installation of large moieties.
Covalent target engagement using UV-mediated photo-crosslinking.
Antibodies containing only canonical amino acids rarely engage in covalent interactions with target antigens. Given the well-known photo-crosslinking capabilities of p-azido-L-phenylalanine (AzF),64–66 we investigated whether presentation of AzF within synthetic antibodies could facilitate covalent crosslinking on the yeast surface and in solution (Figure 6). Figure 6A depicts our general strategy for evaluating covalent crosslinking on the yeast surface using a competition assay. In this three-step approach, scFvs are treated with unmodified IgGs to allow binding between the antigen and antibodies. Next, the samples are subjected to UV irradiation to initiate crosslinking. Lastly, the samples are incubated with a higher concentration of the IgG, but this time using the biotinylated form. Flow cytometry-based biotin detection is then used to evaluate the extent to which the biotinylated form of the antigen has competed off the unmodified form (Figure 6A). Accompanying each of these steps are controls to elucidate wild-type and non-crosslinking behavior. These experiments provide a means of evaluating the extent to which AzF-substituted clones facilitate crosslinking on the yeast surface.
Figure 6.
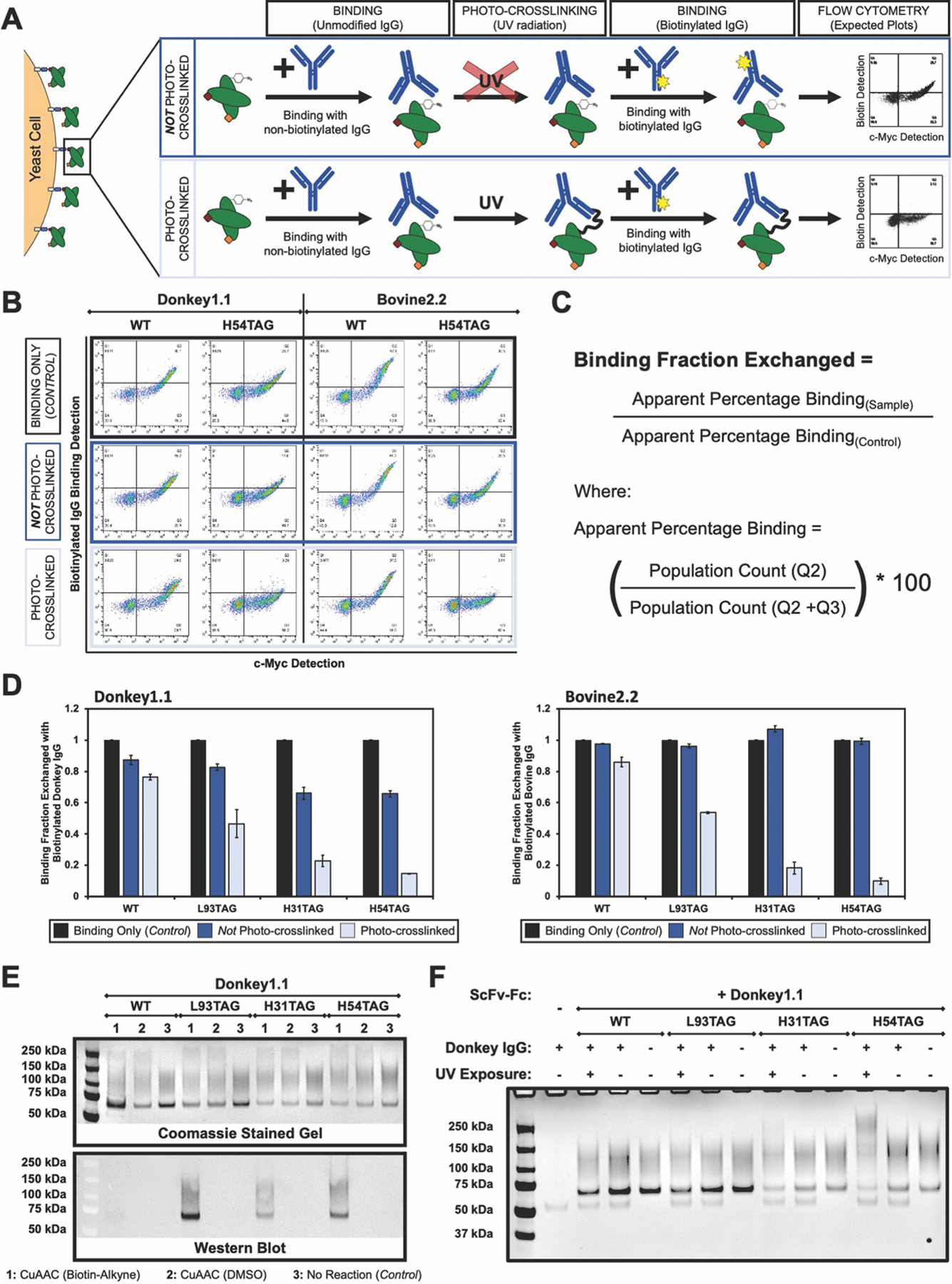
Evaluation of photo-crosslinking on the yeast surface and in solution. A) Strategy for binding competition assay to evaluate photo-crosslinking on the yeast surface. B) Flow cytometry dot plots of competition assay: a decrease in biotinylated IgG detection indicates covalent crosslinking in the presence of UV radiation. “Binding Only (Control)” samples represent binding detection with 500 nM biotinylated IgG in the absence of any competition binding or UV radiation. C) Equations to calculate “Binding Fraction Exchanged,” a determination of the fraction of IgG bound noncovalently to scFvs on the yeast surface following the competition assay described in A. D) Analysis of “Binding Fraction Exchanged” for Donkey1.1 (left) and Bovine2.2 (right) clones during the binding competition assay in the absence and presence of UV radiation (“Not Photo-crosslinked” and “Photo-crosslinked” conditions, respectively). All data reported here are the result of crosslinking assays performed in technical triplicates. E) SDS-PAGE and Western Blot analysis to evaluate scFv-Fc purity and confirm AzF substitutions. Top: gel stained with coomassie SimplyBlue SafeStain for samples subjected to CuAAC conditions or left untreated. Bottom: Western blot probed for the presence of biotinyted scFv-Fcs following CuAAC with biotin-alkyne (refer to Supplementary Figure S24 for full gel images). F) SDS-PAGE analysis of photo-crosslinking in solution using 100 nM donkey IgG and 500 nM scFv-Fc (refer to Supplementary Figure S25 for full gel images).
Figure 6B–6D depict the results of a series of crosslinking experiments on the yeast surface using AzF substitutions in Donkey1.1 and Bovine2.2 clones. Inspection of two-dimensional dot plots of biotinylated IgG binding versus full-length display detection (Figure 6B and Supplementary Figure S22) indicate clear, UV-dependent decreases in antigen binding levels for AzF-substituted Donkey1.1 H54TAG and Bovine2.2 H54TAG. The UV-irradiated samples exhibit very low levels of biotinylated antigen compared to the controls, suggesting irreversible binding between the AzF-substituted clones and the target IgGs. In contrast, the high biotin detection for control samples not subjected to UV irradiation indicates the exchange of unmodified IgGs with biotinylated IgGs during the secondary incubation.
To further investigate the effects of different ncAA incorporation sites on crosslinking, we calculated the fraction of biotinylated IgG exchanged as a measure of photo-crosslinking efficiency (Figure 6C and 6D). Here, the single-step biotinylated IgG binding (control) samples were assumed to exhibit maximum levels of antigen binding, with crosslinked and non-crosslinked sample binding calculated as a fraction of the maximum binding. For the WT samples, we generally observed some loss of biotin detection for both photo-crosslinked and non-photo-crosslinked conditions after the competition assay. This loss in signal may be attributable to some degree of nonspecific crosslinking even in the absence of AzF or to incomplete exchange of unmodified IgG for biotinylated IgG during the competition step of the assay. A comparison of Donkey1.1 and Bovine2.2 non-photo-crosslinked controls also reveals a noticeable difference in the levels of biotinylated IgG detected following the competition step (Figure 6D). Given that Donkey1.1 clones tend to possess stronger target antigen binding affinities than Bovine2.2 clones (Table 3), it is plausible that IgG exchange, although not complete for either set of clones, is more complete for Bovine2.2 clones than for Donkey1.1 clones. For the AzF-containing, photo-crosslinked samples (L93TAG, H31TAG and H54TAG), the biotin detection is generally reduced compared to non-photo-crosslinked samples; the detection of some biotinylated antigen even in UV-irradiated samples suggests that the crosslinking may not be complete. The crosslinking data suggest a clear dependency on the ncAA incorporation site (Figure 6D and Supplementary Figure S22) that is distinct from site-dependent changes in binding affinity. Most prominently, although clones substituted at L93 retain higher affinities (Figure 4C), photo-crosslinking with cells displaying these clones appears to be inefficient. Low efficiency crosslinking may be the result of L93 residing too far from the antigen bound to the CDR-H3-driven antibody paratope to promote efficient crosslinking. This is in contrast to the substantial levels of crosslinking observed on cells displaying clones with AzF substitutions at positions H31 or H54 despite greater reductions in antigen binding affinities.
We next investigated whether the trends in photo-crosslinking efficiency observed on the yeast surface would hold when we evaluated crosslinking in solution. To examine this, we prepared scFv-Fc forms of WT and AzF-substituted L93TAG, H31TAG, and H54TAG Donkey1.1 using secretion constructs similar to those described in Van Deventer et al37 (with modifications as detailed in Materials and Methods). We confirmed the proper expression of the scFv-Fcs using SDS-PAGE analysis and the presence (or absence) of AzF residues in the purified clones using CuAAC with a biotin-alkyne probe and western blotting (Figure 6E, Supplementary Figures S23 and S24). The protein gel staining patterns of purified samples observed here are extremely similar to those observed previously, with the multiple bands being attributable to glycosylated and aglycosylated forms of the scFv-Fcs.37 As shown in Figure 6E, biotin detection on the western blot is only observed for AzF-containing samples that have been subjected to CuAAC, while samples not subjected to CuAAC conditions and all WT samples (irrespective of CuAAC treatments) result in no biotin detection (full gel images reported in Supplementary Figure S24). Thus, CuAAC reactivity of scFv-Fcs expressed in solution is consistent with the results obtained using yeast display: scFv-Fcs retain their ability to selectively undergo CuAAC reactions only when prepared from yeast containing a TAG-substituted scFv-Fc and aaRS/tRNA pair and induction with AzF in the media, thus providing strong evidence for the presence of AzF in these secreted constructs.
With these purified constructs in hand, we performed photo-crosslinking by coincubating the scFv-Fcs with donkey IgG and then exposing them to UV radiation at 365 nm. The resulting samples were then analyzed using SDS-PAGE under reducing conditions to identify potential crosslinking events. Figure 6F depicts the analysis of a series of samples subjected to UV irradiation and corresponding controls (full gel images reported in Supplementary Figure S25). Bands larger than both the individual scFv-Fc and the IgG heavy chain band were detected only for the H31TAG and H54TAG photo-crosslinked samples. These observations and the absence of corresponding bands in control samples strongly suggest that the scFv-Fc and IgG have been covalently crosslinked under the conditions used in these experiments. In contrast, there is no detectable band present in samples containing AzF-substituted Donkey1.1 L93TAG. The trends observed in solution therefore appear to be consistent with the trends observed on the yeast surface: crosslinking reactions are readily detectable for clones substituted with AzF at H31 and H54, while AzF substitution at L93 exhibits the lowest apparent amount of crosslinking on the yeast surface and undetectable levels of crosslinking in solution.
These crosslinking experiments demonstrate the preparation and evaluation of covalently crosslinkable antibodies on the yeast surface and in solution. The consistent trends observed between yeast-based and solution-based experiments confirm the utility of conducting initial experiments on the yeast surface prior to initiating more technically demanding characterizations in solution. Moreover, the covalent scFv-antigen linkages formed here are a clear example of the presentation of unique functionality within synthetic antibodies that is not accessible through the side chains of the canonical amino acids.
CONCLUSIONS
In this work, we investigated the functionality of a minimalist synthetic antibody library and identified strategies for chemically diversifying clones emerging from the library. The single-framework, single-CDR design used in this work is one of the simplest possible diversification schemes, and may be the simplest antibody library reported in yeast display format (at least to our knowledge). Despite such constrained diversity, we isolated moderate to high affinity antibody binders against several animal IgGs using high throughput screening. The affinities of these clones are comparable to the affinities of clones isolated from minimalist phage-based synthetic antibody libraries.8, 11 Several clones we isolated readily discriminate between donkey, bovine, and rabbit IgGs, indicating that the sequence of a single CDR loop is sufficient to enable identification of specific clones.
Limiting the location of antibody diversity to CDR-H3 enabled us to systematically explore the effects of installing chemical groups within additional CDRs of several antibody clones. Using previously characterized aminoacyl-tRNA synthetase/tRNA pairs,34 we identified multiple clones that retained binding function following the incorporation of structurally diverse noncanonical amino acids at locations within CDR-H1, CDR-H2, and CDR-L3. Reductions in antigen binding could be partially explained by the reduction in display levels accompanying the stop codon suppression systems (translation efficiencies at stop codons were less efficient than wild-type translation efficiency under the conditions used here). Titrations of several clones indicated that ncAA substitutions tend to decrease apparent antigen binding affinity, with reductions in affinity ranging from approximately 4-fold to greater than an order of magnitude (Table 3). This observation suggests that ncAA substitutions outside of CDR-H3 affect binding affinity even though these antibody regions were not included in the diversification scheme. However, because changes in display level also change clone avidity on the yeast surface,67, 68 we cannot rule out potential avidity effects without further studies. Given the variability in binding observed between different clones, and the near complete loss of binding in some cases, it is clear that the extent to which ncAA incorporation is tolerated can depend on the particular clone and substitution site of interest. Further studies are needed to determine whether these effects are only due to changes in the stabilities of substituted antibodies, or if they are also influenced by inefficiencies in stop codon readthrough that result in improperly folded proteins. We expect that a better understanding of these questions will help minimize losses in function in future work. In particular, substitution sites beyond the three sites studied here, especially at positions resulting in more conservative substitutions than serine to ncAA substitutions, may lead to a broader set of clones tolerant to ncAA substitutions. More sophisticated antibody libraries may also be valuable in this regard; incorporating ncAAs into antibody library design and screening could provide an empirical route to identifying substitution sites that are well-tolerated in a broad range of antibodies. In any case, this initial work identified multiple substitutions that were reasonably well-tolerated.
Successful yeast display of functional clones containing ncAAs near antibody binding interfaces enabled us to investigate both bioorthogonal chemistries and photo-crosslinking for exploiting these “chemically diversified” clones. The versatility of the display format supported efficient investigation of each these schemes. Bioorthogonal CuAAC and SPAAC reactions were readily achieved on the yeast surface, in line with previous functionalization results from our group and others on yeast33, 34, 69, 70 and E. coli25, 71, 72. Strikingly, in most substituted clones, installation of bulky probes near the antibody binding interface did not appear to overtly affect binding function. Photo-crosslinking experiments with p-azido-L-phenylalanine on the yeast surface strongly suggest site-dependent formation of covalent linkages with target antigens. Importantly, we were able to confirm key observations made on the yeast surface with soluble versions of ncAA-substituted clones. Assays of both CuAAC reactivity and photo-crosslinking in solution yielded results consistent with our initial yeast display-based characterizations. The confirmation that ncAA-mediated properties are retained when displayed clones are converted to soluble form is critical, indicating that yeast-based evaluations of ncAA-containing proteins are predictive of solution behavior. Our observation that key properties of chemically diversified antibodies, including binding affinity, chemical reactivity, and crosslinking, can be efficiently evaluated on the yeast surface raises the possibility of performing detailed structure-activity relationship analyses in future work. This could reveal mechanisms by which clones retain (or lose) affinity, stability, specificity, and other key properties relevant for therapeutic lead discovery and development.
Overall, our findings elucidate new routes to leveraging an expanded chemical dimension in engineering the properties of synthetic antibodies. The antibody diversification strategy used here supports chemical diversification within many portions of the antibody variable domain; we expect this approach to be transferrable to other binding protein scaffolds.46, 73–77 We note that the careful introduction of additional antibody diversity, such as using multiple frameworks,9, 11, 36 may enhance antibody library performance while still supporting rapid chemical diversification. In any case, the reactivities accessed through the clickable and photo-active ncAAs used in this work would be challenging or impossible to access using canonical amino acids alone. Moreover, the use of yeast display streamlines the evaluation of ncAA-substituted clones in comparison to soluble antibody production and characterization. The binding and reactive properties of clones identified in this study highlight the potentially broad scope of antibody modifications achievable with additional chemical functionalization. However, we recognize that there are numerous effective bioconjugation strategies that may be applied to prepare chemically diversified antibodies, including thiol modification strategies,22, 78 enzymatic modification strategies,23 or emerging conjugation reactions at other canonical amino acids.79, 80 In this regard, the simple synthetic antibodies and characterization strategies elucidated in this work could facilitate side-by-side evaluations of multiple chemical modification strategies. Finally, while many routes to prepare chemically modified peptides in display formats are now available,81–89 very few analogous approaches for the chemical diversification of displayed proteins have been described.25, 31 Realization of these strategies expands the molecular recognition capabilities of antibodies and other binding proteins to include features such as enzyme active site-targeting groups and covalent targeting moieties that are not normally found within proteins. As such, the efficient preparation and chemical diversification of antibodies on the yeast surface opens up new possibilities for discovering “drug-like” protein leads in high throughput.
MATERIALS AND METHODS
Please refer to the Supporting Information for experimental methods.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA214239, Tufts startup funds, and an award from the Tufts Faculty Research Awards Committee. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Tufts University. The authors also thank J. Stieglitz for providing modified suppression plasmids for use in ncAA incorporation experiments.
ABBREVIATIONS
- CDR
complementarity determining region
- scFv
single-chain variable fragment
- FACS
fluorescence-activated cell sorting
- aaRS
aminoacyl-tRNA synthetase
- OTS
orthogonal translation system
- ncAA
noncanonical amino acid (also unnatural amino acid, nonstandard amino acid, non-native amino acid, or non-natural amino acid
- RRE
relative readthrough efficiency
- MMF
maximum misincorporation frequency
- AzF
p-azido-L-phenylalanine
- OPG
p-propargyloxyphenylalanine
- AzMF
p-azidomethyl-L-phenylalanine
- LysN3
H-L-Lys(EO-N3)-OH
- CuAAC
copper-catalyzed azide-alkyne cycloaddition
- SPAAC
strain-promoted azide-alkyne cycloaddition
- DBCO
dibenzocyclooctyne
- DMSO
dimethyl sulfoxide
Footnotes
Supporting Information
Supporting Information Available: This material is available free of charge via the Internet.
Supplementary methods include all materials and experimental methods associated with this work. Supplementary figures provide in depth library characterizations and binding, click chemistry and photo-crosslinking data for additional clones. Supplementary tables provide detailed information on experimental conditions used throughout this work, additional library characterizations, and relative readthrough efficiency and maximum misincorporation frequency data for noncanonical amino acid incorporation.
The authors declare no competing financial interest.
REFERENCES
- [1].Boder ET, and Jiang W (2011) Engineering antibodies for cancer therapy, Annu Rev Chem Biomol Eng 2, 53–75. [DOI] [PubMed] [Google Scholar]
- [2].Carter PJ, and Lazar GA (2018) Next generation antibody drugs: pursuit of the ‘high-hanging fruit’, Nat Rev Drug Discov 17, 197–223. [DOI] [PubMed] [Google Scholar]
- [3].Bradbury ARM, Sidhu S, Dübel S, and McCafferty J (2011) Beyond natural antibodies: the power of in vitro display technologies, Nature Biotechnology 29, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bowley DR, Labrijn AF, Zwick MB, and Burton DR (2007) Antigen selection from an HIV-1 immune antibody library displayed on yeast yields many novel antibodies compared to selection from the same library displayed on phage, Protein Engineering, Design and Selection 20, 81–90. [DOI] [PubMed] [Google Scholar]
- [5].Cherf GM, and Cochran JR (2015) Applications of Yeast Surface Display for Protein Engineering, In Yeast Surface Display: Methods, Protocols, and Applications (Liu B, Ed.), pp 155–175, Springer New York, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miersch S, and Sidhu SS (2012) Synthetic antibodies: concepts, potential and practical considerations, Methods 57, 486–498. [DOI] [PubMed] [Google Scholar]
- [7].Adams JJ, and Sidhu SS (2014) Synthetic antibody technologies, Curr Opin Struct Biol 24, 1–9. [DOI] [PubMed] [Google Scholar]
- [8].Mahon CM, Lambert MA, Glanville J, Wade JM, Fennell BJ, Krebs MR, Armellino D, Yang S, Liu X, O’Sullivan CM, Autin B, Oficjalska K, Bloom L, Paulsen J, Gill D, Damelin M, Cunningham O, and Finlay WJJ (2013) Comprehensive Interrogation of a Minimalist Synthetic CDR-H3 Library and Its Ability to Generate Antibodies with Therapeutic Potential, Journal of Molecular Biology 425, 1712–1730. [DOI] [PubMed] [Google Scholar]
- [9].Prassler J, Thiel S, Pracht C, Polzer A, Peters S, Bauer M, Nörenberg S, Stark Y, Kölln J, Popp A, Urlinger S, and Enzelberger M (2011) HuCAL PLATINUM, a Synthetic Fab Library Optimized for Sequence Diversity and Superior Performance in Mammalian Expression Systems, Journal of Molecular Biology 413, 261–278. [DOI] [PubMed] [Google Scholar]
- [10].Persson H, Ye W, Wernimont A, Adams JJ, Koide A, Koide S, Lam R, and Sidhu SS (2013) CDR-H3 Diversity Is Not Required for Antigen Recognition by Synthetic Antibodies, Journal of Molecular Biology 425, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fellouse FA, Esaki K, Birtalan S, Raptis D, Cancasci VJ, Koide A, Jhurani P, Vasser M, Wiesmann C, Kossiakoff AA, Koide S, and Sidhu SS (2007) High-throughput Generation of Synthetic Antibodies from Highly Functional Minimalist Phage-displayed Libraries, Journal of Molecular Biology 373, 924–940. [DOI] [PubMed] [Google Scholar]
- [12].Fellouse FA, Li B, Compaan DM, Peden AA, Hymowitz SG, and Sidhu SS (2005) Molecular Recognition by a Binary Code, Journal of Molecular Biology 348, 1153–1162. [DOI] [PubMed] [Google Scholar]
- [13].Rezhdo A, Islam M, Huang M, and Van Deventer JA (2019) Future prospects for noncanonical amino acids in biological therapeutics, Current opinion in biotechnology 60, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tjhung KF, Kitov PI, Ng S, Kitova EN, Deng L, Klassen JS, and Derda R (2016) Silent Encoding of Chemical Post-Translational Modifications in Phage-Displayed Libraries, Journal of the American Chemical Society 138, 32–35. [DOI] [PubMed] [Google Scholar]
- [15].Krall N, da Cruz FP, Boutureira O, and Bernardes GJ (2016) Site-selective protein-modification chemistry for basic biology and drug development, Nat Chem 8, 103–113. [DOI] [PubMed] [Google Scholar]
- [16].Wu P, Shui WQ, Carlson BL, Hu N, Rabuka D, Lee J, and Bertozzi CR (2009) Site-specific chemical modification of recombinant proteins produced in mammalian cells by using the genetically encoded aldehyde tag, Proceedings of the National Academy of Sciences of the United States of America 106, 3000–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lindstedt PR, Aprile FA, Sormanni P, Rakoto R, Dobson CM, Bernardes GJL, and Vendruscolo M (2020) Systematic Activity Maturation of a Single-Domain Antibody with Non-canonical Amino Acids through Chemical Mutagenesis, Cell Chemical Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hayashi A, Haruna K. i., Sato H, Ito K, Makino C, Ito T, and Sakamoto K (2021) Incorporation of Halogenated Amino Acids into Antibody Fragments at Multiple Specific Sites Enhances Antigen Binding, ChemBioChem 22, 120–123. [DOI] [PubMed] [Google Scholar]
- [19].Agarwal P, and Bertozzi CR (2015) Site-Specific Antibody–Drug Conjugates: The Nexus of Bioorthogonal Chemistry, Protein Engineering, and Drug Development, Bioconjugate Chemistry 26, 176–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sievers EL, and Senter PD (2013) Antibody-drug conjugates in cancer therapy, Annu Rev Med 64, 15–29. [DOI] [PubMed] [Google Scholar]
- [21].Tian F, Lu Y, Manibusan A, Sellers A, Tran H, Sun Y, Phuong T, Barnett R, Hehli B, Song F, DeGuzman MJ, Ensari S, Pinkstaff JK, Sullivan LM, Biroc SL, Cho H, Schultz PG, DiJoseph J, Dougher M, Ma D, Dushin R, Leal M, Tchistiakova L, Feyfant E, Gerber H-P, and Sapra P (2014) A general approach to site-specific antibody drug conjugates, Proceedings of the National Academy of Sciences 111, 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ohri R, Bhakta S, Fourie-O’Donohue A, Dela Cruz-Chuh J, Tsai SP, Cook R, Wei B, Ng C, Wong AW, Bos AB, Farahi F, Bhakta J, Pillow TH, Raab H, Vandlen R, Polakis P, Liu Y, Erickson H, Junutula JR, and Kozak KR (2018) High-Throughput Cysteine Scanning To Identify Stable Antibody Conjugation Sites for Maleimide- and Disulfide-Based Linkers, Bioconjug Chem 29, 473–485. [DOI] [PubMed] [Google Scholar]
- [23].Yamazoe S, Hogan JM, West SM, Deng XA, Kotapati S, Shao X, Holder P, Lamba V, Huber M, Qiang C, Gangwar S, Rao C, Dollinger G, Rajpal A, and Strop P (2020) High-Throughput Platform to Identify Antibody Conjugation Sites from Antibody–Drug Conjugate Libraries, Bioconjugate Chemistry 31, 1199–1208. [DOI] [PubMed] [Google Scholar]
- [24].Coumans RGE, Ariaans GJA, Spijker HJ, Renart Verkerk P, Beusker PH, Kokke BPA, Schouten J, Blomenröhr M, van der Lee MMC, Groothuis PG, Ubink R, Dokter WHA, and Timmers CM (2020) A Platform for the Generation of Site-Specific Antibody–Drug Conjugates That Allows for Selective Reduction of Engineered Cysteines, Bioconjugate Chemistry 31, 2136–2146. [DOI] [PubMed] [Google Scholar]
- [25].Van Deventer JA, Yuet KP, Yoo TH, and Tirrell DA (2014) Cell surface display yields evolvable, clickable antibody fragments, Chembiochem 15, 1777–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu CC, Mack AV, Tsao M-L, Mills JH, Lee HS, Choe H, Farzan M, Schultz PG, and Smider VV (2008) Protein evolution with an expanded genetic code, Proceedings of the National Academy of Sciences 105, 17688–17693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu CC, Mack AV, Brustad EM, Mills JH, Groff D, Smider VV, and Schultz PG (2009) Evolution of Proteins with Genetically Encoded “Chemical Warheads”, Journal of the American Chemical Society 131, 9616–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li X, Hitomi J, and Liu CC (2018) Characterization of a Sulfated Anti-HIV Antibody Using an Expanded Genetic Code, Biochemistry 57, 2903–2907. [DOI] [PubMed] [Google Scholar]
- [29].Rader C, Sinha SC, Popkov M, Lerner RA, and Barbas CF (2003) Chemically programmed monoclonal antibodies for cancer therapy: Adaptor immunotherapy based on a covalent antibody catalyst, Proceedings of the National Academy of Sciences 100, 5396–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, Desharnais J, Hagen C, Levin NJ, Shields MJ, Parish M, Murphy RE, Del Rosario J, Oates BD, Lai J-Y, Matin MJ, Ainekulu Z, Bhat A, Bradshaw CW, Woodnutt G, Lerner RA, and Lappe RW (2010) Chemical generation of bispecific antibodies, Proceedings of the National Academy of Sciences 107, 22611–22616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jespers L, Bonnert TP, and Winter G (2004) Selection of optical biosensors from chemisynthetic antibody libraries, Protein Eng Des Sel 17, 709–713. [DOI] [PubMed] [Google Scholar]
- [32].Cheng AC, Doherty EM, Johnstone S, DiMauro EF, Dao J, Luthra A, Ye J, Tang J, Nixey T, Min X, Tagari P, Miranda LP, and Wang Z (2018) Structure-guided Discovery of Dual-recognition Chemibodies, Scientific Reports 8, 7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Van Deventer JA, Le DN, Zhao J, Kehoe HP, and Kelly RL (2016) A platform for constructing, evaluating, and screening bioconjugates on the yeast surface, Protein Eng Des Sel 29, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stieglitz JT, Kehoe HP, Lei M, and Van Deventer JA (2018) A Robust and Quantitative Reporter System To Evaluate Noncanonical Amino Acid Incorporation in Yeast, ACS synthetic biology 7, 2256–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Potts KA, Stieglitz JT, Lei M, and Van Deventer JA (2020) Reporter system architecture affects measurements of noncanonical amino acid incorporation efficiency and fidelity, Molecular Systems Design & Engineering 5, 573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kelly RL, Le D, Zhao J, and Wittrup KD (2018) Reduction of Nonspecificity Motifs in Synthetic Antibody Libraries, J Mol Biol 430, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Van Deventer JA, Kelly RL, Rajan S, Wittrup KD, and Sidhu SS (2015) A switchable yeast display/secretion system, Protein Engineering, Design and Selection 28, 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Adams JJ, Nelson B, and Sidhu SS (2014) Recombinant genetic libraries and human monoclonal antibodies, Methods Mol Biol 1060, 149–170. [DOI] [PubMed] [Google Scholar]
- [39].Woldring DR, Holec PV, Zhou H, and Hackel BJ (2015) High-Throughput Ligand Discovery Reveals a Sitewise Gradient of Diversity in Broadly Evolved Hydrophilic Fibronectin Domains, PLoS One 10, e0138956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kabat EA, and Wu TT (1991) Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites, The Journal of Immunology 147, 1709–1719. [PubMed] [Google Scholar]
- [41].Eigenbrot C, Randal M, Presta L, Carter P, and Kossiakoff AA (1993) X-ray Structures of the Antigen-binding Domains from Three Variants of Humanized anti-p185HER2 Antibody 4D5 and Comparison with Molecular Modeling, Journal of Molecular Biology 229, 969–995. [DOI] [PubMed] [Google Scholar]
- [42].Van Deventer JA, and Wittrup KD (2014) Yeast surface display for antibody isolation: library construction, library screening, and affinity maturation, Methods Mol Biol 1131, 151–181. [DOI] [PubMed] [Google Scholar]
- [43].Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, and Wittrup KD (2006) Isolating and engineering human antibodies using yeast surface display, Nature Protocols 1, 755–768. [DOI] [PubMed] [Google Scholar]
- [44].Nam DH, Rodriguez C, Remacle AG, Strongin AY, and Ge X (2016) Active-site MMP-selective antibody inhibitors discovered from convex paratope synthetic libraries, Proceedings of the National Academy of Sciences 113, 14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Quartararo AJ, Gates ZP, Somsen BA, Hartrampf N, Ye X, Shimada A, Kajihara Y, Ottmann C, and Pentelute BL (2020) Ultra-large chemical libraries for the discovery of high-affinity peptide binders, Nat Commun 11, 3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hackel BJ, and Wittrup KD (2010) The full amino acid repertoire is superior to serine/tyrosine for selection of high affinity immunoglobulin G binders from the fibronectin scaffold, Protein Engineering, Design and Selection 23, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kruziki Max A., Bhatnagar S, Woldring Daniel R., Duong Vandon T., and Hackel Benjamin J. (2015) A 45-Amino-Acid Scaffold Mined from the PDB for High-Affinity Ligand Engineering, Chemistry & Biology 22, 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chin JW, Cropp TA, Anderson JC, Mukherji M, Zhang Z, and Schultz PG (2003) An Expanded Eukaryotic Genetic Code, Science 301, 964. [DOI] [PubMed] [Google Scholar]
- [49].Chin JW (2017) Expanding and reprogramming the genetic code, Nature 550, 53–60. [DOI] [PubMed] [Google Scholar]
- [50].Dunbar J, Krawczyk K, Leem J, Baker T, Fuchs A, Georges G, Shi J, and Deane CM (2014) SAbDab: the structural antibody database, Nucleic Acids Research 42, D1140–D1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yu CM, Peng HP, Chen IC, Lee YC, Chen JB, Tsai KC, Chen CT, Chang JY, Yang EW, Hsu PC, Jian JW, Hsu HJ, Chang HJ, Hsu WL, Huang KF, Ma AC, and Yang AS (2012) Rationalization and design of the complementarity determining region sequences in an antibody-antigen recognition interface, PLoS One 7, e33340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wilkinson IC, Hall CJ, Veverka V, Shi JY, Muskett FW, Stephens PE, Taylor RJ, Henry AJ, and Carr MD (2009) High Resolution NMR-based Model for the Structure of a scFv-IL-1 beta Complex POTENTIAL FOR NMR AS A KEY TOOL IN THERAPEUTIC ANTIBODY DESIGN AND DEVELOPMENT, J Biol Chem 284, 31928–31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ganesan R, Eigenbrot C, Wu Y, Liang WC, Shia S, Lipari MT, and Kirchhofer D (2009) Unraveling the allosteric mechanism of serine protease inhibition by an antibody, Structure 17, 1614–1624. [DOI] [PubMed] [Google Scholar]
- [54].Kudo S, Caaveiro JM, Nagatoishi S, Miyafusa T, Matsuura T, Sudou Y, and Tsumoto K (2017) Disruption of cell adhesion by an antibody targeting the cell-adhesive intermediate (X-dimer) of human P-cadherin, Sci Rep 7, 39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wu N, Deiters A, Cropp TA, King D, and Schultz PG (2004) A genetically encoded photocaged amino acid, J Am Chem Soc 126, 14306–14307. [DOI] [PubMed] [Google Scholar]
- [56].Lemke EA, Summerer D, Geierstanger BH, Brittain SM, and Schultz PG (2007) Control of protein phosphorylation with a genetically encoded photocaged amino acid, Nat Chem Biol 3, 769–772. [DOI] [PubMed] [Google Scholar]
- [57].Pott M, Schmidt MJ, and Summerer D (2014) Evolved Sequence Contexts for Highly Efficient Amber Suppression with Noncanonical Amino Acids, ACS Chemical Biology 9, 2815–2822. [DOI] [PubMed] [Google Scholar]
- [58].Lang K, and Chin JW (2014) Bioorthogonal reactions for labeling proteins, ACS Chem Biol 9, 16–20. [DOI] [PubMed] [Google Scholar]
- [59].McKay CS, and Finn MG (2014) Click chemistry in complex mixtures: bioorthogonal bioconjugation, Chem Biol 21, 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Agard NJ, Baskin JM, Prescher JA, Lo A, and Bertozzi CR (2006) A comparative study of bioorthogonal reactions with azides, ACS Chem Biol 1, 644–648. [DOI] [PubMed] [Google Scholar]
- [61].Hong V, Steinmetz NF, Manchester M, and Finn MG (2010) Labeling live cells by copper-catalyzed alkyne--azide click chemistry, Bioconjug Chem 21, 1912–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kislukhin AA, Hong VP, Breitenkamp KE, and Finn MG (2013) Relative performance of alkynes in copper-catalyzed azide-alkyne cycloaddition, Bioconjug Chem 24, 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zimmerman ES, Heibeck TH, Gill A, Li XF, Murray CJ, Madlansacay MR, Tran C, Uter NT, Yin G, Rivers PJ, Yam AY, Wang WD, Steiner AR, Bajad SU, Penta K, Yang WJ, Hallam TJ, Thanos CD, and Sato AK (2014) Production of Site-Specific Antibody-Drug Conjugates Using Optimized Non-Natural Amino Acids in a Cell-Free Expression System, Bioconjugate Chemistry 25, 351–361. [DOI] [PubMed] [Google Scholar]
- [64].Coin I (2018) Application of non-canonical crosslinking amino acids to study protein–protein interactions in live cells, Current Opinion in Chemical Biology 46, 156–163. [DOI] [PubMed] [Google Scholar]
- [65].Mishra PK, Yoo CM, Hong E, and Rhee HW (2020) Photo-crosslinking: An Emerging Chemical Tool for Investigating Molecular Networks in Live Cells, Chembiochem 21, 924–932. [DOI] [PubMed] [Google Scholar]
- [66].Futran AS, Kyin S, Shvartsman SY, and Link AJ (2015) Mapping the binding interface of ERK and transcriptional repressor Capicua using photocrosslinking, Proceedings of the National Academy of Sciences 112, 8590–8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ackerman M, Levary D, Tobon G, Hackel B, Orcutt KD, and Wittrup KD (2009) Highly avid magnetic bead capture: An efficient selection method for de novo protein engineering utilizing yeast surface display, Biotechnology Progress 25, 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stern LA, Csizmar CM, Woldring DR, Wagner CR, and Hackel BJ (2017) Titratable Avidity Reduction Enhances Affinity Discrimination in Mammalian Cellular Selections of Yeast-Displayed Ligands, ACS Combinatorial Science 19, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lim S, Glasgow JE, Filsinger Interrante M, Storm EM, and Cochran JR (2017) Dual display of proteins on the yeast cell surface simplifies quantification of binding interactions and enzymatic bioconjugation reactions, Biotechnology Journal 12, 1600696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Niquille DL, Hansen DA, Mori T, Fercher D, Kries H, and Hilvert D (2018) Nonribosomal biosynthesis of backbone-modified peptides, Nature Chemistry 10, 282–287. [DOI] [PubMed] [Google Scholar]
- [71].Link AJ, and Tirrell DA (2003) Cell Surface Labeling of Escherichia coli via Copper(I)-Catalyzed [3+2] Cycloaddition, Journal of the American Chemical Society 125, 11164–11165. [DOI] [PubMed] [Google Scholar]
- [72].Tanrikulu IC, Schmitt E, Mechulam Y, Goddard WA 3rd, and Tirrell DA (2009) Discovery of Escherichia coli methionyl-tRNA synthetase mutants for efficient labeling of proteins with azidonorleucine in vivo, Proc Natl Acad Sci U S A 106, 15285–15290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Golinski AW, Holec PV, Mischler KM, and Hackel BJ (2019) Biophysical Characterization Platform Informs Protein Scaffold Evolvability, ACS Combinatorial Science 21, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Silverman AP, Levin AM, Lahti JL, and Cochran JR (2009) Engineered Cystine-Knot Peptides that Bind αvβ3 Integrin with Antibody-Like Affinities, Journal of Molecular Biology 385, 1064–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Miller EA, Sung K-J, Kongsuphol P, Baniya S, Aw-Yong HQ, Tay V, Tan Y, Kabir FM, Pang-Yeo K, Kaspriskie IG, and Sikes HD (2020) Beyond Epitope Binning: Directed in Vitro Selection of Complementary Pairs of Binding Proteins, ACS Combinatorial Science 22, 49–60. [DOI] [PubMed] [Google Scholar]
- [76].Plückthun A (2015) Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy, Annual Review of Pharmacology and Toxicology 55, 489–511. [DOI] [PubMed] [Google Scholar]
- [77].McMahon C, Baier AS, Pascolutti R, Wegrecki M, Zheng S, Ong JX, Erlandson SC, Hilger D, Rasmussen SGF, Ring AM, Manglik A, and Kruse AC (2018) Yeast surface display platform for rapid discovery of conformationally selective nanobodies, Nature Structural & Molecular Biology 25, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Junutula JR, Bhakta S, Raab H, Ervin KE, Eigenbrot C, Vandlen R, Scheller RH, and Lowman HB (2008) Rapid identification of reactive cysteine residues for site-specific labeling of antibody-Fabs, J Immunol Methods 332, 41–52. [DOI] [PubMed] [Google Scholar]
- [79].Elledge SK, Tran HL, Christian AH, Steri V, Hann B, Toste FD, Chang CJ, and Wells JA (2020) Systematic identification of engineered methionines and oxaziridines for efficient, stable, and site-specific antibody bioconjugation, Proc Natl Acad Sci U S A 117, 5733–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hoyt EA, Cal PMSD, Oliveira BL, and Bernardes GJL (2019) Contemporary approaches to site-selective protein modification, Nature Reviews Chemistry 3, 147–171. [Google Scholar]
- [81].Chen S, Lovell S, Lee S, Fellner M, Mace PD, and Bogyo M (2020) Identification of highly selective covalent inhibitors by phage display, Nat Biotechnol DOI: 10.1038/s41587-020-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tharp JM, Hampton JT, Reed CA, Ehnbom A, Chen P-HC, Morse JS, Kurra Y, Pérez LM, Xu S, and Liu WR (2020) An amber obligate active site-directed ligand evolution technique for phage display, Nature Communications 11, 1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang XS, Chen P-HC, Hampton JT, Tharp JM, Reed CA, Das SK, Wang D-S, Hayatshahi HS, Shen Y, Liu J, and Liu WR (2019) A Genetically Encoded, Phage-Displayed Cyclic-Peptide Library, Angewandte Chemie International Edition 58, 15904–15909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Arunika E, Lena S, Jihea Y, Nicholas J. B, Raja M, Atul B, Frank W, and Ratmir D (2020) Genetically Encoded Fragment-Based Discovery (GE-FBD) from Phage-Displayed Macrocyclic Libraries with Genetically-Encoded Unnatural Pharmacophores, ChemRxiv DOI: 10.26434/chemrxiv.12644225.v1. [DOI] [Google Scholar]
- [85].Passioura T, Liu W, Dunkelmann D, Higuchi T, and Suga H (2018) Display Selection of Exotic Macrocyclic Peptides Expressed under a Radically Reprogrammed 23 Amino Acid Genetic Code, Journal of the American Chemical Society 140, 11551–11555. [DOI] [PubMed] [Google Scholar]
- [86].Owens AE, Iannuzzelli JA, Gu Y, and Fasan R (2020) MOrPH-PhD: An Integrated Phage Display Platform for the Discovery of Functional Genetically Encoded Peptide Macrocycles, ACS Central Science 6, 368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bacon K, Blain A, Burroughs M, McArthrur N, Rao BM, and Menegatti S (2020) Isolation of Chemically Cyclized Peptide Binders Using Yeast Surface Display, ACS Combinatorial Science 22, 519–532. [DOI] [PubMed] [Google Scholar]
- [88].Ng S, Lin E, Kitov PI, Tjhung KF, Gerlits OO, Deng L, Kasper B, Sood A, Paschal BM, Zhang P, Ling C-C, Klassen JS, Noren CJ, Mahal LK, Woods RJ, Coates L, and Derda R (2015) Genetically Encoded Fragment-Based Discovery of Glycopeptide Ligands for Carbohydrate-Binding Proteins, Journal of the American Chemical Society 137, 5248–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Deyle K, Kong X-D, and Heinis C (2017) Phage Selection of Cyclic Peptides for Application in Research and Drug Development, Accounts of Chemical Research 50, 1866–1874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


