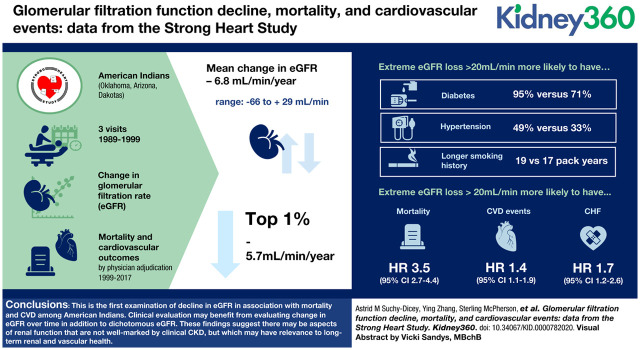
Visual Abstract
Keywords: clinical nephrology, cardiovascular diseases, chronic renal insufficiency, glomerular filtration rate, hypertensive nephropathy, nephritis, North American Indians
Abstract
Background
Rapid kidney decline is associated with mortality and cardiovascular disease, even in the absence of CKD. American Indians (AI) have particularly high burden of kidney disease, cardiovascular disease, and stroke. This study aims to examine extreme loss in glomerular function in this population in association with clinical outcomes.
Methods
The Strong Heart Study, a large longitudinal cohort of adult AI participants, collected plasma creatinine at three examination visits between 1989 and 1999. Intraindividual regressions of eGFR provided linear estimates of the change in kidney function over this time. Surveillance with physician adjudication identified mortality and cardiovascular events between visit three through to 2017.
Results
Mean change in eGFR was loss 6.8 ml/min over the 10-year baseline (range: −66.0 to +28.9 ml/min). The top 1 percentile lost approximately 5.7 ml/min per year. Participants with extreme eGFR loss were more likely to have diabetes (95% versus 71%), hypertension (49% versus 33%), or longer smoking history, among smokers (19 pack-years versus 17 pack-years). CKD (eGFR <60 ml/min) was associated only with mortality, independent of slope: HR, 1.1; 95% CI, 1.0 to 1.3. However, extreme loss in eGFR (>20 ml/min over baseline period) was associated with mortality, independent of baseline eGFR: HR, 3.5; 95% CI, 2.7 to 4.4, and independently associated with composite CVD events and CHF: HR, 1.4 and 1.7; 95% CI, 1.1 to 1.9 and 1.2 to 2.6, respectively.
Conclusions
This is the first examination of decline in eGFR in association with mortality and CVD among AIs. The implications of these findings are broad: clinical evaluation may benefit from evaluating change in eGFR over time in addition to dichotomous eGFR. Also, these findings suggest there may be aspects of renal function that are not well marked by clinical CKD, but which may have particular relevance to long-term renal and vascular health.
Introduction
Decline in renal glomerular filtration function is expected at some basal rate, generally following a linear form, and tends to result from accumulated metabolic, hemodynamic, and dietary stresses (1). Typical decline is defined as a loss of approximately 1% in eGFR per year after age 40 (2,3). However, decline can be accelerated by singular or repeated AKI, underlying CKD, or other environmental or biologic factors (2). Precise quantitative categorization of rapid glomerular decline has been somewhat controversial, but generally defined as a linear loss of more than 1.2 ml/min per year (4) or a dichotomous of loss more than 40%–50% function over a few years (5,6). Rapid glomerular decline, so delineated, has been associated with risk of mortality and cardiovascular disease (CVD) (7,8), even in the absence of defined CKD (9–11), although controversy persists on its clinical definition and relevance.
American Indians (AI) have a particularly high burden of both CKD (12,13) and its clinical comorbidities and sequelae, including diabetes, CVD, and stroke (14–24). Glomerular function decline is also likely to differ by ethnic background (10), but no study has examined patterns or rates of decline in AI, and little is known about how rapid glomerular decline might affect mortality, cardiovascular events, and stroke in this population.
Our study aims to establish the first examination of glomerular filtration function loss over several years, including the prevalence of rapid glomerular loss and whether such loss is independently associated with clinical outcomes, in a large, population-based, geographically heterogeneous, longitudinal cohort study of adult AI. The renal, endocrine, and cardiovascular burden of this population is clinically significant, and the findings of this work are likely to have relevance to patient and public health practice, and to future research in these areas.
Materials and Methods
Study Setting
The data for this study were collected by the longitudinal Strong Heart Study (SHS) cohort, which has been previously described in detail (25). Study field centers included multiple communities and tribes in each of the US Northern Plains, Southern Plains, and Southwest. The SHS baseline visit was in 1989–1991; of this, approximately 89% of patients participated in a second visit in 1993–1995, and a third visit in 1997–1999. Participating cohort members with usable creatinine assays at all three visits were eligible for inclusion in these analyses (N=2114). A few assays (N=22) were excluded due to uncertainty in assay values (calculated GFR >200 ml/min). None of the participants was receiving maintenance dialysis at the time of the first visit. The resulting number available for analysis was N=2092 (Supplemental Material). Approval for study procedures has been obtained from all participating Institutional Research Board, Indian Health Service, and tribal council organizations; all participants provided written, informed consent. This report has been reviewed and received approval from all required tribal council and Indian Health Service sites.
Plasma Creatinine
All examination visits collected fasting blood samples for plasma creatinine assay. Aliquoted samples were immediately frozen at −80°C and shipped to Penn Medical Laboratory (Hyattsville, MD) for storage and assay. Serum creatinine was assayed using enzymatic rate methods, both on the Vitros platform (Ortho Clinical Diagnostics, Rochester, NY) and traceable to isotope dilution mass spectrometry calibration standards. Calibration samples included (1) random selection of N=200 samples from each examination visit and (2) all samples with measured plasma creatinine >2.0 mg/dl. These samples were evaluated in comparison with uncalibrated samples, using weighted Deming regressions to estimate best-fit models for standardizing the remaining samples for each examination visit. Regression assumptions were evaluated using Bland-Altman, residual-fitted, and quantile-quantile plots; outliers with extreme Cook values were excluded to avoid unnecessary influence on model fit. The final calibration equations for each examination visit were (1) α: −0.197, β: 0.998; (2) α: −0.137, β: 0.908; and (3) α: −0.099, β: 1.003. These equations were very similar to a previous calibration carried out for the first examination only (α: −0.159, β: 0.980) (26). Additional details for the plasma creatinine calibrations are included in the Supplemental Material.
Glomerular Function Decline
Glomerular function was quantified at each of the three visits using the CKD Epidemiology Consortium 2009 equation for eGFR. Acquired glomerular dysfunction was defined using intraindividual regressions of eGFR over the three examination visits, producing β coefficient estimates for “slope,” or linear change in eGFR over the time between visit one and visit three (mean time between examination one and examination three: 8.0 years; range 5.5–10.2). Extreme loss in eGFR over this period was defined on the basis of the “slope” variable distribution (mean −6.8, SD 9.4) as >2× SD, or 20+ ml/min loss. Such a cumulative loss, if linear, corresponds to loss of eGFR ≥2.5 ml/min per year, which is more than two times the expected rate (1,4).
Cardiovascular Outcomes
Surveillance and adjudication procedures were used to identify and confirm cardiovascular events, including any-cause mortality, myocardial infarction, coronary heart disease, congestive heart failure (CHF), and stroke, occurring after visit three (1997–1999) until December 31, 2017, a follow-up period of approximately 18–20 years. These procedures have been described in detail (27). In short, field staff identified possible events using a combination of telephone and postal mail contact and electronic medical record review; a convened panel of study clinicians then reviewed a packet of medical records, making a collective determination of “definite,” “probable,” or “possible.” Outcomes used in our analyses included death, a composite of any definite CVD event, definite CHF, and definite stroke.
Demographics and Clinical Data
At each visit, participants self-reported age, sex, years of education, annual household income, lifetime smoking habits, and current alcohol use habits. Body mass index was measured as weight in kilograms divided by height in meters squared. Seated BP was measured by sphygmomanometry, and hypertension was defined as average systolic BP ≥140 mm Hg, average diastolic BP ≥90 mm Hg, or use of antihypertensive medications. Glucose and LDL were assayed in plasma on the Vitros platform; diabetes was defined as fasting glucose ≥126 mg/dl or use of antihyperglycemic medications, and hyperlipidemia was defined as LDL ≥140 mg/dl or use of statins. Covariates that have values that may vary over time were defined in these analyses at the time of the first visit (examination one).
Statistical Analyses
We summarized eGFR over the three examination visits and centers using histograms and dot plots, and quantified the intraindividual changes over these visits using linear regressions of each individual participant’s eGFR data over the time of the three examination visits. We examined the association between baseline (intercept) eGFR and change in eGFR (slope) using scatter plots with Loess and 95% confidence intervals (95% CI). We quantified differences between participants with extreme eGFR decline (>20 ml/min between examination one and three), and those without such loss using summary measures including mean and SD, or count and percent. We examined associations of time to mortality or cardiovascular, stroke, or CHF event using Cox proportional-hazard models, with follow-up time starting after the end of the defined baseline period used to measure change in eGFR (examinations 1–3) and excluding those with prevalent events as of the time of the first examination. The Cox model exposures were either CKD (eGFR <60 ml/min) by the time of the third visit or loss of >20 ml/min between visits one and three; these models were adjusted for either baseline eGFR or slope eGFR (as relevant), and for age, sex, and field center. We conducted models both with and without adjustment for hypertension and diabetes, as theoretical confounders and/or features within the causal pathway. Cox modeling assumptions, including proportionality of hazards, were checked using graphical examination of modeling parameters. The total number available for any analysis was 2092; participants with events before the beginning of the follow-up period were additionally excluded from those Cox models.
Results
Change in eGFR
Over the mean 8 years between examinations one and three, eGFR generally decreased, although some variation exists. Mean change in eGFR was loss of 6.8 ml/min, with a range of −66.0 ml/min to +28.9 ml/min (interquartile range, IQR, −52.5 to +14.3). The top 1 percentile lost, on average, 45.4 ml/min over the mean 8 years between examinations one and three, corresponding to approximately 5.7 ml/min per year. Distributions also shifted somewhat, with slight changes to mean and substantial changes to the lower ranges (Figure 1).
Figure 1.
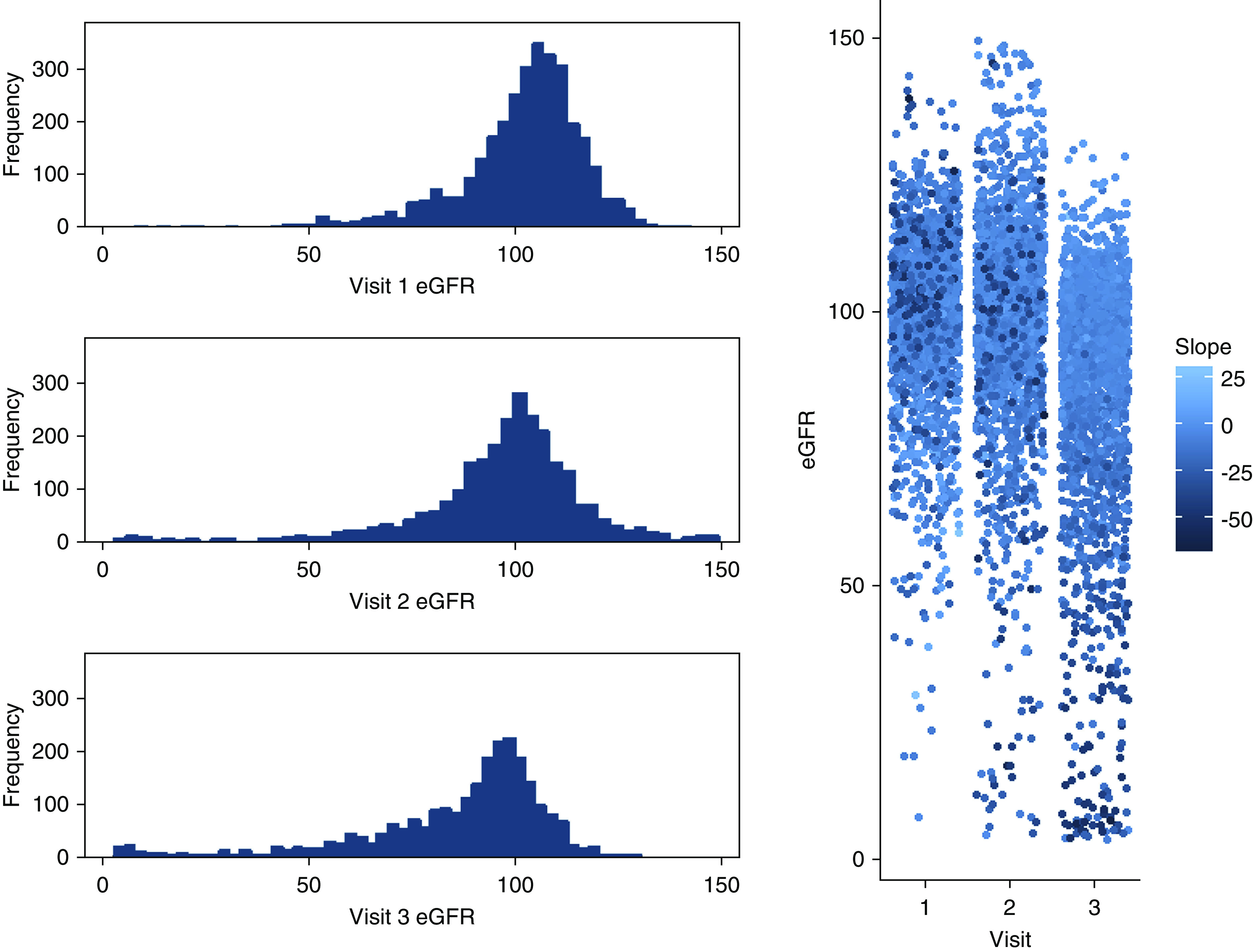
Change in eGFR over three examinations in the Strong Heart Study (1989–1999). Histograms (left) and dot-plots (right) showing distribution of measured eGFR at examination visits 1–3, with coloring (right) by intraindividual change in eGFR over time (slope).
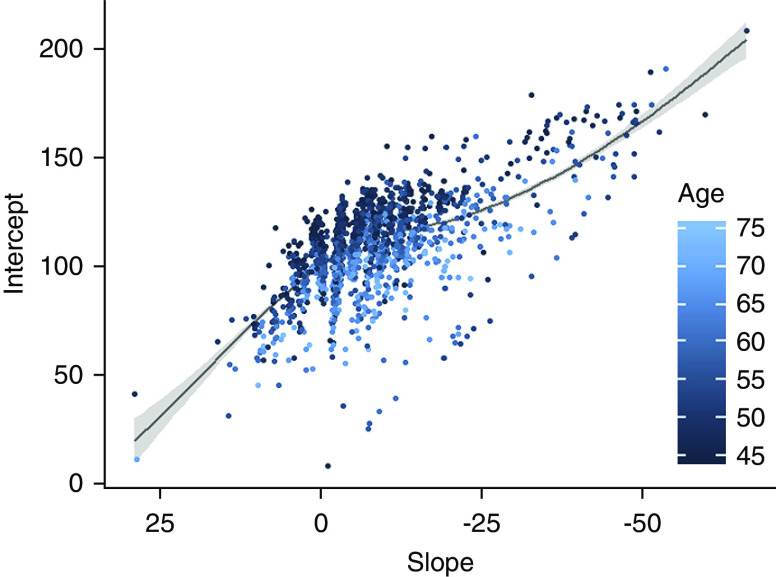
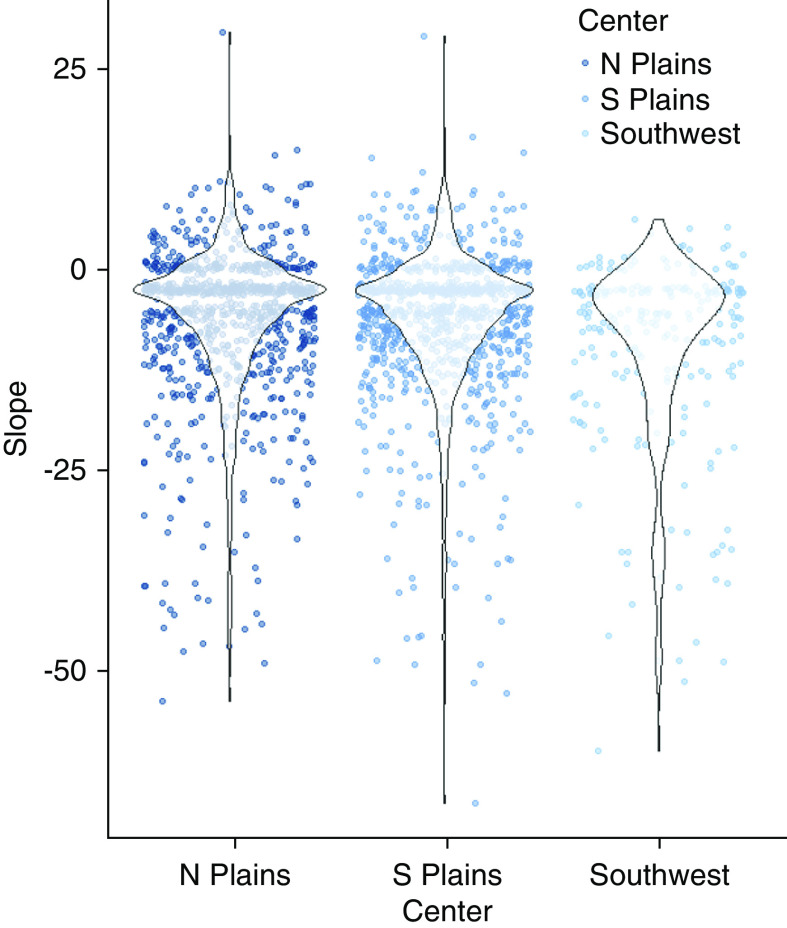
Initial eGFR (mean 100.0 ml/min, SD, 14.6; IQR, 23.4–138.3) was positively associated with the change in eGFR (Figure 2); participants with a higher starting eGFR had, on average, larger declines in eGFR over the study period, despite substantial variability. Change in eGFR was also associated with field center (Figure 3), with the Southwest site having almost no participants with an increase and greater average loss, compared with the other two sites.
Figure 2.
Initial eGFR versus change in eGFR over three examinations in the Strong Heart Study (1989–1999). Scatterplot of modeled baseline eGFR (eGFR, intercept) over intraindividual change in eGFR for examination visits 1–3 (slope), with Loess and 95% confidence intervals and coloration by age.
Figure 3.
Change in eGFR by field center in the Strong Heart Study (1989–1999). Dot-plots and violin plots for intraindividual change in estimate GFR (eGFR, slope) from examination visits 1–3, over field centers.
Extreme eGFR Loss
Despite the association in continuous values for examination 1 eGFR and change in eGFR (“slope”) between examination one and examination three, there was no mean difference in baseline eGFR between participants who had extreme eGFR decline (>20 ml/min loss) and those who did not (Table 1); such differences were evident in mean eGFR for examination two and examination three. Participants with extreme eGFR decline also had approximately 1 year less of formal education, were twice as likely to be from the Southwest center, and were more likely to have a low income (77% versus 60%), diabetes (95% versus 71%), hypertension (49% versus 33%), and longer smoking history among smokers (19 pack-years versus 17 pack-years). Participants with extreme eGFR decline were less likely to have hyperlipidemia (17% versus 28%) or to be a current drinker at any of the three examinations (38% versus 48%), and were not different in age, sex, or body mass index, compared with those who had less extreme eGFR changes over time. Cox proportional-hazard models for mortality, CVD, stroke, and CHF events detected similar associations for cumulative dichotomous exposure (CKD) or for extreme cumulative loss in eGFR (Table 2), adjusted for sociodemographic and clinical characteristics, as well as with mutual adjustment. Mortality events occurred at a rate of 41.8 per 1000 person-years over a mean 13.7 years follow-up; CVD events at a rate of 19.6 per 1000 person-years over a mean 17.7 years follow-up; stroke events at a rate of 4.4 per 1000 person-years over a mean 19.0 years follow-up; and CHF events at a rate of 6.7 per 1000 person-years over a mean 18.7 years follow-up. CKD (dichotomous exposure) was associated with mortality and stroke, independent of extreme loss in eGFR over time: hazard ratios (HR) of 1.6 (95% CI, 1.3 to 2.1) (mortality) and 1.9 (95% CI, 1.2 to 3.2) (stroke), respectively. However, extreme loss in eGFR (cumulative exposure) was more strongly associated with mortality, independent of baseline eGFR: HR, 3.4; 95% CI, 2.7 to 4.2; and was additionally associated with composite CVD events: HR, 1.4; 95% CI, 1.1 to 1.9; and with CHF: HR, 1.7; 95% CI, 1.1 to 2.6. Additional adjustment for hypertension and diabetes, which may represent either or both confounders and/or features within the causal pathway, had similar patterns in findings in terms of effect size, with the exception of slope (Table 3). Mortality was associated with both CKD (dichotomous exposure: HR, 1.41; 95% CI, 0.95 to 2.08) and extreme loss in eGFR (cumulative exposure HR, 1.72; 95% CI, 0.99 to 3.00), with only the latter being at the threshold of significant; none of the other findings remained significant with these confounders/mediators in the model. Extreme loss in eGFR was not statistically independently associated with stroke. Sensitivity analyses (Supplemental Material) for CKD exposure without adjustment for extreme loss in eGFR detected expected associations for mortality (HR, 2.4; 95% CI, 2.0 to 3.0), CVD (HR, 1.2; 95% CI, 1.0 to 1.6), stroke (HR, 1.7; 95% CI, 1.2 to 2.6), and CHF (HR, 1.5; 95% CI, 1.0 to 2.1).
Table 1.
Selected participant characteristics from American Indians in the Strong Heart Study (1989–1999)
| Characteristics | No Extreme eGFR Declinea | Extreme eGFR Declinea |
| N=1930 | N=162 | |
| Examination 1 eGFR, ml/min, mean (SD) | 100.0 (14.2) | 99.5 (18.2) |
| Examination 2 eGFR, ml/min, mean (SD) | 99.6 (16.8) | 80.5 (32.0) |
| Examination 3 eGFR, ml/min, mean (SD) | 90.6 (16.2) | 36.2 (21.4) |
| Age, yr, mean (SD) | 55.6 (7.7) | 56.7 (7.6) |
| Male, % | 38.7 | 36.4 |
| Formal education, yr, mean (SD) | 11.8 (2.9) | 10.9 (2.8) |
| Household income <$15K/yr, % | 60 | 77 |
| Diabetes, % | 71 | 95 |
| Hypertension, % | 33 | 50 |
| Systolic BP, mean (SD) | 124.4 (17.6) | 131.5 (19.1) |
| Hyperlipidemia, % | 28 | 17 |
| BMI, mean (SD) | 30.7 (5.9) | 31.1 (4.9) |
| Lifetime smoking pack-yr, mean (SD)b | 16.7 (19.5) | 18.6 (23.7) |
| Current drinker at any of visits 1–3, % | 48 | 378 |
| Southwest field center (N=244), % | 11 | 23 |
| Southern Plains field center (N=932), % | 45 | 36 |
| Northern Plains field center (N=916), % | 44 | 41 |
Extreme eGFR decline defined as loss in eGFR >20 ml/min between examination 1 and 3 (1989–1999).
Lifetime smoking pack yr calculated only among current and prior smokers.
Table 2.
Cox proportional hazard model for mortality, cardiovascular, stroke, and coronary events in American Indians from the Strong Heart Study (1989–1999)
| Event | Exposure: CKD | Exposure: Loss >20 ml/min | |||||||
| Yr FU, Mean (SD) | New Events | Incidence Rate | Hazard Ratio | 95% Confidence Interval | P value | Hazard Ratio | 95% Confidence Interval | P value | |
| Mortality | 13.7 (6.4) | 1198 | 0.0418 | 1.63 | 1.27 to 2.09 | <0.001 | 3.37 | 2.70 to 4.19 | <0.001 |
| N=2098 | |||||||||
| All CVD | 17.7 (4.5) | 683 | 0.0196 | 1.16 | 0.84 to 1.59 | 0.37 | 1.43 | 1.09 to 1.88 | 0.01 |
| N=1971 | |||||||||
| Stroke | 19.0 (2.3) | 174 | 0.0044 | 1.91 | 1.15 to 3.16 | 0.01 | 1.42 | 0.86 to 2.34 | 0.17 |
| N=2081 | |||||||||
| CHF | 18.7 (2.9) | 256 | 0.0067 | 1.14 | 0.68 to 1.88 | 0.62 | 1.68 | 1.10 to 2.55 | 0.02 |
| N=2035 | |||||||||
Models excluding prevalent CVD, stroke, and/or CHF as relevant; adjusted for baseline eGFR and/or eGFR slope as relevant; all adjusted for age, sex, field center, smoking, alcohol use, BMI, education, income. FU, follow up; CVD, cardiovascular disease; BMI, body mass index.
Table 3.
Cox proportional hazard model for mortality, cardiovascular, stroke, and coronary events in American Indians from the Strong Heart Study (1989–1999) with addition of clinical features as confounders/mediators
| Event | Exposure: CKD | Exposure: Loss >20 ml/min | |||||||
| Yr FU, Mean (SD) | New Events | Incidence Rate | Hazard Ratio | 95% Confidence Interval | P value | Hazard Ratio | 95% Confidence Interval | P value | |
| Mortality | 13.7 (6.4) | 1198 | 0.0418 | 1.41 | 0.95 to 2.08 | 0.08 | 1.72 | 0.99 to 3.00 | 0.05 |
| N=2098 | |||||||||
| All CVD | 17.7 (4.5) | 683 | 0.0196 | 1.02 | 0.64 to 1.64 | 0.93 | 1.72 | 0.90 to 3.32 | 0.10 |
| N=1971 | |||||||||
| Stroke | 19.0 (2.3) | 174 | 0.0044 | 1.93 | 0.92 to 4.04 | 0.08 | 1.64 | 0.52 to 5.16 | 0.40 |
| N=2081 | |||||||||
| CHF | 18.7 (2.9) | 256 | 0.0067 | 1.31 | 0.57 to 3.01 | 0.52 | 2.70 | 0.90 to 8.01 | 0.08 |
| N=2035 | |||||||||
Models excluding prevalent CVD, stroke, and/or CHF as relevant; adjusted for baseline eGFR and/or eGFR slope as relevant; all adjusted for age, sex, field center, smoking, alcohol use, BMI, education, income, hypertension, and diabetes. FU, follow up; CVD, cardiovascular disease; BMI, body mass index.
Discussion
We found that extreme loss in glomerular filtration function was associated more strongly with cardiovascular and mortality events than the more conventional dichotomous measure of chronic renal dysfunction. These findings suggest that evaluating change in glomerular filtration function, namely, eGFR, over time could be a powerful, independent indicator for vascular pathology. Biologically, patients with rapid functional decline are likely to have significant pathologic changes in multiple systems, although it is unclear whether such rapid decline is reflective or causal of such changes. Clinically, these findings are meaningful because they suggest that evaluating dichotomous measures of kidney function alone may be inadequate, and additional definitions to identify renal pathology may be needed. Furthermore, because extreme loss patterns in GFR (e.g., >3–5 ml/min per year) may be detectable before the conventional threshold definition for CKD (i.e., GFR <60 ml/min) is reached, these findings suggest additional clinical tools to define early renal pathology may be useful for patient prognostics.
Other clinical fields have made similar discoveries, wherein time-dependent variability or decline convey meaningful independent forms of risk. Evaluation of BP (28,29), for example, where a variability and/or steep increase in BP over multiple visits has been demonstrated to convey predictive capacity for mortality (30–33), cardiovascular, and cerebrovascular events (34–39), and related outcomes (40–42), independent of mean BP or presence of hypertension. Further research to establish patterns of time-varying clinical measurements in AI and their clinical consequences may identify further associations with meaningful, independent patterns of risk. Additionally, alternate measures of renal function should be measured and assessed for associations with risk, such as proximal tubular secretion.
Overall, our findings of average eGFR loss in AI appear lower than for other populations. One study reported that the top 1 percentile of Black patients reportedly lose 23.6 ml/min per year; Hispanics and non-Hispanic Whites lose approximately 20 ml/min per year; and Asians lose 17.6 ml/min per year (10). However, study methodologies may not be comparable; previous findings included an health maintenance organization population with a broader age range, and no selection pressure, whereas this study included a middle-aged and elderly cohort of survivors. A biethnic study comparing US middle-aged non-Hispanic Whites and Blacks reported mean percentage annual loss in eGFR as loss 2.5% (IQR, 0.3%–5.7%), with Black patients having steeper declines, although the findings were not reported stratified by race (11). We did find that age was not associated with degree of change in eGFR (Table 1), suggesting that selective survival is not likely to explain our findings. Nonetheless, future research may examine broader age ranges to derive more generalizable estimates of both typical and extreme eGFR loss patterns.
Our findings of association with cardiovascular events are consistent with previous reports of dichotomous glomerular dysfunction in this same cohort population, in particular with one study that estimated hazard ratios comparing participants with CKD stage III (eGFR 30–60 ml/min) and CKD stage IV and V (eGFR <30 ml/min) with those who had normal function (eGFR 90–120 ml/min) for outcomes of CHD (HR, 2.2 [95% CI, 1.6 to 3.0] and 3.1 [95% CI, 1.9 to 5.1], respectively), CVD (HR, 1.8 [95% CI, 1.4 to 2.4] and 2.8 [95% CI, 1.9 to 4.3], respectively), and stroke (HR, 1.7 [95% CI, 0.9 to 2.9] and 1.9 [95% CI, 0.7 to 5.2], respectively) (26). Our analyses expand this prior work by examining multiple measures of eGFR, to examine patterns of change in glomerular filtration over time as an independent feature of renal function and risk assessment.
One key finding that warrants additional consideration is the possible contribution of diabetic nephropathy or other pathology to these results. Diabetics were more common in the extreme eGFR loss group (95% versus 71%). When Cox models for mortality were additionally adjusted for potential mediators that could be part of the causal pathway (diabetes, hypertension, hyperlipidemia), findings for slope were somewhat attenuated; most notably, HR 3.4 (95% CI, 2.7 to 4.2) changed to HR 1.7 (95% CI, 1.0 to 3.0) for mortality. These findings suggest there may be confounding and/or mediation by these clinical features, and therefore future research may be needed to examine the contribution of diabetes, hypertension, or hyperlipidemia on these associations.
Other interpretations of these findings could explain our results. Because participants were required to contribute data to multiple examination visits as an inclusion criterion, selective survival could influence the balance of latent health characteristics among those remaining, such as for CKD, which lead to measurement error and bias. However, because our estimates of risk are similar to prior studies that were not be subject to similar confounding or bias, it is not likely that these issues could solely account for our reported associations. In any case, interpretation of our findings should be focused on individuals who survive at least 8 years after a first measurement of GFR.
This project has some other notable strengths and limitations. Chiefly, we cannot distinguish in these data between participants who had acute or nonlinear losses in glomerular function, which can contribute to measurement error, perhaps accounting for some of observed increases in eGFR over time. Future research should examine more finely tuned patterns of change using additional data-collection points. The strengths of this study include the high-quality, standardized study protocol that systematically evaluated clinical factors and endpoints; highly accurate and precise quantification of serum creatinine and standard utilization of a well-characterized estimating equation for glomerular filtration function; and focus on a population disproportionately affected by hypertension, diabetes, kidney disease, CVD, and stroke, with interpretability that may be informative for other populations experiencing similar exposure patterns. Additionally, we were unable to evaluate diagnosed ESKD as an adjudicated time-to-event outcome, which may be a focus for future research.
In summary, this study comprises the first examination of patterns of change in glomerular filtration function in a highly burdened minority population, and associations of such patterns with mortality and cardiovascular outcomes. The implications of these findings are broad: first, clinical and risk evaluation of patients may benefit from evaluating change in eGFR over time. Future research should evaluate the clinical utility of incorporating such measures into standard practice. Also, these findings of associations of mortality, CVD, and CHF with extreme loss warrant additional consideration. The findings for cardiovascular outcomes suggest there may be aspects of renal function that are not well marked by clinical CKD but may have particular relevance to long-term health.
Disclosures
S. McPherson has received research funding from the Bristol-Myers Squibb Foundation outside the submitted work. K.R. Tuttle reports personal fees from AstraZeneca, Bayer, Eli Lilly and Company, Gilead, Goldfinch Bio, and Novo Nordisk, outside the submitted work. All remaining authors have nothing to disclose.
Funding
This work has been funded in whole or in part with federal funds from the National Institutes of Health, from research grants K01AG057821, P50AG005136, R01HL109315, R01HL109301, R01HL109284, R01HL109282 and R01HL109319; cooperative agreements U01HL41642, U01HL41652, U01HL41654, U01HL65520 and U01HL65521; and contract numbers 75N92019D00027, 75N92019D00028, 75N92019D00029 and 75N92019D00030.
Acknowledgements
We wish to thank all SHS participants and communities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
Author Contributions
A.M. Suchy-Dicey was responsible for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, visualization, writing the original draft, and writing review and editing. B.V. Howard was responsible for data curation, resources, supervision, and writing review and editing. D.S. Buchwald was responsible for data curation, funding acquisition, resources, supervision, and writing review and editing. J. Umans was responsible for data curation, methodology, resources, and writing review and editing. K.R. Tuttle was responsible for the investigation and writing review and editing. S. McPherson was responsible for the methodology and writing review and editing. Y. Zhang was responsible for data curation and writing review and editing.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0000782020/-/DCSupplemental.
Download Supplemental Material, PDF file, 52 KB (60.5KB, pdf)
References
- 1.Hazzard WR, Halter JB: Hazzard’s Geriatric Medicine and Gerontology, New York, McGraw-Hill Medical, 2009 [Google Scholar]
- 2.Kelly KJ, Dominguez JH: Rapid progression of diabetic nephropathy is linked to inflammation and episodes of acute renal failure. Am J Nephrol 32: 469–475, 2010. 10.1159/000320749 [DOI] [PubMed] [Google Scholar]
- 3.Weng SC, Tarng DC, Chen CM, Cheng CH, Wu MJ, Chen CH, Yu TM, Shu KH; CKDBHPDH investigators: Estimated glomerular filtration rate decline is a better risk factor for outcomes of systemic disease-related nephropathy than for outcomes of primary renal diseases. PLoS One 9: e92881, 2014. 10.1371/journal.pone.0092881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eftimovska N, Stojceva-Taneva O, Polenakovic M: Slow progression of chronic kidney disease and what it is associated with. Prilozi 29: 153–165, 2008 [PubMed] [Google Scholar]
- 5.Looker HC, Colombo M, Hess S, Brosnan MJ, Farran B, Dalton RN, Wong MC, Turner C, Palmer CN, Nogoceke E, Groop L, Salomaa V, Dunger DB, Agakov F, McKeigue PM, Colhoun HM; SUMMIT Investigators: Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int 88: 888–896, 2015. 10.1038/ki.2015.199 [DOI] [PubMed] [Google Scholar]
- 6.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, Mitsnefes M, Kaskel F, Greenbaum LA, Mak RH, Flynn J, Moxey-Mims MM, Furth S: Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015. 10.1053/j.ajkd.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, Balasubramanian S, Nurutdinova D, Xian H, Stroupe K, Abbott KC, Eisen S: Rate of kidney function decline associates with mortality. J Am Soc Nephrol 21: 1961–1969, 2010. 10.1681/ASN.2009121210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naimark DM, Grams ME, Matsushita K, Black C, Drion I, Fox CS, Inker LA, Ishani A, Jee SH, Kitamura A, Lea JP, Nally J, Peralta CA, Rothenbacher D, Ryu S, Tonelli M, Yatsuya H, Coresh J, Gansevoort RT, Warnock DG, Woodward M, de Jong PE; CKD Prognosis Consortium: Past decline versus current eGFR and subsequent mortality risk. J Am Soc Nephrol 27: 2456–2466, 2016. 10.1681/ASN.2015060688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins RM, Tang X, Bengier AC, Kirchner HL, Bucaloiu ID: Variability in estimated glomerular filtration rate is an independent risk factor for death among patients with stage 3 chronic kidney disease. Kidney Int 82: 1332–1338, 2012. 10.1038/ki.2012.281 [DOI] [PubMed] [Google Scholar]
- 10.Derose SF, Rutkowski MP, Crooks PW, Shi JM, Wang JQ, Kalantar-Zadeh K, Kovesdy CP, Levin NW, Jacobsen SJ: Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis 62: 236–244, 2013. 10.1053/j.ajkd.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J: Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 20: 2617–2624, 2009. 10.1681/ASN.2009010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Lee ET, Howard BV, Best LG, Umans JG, Yeh J, Wang W, Yeh F, Ali T, Devereux RB, de Simone G: Insulin resistance, incident cardiovascular diseases, and decreased kidney function among nondiabetic American Indians: The Strong heart study. Diabetes Care 36: 3195–3200, 2013. 10.2337/dc12-2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connell J, Yi R, Wilson C, Manson SM, Acton KJ: Racial disparities in health status: A comparison of the morbidity among American Indian and U.S. adults with diabetes. Diabetes Care 33: 1463–1470, 2010. 10.2337/dc09-1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makin SDJ, Cook FAB, Dennis MS, Wardlaw JM: Cerebral small vessel disease and renal function: Systematic review and meta-analysis. Cerebrovasc Dis 39: 39–52, 2015. 10.1159/000369777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo S, Qi RF, Wen JQ, Zhong JH, Kong X, Liang X, Xu Q, Zheng G, Zhang Z, Zhang LJ, Lu GM: Abnormal intrinsic brain activity patterns in patients with end-stage renal disease undergoing peritoneal dialysis: A resting-state functional MR imaging study. Radiology 278: 181–189, 2016. 10.1148/radiol.2015141913 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Hirawa N, Yatsu K, Kobayashi Y, Yamamoto Y, Saka S, Andoh D, Toya Y, Yasuda G, Umemura S: Relationship between silent brain infarction and chronic kidney disease. Nephrol Dial Transplant 24: 201–207, 2009. 10.1093/ndt/gfn419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akoudad S, Sedaghat S, Hofman A, Koudstaal PJ, van der Lugt A, Ikram MA, Vernooij MW: Kidney function and cerebral small vessel disease in the general population. Int J Stroke 10: 603–608, 2015. 10.1111/ijs.12465 [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Vea A, Salvadó E, Bardají A, Gutierrez C, Ramos A, García C, Compte T, Peralta C, Broch M, Pastor R, Angelet P, Marcas L, Saurí A, Oliver JA: Silent cerebral white matter lesions and their relationship with vascular risk factors in middle-aged predialysis patients with CKD. Am J Kidney Dis 47: 241–250, 2006. 10.1053/j.ajkd.2005.10.029 [DOI] [PubMed] [Google Scholar]
- 19.Nagai M, Hoshide S, Takahashi M, Shimpo M, Kario K: Sleep duration, kidney function, and their effects on cerebral small vessel disease in elderly hypertensive patients. Am J Hypertens 28: 884–893, 2015. 10.1093/ajh/hpu243 [DOI] [PubMed] [Google Scholar]
- 20.Sedaghat S, Vernooij MW, Loehrer E, Mattace-Raso FU, Hofman A, van der Lugt A, Franco OH, Dehghan A, Ikram MA: Kidney function and cerebral blood flow: The rotterdam study. J Am Soc Nephrol 27: 715–721, 2016. 10.1681/ASN.2014111118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogels SC, Emmelot-Vonk MH, Verhaar HJ, Koek HL: The association of chronic kidney disease with brain lesions on MRI or CT: A systematic review. Maturitas 71: 331–336, 2012. 10.1016/j.maturitas.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 22.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW: Ethnic disparities in stroke: Epidemiology, acute care, and postacute outcomes. Stroke 36: 374–386, 2005. 10.1161/01.STR.0000153065.39325.fd [DOI] [PubMed] [Google Scholar]
- 23.Harwell TS, Oser CS, Okon NJ, Fogle CC, Helgerson SD, Gohdes D: Defining disparities in cardiovascular disease for American Indians: Trends in heart disease and stroke mortality among American Indians and whites in Montana, 1991 to 2000. Circulation 112: 2263–2267, 2005. 10.1161/CIRCULATIONAHA.105.560607 [DOI] [PubMed] [Google Scholar]
- 24.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics-2017 update: A report from the American Heart Association [published correction appears in Circulation 135: e646, 2017 10.1161/CIR.0000000000000491]. Circulation 135: e146–e603, 2017. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV: The Strong Heart Study. A study of cardiovascular disease in American Indians: Design and methods. Am J Epidemiol 132: 1141–1155, 1990. 10.1093/oxfordjournals.aje.a115757 [DOI] [PubMed] [Google Scholar]
- 26.Shara NM, Wang H, Mete M, Al-Balha YR, Azalddin N, Lee ET, Franceschini N, Jolly SE, Howard BV, Umans JG: Estimated GFR and incident cardiovascular disease events in American Indians: The Strong Heart Study. Am J Kidney Dis 60: 795–803, 2012. 10.1053/j.ajkd.2012.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, Kizer JR, Howard BV, Cowan LD, Yeh J, Howard WJ, Wang W, Best L, Lee ET: Incidence and risk factors for stroke in American Indians: The Strong Heart Study. Circulation 118: 1577–1584, 2008. 10.1161/CIRCULATIONAHA.108.772285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancia G: Visit-to-visit blood pressure variability: An insight into the mechanisms. Hypertension 68: 32–33, 2016. 10.1161/HYPERTENSIONAHA.116.07139 [DOI] [PubMed] [Google Scholar]
- 29.Muntner P, Levitan EB: Visit-to-visit variability of blood pressure: Current knowledge and future research directions. Blood Press Monit 18: 232–238, 2013. 10.1097/MBP.0b013e3283624b24 [DOI] [PubMed] [Google Scholar]
- 30.Suchy-Dicey AM, Wallace ER, Mitchell SV, Aguilar M, Gottesman RF, Rice K, Kronmal R, Psaty BM, Longstreth WT Jr: Blood pressure variability and the risk of all-cause mortality, incident myocardial infarction, and incident stroke in the cardiovascular health study. Am J Hypertens 26: 1210–1217, 2013. 10.1093/ajh/hpt092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang TI, Tabada GH, Yang J, Tan TC, Go AS: Visit-to-visit variability of blood pressure and death, end-stage renal disease, and cardiovascular events in patients with chronic kidney disease. J Hypertens 34: 244–252, 2016. 10.1097/HJH.0000000000000779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC: Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: A 5·5-year prospective analysis. Eur J Clin Invest 42: 245–253, 2012. 10.1111/j.1365-2362.2011.02574.x [DOI] [PubMed] [Google Scholar]
- 33.Lau KK, Wong YK, Chang RS, Teo KC, Hon SF, Chan KH, Wat KL, Cheung RT, Li LS, Siu CW, Ho SL, Tse HF: Visit-to-visit systolic blood pressure variability predicts all-cause and cardiovascular mortality after lacunar infarct. Eur J Neurol 21: 319–325, 2014. 10.1111/ene.12310 [DOI] [PubMed] [Google Scholar]
- 34.Hussein WF, Chang TI: Visit-to-visit variability of systolic blood pressure and cardiovascular disease. Curr Hypertens Rep 17: 14, 2015. 10.1007/s11906-014-0527-8 [DOI] [PubMed] [Google Scholar]
- 35.Mallamaci F, Minutolo R, Leonardis D, D’Arrigo G, Tripepi G, Rapisarda F, Cicchetti T, Maimone I, Enia G, Postorino M, Santoro D, Fuiano G, De Nicola L, Conte G, Zoccali C: Long-term visit-to-visit office blood pressure variability increases the risk of adverse cardiovascular outcomes in patients with chronic kidney disease. Kidney Int 84: 381–389, 2013. 10.1038/ki.2013.132 [DOI] [PubMed] [Google Scholar]
- 36.Nagai M, Kario K: Visit-to-visit blood pressure variability, silent cerebral injury, and risk of stroke. Am J Hypertens 26: 1369–1376, 2013. 10.1093/ajh/hpt167 [DOI] [PubMed] [Google Scholar]
- 37.Okada R, Okada A, Okada T, Nanasato M, Wakai K: Visit-to-visit blood pressure variability is a marker of cardiac diastolic function and carotid atherosclerosis. BMC Cardiovasc Disord 14: 188, 2014. 10.1186/1471-2261-14-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossignol P, Kessler M, Zannad F: Visit-to-visit blood pressure variability and risk for progression of cardiovascular and renal diseases. Curr Opin Nephrol Hypertens 22: 59–64, 2013. 10.1097/MNH.0b013e32835b489f [DOI] [PubMed] [Google Scholar]
- 39.Song H, Wei F, Liu Z, Zhao Y, Ye L, Lu F, Zhang H, Diao Y, Qi Z, Xu J: Visit-to-visit variability in systolic blood pressure: Correlated with the changes of arterial stiffness and myocardial perfusion in on-treated hypertensive patients. Clin Exp Hypertens 37: 63–69, 2015. 10.3109/10641963.2014.897724 [DOI] [PubMed] [Google Scholar]
- 40.Lattanzi S, Viticchi G, Falsetti L, Buratti L, Luzzi S, Provinciali L, Silvestrini M: Visit-to-visit blood pressure variability in Alzheimer disease. Alzheimer Dis Assoc Disord 28: 347–351, 2014. 10.1097/WAD.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 41.Ogliari G, Smit RA, Westendorp RG, Jukema JW, de Craen AJ, Sabayan B: Visit-to-visit blood pressure variability and future functional decline in old age. J Hypertens 34: 1544–1550, 2016. 10.1097/HJH.0000000000000979 [DOI] [PubMed] [Google Scholar]
- 42.Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, Sattar N, Jukema JW, van Osch MJ, van der Grond J, van Buchem MA, Westendorp RG, de Craen AJ, Mooijaart SP: Association of visit-to-visit variability in blood pressure with cognitive function in old age: Prospective cohort study. BMJ 347: f4600, 2013. 10.1136/bmj.f4600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Download Supplemental Material, PDF file, 52 KB (60.5KB, pdf)