Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, multisystem inflammatory syndrome in children, pediatric intensive care, pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2, severe acute respiratory syndrome coronavirus-2
OBJECTIVES:
To 1) analyze the short-term biochemical improvements and clinical outcomes following treatment of children with post-severe acute respiratory syndrome coronavirus-2 inflammatory syndrome (multisystem inflammatory syndrome in children/pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2) admitted to U.K. PICUs and 2) collate current treatment guidance from U.K. PICUs.
DESIGN:
Multicenter observational study.
SETTING:
Twenty-one U.K. PICUs.
PATIENTS:
Children (< 18 yr) admitted to U.K. PICUs between April 1, 2020, and May 10, 2020, fulfilling the U.K. case definition of pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Routinely collected, deidentified data were analyzed. Propensity score and linear mixed effects models were used to analyze the effect of steroids, IV immunoglobulin, and biologic agents on changes in C-reactive protein, platelet counts, and lymphocyte counts over the course of PICU stay. Treatment recommendations from U.K. clinical guidelines were analyzed. Over the 6-week study period, 59 of 78 children (76%) received IV immunoglobulin, 57 of 78 (73%) steroids, and 18 of 78 (24%) a biologic agent. We found no evidence of a difference in response in clinical markers of inflammation between patients with multisystem inflammatory syndrome in children/pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 who were treated with IV immunoglobulin, steroids, or biologics, compared with those who were not. By the end of the study period, most patients had received immunomodulation. The 12 patients who did not receive any immunomodulators had similar decrease in inflammatory markers as those treated. Of the 14 guidelines analyzed, the use of IV immunoglobulin, steroids, and biologics was universally recommended.
CONCLUSIONS:
We were unable to identify any short-term benefit from any of the treatments, or treatment combinations, administered. Despite a lack of evidence, treatment guidelines for multisystem inflammatory syndrome in children/pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 have become very similar in advising step-wise treatments. Retaining clinical equipoise regarding treatment will allow clinicians to enroll children in robust clinical trials to determine the optimal treatment for this novel important condition.
Following the first wave of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections in the United Kingdom, cases emerged of children presenting to PICUs with multisystem inflammation, a novel condition which has now been termed as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the United Kingdom (1) and multisystem inflammatory syndrome in children (MIS-C) in the United States (2).
The definitions of PIMS-TS and MIS-C are very similar (3). The major difference is that the diagnosis of MIS-C requires antibody or polymerase chain reaction evidence of SARS-CoV-2 infection, or known close contact with a SARS-CoV2 patient, whereas PIMS-TS merely needs to be “temporally associated with the SARS-CoV-2 pandemic.” We use the nomenclature MIS-C/PIMS-TS throughout this article.
A number of studies have described the presenting clinical and laboratory features of MIS-C/PIMS-TS (4–6). In our U.K. cohort study of 78 critical care patients with MIS-C/PIMS-TS (7), patients received various immunomodulatory treatments including IV immunoglobulin (IVIG), steroids, biological agents, and antiplatelet therapy; by the end of the study period, treatment choices rapidly coalesced toward an orthodoxy, with the majority of patients receiving such treatments. This has continued, with Centers for Disease Control and Prevention data showing 88% of severely affected MIS-C patients receiving IVIG, and 73% received steroids (8). Although there is a clinical overlap with other childhood conditions such as Kawasaki disease (KD) and toxic shock syndrome (4), the effectiveness of using a similar management strategy for this distinct emerging inflammatory condition remains unknown, and outcome data are urgently required. To date, there have been no published clinical trials of any of these treatments in this novel condition, although the Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial began recruiting children with MIS-C/PIMS-TS in August 2020, after our data collection period (9).
Even though long-term outcome data for children with MIS-C/PIMS-TS are lacking, given the biochemical evidence of intense inflammation at presentation, it is plausible that normalization of inflammatory markers may represent surrogate endpoints for treatment efficacy. Although there is currently no evidence in MIS-C/PIMS-TS, persistence of inflammation in KD correlates with increased risk of coronary artery aneurysms, the most severe sequelae, and the aim of treatment in KD is to suppress inflammation, as measured by biochemical markers including C-reactive protein (CRP) and neutrophil count (10, 11). Lymphopenia (12) or abnormal platelet counts (13) are poor prognostic factors in sepsis.
Our primary objective was to identify whether the use of immunomodulatory therapies was associated with improvement in biochemical markers of inflammation (normalization of CRP, lymphocyte count, and platelet count) in critical care patients with MIS-C/PIMS-TS. We analyzed associations without the ability to test causal inference. Our secondary objective was to review the available MIS-C/PIMS-TS guidance published by U.K. PICUs with regard to immunomodulation and anticoagulation in order to provide context and understanding of the evolution of treatment in this emerging condition.
MATERIALS AND METHODS
We performed a multicenter observational study of children less than 18 years old, admitted to U.K. PICUs over a 40-day period (April 1, 2020, to May 10, 2020), who fulfilled the U.K. Royal College of Paediatrics and Child Health case definition of PIMS-TS (1). This is a secondary analysis of a previously described cohort (7, 14). Initial presenting features of 29 of these patients have been previously reported focusing on the definition of this novel condition (4) (eight of these 29 patients had previously been described in a correspondence article [6]). Cardiac features in six patients (15), renal features in 23 patients (16), and critical care course in 10 patients (17) have also been presented in single-center reports. The project was classified as a service evaluation by the Nottingham Research and Innovation team (Nottingham Clinical Effectiveness Team ref: 20-235C), and ethics approval was not required. The study team analyzed routinely collected deidentified data submitted by clinicians from the individual PICUs as a local service evaluation. Clinicians obtained informed parental consent if required locally. Data were submitted for central analysis using a secure, web-based survey tool (Surveymonkey, San Mateo, CA) and included demographic details, presenting clinical features, underlying co-morbidities, laboratory markers during the first 5 days of critical care admission, echocardiographic findings, interventions, treatments, and outcome (length of PICU stay). SARS-CoV-2 serology information was collected if available.
In this study, we focused on IVIG, steroids, and biologics as these are immunomodulatory therapies which were most likely to affect the clinical and laboratory outcomes of interest. We divided children into three groups (no treatment, one or two treatments, or all three treatments based on receipt of steroids, IVIG or biologics) and compared baseline data between the groups using a Kruskal-Wallis test (continuous statistics) or a three-sample test for equality of proportions (categorical statistics). The outcome measures of interest were the length of PICU stay and the trajectories of CRP, platelet counts, and lymphocyte count. We evaluated the overall association of the treatment groups using the Fisher exact test and length of stay using the Mann-Whitney U test. We used several strategies to model the response of the inflammatory markers (CRP, lymphocyte and platelet counts) depending upon the treatment administered (steroids, IVIG, or biologics; the explanatory variables). We undertook an analysis, matching treatment to no treatment by propensity score. However, this approach had several limitations including a high risk of type II error (supplementary file 1, http://links.lww.com/PCC/B739). We therefore used linear mixed effects models (LMEMs) to compare the effects of treatments on the trajectories of the patients’ CRP, lymphocyte, and platelet counts over the first 5 days of the admission. LMEMs allowed flexible modeling of multiple measurements per participant. We developed two models for each outcome measure, one which investigated the association of treatment with outcome, and a second which also adjusted for baseline age, sex, and inotrope prescription. Model fit was assessed by either likelihood ratio test (considering p < 0.05 as significant) or a bootstrap likelihood ratio test. Data analysis used R (R project for statistical computing, Vienna, Austria). Full model specifications, including the R code, is in supplementary file 1 (http://links.lww.com/PCC/B739).
All pediatric critical care units that reported having patients with MIS-C/PIMS-TS in the United Kingdom were contacted on July 29, 2020, to share any guidelines which had been produced to date. Two investigators independently extracted guideline statements or recommendations. Treatment advice including doses, frequency, and eligible patient groups (including whether therapies should only be given as part of a trial) were summarized. Dates of internal or website publication for each guideline were recorded.
RESULTS
During the study period, data on 78 patients admitted to PICUs and meeting the case definition for PIMS-TS were submitted. Data are reported for the first 5 days of admission to the PICU. Survival to PICU discharge was 76 of 78 (97%).
Over the 6-week study period, 59 of 78 (76%) children received IVIG, 57 of 78 (73%) steroids, and 19 of 78 (24%) biologic agents. Fifty-one of 78 (65%) received both IVIG and steroids. Fourteen received all three therapies, and 12 patients received none of the above treatments. Biological agents received were anakinra (8 patients), infliximab (9 patients), tocilizumab (3 patients), and rituximab (1 patient) with two patients having two agents (anakinra/infliximab and anakinra/rituximab). One child was treated with remdesivir. Baseline characteristics of patients based on the treatment group are shown in Table 1. PICU survival was 76 of 78 (97%). Median overall length of stay was 5.0 days (IQR, 3.0–6.5 d). There was a difference in median length of intensive care stay between those not given biologics (5 d) and those given (6 d), but all the other groups showed no difference (Table 2).
TABLE 1.
Baseline Characteristics
| Variables | None of IVIG, Steroids, or Biologics, n = 12 | One or Two of IVIG, Steroids, or Biologics, n = 52 | All of IVIG, Steroids, and Biologics, n = 14 | p |
|---|---|---|---|---|
| Age, yr, median (IQR) | 10.3 (8.0–15.0) | 10.8 (7.0–13.0) | 12.0 (9.5–14.8) | 0.24 |
| Female, n (%) | 7/12 (58.3) | 17/52 (32.7) | 2/14 (14.3) | 0.06 |
| C-reactive protein (mg/L), median (IQR) | 213 (123–268) | 251 (168–312) | 292 (187–316) | 0.38 |
| Platelet count (cells × 109/L), median (IQR) | 177 (91–204) | 161 (111–232) | 183 (129–225) | 0.38 |
| Lymphocyte count (cells × 109/L), median (IQR) | 0.8 (0.7–0.9) | 1.1 (0.7–1.8) | 0.8 (0.4–0.9) | 0.03 |
| Received invasive ventilation, n (%) | 5/12 (42) | 27/52 (52) | 4/14 (29) | 0.28 |
| Received inotropes, n (%) | 7/12 (58) | 47/52 (88) | 12/14 (86) | 0.04 |
IQR = interquartile range, IVIG = IV immunoglobulin.
Categorical variables were compared with a three-sample test for equality of proportions, and continuous variables were compared with a Kruskal-Wallis test.
Boldface values indicate p < 0.05.
TABLE 2.
Associations Between Treatments and Length of Stay
| Treatment Given | PICU Length of Stay (d) (IQR) | p | |
|---|---|---|---|
| Whole cohort | 5 (3.0–6.5) | ||
| IV immunoglobulin | Given | 5 (3-6.5) | 0.693 |
| Not given | 5 (3.25–7) | ||
| Steroids | Given | 5 (3.25–7) | 0.414 |
| Not given | 5 (3–6) | ||
| Biologics | Given | 6 (5–7.5) | 0.0098 |
| Not given | 5 (3–6) | ||
| None | None given | 4 (2.5–5) | 0.0672 |
| ≥ 1 given | 5 (4–7) | ||
| One or two | One or two | 5 (3–7) | 0.866 |
| None or all 3 | 5 (3.75–6.25) | ||
| All three treatments | All 3 given | 6 (5–7) | 0.0535 |
| None given | 5 (3–6.25) | ||
IQR = interquartile range.
p values by two-tailed Mann-Whitney U test.
The propensity score analysis showed no treatment effect on final predischarge CRP, platelet, or lymphocyte counts (supplementary file 1, http://links.lww.com/PCC/B739). We went on to fit LMEMs for all three response variables (CRP, platelets, and lymphocytes), including models adjusted for baseline covariates. Incorporating treatments did not improve model fit (by either likelihood ratio test (considering p < 0.05 as significant) or a bootstrap likelihood ratio test), indicating no evidence that the treatments were associated with the outcome measures of patient trends in CRP, platelets, or lymphocyte count in the first 5 days of therapy. The p values for likelihood ratio tests for the unadjusted models were 0.846, 0.488, and 0.07 for CRP, lymphocyte, and platelet trajectory, respectively. For the adjusted models, the p values were 0.801, 0.393, and 0.055, respectively. We note that although the platelet model is close to our prespecified threshold of 0.05, the bootstrap likelihood ratio 95% CI includes zero (–33.06 to 101.38), and therefore, we do not consider this to be a statistically significant result (supplementary file 1, http://links.lww.com/PCC/B739).
We plotted and compared mean fitted values and 95% CIs for participants receiving: no treatment, each individual therapy of steroids, IVIG, biologics, the combination of steroids and IVIG, and all three treatments for the unadjusted model (Fig. 1) and adjusted model (Supplementary Fig. 1, http://links.lww.com/PCC/B740; legend, http://links.lww.com/PCC/B743). These plots do not show evidence of differences between the response variables for patients receiving or not receiving any of the therapies There was some suggestion of separation of curves in both adjusted and unadjusted models for the combination of IVIG and steroids, but the differences were not statistically significant.
Figure 1.
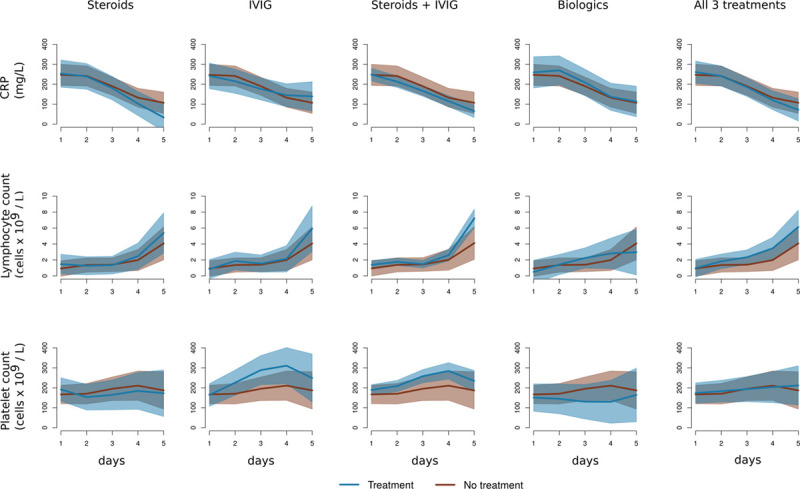
Linear mixed effect model estimates of 5 d progression of C-reactive protein (CRP), lymphocyte count, and platelet count dependent on whether the patient had been given steroids, IV immunoglobulin (IVIG), the combination of steroids plus IVIG, biologic agents, or all three treatments, compared with no treatment. Brown lines are average (mean) response with 95% CIs of no treatment, blue lines are mean and 95% CIs of treatment. The model estimates are not adjusted for baseline risk; adjusted estimates are plotted in Supplementary Figure 1 (http://links.lww.com/PCC/B740; legend, http://links.lww.com/PCC/B743).
Exploratory subgroup analysis for each individual biologic agent was limited by the number in each group; however, compared with all patient not on a specific biologic, only tocilizumab was associated with a trajectory of more rapid resolution of CRP, otherwise we found no evidence for a difference (Supplementary file 2, http://links.lww.com/PCC/B741). We caution against overinterpretation of these results.
Thirteen of 16 centers (81%) who had treated patients with MIS-C/PIMS-TS produced guidance on management between May 4, and July 23, 2020, with a 14th guideline published by a collaborative U.K. group (supplement 2, http://links.lww.com/PCC/B742). The publication of guidelines after patients had been treated may be due to the rapid nature of the emergence of the condition and the need to discuss, refine, and agree institutional approaches. The majority of guidelines advised that IVIG, steroids, and antiplatelet agents should be given to all MIS-C/PIMS-TS patients requiring PICU admission (Table 3). No guideline advised biologic agents for all PICU patients, suggesting that these should be considered for specific patient groups or within a clinical trial. There was variability in suggested therapies, dosing, and strength of recommendation between centers.
TABLE 3.
Advice Given in 14 U.K. Multisystem Inflammatory Syndrome In Children/Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2 Guidelines
| Treatment | Give to all PICU Patients, n (%) | Consider or to Specific Patients, n (%) | Only as Part of a Trial, n (%) | Do not Give, n (%) | Not Reported, n (%) |
|---|---|---|---|---|---|
| IV immunoglobulin | 9/14 (64) | 5/14 (36) | 0 | 0 | 0 |
| Steroids | 7/14 (50) | 6/14 (43) | 1/14 (7) | 0 | 0 |
| Biologics | 0 | 9/14 (64) | 2/14 (14) | 0 | 3/14 (21) |
| Antiplatelet | 11/14(79) | 3/14 (21) | 0 | 0 | 0 |
| Anticoagulation | 5/14 (50) | 5/14 (36) | 0 | 1/14 (7) | 3/14 (21) |
| Antivirals | 0 | 4/14 (28) | 0 | 0 | 10/14 (71) |
DISCUSSION
We analyzed the impact of immunomodulatory treatments on the resolution of markers of inflammation and length of stay in a large U.K. cohort of critical care admissions with PIMS-TS. We were unable to identify any clinically or statistically significant change in trajectory of selected inflammatory markers (CRP, lymphocyte count, platelet count) in patients who had individual or combination immunomodulatory treatments compared with no treatment. In a condition now widely described as inflammatory, it is biologically plausible that immunomodulation would lead to a change in trajectories of markers of inflammation, which we have chosen as our outcome measure. We have used propensity score and unadjusted and adjusted LMEMs and have not been able to demonstrate a convincing association between treatment and outcome. We do however note a trend to separation of trajectories of CRP, leucocytes, and platelet counts in the group of patients who received both steroids and IVIG compared with no treatment. However, in our view, this is hypothesis generating, and we strongly advocate testing these treatments formally in randomized trials.
Within a short duration of this condition being recognized, significant “treatment orthodoxy” occurred, where almost all treating clinicians were giving the same IVIG-steroids ± biologics combination, with increasing numbers giving biologic agents (7). This is likely due to the rapid and worldwide dissemination of opinions on treatment early in the identification of this disease with multiple international webinars (18, 19) on discussing this condition attracting several hundred attendees. Webinars and personal communication are likely to be responsible for the similarity in our management rather than medical literature as the first peer review publication describing MIS-C/PIMS-TS was published just 2 days before our last patient in the cohort was admitted to PICU (6), and only one of the guidelines was published before this date. The recommendations from the United Kingdom reflect those published elsewhere in the United States (20, 21). As this recommended approach became ingrained, a lack of perceived equipoise can make randomized trials difficult. Furthermore, the regulatory, funding, and logistical complexities of launching a high-quality randomized trial create difficulties in responding to a clinical situation moving at such high speed. Guidelines can be published immediately, whereas the construction of the much more valuable careful clinical trial usually takes significant time. One trial to date is randomizing MIS-C/PIMS-TS patient treatments (9), including a “no treatment” arm, which is fundamental to finding the effective treatments for this condition. This trial started recruiting children remarkably only 4 months after the discovery of PIMS-TS/MIS-C. Interpreting observational data is difficult as there is no control untreated arm (22).
IVIG is used in MIS-C/PIMS-TS due to the clinical overlap with KD (4). The major long-term complication of KD is coronary artery abnormalities (10, 11); a subset of the cohort with MIS-C/PIMS-TS have been shown to have coronary artery abnormalities in the short and medium term, with long-term data pending (4, 7, 21). Although there are clinical and immunologic differences between KD and MIS-C/PIMS-TS (4, 23), there is clinical overlap in presentations, and IVIG, the first-line treatment for KD, has been widely used in MIS-C/PIMS-TS. However, the evidence base for using IVIG in KD is limited, with the Cochrane review showing no significant benefit beyond the short term in the small number of randomized trials for medium-long term risk of developing coronary artery aneurysms (24). No randomized clinical trial of long-term effects of IVIG in KD has been performed since 1990 (24, 25). The use of steroids has greater evidence base (25), with a lack of evidence for salicylates (26).
Antiplatelet agents are commonly used in KD, where thrombocytosis is a common late effect (10). Thrombocytosis has not, to date, been reported in MIS-C/PIMS-TS patients. Biologic agents have been used to reduce the multisystem inflammatory response, but the variability in choice of biologics indicates uncertainty on which part of the inflammatory pathway is pathologic in MIS-C/PIMS-TS, although ongoing research is beginning to explore this area (27). Many of these treatments are very high cost and have limited availability, especially in low- and middle-income countries. Advising widespread use of these agents may cause a significant burden on such healthcare systems.
Our cohort is important in that it describes patients at the emergence of this condition, before the development of the treatment orthodoxy we described. Prior to the well-publicized case definitions, therapies given were aligned to “culture negative sepsis,” presumed viral cardiomyopathies, or other undiagnosed diseases. This gives us an uncontrolled group, albeit small, who did not receive immunomodulation to permit comparisons between treatment, course and outcome.
Our study has limitations. First, this is observational data, and we therefore cannot draw robust conclusion about the efficacy of treatments. Within the coronavirus disease 2019 pandemic, multiple observational studies have been cited when advocating for specific treatments. In contrast, we urge caution against using specific unproven treatments outside of clinical trials. We present analysis of early U.K. experience of MIS-C/PIMS-TS, where some patients tended to receive supportive care alone. Assuming that the condition did not change over the 2-month period, patients in our cohort received treatment according to the prevailing wisdom which changed over time, and we therefore present data comparing children who received no treatment against those who did. We are unable to eliminate the possibilities of confounding by indication or time.
Second, we did not have data on the timing of the treatments within the intensive care course. It is possible that the same treatment given early in the intensive care course may have different effects to if it were given late. As treatments became more standard however, it is likely that the initial treatments (except perhaps biologics) were given early in the disease.
Third, we have a relatively small cohort. Therefore, we may not be powered to detect differences in outcomes, if they exist, and our analysis is susceptible to type II error. Additionally, as we have used a fairly flexible modeling approach, given our sample size, our models may be overfitted to the data, leading to chance findings. We have undertaken multiple analyses (propensity score with genetic matching) and LMEMs both adjusted and unadjusted for baseline risk, and none of these have demonstrated convincing evidence for treatment influencing short-term outcomes.
Fourth, our “biologics” group was made up of a diverse group of medicines with differing immunologic targets. It is plausible that an effective treatment is not evident due to grouping. Our exploratory subgroup analysis did however not uncover any associations, apart from the expected drop in CRP with tocilizumab.
Finally, the mechanisms of this syndrome are not yet fully understood and the impact of such treatments on other clinical markers of treatment response (length of ventilation, change in inotrope score) as well as other markers of inflammation described in MIS-C/PIMSTS such as ferritin, troponin, brain natriuretic peptides was not examined in this analysis.
CONCLUSIONS
We found no evidence of a difference in response in biochemical markers of inflammation between patients with MIS-C/PIMS-TS who were treated with IVIG, steroids, or biologics, compared with those who were not. Despite a lack of evidence, U.K. and U.S. treatment guidelines for MIS-C/PIMS-TS have become very similar.
Clinicians treating MIS-C/PIMS-TS should retain equipoise and participate in clinical trials of treatments currently being used for this important condition, especially those with a treatment arm of supportive care alone (e.g., the RECOVERY trial).
Supplementary Material
Footnotes
Requests for data sharing to the corresponding author.
The corresponding author confirms that he had full access to all the data in the study and has final responsibility for the decision to submit for publication.
Drs. Ramnarayan and Scholefield are joint senior authors. Drs. Davies and Lillie contributed to literature search, figures, study design, data collection, data analysis, data interpretation, and writing. Dr. Prayle contributed to literature search, figures, study design, data analysis, data interpretation, and writing. Dr. Evans contributed to literature search, study design, data collection, data interpretation, and writing. Dr. Griffiths contributed to literature search, figures, data collection, data interpretation, and writing. Drs. du Pré, Johnson, Krishnan Kanthimathinathan, Playfor, Deep, Brierley, Waters, Mohammad, Singh, Jardine, Ross, Shetty, Worrall, Sinha, and Koul contributed to literature search, data collection, and writing. Drs. Whittaker and Vyas contributed to literature search, data interpretation, and writing. Drs. Padmanabhan and Scholefield contributed to literature search, study design, data collection, data analysis, data interpretation, and writing.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Dr. Prayle’s institution received funding from the Thrasher Research Fund, Action for Ataxia-Telangiectasia, and the Sir Jules Thorn Trust, and he disclosed the off-label product use of drugs for multisystem inflammatory syndrome in children/pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2. All authors have completed the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Royal College of Paediatrics and Child Health (RCPCH): Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated With COVID-19. 2020. Available at: https://%20inflammatory%20syndrome-20200501.pdf. Accessed July 30, 2020
- 2.Centers for Disease Control and Prevention: Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). CDCHAN-00432, 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed July 30, 2020
- 3.Kanthimathinathan HK, Scholefield BR: Pediatric inflammatory multisystem syndrome: Time to collaborate. J Pediatric Infect Dis Soc 2020. Sep 18. [online ahead of print 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia: Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team: Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020; 383:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. : Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies P, Evans C, Kanthimathinathan HK, et al. : Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc Health 2020; 4:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfred-Cato S, Bryant B, Leung J, et al. ; California MIS-C Response Team: COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RECOVERY: Randomised Evaluation of COVID-19 Therapy. 2020. Available at: https://www.recoverytrial.net/files/recovery-protocol-v8-0-2020-07-08.pdf. Accessed September 10, 2020
- 10.Yeung R: Elzouki A, Harfi H, Nazer H, et al. (Eds). Kawasaki disease. In: Textbook of Clinical Pediatrics. 2012, pp Berlin, Heidelberg, Springer-Verlag, 1675–1684 [Google Scholar]
- 11.McCrindle BW, Rowley AH, Newburger JW, et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention: Diagnosis, treatment, and long-term management of kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–e999 [DOI] [PubMed] [Google Scholar]
- 12.Drewry AM, Samra N, Skrupky LP, et al. : Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014; 42:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Zhao L, Wang YY, et al. : Platelets as a prognostic marker for sepsis: A cohort study from the MIMIC-III database. Medicine (Baltimore) 2020; 99:e23151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deep A, Upadhyay G, du Pré P, et al. : Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 pandemic: Experience from PICUs across United Kingdom. Crit Care Med 2020; 48:1809–1818 [DOI] [PubMed] [Google Scholar]
- 15.Ramcharan T, Nolan O, Lai CY, et al. : Paediatric inflammatory multisystem syndrome: Temporally associated with SARS-CoV-2 (PIMS-TS): Cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 2020; 41:1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart D, Harley J, Johnson M, et al. : Renal dysfunction in hospitalised children with COVID-19. Lancet Child Adolesc Health 2020; 4:e28–e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richens N, Kanthimathinathan H, Sontakke S, et al. : Critical care course of pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 and response to immunomodulation. J Pediatr Intensive Care December 2020, DOI: 10.1055/s-0040-1721456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.South Thames Paediatric Network [webinar]: Paediatric Multisystem Inflammatory Syndrome Associated With COVID-19, 2020
- 19.Paediatric Intensive Care Society [webinar]: Paediatric Inflammatory Multi-System Syndrome (MIS-C/PIMS-TS), 2020
- 20.Henderson LA, Canna SW, Friedman KG, et al. : American College of Rheumatology Clinical Guidance for pediatric patients with multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 and hyperinflammation in COVID-19. Version 1. 2020 Jul 23. [online ahead of print] Arthritis Rheumatol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennon T, Penque M, Hicar M, et al. : COVID-19 associated multisystem inflammatory syndrome in children (MIS-C) guidelines; a Western New York approach. Prog Pediatr Cardiol 2020; 101232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bates D, Machler M, Bolker B, et al. : Fitting linear mixed effects models using lme4. J Stat Softw 2015; 67:1–48 [Google Scholar]
- 23.Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team: The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell 2020; 183:968–981.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oates-Whitehead RM, Baumer JH, Haines L, et al. : Intravenous immunoglobulin for the treatment ofKawasaki disease in children. Cochrane Database Syst Rev 2003; 2003:CD004000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wardle AJ, Connolly GM, Seager MJ, et al. : Corticosteroids for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev 2017; 1:CD011188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumer JH, Love S, Gupta A, et al. : Salicylate for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev 2006; 4:CD004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter MJ, Fish M, Jennings A, et al. : Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 2020; 26:1701–1707 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


