Abstract
Background
The dental office potentially possesses all transmission risk factors for severe acute respiratory syndrome coronavirus 2. Anticipating the future widespread use of COVID-19 testing in dental offices, the authors wrote this article as a proactive effort to provide dental health care providers with current and necessary information surrounding the topic.
Methods
The authors consulted all relevant and current guidelines from the Centers for Disease Control and Prevention and the US Food and Drug Administration, as well as online resources and review articles.
Results
Routine COVID-19 screening and triage protocols are unable to detect all infected people. With the advancements in diagnostic tools and techniques, COVID-19 testing at home or in the dental office may provide dentists with the ability to evaluate the disease status of their patients. At-home or point-of-care (POC) tests, providing results within minutes of being administered, would allow for appropriate measures and rapid decisions about dental patients' care process. In this review, the authors provide information about available laboratory and POC COVID-19 screening methods and identify and elaborate on the options available for use by dentists as well as the regulatory requirements of test administration.
Conclusions
Dentists need to be familiar with COVID-19 POC testing options. In addition to contributing to public health, such tests may deliver rapid, accurate, and actionable results to clinical and infection control teams to enhance the safe patient flow in dental practices.
Practical Implications
Oral health care must continue to offer safety in this or any future pandemics. Testing for severe acute respiratory syndrome coronavirus 2 at the POC offers a control mechanism contributing to and enhancing the real and perceived safety of care in the dental office setting.
Key Words: SARS-CoV-2, point-of-care testing, COVID-19 testing, oral fluids, antigen, antibody, saliva, aerosols, surveillance, dentistry
Abbreviation Key: ADA, American Dental Association; CDC, Centers for Disease Control and Prevention; cDNA, Complementary DNA; CLIA, Clinical Laboratory Improvement Amendments; FDA, Food and Drug Administration; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; NAAT, Nucleic acid amplification test; PCR, Polymerase chain reaction; POC, Point-of-care; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
The dental setting is a unique environment in the COVID-19 pandemic because it potentially possesses all transmission risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as stated by the Centers for Diseases Control and Prevention (CDC).1 The infection occurs when the virus is inhaled or deposited on mucous membranes including the nose and mouth.2 As of April 2021, there are no direct data available to assess the risk of experiencing SARS-CoV-2 transmission in dental practice (that is, patient to patient, provider to patient, patient to provider, different procedures). Irrespective of the path of transmission, an estimated 0.9% of US dentists3 and an estimated 3.1% of US dental hygienists tested postive for SARS-CoV-2.4
In view of the advancements in diagnostic and screening tools and techniques for COVID-19, dental practices may directly benefit from the ability to evaluate the disease status of patients while contributing to broader public health efforts. Rapid point-of-care (POC) tests provide results within minutes, allowing for appropriate measures and rapid decisions about next steps in the care process. SARS-CoV-2–induced COVID-19 represents only the latest manifestation of a 150-year history of epidemic disease patterns and transmission of contagious diseases. As such, the rapid screening and diagnostics testing approaches created or adapted for COVID-19 will be applicable for future viral pathogens, and thus, the dental office should be prepared to adopt standardized work flows as common future practice.
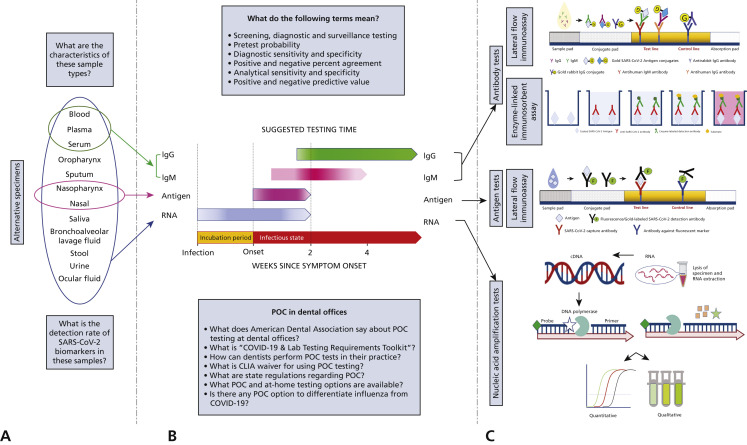
Anticipating the current and future need for pathogen testing in the dental offices, in this report we aim to provide the latest available laboratory methods for COVID-19 screening and diagnosis and to identify and further elaborate on the options available for use by dentists as well as the regulatory requirements of administration of those tests. Fundamentals of testing for COVID-19 detection is summarized in Figure 1 .
Figure 1.
Fundamentals of COVID-19 testing. A. COVID-19 biomolecules can be detected in oral fluids as well as other sites and specimens. A detailed description and comprehensive review of these specimens' characteristics and viral loads and detection rates of COVID-19 is provided by Shirazi and colleagues.2B. Schematic representation of detection window for COVID-19 biomarkers. The best detection and testing time for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA and antigen is up to 14 days from symptom onset. Immunoglobulin M (IgM) antibody against SARS-CoV-2 is detectable days after symptom onset, peaks during week 2, and will start to disappear at approximately week 4. Immunoglobulin G (IgG) against SARS-CoV-2 antibody will be detectable at approximately week 2 and persist for an unknown time. The duration of persistence of IgG antibody is not fully clear yet. C. COVID-19 biomarkers are detected with various laboratory methods that have different mechanism of action. Figure 2, Figure 3, Figure 4 include detailed description of each method. POC: Point-of-care.
Essential terminology to better understand and use COVID-19 resources
Screening testing
This type of testing is performed to identify infected people before development of symptoms or infected people without signs or symptoms (asymptomatic) or suspected exposure to COVID-19, who may be contagious to prevent the spread of virus. Examples of screening include testing plans developed by a workplace to test all employees returning to the workplace regardless of exposure or signs and symptoms. These tests are designed to have a higher false-positive rate (lower specificity) but high sensitivity (low false-negative rates) with the desire to screen for and capture those with the disease at the risk of involving those who are uninfected with more invasive testing.
Diagnostic testing
These types of tests also look for occurrence at the individual level but are performed when there is a particular reason to suspect that a person may be infected. They are performed when a person has signs or symptoms consistent with COVID-19, when a person is asymptomatic but has a recent known or suspected exposure to COVID-19, or when testing to determine resolution of infection. These are designed with high sensitivity (high true positive and low false negative) and high specificity (high true negative and low false positive) in populations considered at high risk of developing the disease. As implied, if the test is applied to a high-risk population, then a positive test will have a greater positive predictive value than the same result in a population in which there is a low disease prevalence (and thus a much higher rate of false-positive values).
Surveillance testing
This type of test is used to monitor population- or community-level outbreaks of COVID-19 or to characterize the incidence and prevalence of COVID-19. These are performed on deidentified specimens (typically, pooled), and the results are not linked to individual people. Thus, surveillance testing is used for health policy and not patient care. It may be a random sampling of a certain percentage of a specific population to monitor for increasing or decreasing prevalence and determining the population effect from community interventions such as social distancing. Table 1 provides a summarized comparison of different testing strategies.
Table 1.
Comparison of different testing strategies for COVID-19.
| CONSIDERATIONS FOR EACH STRATEGY |
TESTING STRATEGY |
||
|---|---|---|---|
| Screening | Diagnostic | Surveillance | |
| For asymptomatic person without known or suspected exposure | Yes | No | NA∗ |
| For symptomatic person or known or suspected exposure | No | Yes | NA |
| To characterize incidence and prevalence in the community | NA | NA | Yes |
| Results may be returned to people | Yes | Yes | No |
| Results are returned in aggregate to requesting institution | No | No | Yes |
| Results are reported to state public health department | Yes | Yes | If requested |
| Testing can be performed in a Clinical Laboratory Improvement Amendments-certified laboratory | Yes | Yes | Yes |
| Testing can be performed in a non Clinical Laboratory Improvement Amendments-certified laboratory | No | No | Yes |
| Test system must be Food and Drug Administration authorized or be offered under the policies in Food and Drug Administration’s guidance | Yes | Yes | No |
NA: Not applicable.
Pretest probability
A Bayesian statistical approach uses the likelihood the person being tested really has the infection before the test, a test is performed, and the pre- and posttest probabilities are compared creating a likelihood index. Thus, a diagnostic test performs optimally for detecting an “infection” when the pretest probability is high (meaning that in the test population there is already a high rate of disease). The pretest probability increases with increasing prevalence in the population, and clinical indications or symptoms of illness in the person being tested. In contrast, tests typically perform best for excluding an infection when the pretest probability is low.
Diagnostic sensitivity and specificity
Sensitivity is the ability of a test to correctly identify people with infection and is the proportion of true positive results to the sum of true-positive and true-negative results. Specificity is the ability to correctly rule out infection and is the proportion of truenegative results to the sum of true-negative and false-positive results. False-negative results are the most consequential, and thus screening tests are based to capture those at risk.
Positive and negative percentage agreement
In new situations like the COVID-19 pandemic in which there is no clinical reference standard test with 100% sensitivity and specificity, the sensitivity and specificity of a new test will be evaluated against an imperfect reference standard and will be respectively reported as positive percentage agreement (the proportion of people with the target condition by the imperfect reference standard who test positive) and negative percentage agreement (the proportion of people free of the target condition by imperfect reference standard who test negative). The challenge is that the benchmark for the positive percentage agreement will change over time, so this is at best a field measure supporting just-in-time clinical decision making.
Analytical sensitivity
Analytic sensitivity is also referred to as limit of detection and is the minimum amount of SARS-CoV-2 viral particle count detected or quantitatively determined greater than 95% of the time by means of the used test.
Analytical specificity
Analytic specificity is the ability of a specific test platform to detect its intended target (that is, SARS-CoV-2). Cross-reactivity and target interference affect analytical specificities. Cross-reactivity occurs when a patient’s sample contains SARS-CoV-2 as well as other genetically related organisms, and the assay’s analytic reagents (for example, polymerase chain reaction primers or antibodies) cross-react or anneal to sequences or antigens of these organisms, generating a false-positive results. Interference occurs when the specimen is contaminated with endogenous elements, such as hemoglobin or medications, or exogenous inhibitory substances, such as lipstick, hand cream, glove powder, and serum separators. These inhibit chemical and biochemical reactions essential to the test and increase false-negative results.
Positive predictive value
Positive predictive value is the probability a patient with a positive test result most likely has the infection (or the test target). It is affected by general prevalence, test sensitivity, and pretest probability in the population of interest. In a high-prevalence setting, the positive predictive value increases, meaning it is more likely people testing positive are truly positive for disease (or appropriately reactive in the case of an antibody test platform). In turn, as prevalence in the population declines, false-positive rates will increase as they are inversely related. Test specificity and pretest probability have the greatest impact on false-positive rates. As the pretest probability and the specificity of the test increases, the false-positive rate decreases and the positive predictive value increases. When a test is used in a population in which prevalence is low, the positive predictive value drops because there are more false-positive results, since the pretest probability is low.
Negative predictive value
Negative predictive value is the possibility that a patient with a negative test result most likely does not have the infection. It is affected by pretest probability and prevalence. In a high-prevalence setting, the negative predictive value declines, whereas in a low-prevalence setting it increases. Pretest probability and test sensitivity have the greatest impact on false-negative rates. As the pretest probability decreases, the false-negative rate decreases and the negative predictive value increases. As the sensitivity of the test increases, the false-negative rate decreases and the negative predictive value increases. Table 2 shows the relationship between test characteristics and test outcome.
Table 2.
Crosstalk between test characteristics and test results.
| PRETEST PROBABILITY∗ | or | POSITIVE PREDICTIVE VALUE† | or | NEGATIVE PREDICTIVE VALUE† | OUTCOME |
|---|---|---|---|---|---|
| Low | Low | High | Increased chance of true-negative or false-positive results | ||
| High | High | Low | Increased chance of true-positive or false-negative results |
Specificity and sensitivity of tests are not affected by the pretest probability.
Pretest probability affects predictive values.
Methods
We reviewed the CDC guidelines on COVID-19 for up-to-date (as of April 5, 2021) information regarding testing options and related matters.5 , 6 We consulted the US Food and Drug Administration (FDA) website for the latest authorized in vitro diagnostic tests.7, 8, 9 We searched PubMed (MEDLINE) for review articles regarding mechanism of action of different testing options.
Alternative Methods for Laboratory and POC Detection of SARS-CoV-2
Laboratory testing for SARS-CoV-2 involves detection of the viral RNA using nucleic acid amplification tests (NAATs), of the viral structural (or nucleocapsid) proteins using antigen tests or of the virus-specific human adaptive immune response (antibodies) using immunoassays ( Figure 1C, Figure 2, Figure 3, Figure 4 ). An optimum test for direct diagnosis of SARS-CoV-2 infection must have low infrastructural requirements, short turnaround time, minimum batching, high throughput, high accuracy, and low cost to allow public access.10 Table 3 summarizes the important aspects of each testing method.
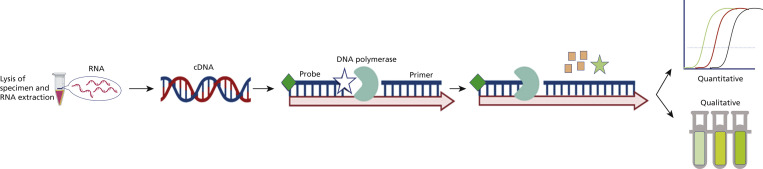
Figure 2.
Mechanism of action of nucleic acid amplification tests (NAATs). NAATs generally require 5 steps, which are modified or merged in different NAATs. The 5 steps are lysis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral particles in the sample; purification of the viral RNA; reverse transcription of the RNA to complementary DNA (cDNA), which produces DNA templates for the next step; thermocyclic or isothermal amplification of specific regions (target genes) of the cDNA using gene-specific primers, DNA polymerases, and probes to amplify only the selected region; and detection of the amplified cDNA, which can happen during or after step 4. The difference among various NAATs arise from modification of these steps. Some NAATs do not require separate lysis and purification steps, whereas some others merge reverse transcription and amplification. Analytical accuracy of COVID-19 NAATs depends mainly on the primer or probe design, and different assays use different primer or probe sets targeting different regions of the SARS-CoV-2 genome. Some NAATs, including isothermal methods, are authorized for point-of-care COVID-19 diagnostics and provide fast, sensitive, high-efficiency, and cost-effective results without need for specialized equipment.
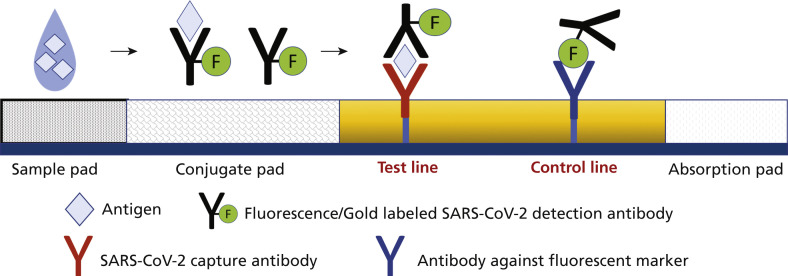
Figure 3.
Mechanism of action of antigen tests. Point-of-care antigen tests typically are lateral flow assays and come with a test strip or cassette composed of multiple overlapping pads. The clinical sample needs to be placed in a tube containing the extraction reagent after collection, which disrupts the viral particle and exposes the viral proteins. Next, the sample is added to the sample pad, where it starts to travel via capillary action through the conjugate pad and conjugates with the fluorescence-labeled severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection antibody or the detector-antibody gold conjugate. Next, it flows through an analytical membrane striped with a capture antibody specified as the test line. Most tests include a control line after the test line to validate proper fluid flow and the activity of assay reagents. The final outcome might be read via an instrument or displayed as colored lines for naked-eye reading.
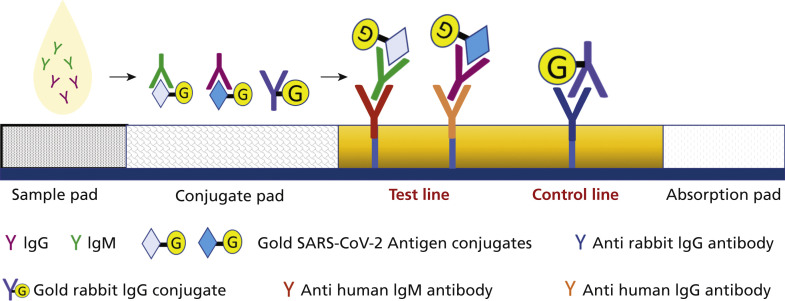
Figure 4.
Mechanism of action of antibody tests. The authorized point-of-care serologic tests commonly are based on lateral flow immunoassay, which detect binding antibodies. Lateral-flow immunoassays are composed of a cassette that encloses strip membranes. After the specimen is added to the sample pad, it moves forward by capillary action through the conjugated pad, where the present immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in the sample interact with impeded gold conjugated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens and rabbit-gold conjugated antibodies. The created antigen-antibody complexes move forward to the analytical membrane and bind antihuman IgG and IgM antibodies (capture antibodies) at the test line and get immobilized, whereas the rabbit-gold conjugate antibodies bind to antirabbit IgG antibodies at the control line and get immobilized. The result will be visible as colored lines owing to the accumulation of gold particles. Depending on whether the test is for IgM- or IgG-class antibodies or both, the test may show 1, 2, or 3 stripes.
Table 3.
Comparison of different testing options for COVID-19.
| DESCRIPTION | NUCLEIC ACID AMPLIFICATION TEST | ANTIGENT TEST | ANTIBODY TEST |
|---|---|---|---|
| Test Type | Viral, molecular | Viral, immunoassay | Serologic, immunoassay |
| Purpose | Diagnostic | Diagnostic, screening | Screening |
| Intended Use | Detect current infection | Detect current infection | Detect prior or recent infection |
| Analyte Detected | Viral RNA | Viral structural proteins | Virus-specific antibodies |
| Sensitivity | Generally high | Moderate to high | Moderate to high |
| Specificity | High | High | High |
| Test Complexity | Differs by test | Relatively easy to use | Relatively easy to use |
| Turnaround Time | 15 min to > 2 d | 15 min to > 2 d | 15 min to > 2 d |
| Operating Temperature | Need thermal cycling—some are isothermal | Ambient | Ambient |
| Results Presentation | Quantitative or qualitative | Visual or instrument read | Visual or instrument read |
| Authorized for Point-of-careUse | Some tests | Most tests | Some tests |
| Authorized for Use at Home | Very few, sample collection is possible at home for many tests | Some tests | No |
| Suggested Test Time | 1 d after infection to 14 d from symptom onset | 5-7 d from symptom onset | ≥ 15 d from symptom onset |
| Cost per Test | Moderate (≈ $100-$200) | Low (≈ $5-$50) | Moderate (≈ $100-$200) |
| Authorized Specimen Type | Nasopharyngeal, oropharyngeal, nasal, sputum, saliva | Nasopharyngeal, nasal | Whole blood, serum, plasma, fingerstick blood |
| Transport Medium | Viral transport medium or saline—nothing needed for some tests | Extraction medium | Anticoagulant—nothing needed for fingerstick tests |
| Disadvantages | High requirements for operators and laboratory conditions. High cost, complexity, and turnaround time | Less sensitive in the early stage of disease | Lower sensitivity and specificity. Cross-reactivity. Long window period |
Molecular diagnostic tests
The recommended test platform for diagnosis of SARS-CoV-2 infection involves detection of viral RNA using NAATs. The FDA-authorized methods in this category use a variety of platforms. A detailed description of NAAT-based techniques has been widely discussed in the literature.11, 12, 13, 14, 15, 16, 17, 18 Figure 2 shows a simplified and general process of NAATs.
A single negative NAAT result does not exclude infection, and factors such as exposure risk and potential laboratory errors must be considered. False-negative results may occur if the specimen was obtained too early after exposure or was not collected, transported, handled, or treated properly; if the nucleic acid extraction step was improper; or if the sample contained amplification inhibitors or an insufficient amount of virus. The sensitivity of NAATs depends greatly on the sample type (that is, nasopharyngeal, saliva), stage of the infection, viral load, and time of collection in relation to the course of disease.15 , 19 Figure 1B depicts the suggested time for the use of different tests.
A positive result is only indicative of the presence of SARS-CoV-2 RNA (including degraded viral RNA) and not an infectious (contagious) state. The viral RNA may be detectable for several days to several weeks after the entry of live virus. Assays using droplet digital polymerase chain reaction (PCR), a method enabling high throughput and absolute quantification of rare targets within a biological sample, detect intact SARS-CoV-2 RNA and can detect potentially infectious clinical COVID-19 samples. A false-positive result may occur in which the pretest probability or the prevalence of infection is low, or the false-positive result may be due to technical errors, the cross-reactivity of primers, cross-contamination of the sample during its handling or preparation, or contamination of reagents in the laboratory.14 , 15 , 19, 20, 21 Thus, clinical correlations with signs and symptoms, patient history, days from symptom onset, and relevant diagnostic information are necessary to evaluate a patient's infection status and risk. Nevertheless, the infectivity period has been established at 10 days from the first positive molecular test in asymptomatic patients or an additional 3 days without symptoms in patients with clinical manifestations.22 None of the COVID-19 vaccines authorized or in development as of April 2021 will result in a positive result in NAATs because they do not contain live virus or its RNA fragments.
Antigen diagnostic tests
Antigen tests directly detect viral structural proteins without any amplification steps and do not require trained personnel and special laboratory instruments or reagents. The tests use antibodies reactive to SARS Co-V-2 proteins to detect infection. Although they are rapid, simple, and relatively inexpensive immunoassays, they only reveal the active, current COVID-19 infection and level of contagiousness possibility. A description of methods for antigen detection has been described in the literature.14, 15, 16, 17, 18
The authorized antigen tests are performed only on nasopharyngeal or nasal swab specimens. They are not restricted to specific age groups, and many can be used at POC with results in about 15 minutes. They can be used in screening or diagnostic testing situations in which a person is suspected for COVID-19 or has had a known exposure to someone with COVID-19. They also can be used as screening tests on asymptomatic people to detect or exclude COVID-19 or to determine whether a person who previously was diagnosed with COVID-19 remains infectious. Screening testing with antigen tests is also beneficial when repeat testing could identify quickly people with a SARS-CoV-2 infection, thus alerting health care workers to institute prevention and control measures. There is value in providing immediate results with antigen tests when a rapid test turnaround time is required, even though they may have lower sensitivity than NAATs.23 Figure 3 describes a simplified process of antigen tests.
Inherently, antigen-screening platforms are less sensitive than genetic PCR-based detection platforms (for example, NAATs) but have a similarly high specificity. They are less reliable in the clinical diagnosis of COVID-19 patients with low viral load. The antigen test’s best clinical performance is during early symptomatic infection, when the viral load is considered to be the highest (Figure 1B).14 , 18
One limitation of antigen testing is the potential for higher false-negative results. False-negative results occur owing to inherent low sensitivity, if the antigen level in specimens collected is below the detection limit of the assay, or if the time of collection is before or much later after symptom onset. Also, the stability of antigens in biological samples can degrade as a function of time and temperature after sample collection. Because positive and negative predictive values are highly dependent on communal COVID-19 prevalence, false-negative test results are statistically more likely during peak activity when prevalence of disease is high. Negative antigen test results should be handled differently depending on the test, its stated performance characteristics, and intended application (for example, clinical diagnosis, screening). In general, negative antigen test results should be considered presumptive, meaning that they are preliminary results. Thus, an antigen test result should be confirmed with an NAAT, especially if the result of the antigen test is inconsistent with the clinical context.23 More information is provided in the Appendix (available online at the end of this article).
False-positive test results are unlikely when an antigen test is used according to the manufacturer’s instructions, given that the specificity of antigen tests is generally as high as most NAATs.23 However, antigen tests detect both viable and nonviable SARS-CoV-2 viral antigens and may yield a positive result in the absence of live virus (as is the case with the NAATs). The potential for a false-positive test result is more likely when the prevalence of infection or pretest probability is low or if samples are cross-contaminated. Nevertheless, clinicians can rely on a positive antigen test result for a symptomatic patient.23 None of the COVID-19 vaccines authorized (or in development) will cause a positive result in antigen tests because they do not contain viral antigens. Table 4 summarizes the relationship between the results of antigen tests, NAATs, and clinical manifestation of COVID-19. An antigen testing algorithm based on the recommendation of the CDC is provided in the Appendix (available online at the end of this article).
Table 4.
Relationship between the results of antibody tests, antigen tests, NAATs,∗ and clinical manifestation of COVID-19.
| CLINICAL MANIFESTATION |
NAAT RESULT |
ANTIGEN TEST RESULT |
SEROLOGIC TEST RESULT |
RISK OF EXPERIENCING INFECTION TRANSMISSION |
|
|---|---|---|---|---|---|
| Immunoglobulin M | Immunoglobulin G | ||||
| Early Stage of Infection | + | + | + | − | High |
| Active Phase of Infection | + | + | + | + | High |
| Late Stage of Infection, Possible Recurrent Infection | + | + | − | + | High |
| Window Period | + | + | − | − | High |
| Early Stage of Infection, False-Negative NAAT Result | − | + | + | − | High |
| Late Stage of Infection, False-Negative NAAT Result | − | + | − | + | High |
| Window Period, False-Negative NAAT Result | − | + | − | − | High |
| Asymptomatic Infection, False-Positive NAAT Result, False-Positive SerologicTest Result | + | − | + | + | Low |
| Window Period with Asymptomatic Infection, False-Positive NAAT Test Result | + | − | − | − | Low |
| Past Infection | − | − | − | + | Low |
| Convalescence | − | − | + | + | Low |
NAAT: Nucleic acid amplification test.
Dental offices must follow the manufacturer’s instructions and package inserts when using these tests to obtain valid and reliable results. The performance of antigen tests can be affected if the test components are not stored and handled properly. They should remain in the sealed pouch until immediately before use, never be frozen, and always be brought to room temperature (15-30 °C) before use. The appropriate incubation time before reading the results and whether the results should be interpreted visually or with an instrument analyzer should be considered. Reading the test before or after the specified time could result in false-positive or false-negative test results.23 More information regarding antigen tests is provided in the Appendix (available online at the end of this article).
Serologic tests
Serologic tests use antibody-antigen recognition by using cloned or recombinant SARS-CoV-2 antigens to detect patient antibodies generated against the virus. Serologic tests authorized for emergency use by the FDA detect immunoglobulin M (IgM), immunoglobulin G (IgG), or total antibody. A detailed description of these techniques is reported elsewhere.10 , 12 , 24 , 25 Figure 4 depicts a simplified process of antibody detection tests.
FDA-authorized serologic tests are performed using whole venous or fingerstick blood, serum, or plasma, with comparable sensitivity and specificity between whole blood and serum. They are not costly or restricted to any certain age, are relatively easy to use, and generally return results in approximately 15 through 90 minutes depending on the assay, some of which may require laboratory technicians and specialized instruments. Almost all the tests are qualitative (providing a result that is positive, negative, or indeterminate) rather than quantitative (providing a quantitative assessment of antibody levels). Some serologic tests are available for POC use and use lateral flow immunoassay with fingerstick blood collection. Serologic testing, by itself, should not be used to establish the presence or absence of SARS-CoV-2 infection or reinfection. COVID-19 vaccines might result in positive antibody test results because they trigger generation of antibody against SARS-CoV-2.
Serologic tests are both less sensitive and less specific than NAATs and most antigen tests must contend with the inherent variability of the human polyclonal antibody response. In addition, they inherently measure a response to an infection, not the infection itself. The delay required for plasma B cells to generate a humoral antibody response and for the body to mount a full polyclonal humoral immunity does not allow for the use of serologic tests early in the course of infection.14 , 19 Serologic tests can be used to determine different aspects of immune response and functionality of antibodies. They also are indicated in epidemiologic and surveillance studies to mass screen the prevalence of COVID-19 and identify asymptomatic patients or carriers and to detect the presence, nature, and extent of the humoral immune response against SARS-CoV-2 infection, which also allows for differentiating between past and current infection as well as its progress. Serologic tests are also useful in vaccine studies and investigation of late complications, whereas NAATs may yield false-negative results owing to low viral loads in the tested samples. They also can be offered in adjunction to NAATs or antigen tests for people who seek treatment 9 through 14 days after illness onset, during which the sensitivity of NAATs is decreasing and the sensitivity of serologic testing is increasing.14 , 15 , 19 , 20 , 25 Table 4 summarizes the relationship between the results of antibody tests, NAATs, and clinical manifestation of COVID-19. The immunologic response to SARS-CoV-2 infection is not limited to antibody production; the cellular responses of T cells and macrophages against the invading virus may play a significant, yet currently underexamined, role in disease progression. Potential cellular assessments may prove to be of additional value.26
As of April 2021, data are limited regarding the nature, kinetics, and dynamics of immunologic responses to SARS-CoV-2 infection,27 which can vary with age, sex, and presence of comorbidities, as well as characteristics of the viral load and variant infectivity and transmissibility. It has been suggested that seroconversion after exposure to SARS-CoV-2 is similar to other acute viral infections, with IgG concentration beginning to rise as IgM concentration reaches a plateau and then declines. IgM usually becomes undetectable weeks to months after infection, whereas IgG may remain detectable for months or years (Figure 1B). Therefore, IgM and IgG are indicative of early or recent and late or past stage responses to the infection. Nevertheless, the role of virus-specific immunoglobulin A (IgA) against SARS-CoV-2 infection in humans should be emphasized.14 , 15 , 28 It is suggested that IgA-mediated mucosal immunity is a critical defense mechanism against SARS-CoV-2 at the individual level, which may reduce infectivity of human secretions and viral transmission. Secretory IgA can be measured easily in saliva. This also may encourage the development of vaccines that induce specific mucosal IgA responses against SARS-CoV-2.29 In addition, it provides the chance to test for antibodies in samples, including saliva, respiratory tract secretions, and other body fluids in addition to blood products.15 More information regarding serologic tests is provided in the Appendix (available online at the end of this article).
The timing of sample collection, host immune factors, and test characteristics should be considered when interpreting antibody test results. There is a possibility of false-negative results and false-positive results considering the overall sensitivity and specificity of antibody tests. Negative antibody results do not exclude SARS-CoV-2 infection, and false-negative results may be associated with the time needed to develop an immune response early during the course of disease (window period). Some people do not develop detectable IgG or IgM antibodies after infection. Chronic immunosuppression may cause an impaired immune response and no detectable titer. Alternatively, false-positive cross-reactivity may result in patients who have been infected with related human coronaviruses before, such as those that cause the common cold.12 , 15 , 20 , 30
What dentists need to know about point-of-care testing in the dental office
The American Dental Association (ADA) considers POC testing and screening of patients for medical conditions, including COVID-19, to be within a dentist’s scope of practice if such conditions could complicate oral health care or put the patient and dental staff at risk of developing the disease. The FDA includes dentists among those professionals who can test for COVID-19, and the CDC recommends dental facilities to consider implementing preprocedure testing for COVID-19.6 , 31
The ADA has released the COVID-19 & Lab Testing Requirements Toolkit to help guide dentists interested in offering their patients rapid-response, POC COVID-19 screening and testing within their practices.32 It provides information for dentists on applying for the federal certification required to offer this type of testing. The toolkit also includes compliance requirements, tips for developing a waived testing program in dental practices, COVID-19 test reporting requirements, and guidance on medical benefit plan claim filing for in-office COVID-19 testing. Any dental practice that plans to incorporate testing for COVID-19 into their screening protocol is considered a clinical laboratory and must comply with regulations set by the Clinical Laboratory Improvement Amendments (CLIA) law, which establishes quality standards for all laboratory testing to ensure accuracy, reliability, and timeliness of patient test results regardless of where the test was performed.7 , 32 , 33 Specific requirements for each state and guidance on patient medical benefit plan claim filing for in-office COVID-19 testing are provided in the ADA toolkit.32
Dentists who register for a CLIA certificate of waiver for their practices must only perform tests that have been classified as waived tests, which include some NAATs and antigen and antibody tests, and must follow the manufacturer’s instructions for each test.32 , 33 The CDC has provided comprehensive guidelines and useful educational materials for POC testing and infection control.6 , 33 , 34 According to CDC reporting guidelines, dental offices with CLIA certificates are considered testing sites and must report both the positive and negative results of COVID-19 diagnostic and screening tests that they perform using in-home diagnostic or screening tests to the appropriate state or local public health department.32 , 35 CDC guidelines must be followed for specimen collection and handling of POC tests.33 , 36
The POC testing options and technologies for COVID-19 testing are growing and evolving fast. As of April 2021, multiple POC testing options are authorized, and dental offices with CLIA certificate are eligible to use and benefit from them.7 However, there are no saliva-based POC antigen or antibody tests approved for use. The characteristics of all authorized POC tests for COVID-19 are summarized in eTable 1, eTable 2, eTable 3 (available online at the end of this article). Large-scale assessment of the sensitivity and specificity of these tests are not reported widely.
The FDA has issued an emergency use authorization for an over-the-counter and a prescription-only NAAT-based diagnostic tests that can be fully self-administered and provide rapid results at home, using nasal swab samples in people 2 years or older. They are also authorized for POC use, but samples must be collected by a health care provider. Many other NAAT-based assays also are authorized for at-home specimen collection with subsequent remote laboratory processing.7 There are some POC multiplexed reverse transcription PCR–based assays for simultaneous rapid and qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B viruses in less than 1 hour. There is also an automated POC assay that uses isothermal nucleic acid amplification technology for qualitative detection of SARS-CoV-2 in direct (no need for transport medium) nasal, nasopharyngeal, and throat swabs in less than 13 minutes (eTable 1, available online at the end of this article).
Furthermore, the FDA has issued an emergency use authorization for an over-the-counter fully at-home diagnostic test for COVID-19, which is a lateral flow antigen test that uses a nasal swab sample from anyone 2 years or older and gives the result within 20 minutes. Other fully at-home antigen-based tests are also available with prescription. There are also antigen tests for simultaneous detection and differentiation of SARS-CoV-2, influenza A, and influenza B viruses, which give results within 15 minutes. This is in addition to multiple POC antigen tests that have been authorized for POC use (eTable 2, available online at the end of this article).8
A number of serologic tests are authorized for POC use and detect IgG or IgM from fingerstick blood in approximately 10 to 20 minutes (eTable 3, available online at the end of this article).9
These options, and particularly such tests that can be fully administered entirely outside of a laboratory or health care setting, may deliver rapid, accurate, and actionable results to dental clinical and infection control teams, with the promise of contributing to enhanced infection control in dental and other health care settings.
Discussion
The CDC has reported many people residing in the United States have seen a dentist over the past year but no other medical professional.37 Therefore, dental offices and settings could make a major contribution to public health by being the first-line of COVID-19 screening testing and vaccine advocacy for a large portion of the population. Dental settings may also be important for COVID-19 surveillance studies in the future.
Characteristics and detection rate or viral load of SARS-CoV-2 in different anatomic sites and from various specimens and fluids related to dentistry have been reviewed comprehensively in a 2021 article by Shirazi and colleagues.2 Lamberghini and colleagues38 reported an overall SARS-CoV-2 positivity rate of 2.3% in asymptomatic children attending a high-volume pediatric dental clinic. Conway and colleagues39 reported that the COVID-19 positivity rate was 0.6% (95% CI, 0.4% to 0.8%) in child and adult asymptomatic patients attending multiple oral health care centers. Palla and Callahan40 reported 6.7% of asymptomatic patients attending an emergency department tested positive for COVID-19, 50% of whom visited a dentist within 1 week of consultation.
These findings highlight that basic patient triage protocols alone would not identify everyone who is infected. Therefore, timely, accurate (highly sensitive and specific), and rapid screening and testing that can distinguish people with COVID-19 from those who are healthy or infected with other viruses is an essential need to take required actions, optimize patient care, maintain the safety of dental patients and treatment providers, and contain and prevent disease spread. POC tests that can be fully administered entirely outside of a laboratory or health care setting will deliver rapid, accurate, and actionable results to dental clinical and infection control teams and will contribute greatly to safe patient flow in dental practices. In addition, the ability for detection and differentiation of influenza and COVID-19, which have similar symptoms, will give insights for effective triage and treatment management.
Conclusions
Oral health care must continue to offer safety, despite the COVID-19 pandemic or future pandemics. Mitigation of SARS-CoV-2 transmission requires the application of engineering and administrative controls and the use of personal protective equipment. Although the promise of vaccines may reduce levels of the virus within communities, risks will remain until this virus falls into the annual viral background, and surveillance strategies at the community level remain important. Testing for SARS-CoV-2 at the POC offers another control mechanism contributing to and enhancing the real and perceived safety of care in the dental office setting. Several options for POC testing within the dental office are emerging (with additional responsibilities) and can provide additional guidance to reduce disease transmission in the dental office.
Biographies
Dr. Shirazi is a research specialist, Department of Oral Biology, College of Dentistry, University of Illinois Chicago, 801 S Paulina St, Chicago, IL 60612.
Dr. Stanford is the dean, College of Dentistry, University of Illinois Chicago, Chicago, IL.
Dr. Cooper is the associate dean for research, College of Dentistry, University of Illinois Chicago, Chicago, IL.
Footnotes
Disclosures. None of the authors reported any disclosures.
Supplemental data related to this article can be found at: https://doi.org/10.1016/j.adaj.2021.04.019.
Consideration for Antibody Tests
Serologic tests may detect 2 different types of antibodies. First are binding antibodies, which do not indicate the functionality, extent, or protectiveness of immune response. Current point-of-care tests detect these types of antibodies. Second are neutralizing antibodies, which have a high affinity for SARS-CoV-2, may inactivate the virus and attenuate infection, and can provide the best correlate of protective immunity. Neutralization tests determine the functional ability of antibodies to prevent infection of virus in vitro through incubating serum or plasma with live virus followed by infection and incubation of cells. This will require either biosafety level 3 or 2 laboratories, depending on what form of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is used.
The development of a serologic test that is robust and suitable for routine deployment takes substantially long. The basis is obtaining antigens that can be accurately recognized by specific antibodies because each antibody has sites that can bind only 1 specific type of antigen. It requires detecting distinct proteins that form the virus, finding the most divergent proteins from previous virus proteins among those (to avoid cross-reactivity), then identifying specific polyclonal antibodies generated by human adaptive immune response to the selected proteins, and finally testing to ensure that there is limited cross-reactivity with antibodies developed against other viruses. The protein target of each test determines its cross-reactivity and specificity. Cross-reactivity occurs when synthetic antibodies bind with an antigen different from the target antigen and is observed between human coronaviruses, such as SARS-CoV-2, SARS, and Middle East respiratory syndrome coronavirus, owning to highly conserved or similar amino acid sequences of viral antigens. S and N proteins are 2 major antigenic targets for SARS-CoV-2 antibody tests. N protein is the most abundantly expressed immunodominant protein, but it is more conserved across coronaviruses than S, and within S, receptor-binding domain is more conserved than S1 domain. SARS-CoV-2 antigens used for serologic tests are usually specific labeled antigens or synthetic or recombinant epitopes mainly prepared via genetic engineering technology. Nevertheless, developing tests to detect human polyclonal antibodies against SARS-CoV-2 using cloned antigens (serologic tests) are less time consuming and easier than developing tests to detect the viral antigens using monoclonal antibodies (antigen tests).
It is proposed that detecting solely the immunoglobulin M class of antibodies might be helpful for diagnostic purposes. However, there is no advantage for the use of one over another. The accurate detection of antibodies is possible approximately 10 through 14 days, up to 3 weeks in some cases, after the first symptom onset. But it might not prove infectiousness. Also, detection of 1 or 2 antibodies (immunoglobulin M or immunoglobulin G) does not necessarily guarantee immunity against reinfection. The factors associated with development of a protective antibody response; the longevity of antibodies; the existence, duration, and extent of protective immunity and the needed titer of antibodies to provide such protection; and the correlation of binding antibody titers to neutralization ability are not fully clear yet.
The favorable sensitivity and specificity for an antibody test depends on its purpose and must be considered before its implementation. High sensitivity (≥ 90%) is required for diagnosis in symptomatic patients. A slight reduction in specificity could be acceptable in this context, as some false-positive results may be tolerated if other potential tests are considered. However, high specificity (≥ 99.5%, according to the Centers for Disease Control and Prevention) is necessary if antibody tests are used to release people from social isolation or as a basis to return to normal activities. Otherwise, false-positive results would return nonimmune people to the risk of being exposed. This is assuming antibody positivity is a correlate of protective immunity, which is not fully clear yet. False-positive test results can be reduced via testing populations with high prevalence (high positive predictive value) and people with an elevated chance of previous exposure to SARS-CoV-2 (high pretest probability). For example, for a point-of-care test with 99.8% specificity and 90% sensitivity, the positive predictive value is 90.2% at 2% seroprevalence and 99.5% at 30% population seroprevalence. Alternatively, an orthogonal testing algorithm, in which people who initially test positive are tested with a second test with different design characteristics, can be used if the positive predictive value of a single test is low.
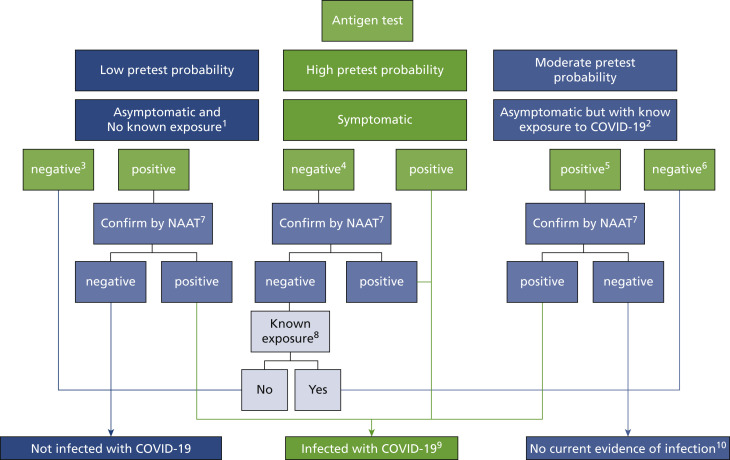
eFigure.
Antigen testing algorithm based on CDC recommendation.
1No known exposure to a person with COVID-19 within the past 14 days. 2Single, multiple, or continuous known exposure to a person with COVID-19 within the past 14 days; perform NAAT first if short turnaround time is available, if the person cannot be effectively and safely quarantined, or if there are barriers to possible confirmatory testing. 3If prevalence of infection is not low in the community, clinical discretion should consider whether this negative antigen result requires confirmation. 4If a symptomatic person has a low likelihood of SARS-CoV-2 infection, clinical discretion should determine if this negative antigen test result requires confirmatory testing. 5In instances of higher pretest probability, such as high incidence of infection in the community, clinical discretion should determine if this positive antigen result requires confirmation. 6In certain settings, serial antigen testing could be considered for those with a negative antigen test result; serial testing may not require confirmation of negative results. The role of a negative antigen test result in ending quarantine depends on when it is performed in the quarantine period. See CDC’s Options to Reduce Quarantine for guidance on use of antigen testing for this purpose and when a negative antigen test result indicates no infection with SARS-CoV-2. 7Nucleic acid amplification test; confirm within 48 hours using an NAAT, such as reverse transcription polymerase chain reaction, that has been evaluated against the Food and Drug Administration’s reference panel for analytical sensitivity. 8Known exposure to a person with COVID-19 within the past 14 days; if unsure, clinical discretion should determine whether isolation is necessary. 9Isolation is necessary. 10Quarantine is necessary. Clinical discretion should determine if and when additional testing is necessary.
eTable 1.
| PRODUCT‡ | TEST AT HOME | ACCESSIBILITY | MULTIANALYTE | METHOD | RESULTS REPRESENTATION§ | SAMPLE TYPE¶ | TARGET | PERFORMANCE EVIDENCE FOR SARS-CoV-2# | TIME TO RESULSTS |
|---|---|---|---|---|---|---|---|---|---|
| Cue COVID-19 Test for Home and OTC∗∗Use | Yes | OTC home testing, health care provider†† at POC | No | RT,‡‡ isothermal amplification | Qualitative, instrument read | Anterior nasal swab in adults (self-swabbing) or children ≥ 2 y of age (swabbed by an adult) | Nucleocapsid gene | LoD§§: 20 genome copies/sample wand PPA¶¶: 97.4% (95% CI, 86.5% to 99.5%) NPA##: 99.1% (95% CI, 96.9% to 99.8%) |
~ 20 min |
| Visby Medical COVID-19 POC Test | No | Health care provider | No | RT-PCR∗∗∗ | Qualitative, visual read | Nasopharyngeal, anterior nasal, or Midturbinate swabs collected by a health care provider or anterior nasal or mid-turbinate swabs self-collected (by people aged ≥ 18 y, under the supervision of a health care provider) | Nucleocapsid gene | LoD: 435 copies/swab PPA: 100.0% (95% CI, 89.0% to 100.0%) NPA: 95.3% (95% CI, 87.1% to 98.4%) |
~ 30 min |
| Xpert Xpress SARS-CoV-2 Test | No | Health care provider | No | Real-time RT-PCR | Qualitative, instrument read | Nasopharyngeal, nasal, or mid-turbinate swab | Nucleocapsid gene, envelope protein gene | LoD: 5.4 x 103 RNA NAAT‡‡‡ detectable units/milliliter PPA: 97.8% (95% CI, 88.4% to 99.6%) NPA: 95.6% (95% CI, 85.2% to 98.8%) |
30 min for positives and ~ 45 min for negatives |
| Accula SARS-Cov-2 Test | No | Health care provider | No | PCR and lateral flow | Qualitative, visual read | Clinician-collected nasal or nasal mid-turbinate swab samples or clinician-instructed self-collected (collected on site) nasal swab | Nucleocapsid gene | LoD: 200 copies/reaction. PPA: 95.8% (95% CI, 78.88% to 99.89%) NPA: 100% (95% CI, 86.77% to 100%) |
~ 30 min |
| BioFire Respiratory Panel 2.1-EZ | No | Health care provider | Yes,16 viruses and 4 bacteria | Real-time, nested multiplexed PCR | Qualitative, instrument read | Nasopharyngeal swab | Spike protein gene and membrane protein gene | Overall, 97.1% sensitivity and 99.3% specificity (prospective specimens) 98.0% sensitivity and 100% specificity (archived specimens) 100% PPA and 100% NPA (contrived specimens) |
~ 45 min |
| Cobas SARS-CoV-2 & Influenza A/B Nucleic Acid Test | No | Health care provider | SARS-CoV-2, influenza A and influenza B virus | Multiplexed real-time RT-PCR | Qualitative, instrument read | Health care provider-collected nasopharyngeal and nasal swabs and self-collected nasal swabs (collected in a health care setting with instruction by a health care provider) | RdRp gene (ORF1ab) and nucleocapsid gene | LoD: 12 copies/mL PPA: 100% NPA: 100% |
~ 20 min |
| Lucira COVID-19 All-in-One Test Kit | Yes | Prescription home testing, health care provider at POC | No | RT-loop mediated isothermal amplification | Qualitative, instrument read | Self-collected nasal swab in people aged ≥14 y and in people aged ≤ 13 y when the specimen is collected by a health care provider at the POC | 2 non overlapping regions of the nucleocapsid gene | LoD: 900 copies/mL PPA: 94.1% (95%CI, 85.5% to 98.4% NPA: 98.0% (95%CI, 89.4% to 99.9%) |
< 30 min |
| ID NOW COVID-19 | No | Health care provider | No | RT, isothermal amplification | Qualitative, instrument read | Direct nasal, nasopharyngeal or throat swabs | RdRp | LoD: 125 genome equivalents/mL | ≤ 13 min |
| Xpert Xpress SARS-CoV-2/Flu/RSV††† | No | Health care provider | SARS-CoV-2, influenza A and influenza B virus, RSV | Multiplexed real-time RT-PCR | Qualitative, instrument read | Nasopharyngeal or nasal swab | Envelope and N-nucleocapsid (N2) | LoD:131 copies/mL for COVID-19 PPA and NPA of 97.9% and 100.0% for SARS-CoV-2, respectively; 100.0% and 100.0% for influenza A, respectively; 100.0% and 99.0% for influenza B, respectively 100.0% and 100.0% for RSV, respectively |
25 min for positive COVID-19 results and 36 min for all 4 pathogens |
| Cue COVID-19 Test | No | Health care provider | No | RT, isothermal amplification | Qualitative, instrument read | Direct nasal swabs | Nucleocapsid gene | LoD: 1,300 copies/mL PPA 100% NPA 92% |
25 min |
POC: Point-of-care.
As of April 5, 2021. An up-to-date list of POC molecular diagnostic tests is available online at the US Food and Drug Administration’s “In Vitro Diagnostics EUAs: Molecular Diagnostic Tests for SARS-CoV-2.”7 Those tests that have W in the Authorized Setting column are authorized for POC use. The link for each product’s user instruction is provided in the Authorization Documents section.
Products are listed according to the authorization date.
All of the mentioned products automate all aspects of nucleic acid testing, from nucleic acid extraction to results.
Nasal and nasopharyngeal swabs are contraindicated in people who are susceptible to nosebleeds or have had recent facial or head injury or surgery.
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
OTC: Over-the-counter.
A health care provider includes, but is not limited to, physicians, dentists, nurses, pharmacists, technologists, laboratory directors, epidemiologists, or any other practitioners or allied health care professionals. The Centers for Disease Control and Prevention provides an online guideline for the collecting and handling of clinical specimens for COVID-19 testing.36
RT: Reverse transcription.
LoD: Limit of detection.
PPA: Positive percentage agreement.
NPA: Negative percentage agreement.
PCR: Polymerase chain reaction.
NAAT: Nucleic acid amplification test.
RSV: Respiratory syncytial virus.
eTable 2.
| PRODUCT‡ | TEST AT HOME | ACCESSIBILITY | MULTIANALYTE | METHOD | RESULTS READING | SAMPLE TYPE§ | TAREGET ANTIGEN¶ | PERFORMANCE EVIDENCE FOR SARS-CoV-2# | TIME TO RESULTS | SUGGESTED TIME POINT FOR TESTING |
|---|---|---|---|---|---|---|---|---|---|---|
| BD Veritor System for Rapid Detection of SARS-CoV-2 & Flu A+B | No | Health care provider ∗∗ | Yes | Chromatographic digital immunoassay | Instrument | Direct anterior nasal swab | SARS-CoV-2 nucleocapsid antigen or influenza A and B nucleoprotein | LoD††: 2.8 x 102 TCID50‡‡/milliliter PPA§§: 86.7% (95% CI, 75.8% to 93.1%) NPA##: 99.5% (95% CI 97.4-99.9%) |
20 min | Within the first 6 d of symptom onset |
| QuickVue At-Home COVID-19 Test | Yes | Prescription home testing, health care provider | No | Lateral flow fluorescence immunoassay | Visual | Direct anterior nares swab | Nucleocapsid protein | LoD: 1.91 x 104 TCID50/mL PPA: 84.8% (95% CI, 71.8% to 92.4%) NPA: 99.1 % (95% CI, 95.2% to 99.8%) |
10 min | Within the first 6 d of the onset of symptoms |
| Ellume COVID-19 Home Test | Yes | Over-the-counter home testing, health care provider | No | Lateral flow fluorescence immunoassay | Instrument | Midturbinate nasal swabs that are self-collected by people aged ≥ 16 y, or are collected by an adult from people aged ≥ 2 yr | Nucleocapsid protein | LoD:103.80 TCID50/mL PPA: 95% (95% CI, 82% to 99%) NPA: 97% (95% CI, 93% to 99%) |
20 min | Intended for use in people with or without symptoms or other reasons to suspect a COVID-19 infection |
| LumiraDx SARS-CoV-2 Ag Test | No | Health care provider | No | Microfluidic immuno-fluorescence assay | Instrument | Direct nasal swab | Nucleocapsid protein | LoD: 32 TCID50/mL PPA: 97.6% (95% CI, 91.6% to 99.3%) NPA: 96.6% (95% CI, 92.7% to 98.4%) |
12 min | Within the first 12 days of symptom onset |
| BD Veritor System for Rapid Detection of SARS-CoV-2 | No | Health care provider | No | Chromatographic digital immunoassay | Instrument | Nasal swabs | Nucleocapsid protein | LoD: 140 TCID50/mL PPA: 84% (95% CI, 67% to 93%) NPA: 100% (95% CI, 98% to 100%) |
15 min | Within the first 5 d of onset of symptoms |
| QuickVue SARS Antigen Test | No | Health care provider | No | Lateral flow immunoassay | Visual | Anterior nares swab | Nucleocapsid protein | LoD: 7.57x103 TCID50/mL PPA: 96.6% (95% CI, 88.3% to 99.0%) NPA: 99.3% (95% CI, 96.0% to 99.9%) |
10 min | Within the first 5 d of the onset of symptoms |
| BinaxNOW COVID-19 Ag Card Home Test | Yes | Over-the-counter home testing; Healthcare provider | No | Lateral flow immunoassay | Visual | Self-collected observed direct anterior nares swab samples from people aged ≥ 15 y. Adult-collected nasal swab samples from people aged ≥ 4 y. |
Nucleocapsid protein | LoD: 140.6 TCID50/mL PPA: 84.6% (95% CI, 76.8% to 90.6%) NPA: 98.5% (95% CI, 96.6% to 99.5%) |
15 min | Within the first 7 d of symptom onset |
| Clip COVID Rapid Antigen Test | No | Health care provider | No | Lateral flow immune-luminescent assay | Instrument | Direct anterior nasal swab | Nucleocapsid protein | LoD: 88 TCID50/mL PPA: 96.9% (95% CI, 83.8% to 99.9%) NPA: 100% (95% CI, 97.3% to 100%) |
30 min | Within the first 5 d of onset of symptoms |
| CareStart COVID-19 Antigen Test | No | Health care provider | No | Lateral flow immunoassay | Visual | Nasopharyngeal swab specimens | Nucleocapsid protein | LoD: 6.4 x 103 TCID50/mL PPA: 83.33% (95% CI, 43.65% to 97.00%). NPA: 100% (95% CI, 81.57% to 100%) |
10 min | Within the first 5 d of onset of symptoms |
| Sofia 2 Flu + SARS Antigen FIA | No | Health care provider | Yes | Lateral flow fluorescence immunoassay | Instrument | Direct nasopharyngeal and nasal swab | Nucleocapsid protein from SARS-CoV-2, influenza A, and influenza B | LoD: 91.7 x 103 TCID50/mL PPA: 95.2% (95% CI, 84.2% to 98.7%) NPA: 100.0% (95% CI, 96.9% to 100.0%) |
15 min | Within the first 5 d of onset of symptoms |
| Sofia SARS Antigen FIA | No | Health care provider | No | Lateral flow fluorescence immunoassay | Instrument | Direct nasopharyngeal and nasal swab | Nucleocapsid protein | LoD: 113 TCID50/mL PPA: 96.7% (95% CI, 83.3% to 99.4%) NPA: 100.0% (95% CI, 97.9% to 100.0%) |
15 min | Within the first 5 d of onset of symptoms |
POC: Point-of-care.
As of April 5, 2021. An up-to-date list of POC antigen diagnostic tests is available online at the US Food and Drug Administration’s “In Vitro Diagnostics EUAs: Antigen Diagnostic Tests for SARS-CoV-2.”8 Those tests that have W in the Authorized Setting column are authorized for POC use. The link for each product’s user instruction is provided in the Authorization Documents section.
Products are listed according to the authorization date.
Nasal and nasopharyngeal swabs are contraindicated in people who are susceptible to nosebleeds or have had recent facial or head injury or surgery.
None of the listed tests differentiate between severe acute respiratory syndrome coronavirus (SARS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Possible false-positive results in patients with history of infection with SARS-CoV-2.
Failure of the user to follow the test procedure correctly may affect the test performance adversely or invalidate the test result. All results are presumptive in asymptomatic people. Positive results indicate the presence of viral antigens, but the clinical correlation with medical history and other diagnostic information are necessary to determine infection status. Negative results are presumptive in symptomatic people.
A health care provider includes, but is not limited to, physicians, dentists, nurses, pharmacists, technologists, laboratory directors, epidemiologists, or any other practitioners or allied health care professionals. The Centers for Disease Control and Prevention provides an online guideline for the collecting and handling of clinical specimens for COVID-19 testing.36
LoD: Limit of detection.
TCID50: Median tissue culture infectious dose.
PPA: Positive percentage agreement.
NPA: Negative percentage agreement.
eTable 3.
| PRODUCT‡ | TEST AT HOME | ACCESSIBILITY | METHOD | RESULTS READING | SAMPLE TYPE | TAREGET ANTIBODY§ | PERFORMANCE EVIDENCE FOR SARS-CoV-2¶ | TIME TO RESULSTS |
|---|---|---|---|---|---|---|---|---|
| MidaSpot COVID-19 Antibody Combo Detection Kit | No | Health care provider# | Lateral flow | Visual | Fingerstick whole blood | IgM and IgG | IgM NPA∗∗: 97.7% (95% CI,†† 91.8% to 100%) IgG NPA: 100% (95% CI, 92.0% to 100%) 8-14 d IgM PPA‡‡: 100.0% (95% CI, 63.1% to 100.0%) IgG PPA: 75% (95% CI, 40.9% to 92.7%) ≥ 15 d IgM PPA: 100.0% (95% CI, 88.4% to 100.0%) IgG PPA: 100% (95% CI, 88.6% to 100%) |
20 min |
| RapCov Rapid COVID-19 Test | No | Health care provider | Lateral flow | Visual | Fingerstick whole blood | IgG | PPA: 90.0% (95% CI, 73.6% to 97.3%) NPA: 95.2% (95% CI, 89.2% to 97.9%) |
15 min |
| Sienna-Clarity COVIBLOCK COVID-19 IgG/IgM Rapid Test Cassette | No | Health care provider | Lateral flow | Visual | Fingerstick whole blood | IgM and IgG | Multiple clinical performance data. See the instructions for use. §§ | 10 min |
| RightSign COVID-19 IgG/IgM Rapid Test Cassette | No | Health care provider | Lateral flow | Visual | Fingerstick whole blood | IgM and IgG | IgM PPA: 88.89% (95% CI, 75.95% to 96.29%) IgM NPA:100.00% (95% CI, 91.06% to 100.00%) IgG PPA: 91.11% (95% CI, 78.78% to 97.52%) IgG NPA:100.00% (95% CI, 91.06% to 100.00%) |
10 min |
| Assure COVID-19 IgG/IgM Rapid Test Device | No | Health care provider | Lateral flow | Visual | Fingerstick whole blood | IgM and IgGs | IgM NPA: 100% (95% CI. 97.7% to 100%) IgG NPA: 100% (95% CI, 97.7% to 100%) 8-14 d IgM PPA: 83.3% (95% CI, 55.2% to 95.3%) IgG PPA: 83.3% (95% CI, 55.2% to 95.3%) ≥ 15 d IgM PPA: 89.3% (95% CI, 72.8% to 96.3%) IgG PPA: 100% (95% CI, 91.2% to 100%) |
15 min |
POC: Point-of-care.
As of April 5, 2021. An up-to-date list of POC antigen diagnostic tests is available online at the US Food and Drug Administration’s “In Vitro Diagnostics EUAs: Serology and Other Adaptive Immune Response Tests for SARS-CoV-2.”9 Those tests that have W in the Authorized Setting column are authorized for POC use. The link for each product’s user instruction is provided in the Authorization Documents section.
Products are listed according to the authorization date.
Results from antibody testing should not be used to diagnose or exclude severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or to inform infection status.
For optimal test performance, proper sample collection is critical. Failure to follow the procedure may give inaccurate results. In the early onset of symptom, anti-SARS-Cov-2 immunoglobulin M (IgM) and immunoglobulin G (IgG) antibody concentrations may be below detectable levels. SARS-CoV-2 IgG antibodies may be below detectable levels in patients who have been exhibiting symptoms for less than 15 days. Results from immunosuppressed patients should be interpreted with caution. False-positive results may be due to cross-reactivity with past or present infection with non-SARS-CoV-2 strains, such as coronaviruses HKU1, NL63, OC43, or 229E. Other information should be considered, including clinical history and local disease prevalence, in assessing the need for a second but different serology test to confirm an immune response.
A health care provider includes, but is not limited to, physicians, dentists, nurses, pharmacists, technologists, laboratory directors, epidemiologists, or any other practitioners or allied health care professionals. The Centers for Disease Control and Prevention provides an online guideline for the collecting and handling of clinical specimens for COVID-19 testing.36
NPA: Negative percentage agreement.
CI: Confidence interval.
PPA: Positive percentage agreement.
Refer to product catalog for further information.
Appendix. Considerations for Antigen Tests
The development of highly sensitive and specific antigen tests is challenging, despite polymerase chain reaction–based methods that only needed the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequence to be identified in order to generate specific primers, which is not a difficult process. First, the selected antibodies must have high affinity toward the antigen in separate sites, may not interfere with each other, and may not cross-react with antigens of other viruses. Second, antigen tests do not amplify the target molecule, and the intensity of the signal may be low if there is not enough antigen.
Positive and negative predictive values of all in vitro diagnostic tests vary depending on the pretest probability. For example, a test with 98% specificity would have a positive predictive value of approximately 80% in a population with 10% prevalence, meaning 20 out of 100 positive results would be false-positive. Nevertheless, clinicians can rely on a positive antigen test result for a symptomatic patient. The Centers for Disease Control and Prevention (CDC) considers low prevalence to be when nucleic acid amplification test (NAAT) positivity over the previous 14 days is less than 5% or when there are fewer than 20 new cases of coronavirus disease 2019 (COVID-19) per 100,000 people within the previous 14 days. The figure shows an antigen testing algorithm based on the CDC recommendation.
References
- 1.Centers for Disease Control and Prevention How COVID-19 spreads. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html Accessed March 31, 2021. [PubMed]
- 2.Shirazi S., Stanford C.M., Cooper L.F. Characteristics and detection rate of SARS-CoV-2 in alternative sites and specimens pertaining to dental practice: an evidence summary. J Clin Med. 2021;10(6):1158. doi: 10.3390/jcm10061158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estrich C.G., Mikkelsen M., Morrissey R. Estimating COVID-19 prevalence and infection control practices among US dentists. JADA. 2020;151(11):815–824. doi: 10.1016/j.adaj.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estrich C.G., Gurenlian J.R., Battrell A. COVID-19 prevalence and related practices among dental hygienists in the United States. J Am Dent Hyg Assoc. 2021;95(1):6–16. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/index.html Accessed March 31, 2021.
- 6.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19)-guidance for dental settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html Accessed June 9, 2021.
- 7.Food and Drug Administration In vitro diagnostic EUAs: molecular diagnostic tests for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2 Accessed March 31, 2021.
- 8.US Food and Drug Administration In vitro diagnostic EUAs: antigen diagnostic tests for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2 Accessed March 31, 2021.
- 9.US Food and Drug Administration In vitro diagnostic EUAs: serology and other adaptive immune response tests for SARS-CoV-2. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-serology-and-other-adaptive-immune-response-tests-sars-cov-2 Accessed March 31, 2021.
- 10.Russo A., Minichini C., Starace M., Astorri R., Calò F., Coppola N. Current status of laboratory diagnosis for COVID-19: a narrative review. Infect Drug Resist. 2020;13:2657–2665. doi: 10.2147/IDR.S264020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini A, Pandey R, Osman E, et al. Roadmap to the bioanalytical testing of COVID-19: from sample collection to disease surveillance [published online ahead of print October 30, 2020]. ACS Sens. 10.1021/acssensors.0c01377. [DOI] [PubMed]
- 12.Dhamad A.E., Abdal Rhida M.A. COVID-19: molecular and serological detection methods. PeerJ. 2020;8 doi: 10.7717/peerj.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Lee Y, Yang T, Sun J, Shen C, Cheng C. Current diagnostic tools for coronaviruses: from laboratory diagnosis to POC diagnosis for COVID-19 [published online ahead of print August 13, 2020]. Bioeng Transl Med. 10.1002/btm2.10177. [DOI] [PMC free article] [PubMed]
- 14.Jayamohan H, Lambert CJ, Sant HJ, et al. SARS-CoV-2 pandemic: a review of molecular diagnostic tools including sample collection and commercial response with associated advantages and limitations [published online ahead of print October 18, 2020]. Anal Bioanal Chem. 10.1007/s00216-020-02958-1. [DOI] [PMC free article] [PubMed]
- 15.Kubina R., Dziedzic A. Molecular and serological tests for COVID-19. A comparative review of SARS-CoV-2 coronavirus laboratory and point-of-care diagnostics. Diagnostics (Basel) 2020;10(6):434. doi: 10.3390/diagnostics10060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpdagtas S., Ilhan E., Uysal E., Sengor M., Ustundag C.B., Gunduz O. Evaluation of current diagnostic methods for COVID-19. APL Bioeng. 2020;4(4) doi: 10.1063/5.0021554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji T., Liu Z., Wang G. Detection of COVID-19: a review of the current literature and future perspectives. Biosens Bioelectron. 2020;166:112455. doi: 10.1016/j.bios.2020.112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taleghani N, Taghipour F. Diagnosis of COVID-19 for controlling the pandemic: a review of the state-of-the-art [published online ahead of print November 27, 2020]. Biosens Bioelectron. 10.1016/j.bios.2020.112830. [DOI] [PMC free article] [PubMed]
- 19.dos Santos C.C., Zehnbauer B.A., Trahtemberg U., Marshall J. Molecular diagnosis of coronavirus disease 2019. Crit Care Explor. 2020;2(9) doi: 10.1097/CCE.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Cruz R.J., Currier A.W., Sampson V.B. Laboratory testing methods for novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) Front Cell Dev Biol. 2020;8:468. doi: 10.3389/fcell.2020.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Candel F.J., Barreiro P., Román J.S. Recommendations for use of antigenic tests in the diagnosis of acute SARS-CoV-2 infection in the second pandemic wave: attitude in different clinical settings. Rev Esp Quimioter. 2020;33(6):466–484. doi: 10.37201/req/120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization Criteria for releasing COVID-19 patients from isolation. https://www.who.int/publications-detail-redirect/criteria-for-releasing-covid-19-patients-from-isolation Accessed April 4, 2021.
- 23.Centers for Disease Control and Prevention Interim guidance for antigen testing for SARS-CoV-2. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html Accessed March 31, 2021.
- 24.Ravi N., Cortade D.L., Ng E., Wang S.X. Diagnostics for SARS-CoV-2 detection: a comprehensive review of the FDA-EUA COVID-19 testing landscape. Biosens Bioelectron. 2020;165:112454. doi: 10.1016/j.bios.2020.112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yüce M., Filiztekin E., Özkaya K.G. COVID-19 diagnosis: a review of current methods. Biosens Bioelectron. 2021;172:112752. doi: 10.1016/j.bios.2020.112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights [published online ahead of print March 15, 2021]. Nat Rev Immunol. 10.1038/s41577-021-00522-1. [DOI] [PMC free article] [PubMed]
- 28.La Marca A., Capuzzo M., Paglia T., Roli L., Trenti T., Nelson S.M. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod Biomed Online. 2020;41(3):483–499. doi: 10.1016/j.rbmo.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2 [published online ahead of print December 7, 2020]. Sci Transl Med. 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed]
- 30.Krajewski R., Gołe¸biowska J., Makuch S., Mazur G., Agrawal S. Update on serologic testing in COVID-19. Clin Chim Acta. 2020;510:746–750. doi: 10.1016/j.cca.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Dental Association ADA supports point of care COVID-19 testing by dentists. https://www.ada.org/en/press-room/news-releases/2020-archives/november/ada-supports-point-of-care-covid-19-testing-by-dentists Accessed December 30, 2020.
- 32.American Dental Association COVID-19 & lab testing requirements toolkit. http://www.ada.org/∼/media/CPS/Files/Articles/Toolkits/ADA_CLIA_Toolkit.pdf Accessed November 11, 2020.
- 33.Centers for Disease Control and Prevention Guidance for SARS-CoV-2 point-of-care and rapid testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/point-of-care-testing.html Accessed March 31, 2021.
- 34.Centers for Disease Control and Prevention Waived tests. https://www.cdc.gov/labquality/waived-tests.html Accessed March 31, 2021.
- 35.Centers for Disease Control and Prevention How to report COVID-19 laboratory data. https://www.cdc.gov/coronavirus/2019-ncov/lab/reporting-lab-data.html Accessed March 31, 2021.
- 36.Centers for Disease Control and Prevention Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html Accessed March 31, 2021.
- 37.National Center for Health Statistics NHANES: National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index.htm
- 38.Lamberghini F, Trifan G, Testai FD. SARS-CoV-2 infection in asymptomatic pediatric dental patients [published online ahead of print January 19, 2021]. JADA. 10.1016/j.adaj.2021.01.006. [DOI] [PMC free article] [PubMed]
- 39.Conway DI, Culshaw S, Edwards M, et al; Dental COVID-19 Surveillance Survey Group. SARS-CoV-2 positivity in asymptomatic-screened dental patients [published online ahead of print January 1, 2021]. medRxiv. 10.1101/2020.12.30.20248603. [DOI] [PMC free article] [PubMed]
- 40.Palla B, Callahan N. What is the rate of COVID-19 infection in a population seeking dental care [published online ahead of print March 2, 2021]? JADA. 10.1016/j.adaj.2021.02.009. [DOI] [PMC free article] [PubMed]