Abstract
Objective:
Rapid fluid resuscitation has become standard in sepsis care, despite “low-quality” evidence and absence of guidelines for populations ‘at risk’ for volume overload. Our objectives include: 1) identify predictors of reaching a 30 mL/kg crystalloid bolus within three hours of sepsis onset (30by3); 2) assess the impact of 30by3 and fluid dosing on clinical outcomes; 3) examine differences in perceived ‘at-risk’ volume-sensitive populations, including: end-stage renal disease (ESRD), heart failure (HF), obesity, advanced age, or with documentation of volume “overload” by bedside examination.
Design:
Retrospective cohort study. All outcome analyses controlled for sex, ESRD, HF, sepsis severity (severe sepsis vs septic shock), obesity, Mortality in Emergency Department Sepsis score, and time to antibiotics.
Setting:
Urban, tertiary care center between January 1, 2014—May 31, 2017.
Patients:
Emergency Department treated adults (age ≥18 years; n=1,032) with severe sepsis or septic shock.
Measurements and Main Results:
In total, 509 patients received 30by3 (49.3%). Overall mortality was 17.1% (n=176), with 20.4% mortality in the shock group. Patients who were elderly (OR=0.62, 95% CI 0.46–0.83), male (OR=0.66, CI 0.49–0.87), obese (OR=0.18, CI 0.13–0.25), or with ESRD (OR=0.23, CI 0.13–0.40), HF (OR=0.42, CI 0.29–0.60), or documented volume “overload” (OR=0.30, CI 0.20–0.45) were less likely to achieve 30by3. Failure to meet 30by3 had increased odds of mortality (OR=1.52, CI 1.03–2.24), delayed hypotension (OR=1.42, CI 1.02–1.99), and increased ICU stay (~2 days) (β=2.0, CI 0.5–3.6), without differential effects for ‘at-risk’ groups. Higher fluid volumes administered by three hours correlated with decreased mortality, with a plateau effect between 35–45 mL/kg (p<0.05).
Conclusions:
Failure to reach 30by3 was associated with increased odds of in-hospital mortality, irrespective of comorbidities. Predictors of inadequate resuscitation can be identified, potentially leading to interventions to improve survival. These findings are retrospective and require future validation.
Keywords: Sepsis, Shock, Septic, Fluids, Resuscitation, Mortality, Heart Failure, Renal Failure, Elderly, Obesity, Overload
INTRODUCTION
Despite significant improvements in care, mortality from sepsis remains high (1–5). The Surviving Sepsis Campaign (SSC) recommends that patients with “sepsis-induced hypoperfusion” receive a 30 mL/kg intravenous (IV) bolus of crystalloid fluids within three hours of identification, which has become a mandate by the Centers for Medicare & Medicaid Services (CMS) (5). The 30 mL/kg approach was derived based on average fluid volumes received in larger sepsis trials, though the evidence behind this recommendation from the SSC is listed as “low-quality” and does not address exceptions for medical comorbidities or volume status (5).
Numerous studies support the administration of early, aggressive fluid resuscitation (6–9); however, the topic remains controversial. A large, multicenter study found no mortality benefit of early fluids in patients with septic shock (10). Further, studies evaluating a more extended time course have highlighted deleterious effects of fluids in critically-ill populations (11–15). There were noted benefits with a negative-fluid-balance strategy for intensive care unit (ICU) patients requiring renal replacement therapy or with acute lung injury; however, these were not exclusive to septic patients, nor do they evaluate the effects of early fluid resuscitation (12, 13). Additionally, significant increases in mortality have also been noted when the total volume of fluids received during the first 24 hours of resuscitation is seven liters or greater (14). Others have further demonstrated that greater fluid balances are associated with an increased hazard of death on the third day of ICU admission, but not the first 24 hours (15).
Data on fluid resuscitation in septic patients who are perceived to be ‘at risk’ of volume overload remains inconclusive. Patients who are obese receive a lower weight-based fluid dose relative to non-obese patients, yet demonstrate improved survival (17, 18). Additionally, patients with end-stage renal disease (ESRD), heart failure (HF), and advanced age often receive less fluids or have significant delays to fluid administration with differing effects on mortality (9, 19–21). These findings may offer some explanation for why practitioners hesitate to order fluids in septic patients, particularly in these perceived ‘at-risk’ populations.
The objectives of this study include: 1) identify predictors of reaching a 30 mL/kg crystalloid bolus within three hours of sepsis onset (30by3); 2) assess the impact of receiving 30by3 and dosing of fluids on various clinical outcomes; 3) examine differences in these perceived ‘at-risk’ populations, including: ESRD, HF, obesity, advanced age, or with documentation of volume “overload” by bedside examination.
MATERIALS AND METHODS
Study Design and Population:
This was a retrospective cohort study from January 1, 2014 to April 30, 2015 and from February 1, 2016 to May 31, 2017 for patients treated in the Emergency Department (ED) at an urban, tertiary care center with ~70,000 visits per year, predominantly serving African-American patients (~73% of visits). Data identifying positive sepsis cases were collected prospectively as part of a quality improvement initiative. Data were not available between April 2015 and February 2016 secondary to ICD-coding changes. This study was granted approval by the Institutional Review Board.
Selecting a Sepsis Cohort:
The study population was evaluated for the presence of severe sepsis and septic shock within the first eight hours of ED arrival. All ED encounters with the International Classification of Disease-Revision 9 codes (ICD-9) (prior to October 1, 2015) or ICD-10 (on and after October 1, 2015) codes related to sepsis and are the same used for case selection by CMS (Supplementary 1A) (22). To further capture instances where there was concern for infection, patients with orders for blood cultures, urine cultures, or broad-spectrum antibiotics within 12 hours of ED arrival were also flagged. A physician reviewed each chart using a standardized process to determine whether infection-related organ dysfunction occurred within eight hours of ED arrival. Identified cases were confirmed with the treating providers and a multidisciplinary quality improvement group. Computational algorithms were used to identify cases and demographics and, therefore, blinded to input variables for sepsis management and clinical endpoints. The physician chart reviewer was not blinded, however, cases were not originally identified for the purpose of this study.
Sepsis Definitions:
Severe sepsis and septic shock were defined using Sepsis-2 criteria (Supplementary 1B) (1, 2). Time of onset was determined when all the above criteria were met and/or when laboratory values resulted.
Inclusion and Exclusion Criteria:
Patients aged ≥18 years were included. Patients with a left ventricular assist device were excluded, unless the initial serum lactic acid level was greater than 2.0 mmol/L. Additionally, patients who were made do-not-resuscitate or comfort care within 12 hours of sepsis onset, having received IV antibiotics 24 hours prior to arrival, or transferred to the operating room or cardiac catheterization laboratory within three hours of sepsis onset were also excluded. For patients with multiple encounters, a single visit was randomly selected for inclusion in the analytic dataset (Supplementary Figure 3).
Data Collection and Accuracy:
Data was abstracted from the electronic medical record (Epic©, Verona, WI). Sepsis-relevant interventions and clinical factors, including time to 30mL/kg bolus were manually collected. If a start time to fluid resuscitation was documented without an end time, then fluids were assumed to infuse at 1L/hr. Additionally, the actual body weight was used for the dosing of fluids in this cohort. The mortality in ED sepsis (MEDS) scores were calculated to help control for disease severity (23). Abstractors were trained using strict definitions and protocols and were monitored to ensure accuracy of data. Thirty-five charts (~5 charts/abstractor) total were reviewed with a raw agreement of 95.1% and an inter-rater reliability, κ = 0.93 (24). Abstractors were not blinded to the purpose of this study.
Statistical Analysis:
Analyses were performed using Stata 15 (StataCorp., College Station, TX). Demographics and other characteristics were summarized using means and standard deviations, medians and interquartile ranges, or frequency counts and percentages. Median time to reaching fluid goals was estimated, along with 95% confidence intervals, using Kaplan-Meier methods. Multivariable logistic regression, with covariables chosen a priori, was used to determine factors associated with reaching fluid goals. Logistic regression was also utilized to assess the relationship between not reaching fluid goals by three hours and various outcomes, including: in-hospital mortality, delayed hypotension, ICU admission, and intubation (excluding those already intubated at time of sepsis diagnosis). In these models, adjustment was made for sex, ESRD, HF, disease severity (severe sepsis versus septic shock), obesity, MEDS score, and time to antibiotics. ICU length of stay (among those admitted to the ICU) was analyzed using the pseudo-observation approach with a linear regression model (25). This method accounts for the censoring due to in-hospital deaths. Tests of the differences in the effect of not reaching fluid goals by three hours across subgroups were performed by fitting a model to the entire sample containing interaction terms between each covariate and the subgroup indicator. The interaction term involving not reaching fluid goals by three hours was then tested using a Wald test. For further examination of dose effects, a separate series of models using amount of fluids received at three hours as the independent variable of interest were fit. To allow for non-linear effects of dose, a quadratic term was included and the overall test of the association between dose and outcome was the resulting two degree-of-freedom test. Plots were generated based on average predicted values from these models. In order to make the plot for ICU LOS most informative, a negative binomial regression model was fit to provide an estimate of the number of days spent in the ICU. Statistical significance was defined as p<0.05.
RESULTS
Demographics, Special Populations, and Sepsis Metric Measurements:
In total, 1,704 patient encounters were included. After exclusions, the new sample included 1,032 patients (Supplementary Figure 3). The cohort was dichotomized into two groups: those which received a 30 mL/kg bolus of IV fluids by three hours (30by3) vs. those which received the 30 mL/kg beyond three hours or never at all (30after3). Seventy-eight patients received no fluids within 3 hours and were included in the 30after3 group. Baseline demographics, special populations, and sepsis-specific parameters are outlined in Table 1 and in Supplementary Table 4.
Table 1. Demographics, Special Populations, and Outcome Measurements.
Table 1 describes the study cohort, dichotomized into those which received 30 mL/kg by the first three hours of resuscitation (30by3) vs. those who had reached 30mL/kg after 3 hours (30after3). Seventy-eight patients total received no fluids within 3 hours of sepsis onset and were included in the ‘30after3’ group.
| Total | 30by3 | 30after3 | |
|---|---|---|---|
| Total Patients | 1,032 | 509 | 523 |
| Demographics | |||
| Age (years), mean±SD | 63.4 ± 17.0 | 62.7 ± 17.7 | 64.0 ± 16.3 |
| Male sex, n(%) | 510 (49.4%) | 236 (46.4%) | 274 (52.4%) |
| BMI (kg/m2), mean±SD | 27.2 ± 8.8 | 23.9 ± 6.2 | 30.5 ± 9.8 |
| MEDS score, mean±SD | 10.6 ± 4.7 | 10.8 ± 4.5 | 10.4 ± 4.8 |
| Time to diagnosis (hr), mean±SD | 1.7 ± 1.5 | 1.7 ± 1.6 | 1.6 ± 1.5 |
| Special Populations, n(%) | |||
| Elderly | 488 (47.3%) | 226 (44.4%) | 262 (50.1 %) |
| Obesity | 292 (28.3%) | 69 (13.6%) | 223 (42.6%) |
| ESRD | 101 (9.8%) | 19 (3.7%) | 82 (15.7%) |
| HF | 245 (23.7%) | 67 (13.2%) | 178 (34.0%) |
| “Overload” | 208 (20.2%) | 42 (8.3%) | 166 (31.7%) |
| Shock | 653 (63.3%) | 359 (70.5%) | 294 (56.2%) |
| In-Hospital Metrics | |||
| ED LOS (hr), mean±SD | 9.4 ± 4.7 | 9.1 ± 4.4 | 9.7 ± 5.0 |
| ICU Admission, n(%) | 606 (58.7%) | 315 (61.9%) | 291 (55.6%) |
| ICU LOS (days), mean±SD | 2.6 ± 4.3 | 2.3 ± 3.8 | 2.9 ± 4.7 |
| Hospital LOS (days), mean±SD | 9.9 ± 9.4 | 9.2 ± 7.9 | 10.6 ± 10.6 |
| Mortality | 176 (17.1%) | 74 (14.5%) | 102 (19.5%) |
| Severe Sepsis (% of severe group) | 43 (11.3%) | 16 (10.7%) | 27 (11.8%) |
| Shock (% of shock group) | 133 (20.4%) | 58 (16.2%) | 75 (25.5%) |
| Sepsis Parameters | |||
| ≥2 SIRS Criteria, n(%) | 881 (85.4%) | 445 (87.4%) | 436 (83.4%) |
| ≥2 qSOFA Criteria, n(%) | 345 (33.4%) | 188 (36.9%) | 157 (30.0%) |
| Volume IVF by 3h (L), median (IQR) | 3.0 (1.0–2.0) | 3.0 (2.0–3.5) | 1.0 (0.5–2.0) |
| MAP mmHg, median (IQR) | 79.5 (66.7–95) | 79.3 (66.7–94.7) | 80.0 (52–79) |
| Lactate mmol/L, mean±SD | 3.5 ± 2.7 | 3.8 ± 2.8 | 3.2 ± 2.4 |
| Time to ABx (hr), mean±SD | 1.3 ± 3.3 | 0.7 ± 3.4 | 1.8 ± 3.1 |
| Intubation, n (%) | 158 (15.3%) | 77 (15.1%) | 81 (15.5%) |
| Vasopressor, n (%) | 215 (20.8%) | 108 (21.2%) | 107 (20.5%) |
| Positive blood cultures, n (%) | 325 (31.5%) | 165 (32.4%) | 160 (30.6%) |
| Sepsis Source, n (%) | |||
| Pneumonia | 313 (30.3%) | 162 (31.8%) | 151 (28.9%) |
| Genitourinary | 248 (24.0%) | 132 (25.9%) | 116 (22.2%) |
| Abdominal | 151 (14.6%) | 75 (14.7%) | 76 (14.5%) |
| Skin/Soft Tissue | 143 (13.9%) | 53 (10.4%) | 90 (17.2%) |
| Indwelling IV catheter | 53 (5.1%) | 22 (4.3%) | 31 (5.9%) |
| Other/Unknown | 124 (12.0%) | 65 (12.8%) | 59 (11.3%) |
Legend: SD = standard deviation, IQR = interquartile range, BMI = body mass index, MEDS = Mortality in Emergency Department, Special Populations: Elderly = age ≥65 years, obesity = BMI >30 kg/m2, ESRD = end-stage renal disease, on peritoneal or hemodialysis prior to hospitalization, HF = heart failure, defined as echocardiogram with ejection fraction <45% within one year of hospitalization or as documented in the medical chart (including those with preserved ejection fraction), “Overload” = physician documentation of volume overload status precluding fluid administration. ED LOS = Emergency Department length of stay (hours), ICU = intensive care unit, Sepsis, SIRS = systemic inflammatory response syndrome (defined in Supplementary 1b), qSOFA = quick sequential organ failure assessment (defined in Supplementary 1b), IVF = intravenous fluids administered by bolus, MAP = mean arterial pressure, ABx = antibiotics.
Factors Associated with Failure to Reach 30by3:
Median times to fluid goals and volumes administered are outlined in Table 2. Patients who were elderly (OR=0.62, 95% CI 0.46–0.83), male (OR=0.66, 95% CI 0.49–0.87), obese (OR=0.18, 95% CI 0.13–0.25), with documentation of volume “overload” (OR=0.30, 95% CI 0.20–0.45), or with co-morbid ESRD (OR=0.23, 95% CI 0.13–0.40) or HF (OR=0.42, 95% CI 0.29–0.60) demonstrated decreased odds in achieving 30by3, while patients with septic shock were at increased odds of achieving 30by3 (OR=2.18, 95% CI 1.62–2.93).
Table 2. Special Populations: Identification of Factors Associated with Failure to Meet 30by3.
Table 2 describes the median resuscitation volumes reported in mL/kg (by 3 hours) as well as the median time to the 30 mL/kg goal. It was demonstrated that patients who were elderly, male sex, obese, a reported history of ESRD or HF, and with documentation of fluid “overload” were at decreased odds of meeting 30by3 while controlling for age, sex, ESRD, HF, disease severity (severe sepsis vs. septic shock), obesity, and fluid “overload”.
| Population | Resuscitation Volume (mL/kg), median (IQR) | Resuscitation Volume (L), mean ± SD | Time to 30 mL/kg (hr), median (95% CI)* | Odds Ratio of Reaching 30by3 (95% CI)** |
|---|---|---|---|---|
| Elderly | ||||
| Yes | 27.0 (13.6–36.7) | 1.9 ± 1.2 | 3.6 (3.0–4.3) | 0.62 (0.46–0.83) |
| No | 29.4 (13.2–41.7) | 2.2 ± 1.4 | 2.9 (2.7–3.4) | |
| Male Sex | ||||
| Yes | 27.6 (12.7–38.3) | 2.1 ± 1.3 | 3.6 (3.0–4.4) | 0.66 (0.49–0.87) |
| No | 29.4 (14.7–40.1) | 2.0 ± 1.3 | 2.9 (2.6–3.4) | |
| Obesity | ||||
| Yes | 16.5 (6.9–28.0) | 1.8 ± 1.3 | 11.5 (9.3–15.6) | 0.18 (0.13–0.25) |
| No | 30.2 (19.2–44.1) | 2.1 ± 1.3 | 2.5 (2.3–2.8) | |
| ESRD | ||||
| Yes | 10.7 (3.3–23.0) | 1.1 ± 1.1 | Unable to Estimate | 0.23 (0.13–0.40) |
| No | 29.4 (15.7–40.1) | 2.2 ± 1.3 | 2.9 (2.8–3.2) | |
| HF | ||||
| Yes | 14.3 (5.4–30.0) | 1.4 ± 1.3 | 10.7 (6.9–17.3) | 0.42 (0.29–0.60) |
| No | 30.0 (18.2–41.3) | 2.2 ± 1.2 | 2.7 (2.5–2.9) | |
| “Overload” | ||||
| Yes | 12.2 (2.8–25.6) | 1.2 ± 1.1 | Unable to Estimate | 0.30 (0.20–0.45) |
| No | 30.0 (17.9–42.2) | 2.3 ± 1.2 | 2.7 (2.5–2.9) | |
| Shock | ||||
| Yes | 30.0 (15.3–44.1) | 2.2 ± 1.4 | 2.8 (2.5–3.0) | 2.18 (1.62–2.93) |
| No | 25.0 (11.1–32.0) | 1.7 ± 1.0 | 4.5 (3.6–5.8) | |
Estimated using Kaplan-Meier method
Based on multivariable logistic regression model with 30by3 as the dependent variable.
Regression Analyses and Dose-Effect Curves:
Regression analyses and dose-effect curves are outlined in Table 3 and Figure 1, respectively. Failure to achieve 30by3 demonstrated increased odds of in-hospital mortality (OR=1.52, 95% CI 1.03–2.24), delayed hypotension (OR=1.42, 95% CI 1.02–1.99), and increased ICU LOS amongst those who required ICU admission (~mean 2 days, β=2.0, 95% CI 0.5–3.6). Higher fluid volumes administered by three hours correlated with decreased mortality, with a plateau effect between 35–45 mL/kg (p=0.049), increased ICU admission (p=0.023), and decreased ICU LOS in those requiring ICU admission (p=0.005). There was a higher risk of intubation at both low and high fluid volumes, with a trough effect at ~30–35 mL/kg (p=0.018).
Table 3. Multivariable Regression Analyses.
Table 3 demonstrates various regression analyses across outcomes, including mortality, delayed hypotension, ICU admit and LOS, and intubation. It was demonstrated that 30after3 was associated with significant increased odds of mortality, delayed hypotension, and longer ICU LOS, while controlling for sex, ESRD, HF, disease severity (severe sepsis versus septic shock), obesity, MEDS score, and time to antibiotics.
| Variable | Mortality | Delayed Hypotension | ICU Admit | ICU LOS | Intubation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | β (95% CI) | p | OR (95% CI) | p | |
| 30after3a | 1.52 (1.03–2.24) | 0.035 | 1.42 (1.02–1.99) | 0.040 | 0.77 (0.6–1.0) | 0.088 | 2.0 (0.5–3.6) | 0.010 | 1.20 (0.78–1.84) | 0.416 |
| MEDS scoreb | 1.18 (1.13–1.23) | <0.001 | 1.01 (0.98–1.05) | 0.546 | 1.08 (1.0–1.1) | <0.001 | 0.2 (0.1–0.4) | 0.002 | 1.03 (0.99–1.08) | 0.172 |
| Time to ABxc | 1.02 (0.98–1.07) | 0.362 | 1.02 (0.97–1.08) | 0.392 | 1.01 (1.0–1.1) | 0.695 | 0.02 (−0.2–0.3) | 0.886 | 1.00 (0.95–1.06) | 0.919 |
| Obesity | 0.74 (0.48–1.13) | 0.158 | 1.03 (0.72–1.46) | 0.882 | 1.11 (0.8–1.5) | 0.527 | −0.2 (−1.8–1.4) | 0.816 | 1.46 (0.95–2.26) | 0.088 |
| Male Sex | 0.63 (0.44–0.89) | 0.009 | 0.75 (0.56–1.00) | 0.053 | 0.93 (0.7–1.2) | 0.580 | −0.7 (−2.0–0.6) | 0.305 | 1.06 (0.72–1.56) | 0.762 |
| ESRD | 1.23 (0.70–2.17) | 0.471 | 1.62 (0.94–2.80) | 0.084 | 1.35 (0.8–2.2) | 0.222 | 1.0 (−1.2–3.2) | 0.371 | 0.83 (0.42–1.64) | 0.583 |
| HF | 1.34 (0.90–2.00) | 0.149 | 1.48 (1.02–2.16) | 0.038 | 1.71 (1.2–2.4) | 0.002 | 0.4 (−1.2–1.9) | 0.653 | 0.88 (0.55–1.40) | 0.590 |
| Shock | 1.05 (0.69–1.60) | 0.822 | 7.80 (5.59–10.90) | <0.001 | 2.98 (2.2–4.0) | <0.001 | −1.0 (−2.7–0.7) | 0.234 | 1.98 (1.23–3.21) | 0.005 |
Legend: All estimated by Multivariable Logistic Regression analyses, except for ICU LOS, which was analyzed by the methods described in Andersen et al. (25). β represents a regression coefficient from a linear model, 2.0 represents an ICU LOS of ~2 days longer in the 30after3 group, after adjustment for the other covariates in the model.
vs. 30by3
per 1-point increase in the MEDS score
per 1hour increase in time to ABx.
Figure 1: Dose-Effect Curves.
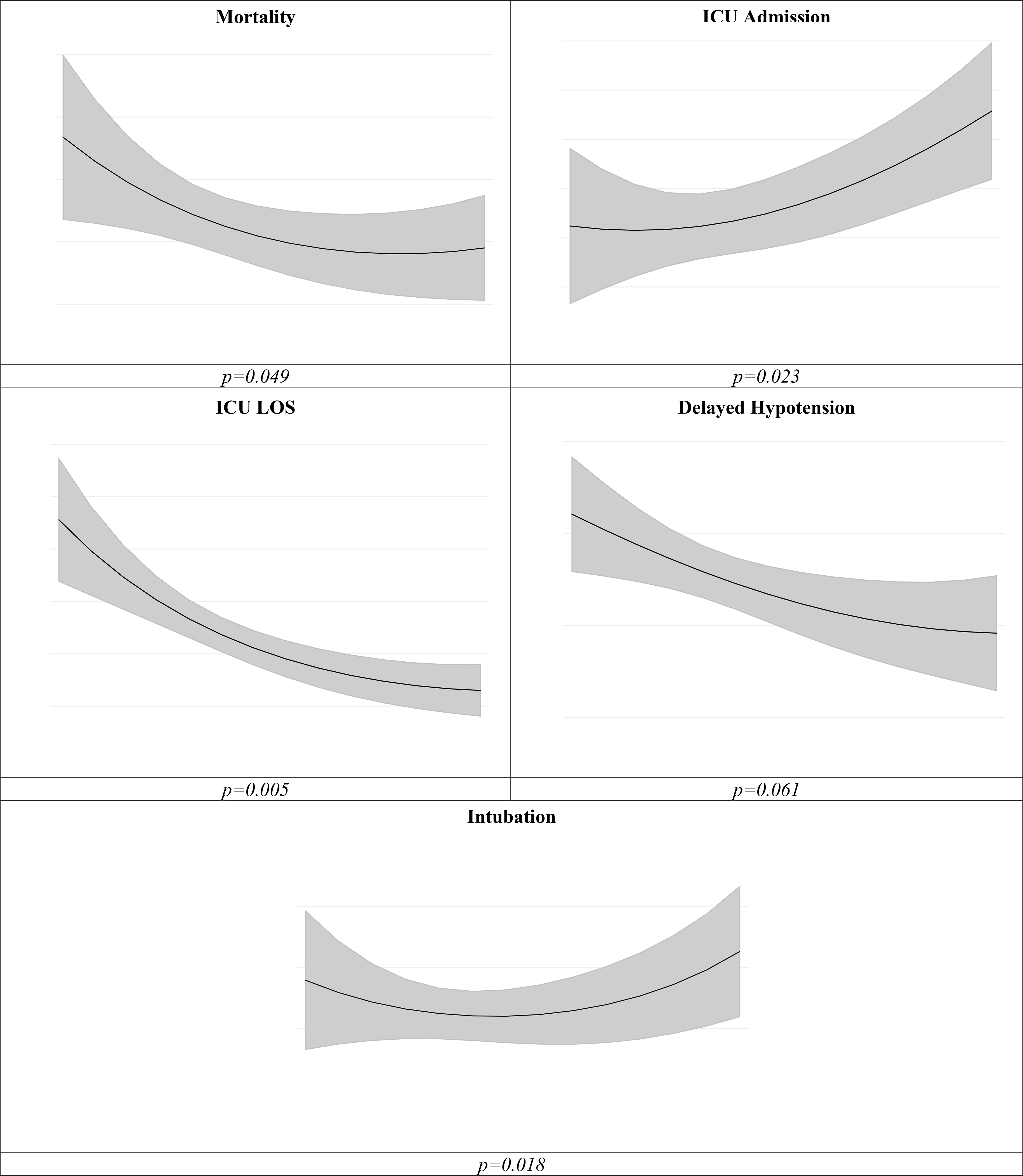
Figure 1 estimates from the multivariable logistic or negative binomial regression (ICU LOS) models including a quadratic term for volume administered by 3 hours. Gray shaded area indicate 95% CI.
Subgroup Analysis:
Figure 2 demonstrates a subgroup analysis comparing the effects of 30after3 on mortality across various subgroups of patients. No significant 30after3 by subgroup interactions were noted.
Figure 2. Comparison of the Effect of 30after3 on Mortality Across Subgroups.
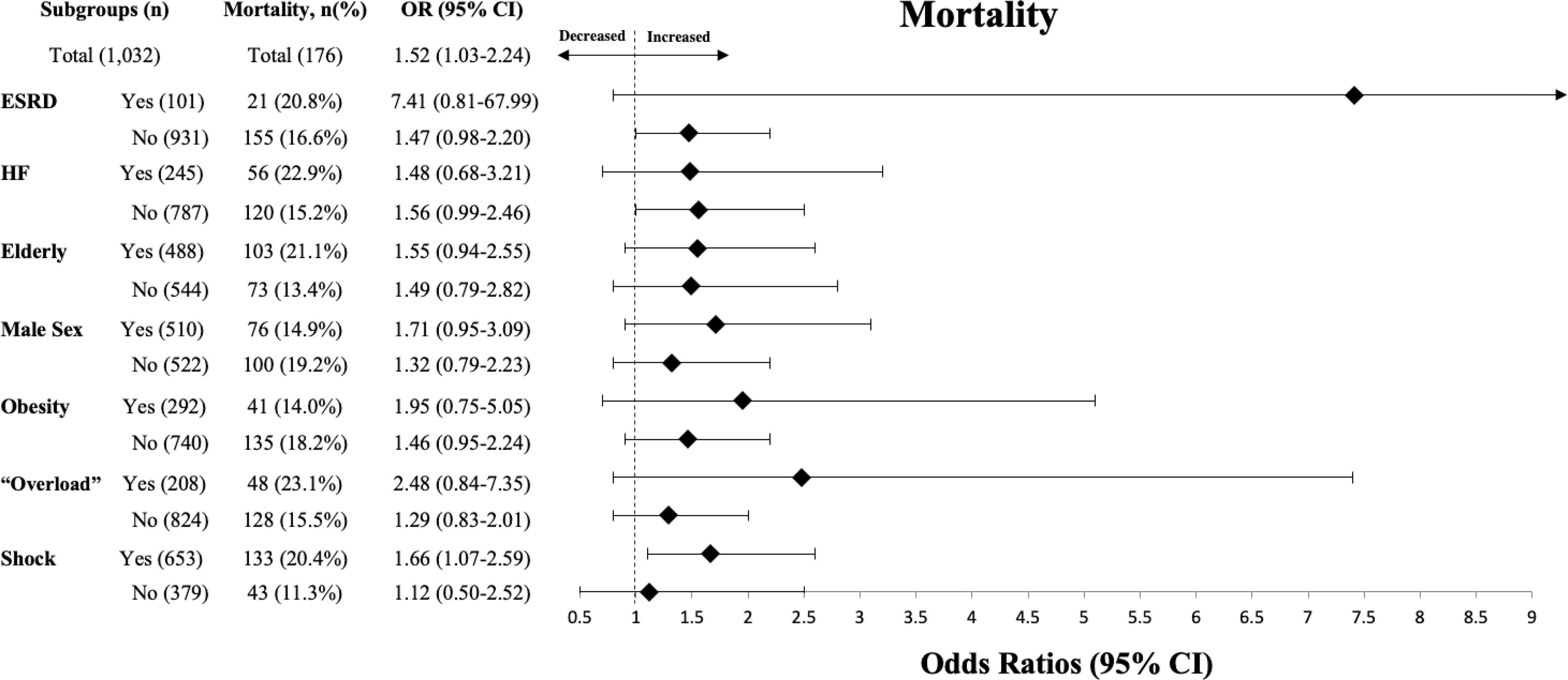
Figure 2 demonstrates a subgroup analysis comparing the effects of 30after3 on mortality across various subgroups of patients. An odds ratio >1 indicating increased odds of mortality for those failing to meet fluid goals when compared to those meeting fluid goals. When evaluating specific subgroups, no significant interaction effects were found.
DISCUSSION
Our study demonstrated that patients with severe sepsis and septic shock who were elderly, male, obese, with documented volume “overload” by bedside examination, and with a medical history of HF and ESRD were less likely to achieve 30by3. When controlling for various confounders, individuals who did not receive 30by3 were at increased odds of mortality, delayed hypotension, and increased ICU LOS. This study adds to growing literature supporting early, rapid fluid resuscitation in patients with severe sepsis and septic shock, regardless of their perceived risk of becoming volume overloaded.
Preexisting literature on fluid resuscitation in septic patients has not provided consistent results (6–15, 26). Subsequently, there exists some controversy—with some describing recommendations for early, rapid crystalloid resuscitation as “iatrogenic salt water drowning” (27). However, there are important differences in some of the cited evidence relative to our study. Some studies do not use a weight-based approach, while the interpretation of others has been extrapolated from cohorts not exclusive to septic patients or from findings based on fluid status surrogates, such as CVP, which is now known to be an unreliable predictor of fluid responsiveness (28, 29). Fewer studies focus on fluid resuscitation within the first several hours of presentation.
The timing of fluids in the setting of sepsis may have a significant impact on outcomes. Similar to our findings, studies evaluating early fluid resuscitation show mortality benefits (6–9, 30). This is counter to studies showing deleterious effects of fluids administered over more extended periods (within 12 hours to seven days) (11–15). There may be a “critical window” for intervention before a hypothesized irreversible degree of endothelial dysfunction has ensued (8, 31, 32). Leisman et al. demonstrated that patients with delayed fluid resuscitation were more likely to be fluid refractory compared to those who received earlier resuscitation (8). Our findings show that patients not receiving 30by3 have increased odds of mortality, as well as delayed hypotension. However, optimal targets for resuscitation remain unknown and will require further investigation.
Contrary to our findings, Seymour et al. did not find an association between reaching early fluid goals and mortality. However, this study’s definition of ‘time-zero’ was not standardized (i.e. triage vs. entry of an order set) and varied widely between participating sites. Furthermore, patients were excluded if they had not met fluid goals by 12 hours. When these patients were included in their analysis, a mortality benefit was found (10). Median times for reaching 30by3 in our study were near or greater than 12 hours for patients with obesity, HF, ESRD, or documentation of “overload,” which accounted for >50% of our cohort. The median fluid volumes at three hours for these patients ranged from 10.7–16.5 mL/kg, which was shown to have a more dramatic effect on mortality relative to higher volumes (Figure 1). In other studies involving the implementation of sepsis bundles, mortality benefits were found to be driven by an increase in fluid volumes given to patients with HF and ESRD specifically (33).
The SSC recommends a 30 mL/kg bolus of IV crystalloid be given to all patients with “sepsis-induced hypoperfusion” (5). This volume of fluid is thought to initiate resuscitation while awaiting more precise measurements of hemodynamic status. Despite this, patients continue to receive less than this recommended amount and often fluids are delayed in patients with perceived ‘at-risk’ conditions, such as HF or ESRD (9, 14). Our study expands on this, demonstrating that patients who are also elderly, obese, male, or with documentation of volume “overload” by bedside examination are under resuscitated in a similar manner. Our regression analyses demonstrate that failure to achieve the recommended 30by3 increased the odds of in-hospital mortality, delayed hypotension, and increased ICU LOS, irrespective of these conditions. However, a subgroup analysis examining the odds of mortality in patients failing to reach 30by3 demonstrated no interactive effects but was very likely underpowered to appropriately measure these effects in greater detail.
Finally, we attempted to examine what may be an optimal fluid volume. Our dose effect curves suggest survival benefit with increased doses of fluids, plateauing between 35–45 mL/kg (Figure 1), supporting SSC recommendations. A more dramatic decrease in mortality is observed for patients receiving 0–10mL/kg relative to the higher volumes, suggesting that a relationship between dosing of fluids, in addition to the timing, may influence odds of mortality. Interestingly, the same analysis also shows that the ~30–35 mL/kg volume to be the point at which the risk of intubation is the lowest, similar to a previous finding (9).
This study has several key limitations important to highlight. The findings in this study are subject to issues surrounding endogeneity. That is, in a retrospective analysis, is impossible to disentangle the relationship between fluid volume and mortality beyond accounting for potential confounders (i.e., septic phenotypes where patients are more likely to survive may receive more fluids and additionally, fluids may be withheld from patients perceived to have a higher likelihood of death). Further, the 30by3 group had inherent differences compared to the other group. Specifically, this group had higher incidence of septic shock, reduced time to antibiotics by ~1 hour, and likely increased recognition of sepsis with slightly higher rates of SIRS and qSOFA upon arrival. In addition, there may be additional confounding variables not controlled for in our regression analyses. Further, our data relied heavily on accurate documentation by providers and nurses (i.e., there was no objective way to determine when a fluid bolus was completed, other than nursing documentation). Next, our patient population was selected prospectively as part of a CMS-driven, quality-based initiative using Sepsis-2 criteria and using ICD codes which may differ from more recent analyses or miss atypical presentations. Finally, a MEDS score, versus more traditional tools like APACHE, was used to control for sepsis-related risk. However, the MEDS score was derived to predict risk of mortality for patients with sepsis in the ED and may outperform other scoring systems when applied to an ED population (34). Ultimately, causal inference will need to be determined in large, randomized, multicenter clinical trials to better understand and validate our findings.
CONCLUSION
Patients with severe sepsis and septic shock who do not receive the recommended 30 mL/kg IV fluid bolus within the first three hours of severe sepsis or septic shock diagnosis were at increased risk of in-hospital mortality, delayed hypotension, and increased ICU LOS. Predictors of inadequate resuscitation can be identified, potentially leading to actionable interventions to improve patient outcomes. However, these findings are retrospective, and future, prospective investigations specific to these special populations are required.
Supplementary Material
Acknowledgments
Financial support: This project was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) through Grant Number 5UL1TR002389–02 that funds the Institute for Translational Medicine (ITM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rivers E, Nguyen B, Havstad S, et al. : Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N Engl J Med 2001; 345:1368–1377 [DOI] [PubMed] [Google Scholar]
- 2.ProCESS Investigators, Yealy DM, Kellum JA, et al. : A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peake SL, Delaney A, Bailey M, et al. : Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506 [DOI] [PubMed] [Google Scholar]
- 4.Mouncey PR, Osborn TM, Power GS, et al. : Trial of Early, Goal-Directed Resuscitation for Septic Shock. N Engl J Med 2015; 372:1301–1311 [DOI] [PubMed] [Google Scholar]
- 5.Levy MM, Rhodes A, Phillips GS, et al. : Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015; 43:3–12 [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Ramar K, Park JG, et al. : Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: a retrospective cohort study. Chest 2014; 146:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leisman D, Wie B, Doerfler M, et al. : Association of Fluid Resuscitation Initiation Within 30 Minutes of Severe Sepsis and Septic Shock Recognition With Reduced Mortality and Length of Stay. Ann Emerg Med 2016; 68:298–311 [DOI] [PubMed] [Google Scholar]
- 8.Leisman DE, Doerfler ME, Schneider SM, et al. : Predictors, Prevalence, and Outcomes of Early Crystalloid Responsiveness Among Initially Hypotensive Patients With Sepsis and Septic Shock. Crit Care Med 2018; 46:189–198 [DOI] [PubMed] [Google Scholar]
- 9.Leisman DE, Goldman C, Doerfler ME, et al. : Patterns and Outcomes Associated With Timeliness of Initial Crystalloid Resuscitation in a Prospective Sepsis and Septic Shock Cohort. Crit Care Med 2017; 45:1596–1606 [DOI] [PubMed] [Google Scholar]
- 10.Seymour CW, Gesten F, Prescott HC, et al. : Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017; 376:2235–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd JH, Forbes J, Nakada T, et al. : Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39:259–265 [DOI] [PubMed] [Google Scholar]
- 12.RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, et al. : An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med 2012; 40:1753–1760 [DOI] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, et al. : Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575 [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Linde-Zwirble WT, Bittner EA, et al. : Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med 2017; 43:625–632 [DOI] [PubMed] [Google Scholar]
- 15.Sakr Y, Rubatto Birri PN, Kotfis K, et al. : Higher Fluid Balance Increases the Risk of Death From Sepsis: Results From a Large International Audit. Crit Care Med 2017; 45:386–394 [DOI] [PubMed] [Google Scholar]
- 16.Akhter M, Hallare M, Roontiva A, et al. : Fluid Resuscitation of Septic Patients at Risk for Fluid Overload. Ann Emerg Med 2017; 70:S61–S62 [Google Scholar]
- 17.Wacharasint P, Boyd JH, Russell JA, et al. : One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Crit Care Lond Engl 2013; 17:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arabi YM, Dara SI, Tamim HM, et al. : Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit Care Lond Engl 2013; 17:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou Dagher G, Harmouche E, Jabbour E, et al. : Sepsis in hemodialysis patients [Internet]. BMC Emerg Med 2015; 15 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4606908/ [DOI] [PMC free article] [PubMed]
- 20.Duttuluri M, Rose K, Shapiro J, et al. : Fluid Resuscitation Dilemma in Patients with Congestive Heart Failure Presenting with Severe Sepsis/Septic Shock [Internet]. In: D45. CRITICAL CARE: CIRCULATORY HEMODYMANICS, SHOCK, CARDIOVASCULAR DISEASE, AND FLUID MANAGEMENT. American Thoracic Society; 2016. p. A7048–A7048.Available from: 10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A7048 [DOI] [Google Scholar]
- 21.Palomba H, Corrêa TD, Silva E, et al. : Comparative analysis of survival between elderly and non-elderly severe sepsis and septic shock resuscitated patients. Einstein Sao Paulo Braz 2015; 13:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Specifications Manual for National Hospital Inpatient Quality Measures | Joint Commission [Internet]. [cited 2018 Nov 21] Available from: http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx
- 23.Shapiro NI, Wolfe RE, Moore RB, et al. : Mortality in Emergency Department Sepsis (MEDS) score: a prospectively derived and validated clinical prediction rule. Crit Care Med 2003; 31:670–675 [DOI] [PubMed] [Google Scholar]
- 24.Kaji AH, Schriger D, Green S: Looking Through the Retrospectoscope: Reducing Bias in Emergency Medicine Chart Review Studies. Ann Emerg Med 2014; 64:292–298 [DOI] [PubMed] [Google Scholar]
- 25.Andersen PK, Syriopoulou E, Parner ET: Causal inference in survival analysis using pseudo-observations. Stat Med 2017; 36:2669–2681 [DOI] [PubMed] [Google Scholar]
- 26.Andrews B, Semler MW, Muchemwa L, et al. : Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA 2017; 318:1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marik PE: Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care 2014; 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kox M, Pickkers P: “Less is more” in critically ill patients: not too intensive. JAMA Intern Med 2013; 173:1369–1372 [DOI] [PubMed] [Google Scholar]
- 29.Marik PE, Baram M, Vahid B: Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008; 134:172–178 [DOI] [PubMed] [Google Scholar]
- 30.Lane DJ, Wunsch H, Saskin R, et al. : Association Between Early Intravenous Fluids Provided by Paramedics and Subsequent In-Hospital Mortality Among Patients With Sepsis. JAMA Netw Open 2018; 1:e185845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson P, Stensballe J, Ostrowski S: Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism [Internet]. Crit Care 2017; 21 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5299749/ [DOI] [PMC free article] [PubMed]
- 32.Goldenberg NM, Steinberg BE, Slutsky AS, et al. : Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 2011; 3:88ps25. [DOI] [PubMed] [Google Scholar]
- 33.Liu VX, Morehouse JW, Marelich GP, et al. : Multicenter Implementation of a Treatment Bundle for Patients with Sepsis and Intermediate Lactate Values. Am J Respir Crit Care Med 2016; 193:1264–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JM, Greenslade JH, Chu K, et al. : Severity Scores in Emergency Department Patients With Presumed Infection: A Prospective Validation Study. Crit Care Med 2016; 44:539–547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


