Abstract
Background
Subarachnoid neurocysticercosis (SANCC) represents the most severe and difficult to treat form of neurocysticercosis. The inflammatory response contributes significantly to the morbidity and mortality of the disease. This study sought to understand the nature and evolution of the inflammation associated with SANCC, and evaluate for predictors of time to cure.
Methods
There were 16 subjects with SANCC (basilar cistern, sylvian fissure, and/or spinal involvement) during active infection who had cerebrospinal fluid (CSF) cytokine and chemokine profiling, of whom 9 had a second CSF sample at (or following) the time of cure. The relationships between clinical parameters and cytokine/chemokine results were assessed.
Results
Compared to pools of healthy donor CSF, those with active SANCC showed a significant (P < .05) increase in chemokines and cytokines associated with Type 1 immunity (interferon [IFN] γ, interleukin [IL] 12p70, C-X-C Motif Ligand 10 CXCL-10); Type 2 immunity (IL-10, IL-13); IFNα2; and the chemokines Macrophage inflammatory protein MIP-1α/CCL3, MIP-1ß/CCL4, and Vascular Endothelial Growth Factor VEGF that appears to be locally (central nervous system [CNS]) produced. Compared to those with active disease, those with CSF taken at the time of cure showed a significant decrease in most of these chemokines and cytokines. Despite this, CSF from cured SANCC patients had levels of IL-10 (P = .039), CXCL-10 (P = .039), and IL-12p70 (P = .044) above those seen in CSF from uninfected subjects. High ratios of IL-12p70/IL-10 early in infections were associated with a shorter time to cure (r = −0.559; P = .027), and a high Taenia solium burden (by quantitative polymerase chain reaction) was associated with longer times to cure (r = 0.84; P = .003).
Conclusions
SANCC is associated with a marked, CNS-localized cytokine-/chemokine-driven inflammatory response that largely decreases with curative therapy, though some analytes persisted above the normal range. The relative balance between proinflammatory and regulatory cytokines may be an important determinant for a cure in SANCC.
Keywords: neurocysticercosis, subarachnoid, extra-parenchymal, cerebral spinal fluid, inflammation
Cerebrospinal fluid cytokine and chemokine elevations in subjects with subarachnoid neurocysticercosis (SANCC) early in treatment and changes that occur following cure are correlated with clinical parameters. The balance of Type 1/regulatory cytokines may be important in achieving cure in SANCC.
Neurocysticercosis (NCC) is a parasitic infection characterized by the presence of the larval stage of the pork tapeworm Taenia solium in the central nervous system (CNS). NCC is of significant global health importance, as it is responsible for up to 60% of all epilepsy in endemic areas [1]. In recent years, nonendemic areas have recognized the increased burden of imported cases, including the United States [2, 3].
Symptoms of NCC are caused by T. solium cysts in the CNS that provoke an inflammatory response once their cyst wall is damaged due to natural involution or to anthelmintic therapy [4]. Patients with parenchymal NCC most commonly present with seizures. Less commonly, cysts can grow outside of the brain parenchyma. Subarachnoid NCC (SANCC), the most severe form of extra-parenchymal NCC, is characterized by the presence of an aberrant, proliferative form of the parasite (racemose) in the subarachnoid space of the brain. The mass effect exerted by the cysts, aseptic meningitis caused by the exuberant inflammatory response, and communicating hydrocephalus from the inflammatory scarring of the meninges are responsible for the majority of acute symptoms, the control of which is paramount in successful clinical care [5]. The treatment of SANCC requires prolonged antiparasitic drugs, paired with anti-inflammatory therapy, most commonly corticosteroids, to target both the parasitic infection and the host inflammatory response [6].
Previous studies have found that active NCC disease produces local inflammation in the brain.
Degenerating parenchymal lesions have been associated with an eosinophil-rich granulomatous inflammation, with high tissue levels of interferon (IFN) γ, interleukin (IL) 18, IL-4, Transforming Growth Factor (TGFβ) [7], and IL-12 [4], and low levels of IL-10 and IL-13 [7]. Cerebrospinal fluid (CSF) from SANCC patients, when compared to CSF from those with parenchymal NCC, has been shown to have elevated levels of IL-5, IL-6, and IL-10 [8, 9].
This study aimed to understand the nature of the inflammation in the CSF of subjects with active SANCC in a more comprehensive way, and to understand how the inflammation evolves with cure. By examining the interrelationships between these drivers of inflammation and some of the critical clinical parameters, we may be better able to target specific host pathways with anti-inflammatory ancillary treatment, in concert with anthelmintics.
METHODS
Samples
This was a retrospective analysis on stored patient samples. Patient CSF and serum samples were obtained as part of National Institute of Allergy and Infectious Diseases’ institutional review board–approved protocols (85-I-0127 for NCC). In accordance with routine clinical care, the CSF, serum, and plasma samples of patients with NCC are collected and stored at −80°C. Informed written consent was obtained from all patients. Because patients are referred from outside facilities, they are often on antiparasitics at the time of initial evaluation, when a lumbar puncture (LP) is performed (Table 1).
Table 1.
Clinical Characteristics of Subjects and Cerebrospinal Fluid Parameters
| Subject number | Cluster, Fig 1 | Sex, M/F | Age, active | Subarachnoid cyst location | Paired/unpaired | Active time point LP | Cure time point LP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total anthelmintics previously, months | Time to cure, months | Steroid dose, prednisone equivalent | Other immuno-modulator | CSF WBC, cells/mm3 | CSF glucose, mg/dL | CSF TsAg, ng/mL | CSF qPCR, Dq value | Steroid dose, prednisone equivalent | Other immuno-modulator | CSF WBC, cells/mm3 | CSF glucose, mg/dL | CSF TsAg, ng/mL | CSF qPCR, Cq value | Follow-up, years | ||||||
| 1 | C | F | 41 | Spine | Paired | 0 | 16.8 | 35 | None | 30 | 47 | 4000 | 30.0 | 8.75 | Etanercept, anakinra | 18 | 54 | 0 | No amplification | 1 |
| 2 | B | M | 39 | Basilar cistern | Paired | 6 | 50.8 | 40 | None | 174 | 89 | 751 | 20.1 | 0 | None | 10 | 116 | 0 | No amplification | 4 |
| 3 | C | M | 51 | Basilar cistern, sylvian fissurea | Paired | .25 | 9.3 | 53.3 | None | 16 | 54 | 529 | 29.6 | 0 | Methotrexate | 2 | 53 | 0 | No amplification | 5.3 |
| 4 | B | F | 41 | Basilar cistern, sylvian fissure | Paired | 0 | 47.1 | 53.3 | None | 15 | 48 | 2624 | 27.4 | 0 | None | 5 | 53 | 0 | No amplification | 2.5 |
| 5 | B | M | 31 | Basilar cistern, sylvian fissureb | Paired | 4 | 21.7 | 0 | Methotrexate | 135 | 28 | 101 | 26.6 | 0 | None | 9 | 52 | 1.5c | 35.5 | 1.6 |
| 6 | B | M | 36 | Spine | Paired | 5 | 13.2 | 0 | Methotrexate | 51 | 43 | 440 | 30.4 | 0 | Etanercept | 3 | 37 | 0 | 36.0 | 8.3 |
| 7 | B | M | 41 | Sylvian fissure, basilar cistern | Paired | 4 | 12.2 | 7.5 | None | 7 | 52 | 11 | 30.3 | 0 | None | 45 | 50 | 0 | 35.2 | 2.7 |
| 8 | A | M | 51 | Spine, basilar cistern | Paired | 15 | 18.4 | 0 | None | 34 | 43 | 157 | 26.8 | 0 | Methotrexate | 3 | 62 | 0 | 36.0 | 8.3 |
| 9 | A | M | 57 | Sylvian fissure, spine | Paired | 14 | 13.7 | 53.3 | None | 3 | 29 | 119 | 29.5 | 10 | Methotrexate, etanercept | 5 | 55 | 0 | No amplification | 5 |
| 10 | B | F | 28 | Spine | Unpaired | 13 | 4.7 | 0 | None | 12 | 44 | 578 | 36.9 | … | … | … | … | … | … | … |
| 11 | C | M | 33 | Basilar cistern, sylvian fissurea | Unpaired | .25 | 6.4 | 0 | None | 44 | 36 | 871 | 28.8 | … | … | … | … | … | … | … |
| 12 | C | M | 37 | Basilar cistern, sylvian fissureb | Unpaired | 2 | 15.1 | 106.7 | None | 7 | 95 | 147 | 26.5 | … | … | … | … | … | … | … |
| 13 | B | F | 42 | Basilar cistern, spinea,b | Unpaired | 1 | 23.0 | 133.3 | Methotrexate | 23 | 0 | 139 | 27.0 | … | … | … | … | … | … | … |
| 14 | C | F | 44 | Basilar cisternb | Unpaired | 2 | 79.0 | 10 | Methotrexate | 153 | 60 | 7600 | 23.1 | … | … | … | … | … | … | … |
| 15 | B | M | 40 | Basilar cistern, sylvian fissure, spine | Unpaired | 11 | 115.5 | 0 | Methotrexate | 55 | 38 | 20000 | 23.5 | … | … | … | … | … | … | … |
| 16 | C | M | 26 | Sylvian fissurea,b | Unpaired | 0 | 6.3 | 0 | None | 4 | 55 | 38 | No amplification | … | … | … | … | … | … | … |
| 17 | … | M | … | Basilar cistern | Unpaired | … | … | … | … | … | … | … | … | 0 | None | 6 | 63 | 0 | No amplification | 3 |
| 18 | … | F | … | Basilar cistern | Unpaired | … | … | … | … | … | … | … | … | 5 | None | 27 | 41 | 5.4c | 33.2 | 3.4 |
Abbreviations: CSF, cerebrospinal fluid; F, female; LP, lumbar puncture; M, male; qPCR, quantitative polymerase chain reaction; TsAg, Taenia solium antigen; WBC, white blood cell.
aAlso had ventricular disease.
bAlso had calcified parenchymal disease.
cTsAg negative at next LP without the use of anthelmintics.
The CSF from 16 patients with active SANCC was assessed. SANCC was defined by magnetic resonance imaging (MRI) demonstration of cysts in either the basilar cisterns, sylvian fissures, or subarachnoid space around the spinal cord. “Active” disease was defined as both requiring treatment (due to a combination of symptoms, MRI findings, and/or high CSF white blood cell [WBC] count) and having a positive Taenia solium antigen (TsAg) in the CSF [10]. All patients were either on anthelmintic treatment (albendazole and/or praziquantel) or were started on therapy following the LP for the active time point. There were 11 subjects that had CSF obtained at the time of cure; 9 of these subjects had paired samples from the active time point. “Cured” CSF was defined as CSF obtained at the time of or following discontinuation of anthelmintics. All cure samples had to either have undetectable TsAg or have become negative on subsequent LPs without further anthelmintic drugs. All cured patients studied were shown to have a durable cure through a yearly follow-up exam and an MRI, with a minimum follow-up of 1 year. To assess relative concentrations of analytes in the CSF compartment as compared to the peripheral blood, for each of the 9 patients with paired CSF samples, serum samples from the same time points also underwent cytokine and chemokine detection.
A total of 9 CSF samples from healthy individual donors (provided courtesy of Dr Avindra Nath) were combined into 3 pools, containing 3 different healthy donor CSF samples each. The 3 CSF pools were used as “healthy control” comparators.
Cytokine and Chemokine Concentrations
The Milliplex MAP Multiplex assay (MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel [HCYTMAG-60K-PX29]; Merck KGaA, Darmstadt, Germany) was run on the Luminex system to determine the CSF and serum levels of EGF, Eotaxin-1, G-CSF, GM-CSF, IFN-α2, IFN-γ, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17a, IL-1RA, IL-1α, IL-1ß, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, CXCL-10, MCP-1 (CCL2), MIP-1α (CCL3), and MIP-1ß (CCL4), VEGF, TGFß1, TGFß2, and TGFß3 in patients with SANCC. See the Supplementary Data for more information.
Clinical and Laboratory Data
All CSF samples were subjected to DNA extraction and quantitative polymerase chain reaction (qPCR) for T. solium, as previously described [11]. Samples were also tested for TsAg [10], as per the manufacturer’s instructions (ApDia, Turnhout, Belgium). All subjects underwent a chart review for additional clinical parameters. The total time that subjects had been on anthelmintic (albendazole and/or praziquantel) therapy was calculated in months (and included the time prior to National Institutes of Health admission). The time to clinical cure was calculated from the date of CSF collection and when anthelmintics were stopped. WBC counts and glucose from the analyzed CSF samples were extracted from the chart. Total steroid dosage, converted to prednisone equivalent, at the time of CSF collection was recorded, as well as use of steroid-sparing immunomodulators.
Statistical Analysis
The statistical analysis was performed using Prism 7 (GraphPad Software; San Diego, CA). Nonparametric Mann-Whitney tests were used to separately compare unpaired samples between the (unpaired) viable disease samples with normal pools and the cured samples with normal pools. Nonparametric Wilcoxon tests were used to compare both CSF and serum samples, paired over the course of disease. For samples from the active time point with paired serum and CSF, a protein correction factor for analytes measured from CSF was calculated based on a ratio of CSF total protein to serum total protein. All P values less than .05 were considered to be statistically significant. Correlation matrices were constructed using the cytokine/chemokines that were most elevated during the active time period (G-CSF, GM-CSF, IFN-α2, IFN-γ, IL-10, IL-12p40, IL-12p70, IL-13, IL-17A, IL-4, IL-5, IL-6, IL-8, CXCL-10, MCP-1, MIP-1a, MIP1b, VEGF, IL-1RA) and were correlated with TsAg, qPCR (samples with no amplification were considered to have a quantification cycle (Cq) value of 40), CSF WBC, CSF glucose, steroid dose, time on anthelmintic therapy, and time to clinical cure. Correlation matrix P values were only considered significant after Bonferroni-Dunn correction for multiple comparisons. Cytokine concentration standardization and hierarchical clustering of cytokine concentrations were created using JMP (SAS Institute Inc., Cary, NC). The correlation heat map, relating cytokine concentrations and additional factors of interest, was creating using JMP Genomics (SAS Institute Inc., Cary, NC). Additionally, TGFß-1 and TGFß-2 concentrations were correlated with the time to cure.
RESULTS
Clinical and Laboratory Data
The clinical and laboratory parameters at the time of the active disease LP are shown in Table 1. At the active time point, there was 1 patient with a negative qPCR CSF result for T. solium DNA who was asymptomatic at the time of incidental discovery of disease. At the time of cure, there were 2 subjects with detectable TsAg levels (Table 1). These 2 subjects remained off anthelmintics but had subsequent CSF samples that demonstrated negative TsAg, and were considered to be cured from the time anthelmintics were stopped. Concurrent immunomodulators are listed in Table 1. Just over half (9 of 16) of patients were on corticosteroids at the time of active disease LP.
CSF Cytokine and Chemokine Levels in Active SANCC
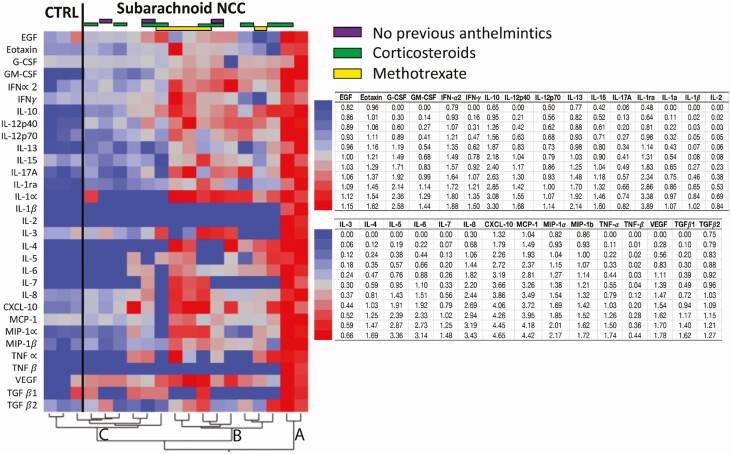
Overall, there was significant heterogeneity between the degree to which those with active SANCC and healthy controls elaborated cytokines and chemokines (Figure 1). As demonstrated in the heat map and dendrogram (Figure 1), all measured analytes across the SANCC subjects and 9 uninfected CSF samples (3 pools) could be described by 3 clusters. Cluster A includes 2 subjects that had high concentrations of all tested analytes relative to all other subjects. Cluster C includes the 3 uninfected pools and 6 other SANCC patients (including an asymptomatic subject diagnosed incidentally on imaging) that have the lowest CSF concentrations of all analytes. Cluster B consists of 8 SANCC subjects that fall in between these “low” and “high” responders. Interestingly, the CSF WBC levels did not predict those subjects with the highest cytokine levels. For instance, the 2 “high responders” had CSF WBC counts of 3 and 34 cells/mm3, respectively, whereas the median CSF WBC count for all CSF samples was 26.5 during active disease. While overall the “low responders” cluster did have a median CSF WBC count of 16 WBC/mm3, less than the overall median, 1 subject in this group had a CSF WBC count of 153 WBC/mm3. Thus, the WBC count generally appeared to be an unreliable predictor of the degrees of cytokine/chemokine elevations in the CSF.
Figure 1.
Heat map of differential expression of CSF cytokines in active SANCC and healthy donors. The heat map and corresponding dendrogram show the hierarchical clustering of log transformed and standardized cytokine and chemokine concentrations in the CSF of patients with active disease and of healthy individuals. The cytokines input into the clustering are shown and includes any analytes where there were detectable results. CSF of subjects with active SANCC clustered into 3 groups: Group A are the high, Group B the intermediate, and Group C the low cytokine/chemokine producers. Group C includes the uninfected healthy controls, as well as an asymptomatic incidentally discovered subject with SANCC. Abbreviations: CSF, cerebrospinal fluid; CTRL, control group; IFN, interferon; IL, interleukin; NCC, neurocysticercosis; SANCC, subarachnoid neurocysticercosis; TNF, tumor necrosis factor.
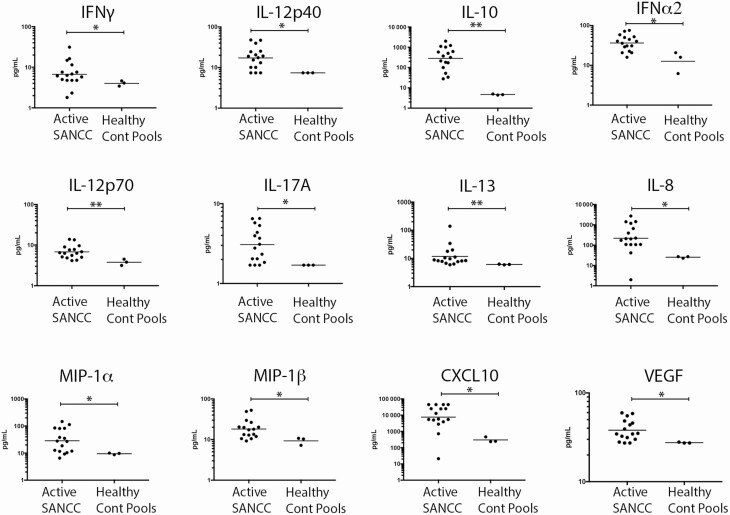
CSF samples from those with active SANCC (Figure 2) showed marked increases in the Type 1–associated analytes IFN-γ (P = .046), IL-12p70 (P = .008), IL-12p40 (P = .032), and CXCL-10 (P = .011). Moreover, the regulatory cytokine IL-10 (P = .002), as well as Type 2 cytokine IL-13 (P = .008), were similarly elevated in samples with active SANCC. Proinflammatory cytokines IL-17A (P = .032) and IFNα2 (P = .011); the chemokines IL-8 (P = .013), MIP-1α/CCL3 (P = .033), and MIP-1ß/CCL4 (P = .022); and the growth factor VEGF (P = .028) were also significantly elevated during active disease as compared with CSF samples of uninfected donors. All other analytes tested showed no significant difference between active SANCC CSF compared to healthy donor CSF.
Figure 2.
There were significantly elevated CSF cytokine concentrations in subjects with active SANCC, compared to healthy donors. Concentrations (pg/ml) of analytes are shown from 16 individual, unpaired CSF samples from patients with active SANCC and 3 pools (9 total subjects) of healthy donor CSF. Only shown are the cytokines that have significantly different concentrations between active subarachnoid disease CSF samples and the healthy controls. The horizontal line denotes the geometric mean. Concentrations are on a log scale. *P = .01–.05; **P < .01. Abbreviations: Cont, control; CSF, cerebrospinal fluid; IFN, interferon; IL, interleukin; SANCC, subarachnoid neurocysticercosis.
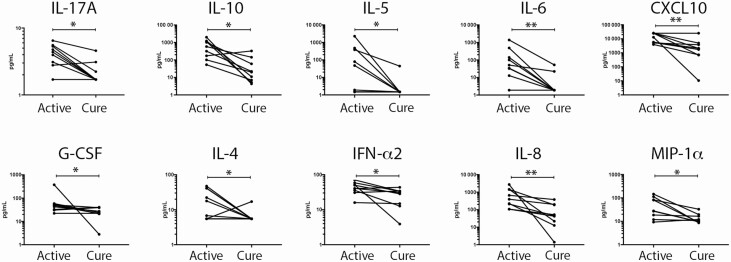
CSF Cytokine/Chemokine Changes in SANCC With Cure
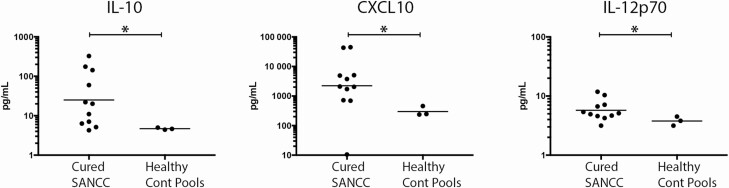
Using paired CSF samples that were available for subjects both at the active disease time point and cure (n = 9), we were able to demonstrate that the cure of SANCC was associated with significant decreases in G-CSF (P = .039), IFN-α2 (P = .039), IL-10 (P = .02), IL-17a (P = .016), IL-4 (P = .047), IL-5 (P = .031), IL-6 (P = .008), IL-8 (P = .004), CXCL-10 (P = .004), and MIP-1α (P = .02; Figure 3). This suggests, not surprisingly, that a cure is associated with normalization of many cytokines. However, not all analytes completely normalized, and in fact some stayed quite elevated. As shown in Figure 4 CXCL-10 (P = .039), IL-12p70 (P = .041), and (despite its significant fall) IL-10 (P = .039) were still elevated above the normal range when compared to healthy donor CSF samples. These elevations likely reflect persistent inflammation despite the cure.
Figure 3.
Cytokines and chemokines significantly change in SANCC CSF over time. There were 9 SANCC subjects with paired CSF samples measured for all analytes at both an active and cured disease time point. Each individual’s active and cured CSF analyte concentrations are connected by a horizontal line. Concentrations (pg/ml) of analytes that significantly change over time are shown (log scale). *P = .01–.05; **P < .01. Abbreviations: Cont, control; CSF, cerebrospinal fluid; IFN, interferon; IL, interleukin; SANCC, subarachnoid neurocysticercosis.
Figure 4.
CSF cytokines were significantly elevated following cure. Unpaired CSF from 11 SANCC subjects following cure, as compared with 3 pools of healthy donor CSF (9 total subjects), demonstrate significant elevations in IL-10, CXCL-10, and IL12p70 following cure. The horizontal line denotes the geometric mean. *P < .05. Abbreviations: Cont, control; CSF, cerebrospinal fluid; IL, interleukin; SANCC, subarachnoid neurocysticercosis.
Cytokine/Chemokines are Locally Produced in the CNS
Serum was obtained at the same time as CSF and was likewise tested from the 9 subjects with paired CSF samples (Supplementary Figure 2). On unadjusted measurement, all but a few analytes (EGF, Eotaxin-1, TGFß1, and TGFß2) had significantly higher concentrations in the CSF samples compared to those levels found in the serum, suggesting that each of these analytes were being produced locally and that the levels do not merely show a transudation from the vascular compartment (see Supplementary Figure 1). The relative increase in CSF compared with serum levels during active disease was most pronounced (P < .01) for IL-10, IL1Rα, IL-13, IL-12p40, IFN-γ, G-CSF, IFN-α2, IL-6, IL-8, CXCL-10, and MCP-1. When CSF cytokine concentrations were normalized to total protein for comparison to serum levels, the CSF concentrations of GM-CSF, IL-12p70, IL-15, IL-17A, MIP-1α, MIP1ß, VEGF, and TGFb2 were additionally very significantly elevated (P < .01) compared to serum. The aforementioned analytes were between 30 to 105 times more concentrated in CSF compared with serum. Moreover, the paired serum samples did not show any changes in cytokine or chemokine levels over time. Thus, it appears the increased chemokine and cytokine concentrations seen during active SANCC are locally produced in the CNS.
Clinical and Laboratory Data Correlations
To understand the interrelationship among the cytokines/chemokines measured and the clinical parameters defined by continuous variables (WBC, glucose, CNS parasite DNA, time to cure), correlation matrices were examined. The parasite burden, as determined by the reciprocal qPCR Cq value from the CSF, was significantly correlated with the time to cure (where a higher parasite burden required more time until a clinical cure; R = 0.84; P = .003). The CSF glucose levels were inversely associated with levels of CXCL-10 (R = −0.79; P = .012), MIP-1α (R = −0.77; P = .018), and MIP-1ß (R = −0.73; P = .044). The CSF WBC count weakly correlated with the parasite burden (r = −0.61), time to cure (r = 0.6), and IL-10 levels (r = 0.52), but these correlations were not significant after correcting for multiple comparisons (P > .05). The total duration of anthelmintics prior to obtaining CSF (including all previous courses) was significantly associated with levels of IL-4 (R = 0.91; P < .001), IL-5 (R = 0.81; P = .009), and IL-10 (R = 0.77; P = .021). The Spearman correlation showed no relationship between TGFß-1 or TGFß-2 and the time to cure. The steroid dosage and TsAg concentration did not correlate with any parameter studied.
Given the observation that IL-10 levels significantly fell during treatment, while IL-12p70 and, to a lesser extent, IFN-γ remained elevated, we did a simple correlation analysis between the ration of the levels of IL-12p70/IL-10 or of IFN-γ /IL-10 and the time to cure. We found that IL-12p70/IL10 levels were inversely correlated with the time to cure (r = −0.559; P = .026), whereas IFN-γ /IL-10 levels (r = −0.468; P = .07) were not.
Cytokine-to-Cytokine Correlations
A correlation analyses of the most elevated cytokines and chemokines (from Figures 2 and 3) revealed cytokine/chemokine interrelationships and provides potential insight into their regulation. The levels of IL-10 were highly correlated (P < .01) with the levels of IL-5, IL-4, and IL-6, and moderately correlated (P < .05) with the levels of IL-1RA, IL-13, and IL-12p70. The CSF levels of IL-12p70 were highly correlated with the levels of IL-17A (P < .001; and IL-12p40, as expected), as well as IL-6 (P = .001), IL-4 (P = .001), and IFN-α2 (P = .002), and were statistically significantly correlated (P < .05), with MIP-1b, MCP-1, GM-CSF, and IL-10. MIP-1α was most highly correlated with IL-8 (P < .001), CXCL-10 (P < .01), and IL-1ra (P < .01), and less so with IFN-γ and MIP-1b (P < .025). Full correlation matrices data can be found in Supplementary Figure 2.
DISCUSSION
In this study, we focused on the CSF compartment of those with SANCC (ie, involving the basilar cisterns, sylvian fissures, or spine) to define in a comprehensive way the cytokine and chemokine profile during active disease and the changes that occur with a cure. In the course of clinical care of these patients, controlling the inflammatory response is crucial, as inflammation is a large contributor to morbidity and mortality [5, 6]. Currently, corticosteroids are most often used to decrease this inflammation. However, their use is associated with significant side effects and may contribute to sustained parasite viability [12]. A better understanding of what inflammation controls the growth of the organism and is associated with a successful cure is essential to developing targeted immunomodulatory therapies.
Among those with active SANCC, there was significant heterogeneity in CSF cytokine and chemokine levels (Figure 1). Heterogeneity in markers of inflammation has been found in other studies [8], and may reflect patients being at different points on a cyclic waxing and waning of the CSF inflammation [5], inter-patient set-point variability, or both. Notably, a correlation analyses failed to find a relationship between CSF WBC counts and any particular biomarker; indeed, some of the CSF with the highest cytokine/chemokine levels had relatively lower WBC counts. Nonetheless, as a group, SANCC patients had significant elevations of Type 1 and Type 2 cytokines, macrophage and cytotoxic T cell chemokines, IFNα2, and VEGF. Interestingly, at the time of cure, above-normal CSF levels persisted for IL-12p70, CXCL-10, IL-10, and IFN-γ. The persistence of high IL-12 and IFN-γ levels in the CSF of those with SANCC is similar to the cytokine elevations seen in antigen-driven PBMCs peripheral blood mononuclear cells from patients with SANCC [13]. For IL-10, it has been suggested that regulatory T cells are the primary source of IL-10 in SANCC [14, 15] and that IL-10–producing regulatory CD4+ T cells are increased in treatment-refractory subjects [16]. Our observation that a higher IL-12/IL-10 balance correlates with less time to a cure supports these findings and suggests that the balance between T helper type 1 Th1-associated cytokines and certain regulatory cytokines may dictate the level of refractoriness to treatment in SANCC. While TGFß elevations have been found in those with less treatment-responsive SANCC [17, 18], we were unable to find a relationship between TGFß1 or TGFß2 concentrations and the duration of anthelmintics needed to achieve cure. This may be reflective of differences in defining a “response” in the literature and our definition of durable cure and/or the treatment experience in our patient group.
The correlation of CSF IL-4, IL-5, and IL-10 levels with total previous anthelmintic treatment duration may reflect a treatment effect. CSF antigen levels spike and fall with recurrence and treatment cycles [5]; in SANCC, IL-10 production by PBMCs has been shown to be dependent on the parasite antigen concentration [13]. These data raise the question as to whether the IL-12/IL-10 ratio is a potentially modifiable risk factor for treatment-refractory disease, which could be mitigated with initial aggressive treatment approaches so as to avoid repeated short cycles that expose the CNS to repeated bursts of antigen.
Interestingly, while no single analyte measured could predict the time to cure, the relative T. solium DNA concentration was highly inversely correlated with the time to cure, suggesting that qPCR may be a more dynamic reflection of treatment response than previously appreciated [5].
There are several limitations to this study. The sample size is small. Some CSF samples may have undergone previous freeze-thaw cycles, thereby underestimating the importance of some heat-labile analytes. The use of methotrexate and etanercept in subjects may have affected cytokine concentrations. Etanercept has been shown to decrease some of the cytokines assessed here (MIP-1a, IL-17A, IL-8, CXCL-10, IL-6) in the blood of subjects receiving this drug [19], but whether this affects CSF local production is not proven. Importantly, blood levels of IL-10, IL-12p70, GM-CSF, and IFN-γ have been shown to not be significantly altered in patients taking etanercept, with or without methotrexate [20]. If the IL-12/IL-10 balance is important in controlling the parasite, the persistent elevations in these cytokines despite the use of etanercept may contribute to the reported success of using this agent in SANCC [5].
Overall, proinflammatory and regulatory cytokine and chemokine concentrations were found to be increased in the CSF of patients with active SANCC, and decreased over the course of treatment. However, some low-grade CNS inflammation remained at the time of cure. This fairly comprehensive profiling of local inflammation in SANCC helps provide a basis for exploring the associations and roles of relative cytokine changes over time in treatment success. The strengths of this analysis include the longitudinal cytokine changes over time, the high degree of certainty of cure, and the robust correlations of various factors with the time to achieving cure. Significant knowledge gaps remain in terms of what creates the most collateral damage for the patient in this inflammatory milieu, and how best to control it. Beyond parasite control, given that systemic IFN-α induces neuropsychiatric symptoms [21] and the Type 1 [22] and regulatory/Type 2 CSF cytokine increases [23] seen here have been suggested to be associated with neurodegeneration, delineating how long these perturbations persist in the CNS and what drugs alter this course may be important questions to answer in improving outcomes in SANCC.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Debacq G, Moyano LM, Garcia HH, et al. Systematic review and meta-analysis estimating association of cysticercosis and neurocysticercosis with epilepsy. PLOS Negl Trop Dis 2017; 11:e0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O’Neal SE, Flecker RH. Hospitalization frequency and charges for neurocysticercosis, United States, 2003–2012. Emerg Infect Dis 2015; 21: 969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Croker C, Redelings M, Reporter R, Sorvillo F, Mascola L, Wilkins P. The impact of neurocysticercosis in California: a review of hospitalized cases. PLOS Negl Trop Dis 2012; 6:e1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Restrepo BI, Llaguno P, Sandoval MA, Enciso JA, Teale JM. Analysis of immune lesions in neurocysticercosis patients: central nervous system response to helminth appears Th1-like instead of Th2. J Neuroimmunol 1998; 89:64–72. [DOI] [PubMed] [Google Scholar]
- 5. Nash TE, O’Connell EM, Hammoud DA, Wetzler L, Ware JM, Mahanty S. Natural history of treated subarachnoid neurocysticercosis. Am J Trop Med Hyg 2020; 102:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White AC, Coyle CM, Rajshekhar V, et al. Diagnosis and treatment of neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg 2018; 98:945–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Restrepo BI, Alvarez JI, Castaño JA, et al. Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile. Infect Immun 2001; 69:4554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chavarría A, Fleury A, García E, Márquez C, Fragoso G, Sciutto E. Relationship between the clinical heterogeneity of neurocysticercosis and the immune-inflammatory profiles. Clin Immunol 2005; 116:271–8. [DOI] [PubMed] [Google Scholar]
- 9. Sáenz B, Fleury A, Chavarría A, et al. Neurocysticercosis: local and systemic immune-inflammatory features related to severity. Med Microbiol Immunol 2012; 201:73–80. [DOI] [PubMed] [Google Scholar]
- 10. Brandt JR, Geerts S, De Deken R, et al. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol 1992; 22:471–7. [DOI] [PubMed] [Google Scholar]
- 11. O’Connell EM, Harrison S, Dahlstrom E, Nash T, Nutman TB. A novel, highly sensitive qPCR assay for the diagnosis of subarachnoid and ventricular neurocysticercosis and for assessing response to treatment. Clin Infect Dis 2019; 9:1875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palomares-Alonso F, Toledo A, Palencia Hernández G, Jung-Cook H, Fleury A. Effect of dexamethasone on albendazole cysticidal activity in experimental cysticercosis by Taenia crassiceps in BALB/c mice: in vitro and in vivo evaluation. Exp Parasitol 2020; 208:107801. [DOI] [PubMed] [Google Scholar]
- 13. Tuero I, Palma S, Cabeza F, et al. ; Cysticercosis Working Group in Perú . A comparative study of peripheral immune responses to Taenia solium in individuals with parenchymal and subarachnoid neurocysticercosis. PLOS Negl Trop Dis 2015; 9:e0004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adalid-Peralta L, Fleury A, García-Ibarra TM, et al. Human neurocysticercosis: in vivo expansion of peripheral regulatory T cells and their recruitment in the central nervous system. J Parasitol 2012; 98:142–8. [DOI] [PubMed] [Google Scholar]
- 15. Arce-Sillas A, Alvarez-Luquin DD, Cardenas G, et al. Interleukin 10 and dendritic cells are the main suppression mediators of regulatory T cells in human neurocysticercosis. Clin Exp Immunol 2016; 183:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arce-Sillas A, Cárdenas G, Álvarez-Luquín D, et al. Treatment-resistant human extraparenchymal neurocysticercosis: an immune-inflammatory approach to cysticidal treatment outcome. Neuroimmunomodulation 2018; 25:103–9. [DOI] [PubMed] [Google Scholar]
- 17. Adalid-Peralta L, Rosas G, Arce-Sillas A, et al. Effect of transforming growth factor-β upon Taenia solium and Taenia crassiceps cysticerci. Sci Rep 2017; 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cárdenas G, Fragoso G, Rosetti M, et al. Neurocysticercosis: the effectiveness of the cysticidal treatment could be influenced by the host immunity. Med Microbiol Immunol 2014; 203:373–81. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Tomalin L, Lee J, et al. Reduction of inflammatory and cardiovascular proteins in the blood of patients with psoriasis: differential responses between tofacitinib and etanercept after 4 weeks of treatment. J Invest Dermatol 2018; 138:273–81. [DOI] [PubMed] [Google Scholar]
- 20. Takeshita M, Suzuki K, Kikuchi J, et al. Infliximab and etanercept have distinct actions but similar effects on cytokine profiles in rheumatoid arthritis. Cytokine 2015; 75:222–7. [DOI] [PubMed] [Google Scholar]
- 21. Wang BY, Amolat MJ, Woo P, Brandwein-Gensler M. Atypical mycobacteriosis of the larynx: an unusual clinical presentation secondary to steroids inhalation. Ann Diagn Pathol 2008; 12:426–9. [DOI] [PubMed] [Google Scholar]
- 22. Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol 2006; 112:195–204. [DOI] [PubMed] [Google Scholar]
- 23. Hu WT, Howell JC, Ozturk T, et al. CSF cytokines in aging, multiple sclerosis, and dementia. Front Immunol 2019; 10:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.