Abstract
Background
Stenotrophomonas maltophilia is increasingly common in patients with acute myeloid leukemia (AML). Little is known about factors that drive S. maltophilia infection. We evaluated the microbiome and cumulative antibiotic use as predictors of S. maltophilia infection in AML patients receiving remission induction chemotherapy (RIC).
Methods
Subanalysis of a prospective, observational cohort of patients with AML receiving RIC between September 2013 and August 2015 was performed. Fecal and oral microbiome samples collected from initiation of RIC until neutrophil recovery were assessed for the relative abundance of Stenotrophomonas via 16S rRNA gene quantitation. The primary outcome, microbiologically proven S. maltophilia infection, was analyzed using a time-varying Cox proportional hazards model.
Results
Of 90 included patients, 8 (9%) developed S. maltophilia infection (pneumonia, n = 6; skin–soft tissue, n = 2); 4/8 (50%) patients were bacteremic; and 7/8 (88%) patients with S. maltophilia infection had detectable levels of Stenotrophomonas vs 22/82 (27%) without infection (P < .01). An oral Stenotrophomonas relative abundance of 36% predicted infection (sensitivity, 96%; specificity, 93%). No association of S. maltophilia infection with fecal relative abundance was found. Cumulative meropenem exposure was associated with increased infection risk (hazard ratio, 1.17; 95% confidence interval, 1.01–1.35; P = .03).
Conclusions
Here, we identify the oral microbiome as a potential source for S. maltophilia infection and highlight cumulative carbapenem use as a risk factor for S. maltophilia in leukemia patients. These data suggest that real-time monitoring of the oral cavity might identify patients at risk for S. maltophilia infection.
Keywords: pneumonia, bacteremia, risk factors, colonization, meropenem
In this prospective cohort study, we identify the oral microbiome as a potential source for Stenotrophomonas maltophilia and highlight the role of cumulative antibiotic exposure, particularly carbapenems, as a risk factor for S. maltophilia infections in patients with leukemia.
(See the Editorial Commentary by Fredricks on pages 1514–6.)
Stenotrophomonas maltophilia is an intrinsically multidrug-resistant (MDR) gram-negative bacteria and the most frequently identified carbapenem-resistant gram-negative species in hospitalized patients with pneumonia [1, 2]. Stenotrophomonas maltophilia is increasingly identified in patients with cancer and is associated with high morbidity and mortality in this highly vulnerable population [2, 3]. Patients with acute myeloid leukemia (AML) are at particularly high risk for poor outcomes, with overall mortality in excess of 20% in patients with primary bacteremia and 60% for patients with pneumonia [4–7]. In its most devastating form, S. maltophilia infection manifests as hemorrhagic pneumonia with a case fatality rate approaching 100% [4]. As S. maltophilia is intrinsically resistant to the majority of antibiotics used to empirically treat febrile neutropenia in patients with AML and delayed appropriate antibiotic treatment is associated with increased mortality, identification of patients at risk for S. maltophilia infection is of paramount importance [8].
Due to intrinsic carbapenem resistance, prior carbapenem use appears to be the predominant risk factor for infection with S. maltophilia, and prior studies have identified carbapenem use, among other common factors such as prolonged hospital stay and intensive care unit admission, as a key risk factor [9–12]. Empiric carbapenem use is increasingly common in patients with AML due to rising rates of infections caused by extended-spectrum β-lactamase–producing organisms; therefore, an in-depth understanding of the risk–benefit profile of widespread carbapenem use is of high importance [13, 14]. However, prior studies have largely evaluated carbapenem exposure as a dichotomous variable or in arbitrarily categorized numbers of days, preventing an understanding of how cumulative carbapenem exposure modifies risk for subsequent S. maltophilia infection [2].
Colonization with MDR organisms, detected through either traditional means or microbiome analysis, is clearly linked to subsequent infection in patients with hematologic malignancies [15–17]. Indeed, a recent study performed in hematopoietic stem cell transplant recipients identified oral colonization with S. maltophilia as being significantly associated with S. maltophilia infection [18]. That study did not, however, integrate antimicrobial exposure or allow for a quantitative assessment of S. maltophilia burden in relation to infection. Thus, we sought to characterize cumulative antibiotic exposure and the relative abundance of S. maltophilia in patients with AML in order to identify patients at increased risk for S. maltophilia infections.
METHODS
Patient Enrollment and Antibiotic Use Assessment
This was a S. maltophilia-focused substudy of a previously published microbiome-based prospective, observational, cohort study of patients with a new diagnosis of AML who were receiving remission-induction chemotherapy (RIC) between September 2013 and August 2015. Details on the cohort have been previously published [19, 20]. Seven patients from the original cohort were excluded from this analysis due to incomplete clinical and antimicrobial exposure data. Fecal and buccal microbiome samples were collected from each patient prior to the start of RIC and every 96 hours thereafter until the resolution of neutropenia (absolute neutrophil count >500 cells/mm3). The University of Texas MD Anderson Cancer Center Institutional Review Board approved this study. All patients provided written, informed consent prior to enrollment in accordance with the Declaration of Helsinki.
Sample Collection and Microbiome Analysis
Buccal samples were collected using the Catch-All Sample Collection Swab (Epicentre) and placed in sterile 2-mL cryovials. Inpatient stool samples were collected in a stool hat and aliquoted into sterile 2-mL cryovials, while outpatient stool samples were collected using the BBL CultureSwab (BD Diagnostics). All samples were stored at −80o C until processing. Samples were submitted to the Alkek Center for Metagenomics and Microbiome Research (CMMR) of Baylor College of Medicine in 3 batches for microbial DNA extraction and microbiome profiling gene via 16S rRNA V4 gene sequencing. The CMMR is a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory that specializes in microbiome profiling and uses a set of controls to evaluate the performance of each step and determine potential contamination events throughout sample processing, library preparation, and data generation. Extraction controls are reagent controls (negative) and previously characterized samples (positive) that were subjected to the same procedures as the study samples. The 16S library preparation controls include a nontemplate control (negative) and purified DNA extracted from a pure culture of Francisella tularensis (positive). For the positive controls, 99% of reads are required to map to the F. tularensis reference strain in order to pass quality control. Both extraction and library preparation controls are carried through sequencing. For this study, data from extraction controls were not available due to the historical nature of the data. Additional information on control methods used for the microbiome analysis are presented in the Supplementary Methods. Bacterial DNA was extracted using the MO BIO PowerSoil DNA Isolation Kit (MO BIO Laboratories), and 16Sv4 rRNA gene libraries were generated following a protocol adapted from the Earth Microbiome Project [21, 22]. Briefly, the 16S rRNA V4 gene region was amplified and sequenced using Illumina MiSeq using a 2 × 250 paired-end protocol. The 16S rRNA V4 gene sequences were assigned to operational taxonomic units (OTUs) using the UPARSE pipeline, and taxonomic classifications were derived from alignments to the SILVA SSURef_NR99_119 database.
Antibiotic Use Assessment, Definitions, and Statistical Analyses
All antibiotic use for each patient from the time of enrollment to completion of follow-up was extracted from a database maintained by the pharmacy informatics. An antimicrobial therapy day was defined as any single calendar day on which an antibiotic was administered, regardless of dose or dosing frequency. Antibiotic use was assessed at the individual drug level and considered as both any use (ie, 1 or more days of therapy) and cumulative use (ie, total days of therapy during the study period). Only antibiotics commonly used empirically to treat or prevent neutropenic fever were assessed to minimize selection bias. Prophylactic agents were ciprofloxacin, levofloxacin, and cefpodoxime; treatment antibiotics were cefepime, piperacillin-tazobactam, meropenem, linezolid, and vancomycin. As ceftazidime and tigecycline are rarely used during RIC and generally in patients at high risk for S. maltophilia infection at our institution, these agents were specifically not assessed. Patients were evaluated for infection and antibiotic use from start of chemotherapy until neutrophil recovery. Cultures were obtained following routine clinical practice. Stenotrophomonas maltophilia bacteremia was defined as growth of S. maltophilia from blood regardless of clinical symptoms or concomitant growth from any site other than blood. Stenotrophomonas maltophilia pneumonia was defined as growth of S. maltophilia from sputum or bronchoalveolar lavage (BAL) in the presence of new or changing pulmonary infiltrates and respiratory symptoms or a positive blood culture if no respiratory cultures were obtained. Stenotrophomonas maltophilia skin–soft tissue infection (SSTI) was defined as skin erythema or swelling with growth of S. maltophilia from skin biopsy. Both S. maltophilia pneumonia and SSTI could exist independently of or concurrently with bacteremia.
The primary outcome was microbiologically documented infection with S. maltophilia (inclusive of bacteremia, pneumonia, or SSTI). Bivariate comparisons of patients with and without S. maltophilia infection were made using the Fisher exact test and the Mann-Whitney U test. A potential “best” predictive value of S. maltophilia relative abundance was determined by visually inspecting the receiver operator characteristics of each potential cut-point in order to maximize both sensitivity and specificity. In order to account for the time-varying nature of both S. maltophilia relative abundance and antibiotic use, a time-varying Cox proportional hazards model was used, with patients censored at neutrophil recovery or death. The time-varying Cox proportional hazards model accounts for immortal time bias and allows for an assessment of risk associated with each additional day of antibiotic exposure [23]. The last measured value was carried forward for patients with missing microbiome samples. A multivariable Cox proportional hazards model was constructed by starting with a full model and iteratively removing the least relevant predictors until an increase in the Akaike information criterion was observed. However, due to the limited sample size and likely overfitting, this model should be viewed as purely hypothesis-generating. All statistical analyses were performed using Stata v13.1 (StataCorp LP, College Station, TX).
RESULTS
Infection Characteristics
A total of 90 patients were included, 8 (8.9%) of whom developed microbiologically confirmed infection caused by S. maltophilia. Six patients had S. maltophilia pneumonia, 1 had ecthyma gangrenosum, and 1 had a complicated SSTI of the right lower extremity. One of 6 patients with pneumonia was diagnosed solely on the basis of a positive blood culture and development of nodular pulmonary infiltrates consistent with S. maltophilia infection. The remainder were diagnosed on the basis of bronchoalveolar lavage and/or respiratory cultures in addition to new or changing pulmonary infiltrates. Bacteremia was documented in 4 of 8 (50%) patients, including in 3 of 6 (50%) patients with pneumonia and in the patient with right lower extremity SSTI. Primary infection developed a median of 17.5 days (range, 11–28) following the start of induction chemotherapy. Clinical characteristics of patients with and without S. maltophilia infection are presented in Table 1, with no characteristics being significantly associated with S. maltophilia infection. The antimicrobial susceptibility profiles of the 8 diagnostic cultures are presented in Supplementary Table 1.
Table 1.
Baseline Characteristics of Patients With and Without Stenotrophomonas maltophilia Infection
| Characteristic | No Infection (n = 82) | Infection (n = 8) | P Value |
|---|---|---|---|
| Age,a y | 58 (46–68) | 59 (56–72) | .27 |
| Male sex | 42 (51) | 5 (63) | .72 |
| High-intensity chemotherapy | 55 (67) | 5 (63) | 1.00 |
| Complex cytogenetics | 10 (13) | 3 (43) | .15 |
| Eastern Cooperative Oncology Group (ECOG) performance statusa | 1 (1–1) | 1 (1–2) | .20 |
| Duration of neutropenia (days)a | 26 (21–34) | 29 (24–45) | .21 |
All reported as n (%) and tested with the Fisher exact test unless otherwise specified.
aMedian (interquartile range); tested using the Wilcoxon rank sum test.
Stenotrophomonas maltophilia Microbiome Description and Relative Abundance
DNA extraction, 16Sv4 libraries, and 16Sv4 sequences were successfully generated for all the samples included in this analysis (438 stool and 556 oral). The 16S library polymerase chain reaction (PCR) nontemplate control yielded 75 sequencing reads; more than 75% mapped to Methylobacterium, a commonly identified laboratory and reagent contaminant [24], and none mapped to Stenotrophomonas or closely related genera (Supplementary Table 2).
Taxonomic classification and relative abundances of OTUs that mapped to the genus Stenotrophomonas were derived from the taxonomic classification table generated by the CMMR 16S pipeline. Stenotrophomonas spp. relative abundance was calculated as the percent of OTUs assigned to the genus Stenotrophomonas relative to all other assigned OTUs. Although there are at least 12 known species in the genus Stenotrophomonas, only 2 named species are included in the SILVA database (Stenotrophomonas maltophilia and Stenotrophomonas pictorum). A BLASTn [25] analysis of the OTU sequences (2) mapping to the genus Stenotrophomonas in our data revealed 100% identity to S. maltophilia but also to Stenotrophomonas pavanii. Although v4 amplicons mapped to both S. maltophilia and another Stenotrophomonas species, S. maltophilia is the only member of this genera routinely identified in humans [26, 27].
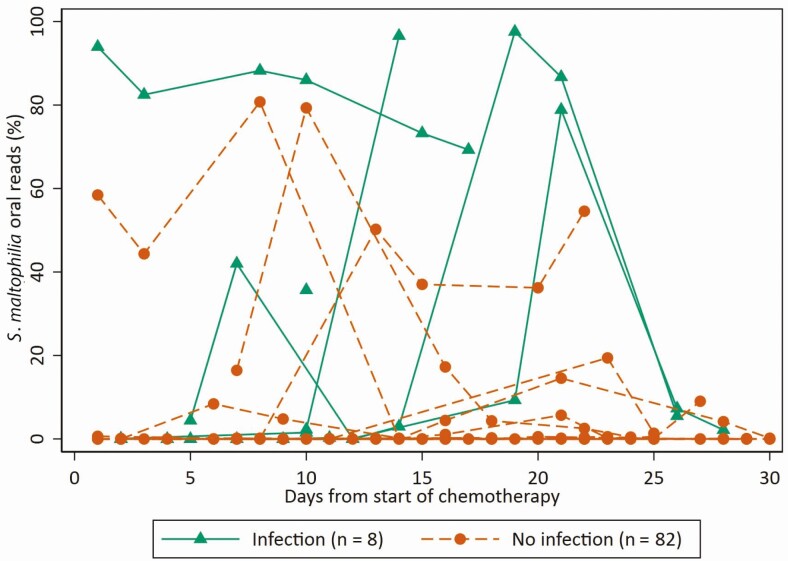
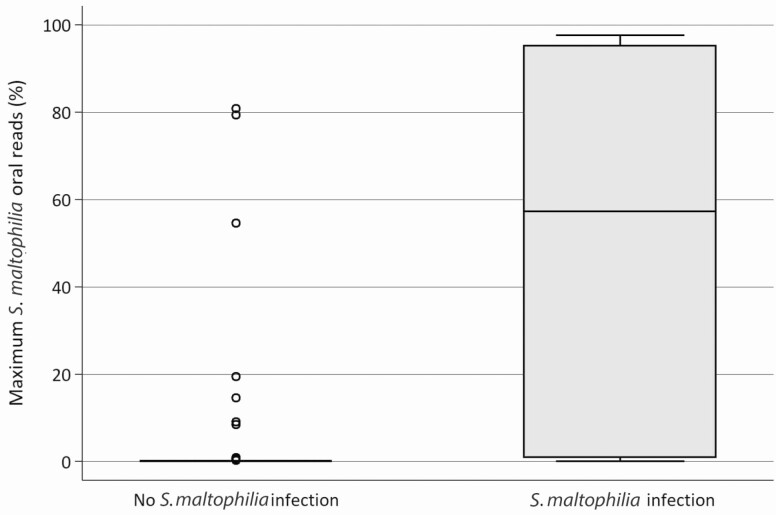
Stenotrophomonas was detected in the oral or stool microbiome of only 3 (3.3%) and in none of the patients at baseline, respectively. Stenotrophomonas was detected at any point during the risk period (ie, the period between chemotherapy start and neutrophil recovery) in the oral and stool microbiome of 29 (32%) and 8 (9%) patients, respectively. Seven of 8 (88%) patients with S. maltophilia infection had oral microbiome detection of Stenotrophomonas prior to onset of infection in contrast to 22/82 (27%) without S. maltophilia infection (P < .01). The sole patient in whom Stenotrophomonas was not detected in the oral microbiome prior to infection had the last sample obtained 2 days prior to a diagnostic BAL; the oral sample obtained 2 days later had a relative abundance (ie, percentage of reads mapping to Stenotrophomonas relative to total number of reads) of 43%. The relative abundance of Stenotrophomonas in the oral microbiome varied over the duration of the risk period (Table 2) and tended to decrease after an initial peak (Figure 1). The median (interquartile range) maximum relative oral abundance of Stenotrophomonas was higher in patients with S. maltophilia infection (57% [1%–95%] compared with those with no infection (0% [0%–0%]; Figure 2). A peak oral Stenotrophomonas relative abundance of >36% appeared to best predict infection (sensitivity, 63%; specificity, 96%; likelihood ratio +, 17.08; likelihood ratio −, 0.39; positive predictive value, 61%; negative predictive value, 96%; 93% correctly classified). In contrast, any detection of Stenotrophomonas in the oral microbiome was a relatively poor predictor of S. maltophilia infection (sensitivity, 88%; specificity, 74%; likelihood ratio +, 3.26; likelihood ratio −, 0.17; positive predictive value, 24%; negative predictive value, 98%). Overall, 7/29 (24%) patients with any detection in the oral microbiome developed S. maltophilia infection. In contrast to the oral microbiome, there was no clear association between stool Stenotrophomonas detection and S. maltophilia infection, with 2/8 (25%) patients with infection having stool detection vs 6/82 (7%) without infection (P = .15). Further, the appearance of Stenotrophomonas in the fecal microbiome always followed its appearance in the oral microbiome (data not shown). When the time-varying relative abundance of Stenotrophomonas was considered, an increasing relative abundance of oral Stenotrophomonas colonization significantly correlated with S. maltophilia infection (Table 3).
Table 2.
Oral and Fecal Microbiome Stenotrophomonas Relative Abundance in Patients With and Without Stenotrophomonas maltophilia Infection
| Sample Site | No Infection (n = 82) | Infection (n = 8) | P Value |
|---|---|---|---|
| Oral | |||
| Peak abundance (%) | 0.00 (0.00–80.76) | 57.27 (0.00–97.56) | <.01 |
| Last abundance (%) | 0.00 (0.00–54.56) | 3.84 (0.00–96.57) | <.01 |
| Baseline detection (n, %) | 2 (2) | 1 (13) | .25 |
| Any detection (n, %) | 22 (27) | 7 (88) | <.01 |
| Stool | |||
| Peak abundance (%) | 0.00 (0.00–9.85) | 0.00 (0.00–92.14) | .07 |
| Last abundance (%) | 0.00 (0.00–9.85) | 0.00 (0.00–0.63) | .24 |
| Baseline detection (n, %) | 0 (0) | 0 (0) | 1.00 |
| Any detection (n, %) | 6 (7) | 2 (25) | .15 |
Values reported as median (range) unless otherwise reported. P values calculated using the Wilcoxon rank sum test (percent relative abundance) and Fisher exact test (percent detectable).
Figure 1.
Relative abundance of Stenotrophomonas in patients with and without Stenotrophomonas maltophilia infection. All lines originate at the time of first sampling and end at the end of the risk period (time of S. maltophilia infection or neutrophil recovery). The x-axis is right-truncated at 30 days for clarity; 71/90 (88%) remained at 0% detectable throughout the risk period.
Figure 2.
Maximum Stenotrophomonas oral abundance in patients with and without Stenotrophomonas maltophilia infection. Horizontal bars indicate median and upper and lower quartiles. Solid dots indicate outlier values, as applicable.
Table 3.
Time-varying Antibiotic Exposure and Oral Microbiome Relative Abundance as Predictors of Stenotrophomonas maltophilia Infection
| Antibiotic | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Cefepimea | 1.02 | .84–1.23 | .87 |
| Cefpodoximea | 1.00 | .75–1.34 | 1.00 |
| Ciprofloxacina | 0.75 | .51–1.10 | .15 |
| Levofloxacina | 0.83 | .66–1.04 | .10 |
| Linezolida | 1.12 | .99–1.27 | .06 |
| Meropenema | 1.17 | 1.01–1.35 | .03 |
| Piperacillin-tazobactama | 1.07 | .86–1.33 | .55 |
| Stenotrophomonas maltophilia oral abundanceb | 1.04 | 1.03–1.05 | <.01 |
aHazard ratios refer to hazard associated with each additional day of antibiotic exposure.
bHazard ratios refer to hazard associated with 1% increase in S. maltophilia relative abundance.
Antimicrobial Use Assessment
The use of antibiotics generally as initial treatment for or prophylaxis against neutropenic fever is presented in Table 4. When treated as a time-varying covariate, each additional day of meropenem use increased the hazard of S. maltophilia infection by 17% (hazard ratio [HR], 1.17; 95% confidence interval [CI], 1.01–1.35; P = .03). No other β-lactam antibiotic was significantly correlated with S. maltophilia infection (Table 3). Linezolid use also correlated with S. maltophilia infection (HR, 1.12; 95% CI, .99–1.27; P = .06), although this may be because linezolid receipt is highly correlated with meropenem. Indeed, in the exploratory multivariable model, meropenem and the relative oral abundance of S. maltophilia appear to be associated with increased risk of S. maltophilia infection, while ciprofloxacin and levofloxacin are associated with decreased risk (Table 5).
Table 4.
Antibiotic Use in Patients With and Without Stenotrophomonas maltophilia Infection
| Antibiotic | No Infection (n = 82) | Infection (n = 8) |
|---|---|---|
| Cefepime | ||
| Median (IQR) number of days | 3 (0–7) | 2 (0–5) |
| Any use (n, %) | 49 (60) | 4 (50) |
| Cefpodoxime | ||
| Median (IQR) number of days | 0 (0–2) | 0 (0–0) |
| Any use (n, %) | 25 (30) | 1 (13) |
| Ciprofloxacin | ||
| Median (IQR) number of days | 0 (0–2) | 0 (0–2) |
| Any use (n, %) | 24 (30) | 3 (30) |
| Levofloxacin | ||
| Median (IQR) number of days | 5 (0–10) | 0 (0–2) |
| Any use (n, %) | 55 (67) | 3 (38) |
| Linezolid | ||
| Median (IQR) number of days | 6 (2–10) | 8 (4–9) |
| Any use (n, %) | 62 (76) | 8 (100) |
| Meropenem | ||
| Median (IQR) number of days | 2 (0–8) | 5 (3–10) |
| Any use (n, %) | 43 (52) | 7 (88) |
| Piperacillin-tazobactam | ||
| Median (IQR) number of days | 0 (0–2) | 0 (0–3) |
| Any use (n, %) | 23 (28) | 2 (25) |
Abbreviation: IQR, interquartile range.
Table 5.
Exploratory Multivariable Analysis of Risk Factors for Stenotrophomonas maltophilia Infection
| Factor | Adjusted Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Ciprofloxacina | 0.59 | .40–.87 | <.01 |
| Levofloxacina | 0.83 | .62–1.12 | .23 |
| Meropenema | 1.10 | .97–1.26 | .12 |
| Stenotrophomonas maltophilia oral abundanceb | 1.03 | 1.02–1.05 | <.01 |
aHazard ratios refer to hazard associated with each additional day of antibiotic exposure.
bHazard ratios refer to hazard associated with 1% increase in S. maltophilia relative abundance.
DISCUSSION
In this prospective, observational study, we identified the oral microbiome as a potential predictor of S. maltophilia infection in patients with AML who are receiving chemotherapy. Patients with S. maltophilia infection more frequently had detection of Stenotrophomonas in oral microbiome samples and had a higher relative abundance of Stenotrophomonas than patients without infection. Notably, this finding includes 2 patients with SSTIs caused by S. maltophilia, indicating that the oral microbiome may serve either as a potential reservoir for pathogenic S. maltophilia or as an indicator of multisite colonization pressure in these patients. Additionally, we confirm and expand on findings that carbapenems are a significant risk factor for S. maltophilia infection, identifying that each additional day of use further increases the risk of S. maltophilia infection [2, 9, 10].
Previous studies have clearly found that microbiome domination events precede infection with pathogenic microorganisms in patients with hematologic malignancy [16–18, 28, 29]. Importantly, however, these studies have focused on the fecal, rather than oral, microbiome. Our findings make it evident that the oral microbiome may play an important role in the pathogenesis of certain infections and should be given consideration in studies that link the microbiome with clinically relevant infections. It is worth noting previous studies that have identified associations between the fecal microbiome and subsequent infection have focused predominantly on infections caused by Enterobacteriales and Enterococcus spp., which in neutropenic patients are generally associated with primary bloodstream infection caused by gastrointestinal translocation [30]. In contrast, S. maltophilia infections in this population are generally either primarily respiratory in origin or catheter-related bloodstream infections; therefore, the relationship between the oral microbiome and S. maltophilia infection, rather than fecal microbiome and infection, does seem logical [3]. Whether the same relationship holds true for other organisms more commonly associated with respiratory infections rather than gastrointestinal translocation, such as Pseudomonas aeruginosa and Acinetobacter baumannii, is unclear.
We additionally expand on other studies that have assessed antibiotic exposure as a risk factor for S. maltophilia infection. Previous studies performed in general patient populations and in patients with hematologic malignancy have identified carbapenem use as a significant risk factor for S. maltophilia infection, in agreement with the intrinsic resistance of S. maltophilia to carbapenem antibiotics [2]. However, not all studies have identified carbapenem use as a risk factor for S. maltophilia infection in all situations, particularly when evaluated as a dichotomized (ie, yes/no) variable [7, 10, 31]. In our study, we identified both dichotomized use of carbapenems as a risk factor for S. maltophilia infection as well as cumulative exposure assessed in a time-varying Cox proportional hazards model. It is reasonable to expect that a larger cumulative antibiotic exposure would increase risk to a greater extent than a smaller cumulative exposure. Indeed, the critical need to assess antibiotic exposure as a cumulative measure in a time-varying model has recently been demonstrated [23, 32, 33]. While it is possible and likely that prolonged length of stay is associated with more antibiotic use and exposure to hospital-acquired pathogens and could therefore potentially explain the observed association with cumulative antibiotic exposure, the differential cumulative risk of different antibiotics argues against this point. Of note, no other β-lactam antibiotic was associated with increased risk of S. maltophilia infection. As organisms that require the use of carbapenems are increasingly common in patients with AML, the risk–benefit trade-off for continued empiric carbapenem use relative to other β-lactams must be carefully considered in each patient [13].
The prevalence of S. maltophilia in patients with AML receiving induction chemotherapy is both surprising and concerning. While S. maltophilia is a known pathogen in cancer patients, it is generally perceived to be a pathogen that appears later in a patient’s treatment course due to low virulence potential [10]. However, in this cohort of newly diagnosed patients with AML receiving induction chemotherapy, 9% of patients had microbiologically confirmed S. maltophilia infection and all infections occurred following receipt of a carbapenem. These data suggest that S. maltophilia infection must be considered in any patient with AML who has received treatment with a carbapenem, with patients who have received longer courses of carbapenems being at higher risk. Additionally, these data highlight the potential harms of early carbapenem use leading to microbiome dysbiosis and selection pressure for carbapenem-resistant organisms, such as S. maltophilia. As S. maltophilia is a fairly ubiquitous environmental organism and our data indicate that acquisition of S. maltophilia occurs over time, infection control measures or environmental screening may also be plausible methods to mitigate against the risk of early S. maltophilia infection [34].
There are several limitations to our study. First, the relatively small sample size and single-site nature of the study preclude the development of a multivariable risk prediction model, although preliminary findings indicate that such a model is feasible at scale. Due to the limited sample size, all results should be viewed as hypothesis-generating. Second, misclassification bias of our primary outcome, S. maltophilia infection, is possible as the diagnosis in many cases relied on respiratory cultures. In patients with heavy oral colonization by Stenotrophomonas, this may have led to contamination of the diagnostic respiratory culture. However, as half of the patients with pneumonia also had concomitant bacteremia and all patients had clinical signs and symptoms compatible with pneumonia, this seems less likely. It is unknown if these findings are applicable at other centers caring for patients with AML, and it is also not clear if the relationships between the microbiome, antibiotic exposure, and S. maltophilia infection are relevant in other patient populations. Additionally, the tremendous genetic heterogeneity of S. maltophilia is just beginning to be understood, and how interstrain variability may influence these findings is unknown [35]. Therefore, these findings require validation in a multicenter study. Finally, while the relationship between the oral microbiome relative abundance and S. maltophilia infection appears to be quite strong, the applicability of this finding is limited until longitudinal microbiome sampling on clinical samples becomes feasible as baseline detection does not appear to predict subsequent infection. However, for centers with a high prevalence of S. maltophilia infection, development of dynamic PCR-based screening methods may have utility in directing empiric treatment for patients with suspected infections, and the performance characteristics of such screening should be evaluated in future studies. Last, the lack of data on the extraction controls limited our ability to exclude the potential of a reagent or processing contamination event during DNA extraction or any processes upstream of it. In addition, techniques used for microbiome evaluation did not allow us to specifically determine that all sequencing reads that map to the genus Stenotrophomonas are, in fact, S. maltophilia. However, the absence of reads that map to Stenotrophomonas in the library preparation controls and the validation with species-specific PCR (data not shown) partially mitigate these concerns.
Despite these limitations, there are several notable strengths to our study. First, the prospective design and longitudinal microbiome sampling allowed for an assessment in the relative abundance of Stenotrophomonas as a function of time. Additionally, this cohort is the largest of its kind to date and can therefore provide insight on relatively rare individual events, such as S. maltophilia infection. Finally, incorporation of microbiome data and antibiotic use data represents a step forward in understanding how the interaction of the microbiome and antibiotic use may affect downstream infection risk.
In conclusion, the oral microbiome and cumulative antibiotic use appear to be important factors in the development of S. maltophilia infection in patients with AML receiving chemotherapy. Multicenter studies are needed to validate and expand on these findings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors gratefully acknowledge the support of Vanessa Stevens, PhD, for technical assistance and advice in developing the time-varying Cox proportional hazards model.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH; R01 AI134637, R21 AI143229, and K24 AI121296 to C. A. A.; U01 AI124290 to T. C. S.; K01 AI143881-01 to J. G. P.), the National Institute of Diabetes and Digestive and Kidney Disease at the NIH (P30 DK56338) to T. C. S., the MD Anderson Odyssey Fellowship Program (to J. G. P.), the CFP Foundation (to J. G. P.), the UTHealth Presidential Award (to C. A. A.), the University of Texas STARS Award (to C. A. A.), and the Texas Medical Center Health Policy Institute Funding Program (to C. A. A.).
Potential conflicts of interest. S. L. A. has received research support from Melinta Therapeutics and Merck and has served on advisory boards for Shionogi, Paratek, and Merck. T. C. S. has received research support from Merck, Nivalis, Cubist, Mead Johnson, Rebiotix, BioFire, and Assembly BioSciences and has served on advisory boards for Rebiotix and BioFire. C. A. A. has received research support from Merck Inc, MeMed Diagnostics, and Entasis Therapeutics; chapter royalties from UptoDate, Harrison Principles of Internal Medicine, and Mandell Principles and Practice of Infectious Diseases; study section member and grant reviewer fees from NIH/NIAID; reimbursement for traveling to IDWeek and ID Program Committee meetings as IDWeek chair from the Infectious Diseases Society of America; reimbursement for traveling to ASM Microbe from the American Society for Microbiology; and Antimicrobial Agents and Chemotherapy editor’s stipend from the American Society for Microbiology outside the submitted work. D. P. K. has received support and consultancy fees from Astellas Pharma, Cidara, Amplyx, Pulmocide and Mayne, Gilead, and United Medical; served on the advisory board of Merck; and has received the Texas 4000 Distinguished Professorship for Cancer Research and NIH-NCI Cancer Center CORE Support grant no. 16672 outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. A novel algorithm to analyze epidemiology and outcomes of carbapenem resistance among patients with hospital-acquired and ventilator-associated pneumonia: a retrospective cohort study. Chest 2019; 155:1119–30. [DOI] [PubMed] [Google Scholar]
- 2. Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev 2012; 25:2–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Safdar A, Rolston KV. Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clin Infect Dis 2007; 45:1602–9. [DOI] [PubMed] [Google Scholar]
- 4. Kim SH, Cha MK, Kang CI, et al. Pathogenic significance of hemorrhagic pneumonia in hematologic malignancy patients with Stenotrophomonas maltophilia bacteremia: clinical and microbiological analysis. Eur J Clin Microbiol Infect Dis 2019; 38:285–95. [DOI] [PubMed] [Google Scholar]
- 5. Ko JH, Kang CI, Cornejo-Juárez P, et al. Fluoroquinolones versus trimethoprim-sulfamethoxazole for the treatment of Stenotrophomonas maltophilia infections: a systematic review and meta-analysis. Clin Microbiol Infect 2019; 25:546–54. [DOI] [PubMed] [Google Scholar]
- 6. Jeon YD, Jeong WY, Kim MH, et al. Risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. Medicine (Baltimore) 2016; 95:e4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sumida K, Chong Y, Miyake N, et al. Risk factors associated with Stenotrophomonas maltophilia bacteremia: a matched case-control study. PLoS One 2015; 10:e0133731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Micozzi A, Venditti M, Monaco M, et al. Bacteremia due to Stenotrophomonas maltophilia in patients with hematologic malignancies. Clin Infect Dis 2000; 31:705–11. [DOI] [PubMed] [Google Scholar]
- 9. Boktour M, Hanna H, Ansari S, et al. Central venous catheter and Stenotrophomonas maltophilia bacteremia in cancer patients. Cancer 2006; 106:1967–73. [DOI] [PubMed] [Google Scholar]
- 10. Aisenberg G, Rolston KV, Dickey BF, Kontoyiannis DP, Raad II, Safdar A. Stenotrophomonas maltophilia pneumonia in cancer patients without traditional risk factors for infection, 1997–2004. Eur J Clin Microbiol Infect Dis 2007; 26:13–20. [DOI] [PubMed] [Google Scholar]
- 11. Ansari SR, Hanna H, Hachem R, Jiang Y, Rolston K, Raad I. Risk factors for infections with multidrug-resistant Stenotrophomonas maltophilia in patients with cancer. Cancer 2007; 109:2615–22. [DOI] [PubMed] [Google Scholar]
- 12. Armand-Lefèvre L, Angebault C, Barbier F, et al. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 2013; 57:1488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker TM, Satlin MJ. The growing threat of multidrug-resistant gram-negative infections in patients with hematologic malignancies. Leuk Lymphoma 2016; 57:2245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blennow O, Ljungman P. The challenge of antibiotic resistance in haematology patients. Br J Haematol 2016; 172:497–511. [DOI] [PubMed] [Google Scholar]
- 15. Satlin MJ, Chavda KD, Baker TM, et al. Colonization with levofloxacin-resistant extended-spectrum β-lactamase-producing Enterobacteriaceae and risk of bacteremia in hematopoietic stem cell transplant recipients. Clin Infect Dis 2018; 67:1720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 2010; 120:4332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheich S, Koenig R, Wilke AC, et al. Stenotrophomonas maltophilia colonization during allogeneic hematopoietic stem cell transplantation is associated with impaired survival. PLoS One 2018; 13:e0201169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galloway-Pena JR, Shi Y, Peterson CB, et al. Gut microbiome signatures are predictive of infectious risk following induction therapy for acute myeloid leukemia. Clin Infect Dis 2020; 71(1):63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galloway-Peña JR, Smith DP, Sahasrabhojane P, et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer 2016; 122:2186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 2011; 108 Suppl 1:4516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 2012; 6:1621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011; 53:42–8. [DOI] [PubMed] [Google Scholar]
- 24. Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 2014; 12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403–10. [DOI] [PubMed] [Google Scholar]
- 26. Ryan RP, Monchy S, Cardinale M, et al. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat Rev Microbiol 2009; 7:514–25. [DOI] [PubMed] [Google Scholar]
- 27. Patil PP, Midha S, Kumar S, Patil PB. Genome sequence of type strains of genus Stenotrophomonas. Front Microbiol 2016; 7:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hakim H, Dallas R, Wolf J, et al. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin Infect Dis 2018; 67:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taur Y, Jenq RR, Ubeda C, van den Brink M, Pamer EG. Role of intestinal microbiota in transplantation outcomes. Best Pract Res Clin Haematol 2015; 28:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Velden WJ, Herbers AH, Netea MG, Blijlevens NM. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: introducing the paradigm febrile mucositis. Br J Haematol 2014; 167:441–52. [DOI] [PubMed] [Google Scholar]
- 31. Nseir S, Di Pompeo C, Brisson H, et al. Intensive care unit-acquired Stenotrophomonas maltophilia: incidence, risk factors, and outcome. Crit Care 2006; 10:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal β-lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy 2019; 39:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Munoz-Price LS, Frencken JF, Tarima S, Bonten M. Handling time-dependent variables: antibiotics and antibiotic resistance. Clin Infect Dis 2016; 62:1558–63. [DOI] [PubMed] [Google Scholar]
- 34. Adegoke AA, Stenström TA, Okoh AI. Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: looking beyond contemporary antibiotic therapy. Front Microbiol 2017; 8:2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mojica MF, Rutter JD, Taracila M, et al. Population structure, molecular epidemiology, and beta-lactamase diversity among Stenotrophomonas maltophilia isolates in the United States. MBio 2019; 10(4):e00405-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.