Abstract
Background
Although the United States Food and Drug Administration recently approved the human papillomavirus (HPV) vaccine for individuals aged 27–45 years, the Centers for Disease Control and Prevention did not change its guidelines for routine HPV vaccination. Since recommendations for adult vaccination emphasize shared clinical decision-making based on risk of new infections, we examined the relationship between HPV prevalence and sexual behavior.
Methods
This study was conducted among 5093 HPV-unvaccinated, sexually experienced adults aged 18–59 years in the National Health and Nutrition Examination Surveys (2013–2016). For each sex and age group, adjusted prevalences of 9-valent vaccine–specific, high-risk, and any HPV infection were estimated by number of lifetime sexual partners (LTSPs) using logistic regression. An analysis restricted to persons who did not have a new sexual partner in the past year (ie, removing those at highest risk of newly acquired HPV) was also conducted.
Results
In each age group, genital HPV prevalence was higher among persons with >5 LTSPs compared with 1–5 LTSPs in both males and females. There were only slight reductions in HPV prevalence after removing participants who reported a new sexual partner in the past year. For example, among females aged 27–45 years with >5 LTSPs, the adjusted prevalence of 9-valent vaccine–type HPV infection was 13.4% (95% confidence interval [CI], 9.9%–17.0%) in the full population compared to 12.1% (95% CI, 8.8%–15.4%) among those with no new sexual partners.
Conclusions
Prevalent HPV infection was primarily reflective of cumulative exposures over time (higher LTSPs). New exposures had limited impact, emphasizing the need to consider sexual history in the decision-making process for adult HPV vaccination.
Keywords: genital HPV, HPV vaccination, NHANES, sexual behavior, HPV prevalence
Among sexually experienced adults in the National Health and Nutrition Examination Survey, human papillomavirus (HPV) prevalence primarily reflected cumulative exposures (higher lifetime sexual partners); new exposures had limited impact. Lifetime sexual history is important when considering adult HPV vaccination.
Infection with high-risk (HR) human papillomavirus (HPV) can lead to the development of anogenital and oropharyngeal cancers in both males and females. A safe and highly effective prophylactic vaccine is available that covers 9 HPV types (7 HR-HPV and 2 low-risk HPV types). The United States (US) Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) currently recommends routine HPV vaccination at age 11 or 12 years or as young as age 9 years as the vaccine provides the most protection before an individual is exposed to HPV [1]. The ACIP also recommends catch-up vaccination for males and females through age 26 years who were not previously vaccinated.
The US Food and Drug Administration (FDA) approved the use of Gardasil 9 in 2018 for individuals aged 27–45 years [2]. However, the ACIP did not change its recommendations for routine HPV vaccination. Rather, ACIP concluded that the public health benefit of HPV vaccination among adults aged 27–45 years is minimal, and thus recommended a strategy of shared clinical decision-making because some persons who are not adequately vaccinated may benefit from vaccination [3]. The CDC suggests that clinicians discuss HPV vaccination with individuals who are most likely to benefit. The American College of Obstetricians and Gynecologists (ACOG) recommends that vaccination for women aged 27–45 years should be individually based with consideration of patient circumstances, preferences, and concerns, including the possibility of acquiring a new HPV infections [4].
Newly acquired HPV infections are strongly linked to new sex partners, whereas persistent or reactivated infections are more strongly associated with cumulative exposure (ie, higher lifetime number of sexual partners) [5–7]. Therefore, we sought to examine the relationship between current and past sexual behaviors on prevalent burden of genital HPV infection by age and sex in a nationally representative sample of sexually experienced adults in the US. Since current recommendations for adult HPV vaccination emphasize shared clinical decision-making and the role of new HPV infections, the results from this study will provide critical risk information to guide patient–provider discussions regarding HPV vaccination decisions.
METHODS
Study Design and Population
The analysis uses data from the 2013–2016 National Health and Nutrition Examination Surveys (NHANES) [8]. NHANES is a stratified, multistage probability sampling design conducted by the National Center of Health Statistics (NCHS) of the CDC that is nationally representative of the US noninstitutionalized population. The survey collect information on demographics, as well as a variety of health-related information through in-person computer-assisted personal interviews (CAPIs) in the participant’s household, and audio computer-assisted self-interviews (ACASIs), physical examinations, and laboratory tests at a medical examination center (MEC). The overall unconditional medical examination response rate in 2013–2014 and 2015–2016 was 68.5% and 58.7%, respectively [9]. A total of 8065 persons aged 18–59 years participated in the household interview and medical examination.
Of the 8065 age-eligible persons examined, 2366 participants were excluded since they declined or had an unevaluable HPV test or were missing information on any covariates of interest (Supplementary Figure 1). Of the remaining 5699 participants who had information on all variables, 606 participants were excluded because they either reported not being sexually experienced or had least 1 dose of an HPV vaccine. Thus, this analysis was performed among 5093 participants aged 18–59 years who had an evaluable HPV test, reported at least 1 lifetime sexual partner, and had not received an HPV vaccination. The distributions of characteristics stratified by those who were excluded due to missing data (ie, item nonresponse) were examined (Supplementary Table 1). In brief, participants aged 18–26 years and minority participants were more likely to be excluded from the analysis than older or white participants.
Data collection and study procedures were approved by the NCHS Research Ethics Board. Data were de-identified and made publicly available in 2-year cycles. This secondary analysis was deemed exempt from review by the Johns Hopkins University School of Medicine Institutional Review Board.
Questionnaire Data
Demographic information (age, sex, race/ethnicity, and marital status) was collected in the participant’s household, as was data on immunization history and cigarette smoking history. Data on marital status was only collected among participants aged 20 years or older, and categorized as either “married/living with partner” or “not married/living with partner,” which includes participants who are never married, divorced, separated, or widowed. To avoid excluding participants aged 18–19 years from the primary analysis, these participants had their marital status categorized as “not asked.” HPV vaccination status was asked at home via CAPI, and was defined as having received at least 1 dose of any HPV vaccine [10]. Self-reported cigarette smoking status was ascertained via CAPI and was categorized as “current smoker” (≥100 cigarettes in lifetime and currently smoking either some days or every day), “former smoker” (≥100 cigarettes in lifetime but do not currently smoke), or “never smoker” (<100 cigarettes in lifetime).
Sexual behavior data were collected by ACASI at the MEC, and this module was offered in several languages [11]. Participants were asked about their lifetime and past-year sexual history. The total number of lifetime sexual partners (LTSPs) was calculated as the total self-reported number of both opposite and same sex vaginal, oral, and anal sex partners. Participants were considered “sexually experienced” if they reported ≥1 LTSPs. Participants who reported ≥1 opposite or same sex vaginal, oral, or anal sex partner in the past 12 months were considered “sexually active.” Sexually active participants were also asked if they had a new sexual partnership in the past 12 months (no vs yes). For individuals who were not sexually active, we imputed a “no” response for the new sexual partnership variable.
Laboratory Testing
Participants aged 14–59 years were asked to self-collect a penile or vaginal swab at the MEC. Specimens were stored at 4°C and shipped to the CDC for DNA extraction, which took place within 3–4 weeks, and analysis [12, 13]. Polymerase chain reaction genotyping for HPV was performed on penile and vaginal swabs using the Linear Array HPV Genotype Test (Roche Molecular Diagnostics, Indianapolis, Indiana) which detects 37 genotypes [13, 14]. Samples were determined to be inadequate if β-globin was not detected and the participant was negative for all HPV types as well as low and high positive globin. All inadequate samples were recoded as missing for this analysis [15].
The prevalence of a genital vaccine-type HPV infection was defined as the detection of ≥1 genotype in the Gardasil 9 vaccine (6, 11, 16, 18, 31, 33, 45, 52, and/or 58) [16]. The prevalence of a genital HR-HPV infection was defined as the detection of 1 or more HR-HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and/or 68). The prevalence of any genital HPV infection was defined as the detection of ≥1 of the 37 possible genotypes.
Statistical Analysis
All analyses were weighted unless specified otherwise. MEC weights were precalculated by NCHS to account for unequal probability of selection and adjusted for nonresponse both for the at-home interview and the MEC portion, as well as being poststratified to match the Census Bureau’s American Community Survey total population count. The MEC weights were pooled across the 2 NHANES cycles and applied accordingly [17]. Data were examined using descriptive statistics. Taylor series linearization was used to calculate standard errors (SEs), and Korn and Graubard confidence intervals (CIs) were calculated for crude prevalence estimates.
The primary outcomes included the crude prevalence of genital vaccine-type HPV, HR-HPV, and any HPV stratified by sex, age group, and number of LTSPs. Age was categorized based on vaccination guidelines: ages 18–26, 27–45, and 46–59 years. LTSPs were dichotomized as those with 1–5 LTSPs and those with >5 LTSPs. LTSPs was dichotomized at 6—the median number of LTSPs in the overall sample of males and females combined—due to ease of interpretation and to maximize the stability of prevalence estimates. Since the HPV vaccine is presumed to prevent only newly acquired infections, the crude prevalence of each outcome was also calculated after excluding participants at highest risk for newly acquired infection, defined as those who reported a new sexual partner within the past 12 months.
In both the full analytic sample and in the sample restricted to participants who reported no new sexual partnership, predictive margin estimates of each HPV outcome were calculated using sex-stratified multivariable logistic regression models. These estimates, commonly referred to as adjusted prevalence estimates, are the average predicted probability of HPV infection if all participants in the sample had been in that group (ie, marginal standardization), while adjusting for all variables in the model [18]. The multivariable models included terms for age group, binary LTSP group, an interaction between age group and binary LTSP group, race/ethnicity, marital status, cigarette smoking status, and report of a new sexual partner in the past 12 months. To mitigate residual confounding, the models also included a continuous term for the individual number of LTSPs. A sensitivity analysis was conducted excluding participants aged 18–19 years since marital status among this group was unknown.
Data management and visualization was done in R version 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical analyses were conducted in Stata MP, version 15 (StataCorp LP, College Station, Texas). The svy command package was used to account for the complex survey design. The user written command “kg_nchs” was used to calculate Korn and Graubard 95% CI and predictive margins were estimated using the “margins” command [19, 20].
RESULTS
Study Population
Among both males (n = 2495) and females (n = 2598), the distribution of race/ethnicity, marital status, and cigarette smoking status varied substantially by age group (Table 1). The majority of both males and females aged 27–45 and 46–59 years reported either being married or living with a partner. Among males, the median number of LTSPs increased with age, ranging from 5 (interquartile range [IQR], 3–12) LTSPs among 18- to 26-year-olds to 8 (IQR, 3–19) LTSPs among 27- to 45-year-olds and 10 (IQR, 5–20) LTSPs among 45- to 59-year-olds. Among females, the median number of LTSPs was 5 across all age groups (IQR, 2–8 LTSPs for 18- to 26-year-olds, 2–10 LTSPs for 27- to 47-year-olds, and 3–10 LTSPs for 45- to 59-year-olds). In contrast, the proportion reporting a new sexual partner in the past 12 months substantially decreased with older age. Among males, the percentage reporting a new sexual partner declined from 40.7% among 18- to 26-year-olds to 16.6% among 27- to 45-year-olds and 9.3% among 45- to 59-year-olds. Among females, the percentage reporting a new sexual partner declined from 28.4% among 18- to 26-year-olds to 10.5% among 27- to 45-year-olds and 3.5% among 45- to 59-year-olds.
Table 1.
Characteristics of Sexually Experienced Individuals Aged 18–59 Years Who Were Unvaccinated for Human Papillomavirus Infection, Stratified by Sex and Age Group—National Health and Nutrition Examination Surveys, 2013–2016
| Characteristic | Males | Females | ||||
|---|---|---|---|---|---|---|
| 18–26 y (n = 440) | 27–45 y (n = 1193) | 46–59 y (n = 862) | 18–26 y (n = 331) | 27–45 y (n = 1265) | 46–59 y (n = 1002) | |
| Race/ethnicity | ||||||
| Non-Hispanic white | 58.3 (3.4) | 59.4 (3.1) | 71.0 (3.0) | 55.3 (4.6) | 58.7 (3.2) | 72.1 (2.6) |
| Non-Hispanic black | 13.4 (1.9) | 10.5 (1.4) | 10.3 (1.4) | 15.1 (3.0) | 12.2 (1.8) | 10.5 (1.5) |
| Mexican American | 13.0 (2.2) | 12.0 (1.7) | 7.2 (1.4) | 11.7 (2.3) | 12.5 (1.9) | 6.3 (1.1) |
| Other Hispanic | 7.1 (1.3) | 8.2 (1.2) | 5.1 (1.0) | 9.2 (1.4) | 7.3 (1.1) | 5.3 (1.1) |
| Non-Hispanic Asian | 3.6 (0.7) | 5.5 (1.0) | 3.5 (0.6) | 4.0 (0.9) | 5.7 (0.9) | 3.3 (0.6) |
| Other/multiracial | 4.7 (1.4)a | 4.2 (0.8) | 3.0 (0.9) | 4.7 (1.2) | 3.6 (0.5) | 2.5 (0.9)a |
| Married or living with partner | ||||||
| No | 56.2 (3.0) | 27.3 (1.5) | 25.6 (1.9) | 45.1 (3.2) | 29.6 (1.8) | 33.5 (2.1) |
| Yes | 32.4 (2.9) | 72.7 (1.5) | 74.4 (1.9) | 42.1 (3.5) | 70.4 (1.8) | 66.5 (2.1) |
| Not askedb | 11.4 (1.6) | … | … | 12.8 (1.8) | … | … |
| Cigarette smoking status | ||||||
| Never | 58.4 (2.8) | 52.5 (1.7) | 46.9 (2.5) | 67.7 (3.4) | 66.2 (1.7) | 56.9 (2.3) |
| Former | 12.8 (2.3) | 21.3 (1.5) | 30.2 (2.3) | 5.8 (1.5) | 15.1 (1.2) | 21.9 (1.3) |
| Current | 28.8 (2.8) | 26.1 (1.3) | 22.9 (2.0) | 26.5 (2.9) | 18.7 (1.3) | 21.3 (2.2) |
| Age at first sex, median (IQR) | 16 (15–18) | 17 (15–19) | 16 (15–18) | 16 (15–18) | 17 (16–19) | 17 (16–19) |
| Sexually active last 12 mo | ||||||
| No | 7.3 (1.4) | 5.2 (0.7) | 11.9 (1.5) | 4.9 (1.7)a | 6.8 (1.0) | 25.8 (1.8) |
| Yes | 92.7 (1.4) | 94.8 (0.7) | 88.1 (1.5) | 95.1 (1.7) | 93.2 (1.0) | 74.2 (1.8) |
| New sexual partner last 12 mo | ||||||
| No | 59.3 (3.5) | 83.4 (1.4) | 90.7 (1.6) | 71.6 (2.5) | 89.5 (1.1) | 96.5 (0.7) |
| Yes | 40.7 (3.5) | 16.6 (1.4) | 9.3 (1.6) | 28.4 (2.5) | 10.5 (1.1) | 3.5 (0.7) |
| No. of LTSPs | ||||||
| 1–5 | 53.0 (3.5) | 38.2 (1.8) | 29.9 (2.6) | 57.5 (3.5) | 50.8 (2.5) | 57.5 (2.2) |
| >5 | 47.0 (3.5) | 61.8 (1.8) | 70.1 (2.6) | 42.5 (3.5) | 49.2 (2.5) | 42.5 (2.2) |
| No. of LTSPs, median (IQR) | 5 (3–12) | 8 (3–19) | 10 (5–20) | 5 (2–8) | 5 (2–10) | 5 (3–10) |
Data are presented as weighted column percentages and corresponding standard errors, unless otherwise indicated.
Abbreviations: IQR, interquartile range; LTSP, lifetime sexual partner.
aResidual standard error >30%. Estimate should be interpreted with caution.
bData on marital status were not collected for participants aged 18–19 years.
Prevalence of Penile HPV Infection by Age and Sexual Behavior
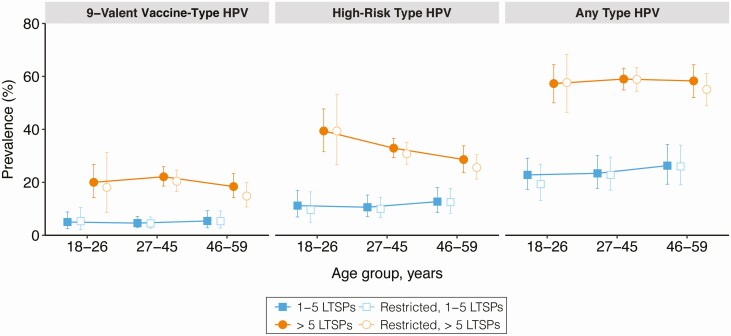
For each age group, the detection of 9-valent vaccine type, high-risk type, and any type penile HPV infection was significantly higher among males with >5 LTSPs than males with 1–5 LTSPs (Figure 1). These differences remained significant after multivariable adjustment and when restricting the analysis to males who did not have a new sexual partner in the past 12 months, with the exception of 9-valent vaccine–type HPV infection among 18- to 26 year-olds, for whom data were sparse (Figure 1; Table 2). Furthermore, when comparing the full and restricted samples, there were only slight reductions in the burden of prevalent HPV infection after removing participants who reported a new sexual partner in the past 12 months (ie, those at highest risk of newly acquired HPV). For example, in the expanded age group for FDA-approved vaccine (27–45 years), the adjusted prevalence of 9-valent vaccine–type HPV infection among all males with 1–5 LTSPs was 6.2% (95% CI, 3.6%–8.7%) and 5.1% (95% CI, 2.9%–7.3%) when excluding participants who reported a new sexual partner in the past 12 months (Table 2). Among males with >5 LTSPs, the adjusted prevalence of 9-valent vaccine type HPV infection was 20.0% (95% CI, 16.6%–23.3%) and 18.8% (95% CI, 15.0%–22.6%) in the full and restricted samples, respectively.
Figure 1.
Detection of penile human papillomavirus (HPV) infection among unvaccinated, sexually experienced males, stratified by age and number of lifetime sexual partners (LTSPs), and restricted among those who reported no new sexual partners in the previous 12 months. Data are crude weighted prevalence estimates; corresponding error bars are Korn and Graubard 95% confidence intervals. Estimates for vaccine-type HPV among males, age 18–26 years, reporting 1–5 LTSPs and no new partner over the past 12 months have a relative confidence interval width >130% and should be interpreted with caution.
Table 2.
Adjusted Prevalence Estimates (Predictive Margins) of Penile Human Papillomavirus Infection Among Unvaccinated, Sexually Experienced Males, Stratified by Age and Number of Lifetime Sexual Partners
| HPV Type and Age Group | Adjusted HPV Prevalence, % (95% CI) | |
|---|---|---|
| Overall Male Population (n = 2495)a | Males Without a New Partner in the Past 12 mo (n = 2025)b | |
| 9-valent vaccine typec | ||
| Age 18–26 y | ||
| 1–5 LTSPs | 5.4 (2.1–8.6) | 5.7 (1.3–10.2) |
| >5 LTSPs | 15.6 (9.1–22.1) | 16.4 (5.8–26.9) |
| Age 27–45 y | ||
| 1–5 LTSPs | 6.2 (3.6–8.7) | 5.1 (2.9–7.3) |
| >5 LTSPs | 20.0 (16.6–23.3) | 18.8 (15.0–22.6) |
| Age 46–59 y | ||
| 1–5 LTSPs | 7.3 (3.0–11.5) | 6.2 (2.6–9.8) |
| >5 LTSPs | 17.6 (13.6–21.7) | 14.0 (9.7–18.3) |
| High-risk typed | ||
| Age 18–26 y | ||
| 1–5 LTSPs | 11.9 (6.9–17.0) | 10.6 (4.3–16.9) |
| >5 LTSPs | 33.6 (24.9–42.4) | 37.4 (24.5–50.2) |
| Age 27–45 y | ||
| 1–5 LTSPs | 13.7 (8.6–18.7) | 11.9 (7.6–16.2) |
| >5 LTSPs | 30.5 (27.3–33.8) | 29.0 (25.3–32.8) |
| Age 46–59 y | ||
| 1–5 LTSPs | 15.9 (10.9–20.9) | 14.2 (9.6–18.9) |
| >5 LTSPs | 26.9 (22.4–31.3) | 23.3 (19.3–27.4) |
| Any typee | ||
| Age 18–26 y | ||
| 1–5 LTSPs | 24.2 (18.4–30.1) | 21.7 (13.7–29.6) |
| >5 LTSPs | 51.7 (43.3–60.1) | 55.7 (44.8–66.6) |
| Age 27–45 y | ||
| 1–5 LTSPs | 27.9 (20.5–35.2) | 26.3 (19.4–33.3) |
| >5 LTSPs | 56.1 (51.7–60.5) | 55.6 (50.4–60.9) |
| Age 46–59 y | ||
| 1–5 LTSPs | 30.9 (23.2–38.7) | 29.6 (21.9–37.4) |
| >5 LTSPs | 56.8 (51.3–62.4) | 52.2 (46.7–57.7) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; LTSP, lifetime sexual partner.
aFor each HPV outcome, adjusted prevalence estimates (predictive margins) of HPV detection were calculated by weighted multivariable logistic regression models, which included adjustment for race/ethnicity, marital status, cigarette smoking status, lifetime number of sexual partners (continuous), and report of a new sexual partnership in the past 12 months.
bFor each HPV outcome, adjusted prevalence estimates (predictive margins) of HPV detection were calculated by weighted multivariable logistic regression models, which included adjustment for race/ethnicity, marital status, cigarette smoking status, and lifetime number of sexual partners (continuous).
cNine-valent vaccine types: 6, 11, 16, 18, 31, 33, 45, 52, 58.
dHigh-risk types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68.
eAny type HPV: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, IS39.
Crude and adjusted prevalence estimates of penile HPV infection by other risk factors are presented in Supplementary Tables 2and 3.
Prevalence of Cervicovaginal HPV Infection by Age and Sexual Behavior
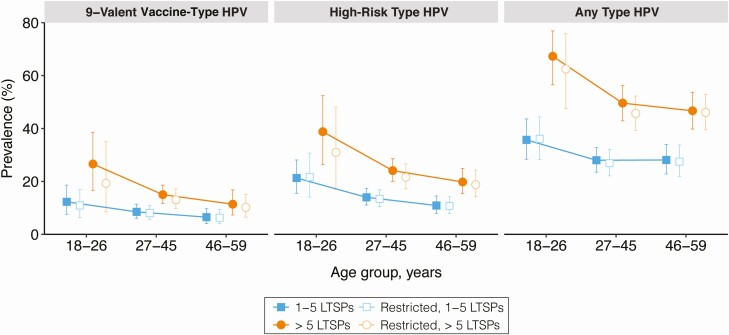
For some age groups, the prevalence of 9-valent, high-risk, and any type of cervicovaginal HPV infection was significantly higher among females with >5 LTSPs than females with 1–5 LTSPs (Figure 2). Similar inferences were obtained after adjusting for race/ethnicity, marital status, cigarette smoking status, and the report of a new sexual partner in the past 12 months (Table 3). Restricting the analysis to persons who did not report a new sexual partner in the past 12 months also did not appreciably change the crude or adjusted point estimates or inferences of interest (Figure 2; Table 3). For example, in the expanded age group for FDA-approved vaccine (27–45 years), the adjusted prevalence of 9-valent vaccine type HPV infection among all females with 1–5 LTSPs was 9.9% (95% CI, 7.1%–12.8%) and 9.2% (95% CI, 6.1%–12.3%) when excluding participants who reported a new sexual partner in the past 12 months (Table 3). Among females with >5 LTSPs, the adjusted prevalence of 9-valent vaccine type HPV infection was 13.4% (95% CI, 9.9%–17.0%) and 12.1% (95% CI, 8.8%–15.4%) in the full and restricted samples, respectively.
Figure 2.
Detection of cervicovaginal human papillomavirus (HPV) infection among unvaccinated, sexually experienced females, stratified by age and number of lifetime sexual partners (LTSPs), and restricted among those who reported no new sexual partners in the past 12 months. Data are crude weighted prevalence estimates; corresponding error bars are Korn and Graubard 95% confidence intervals. Estimates for 9-valent vaccine-type HPV among females age 18–26, reporting >5 LTSPs, and no new partner over the past 12 months have a relative confidence interval width >130% and should be interpreted with caution.
Table 3.
Adjusted Prevalence Estimates (Predictive Margins) of Cervicovaginal Human Papillomavirus Infection Among Unvaccinated, Sexually Experienced Females, Stratified by Age and Number of Lifetime Sexual Partners
| Adjusted HPV Prevalence, % (95% CI) | ||
|---|---|---|
| HPV Type and Age Group | Overall Female Population (n = 2598)a | Females Without a New Partner in the Past 12 Months (n = 2337)b |
| 9-valent vaccine typec | ||
| Age 18–26 y | ||
| 1–5 LTSPs | 11.3 (6.3–16.2) | 10.9 (5.6–16.2) |
| >5 LTSPs | 18.6 (9.3–27.9) | 15.4 (3.3–27.4) |
| Age 27–45 y | ||
| 1–5 LTSPs | 9.9 (7.1–12.8) | 9.2 (6.1–12.3) |
| >5 LTSPs | 13.4 (9.9–17.0) | 12.1 (8.8–15.4) |
| Age 46–59 y | ||
| 1–5 LTSPs | 7.6 (4.4–10.8) | 6.9 (4.1–9.8) |
| >5 LTSPs | 10.9 (7.0–14.7) | 8.9 (5.8–12.1) |
| High-risk typed | ||
| Age 18–26 y | ||
| 1–5 LTSPs | 18.1 (12.2–23.9) | 20.1 (12.4–27.7) |
| >5 LTSPs | 29.9 (17.3–42.5) | 26.1 (11.0–41.2) |
| Age 27–45 y | ||
| 1–5 LTSPs | 15.7 (12.5–19.0) | 14.9 (11.4–18.4) |
| >5 LTSPs | 22.7 (18.6–26.9) | 20.5 (16.3–24.6) |
| Age 46–59 y | ||
| 1–5 LTSPs | 12.3 (8.7–15.9) | 11.6 (8.4–14.9) |
| >5 LTSPs | 19.1 (14.9–23.4) | 17.0 (12.5–21.5) |
| Any typee | ||
| Age 18–26 y | ||
| 1–5 LTSPs | 32.3 (24.8–39.9) | 34.3 (26.1–42.5) |
| >5 LTSPs | 56.4 (44.0–68.7) | 54.9 (39.0–70.8) |
| Age 27–45 y | ||
| 1–5 LTSPs | 32.5 (27.1–37.8) | 31.0 (24.4–37.6) |
| >5 LTSPs | 45.8 (39.6–52.0) | 41.9 (34.8–49.1) |
| Age 46–59 y | ||
| 1–5 LTSPs | 32.4 (26.4–38.3) | 31.0 (24.2–37.8) |
| >5 LTSPs | 43.6 (36.4–50.7) | 40.9 (33.1–48.7) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; LTSP, lifetime sexual partner.
aFor each HPV outcome, adjusted prevalence estimates (predictive margins) of HPV detection were calculated by weighted multivariable logistic regression models, which included adjustment for race/ethnicity, marital status, cigarette smoking status, number of lifetime sexual partners (continuous), and report of a new sexual partnership in the past 12 months.
bFor each HPV outcome, adjusted prevalence estimates (predictive margins) of HPV detection were calculated by weighted multivariable logistic regression models, which included adjustment for race/ethnicity, marital status, cigarette smoking status, and number of lifetime sexual partners (continuous).
cNine-valent vaccine types: 6, 11, 16, 18, 31, 33, 45, 52, 58.
dHigh-risk types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68.
eAny type HPV: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, IS39.
Crude and adjusted prevalence estimates of cervicovaginal HPV infection by other risk factors are presented in Supplementary Tables 4and 5.
Sensitivity Analyses
Similar findings were observed when excluding 18- to 19-year-olds from the analyses due to lack of information on marital status (Supplementary Tables 6 and 7).
Discussion
In the first analysis to evaluate genital HPV burden in a nationally representative sample of unvaccinated US men and women by age and sexual behavior simultaneously, we report higher HPV prevalence in individuals with >5 lifetime sexual partners (vs 1–5 LTSPs) from age 18–59 years. This observation remained after restricting the analysis to individuals with no new sexual partners in the past 12 months. Taken together, this analysis suggests that a significant burden of prevalent genital HPV in adult men and women does not reflect a recent acquisition from a new sex partner, particularly in individuals over the recommended age for catch-up vaccination (>26 years).
Calls for prophylactic HPV vaccination in adults have increased over recent years to address the increasing epidemics of HPV-associated oral and anal cancers, and to accelerate the prevention of cervical precancer and cancer in screened populations [21]. Given our current understanding that HPV vaccines have limited (if any) therapeutic effect [22–25], the success of such adult vaccination strategies presumes that causal infections are occurring later in life, and are thus preventable via immunization. Cervical cancer modeling studies suggest, however, that up to 75% of causal HPV infections occur prior to age 30 [26]. This is supported by NHANES survey data, where new partner acquisition peaks in the 20s and reaches a nadir by age 30 years [27]. Thus, while natural history studies show a high relative risk of new HPV detection with a new sexual partner independent of age, most of the absolute number of newly detected infection is likely recurrent detection of previously acquired infection [28]. Taken together, these data highlight the importance of considering all sexual risk exposures, not just new exposures, when considering the benefits of adult HPV vaccination.
We still lack any quantifiable estimates of the proportion of HPV infections that are “cleared” (ie, eradicated) vs immunologically “controlled” to explain the high prevalence of HPV throughout the lifespan in the absence of significant continuous new acquisition [29]. It is likely that prevalent HPV burden represents a mix of long-term persistent HPV, most of which reflects transient recurrent detection, which will be rapidly controlled again by a robust T-cell–mediated immune response [28, 30–32], except in immunosuppressed populations [33, 34]. While this has been difficult to empirically demonstrate in epidemiological studies, recent simulation models that include transitions between undetectable viral latency and detectable reactivation provide a better fit to observed data in both women and men compared with models that ignore latency [35, 36].
Despite the strengths afforded by this analysis through the utilization of a large nationally representative sample of men and women in the US household population, there are some notable limitations. In addition to unit nonresponse, not all individuals provided genital samples or questionnaire data, which may have biased our results. There is a risk of reporting bias, especially with regard to sexual behavior and vaccination status, which may have influenced our outcomes. Although NHANES is the largest population-based survey of HPV prevalence in the US, sample sizes remained insufficient to permit informative stratification of only individuals with a new sexual partner in the past 12 months, or more granular stratifications of LTSPs and age. It is also worth noting that the DNA extraction method used in NHANES results in a high test sensitivity, especially compared to standard clinical assays [37]. This may explain why HPV prevalence is relatively high in older adults, such as postmenopausal women, who may be more likely to have low viral load infections.
When carefully examining HPV prevalence simultaneously by age and sexual behavior, recent acquisition appears to have a lower overall contribution to current detectable infection burden of HPV in the US population relative to cumulative exposures over a lifetime. Although there is some evidence to support HPV vaccination in some adult subpopulations [38, 39], our observations are generally consistent with prior cost-effectiveness models, suggesting a relatively low population-level benefit to vaccination over the age of 26 years [40, 41]. Thus, when discussing the risks/benefits to vaccination of adult men and women for clinical decision making, it will be important to incorporate the likely ongoing risk of HPV infection due to recurrence of previously acquired and controlled infections as well as risks of new acquisitions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to the National Health and Nutrition Examination Survey study staff and participants, without whom this study would not have been possible.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID); and by extramural support from NIAID (grant numbers R01AI120938 and R01AI128779 to A. A. R. T and T32AI102623 to E. U. P.) and the National Cancer Institute (P50CA098252 to A. F. R).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. Morb Mortal Weekly Rep 2016; 65:1405–8. [DOI] [PubMed] [Google Scholar]
- 2. US Food and Drug Administration. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old.2018. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622715.htm.
- 3. Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. Am J Transplant 2019; 19:3202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American College of Obstetricians and Gynecologists. FDA approval of 9-valent HPV vaccine for use in women and men age 27–45. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-expanded-use-gardasil-9-include-individuals-27-through-45-years-old#:~:text=The%20U.S.%20Food%20and%20Drug,aged%2027%20through%2045%20years. Accessed 25 March 2020.
- 5. Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE. Contributions of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res 2012; 72:6183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fu TC, Carter JJ, Hughes JP, et al. Re-detection vs. new acquisition of high-risk human papillomavirus in mid-adult women. Int J Cancer 2016; 139:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ranjeva SL, Baskerville EB, Dukic V, et al. Recurring infection with ecologically distinct HPV types can explain high prevalence and diversity. Proc Natl Acad Sci U S A 2017; 114:13573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). Available at: https://www.cdc.gov/nchs/nhanes/index.htm. Accessed 25 March 2020.
- 9. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). NHANES response rates and population totals. Available at: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx. Accessed 10 August 2020.
- 10. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). Interviewer procedures manual. Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_Interviewer_Procedures_Manual.pdf. Accessed 10 August 2020.
- 11. National Center for Health and Vital Statistics. National Health and Nutrition Examination Survey. 2015–2016 data documentation, codebook, and frequencies. Data file: SXQ_I.xpt. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/SXQ_I.htm. Accessed 10 August 2020.
- 12. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). Laboratory procedure manual; high-risk human papillomavirus (HPV) genotypes; self collected vaginal swabs; Cobas HPV (Roche Diagnostics). Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/HPVSWC_I_HPVC_I_R_MET.pdf. Accessed 10 August 2020.
- 13. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). Laboratory procedure manual; high-risk human papillomavirus (HPV) genotypes; self collected penile swabs; Cobas HPV (Roche Diagnostics). Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015–2016/labmethods/HPVP_I_MET.pdf. Accessed 10 August 2020.
- 14. Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). Laboratory procedure manual; human papillomavirus (HPV) genotypes; DNA extracted from self collected vaginal swabs; Linear Array HPV Genotyping Assay (Roche Diagnostics). Available at: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/HPVSWR_I_HPVS_I_R_MET.pdf. Accessed 10 August 2020.
- 15. National Centers for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2015–2016 data documentation, codebook, and frequencies. Data file: HPVSWR_I.xpt. Available at: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/HPVSWR_I.htm. Accessed 10 August 2020.
- 16. US Food and Drug Administration. Gardasil 9. Silver Spring, MD: FDA; 2018. [Google Scholar]
- 17. Chen T-C, Parker JD, Clark J, Shin H-C, Rammon JR, Burt VL. National Health and Nutrition Examination Survey: estimation procedures, 2011–2014. Vital Health Stat 2018; 177:1–26. [PubMed] [Google Scholar]
- 18. Graubard BI, Korn EL. Predictive margins with survey data. Biometrics 1999; 55:652–9. [DOI] [PubMed] [Google Scholar]
- 19. Ward BW. kg_nchs: a command for Korn-Graubard confidence intervals and National Center for Health Statistics’ data presentation standards for proportions. Stata J 2019; 19:510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Korn EL, Graubard BI.. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol 1998; 24:193–201. [Google Scholar]
- 21. Bosch FX, Robles C, Díaz M, et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol 2016; 13:119–32. [DOI] [PubMed] [Google Scholar]
- 22. Silverberg MJ, Leyden WA, Lam JO, et al. Effectiveness of catch-up human papillomavirus vaccination on incident cervical neoplasia in a US health-care setting: a population-based case-control study. Lancet Child Adolesc Health 2018; 2:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herweijer E, Sundström K, Ploner A, Uhnoo I, Sparén P, Arnheim-Dahlström L. Quadrivalent HPV vaccine effectiveness against high-grade cervical lesions by age at vaccination: a population-based study. Int J Cancer 2016; 138:2867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dehlendorff C, Sparén P, Baldur-Felskov B, et al. Effectiveness of varying number of doses and timing between doses of quadrivalent HPV vaccine against severe cervical lesions. Vaccine 2018; 36:6373–8. [DOI] [PubMed] [Google Scholar]
- 25. Crowe E, Pandeya N, Brotherton JM, et al. Effectiveness of quadrivalent human papillomavirus vaccine for the prevention of cervical abnormalities: case-control study nested within a population based screening programme in Australia. BMJ 2014; 348:g1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burger EA, Kim JJ, Sy S, Castle PE. Age of acquiring causal human papillomavirus (HPV) infections: leveraging simulation models to explore the natural history of HPV-induced cervical cancer. Clin Infect Dis 2017; 65:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryser MD, Rositch A, Gravitt PE. Modeling of US human papillomavirus (HPV) seroprevalence by age and sexual behavior indicates an increasing trend of HPV infection following the sexual revolution. J Infect Dis 2017; 216:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gravitt PE, Winer RL. Natural history of HPV infection across the lifespan: role of viral latency. Viruses 2017; 9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gravitt PE. The known unknowns of HPV natural history. J Clin Invest 2011; 121:4593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maglennon GA, McIntosh PB, Doorbar J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J Virol 2014; 88:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu SH, Brotman RM, Zenilman JM, Gravitt PE, Cummings DA. Menstrual cycle and detectable human papillomavirus in reproductive-age women: a time series study. J Infect Dis 2013; 208:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu SH, Cummings DA, Zenilman JM, Gravitt PE, Brotman RM. Characterizing the temporal dynamics of human papillomavirus DNA detectability using short-interval sampling. Cancer Epidemiol Biomarkers Prev 2014; 23:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst 2005; 97:577–86. [DOI] [PubMed] [Google Scholar]
- 34. Theiler RN, Farr SL, Karon JM, et al. High-risk human papillomavirus reactivation in human immunodeficiency virus–infected women: risk factors for cervical viral shedding. Obstet Gynecol 2010; 115:1150–8. [DOI] [PubMed] [Google Scholar]
- 35. van Schalkwyk C, Moodley J, Welte A, Johnson LF. Estimated impact of human papillomavirus vaccines on infection burden: the effect of structural assumptions. Vaccine 2019; 37:5460–5. [DOI] [PubMed] [Google Scholar]
- 36. Brouwer AF, Meza R, Eisenberg MC. Integrating measures of viral prevalence and seroprevalence: a mechanistic modelling approach to explaining cohort patterns of human papillomavirus in women in the USA. Philos Trans R Soc Lond B Biol Sci 2019; 374:20180297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unger ER, Steinau M, Lin JM, Patel SS, Swan DC. Impact of HPV assay on observed population prevalence. Diagn Mol Pathol 2011; 20:101–4. [DOI] [PubMed] [Google Scholar]
- 38. Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol 2013; 130:264–8. [DOI] [PubMed] [Google Scholar]
- 39. Ghelardi A, Parazzini F, Martella F, et al. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol Oncol 2018; 151:229–34. [DOI] [PubMed] [Google Scholar]
- 40. Laprise JF, Chesson HW, Markowitz LE, et al. Effectiveness and cost-effectiveness of human papillomavirus vaccination through age 45 years in the United States. Ann Intern Med 2020; 172:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soe NN, Ong JJ, Ma X, et al. Should human papillomavirus vaccination target women over age 26, heterosexual men and men who have sex with men? A targeted literature review of cost-effectiveness. Hum Vaccin Immunother 2018; 14:3010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.