Abstract
Background
The diversity of individuals at risk for Trypanosoma cruzi infection in the United States poses challenges for diagnosis. We evaluated the diagnostic accuracy of Food and Drug Administration (FDA)-cleared tests in the Washington Metropolitan area (WMA).
Methods
In total, 1514 individuals were evaluated (1078 from Mexico, Central and northern South America [TcI-predominant areas], and 436 from southern South America [TcII/V/VI-predominant areas]). Optical density (OD) values from the Hemagen EIA and Chagatest v.3 Wiener, and categorical results of the IgG-TESA-blot (Western blot with trypomastigote excretory-secretory antigen), and the Chagas detect plus (CDP), as well as information of area of origin were used to determine T. cruzi serostatus using latent class analysis.
Results
We detected 2 latent class (LC) of seropositives with low (LC1) and high (LC2) antibody levels. A significantly lower number of seropositives were detected by the Wiener, IgG-TESA-blot, and CDP in LC1 (60.6%, P < .001, 93.1%, P = .014, and 84.9%, P = .002, respectively) as compared to LC2 (100%, 100%, and 98.2%, respectively). LC1 was the main type of seropositives in TcI-predominant areas, representing 65.0% of all seropositives as opposed to 22.8% in TcII/V/VI-predominant areas. The highest sensitivity was observed for the Hemagen (100%, 95% confidence interval [CI]: 96.2–100.0), but this test has a low specificity (90.4%, 95% CI: 88.7–91.9). The best balance between positive (90.9%, 95% CI: 83.5–95.1), and negative (99.9%, 95% CI: 99.4–99.9) predictive values was obtained with the Wiener.
Conclusions
Deficiencies in current FDA-cleared assays were observed. Low antibody levels are the main type of seropositives in individuals from TcI-predominant areas, the most frequent immigrant group in the United States.
Keywords: chronic Chagas disease, Latin American immigrants, serodiagnosis, latent class analysis, test accuracy
We show the limitations of Food and Drug Administration (FDA)-cleared tests in the diagnosis of Chagas disease in a community-based study in Hispanics living in the United States. Two types of seropositive (low and high antibody levels) were detected, which predominance varies by endemic area.
Approximately 40% (18 million) of Latin Americans living in the United States were born in a Chagas-endemic country [1]. Including undocumented immigrants, recent calculations estimate 326 000 to 347 000 cases of Trypanosoma cruzi infections in the United States [2].
T. cruzi is composed of a highly diverse population of 7 discrete typing units (DTUs) whose distribution varies by geographic location [3]. TcI, which includes 5 intra-DTUs (Ia, Ib, Ic, Id, Ie), predominates from the southern United States to northern South America (including Mexico, Central America, Colombia, Venezuela, Ecuador, and Peru). At the same time, TcII/V/VI prevail in southern South America [3, 4]. Differences in the immune response, lower levels of antibodies, and lower sensitivity of diagnostic tests have been observed between TcI- and TcII/V/VI-predominant areas [5–8].
An in vitro study found that infection of human peripheral blood cells with TcI and TcII DTUs induced a different immune response [9]. Compared to TcII, TcI caused a higher monocyte activation, higher production of interleukin (IL)-10 in infected monocytes, and higher levels of IL-17 by CD8+ T cells. Conversely, infection with TcII was associated with higher levels of TNF-alpha and granzyme A by CD8+ T lymphocytes [9]. Differences in the immune response, as well as host-immune factors, may affect the performance of serological tests that have been validated in TcII/V/VI-predominant areas. Lower levels of antibodies to the whole extract of the epimastigote of TcII and recombinant antigens (B13, JL8, and 1F8) was observed in Central Americans compared to South Americans [5].
In the United States, the high diversity of Latin Americans from different endemic countries provides unique challenges in the diagnosis and recognition of clinical manifestations. A recent study in Hispanic blood donors in the United States found that levels of antibodies and sensitivity of some Food and Drug Administration (FDA)-cleared tests were low in Mexicans, intermediate in Central Americans, and high in South Americans [8].
There are four FDA-cleared tests for diagnosis: Hemagen EIA, Wiener ELISA (enzyme-linked immunosorbent assay), lateral flow assay Chagas Detect Plus (CDP), and Ortho ELISA (Supplementary Table 1). The Hemagen, CDP, and Ortho are produced and sold by US manufacturers. The Hemagen and the CDP can be ordered in small numbers, so these tests are the easiest to acquire. The Ortho is only sold in bulk supply and has a short shelf-life. The Wiener is produced by an Argentine laboratory, and without an established distributor in the United States its acquisition is challenging and costly because it involves coordination and payments of freight, expeditors continuous entry bond, local delivery under refrigeration, among others (Sheba Meymandi, personal communication). Due to logistic and costs reasons, most laboratories in the United States use the Hemagen and/or the CDP. However, early findings expressed concerns about the high number of indeterminate and/or false positive results provided by these 2 tests [8, 10, 11]. Furthermore, the Wiener, Hemagen, and CDP have been mainly evaluated in specimens from the Southern cone of Latin America. Evaluations in Mexico and Central America have been done using repository samples that may provide inaccurate results because different countries use a different algorithm of diagnosis and selection of positive individuals depends on the accuracy of screening tests [12]. A Western blot using the Trypomastigote Excretory-Secretory Antigen (TESA-Blot) is one of the confirmatory tests; however, due to the absence of a commercial TESA-blot, the test is only performed by the Centers of Disease Control and Prevention (CDC) for clinical purposes [13].
Latin Americans account for 15.3% (906 00 Latinos) of the population in the Washington Metropolitan Area (WMA) [14, 15]. Different from other areas in the United States, where the predominant Hispanic group is Mexicans, immigrants from El Salvador are the largest immigrant group in the WMA representing 33.3% of the Latin American population, followed by Mexicans (14.6%), Guatemalans (7.6%), Hondurans (2.8%), and Bolivians (2.8%) [15, 16].
We evaluated the accuracy of three of the four FDA-cleared tests in a community-based study of Latin American immigrants in the WMA. We used latent class analysis (LCA) in the absence of a gold standard in this geographically diverse Latin American population. LCA was also used to determine different types of seropositives and their distribution in participants from TcI- and TcII/V/VI-predominant areas.
METHODS
Study Design, Enrollment, and Participants
A cross-sectional study was conducted from February 2016 to April 2018 in the WMA. Individuals >18 years old from any of the 21 endemic countries [17] were enrolled via recruitment in churches, community centers, consulates, and health fairs.
Enrollment was conducted in places where Latin Americans made up >15% of the population [14, 18, 19]. These included areas in the state of Virginia (Manassas Park City, Fairfax City, Fairfax County, Arlington County, Falls Church City, and Alexandria City), the state of Maryland (Montgomery County and Prince George’s County), and Washington, D.C.
The institutional review board of the Johns Hopkins School of Public Health (JHSPH) approved the protocol (IRB 6713).
Serological Tests
Three FDA-cleared tests (2 ELISAs and one lateral flow assay) were run in parallel in all samples following the instructions of the manufacturer. These tests included: The Hemagen Chagas kit (Hemagen Laboratories, Columbia, MD, USA), the Chagatest recombinant v.3.0 (Wiener Laboratories SAIC, Argentina), and the Chagas Detect Plus (CDP, InBios International Inc, Seattle, WA, USA).
The CDP was performed in a community-setting using capillary blood samples obtained by fingerstick. The IgG-TESA-blot was carried out in a subset of serum samples randomly selected and weighted based on their country of origin (TcI vs TcII/V/VI).
The IgG-TESA-blot was done using the TESA from T. cruzi Y strain and was developed following published procedures [20–22] in our laboratory at JHSPH.
Epidemiological Data
A validated questionnaire standardized in Santa Cruz, Bolivia, was used to obtain epidemiological data [23]. The questionnaire was in Spanish and mainly self-administered. In cases of illiterate participants, a study volunteer administered the survey.
Individuals were grouped in TcI- (Mexico, Central America, Peru, Costa Rica, and Venezuela) and TcII/V/VI- (Argentina, Bolivia, Brazil, and Chile) predominant areas based on the predominant T.cruzi DTU of their country of origin. We based our grouping on the results of a meta-analysis of the geographic distribution of T. cruzi DTUs [24]. Peruvians were classified in the TcI-predominant group based on more recent molecular results in circulating vectors in 8 endemic departments [25] and serotyping analysis in infected humans from two endemic cities [26].
Latent Class Analysis
The LCA models included categorical (positive, indeterminate, or negative) or continuous (corrected optical densities, ODs) results obtained in the 2 ELISA tests (Wiener and Hemagen), as well as categorical results of the TESA-blot and the CDP. Different models were evaluated with and without the information of the area of origin. The statistical software Mplus Version 8 (Muthén & Muthén, Los Angeles, CA, USA) was used for LCA. The results of the different models are shown in Supplementary Table 2.
Other Statistical Analysis
Positive and negative predictive values (PPV and NPV, respectively) were calculated using the Bayes theorem. Individuals that had received antitrypanosomal treatment, which may affect antibody levels [27, 28], were excluded. Individuals that had been tested previously for Chagas disease were only excluded for the determination of prevalence, test accuracy, and risk factor analysis but not for LCA modeling. The reasons for this exclusion were (1) Previously diagnosed positive individuals may be more likely to participate in the study, thereby increasing the prevalence; (2) individuals with high antibody levels may be more likely to have received a previous positive diagnosis, thus increasing the number of individuals with high antibody levels; and (3) previously diagnosed Chagas patients may be better informed about Chagas risk factors.
To determine test accuracy, we considered samples with indeterminate results in the ELISAs to be positive. Weighted prevalence was calculated following the distributions of Hispanic immigrants living in the WMA based on the reports of the American Community Survey (ACS) [14]. Statistical analyses were performed using the Stata statistical software package version 15.
RESULTS
A total of 1571 subjects were enrolled, 57 were excluded: 26 because they had received antitrypanosomal treatment and 31 because of missing results in some tests. This analysis included 1514 participants, n = 1078 from TcI-predominant, and 436 from TcII/V/VI-predominant areas. Eighty-seven participants had previously been tested for Chagas disease (TcI-predominant = 11 and TcII/V/VI-predominant = 76) (Table S2).
The evaluation of 4 different LCA showed that the use of 3 latent classes with continuous data of the ELISA tests, categorical values of the IgG-TESA-blot, CDP, and area of origin better explains our data (Supplementary Table 3). Because of that and the hypothesis of >1 type of seropositive reactions possible due to different T. cruzi DTUs, model 4 was used for subsequent analyses.
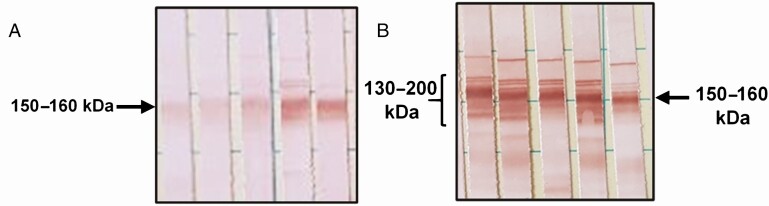
We detected 3 latent classes (LCs) in our model and corresponded to LC1 and LC2 (seropositive classes), and LC3 (seronegative class). The distribution of seropositives by country of origin is shown in Supplementary Table 4. Compared to LC2, LC1 was characterized by having lower OD values or antibody-levels (Ab-levels) in both the Hemagen (P < .001) and Wiener (P < .001). A significantly lower number of positive individuals were detected by the Wiener (P < .001), IgG-TESA-blot (P = .014), and CDP (P = .002) in samples assigned to LC1. Individuals assigned to LC1 were also more likely to react to only the 150–160 kDa band in the IgG-TESA-blot (P < .001) (Figure 1) and more likely to be from TcI-predominant areas (P = .003) (Table 1).
Figure 1.
Band patterns observed in the TESA-blot of seropositive individuals. A, A higher proportion of participants in class 1 reacted only to the 150–160 kDa band (40.0%, 12/30) than participants in latent class 2 (8.0%, 6/75) (P < .001). B, The classical pattern of bands observed in individuals in latent class 2: reaction to the six ladder-like bands of 130–200 kDa (Shed acute phase antigen bands), and the 150–160 kDa band.
Table 1.
Characteristics of Individuals Without a Previous Diagnosis of Chagas Disease Assigned to Each Latent Class
| Characteristic | Seropositive | Seronegative | ||
|---|---|---|---|---|
| Latent Class 1: Low Ab-levels (n = 28, 1.9%) | Latent Class 2: High Ab-levels (n = 70, 4.9%) | P-value (Class 1 vs Class 2) | Latent Class 3: Negatives (n = 1329, 93.1%) | |
| Chagatest v.3, Wiener recombinant | ||||
| Positive, column % (95% CI) | 64.3 (44.1–81.4) | 100.0 (94.9–100.0) | <.001 | .2 (0–.7) |
| Indeterminate, column % (95% CI) | 17.9 (6.0–36.9) | 0.0 (.0–5.1) | <.001 | .2 (0–.5) |
| Corrected OD, median (IQR) | 0.36 (0.03 to 0.66) | 2.17 (1.85 to 2.71) | <.001 | −0.33 (−0.33 to −0.32) |
| Hemagen | ||||
| Positive, column % (95% CI) | 100.0 (87.7–100.0) | 100.0 (94.9–100.0) | 1.000 | 5.6 (4.5–7.0) |
| Indeterminate, column % (95% CI) | 0 (0) | 0 (0) | 1.000 | 3.9 (2.9–5.1) |
| Corrected OD, median (IQR) | 0.41 (0.28–0.62) | 1.47 (1.29–1.66) | <.001 | −0.14 (−0.21 to −0.09) |
| IgG-TESA-blot | ||||
| Positive*, column % (95% CI) | 96.0 (79.6–99.9)a | 100.0 (93.0–100.0)b | .151 | 2.4 (1.6–3.7)c |
| 150 to 160 kDa band only, column % (95% CI) | 41.7 (22.1–63.4) | 9.8 (3.3–21.4) b | .001 | 2.4 (1.5–3.6)c |
| CDP | ||||
| Positives, column % (95% CI) | 82.1 (63.1–93.9) | 100.0 (94.6–100.0)d | <.001 | 6.2 (5.0–7.7)e |
| Other characteristics | ||||
| Subjects from TcI area, column % (95% CI) | 35.7 (18.6–55.9) | 12.9 (11) | .009 | 78.9 (76.6–81.0) |
Corrected OD values correspond to the OD value of the sample minus the cutoff of the plate where the sample was evaluated. For continuous variables, P-values were calculated using the Kruskal-Wallis test. For categorical variables, P-values were calculated using the χ 2 test.
Abbreviations: Ab-levels, antibody levels; CDP, Chagas Detect Plus in whole blood; 95% CI, exact binomial 95% confidence intervals; IgG-TESA-blot, Western blot using the Trypomastigote excretory-secretory antigen for the detection of immunoglobulin G antibodies; IQR, interquartile range; OD, optical density.
*All TESA-blot positives had the 150–160 kDa band; not all had SAPA bands visible.
aOnly 24 of the 28 samples were evaluated in this group.
bOnly 51 of the 70 samples were evaluated in this group.
cOnly 930 of 1329 samples were evaluated in this group.
dOnly 66 of the 70 samples were evaluated in this group.
eOnly 1305 of 1329 samples were evaluated in this group.
The overall weighted seroprevalence was 3.8% (95% confidence interval [CI]: 2.9–5.0%). Specimens assigned to the low (LC1) and high (LC2) Ab-level latent classes were detected in both TcI- and TcII/V/VI-predominant areas. Compared to TcI-predominant areas, the prevalence of seropositives assigned to LC1 was higher in participants from TcII/V/VI-predominant areas (5.2% vs 1.3%). However, the majority of the seropositives (65% of all seropositives) from TcI-predominant areas fell into LC1 (low Ab-level) compared with 22.8% from TcII/V/VI-predominant areas (Table 2).
Table 2.
Weighted Prevalence of the 2 Latent Classes of Seropositive Individuals Detected in the 2 Geographic Areas
| Overall | TcI-predominant Areas | TcII/V/VI-predominant Areas | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Latent Class | % | 95% CI | % Among Positives | % | 95% CI | % Among Positives | % | 95% CI | % Among Positives |
| All seropositives (latent class 1 and 2) | 3.8 | 2.9–5.0 | Reference | 2.1 | 1.3–3.1 | Reference | 22.8 | 18.7–27.6 | Reference |
| Low Ab-levels (latent class 1) | 1.6 | .9–2.6 | 42.1 | 1.3 | .7–2.4 | 65.0 | 5.2 | 3.3–8.1 | 22.8 |
| High Ab-levels (latent class 2) | 2.2 | 1.6–3.0 | 57.9 | 0.8 | .4–1.7 | 40.0 | 17.6 | 14.0–22.0 | 77.2 |
Individuals with a previous diagnosis of Chagas disease were excluded from this calculation.
Weighted prevalence was obtained after assigning weights to participants following the distributions of Hispanic immigrants living in the WMA based on the reports of the American Community Survey.
Abbreviations: Ab, antibody; 95% CI, logit 95% confidence interval.
The Wiener and the CDP had lower sensitivities compared to crude-based assays, but no significant differences in sensitivities were observed between the 2 areas. The highest sensitivity was observed for the Hemagen and the IgG-TESA-blot, but the Hemagen also had a lower specificity. The specificity of all diagnostic tests was similar in the 2 geographic areas. Only the Wiener had a specificity >99%. Significantly lower PPVs were observed in TcI-predominant areas because of the lower prevalence of the infection and specificities <99.0% for most tests. The lowest PPV was found for the Hemagen and the CDP. The best balance between PPV and NPV in TcI-predominant areas was observed with the Wiener (Table 3).
Table 3.
Performance of Each Test in the Diagnosis of Chronic Chagas Disease by Area of Origin in At-risk Individuals Living in the United States
| Overall (Prevalence: 3.8%) | TcI-predominant (Prevalence: 2.1%) | TcII/V/VI-predominant (Prevalence: 22.8%) | |||||
|---|---|---|---|---|---|---|---|
| % (n/N) | 95% CI | % (n/N) | 95% CI | % (n/N) | 95% CI | P-value | |
| Sensitivity | |||||||
| Wiener | 94.9 (93/98) | 88.6–97.8 | 94.7 (18/19) | 75.4–99.1 | 94.9 (75/79) | 87.7–98.0 | .972 |
| Hemagen | 100.0 (98/98) | 96.2–100.0 | 100.0 (19/19) | 88.6–92.2 | 100.0 (79/79) | 95.4–100.0 | 1.000 |
| IgG-TESA-blot | 100.0 (74/74) | 95.1–100.0 | 100.0 (15/15) | 79.6–100.0 | 100.0 (59/59) | 93.9–100.0 | 1.000 |
| CDP | 93.6 (88/94) | 86.8–97.0 | 94.7 (18/19) | 75.4–99.1 | 94.7 (71/75) | 87.1–97.9 | .990 |
| Specificity | |||||||
| Wiener | 99.6 (1323/1328) | 99.1–99.8 | 99.5 (1043/1048) | 98.9–99.8 | 100.0 (280/280) | 98.6–100.0 | .247 |
| Hemagen | 90.4 (1202/1329) | 88.7–91.9 | 90.6 (949/1048) | 88.6–92.2 | 90.0 (253/281) | 86.0–93.0 | .793 |
| IgG-TESA-blot | 98.7 (898/910) | 97.7–99.2 | 99.0 (723/730) | 98.0–99.5 | 97.2 (175/180) | 93.7–98.8 | .055 |
| CDP | 94.7 (1224/1305) | 92.4–95.0 | 93.4 (967/1035) | 91.8–94.8 | 95.2 (257/270) | 91.9–97.2 | .287 |
| Positive predictive value | |||||||
| Wiener | 90.9 | 83.5–95.1 | 80.2 | 60.2–91.6 | 100.0 | 95.1–100.0 | <.001 |
| Hemagen | 29.2 | 23.7–76.3 | 17.8 | 11.9–25.6 | 74.8 | 65.8–82.0 | <.001 |
| IgG-TESA-blot | 75.0 | 64.9–82.9 | 70.1 | 49.2–85.0 | 91.4 | 82.0–96.1 | <.001 |
| CDP | 37.6 | 30.7–45.1 | 22.7 | 16.6–34.5 | 85.3 | 76.2–91.3 | <.001 |
| Negative predictive value | |||||||
| Wiener | 99.9 | 99.4–99.9 | 99.9 | 99.4–100.0 | 98.5 | 96.3–99.4 | .478 |
| Hemagen | 100.0 | 99.6–100.0 | 100.0 | 99.6–100.0 | 100.0 | 98.5–100.0 | 1.000 |
| IgG-TESA-blot | 100.0 | 99.6–100.0 | 100.0 | 99.5–100.0 | 100.0 | 97.9–100.0 | .175 |
| CDP | 99.8 | 99.3–99.9 | 99.9 | 99.4–100.0 | 98.4 | 96.0–99.3 | <.001 |
Abbreviations: CDP, Chagas Detect Plus. Indeterminate results were considered positives to calculate test accuracy; 95% CI, exact binomial 95% confidence intervals.
Indeterminate results were observed in a higher proportion of samples using the Hemagen (3.6%, 52/1427) than in the Wiener (0.5%, 7/1426). Samples with indeterminate results by Wiener were more likely to be classified as seropositive by the LCA (seropositive: 5/7, 71.4% vs seronegative: 2/7, 28.6%, P < .001), whereas those with indeterminate results by Hemagen were more likely to be classified as seronegative (seropositive: 0/52, 0.0% vs seronegative: 52/52, 100%, P < .001).
When compared to seronegative participants, seropositive individuals in both LC1 and LC2 had significant risk factors associated with T. cruzi infection even after adjusting by geographic area. The strongest risk factor in both LC1 and LC2 was having ever seen the vector in an endemic area (range adjusted odds ratio [OR]: 2.19–5.13, P < .05) (Table 4).
Table 4.
Characteristics Associated with T. cruzi Infection in Participants of Each Latent Class in the 2 Geographic Areas
| Characteristic | T.cruzi Seropositivity | Crude OR | Adjusted OR by Area | |||||
|---|---|---|---|---|---|---|---|---|
| % Positive, n/N | % Negative, n/N | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Low antibody levels: Latent class 1 | ||||||||
| Ever lived in rural area | 46.4 (13/28) | 40.4 (537/1329) | 1.28 | .60–2.71 | .522 | 1.90 | .87–4.14 | .107 |
| Ever lived in mud house | 60.7 (17/28) | 35.8 (476/1329) | 2.77 | 1.29–5.96 | .009 | 2.27 | 1.04–4.94 | .040 |
| Ever seen vector in endemic area | 57.1 (16/28) | 25.2 (335/1329) | 3.96 | 1.85–8.44 | <.001 | 2.35 | 1.05–5.27 | .038 |
| Ever bitten by vector in endemic area | 17.9 (5/28) | 13.1 (174/1329) | 1.11 | .74–1.67 | .602 | 0.84 | .54–1.30 | .448 |
| Ever lived in an infested house | 50.0 (13/26) | 22.8 (273/1199) | 1.64 | 1.01–2.66 | .048 | 1.46 | .86–2.48 | .159 |
| Ever heard of Chagas disease | 53.6 (15/28) | 28.5 (378/1327) | 2.90 | 1.37–6.15 | .006 | 1.15 | .48–2.77 | .756 |
| High antibody levels: Latent class 2 | ||||||||
| Ever lived in rural area | 41.4 (29/70) | 40.4 (537/1329) | 1.04 | .64–1.70 | .865 | 1.94 | 1.14–3.31 | .015 |
| Ever lived in mud house | 61.4 (43/70) | 35.8 (476/1329) | 2.85 | 1.74–4.68 | <.001 | 2.10 | 1.24–3.54 | .005 |
| Ever seen vector in endemic area | 61.4 (43/70) | 25.2 (335/1329) | 4.73 | 2.87–7.77 | <.001 | 2.09 | 1.23–3.57 | .007 |
| Ever bitten by vector in endemic area | 42.9 (30/70) | 13.1 (174/1329) | 1.79 | 1.36–2.35 | <.001 | 1.26 | .92–1.71 | .143 |
| Ever lived in an infested house | 57.4 (39/68) | 22.8 (273/1199) | 1.63 | 1.19–2.23 | .002 | 1.38 | .96–1.99 | .083 |
| Ever heard of Chagas disease | 75.4 (52/69) | 28.5 (378/1327) | 7.68 | 4.38–13.45 | <.001 | 2.02 | 1.07–3.82 | .029 |
Abbreviations: 95% CI, 95% confidence interval. Individuals with a previous diagnosis of Chagas disease were excluded from this evaluation. OR, odds ratio. Area of origin corresponds to participants from TcI or TcII/V/VI areas.
DISCUSSION
Through this study, we show the limitations of FDA-cleared Chagas tests. Deficiencies in both recombinant- and lysate- based assays were observed. The Hemagen and CDP had a higher sensitivity in TcI- and TcII/V/VI-predominant areas, but a high number of indeterminate and false-positive results were obtained; this low specificity had a marked impact on PPV in this low-prevalence population. The Wiener had low sensitivity in the 2 areas. Our study also shows the presence of 2 types of seropositives with low and high Ab-levels. The dominance of seropositive individuals with low Ab-levels among positive immigrants from TcI-predominant areas, which are the most frequent immigrant group in the United States [14], could lead to a failure to detect a significant number of infections in this patient population.
Seropositivity with low Ab-levels was not exclusive to Mexico and Central America. The presence of the 2 types of seropositives possible due to TcI or TcII/V/VI infections found in our study correlate with the distribution of T. cruzi DTU I and TcII/V/VI [3]. TcI is the most widespread DTU across Latin America, including the Amazonia (Brazil) and the Andean region (Bolivia and Peru), whereas TcII/V/VI are predominant in southern South America [3, 24–26, 29–32]. The prevalence of LC1 was higher in the Bolivian group than in TcI-predominant areas but corresponded to only 22.8% of all seropositives from Bolivia. Our results closely align with the reports of a meta-analysis, which found that TcI and TcV corresponded to 23.2% and 68.2%, respectively, of T. cruzi isolates in human infections in Bolivia [24].
In the IgG-TESA-blot, individuals with low Ab-levels were more likely to react to only the 150–160 kDa band without reaction to any of the 6 SAPA (shed acute phase antigen) bands, which is different from the typical band-pattern observed in TcII/V/VI-predominant areas (reaction to SAPA bands and the 150–160 kDa complex) [22]. The absence of SAPA bands in the IgG-TESA-blots of patients with low Ab-levels may indicate low levels of parasitemia, which is a characteristic of TcI infections [33–35] or may be due to the low sensitivity of the IgG-TESA-blot due to the low Ab-levels in these patients.
Cross-reactions with leishmaniasis and other parasites were not evaluated in this study. Previous studies showed that the Wiener and the IgG-TESA-blot had high specificity (100%, 0/44) in samples of patients with visceral and cutaneous leishmaniasis, and T. rangeli [22, 36]. The Hemagen, which is based on lysate-based antigen, showed cross-reactivity with leishmaniasis [37], whereas cross-reactivity with leishmaniasis has not been evaluated for the CDP. In this study, all the seropositives reacted to either of the specific assays (Wiener and/or IgG-TESA-blot). Therefore, the probability of having cross-reactions with other parasites is very low.
Our LCA uses the OD values in the ELISAs, OD values may be affected by the antibody levels to the antigens use in the ELISA assays but are less affected by previous assay validations in specific groups of patients. Categorial results of ELISA tests (positive, negative, or indeterminate) are obtained using the OD value of each sample and the cutoff of the assay; the latter may depend on the population in which the assay has been previously evaluated. The use of OD values, instead of the categorical results, in the LCA may decrease the impact of the use of cutoffs that have been validated only in individuals with high antibody levels.
The World Health Organization (WHO) recommends basing the diagnosis of Chagas disease using the results of at least 2 different tests that apply different antigens or platforms. Congruent positive results by at least 2 tests confirms the infection. However, this recommendation may have limitations when 2 tests with low specificity are used. In this study, 0.8% (10/1329) of the samples reacted only to the Hemagen and CDP but were classified as negative in the LCA (Supplementary Table 5). Because both tests lack high specificity, we think that most samples with positive results only to Hemagen and CDP are likely to be false positives.
The advantages of the CDP include that it is a point-of-contact assay, can use whole blood or serum, and provides the results within 20 minutes so that individuals can receive their initial diagnosis during the first visit. Based on our experience, individuals who are notified that they have a positive CDP are more likely to provide accurate contact information and return for follow-up procedures [21]. However, to decrease anxiety, an appropriate explanation of the result, and the probability of inaccurate diagnosis must be provided.
The main strength of this study is that we used samples from Latin Americans living in the United States that were tested in parallel by at least 3 FDA-cleared tests that are based on different antigens, avoiding the selection of samples with high levels of antibodies to a specific antigenic repertoire. Reaching Latin American immigrants through the healthcare system is a challenge due to legal status, language, and cultural barriers. Our study prospectively enrolled individuals in different community centers, increasing the diversity of our participants. Most studies have been performed using repository samples, where the characteristics of the samples depend on the initial tests used for screening.
The main limitations of this study are the small number of positive samples from Mexico due to the lower prevalence of infection in this country. Furthermore, we did not include other FDA-cleared/approved tests such as the ORTHO T. cruzi ELISA test system because this test is difficult to acquire in the United States for population-based diagnostic testing. We cannot conclude that low Ab-levels are the result of TcI infections; future genotyping studies can provide this information. However, molecular testing during the chronic phase is a challenge because it is characterized by low parasitemia levels. Low Ab-levels in southern South America could be the result of mild infections or variations in host antibody response since the main DTUs found in human infections corresponded to TcII/V/VI [3, 38]. Although individuals with low Ab-levels had classic risk factors for T. cruzi infection, we cannot rule out the possibility of exposure without persistent infection or previous antiparasitic treatment at an early age. However, anti-T. cruzi treatment at an early age is not widely distributed in endemic countries where most of our participants come from.
More robust and accurate tests are required if only one test is to be used as a primary screening tool to decrease the number of indeterminate and false-positive results in this low prevalence population. Furthermore, immunoassays need to be adapted to the antigenic repertoire of TcI infections because most immigrants in the United States are from areas where this DTU is prevalent [10]. Although attempts have been made to determine immunodominant TcI antigens [39], this must be a long-term goal. In the short term, there is a need for improved logistics and decreased cost of robust diagnostic tests that are currently FDA-cleared so that they can be used by clinical and research laboratories.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Chagas working group in Peru and the United States: Carol Avila, MD, Fabiola Camacho, MD, Sdenka Herrera, MD, Andres Jimenez, MD, Veronika Lozano, BS, Edith Malaga, MS, Mariel Merida, MD, Carolina Morales, MD, Rodrigo Solis, MD, Fiorella Sotomayor, MD, Alisha Tung, BS, Anna Spector, MD, Manuela Verastegui, PhD, Younghee Yang, BS, Fatima Zapata, MD.
Acknowledgments. The authors thank Dr Susan P. Montgomery for providing positive samples to determine internal sensitivity of the assays, to the consulates of El Salvador, Mexico, Honduras and Guatemala, and the community programs “Ventanilla de Salud,” Urban Strategies and Family Networks in the Washington Metropolitan Area for facilitating access to Hispanic communities.
Financial support. Consejo Nacional de Ciencia, Fondo Nacional de Desarrollo Científico, Tecnológico y de Innovación Tecnológica, FONDECYT, Perú (N084-2016 to Y. E. C.-S.); the National Institutes of Health (D43-TW010074 to R. H. G.), and InBios International, Inc to JHSPH (R. H. G).
Potential conflicts of interest. Y. E. C.-S. reports nonfinancial support from InBios International Inc. during the conduct of the study and outside the submitted work. C. B. reports grants from Mundo Sano Foundation, personal fees from UpToDate, both outside the submitted work. R. H. G. reports grants, nonfinancial support and other from InBios International during the conduct of the study and outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Chagas working group in Peru and the United States are listed in acknowledgement section.
Contributor Information
Chagas Working Group in Peru and the United States:
Carol Avila, Fabiola Camacho, Sdenka Herrera, Andres Jimenez, Veronika Lozano, Edith Malaga, Mariel Merida, Carolina Morales, Rodrigo Solis, Fiorella Sotomayor, Alisha Tung, Anna Spector, Manuela Verastegui, Younghee Yang, and Fatima Zapata
References
- 1. Bureau USC. US Census Bureau, 2016 American Community Survey 1-Year Estimates. 2016. Available at: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk. Accessed 2 October 2019.
- 2. Conners EE, Vinetz JM, Weeks JR, Brouwer KC. A global systematic review of Chagas disease prevalence among migrants. Acta Trop 2016; 156:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zingales B. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop 2018; 184:38–52. [DOI] [PubMed] [Google Scholar]
- 4. Dorn PL, McClure AG, Gallaspy MD, et al. The diversity of the Chagas parasite, Trypanosoma cruzi, infecting the main Central American vector, Triatoma dimidiata, from Mexico to Colombia. PLoS Negl Trop Dis 2017; 11:e0005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Umezawa ES, Bastos SF, Camargo ME, et al. Evaluation of recombinant antigens for serodiagnosis of Chagas disease in South and Central America. J Clin Microbiol 1999; 37:1554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martin DL, Marks M, Galdos-Cardenas G, et al. Regional variation in the correlation of antibody and T-cell responses to Trypanosoma cruzi. Am J Trop Med Hyg 2014; 90:1074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Umezawa ES, Luquetti AO, Levitus G, et al. Serodiagnosis of chronic and acute Chagas disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J Clin Microbiol 2004; 42:449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitman JD, Bulman CA, Gunderson EL, et al. Chagas disease serological test performance in US blood donor specimens. J Clin Microbiol 2019; 57:e01217–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Magalhães LM, Viana A, Chiari E, Galvão LM, Gollob KJ, Dutra WO. Differential activation of human monocytes and lymphocytes by distinct strains of Trypanosoma cruzi. PLoS Negl Trop Dis 2015; 9:e0003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manne-Goehler J, Davis J, Perez JH, et al. 442. The results of a primary care-based screening program for Trypanosoma cruzi in East Boston, Massachusetts. Open Forum Infect Dis 2018; 5:S166. [Google Scholar]
- 11. Mita-Mendoza NK, McMahon E, Kenneson A, et al. Chagas disease in southern coastal ecuador: coinfections with arboviruses and a comparison of serological assays for Chagas disease diagnosis. Am J Trop Med Hyg 2018; 99:1530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otani MM, Vinelli E, Kirchhoff LV, et al. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion 2009; 49:1076–82. [DOI] [PubMed] [Google Scholar]
- 13. Bowling J, Walter EA. Recognizing and meeting the challenge of Chagas disease in the USA. Expert Rev Anti Infect Ther 2009; 7:1223–34. [DOI] [PubMed] [Google Scholar]
- 14. U.S. Census Bureau. Census tabulation detail: Hispanic or Latino origin by specific origin.2017. Available at: https://censusreporter.org/tables/B03001/. Accessed 26 March 2019.
- 15. NW 1615 L. St, Suite 800Washington, Inquiries D 20036USA202-419–4300 | M-857–8562 | F-419–4372 | M. Hispanic population and origin in select US metropolitan areas, 2014. Pew Research Center’s Hispanic Trends Project. Available at: https://www.pewresearch.org/hispanic/interactives/hispanic-population-in-select-u-s-metropolitan-areas/. Accessed 29 June 2020.
- 16. Stepler R, Lopez MH. U.S. Latino population growth and dispersion has slowed since onset of the Great Recession. Pew Research Center’s Hispanic Trends Project.2016; Available at: http://www.pewhispanic.org/2016/09/08/5-ranking-the-latino-population-in-metropolitan-areas/. Accessed 12 May 2017.
- 17. World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Weekly Epidemiological Record 2015; 90:33–34. [PubMed] [Google Scholar]
- 18. Minwuyelet Azimeraw. Hispanic or Latino population in DC MSA and US - October 2014.2014. Available at: https://planning.dc.gov/sites/default/files/dc/sites/op/publication/attachments/Hispanic%20or%20Latino%20Population%20in%20DC%20MSA%20and%20US%20-%20October%202014_2.pdf. Accessed 12 May 2017.
- 19.Singer, Audrey. Metropolitan Washington: a new immigrant gateway. In: Pumar ES, ed., The Hispanic presence in the Washington DC metropolitan region: studies of migrant and urban development. Somerville, MA, USA: Emerald Press, 2012. Available at: https://www.brookings.edu/articles/metropolitan-washington-a-new-immigrant-gateway-2/. Accessed 12 May 2017.
- 20. Messenger LA, Gilman RH, Verastegui M, et al. Toward improving early diagnosis of congenital Chagas disease in an endemic setting. Clin Infect Dis 2017; 65:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bern C, Verastegui M, Gilman RH, et al. Congenital Trypanosoma cruzi transmission in Santa Cruz, Bolivia. Clin Infect Dis 2009; 49:1667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Umezawa ES, Nascimento MS, Kesper N Jr, et al. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas disease. J Clin Microbiol 1996; 34:2143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hidron AI, Gilman RH, Justiniano J, et al. ; Chagas Disease Working Group in Peru and Bolivia . Chagas cardiomyopathy in the context of the chronic disease transition. PLoS Negl Trop Dis 2010; 4:e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brenière SF, Waleckx E, Barnabé C. Over six thousand Trypanosoma cruzi strains classified into discrete typing units (DTUs): attempt at an inventory. PLoS Negl Trop Dis 2016; 10:e0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Padilla CP, Alvarado U, Ventura G, et al. Identifying Trypanosoma cruzi discreet typing units in triatomines collected in different natural regions of Perú. Biomedica 2017; 37:167–79. [DOI] [PubMed] [Google Scholar]
- 26. Bhattacharyya T, Messenger LA, Bern C, et al. ; Chagas Working Group in Bolivia and Peru . Severity of chagasic cardiomyopathy is associated with response to a novel rapid diagnostic test for Trypanosoma cruzi TcII/V/VI. Clin Infect Dis 2018; 67:519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fabbro DL, Danesi E, Olivera V, et al. Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis 2014; 8:e3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sguassero Y, Cuesta CB, Roberts KN, et al. Course of chronic Trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS One 2015; 10:e0139363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abras A, Gállego M, Muñoz C, et al. Identification of Trypanosoma cruzi discrete typing units (DTUs) in Latin-American migrants in Barcelona (Spain). Parasitol Int 2017; 66:83–8. [DOI] [PubMed] [Google Scholar]
- 30. Berry ASF, Salazar-Sánchez R, Castillo-Neyra R, et al. ; Chagas Disease Working Group in Arequipa . Immigration and establishment of Trypanosoma cruzi in Arequipa, Peru. PLoS One 2019; 14:e0221678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez-Perez A, Poveda C, Ramírez JD, et al. Prevalence of Trypanosoma cruzi’s discrete typing units in a cohort of Latin American migrants in Spain. Acta Trop 2016; 157:145–50. [DOI] [PubMed] [Google Scholar]
- 32. de Oliveira MT, Sulleiro E, Gimenez AS, et al. Quantification of parasite burden of Trypanosoma cruzi and identification of discrete typing units (DTUs) in blood samples of Latin American immigrants residing in Barcelona, Spain. PLoS Negl Trop Dis 2020; 14:e0008311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leiby DA, Nguyen ML, Proctor MC, Townsend RL, Stramer SL. Frequency of Trypanosoma cruzi parasitemia among infected blood donors with a potential association between parasite lineage and transfusion transmission. Transfusion 2017; 57:1426–32. [DOI] [PubMed] [Google Scholar]
- 34. Monteiro WM, Margioto Teston AP, Gruendling AP, et al. Trypanosoma cruzi I and IV stocks from Brazilian Amazon are divergent in terms of biological and medical properties in mice. PLoS Negl Trop Dis 2013; 7:e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santana RAG, Magalhães LKC, Magalhães LKC, et al. Trypanosoma cruzi strain TcI is associated with chronic Chagas disease in the Brazilian Amazon. Parasites & Vectors 2014; 7:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caballero ZC, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol 2007; 14:1045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hemagen Diagnostics, Inc. Hemagen Chagas Kit (EIA Method). 2009; Available at: https://www.hemagen.com/product_inserts/66101_06_Chagas_EIA_liquid.pdf. Accessed 23 May 2019.
- 38. Messenger LA, Miles MA, Bern C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Rev Anti Infect Ther 2015; 13:995–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reis-Cunha JL, Mendes TA, de Almeida Lourdes R, et al. Genome-wide screening and identification of new Trypanosoma cruzi antigens with potential application for chronic Chagas disease diagnosis. PLoS One 2014; 9:e106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.