Abstract
Background
Antiretroviral therapy (ART) has reduced mortality among people living with human immunodeficiency virus (HIV), but cancer remains an important cause of death. We characterized cancer-attributable mortality in the HIV population during 2001–2015.
Methods
We used data from population-based HIV and cancer registries in the United States (US). Cox proportional hazards regression models were used to estimate adjusted hazard ratios (HRs) associating cancer diagnoses with overall mortality. Population-attributable fractions (PAFs) were calculated using these HRs and the proportion of deaths preceded by cancer. Cancer-specific PAFs and cancer-attributable mortality rates were calculated for demographic subgroups, AIDS-defining cancers (Kaposi sarcoma [KS], non-Hodgkin lymphoma [NHL], cervical cancer), and non–AIDS-defining cancers.
Results
Cancer-attributable mortality was 386.9 per 100 000 person-years, with 9.2% and 5.0% of deaths attributed to non–AIDS-defining and AIDS-defining cancers, respectively. Leading cancer-attributable deaths were from NHL (3.5%), lung cancer (2.4%), KS (1.3%), liver cancer (1.1%), and anal cancer (0.6%). Overall, cancer-attributable mortality declined from 484.0 per 100 000 person-years during 2001–2005 to 313.6 per 100 000 person-years during 2011–2015, while the PAF increased from 12.6% to 17.1%; the PAF for non–AIDS-defining cancers increased from 7.2% to 11.8% during 2011–2015. Cancer-attributable mortality was highest among those aged ≥60 years (952.2 per 100 000 person-years), with 19.0% of deaths attributed to non–AIDS-defining cancers.
Conclusions
Although cancer-attributable mortality has declined over time, it remains high and represents a growing fraction of deaths in the US HIV population. Mortality from non–AIDS-defining cancers may rise as the HIV population ages. ART access, early cancer detection, and improved cancer treatment are priorities for reducing cancer-attributable mortality.
Keywords: human immunodeficiency virus, AIDS, cancer, population-attributable fraction, mortality
Cancer accounts for a growing fraction of deaths in the US human immunodeficiency virus population. During 2011–2015, 1 in 6 deaths was attributed to cancer. Cancer mortality rates are much higher than in the general population for all age groups.
Among people living with human immunodeficiency virus (HIV; PLWH), the introduction of effective antiretroviral therapy (ART) in 1996 has allowed for long-term suppression of HIV, improved immunity, and extended life expectancy [1, 2]. Immunodeficiency and coinfection with oncogenic viruses place PLWH at elevated risk of certain cancers [3–6]. ART has substantially reduced the incidence of Kaposi sarcoma (KS) and non-Hodgkin lymphoma (NHL), which are malignancies caused by KS-associated herpesvirus and Epstein-Barr virus, respectively, and considered acquired immunodeficiency syndrome (AIDS)-defining cancers [7–9]. Declines for non–AIDS-defining cancers have been more modest, and the burden of these cancers has actually increased with growth and aging of the HIV population [10].
Characterizing evolving patterns of mortality during the current ART era is important for measuring progress toward reducing the impact of cancer and other diseases in the HIV population. With large declines in mortality rates, there have been shifts in the causes of death among PLWH. The proportion of deaths due to non–AIDS-related conditions, including cancer, has increased, while deaths from AIDS-related causes have decreased, though this latter category still comprises the majority of deaths [11–14].
Prior epidemiologic studies have typically adjudicated causes of death to estimate cause-specific mortality [13, 15–17]. However, a major challenge of this approach is assigning an accurate cause of death when there is incomplete information or multiple comorbidities. Alternatively, one can use attributable risk methods to statistically apportion deaths from cancer. This method uses only cancer diagnoses and their associations with overall mortality in a population to estimate population-attributable fractions (PAFs) of cancer-related deaths. Prior studies that used the PAF approach focused on people with AIDS in the HIV/AIDS Cancer Match Study or PLWH in care and on treatment in a consortium of North American cohort studies [18, 19]. In addition to considering the PAF, which is a proportional measure, it is important to calculate the rate of mortality attributable to cancer. The cancer-attributable mortality rate is useful for comparing patterns across populations or calendar periods, while the PAF characterizes the relative importance of cancer as a cause of death.
In the present study, we examine patterns of mortality attributable to cancer in the US HIV population. We consider cancer-attributable mortality rates and PAFs in tandem, reflecting the absolute and proportional contributions of cancer to mortality, respectively. We use population-based registry data that capture a large representative sample of PLWH across the HIV care continuum in the United States.
METHODS
The HIV/AIDS Cancer Match Study is a registry-based study of PLWH in the United States (https://hivmatch.cancer.gov/). HIV diagnoses are ascertained through routine public health surveillance at the state or regional level. Cancer diagnoses are ascertained through linkage to corresponding population-based cancer registries. Vital status is provided from state and national records at the time of linkage.
The current study population included PLWH (aged ≥20 years) followed during 2001–2015 in 10 regions (see Table 1 note). PLWH were followed from cohort entry (defined as the latest of age 20, 1 January 2001, start of name-based HIV registration, 5 years after the start of cancer registry coverage, and earliest of HIV report date or AIDS diagnosis date) through death or the end of cancer registry coverage. Therefore, the cohort includes both people diagnosed with HIV before 2001 who were alive during 2001–2015 and people newly diagnosed during 2001–2015.
Table 1.
Characteristics of People Living With Human Immunodeficiency Virus from 10 States Followed for Mortality During 2001–2015
| Characteristic | Person-years of Follow-up | % |
|---|---|---|
| Total | 3 577 916 | 100.0 |
| Attained year | ||
| 2001–2005 | 914 865 | 25.6 |
| 2006–2010 | 1 546 170 | 43.2 |
| 2011–2015 | 1 116 882 | 31.2 |
| Sex | ||
| Male | 2 603 172 | 72.8 |
| Female | 974 744 | 27.2 |
| Attained age, years | ||
| 20–39 | 1 081 290 | 30.2 |
| 40–59 | 2 206 578 | 61.7 |
| 60+ | 290 048 | 8.1 |
| Race/ethnicity | ||
| Non-Hispanic white | 902 172 | 25.2 |
| Non-Hispanic black | 1 653 374 | 46.2 |
| Hispanic | 963 343 | 26.9 |
| Other | 59 027 | 1.6 |
| HIV transmission risk group | ||
| MSM | 1 296 006 | 36.2 |
| PWID | 672 450 | 18.8 |
| MSM and PWID | 163 791 | 4.6 |
| Heterosexual | 657 633 | 18.4 |
| Other or unknown | 788 036 | 22.0 |
| AIDS status | ||
| AIDS | 2 205 479 | 61.6 |
| HIV only | 1 372 438 | 38.4 |
Participating registries, calendar years, and proportion of person-years of follow-up include: Colorado, 2003–2015 (3.9%); Connecticut, 2005–2010 (1.5%); District of Columbia, 2001–2015 (4.9%); Georgia, 2009–2012 (4.5%); Michigan, 2001–2010 (3.0%); New Jersey, 2001–2012 (9.9%); New York, 2001–2012 (36.5%); North Carolina, 2001–2014 (9.6%); Puerto Rico, 2003–2012 (4.2%); and Texas, 2004–2015 (22.1%). A total of 521 623 people living with human immunodeficiency virus were included in the study.
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men; PWID, people who inject drugs.
Because mortality due to cancer during follow-up can be due to cancers that were diagnosed before follow-up (ie, prevalent) or that arise during follow-up (ie, incident), it is important to capture both from cancer registries. We chose a 5-year window for ascertaining prevalent cancers because most deaths due to cancer were expected to accrue within 5 years of diagnosis and because choosing a longer window would move the start of follow-up later for some regions and thus reduce our cohort size. Cancers were coded according to a standard classification scheme [20].
The PAF was calculated based on the following formula: PAF = pd × [(HR – 1)/HR], where pd is the proportion of deaths preceded by cancer and HR is the hazard ratio for the association between cancer diagnosis and overall mortality [21]. We used Cox proportional hazards regression models to estimate HRs for cancer overall and specific cancers, adjusted for sex, race/ethnicity, HIV transmission risk group, attained calendar year, and time-dependent AIDS status (see Table 2 note). Age was used as the time scale. A cancer diagnosis was treated as a time-dependent variable. The variance of the PAF was calculated using an influence function–based approach [18].
Table 2.
Cancer-attributable Mortality Among People Living With Human Immunodeficiency Virus from 10 States, 2001–2015, by Cancer Site
| Cancer Site | Number of Cancers | Proportion of Deaths Preceded by Cancer, pd (95% CI) | Adjusted Hazard Ratio (95% CI) | Population-attributable Fraction (95% CI) | Cancer-attributable Mortality Rate |
|---|---|---|---|---|---|
| All sites combined | 31 611 | 17.5% (17.3%–17.8%) | 5.79 (5.69–5.89) | 14.5% (13.6%–15.4%) | 386.9 |
| AIDS-defining cancers | 12 315 | 6.5% (6.4%–6.7%) | 4.28 (4.17–4.40) | 5.0% (4.4%–5.6%) | 134.1 |
| Kaposi sarcoma | 4485 | 1.9% (1.8%–2.0%) | 2.99 (2.86–3.14) | 1.3% (.9%–1.6%) | 34.0 |
| Non-Hodgkin lymphoma | 7072 | 4.3% (4.1%–4.4%) | 5.35 (5.18–5.52) | 3.5% (3.0%–3.9%) | 92.6 |
| Cervical | 758 | .4% (.3%–.4%) | 2.56 (2.31–2.85) | .2% (.1%–.4%) | 5.9 |
| Non–AIDS-defining cancers | 19 296 | 11.0% (10.8%–11.2%) | 6.21 (6.08–6.34) | 9.2% (8.5%–9.9%) | 245.7 |
| Anus | 1729 | .8% (.7%–.9%) | 4.02 (3.74–4.31) | .6% (.4%–.8%) | 16.1 |
| Liver | 1331 | 1.1% (1.1%–1.2%) | 19.0 (17.9–20.2) | 1.1% (.8%–1.3%) | 28.4 |
| Hodgkin lymphoma | 1420 | .6% (.5%–.6%) | 3.47 (3.20–3.77) | .4% (.2%–.6%) | 11.3 |
| Breast | 1110 | .4% (.4%–.5%) | 2.97 (2.70–3.27) | .3% (.1%–.4%) | 7.8 |
| Prostate | 2252 | .5% (.5%–.6%) | 1.21 (1.11–1.32) | .1% (−.1%−.2%) | 2.3 |
| Lung | 2878 | 2.5% (2.4%–2.6%) | 16.3 (15.7–17.0) | 2.4% (2.0%–2.7%) | 63.0 |
| Colorectal | 1272 | .6% (.6%–.7%) | 4.00 (3.69–4.34) | .5% (.3%–.7%) | 12.6 |
| Other | 7304 | 4.3% (4.2%–4.5%) | 6.25 (6.06–6.45) | 3.6% (3.2%–4.1%) | 97.4 |
Abbreviation: CI, confidence interval.
Mortality rates are per 100 000 person-years. Cox proportional hazards regression models use age as the time scale and are adjusted for sex, human immunodeficiency virus transmission risk group, race/ethnicity, time-dependent AIDS status, and attained calendar year. The population-attributable fraction (PAF) is calculated as PAF = pd × [(HR − 1)/HR], where pd is the proportion of deaths preceded by a cancer diagnosis and HR is the adjusted hazard ratio for the association between cancer diagnosis and overall mortality. The sum of cancer-specific population-attributable fractions approximates the fractions for all sites combined and for AIDS-defining and non–AIDS-defining cancer categories, which were estimated using separate models.
We calculated the cancer-attributable mortality rate as the product of the PAF and overall mortality rate. Results are presented for all cancer sites combined and for the 10 most common cancers among PLWH (AIDS-defining cancers: KS, NHL, and cervical; non–AIDS-defining cancers: anal, liver, Hodgkin lymphoma, lung, breast, prostate, and colorectal) and other remaining cancers combined. We also present results for subgroups, defined by sex, race/ethnicity, HIV transmission risk group, AIDS status, age, calendar year, and age group–specific patterns across calendar year.
In a sensitivity analysis, we examined the PAF related only to incident cancers by excluding PLWH with prevalent cancers diagnosed before start of follow-up. To assess the extent to which our use of a 5-year window for ascertaining prevalent cancers fully captured cancer-attributable mortality during follow-up, we conducted a second sensitivity analysis in which we ascertained prevalent cancers diagnosed up to 10 years prior to study entry. Analyses were conducted using SAS software, Version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
The study cohort included 521 623 PLWH followed during 2001–2015 for 3 577 916 person-years (median follow-up, 6.8 years; interquartile range [IQR], 3.6–11.5 years; Table 1). Substantial follow-up time accrued during 2006–2010 (43.2%) and among men (72.8%), non-Hispanic blacks (46.2%), and those aged 40–59 years (61.7%). Follow-up time according to specified modes of transmission was predominantly among men who have sex with men (MSM; 36.2%), people who inject drugs (PWID; 18.8%), and heterosexual risk groups (18.4%). Overall, 61.6% of follow-up time occurred after an AIDS diagnosis. Among PLWH, the proportion of follow-up time within the 60+ age group doubled between 2001–2005 and 2011–2015 (5.0% to 10.9%), while the age distribution within each age category remained stable over the calendar period (Supplementary Table 1).
In total, 31 611 participants (6.1%) were diagnosed with cancer (9497 prevalent and 22 114 incident cases). Of these cancers, 12 315 were AIDS-defining and 19 296 were non–AIDS-defining. The most commonly diagnosed cancer types were NHL (22.4%), KS (14.2%), and lung cancer (9.1%; Table 2). Among prevalent cancers, the median time between diagnosis and the start of follow-up was 0.8 years (IQR, 0.0–2.6 years; Supplementary Table 2). For all prevalent cancers combined, 95% had 0 to 4.59 years of survival time from their cancer diagnosis until entering the cohort, and only 5% had a survival time of 4.60 to 5 years (Supplementary Table 2).
There were 95 554 deaths during follow-up (mortality rate, 2671 per 100 000 person-years), and 17.5% of deaths were preceded by cancer (pd = 0.175). The adjusted HR for cancer and death was 5.79 (95% confidence interval [CI], 5.69–5.89), resulting in a PAF of 14.5% (95% CI, 13.6%–15.4%). The corresponding cancer-attributable mortality rate was 386.9 per 100 000 person-years (Table 2).
Non–AIDS-defining cancers collectively contributed a larger fraction of deaths than AIDS-defining cancers (PAFs, 9.2% vs 5.0%; cancer-attributable mortality rates, 245.7 vs 134.1 per 100 000 person-years; Table 2). Individual cancer sites that contributed the most deaths were NHL (3.5%), lung cancer (2.4%), KS (1.3%), liver cancer (1.1%), and anal cancer (0.6%).
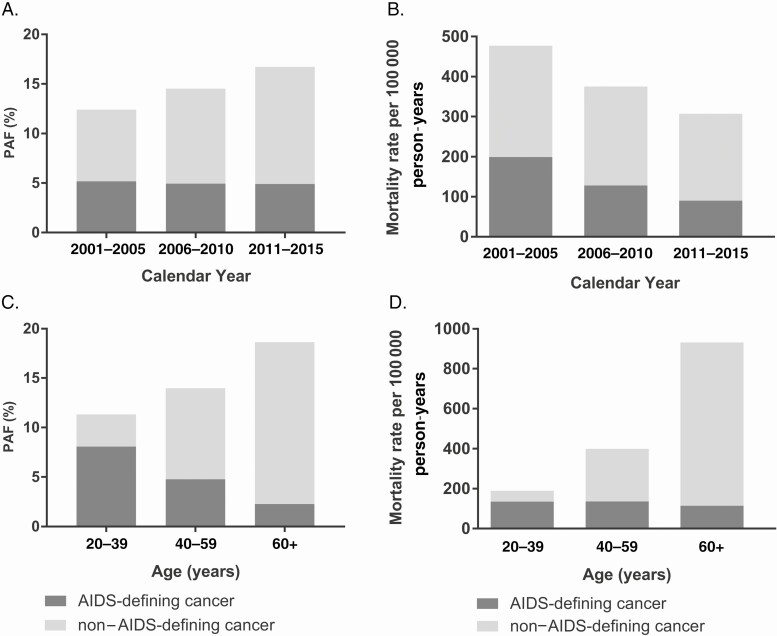
As shown in Table 3, overall mortality among PLWH declined from 3839 per 100 000 person-years during 2001–2005 to 1835 per 100 000 person-years during 2011–2015. Between 2001–2005 and 2011–2015, the cancer-attributable mortality also declined from 484.0 to 313.6 per 100 000 person-years, while the PAF increased from 12.6% during 2001–2005 to 17.1% during 2011–2015 (Table 3). The composition of cancer-attributable deaths shifted such that the proportion of deaths attributable to non–AIDS-defining cancers increased from 7.2% during 2001–2005 to 11.8% during 2011–2015 (Figure 1A), while the proportion attributable to AIDS-defining cancers remained stable (approximately 5%). Among the leading non–AIDS-defining cancers, the PAF for liver, lung, and anal cancers each increased by 0.6%, 0.5%, and 0.5%, respectively, between 2001–2005 and 2011–2015 (Supplementary Table 3). In contrast, the corresponding cancer-attributable mortality rates declined across calendar periods for AIDS-defining and non–AIDS-defining cancer groups (Figure 1B).
Table 3.
Cancer-attributable Mortality Among People Living With Human Immunodeficiency Virus from 10 States, 2001–2015, by Population Subgroup
| Characteristic | Proportion of Deaths Preceded by Cancer, pd (95% CI) | Adjusted Hazard Ratio (95% CI) | Population-attributable Fraction (95% CI) | Crude Mortality Rate | Cancer-attributable Mortality Rate |
|---|---|---|---|---|---|
| Calendar period | |||||
| 2001–2005 | 15.1% (14.8%–15.5%) | 5.99 (5.82–6.17) | 12.6% (11.4%–13.8%) | 3839 | 484.0 |
| 2006–2010 | 18.0% (17.6%–18.3%) | 5.70 (5.55–5.85) | 14.8% (13.4%–16.2%) | 2582 | 382.3 |
| 2011–2015 | 20.7% (20.2%–21.3%) | 5.68 (5.49–5.88) | 17.1% (14.3%–19.9%) | 1835 | 313.6 |
| Sex | |||||
| Male | 18.5% (18.2%–18.8%) | 5.79 (5.68–5.91) | 15.3% (14.1%–16.4%) | 2628 | 401.4 |
| Female | 15.1% (14.7%–15.6%) | 5.76 (5.57–5.95) | 12.5% (10.9%–14.1%) | 2785 | 348.1 |
| Age category, years | |||||
| 20–39 | 13.3% (12.8%–13.8%) | 7.07 (6.77–7.39) | 11.4% (8.9%–13.9%) | 1671 | 191.1 |
| 39–59 | 17.1% (16.8%–17.4%) | 6.01 (5.89–6.14) | 14.2% (13.2%–15.3%) | 2855 | 406.5 |
| 60+ | 24.6% (23.9%–25.3%) | 4.42 (4.25–4.59) | 19.0% (16.9%–21.2%) | 4999 | 952.2 |
| Race/ethnicity | |||||
| Non-Hispanic white | 20.7% (20.1%–21.2%) | 5.74 (5.54–5.94) | 17.1% (14.7%–19.5%) | 2152 | 367.5 |
| Non-Hispanic black | 17.3% (17.0%–17.6%) | 5.78 (5.64–5.92) | 14.3% (13.1%–15.5%) | 3016 | 431.7 |
| Hispanic | 15.5% (15.0%–15.9%) | 5.93 (5.72–6.14) | 12.9% (11.1%–14.6%) | 2632 | 338.9 |
| Other | 17.7% (15.2%–20.2%) | 5.78 (4.85–6.90) | 14.6% (1.4%–27.9%) | 1540 | 225.6 |
| Risk group | |||||
| MSM | 22.5% (22.0%–23.1%) | 6.12 (5.93–6.32) | 18.9% (16.1%–21.6%) | 1683 | 317.3 |
| PWID | 14.1% (13.7%–14.5%) | 5.86 (5.68–6.06) | 11.7% (10.6%–12.8%) | 4602 | 537.5 |
| MSM + PWID | 14.9% (13.9%–15.9%) | 5.73 (5.31–6.20) | 12.3% (8.9%–15.7%) | 3132 | 385.6 |
| Heterosexual | 17.1% (16.5%–17.7%) | 5.97 (5.73–6.23) | 14.2% (11.9%–16.6%) | 2432 | 345.9 |
| Other/unknown | 18.3% (17.8%–18.8%) | 5.19 (5.01–5.38) | 14.8% (12.7%–16.8%) | 2750 | 406.4 |
| AIDS status | |||||
| AIDS | 18.2% (17.9%–18.4%) | 5.61 (5.51–5.71) | 14.9% (14.0%–15.8%) | 3556 | 530.8 |
| Human immunodeficiency virus only | 13.6% (13.1%–14.2%) | 9.04 (8.58–9.52) | 12.1% (8.6%–15.6%) | 1073 | 130.1 |
Mortality rates are per 100 000 person-years. Cox proportional hazards regression models use age as the time scale and are adjusted for sex, human immunodeficiency virus transmission risk group, race/ethnicity, time-dependent AIDS status, and attained calendar year.
Abbreviations: CI, confidence interval; MSM, men who have sex with men; PWID, people who inject drugs.
Figure 1.
PAF and attributable mortality rates for AIDS-defining and non–AIDS-defining cancers across calendar year periods (A, B) and age groups (C, D). Abbreviation: PAF, population-attributable fraction.
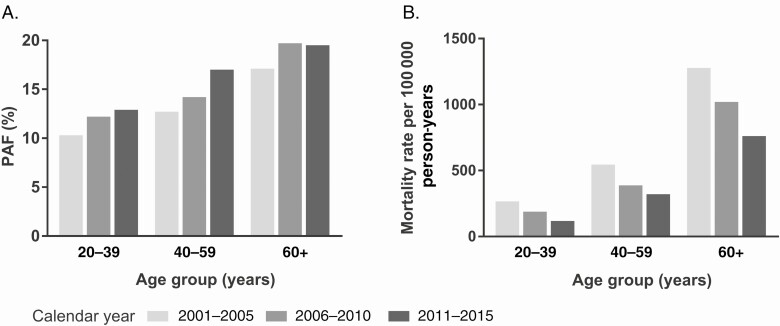
Across demographic groups (Table 3), the PAF was higher among men (15.3%) than women (12.5%). The cancer-attributable mortality rate and PAF increased across age groups (Figure 1C, 1D). Among those aged ≥60 years, 19.0% of deaths were attributed to cancer compared with 11.4% among those aged 20–39 years, and the cancer-attributable mortality rate was 5-fold higher (952.2 vs 191.1 per 100 000 person-years). The composition of cancer-attributable deaths also shifted with age; the proportion of deaths attributable to non–AIDS-defining cancers increased from 3.3% among those aged 20–39 years to 16.4% among those aged ≥60 years, while the proportion attributable to AIDS-defining cancers declined (8.1% vs 2.3%; Figure 1C). The cancer-attributable mortality rate for non–AIDS-defining cancers increased with age from 27.0 per 100 000 person-years in those aged 20–49 years to 818.0 per 100 000 person-years in those aged ≥60 years, while the cancer-attributable mortality rate for AIDS-defining cancers remained stable (Figure 1D). Across calendar periods, all age groups experienced an increase in the PAF (Figure 2A) and substantial declines in the cancer-attributable mortality rate (Figure 2B). The most pronounced increase in the PAF occurred among those aged 40–59 years for whom the PAF increased from 12.7% during 2001–2005 to 17.0% during 2011–2015. Between 2001–2005 and 2011–2015, the cancer-attributable mortality rate declined by 55.6% among those aged 20–39 years and by 40.4%–41.0% among those aged 40–59 years and 60+ years.
Figure 2.
PAF (A) and attributable mortality rates (B) among age groups across calendar year period. Abbreviation: PAF, population-attributable fraction.
Across racial/ethnic groups, the PAF was highest among non-Hispanic whites (17.1%). Although the PAF was only 14.3% in non-Hispanic blacks, this group had the highest cancer-attributable mortality rate (431.7 per 100 000 person-years; Table 3). Across HIV risk groups (Table 3), the largest PAF was among MSM (18.9%), and the PAFs were high for KS (4.3%; 95% CI, 3.0%–5.7%) and anal cancer (1.4%; 95% CI, .6%–2.4%). Although the PAF was only 11.7% among PWID, this group had the highest cancer-attributable mortality rate (537.5 per 100 000 person-years). Among PWID, the PAF for liver cancer was also elevated (1.6%; 95% CI, 1.2%–2.0%) and lung cancer contributed a large proportion of deaths (2.5%; 95% CI, 1.9%–2.9%). Individuals with a prior AIDS diagnosis experienced a larger PAF (14.9%) and cancer-attributable mortality (530.8 per 100 000 person-years) than those with HIV only (PAF = 12.1%; cancer-attributable mortality = 130.1).
In a sensitivity analysis that excluded 9497 people with prevalent cancer, the overall PAF was 12.3% (95% CI, 11.4%–13.2%) and the cancer-attributable mortality rate was 320.2 per 100 000 person-years. PAFs for non–AIDS-defining cancers and AIDS-defining cancers were 8.2% and 3.9%, respectively.
In a second sensitivity analysis that used a 10-year period to ascertain prevalent cancer cases, we evaluated a cohort of N = 431 627 PLWH and 2.4 million person-years of follow-up. Of these, 25 275 (5.9%) were diagnosed with cancer (10 623 prevalent cancers, including 7567 diagnosed 0–5 years and 3056 diagnosed 5–10 years before the start of follow-up, and 14 652 incident cases). There were 56 804 deaths during follow-up (overall mortality rate, 2346.7 per 100 000 person-years), and 19.3% of deaths were preceded by cancer (pd = 0.193). The resulting PAF was 15.7% and the cancer-attributable mortality rate was 368.9 per 100 000 person-years. The patterns in the results for calendar periods, AIDS-defining cancers, and non–AIDS-defining cancers were similar to the primary analysis (data not shown).
DISCUSSION
In the United States, improving access to ART since 1996 has translated to major reductions in overall mortality in the HIV population and, particularly, mortality related to AIDS. In turn, the landscape of causes of deaths has shifted, with cancer responsible for a growing fraction of deaths. In our study, overall mortality in the HIV population dropped from 3839 to 1835 per 100 000 person-years, a 52% decrease between 2001–2004 and 2011–2015, but the cancer-attributable mortality rate declined by only 35% to 313.6 per 100 000 person-years. Cancer-attributable mortality declined dramatically for AIDS-defining cancers, from 199 to 90 per 100 000 person-years over this period, while the declines in mortality attributable to non–AIDS-defining cancers were more modest (278 to 217 per 100 000 person-years).
The cancer-attributable mortality rate is an important measure for assessing the absolute impact of cancer and comparing across populations. Solely considering the PAF, which is a proportional measure, can be misleading because the denominator for the fraction of deaths related to cancer is overall mortality. For example, we found that the PAF increased from 12.6% to 17.1% from 2001–2004 to 2011–2015, but this was because the decline in cancer-attributable mortality was less than for overall mortality. Likewise, it is important to note that cancer-attributable mortality rates among PLWH are alarmingly high compared with cancer mortality in the general US population, especially among young adults. During our most recent study period (2011–2015), the cancer-attributable mortality rate among PLWH who were aged 20–39 years was, remarkably, 12 times that reported for the general population (118 vs 9.8 per 100 000 person-years) [22]. It was also elevated to a lesser extent among PLWH who were aged 40–59 years (2.7 times higher, 322 vs 120 per 100 000 person-years in the general population) and PLWH who were aged 60–69 years (1.7 times higher, 762 vs 429) [22].
The mortality attributable to specific cancer sites in our study reflects the incidence of cancer in the HIV population and the strength of the association with mortality. NHL and KS were the most common cancers, despite decades-long declines in incidence associated with widespread ART use [20, 23]. Consequently, NHL (PAF, 4.3%) and KS (PAF, 1.9%) were leading causes of cancer-attributable deaths. The incidence of non–AIDS-defining cancers of the anus, liver, and lung has declined less sharply over time and remains elevated among PLHW [20, 24]. In addition, mortality associated with liver and lung cancers is very high. As a result, lung (2.4%), liver (1.1%), and anal cancers (0.6%) contributed substantially to mortality.
Cancer-attributable mortality rates increased steeply with age, reflecting the increasing incidence of many non–AIDS-defining cancers among older age groups. In the over-60 age group, we observed that 1 in 5 deaths was attributed to cancer. The distribution of deaths also shifted dramatically with advancing age, showing an increase in the proportion of deaths attributable to non–AIDS-defining cancers. Non-Hispanic whites had the highest PAF (17.1%), but non-Hispanic blacks had the highest rates of both overall and cancer-attributable mortality.
Across transmission risk groups, PWID had the highest cancer-attributable mortality rate, likely reflecting the high prevalence of cancer risk factors in this group and the resulting distribution of cancer types. Approximately 80% of PLWH who inject drugs are coinfected with hepatitis C virus (HCV), and consequently liver cancer contributes a relatively large proportion of deaths (1.6%) [25]. Lung cancer also represents a substantial fraction of deaths among PWID, reflecting the high prevalence of smoking [26]. Similarly, among MSM, for whom the prevalence of infections with human papillomavirus and KS-associated herpesvirus is high, anal cancer (1.4%) and KS (4.3%) comprise a relatively large fraction of deaths [27, 28].
The mortality patterns described here point to opportunities for public health interventions to reduce cancer mortality among PLWH. Early and sustained ART is important for primary prevention of KS and NHL. Improving uptake of HCV screening among all asymptomatic adults without known liver disease and HCV treatment are critical to reduce liver cancer incidence, and primary prevention of lung cancer and other cancers through smoking cessation is important [29, 30]. With the proportion of PLWH aged ≥65 years expected to increase to 21% by 2030, early cancer detection through screening and improved treatment access should be priorities [23]. PLWH have worse cancer survival than those not living with HIV partly due to advanced cancer stage at diagnosis [31–33]. Finally, disparities in cancer treatment among PLWH also contribute to poor survival [34].
We used a statistical approach to estimate mortality attributable to cancer within the HIV population. This approach avoids reliance on determination of causes of death, which can be difficult (eg, based on information on death certificates). For example, we were able to attribute deaths due to KS, NHL, and cervical cancer that might otherwise be coded to AIDS on death certificates. To address the challenge of misclassification of cause of death in the HIV population, other studies have used the CoDe protocol, which requires information from medical charts and other sources to adjudicate causes of death [13, 15–17, 35]. Because comorbid conditions and risk factors are not routinely collected by state registries, implementing CoDe was not feasible in our study. Our statistical method apportions deaths attributable to cancer on a population level. This approach relies on the assumption that the observed association between cancer and death is causal and free of unmeasured confounding. We adjusted for demographic characteristics and time-varying AIDS status, but CD4 cell counts and HIV viral load were not sufficiently available to be used in modeling. We also lacked data on other important cancer risk factors, such as smoking. Nonetheless, the effect of residual confounding would have to be quite large to substantially bias the PAF because, for strong associations such as those we observed between cancer and mortality, the term (HR – 1)/HR approaches 1, and the PAF is very close to pd, that is, the proportion of deaths preceded by cancer.
Our estimates of the fraction of cancer-related deaths are mostly lower than reported from large clinical HIV cohorts in Europe and the United States [13, 17, 18]. Importantly, however, the corresponding cancer mortality rates were similar. In the French mortality survey, which reported the highest fraction of cancer deaths among PLWH (36%), the cancer mortality rate was similar to ours (320 per 100 000 person-years in France in 2010 vs 314 per 100 000 person-years in the United States in 2011–2015, respectively); in contrast, the overall mortality rate was much lower than in our study (888 vs 1835 per 100 000 person-years) [17]. Thus, differences in overall mortality rates contribute to variation in PAFs and similar proportional measures across studies.
Finally, it is unlikely that our PAF estimates are strongly underestimated because the PAF cannot exceed the proportion of deaths that were preceded by a cancer diagnosis (pd). We observed pd = 17.5%, which is not much greater than the estimated PAF of 14.5%. When we expanded the period of ascertainment of prevalent cancers to 10 years before study entry, the PAF increased to 15.7%. The increase was small in this sensitivity analysis because there were relatively few additional prevalent cancers identified and because individuals who live at least 5 years after a prevalent cancer diagnosis are less likely to die from their cancer during follow-up.
Our study cohort was identified from population-based HIV registries in 10 US regions and is thus broadly representative of PLHW in the United States. The overall PAF and cancer-attributable mortality in our study are higher than reported in a consortium of HIV cohorts in North America [18], likely because we included people who are not linked to care and who may have worse immune status. In addition, unlike in some prior studies, our cohort included people who started follow-up with a prior cancer diagnosis [18, 19]. This subgroup is important because estimates that rely solely on incident cancer diagnoses cannot capture the full burden of cancer-related deaths. Indeed, in a sensitivity analysis in which we excluded people with a prevalent cancer, the PAF was noticeably lower (12.3%).
In summary, our study provides new population-based estimates of cancer-attributable mortality for PLWH in the United States. Cancer-attributable mortality remains much higher in the HIV population than the general population, and the fraction of deaths due to cancer has been increasing over time. Although NHL and KS are among the leading causes of cancer deaths in the HIV population, the spectrum of cancer deaths is shifting toward non–AIDS-defining sites, and mortality due to non–AIDS-defining cancers will likely rise as the HIV population ages. The evolving pattern of cancer deaths in the HIV population underscores the continued importance of ART access, early cancer detection, and improved cancer treatment among PLWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors gratefully acknowledge the support and assistance provided by individuals at the following state human immunodeficiency virus (HIV)/AIDS and cancer registries: Colorado, Connecticut, District of Columbia, Georgia, Michigan, New Jersey, New York, North Carolina, Puerto Rico, and Texas. They also thank Timothy McNeel at Information Management Services for programming support.
Disclaimer. The views expressed in this article are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, HIV/AIDS or cancer registries, or their contractors.
Financial support. This research was supported, in part, by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC): Colorado (NU58DP006347-01), Georgia (5U58DP003875-01), Michigan (17NU58DP006334), New Jersey (NU58/DP003931-05-00), New York (U58/DP003879), North Carolina (1NU58DP006281), and Texas (1NU58DP006308). The cancer registry of the District of Columbia was supported by the CDC (DP006302). The following cancer registries were supported by the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute: Connecticut (HHSN261201300019I) and New Jersey (HHSN261201300021I, N01-PC-2013-00021). The New Jersey State Cancer Registry was also supported by the State of New Jersey, and the New York State Cancer Registry was also supported by the State of New York. The following HIV registries were supported by cooperative agreement PS18-1802: Integrated HIV Surveillance and Prevention Programs for Health Departments; National Center for HIV, Viral Hepatitis, STD, and TB Prevention; and CDC: Colorado (NU62PS003960), Connecticut (5U62PS001005-05), Michigan (U62PS004011-02), New Jersey (U62PS004001-2), and New York (NU62PS924546-02-00).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design of IeDEA . Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997; 337:725–33. [DOI] [PubMed] [Google Scholar]
- 3. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008; 123:187–94. [DOI] [PubMed] [Google Scholar]
- 4. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008; 148:728–36. [DOI] [PubMed] [Google Scholar]
- 5. Shiels MS, Engels EA. Increased risk of histologically defined cancer subtypes in human immunodeficiency virus-infected individuals: clues for possible immunosuppression-related or infectious etiology. Cancer 2012; 118:4869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grulich AE, van Leeuwen MT, Falster MO, and Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370:59–67. [DOI] [PubMed] [Google Scholar]
- 7. Shiels MS, Cole SR, Wegner S, et al. Effect of HAART on incident cancer and noncancer AIDS events among male HIV seroconverters. J Acquir Immune Defic Syndr 2008; 48:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franceschi S, Lise M, Clifford GM, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 2010; 103:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pipkin S, Scheer S, Okeigwe I, Schwarcz S, Harris DH, and Hessol NA. The effect of HAART and calendar period on Kaposi’s sarcoma and non-Hodgkin lymphoma: results of a match between an AIDS and cancer registry. AIDS 2011; 25:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Croxford S, Kitching A, Desai S, et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health 2017; 2:e35–46. [DOI] [PubMed] [Google Scholar]
- 12. Adih WK, Selik RM, Hall HI, Babu AS, and Song R. Associations and trends in cause-specific rates of death among persons reported with HIV infection, 23 U.S. jurisdictions, through 2011. Open AIDS J 2016; 10:144–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 14. Palella FJ, Jr., Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43:27–34. [DOI] [PubMed] [Google Scholar]
- 15. Monforte Ad, Abrams D, Pradier C, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs Study Group . HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS 2008; 22:2143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013; 14:195–207. [DOI] [PubMed] [Google Scholar]
- 17. Vandenhende MA, Roussillon C, Henard S, et al. Cancer-related causes of death among HIV-infected patients in France in 2010: evolution since 2000. PLoS One 2015; 10:e0129550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engels EA, Yanik EL, Wheeler W, et al. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis 2017; 65:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis 2010; 51:957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernandez-Ramirez RU, Shiels MS, Dubrow R, and Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017; 4:e495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rockhill B, Newman B, and Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998; 88:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality—All COD, aggregated with State, Total U.S. (1969–2016) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2018. Underlying mortality data provided by NCHS. Available at: http://www.cdc.gov/nchs. Accessed 22 November 2019.
- 23. Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, and Engels EA. Projected cancer incidence rates and burden of incident cancer cases in HIV-infected adults in the United States through 2030. Ann Intern Med 2018; 168:866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colón-López V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 2018; 36:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spralding PR, Richardson JT, Buchacz K, et al. Trends in hepatitis C virus infection among patients in the HIV Outpatient Study, 1996-2007. JAIDS 2010; 53:388–96. [DOI] [PubMed]
- 26. Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162:335–44. [DOI] [PubMed] [Google Scholar]
- 27. Goldstone S, Palefsky JM, Giuliano AR, et al. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J Infect Dis 2011; 203:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engels EA, Atkinson JO, Graubard BI, et al. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis 2007; 196:199–207. [DOI] [PubMed] [Google Scholar]
- 29. Yehia BR, Herati RS, Fleishman JA, et al. ; HIV Research Network . Hepatitis C virus testing in adults living with HIV: a need for improved screening efforts. PLoS One 2014; 9:e102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA 2020; 323:970-5. [DOI] [PubMed]
- 31. Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr 2005; 39:293–9. [DOI] [PubMed] [Google Scholar]
- 32. Coghill AE, Pfeiffer RM, Shiels MS, and Engels EA. Excess mortality among HIV-infected individuals with cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017; 26:1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coghill AE, Han X, Suneja G, Lin CC, Jemal A, and Shiels MS. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer 2019; 125:2868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suneja G, Coghill A. Cancer care disparities in people with HIV in the United States. Curr Opin HIV AIDS 2017; 12:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kowalska JD, Friis-Møller N, Kirk O, et al. ; CoDe Working Group; D:A:D Study Group . The Coding Causes of Death in HIV (CoDe) Project: initial results and evaluation of methodology. Epidemiology 2011; 22:516–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.