Abstract
Background
Human immunodeficiency virus (HIV)–experienced clinicians are critical for positive outcomes along the HIV care continuum. However, access to HIV-experienced clinicians may be limited, particularly in nonmetropolitan areas, where HIV is increasing. We examined HIV clinician workforce capacity, focusing on HIV experience and urban–rural differences, in the Southern United States.
Methods
We used Medicaid claims and clinician characteristics (Medicaid Analytic eXtract [MAX] and MAX Provider Characteristics, 2009–2011), county-level rurality (National Center for Health Statistics, 2013), and diagnosed HIV cases (AIDSVu, 2014) to assess HIV clinician capacity in 14 states. We assumed that clinicians accepting Medicaid approximated the region’s HIV workforce, since three-quarters of clinicians accept Medicaid insurance. HIV-experienced clinicians were defined as those providing care to ≥ 10 Medicaid enrollees over 3 years. We assessed HIV workforce capacity with county-level clinician-to-population ratios, using Wilcoxon-Mann-Whitney tests to compare urban–rural differences.
Results
We identified 5012 clinicians providing routine HIV management, of whom 28% were HIV-experienced. HIV-experienced clinicians were more likely to specialize in infectious diseases (48% vs 6%, P < .001) and practice in urban areas (96% vs 83%, P < .001) compared to non–HIV-experienced clinicians. The median clinician-to-population ratio for all HIV clinicians was 13.3 (interquartile range, 38.0), with no significant urban–rural differences. When considering HIV experience, 81% of counties had no HIV-experienced clinicians, and rural counties generally had fewer HIV-experienced clinicians per 1000 diagnosed HIV cases (P < .001).
Conclusions
Significant urban–rural disparities exist in HIV-experienced workforce capacity for communities in the Southern United States. Policies to improve equity in access to HIV-experienced clinical care for both urban and rural communities are urgently needed.
Keywords: HIV, rural, urban, disparities, health workforce
Human Immunodeficiency Virus (HIV)-experienced clinicians disproportionately practice in metropolitan areas, with significantly lower HIV-experienced clinician workforce capacity in rural vs urban communities. As the HIV epidemic is increasingly represented in rural areas, policies to improve equitable access to HIV-experienced clinical care are essential.
(See Major Articles by Ramirez et al on pages 1608–14 and Budak et al on pages 1623–6 and Editorial Commentary by Armstrong on pages 1627–30).
Achieving national targets for people living with human immunodeficiency virus (PLWH), promoting health equity, and ending the human immunodeficiency virus (HIV) epidemic in the United States (US) by 2030 [1, 2] require renewed efforts to provide accessible, high-quality HIV care. Critical to this effort is a robust and experienced HIV clinician workforce, since greater clinician experience in managing HIV is associated with improved clinical outcomes for PLWH, including viral suppression [3, 4]. However, a significant shortage in HIV care clinicians is anticipated [5, 6]; HIV medical organizations report challenges with physician recruitment and retention [7]; and infectious disease fellowship training slots, a key source for future HIV clinicians, are not being filled [8].
Compounding concerns about HIV clinician supply and experience, questions remain about geographic equity in accessing HIV clinical care. The majority of HIV clinicians currently practice or plan to practice in urban areas [5, 9], mirroring the historic urban concentration of HIV cases. Simultaneously, the epidemic is increasingly shifting toward nonurban areas [10]. PLWH in rural areas experience more limited geographic access to HIV care [11, 12], more advanced HIV disease at diagnosis [13], lower retention in HIV care, and lower rates of viral suppression, compared to those living in the most urban communities [14].
Accessibility of experienced HIV clinicians in rural areas is critical to promote access to care and improve health equity for all PLWH. Leveraging comprehensive administrative claims data from 14 Southern states, we examined urban–rural disparities in HIV clinician workforce capacity, particularly among HIV-experienced clinicians.
METHODS
Data
To identify adults receiving routine HIV care and their clinicians, we used administrative claims data from the Medicaid Analytic eXtract (MAX) for adults aged 19–64 years in 14 Southern states including the District of Columbia (DC), 2009–2011. These states include some of the highest rates of newly diagnosed HIV cases nationally [2]. The MAX data include Medicaid claims for outpatient medical visits and filled prescriptions, enrollee-level demographic information, and national provider identifiers (NPIs) for clinicians providing outpatient services. To identify the clinicians’ practice locations, we used the MAX Provider Characteristics (MAX PC) dataset (2009–2011), which provides demographic and practice-related information for clinicians who submitted medical claims to Medicaid. Data usability of the claims and clinician characteristics was assessed for all 16 states plus DC in the Southern region; 3 states—South Carolina, Texas, and Virginia—did not meet data usability criteria for quality and completeness (eg, correct reporting of NPIs; percentage of enrollees with claims) and were excluded (Supplementary Materials). To estimate HIV clinician capacity and urban–rural differences, we used county-level diagnosed HIV case counts for 2014 for counties reporting at least 5 diagnosed HIV cases in the included states (926 counties including DC) [15].
Approach to Construct Sample
Identifying Enrollees Receiving Routine HIV Care
We first identified, in the administrative claims, Medicaid enrollees living with HIV (Figure 1). We used diagnosis codes from the International Statistical Classification of Diseases and Related Health Problems, Ninth Revision to identify enrollees living with HIV. We verified HIV status using Current Procedural Terminology codes for HIV-related laboratory management (eg, receipt of CD4+ T-lymphocyte or plasma HIV measurements) or National Drug Codes for antiretroviral prescriptions. Enrollees were excluded from the sample and considered not living with HIV if they did not have ≥ 1 HIV diagnosis code and ≥ 1 HIV verification code; if they did not have ≥ 2 HIV diagnosis codes ≥ 30 days apart; if their only HIV verification codes occurred before their first HIV diagnosis code; or if their only prescribed antiretroviral drug was emtricitabine-tenofovir for preexposure prophylaxis.
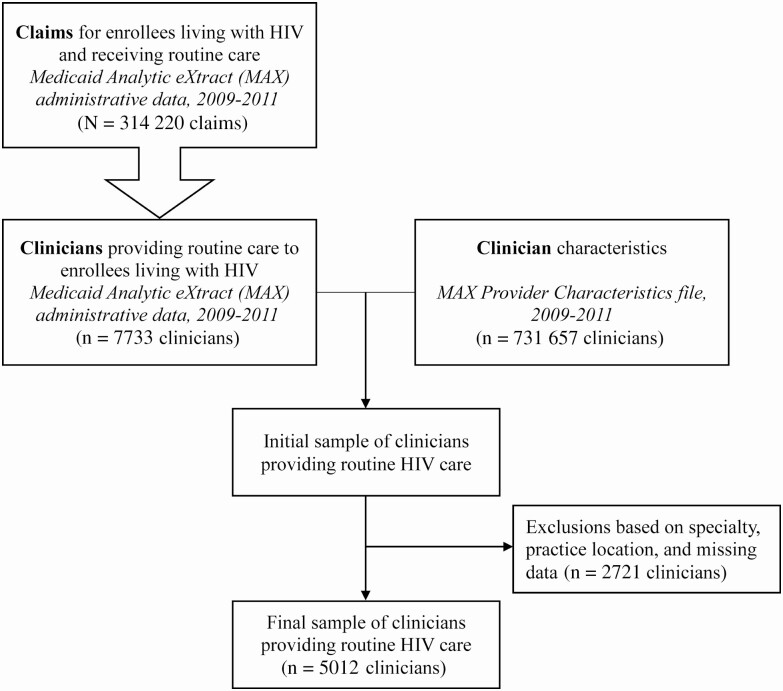
Figure 1.
Process overview for identifying human immunodeficiency virus (HIV) clinicians from claims data. Figure shows an overview of the process for identifying clinicians who provide routine HIV care using administrative claims data. The Medicaid Analytic eXtract (MAX) data used here comprise claims from 14 states for enrollees who qualify for Medicaid based on income or disability criteria, 2009–2011. More detail regarding identification of enrollees living with HIV, claims representing HIV routine care, and HIV clinicians is shown in the Supplementary Materials.
We further limited this sample to enrollees receiving routine HIV care, as routine HIV care claims would later be used to identify HIV clinicians. “Routine HIV care” was defined as office-based or outpatient evaluation and management or preventive care services for which an HIV diagnosis code was in the primary diagnosis position (indicating that the medical visit pertained primarily to HIV management). However, in sensitivity analysis, we relaxed this definition by allowing HIV diagnosis codes to occur in either the primary or secondary position. This relaxed definition captured routine HIV-related or non-HIV-related care for PLWH, plus routine HIV care for which diagnosis codes may have been incorrectly entered due to system-level logistical challenges in the electronic medical record [16]. After all exclusions, the baseline sample included 314 220 routine HIV care claims across 40 453 enrollees with HIV.
Identifying HIV Clinicians and Their Practice Locations
We used the sample of routine HIV care claims to identify clinicians providing routine HIV management. We assumed this sample approximated the region’s HIV workforce, since nearly three-quarters of clinicians accept Medicaid insurance nationally, and Medicaid is the largest single source of insurance for PLWH [17, 18]. We linked NPIs in the claims and the MAX PC dataset to obtain a candidate set of clinicians providing routine HIV care. We removed duplicate NPIs, as well as clinicians in specialties not typically providing routine HIV care (eg, surgeons), who did not practice in the 14 states, who had missing addresses, or whose address could not be matched to a county. We geocoded clinician practice addresses from the MAX PC [19], using a spatial overlay to identify the county in which each clinician practiced. Counties were classified as rural or urban [20]. Urban counties were those in metropolitan statistical areas, federally defined regions containing urban centers of ≥ 50 000 population. Rural counties were nonmetropolitan counties, or those outside of metropolitan statistical areas and with population centers of ≤ 49 999 residents.
Analysis
We summarized the HIV clinician workforce at the clinician and county levels. At the clinician level, we described clinicians by rurality, gender, credential (physician [ie, Doctor of Medicine {MD} or Doctor of Osteopathy {DO}] or advanced practitioner [ie, nurse practitioner or physician assistant]), state, and specialty (physicians only). Physician specialties were categorized as infectious diseases, internal medicine, other primary care (eg, family medicine, pediatrics, geriatrics), and other. We also considered “HIV-experienced clinicians,” or clinicians providing care to ≥ 10 enrollees with HIV across 3 years. While HIV specialty medical societies have used thresholds of 20 or 25 patients with HIV over 3 years to define “HIV specialists” [21, 22], we conservatively opted for a lower patient threshold to reflect availability of claims data from a single payer and to allow comparability with existing literature [4, 5]. However, in sensitivity analysis, we examined a threshold of ≥ 25 enrollees living with HIV across 3 years. We examined differences between HIV-experienced and non–HIV-experienced clinicians for rurality, gender, credential, specialty, and state using χ 2 tests (P < .05).
To assess county-level workforce capacity, we calculated clinician-to-population ratios, defined as the number of clinicians in a county per 1000 diagnosed HIV cases. Diagnosed HIV cases were used as the denominator instead of the general population, as is typical for clinician-to-population ratios, because this better captures the population in need of HIV clinical care. Ratios are reported only for counties with at least 5 diagnosed HIV cases, with smaller clinician-to-population ratios indicating lower workforce capacity relative to HIV burden in a given county. We summarized county-level clinician-to-population ratios using median and interquartile range (IQR), comparing ratios for urban and rural counties using Wilcoxon-Mann-Whitney tests (P < .05). We also considered the special case in which a county has zero HIV clinicians but at least 5 diagnosed HIV cases, and reported the total number of diagnosed HIV cases in those counties. Data were summarized using Stata version 15 and mapped using ArcMap version 10.5 software.
RESULTS
Clinician Characteristics
We identified 5012 clinicians providing routine HIV care in 14 Southern states (Table 1). Thirteen percent practice in rural counties, with > 90% of rural clinicians considered non–HIV-experienced. Nearly 60% of clinicians are men; 82% are physicians and 12% are advanced practitioners. Among physicians (n = 4094), internal medicine (33%) and other primary care (44%) are the most common physician specialties; infectious disease specialists comprise less than a fifth of the sample (18%).
Table 1.
Characteristics of Human Immunodeficiency Virus Clinicians in 14 States, United States, 2009–2011
| Characteristic | Total | Non–HIV-Experienced | HIV-Experienceda | P Valueb |
|---|---|---|---|---|
| All | 5012 | 3615 | 1397 | |
| Practice locationc | < .001 | |||
| Rural | 672 (13) | 613 (17) | 59 (4) | |
| Urban | 4340 (87) | 3002 (83) | 1338 (96) | |
| Gender | .716 | |||
| Women | 1777 (35) | 1268 (35) | 509 (36) | |
| Men | 2881 (57) | 2070 (57) | 811 (58) | |
| Missing | 354 (7) | 277 (8) | 77 (6) | |
| Credentiald | .117 | |||
| Physician | 4094 (82) | 2930 (81) | 1164 (83) | |
| Advanced practitioner | 577 (12) | 431 (12) | 146 (10) | |
| Missing | 341 (7) | 254 (7) | 87 (6) | |
| Physician specialty (n = 4094)e | < .001 | |||
| Infectious diseases | 739 (18) | 179 (6) | 560 (48) | |
| Internal medicine | 1358 (33) | 1045 (36) | 313 (27) | |
| Other primary care | 1819 (44) | 1578 (54) | 241 (21) | |
| Other | 178 (4) | 128 (4) | 50 (4) | |
| State | < .001 | |||
| Alabama | 155 (3) | 105 (3) | 50 (4) | |
| Arkansas | 145 (3) | 110 (3) | 35 (3) | |
| District of Columbia | 94 (2) | 51 (1) | 43 (3) | |
| Delaware | 88 (2) | 61 (2) | 27 (2) | |
| Florida | 1616 (32) | 1069 (30) | 547 (39) | |
| Georgia | 527 (11) | 389 (11) | 138 (10) | |
| Kentucky | 180 (4) | 152 (4) | 28 (2) | |
| Louisiana | 315 (6) | 223 (6) | 92 (7) | |
| Maryland | 235 (5) | 165 (5) | 70 (5) | |
| Mississippi | 291 (6) | 239 (7) | 52 (4) | |
| North Carolina | 683 (14) | 492 (14) | 191 (14) | |
| Oklahoma | 201 (4) | 177 (5) | 24 (2) | |
| Tennessee | 382 (8) | 304 (8) | 78 (6) | |
| West Virginia | 100 (2) | 78 (2) | 22 (2) |
Data are presented as no. (%) unless otherwise indicated. Column percentages may not add to 100% due to rounding.
Abbreviation: HIV, human immunodeficiency virus.
aHIV-experienced clinicians are those who provided HIV care to a minimum of 10 Medicaid enrollees living with HIV between 2009 and 2011.
bStatistically significant differences between HIV-experienced and non–HIV-experienced clinicians were assessed using χ 2 tests (P < .05).
cRurality is defined according to the 2013 National Center for Health Statistics’ Urban-Rural Classification Scheme: “rural” includes counties in nonmetropolitan areas, and “urban” includes counties in metropolitan statistical areas of any size. Delaware has no rural counties, and the District of Columbia is counted as a single county. Three states (South Carolina, Texas, and Virginia) that were originally considered for inclusion were excluded due to data completeness and quality issues.
dPhysicians include MDs (Doctor of Medicine) and DOs (Doctor of Osteopathy). Advanced practitioners include nurse practitioners and physician assistants.
eSpecialties are reported for physicians only (ie, clinicians with MD or DO credentials).
Of all identified HIV clinicians, < 30% are considered HIV-experienced. HIV-experienced clinicians are significantly less likely to practice in rural counties (4%) vs non–HIV-experienced clinicians (17%) (P < .001). There are no significant differences by gender or credential between HIV-experienced and non–HIV-experienced clinicians, though there are differences by state (P < .001). Nearly half of all HIV-experienced physicians specialize in infectious diseases, compared to 6% among non–HIV-experienced physicians (P < .001).
Workforce Capacity
Of 926 counties assessed, > 40% (n = 373) have no HIV clinicians. Counties with no HIV clinicians have an estimated 11 987 diagnosed HIV cases, with nearly 60% in rural counties. Across all counties, the median county-level HIV clinician-to-population ratio is 13.3 (IQR, 38.0) HIV clinicians per 1000 diagnosed HIV cases (Table 2). Ratios vary widely across states, with medians as low as 0 in Arkansas, Kentucky, and West Virginia and as high as 34.5 in Delaware (IQR, 14.5). Overall and within states, rural counties have lower workforce capacity, with overall median clinician-to-population ratios of 7.4 (IQR, 43.5) vs 16.0 (IQR, 32.3) for urban counties; however, these urban–rural differences are not statistically significant with the exception of West Virginia (P < .01).
Table 2.
Number of Counties and Median County-level Human Immunodeficiency Virus (HIV) Clinician-to-Population Ratios (Clinicians per 1000 Diagnosed HIV Cases)
| State | Counties With at Least 5 Diagnosed HIV Cases (Frequency) | All HIV Clinicians, Median (IQR) | P Valuea | HIV-Experienced Clinicians, Median (IQR) | P Valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Rural | Urban | All Counties | Rural Counties | Urban Counties | All Counties | Rural Counties | Urban Counties | |||
| All 14 states | 926 | 531 | 395 | 13.3 (38.0) | 7.4 (43.5) | 16.0 (32.3) | .13 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (4.9) | < .01 |
| Alabama | 67 | 38 | 29 | 11.0 (27.8) | 17.0 (38.5) | 4.3 (14.4) | .05 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (3.2) | < .01 |
| Arkansas | 71 | 51 | 20 | 0.0 (45.5) | 0.0 (52.6) | 15.9 (24.8) | .47 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (5.6) | < .01 |
| DC | 1 | 0 | 1 | 6.2 (–) | … | 6.2 (–) | 2.8 (–) | … | 2.8 (–) | ||
| Delaware | 3 | 0 | 3 | 34.5 (14.5) | … | 34.5 (14.5) | 8.6 (2.2) | … | 8.6 (2.2) | ||
| Florida | 67 | 23 | 44 | 19.3 (20.5) | 27.3 (32.3) | 19.0 (14.6) | .69 | 4.0 (6.7) | 0.0 (6.8) | 5.0 (6.7) | .04 |
| Georgia | 156 | 83 | 73 | 7.6 (28.4) | 0.0 (30.3) | 10.7 (23.7) | .63 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (1.8) | < .01 |
| Kentucky | 99 | 67 | 32 | 0.0 (53.4) | 0.0 (76.9) | 4.4 (37.9) | .62 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | < .01 |
| Louisiana | 62 | 28 | 34 | 13.5 (28.6) | 11.7 (32.8) | 14.5 (21.4) | .71 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (5.2) | < .01 |
| Maryland | 24 | 5 | 19 | 6.9 (12.0) | 0.0 (16.7) | 7.1 (9.4) | .48 | 0.0 (0.4) | 0.0 (0.0) | 0.0 (1.0) | .16 |
| Mississippi | 80 | 63 | 17 | 29.2 (48.0) | 27.3 (56.3) | 33.9 (31.1) | .39 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (3.5) | <.01 |
| North Carolina | 98 | 52 | 46 | 20.0 (41.7) | 17.1 (39.1) | 24.4 (32.5) | .28 | 0.0 (4.1) | 0.0 (0.0) | 0.0 (9.3) | < .01 |
| Oklahoma | 61 | 44 | 17 | 26.3 (47.1) | 26.3 (59.7) | 27.4 (22.4) | .91 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | < .01 |
| Tennessee | 89 | 48 | 41 | 27.0 (56.3) | 30.4 (64.6) | 24.4 (51.3) | .69 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | < .01 |
| West Virginia | 48 | 29 | 19 | 0.0 (48.0) | 0.0 (0.0) | 32.3 (83.3) | < .01 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (14.9) | < .01 |
The left panel of clinician-to-population ratios represents the number of clinicians (physicians and advanced practitioners) providing routine HIV care to any number of Medicaid enrollees living with HIV, per 1000 diagnosed HIV cases in a county. The right panel restricts the sample to HIV-experienced clinicians (ie, those who provide HIV care to ≥10 Medicaid enrollees living with HIV). Rurality is defined according to the 2013 National Center for Health Statistics’ Urban-Rural Classification Scheme: “rural” includes counties in nonmetropolitan areas, and “urban” includes counties in metropolitan statistical areas of any size. Delaware has no rural counties, and DC is counted as a single county. For DC, state-level HIV prevalence data were used, and (–) indicates that no IQR was calculated because DC represents a single geographic entity.
Abbreviations: DC, District of Columbia; IQR, interquartile range.
aAssessed significant urban–rural differences (P < .05) with Wilcoxon-Mann-Whitney tests.
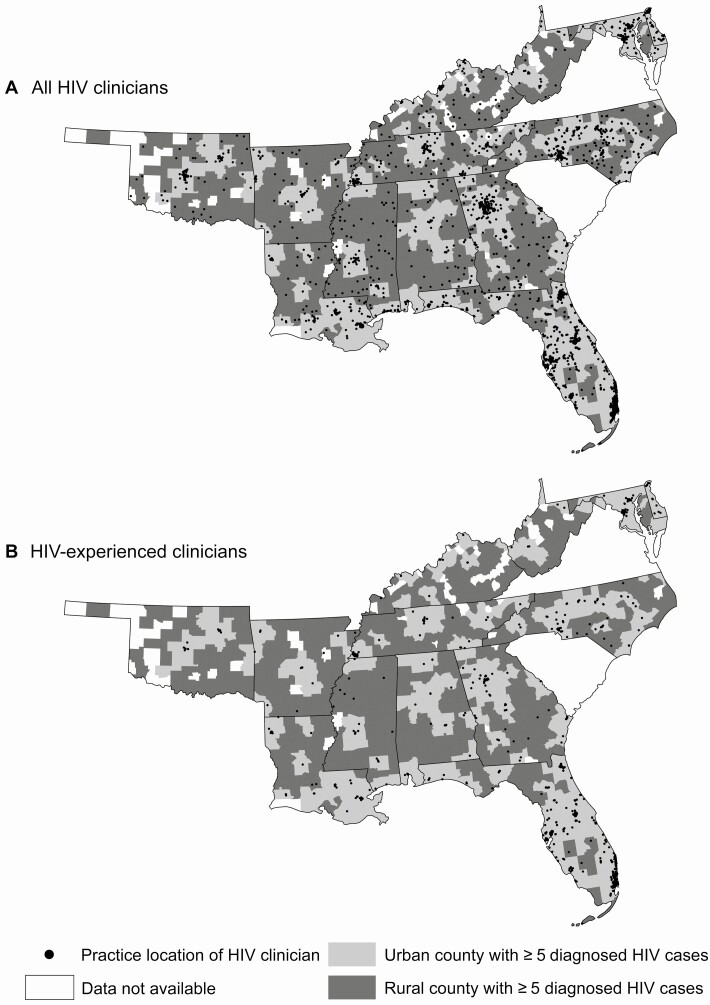
Considering the 1397 HIV-experienced clinicians, 81% of counties have no HIV-experienced clinicians, with rural counties less likely to have HIV-experienced clinicians (94% with no HIV-experienced clinicians) than urban counties (65%) (P < .001; Figure 2). Nearly 43 000 people diagnosed with HIV live in counties with no HIV-experienced clinicians, and 47% of these individuals live in rural counties. There is a median of 0 (IQR, 0) HIV-experienced clinicians per 1000 diagnosed HIV cases across all counties. Overall and in each state except Maryland, ratios are significantly different in rural counties and in urban counties (P < .05).
Figure 2.
County-level rurality and locations of human immunodeficiency virus (HIV) clinicians. Rural counties with ≥5 diagnosed HIV cases in 2014 are shaded in dark gray, and urban counties with ≥5 diagnosed HIV cases are in light gray. Data are not available for counties shaded white. HIV clinicians provide routine HIV care to any number of Medicaid enrollees living with HIV, while HIV-experienced clinicians provide HIV care to ≥10 Medicaid enrollees living with HIV >3 years. HIV clinician workforce capacity is similar across urban and rural counties when considering all HIV clinicians (A), but few HIV-experienced clinicians practice in rural counties (B).
Sensitivity Analyses
Relaxing the definition of routine HIV care used to identify HIV clinicians increased the total number of HIV clinicians (n = 6488) and overall HIV clinician-to-population ratios (median for all counties, 26.0 [IQR, 55.6]). However, few (n = 57) additional HIV-experienced clinicians were identified, and median HIV-experienced clinician-to-population ratios were largely unchanged relative to baseline. Additionally, when defining HIV-experienced clinicians as those with ≥ 25 enrollees living with HIV over 3 years, 15% of HIV clinicians were HIV-experienced, 4% of whom practiced in rural counties. Under this definition, > 56 000 people diagnosed with HIV lived in counties with no HIV-experienced clinicians, with > 60% in urban counties. Overall and in several states, HIV-experienced workforce capacity in rural counties was significantly different from urban counties (P < .01), although differences were not significant for 5 states (Arkansas, Georgia, Kentucky, Maryland, and Mississippi).
DISCUSSION
HIV-experienced clinicians disproportionately practice in metropolitan areas, resulting in significant disparities in HIV-experienced clinician workforce capacity in rural vs urban communities. Less than a third of clinicians who provide routine HIV care are HIV-experienced, and more than 80% of counties across the South lack an HIV-experienced clinician.
Findings highlight geographic barriers to, and disparities in, access to care. Across 14 states, > 20 000 PLWH live in rural counties with no HIV-experienced clinicians and nearly 7000 live in rural counties with no HIV clinicians at all; these PLWH may lack access to high-quality HIV care in their communities. This raises concerns as limited geographic access to care appears associated with poorer linkage to care, retention in care, and viral suppression [23, 24]. While the majority of PLWH live in urban counties, the more limited HIV clinician workforce capacity in rural areas may ultimately contribute to the observed urban–rural disparities in HIV care continuum outcomes [10, 14]. Moreover, as the ongoing opioid epidemic leads to new cases of HIV and hepatitis C in rural communities [25], improving the capacity of clinicians experienced in infectious disease management in rural areas is essential.
This study underscores the limited capacity of HIV-experienced clinicians in both rural and urban communities. Using our conservative baseline definition of HIV-experienced clinicians, we identified a pressing gap in service accessibility, particularly for rural areas. The more restrictive definition of HIV-experienced clinicians in sensitivity analysis additionally emphasizes limited availability in urban communities, where workforce capacity is similar to that of rural communities in some states. A strong body of literature suggests that more experienced HIV clinicians deliver higher quality of care: physicians with fewer HIV patients are less likely to prescribe antiretroviral therapy [26, 27], whereas clinicians with higher caseloads (eg, ≥ 20 or ≥ 50 patients with HIV) are more likely to meet key quality metrics for visit frequency or retention in care, CD4 cell count and HIV RNA viral load testing, viral suppression [3, 4], and certain preventive care measures (eg, tuberculosis testing; lipid panels) [3]. HIV caseload may be more important than physician specialty in predicting quality of care [28], which highlights the implications of limited HIV-experienced clinician capacity, including urban–rural disparities, for achieving national health equity goals and ending the epidemic.
Results are broadly consistent with, yet provide a novel contribution to, the HIV workforce literature. Across the same 14 states we studied, Gilman et al identified a very similar number of HIV clinicians (n = 1484) providing care to ≥ 10 PLWH in a year [5] as did our analysis at baseline (n = 1397) and when relaxing the definition of routine HIV care used to identify clinicians in sensitivity analysis (n = 1454). Our work identified a similar fraction of HIV-experienced clinicians practicing in rural areas (4%) as the previous study (3%) [5]. In a recent survey, 80% of HIV clinicians were physicians and 43% were women [29], compared to 82% physicians and 35% women in our sample. Building on previous work, this study is the first, to our knowledge, to provide a fine-grained analysis of the geographic structure of the US HIV workforce and to quantify urban–rural disparities in HIV workforce capacity, including among HIV-experienced clinicians. Finally, previous analyses relied on a national probability sample of HIV clinicians [29] or on a proprietary all-payer administrative claims database supplemented by surveys to identify a sample of HIV clinicians [5]; this study demonstrates that using Medicaid administrative claims alone yields similar results.
Several approaches exist to increase rural access to experienced HIV clinicians. Remote telehealth alternatives may increase access to care for rural PLWH, with some evaluations indicating high uptake and acceptability [30] and improvements in viral suppression [31], although barriers such as state-specific clinician licensure, limited reimbursement for telemedicine, and limitations on prescribing via telemedicine may hinder widespread implementation of telehealth for HIV management [32]. Another alternative is to expand the HIV care capacity of the existing rural clinician workforce. Programs such as the AIDS Education and Training Centers provide training in HIV management [33, 34], but participation may need to be expanded for clinicians to meet the needs of rural PLWH. State-level policy efforts could involve expanding scope-of-practice laws for nurse practitioners, who provide HIV care of similar quality as physicians [29, 35] but are prohibited from prescribing medications or practicing independently in many states. Alternatively, states could employ HIV-experienced clinicians at local health departments to provide local care or expand transportation assistance when other solutions are not feasible.
LIMITATIONS
We used data from a single insurer (Medicaid, prior to the Affordable Care Act) so we cannot draw definitive conclusions about the total clinicians and clinician caseload or level of HIV training. Using a single source risks underestimating the size of, and geographic disparities within, the clinician workforce. However, findings on the size and geographic distribution of the HIV clinician workforce are largely comparable to previous work representing different geographic areas (eg, state, region, national), and insurance types, including uninsurance [4, 5, 11, 12, 36]. Additionally, current Medicaid claims data are not routinely and widely available, especially since the Affordable Care Act. However, the claims used in the current analysis, while older, represent one of the most comprehensive sources of administrative data for the largest insurer of PLWH and provide important evidence on HIV clinicians’ practice patterns. Current and comprehensive data on Medicaid clinicians—including their characteristics, patient volume, and service locations—are also not routinely available. While current and historical clinician data from other sources are accessible (eg, American Medical Association Physician Masterfile), we used the older Medicaid Provider Characteristics file to remain contemporaneous with our enrollee-level claims data and because the clinician data included additional information (eg, data on advanced practitioners, clinicians practicing in multiple states) not typically available in other data sources. Given recent challenges in HIV workforce recruitment and retention and an increasing rural epidemic, we expect that our estimates represent a conservative upper bound of HIV workforce capacity and urban–rural disparities. Third, assigning HIV clinicians to a single county does not capture PLWH who cross county boundaries for care or clinicians who practice in multiple counties. Our analysis thus may overestimate the number of counties with zero HIV clinicians and underestimate HIV clinician-to-population ratios. Yet our assignment of HIV clinicians to a single geographic unit (here, the county) is consistent with similar literature on US clinician workforce capacity that relies on clinician-to-population ratios [37, 38]. Furthermore, findings from the current study complement emerging evidence on geographic access to HIV care suggesting increased availability of, and shorter travel to, HIV care in urban areas [11, 12, 36]. Fourth, the data draw on the National Plan and Provider Enumeration System, a self-report database that clinicians may not regularly update [39], although this source provides the most accurate physician contact information [40]. Finally, we used data from states in a single geographic region, the Southern US. These states include those with the highest rates of newly diagnosed HIV cases nationally, highlighting the importance of an adequate HIV workforce for those newly diagnosed and diagnosed but uncontrolled. While the rural HIV epidemic is disproportionately represented in the South, we anticipate that our main findings regarding the limited HIV workforce capacity in rural areas would generalize to other regions [5].
CONCLUSIONS
As the US strives to end the HIV epidemic, structural barriers—including clinician workforce capacity—and resulting disparities in access to care have emerged as critical areas of focus. Addressing the needs of rural PLWH has become increasingly important given the shift of the epidemic toward rural communities. HIV-experienced clinicians are more likely to practice in urban areas, and significant urban–rural differences exist in county-level HIV-experienced clinician workforce capacity. However, findings also suggest striking gaps in the workforce capacity of HIV-experienced clinicians across both urban and rural locales. Policies that promote a robust and experienced HIV clinician workforce for PLWH, whether in urban or rural communities, are urgently needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. R. S. B. and A. D. K. designed the study. Y. D. and R. S. B. analyzed the data, and R. S. B., B. D., and A. D. K. aided in data interpretation. R. S. B. and A. D. K. drafted the manuscript, and A. G. R., B. D., D. E. N., F. Z. B., L. E. Y., and L. M. S. revised the manuscript for important intellectual content.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the views of the funder.
Financial support. This work is supported in part by the National Institute on Minority Health and Health Disparities of the National Institutes of Health (award number R01 MD011277) and the Blick Scholars Program at Virginia Commonwealth University.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Office of National AIDS Policy. National HIV/AIDS strategy for the United States: updated to 2020. 2015. Available at: https://www.hiv.gov/federal-response/national-hiv-aids-strategy/overview. Accessed 13 March 2018.
- 2. Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321:844–5. [DOI] [PubMed] [Google Scholar]
- 3. Landovitz RJ, Desmond KA, Gildner JL, Leibowitz AA. Quality of care for HIV/AIDS and for primary prevention by HIV specialists and nonspecialists. AIDS Patient Care STDS 2016; 30:395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Neill M, Karelas GD, Feller DJ, et al. The HIV workforce in New York state: does patient volume correlate with quality? Clin Infect Dis 2015; 61:1871–7. [DOI] [PubMed] [Google Scholar]
- 5. Gilman B, Bouchery E, Barrett K, et al. HIV clinician workforce study. Cambridge, MA: Mathematica Policy Research, 2013. Available at: https://www.mathematica-mpr.com/our-publications-and-findings/publications/hiv-clinician-workforce-study. Accessed 24 April 2017. [Google Scholar]
- 6. Weiser J, Beer L, West BT, Duke CC, Gremel GW, Skarbinski J. Qualifications, demographics, satisfaction, and future capacity of the HIV care provider workforce in the United States, 2013–2014. Clin Infect Dis 2016; 63: 966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carmichael JK, Deckard T, Feinberg J, et al. Averting a crisis in HIV care: a joint statement of the American Academy of HIV Medicine (AAHIVM) and the HIV Medicine Association (HIVMA) on the HIV medical workforce, 2009. Available at: https://www.idsociety.org/uploadedFiles/IDSA/Policy_and_Advocacy/Current_Topics_and_Issues/Workforce_and_Training/Statements/AAHIVM%20HIVMA%20Workforce%20Statement%20062509.pdf.
- 8. Chandrasekar PH. Bad news to worse news: 2015 infectious diseases fellowship match results. Clin Infect Dis 2015; 60:1438. [DOI] [PubMed] [Google Scholar]
- 9. Salsberg E, Mehfoud N, Quigley L, Ericson C, Roberts A. New infectious diseases physicians: results from the survey of infectious disease fellows completing training in 2016: the George Washington University Health Workforce Institute and the Infectious Diseases Society of America, 2016. Available at: http://www.idsociety.org/uploadedFiles/IDSA/Policy_and_Advocacy/Current_Topics_and_Issues/Workforce_and_Training/Background/FULL%20Report%20On%20ID%20Fellows%20Completing%20Training%20in%202016.pdf. Accessed 31 July 2018.
- 10. Schafer KR, Albrecht H, Dillingham R, et al. The continuum of HIV care in rural communities in the United States and Canada: what is known and future research directions. J Acquir Immune Defic Syndr 2017; 75:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimmel AD, Masiano SP, Bono RS, et al. Structural barriers to comprehensive, coordinated HIV care: geographic accessibility in the US South. AIDS Care 2018; 30:1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masiano SP, Martin EG, Bono RS, et al. Suboptimal geographic accessibility to comprehensive HIV care in the US: regional and urban-rural differences. J Int AIDS Soc 2019; 22:e25286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopes BLW, EronJJ, Jr., Mugavero MJ, Miller WC, Napravnik S. HIV care initiation delay among rural residents in the southeastern United States, 1996 to 2012. J Acquir Immune Defic Syndr 2017; 76:171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nelson JA, Kinder A, Johnson AS, et al. Differences in selected HIV care continuum outcomes among people residing in rural, urban, and metropolitan areas-28 US jurisdictions. J Rural Health 2018; 34:63–70. [DOI] [PubMed] [Google Scholar]
- 15. AIDSVu. Understanding HIV where you live. Available at: www.aidsvu.org. Accessed 17 April 2018. [Google Scholar]
- 16. Bowman S. Impact of electronic health record systems on information integrity: quality and safety implications. Perspect Health Inf Manag 2013; 10:1c. [PMC free article] [PubMed] [Google Scholar]
- 17. Hing E, Decker SL, Jamoom E. Acceptance of new patients with public and private insurance by office-based physicians: United States, 2013. NCHS Data Brief. Hyattsville, MD: National Center for Health Statistics, 2015:1–8. [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Behavioral and clinical characteristics of persons with diagnosed HIV infection—Medical Monitoring Project, United States–2015 cycle (June 2015–May 2016). Atlanta, GA: CDC, 2018. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-special-report-number-20.pdf. Accessed 25 January 2019. [Google Scholar]
- 19. Texas A&M Geoservices. Batch geocoding. 2017. Available at: http://geoservices.tamu.edu/Services/Geocode/. Accessed 30 October 2017.
- 20. Ingram DD, Franco SJ. 2013 NCHS urban-rural classification scheme for counties. Vital Health Stat 2 2014:1– 73. [PubMed] [Google Scholar]
- 21. American Academy of HIV Medicine. HIV Specialist. Available at: https://aahivm.org/hiv-specialist/. Accessed 30 July 2018.
- 22. HIV Medicine Association. Identifying providers qualified to manage the longitudinal treatment of patients with HIV infection and resources to support quality HIV care, 2013. Available at: https://www.hivma.org/globalassets/hivma/logos/revised-qualified-hiv-provider-policy-statement-approved-3-16-13-1.pdf. Accessed 30 July 2018.
- 23. Terzian AS, Younes N, Greenberg AE, et al. DC Cohort Executive Committee . Identifying spatial variation along the HIV care continuum: the role of distance to care on retention and viral suppression. AIDS Behav 2018; 22:3009–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ridgway JP, Almirol EA, Schmitt J, Schuble T, Schneider JA. Travel time to clinic but not neighborhood crime rate is associated with retention in care among HIV-positive patients. AIDS Behav 2018; 22:3003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schranz AJ, Barrett J, Hurt CB, Malvestutto C, Miller WC. Challenges facing a rural opioid epidemic: treatment and prevention of HIV and hepatitis C. Curr HIV/AIDS Rep 2018; 15:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landon BE, Wilson IB, McInnes K, et al. Physician specialization and the quality of care for human immunodeficiency virus infection. Arch Intern Med 2005; 165:1133–9. [DOI] [PubMed] [Google Scholar]
- 27. Weiser J, Brooks JT, Skarbinski J, et al. Barriers to universal prescribing of antiretroviral therapy by HIV care providers in the United States, 2013–2014. J Acquir Immune Defic Syndr 2017; 74:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landon BE, Wilson IB, Wenger NS, et al. Specialty training and specialization among physicians who treat HIV/AIDS in the United States. J Gen Intern Med 2002; 17:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weiser J, Chen G, Beer L, et al. Sustaining the HIV care provider workforce: Medical Monitoring Project HIV provider survey, 2013–2014. Health Serv Res 2019; 54:1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohl M, Dillon D, Moeckli J, et al. Mixed-methods evaluation of a telehealth collaborative care program for persons with HIV infection in a rural setting. J Gen Intern Med 2013; 28:1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohl ME, Richardson K, Rodriguez-Barradas MC, et al. Impact of availability of telehealth programs on documented HIV viral suppression: a cluster-randomized program evaluation in the Veterans Health Administration. Open Forum Infect Dis 2019; 6:ofz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on telehealth and telemedicine as applied to the practice of infectious diseases. Clin Infect Dis 2019; 68:1437–43. [DOI] [PubMed] [Google Scholar]
- 33. Culyba RJ, McGee BT, Weyer D. Changing HIV clinical knowledge and skill in context: the impact of longitudinal training in the Southeast United States. J Assoc Nurses AIDS Care 2011; 22:128–39. [DOI] [PubMed] [Google Scholar]
- 34. Portillo CJ, Stringari-Murray S, Fox CB, Monasterio E, Rose CD. The HIV primary care workforce of tomorrow: the UCSF integrated HIV/AIDS primary care capacity nurse practitioner program. J Assoc Nurses AIDS Care 2016; 27:214–22. [DOI] [PubMed] [Google Scholar]
- 35. Wilson IB, Landon BE, Hirschhorn LR, et al. Quality of HIV care provided by nurse practitioners, physician assistants, and physicians. Ann Intern Med 2005; 143:729–36. [DOI] [PubMed] [Google Scholar]
- 36. Ohl ME, Richardson K, Kaboli PJ, Perencevich EN, Vaughan-Sarrazin M. Geographic access and use of infectious diseases specialty and general primary care services by veterans with HIV infection: implications for telehealth and shared care programs. J Rural Health 2014; 30:412–21. [DOI] [PubMed] [Google Scholar]
- 37. Ellis AR, Konrad TR, Thomas KC, Morrissey JP. County-level estimates of mental health professional supply in the United States. Psychiatr Serv 2009; 60:1315–22. [DOI] [PubMed] [Google Scholar]
- 38. Kindig DA, Movassaghi H. The adequacy of physician supply in small rural counties. Health Aff (Millwood) 1989; 8:63–76. [DOI] [PubMed] [Google Scholar]
- 39. Bindman AB. Using the National Provider Identifier for health care workforce evaluation. Medicare Medicaid Res Rev 2013; 3. doi:10.5600/mmrr.003.03.b03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DesRoches CM, Barrett KA, Harvey BE, et al. The results are only as good as the sample: assessing three national physician sampling frames. J Gen Intern Med 2015; 30(Suppl 3):S595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.