Supplemental Digital Content is available in the text.
Key Words: distal pancreatectomy, robot-assisted, robotic, laparoscopic, network meta-analysis
Background:
The efficacy and safety of open distal pancreatectomy (DP), laparoscopic DP, robot-assisted laparoscopic DP, and robotic DP have not been established. The authors aimed to comprehensively compare these 4 surgical methods using a network meta-analysis.
Materials and Methods:
The authors systematically searched MEDLINE, Scopus, Web of Science, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov for studies that evaluated at least 2 of the following pancreatectomy techniques: robot-assisted DP, laparoscopic DP, open DP, and robotic DP. The surface under the cumulative ranking curve (SUCRA) was applied to show the probability that each method would be the best for each outcome.
Results:
Altogether, 46 trials with 8377 patients were included in this network meta-analysis. Robotic DP showed the highest probability of having the least estimated blood loss (SUCRA, 90.9%), the lowest incidences of postoperative pancreatic fistula (SUCRA, 94.5%), clinically related postoperative pancreatic fistula (SUCRA, 94.6%), postoperative bleeding (SUCRA, 75.3%), reoperation (SUCRA, 96.4%), overall complications (SUCRA, 86.9%), and major complications (SUCRA, 99.3%), and the lowest mortality (SUCRA, 83.4%). Robotic DP also proved to be the best approach regarding the attainment of R0 resection (SUCRA, 75.4%) and the number of lymph nodes harvested (SUCRA, 64.1%).
Conclusion:
Robotic DP seems to offer clinical and oncological advantages compared with other DP methods for addressing diseases of the pancreatic body and tail, although it may require a longer operation time and learning curve. The present results require confirmation in future head-to-head randomized controlled trials.
Distal pancreatectomy (DP) has been widely used to treat benign and malignant tumors of the pancreatic body and tail. Minimally invasive DP (MIDP) has been widely reported,1–4 with 3 approaches currently in use: laparoscopic DP (LDP), robot-assisted LDP (RADP), and robotic DP (RDP). The latest guidelines, published in 2020, recommend MIDP over open DP (ODP) for benign and low-grade malignant tumors.5 A recent multicenter randomized controlled trial (RCT) comparing MIDP with ODP showed that MIDP has the advantages of shorter hospital stay, less blood loss, and fewer overall complications (OCs).6 However, we believe that more evidence is needed to confirm that MIDP is preferable to ODP and should be used in the clinical setting. Furthermore, other previous studies, including many meta-analyses, focused on MIDP but did not distinguish RADP, RDP, and LDP as separate entities.7–9 Thus, the safety and efficacy of these individual MIDP methods remain controversial. One meta-analysis that included 17 studies revealed that RADP resulted in fewer OCs than LDP or ODP.10 Conversely, Kamarajah et al8 showed that the OC rate did not significantly differ between RDP and LDP. However, few studies have focused on the differences between RADP and RDP. We hypothesized that the type of DP may play an important role in the efficacy and safety of this surgery. Therefore, we conducted a systematic, aggregate network meta-analysis to assess the value of each of the 4 DP methods (3 minimally invasive and 1 open).
MATERIALS AND METHODS
Study Design
The protocol we used for our data search was derived from the Cochrane Handbook for Systematic Reviews of Interventions.11 We conducted a comprehensive search of MEDLINE, Scopus, Web of Science, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov for relevant studies published between 1990 and 2019. This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The following keywords were used in several logical combinations: distal pancreatectomy, distal pancreatectomies, robotic, robot-assisted, open, laparoscopy, and laparoscopic. The search was limited to RCTs performed with human subjects for which the full-text articles were available, with the publication language restricted to English. We also searched the reference lists of the initially retrieved articles for potentially relevant papers.
IRB approval and informed consent were not needed for this study.
Inclusion and Exclusion Criteria
The inclusion criterion was that the study compared at least 2 of RADP, LDP, ODP, and RDP. ODP, LDP, and RADP were defined as reported previously.12 RDP was defined as completely robotic surgery without laparoscopic assistance, as previously reported.13 Studies that compared total pancreatectomy or pancreaticoduodenectomy were excluded from the present study. We also excluded articles that on-human studies, review articles, letters, abstracts, case reports, and articles that did not report the outcomes of interest that were assessed in the present study.
Outcome Measures
The outcomes analyzed in our study were categorized as intraoperative outcomes, postoperative outcomes, and oncological outcomes. The intraoperative outcomes were: operation time (OT), estimated blood loss (EBL), and spleen preservation (SP). The postoperative outcomes were the presence of postoperative pancreatic fistula (POPF) and/or clinically related POPF (CR-POPF), postoperative bleeding (POBL), reoperation, OCs, major complications (MCs), and mortality. The oncological outcomes were the achievement of R0 margins and the number of lymph nodes harvested (LNH). POPF were classified in accordance with the criteria of the International Study Group of Pancreatic Fistula, with grades B and C regarded as indicating the presence of CR-POPF.14 An MC was defined as grades III and IV in accordance with the Clavien-Dindo grading system.15
Data Extraction and Quality Assessment
Two authors independently conducted comprehensive literature searches to identify articles in multiple electronic databases. Microsoft Excel (Microsoft Corporation, Redmond, WA) was used to identify duplicate studies, which were then removed. The following data were recorded on a standardized form: first author’s name, year of publication, study setting, sample size, and surgical procedure. Disagreements in data abstraction were resolved by consensus, and the methodological quality of the included trials was assessed in accordance with the Newcastle-Ottawa scale.
Statistical Analysis
Data were analyzed using STATA version 13.0 software (Stata Corp, College Station, TX) in a frequentist network meta-analysis. Continuous variables were analyzed using the weighted mean difference and its 95% credible interval. Dichotomous variables were analyzed on the basis of odds ratios with 95% confidence intervals. The percent surface under the cumulative ranking curve (SUCRA) and the mean ranks were calculated for each intervention. The SUCRA index ranges between 0 and 100, with higher values indicating better efficacy.16 Consistency or inconsistency testing was not performed because of the absence of a closed-loop in the network meta-analysis.17 Publication bias of the literature was evaluated using funnel plots.
RESULTS
Study Selection
The initial electronic literature search retrieved 1808 articles, of which 527 were removed after identification as duplicates. Another 1013 trials were excluded for various reasons, and 240 full-text articles were excluded because they had no relevance to our study. A final total of 46 studies met the eligibility criteria and were included in the present analysis.12,13,18–61 A detailed flowchart of the selection process is shown in Figure 1. The characteristics of the included studies are summarized in Table 1. Among the 46 included studies, 4 were 3-armed trials, whereas 42 were 2-armed trials. The sample size ranged from 14 to 1126 patients.
FIGURE 1.
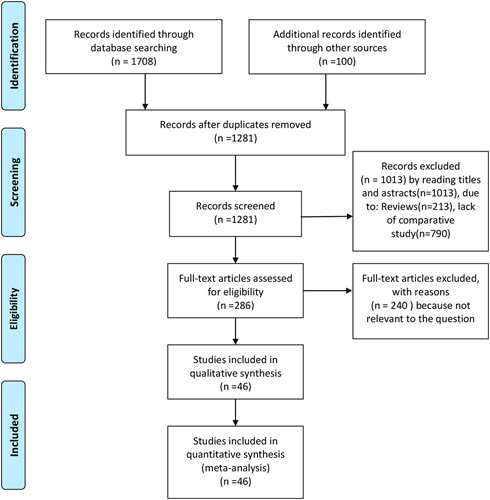
Flow diagram of the published articles evaluated for inclusion in this meta-analysis.
TABLE 1.
Characteristic of Included Studies
| Source | Country | Study Design | Procedure | Sample Size | Age (y) | Sex (F/M) | BMI (kg/m2) | NOS |
|---|---|---|---|---|---|---|---|---|
| Adam et al18 | USA | R | RADP | 61 | 65±14 | 32/29 | NA | 8 |
| LDP | 474 | 64±13 | 255/219 | |||||
| Alfieri et al19 | Italy | R | RADP | 96 | NA | 50/46 | NA | 7 |
| LDP | 85 | 42/43 | ||||||
| Bauman et al55 | USA | R | LDP | 33 | 66±2 | 16/17 | 26.2±0.8 | 7 |
| DPO | 46 | 66±2 | 28/18 | 27.8±0.9 | ||||
| Benizri et al12 | France | R | RADP | 11 | 50±21 | 8/3 | 26±6 | 9 |
| LDP | 23 | 52 ±15 | 13/10 | 27±5 | ||||
| Boggi et al20 | Italy | P | RADP | 11 | 61.8 (50-74) | 5/6 | 24.8 (18.4-35.0) | 8 |
| ODP | 11 | 68.4 (49-78) | 4/7 | 25.0 (17.9-30.8) | ||||
| Braga et al21 | Italy | P | LDP | 100 | 61.4±13.5 | 56/44 | NA | 7 |
| ODP | 100 | 61.0±13.8 | 56/44 | |||||
| Butturini et al22 | Italy | P | RADP | 22 | 54(26-77) | 16/6 | 25 | 8 |
| LDP | 21 | 55(20-71) | 14/7 | 24 | ||||
| Chen et al24 | China | R | RADP | 69 | 56±13 | 46/23 | 25±3 | 9 |
| LDP | 50 | 57±15 | 33/17 | 25±3 | ||||
| Chen et al23 | China | R | LDP | 334 | 60 | 196/138 | 22 | 7 |
| ODP | 48 | 74.5 | 21/27 | 22 | ||||
| Daouadi et al25 | USA | R | RADP | 30 | 59±13 | 21/9 | 28±5 | 7 |
| LDP | 94 | 59±16 | 61/33 | 29±7 | ||||
| Duran et al26 | Spain | R | LDP | 18 | 58±10 | 8/8 | NA | 7 |
| ODP | 13 | 63.8±10.3 | 7/6 | |||||
| RDP | 16 | 61±12 | 7/9 | |||||
| Eckhardt et al27 | Germany | R | RADP | 12 | 49 (29-76) | 9/3 | 23 (20-34) | 8 |
| LDP | 29 | 59 (17-85) | 18/11 | 27 (19-36) | ||||
| Eom et al28 | Korea | R | LDP | 31 | 46.7±16.7 | NA | 22.2±2.2 | 8 |
| ODP | 62 | 47.5±14.9 | 23.0±3.4 | |||||
| Goh et al29 | Singapore | R | RADP | 8 | 57 (21-68) | 6/2 | 28 (22-31) | 9 |
| LDP | 31 | 56 (25-78) | 14/17 | 24 (19-36) | ||||
| Han et al30 | Korea | R | LDP | 42 | 53 (30-75) | 21/21 | 24.64 (18.82-31.53) | 7 |
| ODP | 52 | 54 (36-75) | 18/34 | 23.99 (18.97-31.20) | ||||
| Hong et al31 | Saudi Arabia | R | RADP | 46 | 51.2±13.8 | 32/14 | 24.9±4.1 | 7 |
| LDP | 182 | 60.2±13 | 88/94 | 24.6±3.2 | ||||
| Hu et al32 | China | R | LDP/ODP | 11 | 53.1±13.2 | 4/7 | 23.9±4.2 | 8 |
| 23 | 49.1±9.5 | 10/13 | 25.6±4.0 | |||||
| Huang et al33 | China | R | LDP | 48 | 47.5±17.3 | 22/26 | 23.7±1.9 | 7 |
| ODP | 40 | 51.4±20.3 | 29/11 | 24.1±2.2 | ||||
| Ielpo et al34 | Spain | P | LDP | 26 | 61 (41-79) | 16/10 | 25 (18-32) | 7 |
| RDP | 28 | 60 (35-73) | 15/13 | 24 (19-32) | ||||
| Ito et al61 | Japan | R | LDP | 10 | 42 | NA | NA | 7 |
| RDP | 4 | 52.7 | ||||||
| Jarufe et al56 | Chile | R | LDP | 57 | 49 (13-82) | 44/13 | NA | 7 |
| ODP | 36 | 53 (14-74) | 25/11 | |||||
| Kang et al35 | Korea | R | RADP | 20 | 45±16 | 12/8 | 24±3 | 8 |
| LDP | 25 | 57±14 | 12/13 | 23±3 | ||||
| Khaled et al57 | UK | R | LDP | 22 | 57 (34-78) | 14/8 | 26.5 (21.5-70.2) | 8 |
| ODP | 22 | 59.9 (32-78) | 14/8 | 28.3 (24-36.6) | ||||
| Kooby et al36 | USA | R | LDP | 23 | 64.6±12.3 | 11/12 | 28.5±5.7 | 7 |
| ODP | 189 | 65.9±11.1 | 109/80 | 26.2±6.0 | ||||
| Lai et al58 | China | R | LDP | 18 | 63±18 | 14/4 | 26±3 | 8 |
| RDP | 17 | 61±10 | 6/11 | 24±2 | ||||
| Lee et al37 | USA | R | RADP | 37 | 58±11 | 27/10 | 29 | 8 |
| LDP | 131 | 58±15 | 74/57 | 28 | ||||
| ODP | 637 | 63±13.5 | 351/286 | |||||
| Liu et al13 | China | R | LDP | 102 | 50±15 | 55/47 | NA | 8 |
| RDP | 102 | 48±16 | 68/34 | |||||
| Lyman et al38 | USA | R | RADP | 108 | 53±16.1 | 62/46 | 29.3±6.5 | 8 |
| LDP | 139 | 59.5±15.5 | 6475 | 29±8.5 | ||||
| Marino et al39 | Japan | R | RADP | 35 | 59.3 (40-73) | 15/20 | NA | 7 |
| LDP | 35 | 58.5 (34-69) | 16/19 | |||||
| Matsumoto et al40 | Japan | P | LDP | 32 | 63±14 | 23/9 | 22.5±3.6 | 7 |
| ODP | 35 | 58±17 | 20/15 | 22.9±3.9 | ||||
| Ocuin et al41 | USA | R | ODP | 11 | 63.5±15.0 | 5/6 | 28.1 (24.5-30.3) | 7 |
| RDP | 19 | 62.2±9.6 | 11/8 | 26.0 (23.6-28.4) | ||||
| Qu et al42 | China | R | LDP | 35 | 58±11 | 23/22 | 24±4 | 8 |
| RDP | 35 | 58±11 | 23/22 | 24±3 | ||||
| Raoof et al43 | USA | NCSC | LDP | 563 | NA | 261/302 | NA | 9 |
| ODP | 563 | 259/304 | ||||||
| Raoof et al44 | USA | NCS | RADP | 99 | NA | 54/45 | NA | 8 |
| LDP | 605 | 283/322 | ||||||
| Rehman et al45 | UK | R | LDP | 8 | 64.2 | NA | NA | 8 |
| ODP | 14 | 64 | ||||||
| Rodriguez et al46 | France | R | LDP | 25 | 62.5 (27-83) | 13/12 | 27.3 (20-41) | 7 |
| ODP | 43 | 65 (38-86) | 21/22 | 24.7 (17-34) | ||||
| RDP | 21 | 53 (27-79) | 15/6 | 25 (18-33) | ||||
| Sharpe et al47 | USA | NCS | LDP | 144 | 67.7±10.1 | NA | NA | 7 |
| ODP | 625 | 65.6±10.5 | ||||||
| Shin et al48 | Korea | R | LDP | 70 | 61±7.8 | 23/47 | 24.1±2.1 | 8 |
| ODP | 80 | 65±6 | 32/48 | 23.1±2.2 | ||||
| Soreide et al49 | Norway | NCS | LDP | 327 | 66 (55-72) | 158/169 | NA | 8 |
| ODP | 227 | 66 (55-72) | 126/101 | |||||
| Souche et al50 | France | P | RADP | 23 | 66 (44-83) | 14/9 | 25 (20-34) | 7 |
| LDP | 15 | 57 (34-72) | 12/3 | 23 (19-31) | ||||
| Stauffer et al51 | Italy | R | LDP | 44 | 72±5.8 | 8/26 | 28.3±7.7 | 7 |
| ODP | 28 | 67.3±6.8 | 12/16 | 26.1±4.3 | ||||
| Vijan et al59 | USA | R | LDP | 100 | 59.0±17.3 | 60/40 | 27.4±5.2 | 8 |
| ODP | 100 | 58.6±15.2 | 50/50 | 27.9±5.0 | ||||
| Waters et al52 | USA | R | LDP | 18 | 59 | 9/9 | NA | 7 |
| ODP | 22 | 59 | 12/10 | |||||
| RDP | 17 | 64 | 11/6 | |||||
| Zhang et al54 | China | R | LDP | 17 | 60±7.75 | 6/11 | 23.4±4.7 | 7 |
| ODP | 34 | 64±9 | 15/19 | 23.7±2.4 | ||||
| Zhang et al53 | China | R | LDP | 22 | 55.2±13.1 | 13/9 | 23.9±2.7 | 8 |
| ODP | 76 | 59.8±9.0 | 46/30 | 23.7±3.3 | ||||
| Zhang et al60 | China | R | LDP | 31 | 49±12 | 19/12 | NA | 7 |
| RDP | 43 | 48±11 | 23/20 |
BMI indicates body mass index; LDP, laparoscopic distal pancreatectomy; NA, not available; NCS, nationwide cohort study; ODP, open distal pancreatectomy; P, prospective study; R, retrospective study; RADP, robotic-assisted distal pancreatectomy; RDP, robotic distal pancreatectomy.
Intraoperative Outcomes
The network geometry of the intraoperative outcomes is shown in Figure 2, and the contribution plots are shown in eFigure 1 in the Supplementary Material (Supplemental Digital Content 1, http://links.lww.com/SLE/A252). ODP showed the greatest probability of having the shortest OT (SUCRA, 63.5%), followed by LDP (SUCRA, 63.0%), RADP (SUCRA, 40.4%), and RDP (SUCRA, 33.2%; Table 2). RDP showed the greatest probability of having the least EBL (SUCRA, 90.9%), followed by RADP (SUCRA, 55.8%), LDP (SUCRA, 51.5%), and ODP (SUCRA, 1.9%; Table 2). RADP showed the greatest probability of having the most SPs (SUCRA, 84.1%), followed by RDP (SUCRA, 61.6%), LDP (SUCRA, 41.9%), and ODP (SUCRA, 14.6%; Table 2). The mixed head-to-head comparisons of intraoperative outcomes are shown in eFigure 2 in the Supplementary Material (Supplemental Digital Content 1, http://links.lww.com/SLE/A252).
FIGURE 2.
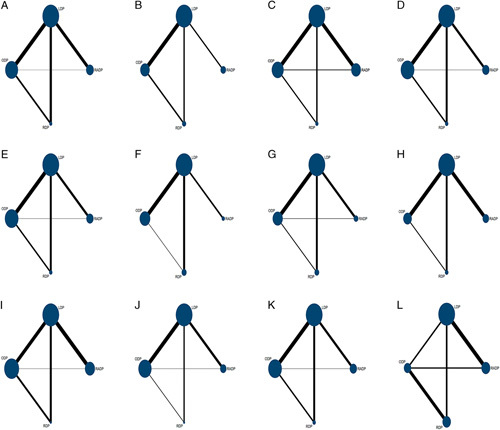
Network geometry of the included studies. A, Operation time (OT). B, Estimated blood loss (EBL). C, Spleen preservation (SP). D, Postoperative pancreatic fistula (POPF). E, Clinically related POPF (CR-POPF). F, Postoperative bleeding (POBL). G, Reoperation. H, Overall complications (OCs). I, Major complications (MCs). J, Mortality. K, R0 resection. L, Number of lymph nodes harvested (LNH). LAP indicates laparoscopic distal pancreatectomy; ODP, open distal pancreatectomy; RADP, robot-assisted laparoscopic pancreatectomy; RDP, robotic distal pancreatectomy.
TABLE 2.
SUCRA Values and Mean Rank of Outcome Measures
| SUCRA (%) | Mean Rank | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Measures | RADP | LDP | ODP | RDP | RADP | LDP | ODP | RDP |
| Intraoperative outcomes | ||||||||
| OT | 40.4 | 63 | 63.5 | 33.2 | 2.8 | 2.1 | 2.1 | 3.0 |
| EBL | 48.7 | 50.4 | 4.7 | 90.6 | 2.5 | 2.5 | 3.9 | 1.1 |
| SP | 84.1 | 41.9 | 14.6 | 59.4 | 1.5 | 2.7 | 3.6 | 2.2 |
| Postoperative outcomes | ||||||||
| POPF | 31.4 | 22.8 | 51.3 | 94.5 | 3.1 | 3.3 | 2.5 | 1.2 |
| CR-POPF | 31.4 | 22.7 | 52.3 | 94.6 | 3.1 | 3.3 | 2.5 | 1.2 |
| POBL | 54.6 | 43.4 | 36.8 | 65.3 | 2.4 | 2.7 | 2.9 | 2.0 |
| Reoperation | 31.8 | 61.3 | 10.5 | 96.4 | 3.0 | 2.2 | 3.7 | 1.1 |
| OC | 45.8 | 44.2 | 23.1 | 86.9 | 2.6 | 2.7 | 3.3 | 1.4 |
| MC | 0.9 | 57 | 42.9 | 99.3 | 4.0 | 2.3 | 2.7 | 1.0 |
| Mortality | 39.2 | 57.8 | 19.6 | 83.4 | 2.8 | 2.3 | 3.4 | 1.5 |
| Oncological outcomes | ||||||||
| R0 | 59.4 | 33.2 | 32 | 75.4 | 2.2 | 3.0 | 3.0 | 1.7 |
| LNH | 58.3 | 23.1 | 54.8 | 63.8 | 2.2 | 3.3 | 2.4 | 2.1 |
CR-POPF indicates clinically related postoperative pancreatic fistula; EBL, estimated blood loss; LDP, laparoscopic distal pancreatectomy; LNH, lymph node harvested; MC, major complication; OC, overall complication; ODP, open distal pancreatectomy; OT, operation time; POBL, postoperative bleeding; POPF, postoperative pancreatic fistula; RADP, robotic-assisted distal pancreatectomy; RDP, robotic distal pancreatectomy; SP, spleen preservation; SUCRA, surface under the cumulative ranking curves.
Postoperative Outcomes
The network geometry of postoperative outcomes is shown in Figure 2, and the contribution plots are shown in eFigure 1 in the Supplementary Material (Supplemental Digital Content 1, http://links.lww.com/SLE/A252). POPF data were provided in 34 studies (4 three-armed studies and 30 two-armed studies); RDP showed the greatest probability of having the lowest incidence of POPF (SUCRA, 94.5%), followed by ODP (SUCRA, 51.3%), RADP (SUCRA, 31.4%), and LDP (SUCRA, 22.8%; Table 2). CR-POPF data were provided in 30 trials; the SUCRA values of RADP, LDP, ODP, and RDP were 31.4%, 22.7%, 52.3%, and 94.6%, respectively (Table 2). POBL data were provided in 17 trials; RDP showed the greatest probability of having the lowest incidence of POBL (SUCRA, 65.3%), followed by RADP (SUCRA, 54.6%), LDP (SUCRA, 43.4%), and ODP (SUCRA, 36.8%; Table 2). Reoperation data were provided in 23 studies; the SUCRA values for RADP, LDP, ODP, and RDP were 31.8%, 61.3%, 10.5%, and 96.4%, respectively (Table 2). OCs were reported in 30 studies (1 three-armed study and 29 two-armed studies); RDP had the greatest probability of having the lowest incidence of OCs (SUCRA, 86.9%), followed by RADP (SUCRA, 45.8%), LDP (SUCRA, 44.2%), and ODP (SUCRA, 23.1%; Table 2). MCs were least likely to occur in RDP (SUCRA, 99.3%), followed by LDP (SUCRA, 57%), ODP (SUCRA, 42.0%), and RADP (SUCRA, 0.9%; Table 2). Mortality data were provided in 35 studies; the SUCRA values for RADP, LDP, ODP, and RDP were 39.2%, 57.8%, 19.6%, and 83.4%, respectively (Table 2). The mixed head-to-head comparisons of intraoperative outcomes are shown in eFigure 2 in the Supplementary Material (Supplemental Digital Content 1, http://links.lww.com/SLE/A252).
Oncological Outcomes
The network geometry of oncological outcomes is shown in Figure 2, and the contribution plots are shown in eFigure 1 in the Supplementary Material (Supplemental Digital Content 1, http://links.lww.com/SLE/A252). The R0 margin data were provided in 28 studies (2 three-armed studies and 26 two-armed studies); RDP tended to be the best procedure for achieving R0 margins (SUCRA, 75.4%), followed by RADP (SUCRA, 59.4%), LDP (SUCRA, 33.2%), and ODP (SUCRA, 32%; Table 2). LNH data were provided in 14 studies (3 three-armed studies and 11 two-armed studies); RDP tended to be the best procedure for LNH (SUCRA, 63.8%), followed by RADP (SUCRA, 58.3%), ODP (SUCRA, 54.8%), and LDP (SUCRA, 23.1%; Table 2). The mixed head-to-head comparisons of intraoperative outcomes are shown in eFigure 1 in the Supplementary Material (Supplemental Digital Content 1, http://links.lww.com/SLE/A252).
Inconsistency, Heterogeneity, and Publication Bias
The inconsistency and heterogeneity for the assessed procedures are shown in Table 3. None of the outcomes had significant local inconsistency within the networks. Heterogeneity was low (τ<0.1) for POPF, CR-POPF, POBL, OC, MC, mortality, and R0 resection. Heterogeneity was high for OT (τ>1), SP (τ=0.52, 0.44), and LNH (τ>1). The comparison-adjusted funnel plots suggested that there was no significant publication bias.
TABLE 3.
Loop Inconsistency and Heterogeneity
| Inconsistency | |||
|---|---|---|---|
| Outcome Measures | Loop | RoR (95% CI) | Heterogeneity, τ |
| Intraoperative outcomes | |||
| OT | B-C-D | 25.23 (1.00-82.24) | >1 |
| A-B-C | 4.95 (1.00-31.92) | >1 | |
| EBL | B-C-D | 4.65 (1.00-42.35) | >1 |
| SP | B-C-D | 2.36 (1.00-111.88) | 0.51 |
| A-B-C | 12.78 (1.00-70.56) | 0.40 | |
| Postoperative outcomes | |||
| POPF | A-B-C | 1.82 (1.00-6.43) | <0.1 |
| B-C-D | 1.17 (1.00-2.90) | <0.1 | |
| CR-POPF | A-B-C | 1.823 (1.00-6.43) | <0.1 |
| B-C-D | 1.175 (1.00-2.90) | <0.1 | |
| POBL | B-C-D | 1.179 (1.00-19.86) | <0.1 |
| OC | B-C-D | 1.645 (1.00-5.86) | <0.1 |
| MC | A-B-C | 1.92 (1.00-4.96) | <0.1 |
| OC | B-C-D | 1.35 (1.00-17.96) | <0.1 |
| Mortality | B-C-D | 3.22 (1.00-37.99) | <0.1 |
| A-B-C | 2.163 (1.00-50.30) | <0.1 | |
| R0 | A-B-C | 1.82 (1.00-6.43) | <0.1 |
| B-C-D | 1.17 (1.00-2.90) | <0.1 | |
| Oncological outcomes | |||
| LNH | B-C-D | 20.52 (1.00-125.19) | >1 |
| A-B-C | 11.73 (1.00-45.28) | >1 | |
A indicates robotic-assisted distal pancreatectomy; B, laparoscopic distal pancreatectomy; C, open distal pancreatectomy; CI, confidence interval; CR-POPF, clinically related postoperative pancreatic fistula; D, robotic distal pancreatectomy; EBL, estimated blood loss; IT, intraoperative transfusion; LNH, lymph node harvested; MC, major complication; OC, overall complication; OT, operation time; POBL, postoperative bleeding; POPF, postoperative pancreatic fistula; RoR, logarithm of the ratio of 2 odds ratios; SSI, surgical site infection.
DISCUSSION
Several studies, including meta-analyses, have compared MIDP with ODP. The 4 DP approaches are ODP, LDP, RADP, and RDP. We believe that the present study is the first network meta-analysis to compare the efficacy and safety of these 4 approaches. The present systematic review and network meta-analysis of 46 studies with 8377 patients showed that RDP was comparable with both other MIDPs and ODP in terms of intraoperative and postoperative outcomes. Regarding the oncological outcomes, RDP showed the greatest probability of achieving R0 resection and optimal LNH. However, the present findings require confirmation in further studies, especially head-to-head RCTs and long-term oncological studies.
EBL was one of the factors evaluated to assess the efficacy of the various DP approaches. Our study showed that RDP had the greatest probability of having the least EBL, followed by RADP and LDP. Most other studies reported that LDP is associated with less EBL than ODP.6,62 However, LDP has limitations, such as 2-dimensional imaging and only a limited area for manipulation. Theoretically, the 3-dimensional (3D) visualization and bionic structure of the robotic surgical field could improve this complex dissection. Compared with traditional LDP, there is less EBL during robotic surgery comprising RADP or RDP. The present head-to-head meta-analysis showed that RDP was associated with less EBL than LDP or RADP.
SP was another factor used to evaluate the efficacy of the various DP methods. SP plays an important role in the human immune system.63 MIDP reportedly increases SP compared with ODP. A recent meta-analysis showed that RADP and LDP are associated with a higher SP rate than ODP, although the SP rate does not significantly differ between RADP and LDP.10 In the present study, RADP and RDP had higher SP rates than either LDP or ODP. The higher SP rate in robotic surgery maybe because of the 3D magnified surgical field. The mixed head-to-head analysis showed that the SP rate did not significantly differ between RADP and RDP. Surgeons prefer to perform MIDP for less invasive, small tumors, and SP is physiologically desirable in patients with benign or low-grade malignancy. However, there is still no consensus on whether the spleen should be preserved. The numbers of patients in the RDP groups were small, and the indication for SP in the presence of various diseases remains controversial. Furthermore, SP is influenced by many factors, including tumor size and surgical expertise.
The incidence of postoperative OCs did not significantly differ between the 4 DP approaches, although RDP tended to have the lowest OC rate, followed by RADP, LDP, and ODP. This finding is consistent with most previous studies.6,7 RDP was associated with fewer MCs than RADP or LDP. Niu et al64 reported that the MC rate does not significantly differ between RADP and LDP. To date, few studies have compared RADP with RDP. In addition, the definition of complications was variable in the included studies, which may have introduced bias. The complication rate of the various DP approaches requires further investigation in head-to-head and scaled RCTs.
POPF is the most common postoperative complication associated with DP. The present study showed that RDP seems to be the best approach for avoiding POPF and CR-POPF, although the subject remains controversial. Alfieri et al19 showed that the incidences of POPF and CR-POPF do not significantly differ between RADP and LDP. Similar results have been reported regarding the minimally invasive treatment of neuroendocrine tumors.30 However, the texture of the pancreatic remnant and the type of pancreatic remnant closure may influence the development of a POPF or CR-POPF. Previous studies have shown that the risk of POPF formation is increased when the pancreas has a soft texture. However, the texture of the pancreas is a subjective judgment, and there is currently no unified objective standard. The included studies did not report adequate details about the texture of the pancreas. More research is needed to determine whether robotic surgery reduces the incidence of POPF.
Regarding the oncological outcomes, our study showed that RDP fared well compared with other procedures. Several previous studies, including a meta-analysis, have suggested that MIDP produces oncological results comparable with those achieved with ODP.65,66 MIDP commonly includes RADP, LDP, and RDP. Our study compared these 3 approaches regarding both the R0 status and LNH. A propensity score-matched study involving multiple European centers showed that MIDP is associated with a higher R0 resection rate and a lower number of LNH.65 However, the required standard of lymphadenectomy in DP is debatable. Although Slidell et al67 demonstrated that at least 12 lymph nodes need removal in DP, most of the studies included in the present analysis reported that the mean number of LNH was >12. Unlike previous reports, our study first showed that RDP may be the best approach in terms of oncological outcomes, although these results must be interpreted with caution because the oncological outcomes may be influenced by several factors. The judgment of an R0 pathologic/clinical outcome depends on the experience of the pathologist and the criteria for R0. In addition, the number of LNH may be related to the nature of the tumor and the scope of the surgery. The International Study Group of Pancreatic Fistula suggests that standard lymph node dissection range and numbers are necessary for tumors located in the tail of the pancreas.68 Theoretically, however, the 3D visual surgical field in RDP may improve the accuracy of judging R0 margins and LNH.
There was high heterogeneity between included studies regarding OT, SP, and LNH. The variation in OT among studies may be related to the method used to calculate the OT, the experience of the surgeon, and the degree of tumor extension. The SP and LNH depend on the surgeon’s experience and the tumor characteristics, among other factors. Furthermore, the sample sizes of the included studies were relatively small, which may contribute to the heterogeneity.
The present study has some limitations. First, the sample sizes of some of the included studies were small and some of the studies were retrospective. Second, the definitions of included outcomes (such as POPF or CR-POPF) varied between studies. Third, although the outcome of surgery may be affected by many factors, such as the texture of the pancreas and the patient’s body mass index, these data were not fully provided in the included studies. Finally, there were few head-to-head and RCT studies.
Despite these limitations, the present study showed that RDP seems to offer clinical and oncological advantages compared with other DP methods for addressing diseases of the pancreatic body and tail, although it may require a longer OT and learning curve. These results require confirmation in a future head-to-head RCT.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.surgical-laparoscopy.com.
ACKNOWLEDGMENTS
The authors thank Kelly Zammit, BVSc, and Nancy Schatken BS, MT(ASCP), from Liwen Bianji, Edanz Group China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
The author declares no conflicts of interest.
REFERENCES
- 1.Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810–819. [DOI] [PubMed] [Google Scholar]
- 2.Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;26:2397–2402. [DOI] [PubMed] [Google Scholar]
- 3.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646–1657. [DOI] [PubMed] [Google Scholar]
- 4.Zureikat AH, Postlewait LM, Liu Y, et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. 2016;264:640–649. [DOI] [PubMed] [Google Scholar]
- 5.Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami International Evidence-based Guidelines on minimally invasive pancreas resection. Ann Surg. 2020;271:1–14. [DOI] [PubMed] [Google Scholar]
- 6.de Rooij T, van Hilst J, van Santvoort H, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 2019;269:2–9. [DOI] [PubMed] [Google Scholar]
- 7.Nigri GR, Rosman AS, Petrucciani N, et al. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc. 2011;25:1642–1651. [DOI] [PubMed] [Google Scholar]
- 8.Kamarajah SK, Sutandi N, Robinson SR, et al. Robotic versus conventional laparoscopic distal pancreatic resection: a systematic review and meta-analysis. HPB (Oxford). 2019;21:1107–1118. [DOI] [PubMed] [Google Scholar]
- 9.Joechle K, Conrad C. Cost-effectiveness of minimally invasive pancreatic resection. J Hepatobiliary Pancreat Sci. 2018;25:291–298. [DOI] [PubMed] [Google Scholar]
- 10.Niu X, Yu B, Yao L, et al. Comparison of surgical outcomes of robot-assisted laparoscopic distal pancreatectomy versus laparoscopic and open resections: a systematic review and meta-analysis. Asian J Surg. 2019;42:32–45. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J, Green SE. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011:S38. [Google Scholar]
- 12.Benizri EI, Germain A, Ayav A, et al. Short-term perioperative outcomes after robot-assisted and laparoscopic distal pancreatectomy. J Robot Surg. 2014;8:125–132. [DOI] [PubMed] [Google Scholar]
- 13.Liu R, Liu Q, Zhao ZM, et al. Robotic versus laparoscopic distal pancreatectomy: a propensity score-matched study. J Surg Oncol. 2017;116:461–469. [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 15.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 16.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. [DOI] [PubMed] [Google Scholar]
- 18.Adam MA, Choudhury K, Goffredo P, et al. Minimally Invasive distal pancreatectomy for cancer: short-term oncologic outcomes in 1,733 patients. World J Surg. 2015;39:2564–2572. [DOI] [PubMed] [Google Scholar]
- 19.Alfieri S, Butturini G, Boggi U, et al. Short-term and long-term outcomes after robot-assisted versus laparoscopic distal pancreatectomy for pancreatic neuroendocrine tumors (pNETs): a multicenter comparative study. Langenbeck Arch Surg. 2019;404:459–468. [DOI] [PubMed] [Google Scholar]
- 20.Boggi U, Palladino S, Massimetti G, et al. Laparoscopic robot-assisted versus open total pancreatectomy: a case-matched study. Surg Endosc. 2015;29:1425–1432. [DOI] [PubMed] [Google Scholar]
- 21.Braga M, Pecorelli N, Ferrari D, et al. Results of 100 consecutive laparoscopic distal pancreatectomies: postoperative outcome, cost-benefit analysis, and quality of life assessment. Surg Endosc. 2015;29:1871–1878. [DOI] [PubMed] [Google Scholar]
- 22.Butturini G, Damoli I, Crepaz L, et al. A prospective non-randomised single-center study comparing laparoscopic and robotic distal pancreatectomy. Surg Endosc. 2015;29:3163–3170. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Pan Y, Mou YP, et al. Surgical outcomes of laparoscopic distal pancreatectomy in elderly and octogenarian patients: a single-center, comparative study. Surg Endosc. 2019;33:2142–2151. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc. 2015;29:3507–3518. [DOI] [PubMed] [Google Scholar]
- 25.Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–132. [DOI] [PubMed] [Google Scholar]
- 26.Duran H, Ielpo B, Caruso R, et al. Does robotic distal pancreatectomy surgery offer similar results as laparoscopic and open approach? A comparative study from a single medical center. Int J Med Robot. 2014;10:280–285. [DOI] [PubMed] [Google Scholar]
- 27.Eckhardt S, Schicker C, Maurer E, et al. Robotic-assisted approach improves vessel preservation in spleen-preserving distal pancreatectomy. Dig Surg. 2016;33:406–413. [DOI] [PubMed] [Google Scholar]
- 28.Eom BW, Jang JY, Lee SE, et al. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surg Endosc. 2008;22:1334–1338. [DOI] [PubMed] [Google Scholar]
- 29.Goh BKP, Chan CY, Soh H-L, et al. A comparison between robotic-assisted laparoscopic distal pancreatectomy versus laparoscopic distal pancreatectomy. Int J Med Robot. 2017;13:115–121. [DOI] [PubMed] [Google Scholar]
- 30.Han SH, Han IW, Heo JS, et al. Laparoscopic versus open distal pancreatectomy for nonfunctioning pancreatic neuroendocrine tumors: a large single-center study. Surg Endosc. 2018;32:443–449. [DOI] [PubMed] [Google Scholar]
- 31.Hong S, Song KB, Madkhali AA, et al. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: a single surgeon's experience of 228 consecutive cases. Surg Endosc. 2019;6:2465–2473. [DOI] [PubMed] [Google Scholar]
- 32.Hu M, Zhao G, Wang F, et al. Laparoscopic versus open distal splenopancreatectomy for the treatment of pancreatic body and tail cancer: a retrospective, mid-term follow-up study at a single academic tertiary care institution. Surg Endosc. 2014;28:2584–2591. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Yadav DK, Xiong C, et al. Laparoscopic Spleen-Preserving Distal Pancreatectomy (LSPDP) versus Open Spleen-Preserving Distal Pancreatectomy (OSPDP): a comparative study. Can J Gastroenterol Hepatol. 2019;2019:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ielpo B, Duran H, Diaz E, et al. Robotic versus laparoscopic distal pancreatectomy: a comparative study of clinical outcomes and costs analysis. Int J Surg (London, England). 2017;48:300–304. [DOI] [PubMed] [Google Scholar]
- 35.Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc. 2011;25:2004–2009. [DOI] [PubMed] [Google Scholar]
- 36.Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010;210:779–785. [DOI] [PubMed] [Google Scholar]
- 37.Lee SY, Allen PJ, Sadot E, et al. Distal pancreatectomy: a single institution's experience in open, laparoscopic, and robotic approaches. J Am Coll Surg. 2015;220:18–27. [DOI] [PubMed] [Google Scholar]
- 38.Lyman WB, Passeri M, Sastry A, et al. Robotic-assisted versus laparoscopic left pancreatectomy at a high-volume, minimally invasive center. Surg Endosc. 2019;33:2991–3000. [DOI] [PubMed] [Google Scholar]
- 39.Marino MV, Mirabella A, Gomez Ruiz M, et al. Robotic-assisted versus laparoscopic distal pancreatectomy: the results of a case-matched analysis from a tertiary care center. Dig Surg. 2019;17:1–11. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto I, Kamei K, Satoi S, et al. Laparoscopic versus open distal pancreatectomy for benign and low-grade malignant lesions of the pancreas: a single-center comparative study. Surg Today. 2019;49:394–400. [DOI] [PubMed] [Google Scholar]
- 41.Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford). 2016;18:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu L, Zhiming Z, Xianglong T, et al. Short- and mid-term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: a retrospective propensity score-matched study. Int J Surg. 2018;55:81–86. [DOI] [PubMed] [Google Scholar]
- 43.Raoof M, Ituarte PHG, Woo Y, et al. Propensity score-matched comparison of oncological outcomes between laparoscopic and open distal pancreatic resection. Br J Surg. 2018;105:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raoof M, Nota CLMA, Melstrom LG, et al. Oncologic outcomes after robot-assisted versus laparoscopic distal pancreatectomy: analysis of the National Cancer Database. J Surg Oncol. 2018;118:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman S, John SK, Lochan R, et al. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single-institution comparative study. World J Surg. 2014;38:476–483. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez M, Memeo R, Leon P, et al. Which method of distal pancreatectomy is cost-effective among open, laparoscopic, or robotic surgery? Hepatobiliary Surg Nutr. 2018;7:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharpe SM, Talamonti MS, Wang E, et al. The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. Am J Surg. 2015;209:557–563. [DOI] [PubMed] [Google Scholar]
- 48.Shin SH, Kim SC, Song KB, et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg. 2015;220:177–185. [DOI] [PubMed] [Google Scholar]
- 49.Soreide K, Olsen F, Nymo LS, et al. A nationwide cohort study of resection rates and short-term outcomes in open and laparoscopic distal pancreatectomy. HPB (Oxford). 2019;21:669–678. [DOI] [PubMed] [Google Scholar]
- 50.Souche R, Herrero A, Bourel G, et al. Robotic versus laparoscopic distal pancreatectomy: a French prospective single-center experience and cost-effectiveness analysis. Surgical Endoscopy. 2018;32:3562–3569. [DOI] [PubMed] [Google Scholar]
- 51.Stauffer JA, Coppola A, Mody K, et al. Laparoscopic versus open distal pancreatectomy for pancreatic adenocarcinoma. World J Surg. 2016;40:1477–1484. [DOI] [PubMed] [Google Scholar]
- 52.Waters JA, Canal DF, Wiebke EA, et al. Robotic distal pancreatectomy: cost effective? Surgery. 2010;148:814–823. [DOI] [PubMed] [Google Scholar]
- 53.Zhang AB, Wang Y, Hu C, et al. Laparoscopic versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a single-center experience. J Zhejiang Univ Sci B. 2017;18:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang M, Fang R, Mou Y, et al. LDP vs ODP for pancreatic adenocarcinoma: a case matched study from a single-institution. BMC Gastroenterol. 2015;15:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauman MD, Becerra DG, Kilbane EM, et al. Laparoscopic distal pancreatectomy for pancreatic cancer is safe and effective. Surg Endosc. 2018;32:53–61. [DOI] [PubMed] [Google Scholar]
- 56.Jarufe N, Soto P, Ahumada V, et al. Laparoscopic Versus Open Distal Pancreatectomy: Comparative Analysis of Clinical Outcomes at a Single Institution. Surg Laparosc Endosc Percutan Tech. 2018;28:62–66. [DOI] [PubMed] [Google Scholar]
- 57.Khaled YS, Malde DJ, Packer J, et al. A case-matched comparative study of laparoscopic versus open distal pancreatectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:363–367. [DOI] [PubMed] [Google Scholar]
- 58.Lai EC, Tang CN. Robotic distal pancreatectomy versus conventional laparoscopic distal pancreatectomy: a comparative study for short-term outcomes. Front Med. 2015;9:356–360. [DOI] [PubMed] [Google Scholar]
- 59.Vijan SS, Ahmed KA, Harmsen WS, et al. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg. 2010;145:616–621. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Jin J, Chen S, et al. Minimally invasive distal pancreatectomy for PNETs: laparoscopic or robotic approach? Oncotarget. 2017;8:33872–33883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito M, Asano Y, Shimizu T, et al. Comparison of standard laparoscopic distal pancreatectomy with minimally invasive distal pancreatectomy using the da Vinci S system. Hepatogastroenterology. 2014;61:493–496. [PubMed] [Google Scholar]
- 62.Gavriilidis P, Roberts KJ, Sutcliffe RP. Laparoscopic versus open distal pancreatectomy for pancreatic adenocarcinoma: a systematic review and meta-analysis. Acta Chirurgica Belgica. 2018;118:278–286. [DOI] [PubMed] [Google Scholar]
- 63.Koukoutsis I, Tamijmarane A, Bellagamba R, et al. The impact of splenectomy on outcomes after distal and total pancreatectomy. World J Surg Oncol. 2007;5:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niu X, Song X, Su A, et al. Low-pressure capnoperitoneum reduces stress responses during pediatric laparoscopic high ligation of indirect inguinal hernia sac: a randomized controlled study. Medicine (Baltimore). 2017;96:e6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Hilst J, de Rooij T, Klompmaker S, et al. Minimally Invasive versus Open Distal Pancreatectomy for Ductal Adenocarcinoma (DIPLOMA): a Pan-European Propensity Score Matched Study. Ann Surg. 2019;269:10–17. [DOI] [PubMed] [Google Scholar]
- 66.Yang D-J, Xiong J-J, Lu H-M, et al. The oncological safety in minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Sci Rep. 2019;9:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. [DOI] [PubMed] [Google Scholar]
- 68.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.surgical-laparoscopy.com.


