BACKGROUND:
Anastomotic leakage might be directly or indirectly related to the prognosis of patients with rectal cancer.
OBJECTIVE:
This study aimed to investigate whether anastomotic leakage affects the oncologic outcomes in patients with rectal cancer.
DESIGN:
This was a retrospective analysis of prospectively collected data.
SETTINGS:
This study was conducted at a teaching hospital between January 2009 and December 2013.
PATIENTS:
Patients who underwent curative resection for primary rectal cancer were included.
MAIN OUTCOME AND MEASURE:
Kaplan–Meier analyses were used to evaluate disease-free survival and overall survival.
RESULTS:
The overall incidence of anastomotic leakage was 2.7% (107/3865). Local recurrence was more frequent in patients with anastomotic leakage than in those without (14.0% vs 6.7%; p = 0.007). By multivariate analysis, anastomotic leakage was associated with increased local recurrence rate (p = 0.014) and poorer overall survival (p = 0.011). In subgroup analysis, compared with other pathologic risk factors, anastomotic leakage was associated with higher occurrence of local and distant recurrence in patients with stage II rectal cancer (p = 0.031 and <0.001). In patients with stage III rectal cancers, adjuvant therapy was more likely to be delayed or canceled in those experiencing anastomotic leakage (63 vs 39 d, p < 0.001; 37.3% vs 66.7%, p < 0.001). In addition, this patient group had the worst survival outcome when compared with those without anastomotic leakage and those with timely adjuvant therapy (5-year disease-free survival rate, p = 0.013; 5-year overall survival rate, p = 0.001).
LIMITATIONS:
This study is limited by its retrospective nature.
CONCLUSIONS:
There was a robust association between anastomotic leakage and local recurrence, while also potentially affect long-term survival of the patient group. Delayed or cancelled adjuvant therapy administration because of anastomotic leakage may partly account for the poorer survival in those patients with advanced rectal cancer. See Video Abstract at http://links.lww.com/DCR/B459.
EFECTOS DE OBSERVANCIA DE TERAPIA ADYUVANTE Y FUGA ANASTOMÓTICA, EN RESULTADOS ONCOLÓGICOS DE PACIENTES CON CÁNCER RECTAL, DESPUÉS DE UNA RESECCIÓN CURATIVA
ANTECEDENTES:
La fuga anastomótica podría estar relacionada directa o indirectamente, con el pronóstico de los pacientes con cáncer de recto.
OBJETIVO:
El estudio tuvo como objetivo investigar si la fuga anastomótica afecta los resultados oncológicos, en pacientes con cáncer de recto.
DISEÑO:
Fue un análisis retrospectivo de datos recolectados prospectivamente.
AJUSTE:
El estudio se realizó en un hospital universitario entre enero de 2009 y diciembre de 2013.
PACIENTES:
Pacientes sometidos a resección curativa por cáncer rectal primario.
PRINCIPALES MEDIDAS DE RESULTADO:
Se utilizaron análisis de Kaplan-Meier para evaluar la supervivencia libre de enfermedad y supervivencia general.
RESULTADOS:
La incidencia global de fuga anastomótica fue del 2,7% (107/3865). La recurrencia local fue más frecuente en pacientes con fuga anastomótica, que en aquellos sin ella (14,0% frente a 6,7%, p = 0,007). Por análisis multivariado, la fuga anastomótica se asoció con una mayor tasa de recurrencia local (p = 0,014) y una peor supervivencia general (p = 0,011). En el análisis de subgrupos, en comparación con otros factores de riesgo patológicos, la fuga anastomótica se asoció con una mayor incidencia de recidiva local y a distancia en pacientes con cáncer rectal en estadio II (p = 0,031 y <0,001, respectivamente). En pacientes con cáncer rectal estadio III, la terapia adyuvante tuvo más probabilidades de retrasarse o cancelarse en aquellos que sufrían fuga anastomótica (63 vs 39 días, p <0,001; 37,3% vs 66,7%, p <0,001). Y este grupo de pacientes tuvo el peor resultado de supervivencia en comparación con aquellos sin fuga anastomótica y aquellos con terapia adyuvante oportuna (tasa de supervivencia libre de enfermedad a 5 años, p = 0,013; tasa de supervivencia global a 5 años, p = 0,001).
LIMITACIONES:
El estudio está limitado por su naturaleza retrospectiva.
CONCLUSIONES:
Hubo una sólida asociación entre la fuga anastomótica y la recurrencia local, mientras que también afecta potencialmente la supervivencia a largo plazo, del grupo de pacientes. La administración de terapia adyuvante retrasada o cancelada debido a una fuga anastomótica, puede explicar en parte, la menor supervivencia en aquellos pacientes con cáncer rectal avanzado. Consulte Video Resumen en http://links.lww.com/DCR/B459.
Keywords: Adjuvant therapy, Anastomotic leak, Local recurrence, Oncological outcomes, Rectal cancer
Rectal cancer (RC) is one of the most commonly diagnosed cancers and causes of cancer-related death worldwide.1–3 With the introduction and dissemination of screening tests, the identification of risk factors, and improvements in treatment regimens, the survival outcomes of patients with RC have been greatly improved over the past few decades.4–6 However, curative surgery is still the key to ensure long-term survival. Postoperative complications, especially anastomotic leakage (AL), could have a significant impact on surgical outcomes and short-term survival. The prognostic effects of AL on long-term survival (LTS) has been reported, however, the effects of AL on oncologic outcomes remain unclear.7,8
As reported in the literature, the incidence of AL varies between 2% and 12% because of the regional economic level, type of surgical procedure, surgical priority, and experience of the surgical team.8–10 Recent studies have suggested that the occurrence of AL may reduce both disease-free survival (DFS) and overall survival (OS) in patients with RC,7,11–13 although this is also influenced by disease recurrence, comorbidities, and other factors.11,14,15 However, it remains unclear whether poor clinical outcomes are influenced solely by the occurrence of AL or by adjusted postoperative adjuvant therapy (AT) that follows, especially in those patients with advanced-stage diseases.
The aim of this single-institution retrospective study was to evaluate the impact of AL on the oncologic outcome among patients with RC in relatively undeveloped western China after curative surgery. In addition, subgroup analysis was conducted to investigate the prognostic influence of AL in patients with stage II diseases and the influence of AL on AT administration in patients with stage III diseases. Additional analysis was conducted to determine the effect of AL on the choice of postoperative AT.
PATIENTS AND METHODS
Patients
The cohort of this single-institutional retrospective study included 3865 consecutive patients with RC in our department who received curative surgery from January 2009 to December 2013. All clinicopathologic and survival information were collected to estimate the associations between AL and LTS. The primary and secondary outcomes were disease recurrence and OS. The relevant institutional ethics committees approved this study.
All of the patients with RC were diagnosed as adenocarcinoma by pathologic biopsy from endoscopy. Patients received abdominal and chest enhanced contrast CT and MRI of pelvis to estimate the clinical disease stage and to make additional treatment decisions. Patients with tumor under clinical T3N+ stage, those with resectable tumor above T3N+ stage but refused neoadjuvant therapy (neoAT), and those finished neoAT received the curative rectal resection with primary anastomosis following the standard of total mesorectal excision. The decision about a protective stoma was decided by surgeon according to clinical consideration during surgery. The curative surgery required an R0 resection margin with a standard of at least 1.5-cm distal margin from tumor through microscopic evaluation, and the result was confirmed by at least 2 pathologists.
The pathologic risk factors were defined as poor differentiated histology, perineural invasion, localized perforation, lymphovascular invasion, bowel obstruction, <12 retrieved lymph nodes, and a positive margin according to the National Comprehensive Cancer Network guidelines for RC. As well, according to Charlson comorbidity index scores, patients with severe or very severe comorbidities were defined as the sicker group, whereas those with normal or moderate comorbidities were defined as control subjects.
In this study, the emergency group was defined as the type of admission, that is, compared with elective admission. The emergency group included the following: 1) patients with complete bowel obstruction receiving diverting stoma, followed by curative surgery with or without neoAT; 2) patients with incomplete obstruction who received surgery after conservative treatment with or without stent canalization; 3) those with bleeding tumor needing medical treatment or emergent surgery; and 4) those with perforation receiving diverting stoma, followed by curative surgery with or without neoAT.
All of the patients were suggested for postoperative treatment and scheduled for periodic follow-ups as recommend by National Comprehensive Cancer Network guidelines.16 The diagnosis of AL was mainly based on clinical symptoms and confirmed by contrast enema or CT. Disease recurrence was defined as systemic or local (hepatic, pulmonary, other organ, or multiorgan) on the basis of clinical, radiologic, and/or endoscopic findings no earlier than 120 days after the initial curative resection. Patients who died in the 120 days after the initial surgery were excluded from the LTS analyses.
Statistical Analysis
Continuous variables are presented as the mean ± SD. Explorative comparisons of groups used the t test for normally distributed data and the Kruskal–Wallis test or the Mann–Whitney U test for nonnormally distributed data. The Pearson χ2 test was used to perform the distribution of nominal- or ordinal-scaled variables. The Kaplan–Meier method and the log-rank (Mantel–Cox) test were used to investigate the time-dependent survival probabilities to compare different subgroups of patients with RC. DFS and OS were used as the primary and secondary outcomes. Univariate and multivariate Cox regression analyses were used to estimate the influence of AL on disease recurrence, DFS, OS, and AT administration. HRs with 95% CIs at a value of >1 indicated an increased likelihood of disease recurrence, death, and AT administration. All of the statistical tests were 2-sided, and a p value of <0.05 was considered statistically significant. All of the statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp, Armonk, NY).
RESULTS
Patient Cohort
The medical charts of 3865 consecutive patients with RC who underwent curative resection at our department between January 2009 and December 2013 were retrospectively reviewed. The overall incidence of AL was 2.7% (107/3865). The median follow-up period was 60 months (interquartile range (IQR), 40–96 mo). The clinicopathologic data of the study cohort are shown in Table 1.
TABLE 1.
Patient characteristics
| Variables | AL, n (%) | No AL, n (%) |
|---|---|---|
| N = 3865 | 107 (2.8) | 3758 (97.2) |
| Sex | ||
| Women | 43 (40.2) | 1476 (39.3) |
| Men | 64 (59.8) | 2281 (60.7) |
| Age group, y | ||
| ≤60 | 56 (52.3) | 1791 (47.7) |
| 60–70 | 35 (32.7) | 1040 (27.7) |
| 70–80 | 13 (12.2) | 638 (17.0) |
| >80 | 3 (2.8) | 289 (7.6) |
| Comorbiditya | ||
| Normal | 67 (62.6) | 2442 (65.0) |
| Moderate | 16 (15.0) | 601 (16.0) |
| Severe | 12 (11.2) | 411 (10.9) |
| Very severe | 12 (11.2) | 225 (5.9) |
| Missing | 0 | 79 (2.2) |
| Tumor stage | ||
| I | 13 (12.1) | 793 (21.1) |
| II | 17 (15.8) | 1254 (33.4) |
| III | 77 (72.0) | 1711 (45.6) |
| Tumor location | ||
| Low rectum | 71 (66.4) | 2253 (60.0) |
| High rectum | 36 (33.6) | 1505 (40.0) |
| Surgical procedure | ||
| Open | 95 (88.8) | 3137 (83.5) |
| Laparoscopy | 12 (11.2) | 621 (16.5) |
| Types of admission | ||
| Elective | 62 (57.9) | 3007 (80.0) |
| Emergency | 45 (42.1) | 751 (20.0) |
| Neoadjuvant therapy | ||
| Yes | 8 (7.5) | 149 (4.0) |
| None | 99 (92.5) | 3609 (96.0) |
| Protective stoma | ||
| Yes | 10 (9.3) | 391 (10.4) |
| None | 97 (90.7) | 3367 (89.6) |
| Organ resection | ||
| None | 87 (81.3) | 3538 (94.1) |
| Other organ | 20 (18.7) | 220 (5.9) |
| Recurrence | ||
| No | 54 (50.5) | 2494 (66.3) |
| Local | 15 (14.0) | 251 (6.7) |
| Distant | 38 (35.5) | 874 (23.3) |
| Missing | 0 | 139 (3.7) |
| Survival status | ||
| Alive | 58 (54.2) | 2612 (69.5) |
| Death | 49 (45.8) | 1060 (28.2) |
| Missing | 0 | 86 (2.3) |
AL = anastomotic leakage.
aComorbidity according to Charlson comorbidity index scores: 0 (normal), 1 (moderate), 2 (severe), and ≥3 (very severe).
AL Is Associated With an Increased Local Recurrence Rate and Could Be Considered a Prognostic Factor for OS
In total, local and distant recurrence were observed in 266 (6.9%) and 912 patients (23.6%). The latter group included 112 patients (2.9%) with both local and distant recurrence. Patients with AL had higher rates of both local and distant recurrence than those without (local: 14.0% vs 6.7%, distant: 35.5% vs 23.3%). The median time to diagnose cancer recurrence in patients with and without AL was 15 (IQR, 6–41 mo) and 20 months (IQR, 5–51 mo).
There was a significant association between the increase in 5-year local recurrence rate and the occurrence of AL by both univariate (p = 0.007; Fig. 1A) and multivariate analyses (HR = 0.501 (95% CI, 0.289–0.870); p = 0.014). In addition, tumor stage, organ resection, and surgical procedure were also significantly associated with the increase in 5-year local recurrence rate by multivariate analysis (Table 2 and Fig. 1A). In contrast, AL was not associated with the increase in 5-year distant recurrence rate (p = 0.143; Fig. 1B). Covariates with a statistically significant influence on 5-year distant recurrence rate were comorbidity, age, tumor stage, and surgical procedure (Table 2).
FIGURE 1.

Kaplan–Meier plots illustrating the association between AL and the rates of local (A) and distant (B) recurrence, DFS (C), and OS (D) at 120 days after curative rectal cancer surgery. AL = anastomotic leakage; DSF = disease-free survival; OS = overall survival.
TABLE 2.
Multivariable Cox regression analyses of long-term outcomes
| Variables | Local recurrence | Distant recurrence | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| AL | 0.014 | 0.081 | 0.011 | ||||||
| No | 1.00 | 1.00 | 1.00 | ||||||
| Yes | 0.501 | 0.289–0.870 | 0.726 | 0.507–1.040 | 0.693 | 0.507–0.929 | |||
| Sex | 0.619 | 0.302 | 0.849 | ||||||
| Women | 1.00 | 1.00 | 1.00 | ||||||
| Men | 0.939 | 0.731–1.205 | 1.076 | 0.936–1.238 | 0.913 | 0.617–1.372 | |||
| Age, y | 0.522 | 0.041 | <0.001 | ||||||
| ≤60 | 1.00 | 1.00 | 1.00 | ||||||
| 60–70 | 1.672 | 0.776–3.604 | 0.190 | 1.012 | 0.699–1.464 | 0.951 | 0.597 | 0.459–0.776 | <0.001 |
| 70–80 | 1.788 | 0.820–3.898 | 0.144 | 1.161 | 0.798–1.691 | 0.435 | 0.691 | 0.528–0.905 | 0.007 |
| >80 | 1.597 | 0.716–3.565 | 0.253 | 1.301 | 0.886–1.911 | 0.180 | 0.860 | 0.651–1.135 | 0.287 |
| Comorbiditya | 0.108 | 0.006 | <0.001 | ||||||
| Normal | 1.00 | 1.00 | 1.00 | ||||||
| Moderate | 1.188 | 0.912–1.396 | 0.061 | 1.041 | 0.812–1.219 | 0.654 | 1.378 | 1.201–1.547 | <0.001 |
| Severe | 1.142 | 0.907–1.519 | 0.254 | 1.208 | 1.000–1.392 | 0.051 | 1.691 | 1.402–1.895 | <0.001 |
| Very severe | 0.834 | 0.514–1.177 | 0.298 | 1.449 | 1.109–1.827 | <0.001 | 2.013 | 1.783–2.349 | <0.001 |
| Tumor stage | 0.001 | <0.001 | <0.001 | ||||||
| I | 1.00 | 1.00 | 1.00 | ||||||
| II | 0.817 | 0.665–1.134 | 0.534 | 1.897 | 1.498–2.379 | <0.001 | 1.056 | 1.029–2.107 | <0.001 |
| III | 1.548 | 1.076–1.946 | <0.001 | 4.176 | 3.307–5.265 | <0.001 | 4.134 | 3.074–6.243 | <0.001 |
| Tumor location | 0.122 | 0.248 | 0.008 | ||||||
| Low rectum | 1.00 | 1.00 | 1.00 | ||||||
| High rectum | 1.236 | 0.945–1.618 | 1.088 | 0.943–1.255 | 1.186 | 1.045–1.35 | |||
| Surgical procedure | 0.048 | 0.023 | 0.044 | ||||||
| Open | 1.00 | 1.00 | 1.00 | ||||||
| Laparoscopy | 1.500 | 1.004–2.243 | 1.263 | 1.032–1.546 | 1.210 | 1.005–1.457 | |||
| Types of admission | 0.112 | 0.646 | 0.003 | ||||||
| Elective | 1.00 | 1.00 | 1.00 | ||||||
| Emergency | 0.755 | 0.534–1.067 | 0.954 | 0.781–1.166 | 0.773 | 0.654–0.914 | |||
| Organ resection | 0.032 | 0.601 | 0.019 | ||||||
| None | 1.00 | 1.00 | 1.00 | ||||||
| Other organ | 0.623 | 0.404–0.961 | 0.925 | 0.690–1.239 | 0.769 | 0.616–0.958 | |||
AL = anastomotic leakage.
aComorbidity according to Charlson comorbidity index scores: 0 (normal), 1 (moderate), 2 (severe), and ≥3 (very severe).
AL was significantly associated with the decrease in 5-year OS rate by both univariate (39.2% vs 60.3%; p < 0.001; Fig. 1D) and multivariate analyses (HR = 0.693; (95% CI, 0.507–0.929); p = 0.011). Other covariates that also had a statistically significant impact on the decrease in 5-year OS rate were age, comorbidity, tumor stage, tumor location, surgical procedure, surgical priority, and organ resection (Table 2).
AL Is a Prognostic Factor for Both Cancer Recurrence and LTS in Patients With Stage II Disease
In additional subgroup analysis, we found that, compared with other pathologic risk factors, AL was associated with a higher occurrence of both local and distant recurrence in stage II RCs (5-year local recurrence for AL, other risk factors, and no risk factor: 17.6% vs 6.4% vs 6.9%, p = 0.031; 5-year distant recurrence for AL, other risk factors, and no risk factor: 60.2% vs 27.1% vs 16.5%, p < 0.001; Figs. 2A and B). Analysis of LTS showed that the occurrence of AL was associated with poorer DFS and OS outcomes than both those with and without pathologic risk factors (5-year DFS rate for AL, other risk factors, and no risk factor: 21.2%, 55.2%, and 64.2%, p < 0.001; 5-year OS rate for AL, other risk factors, and no risk factor: 54.3%, 63.9%, and 77.0%, p < 0.001; Figs. 2C and D).
FIGURE 2.
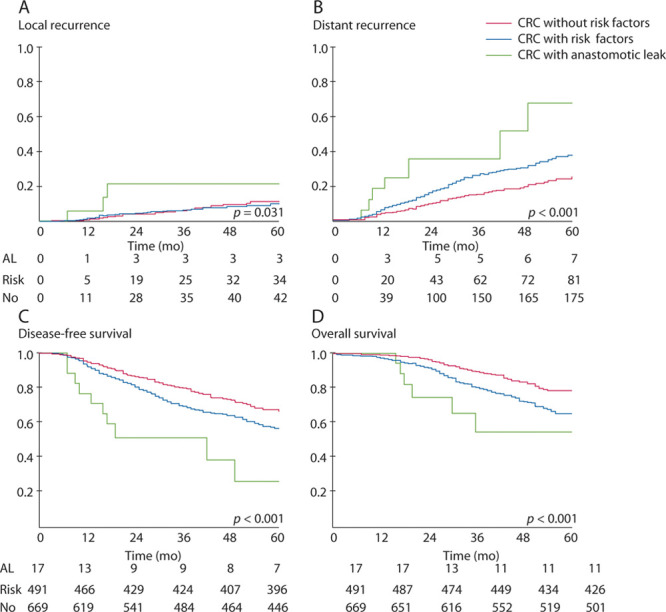
Kaplan–Meier plots illustrating the association between AL and the rates of local (A) and distant (B) recurrence, DFS (C), and OS (D) at 120 days after surgery for stage II rectal cancer. AL = anastomotic leakage; DSF = disease-free survival; OS = overall survival; CRC = colorectal cancer.
Occurrence of AL Affects AT Administration and Results in Poorer LTS of Patients With Stage III Disease
Among patients with stage III disease, AT was administrated in 878 (60.6%) of 1385 patients within 120 days. The proportion of patients receiving AT in the AL group was significantly lower than those without AL (37.3% vs 66.7%; p < 0.001). In addition, the median time to initial AT administration was 63 days (IQR, 41–89 d) after surgery in the AL group and 39 days (IQR, 25–53 d) in the group without AL (p < 0.001). We also found that older and sicker patients tend to receive less AT (old versus young (<65 y): 56.4% vs 63.1%, p = 0.069; sicker versus control: 31.5% vs 69.6%, p < 0.001). Few patients with severe comorbidities who experienced AL received AT after surgery. In subgroup analysis of those experiencing AL, no statistical difference in age and sex was observed between patients receiving AT or not (p = 0.126 and p = 0.238).
Additional survival analysis revealed that patients with AL who did not receive AT had much poorer DFS and OS rates when compared with those who received AT or without AL (5-year DFS rate for AL+AT–, AL+AT+, AL–AT–, and AL–AT+: 25.0%, 42.8%, 43.1%, and 44.0%, p = 0.013; 5-year OS rate for AL+AT–, AL+AT+, AL–AT–, and AL–AT+: 31.0%, 54.3%, 44.6%, and 52.5%, p = 0.001; Fig. 3). It is noteworthy that, in patients with delayed/canceled AT, those with AL had poorer survival than the other group without AL (5-year DFS, p = 0.005; 5-year OS, p = 0.042).
FIGURE 3.

Kaplan–Meier plots illustrating the DFS (A) and OS (B) rates of patients with and without AL after surgery for stage III rectal cancer. AL = anastomotic leakage; DSF = disease-free survival; OS = overall survival; AT = adjuvant therapy.
NeoAT Increased the Incidence of AL and Protective Stoma Improved AT Administration
Among 157 patients who received neoAT, 8 patients experienced AL after surgery, and the incidence was higher than the overall incidence of AL (5.1% vs 2.7%). Among 402 patients who received protective stoma, 10 patients experienced AL after surgery. However, the median time to initial AT administration was 43 days (IQR = 29–73 d) after surgery for patients with protective stoma and 69 days (IQR = 47–89 d) for those without (p < 0.001).
DISCUSSION
AL is a serious postoperative complication occurring in a small portion of patients after reconstructive GI surgery. The occurrence of AL after restorative surgery has been associated with many well-described factors, including poor blood supply, high tension of the anastomotic site, inflammation resulting from radiation or chemotherapy, and other risk factors resulting from the surgical procedure.8,9,17,18 As reported in the literature, AL is not only regarded as a major cause of early postoperative death but also a risk factor on LTS of patients with RC. Our result also supported this, because AL was significantly associated with a decrease in the 5-year OS rate (39.2% vs 60.3%; p < 0.001). However, there is no consensus in the effect of AL on oncologic outcome and whether AL is a risk factor for the administration of AT in patients with advanced-stage diseases.
In this single-institution retrospective study, the overall incidence of AL was 2.7%, and both local and distant recurrence were more common in patients with AL than those without (local: 14.0% vs 6.7%; distant: 35.5% vs 23.3%). These findings are similar to the reported incidence of AL ranging from 2% to 12%. Furthermore, the incidence of AL is reportedly dependent on the diagnostic method and type of anastomosis.8–10
Multivariate analysis of survival found that AL was significantly associated with an increase in local recurrence rate (HR = 0.501 (95% CI, 0.289–0.870); p = 0.014). The significant association between increased local recurrence rate and AL identified in this study is consistent with current literature.11,13,19,20 However, the relationship between AL and an increase in distant recurrence was not suggested in this study. Additional LTS analysis showed that AL was significantly associated with the decreased OS by both univariate (5-y OS rate: 39.2% vs 60.3%; p < 0.001) and multivariate analyses (HR = 0.693 (95% CI, 0.507–0.929); p = 0.011). This result was consistent with the oncologic data in the literature.9,14,15 To assess the influence of AL on OS more accurately, short-term survival must be distinguished from LTS by performing regression analysis separately. After excluding those patients who died within 120 days, AL remained as a risk factor for patient death in this retrospective analysis. It is generally accepted that the LTS was mainly influenced by distant recurrences but not by local ones. Interestingly, here we observed a decrease in OS, as well as an increase in local recurrence simultaneously in the AL group, indicating that local recurrence may also affect LTS in those patients experiencing AL.14,15,19,20
The detailed mechanism of how AL enhances the tumor recurrence rate remains uncertain. Aggarwal et al21 proposed that AL may cause extraluminal implantation of exfoliated cancer cells from the bowel lumen. In addition, the local inflammatory that resulted from the AL could facilitate cancer recurrence. Other retrospective clinical studies indicated that higher local recurrence may be attributed to significantly higher cytokine levels, especially interleukin 8, in the region around the anastomosis.19,20,22,23
AL and other postoperative complications can result in delay or cancellation of postoperative treatment.24 Severe postoperative complications, such as AL, when accompanied by severe comorbidity, were strongly associated with cancellation or delayed AT administration, thus ultimately leading to poorer outcomes.24 In the present study, the proportion of patients receiving AT in the AL group was significantly lower than those without AL (37.3% vs 66.7%; p < 0.001), and very few patients with severe comorbidity experiencing AL received AT after surgery. The secondary influence is the delay in AT attributed to the treatment of AL. In the present study, the median time to initial AT administration after curative surgery was 63 days (IQR, 41–89 d) in the AL group compared with 39 days (IQR, 27–53 d) in the group without AL (p < 0.001). Concerning the consequences of delayed AT, Biagi et al25 reported a 14% decrease in both DFS and OS for every 4-week delay in AT administration. The same result was reported by Krarup et al.26 Effective treatment of AL to reduce its influence on the administration of AT is critical to improve oncologic outcome.
Preventive enterostomy, anal placement of a decompression tube, and other preventive surgical procedures have been reported to relieve the symptoms of AL.14,20,21,26 In this study, we found that protective stoma could reduce the time interval to initiate AT, although it could not eliminate the incidence of AL. In our study, local recurrence was not only affected by AL but also by age, comorbidities, stage, and the type of surgery patient received. So, additional prospective control studies are needed to determine whether age, comorbidities, delayed or cancelled AT, or tumor residue is the determinant of local recurrence.
According to the National Comprehensive Cancer Network guidelines for RC, poor differentiated histology, perineural invasion, localized perforation, lymphovascular invasion, bowel obstruction, <12 retrieved lymph nodes, and a positive margin were risk factors for T3N0M0 RC. Patients with these risk factors should be considered for additional AT.27–29 Other than the factors described above, the occurrence of AL might be considered as a risk factor in patients with stage II RC, because those with AL had poorer survival than patients with any of the other pathologic risk factors. Subgroup analysis also found that AL was associated with increased local and distant recurrence rates in stage II diseases.
As this is a retrospective study, we could only observe the association among the incidences of AL, local recurrence, and the delay or cancellation of AT. It is also noteworthy that this retrospective review and interpretation is limited by the data points when gathered. All of these observed phenomenon need additional prospective study to be proven.
CONCLUSION
The results of this retrospective study revealed a robust association between AL and local recurrence, which also potentially affects LTS in patients experiencing AL. The delay or cancellation of AT administration because of AL may partly account for the poor survival outcome in those with stage III diseases, and AL should be further evaluated as a potential prognostic factor in patients with stage II diseases. Nonetheless, additional prospective studies are needed to evaluate the influence of delayed AT after AL and the prognostic value of AL.
Footnotes
Funding/Support: The work was supported by the Research Foundation of Health Commission of Sichuan Province (grant 19PJ102), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (2016105), and Research Foundation of Outstanding Young Scholars of Sichuan University (grant 2016SCU04B04).
Financial Disclosure: None reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev. 2012;21:411–416. [DOI] [PubMed] [Google Scholar]
- 7.Khan AA, Wheeler JM, Cunningham C, George B, Kettlewell M, Mortensen NJ. The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis. 2008;10:587–592. [DOI] [PubMed] [Google Scholar]
- 8.Bertelsen CA, Andreasen AH, Jørgensen T, Harling H; Danish Colorectal Cancer Group. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Colorectal Dis. 2010;12:37–43. [DOI] [PubMed] [Google Scholar]
- 9.Kube R, Mroczkowski P, Granowski D, et al.; Study group Qualitätssicherung Kolon/Rektum-Karzinome (Primärtumor) (Quality assurance in primary colorectal carcinoma). Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur J Surg Oncol. 2010;36:120–124. [DOI] [PubMed] [Google Scholar]
- 10.Krarup PM, Jorgensen LN, Andreasen AH, Harling H; Danish Colorectal Cancer Group. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis. 2012;14:e661–e667. [DOI] [PubMed] [Google Scholar]
- 11.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–899. [DOI] [PubMed] [Google Scholar]
- 12.Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259:916–923. [DOI] [PubMed] [Google Scholar]
- 13.Nachiappan S, Askari A, Malietzis G, et al. The impact of anastomotic leak and its treatment on cancer recurrence and survival following elective colorectal cancer resection. World J Surg. 2015;39:1052–1058. [DOI] [PubMed] [Google Scholar]
- 14.Bertelsen CA, Andreasen AH, Jørgensen T, Harling H; Danish Colorectal Cancer Group. Anastomotic leakage after curative anterior resection for rectal cancer: short and long-term outcome. Colorectal Dis. 2010;127 onlinee76–e81. [DOI] [PubMed] [Google Scholar]
- 15.den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96:1066–1075. [DOI] [PubMed] [Google Scholar]
- 16.Steele SR, Chang GJ, Hendren S, et al.; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58:713–725. [DOI] [PubMed] [Google Scholar]
- 17.Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg. 2014;101:424–432. [DOI] [PubMed] [Google Scholar]
- 18.Reilly F, Burke JP, Appelmans E, Manzoor T, Deasy J, McNamara DA. Incidence, risks and outcome of radiological leak following early contrast enema after anterior resection. Int J Colorectal Dis. 2014;29:453–458. [DOI] [PubMed] [Google Scholar]
- 19.Alonso S, Pascual M, Salvans S, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. 2015;41:208–214. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt JM, Kiran RP, Lavery IC. The impact of anastomotic leak and intra-abdominal abscess on cancer-related outcomes after resection for colorectal cancer: a case control study. Dis Colon Rectum. 2009;52:380–386. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. [DOI] [PubMed] [Google Scholar]
- 22.Chuang D, Paddison JS, Booth RJ, Hill AG. Differential production of cytokines following colorectal surgery. ANZ J Surg. 2006;76:821–824. [DOI] [PubMed] [Google Scholar]
- 23.Lee YS, Choi I, Ning Y, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Shayeb M, Scarfe A, Yasui Y, Winget M. Reasons physicians do not recommend and patients refuse adjuvant chemotherapy for stage III colon cancer: a population based chart review. BMC Res Notes. 2012;5:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. [DOI] [PubMed] [Google Scholar]
- 26.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930–938. [DOI] [PubMed] [Google Scholar]
- 27.Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51:503–507. [DOI] [PubMed] [Google Scholar]
- 28.Sarli L, Bader G, Iusco D, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272–279. [DOI] [PubMed] [Google Scholar]
- 29.Langner C, Harbaum L, Pollheimer MJ, et al. Mucinous differentiation in colorectal cancer: indicator of poor prognosis? Histopathology. 2012;60:1060–1072. [DOI] [PubMed] [Google Scholar]


