Abstract
Background
In 2012, the United States Preventative Services Task Force (USPSTF) formally recommended against all prostate-specific antigen (PSA) screening for prostate cancer. Our goal was to characterize PSA screening trends in the Veterans Health Administration (VA) before and after the USPSTF recommendation and to determine if PSA screening was more likely to be ordered based on a veteran’s race or age.
Methods
Using the VA Corporate Data Warehouse, we created 10 annual groups of PSA-eligible men covering 2009-2018. We identified all PSA tests performed in the VA to determine yearly rates of PSA screening. All statistical tests were 2-sided.
Results
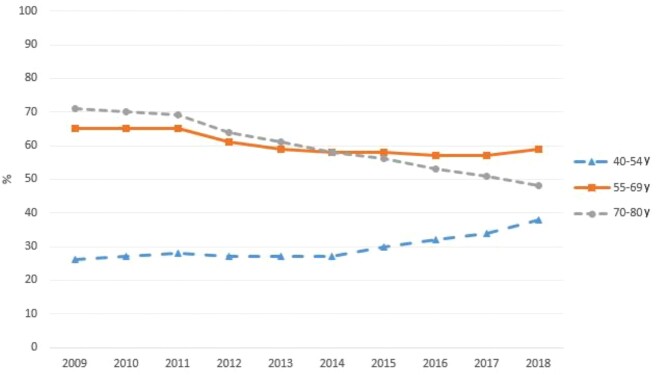
The overall rate of PSA testing in the VA decreased from 63.3% in 2009 to 51.2% in 2018 (P < .001). PSA screening rates varied markedly by age group during our study period, with men aged 70-80 years having the highest initial rate and greatest decline (70.6% in 2009 to 48.4% in 2018, P < .001). Men aged 55-69 years had a smaller decline (65.2% in 2009 to 58.9% in 2018, P < .001) whereas the youngest men, aged 40-54 years, had an increase in PSA screening (26.2% in 2009 to 37.8% in 2018, P < .001).
Conclusions
In this analysis of PSA screening rates among veterans before and after the 2012 USPSTF recommendation against screening, we found that overall PSA screening decreased only modestly, continuing for more than one-half of the men in our study. Veterans of different races had similar screening rates, suggesting that VA care may minimize racial disparities. Veterans of varying ages experienced statistically significantly differences in PSA screening trends.
Since its widespread adoption as a screening test for prostate cancer, the appropriate use of the prostate-specific antigen (PSA) blood test has been controversial. Different expert guidelines recommended substantially different prostate cancer–screening regimens throughout the first 2 decades of the 2000s. Initially, the American Urological Association and American Cancer Society recommended PSA-based prostate cancer screening for all men aged 50 years and older, whereas the United States Preventative Services Task Force (USPSTF) concluded that there was insufficient evidence to recommend for or against screening (1-3). In 2012, however, the USPSTF formally recommended against screening men of all ages, despite continued support for screening from the American Urological Association (4). Notably, the USPSTF recommendations do not specify different recommendations for Black men despite prostate cancer–specific mortality 2-3 times higher than that for White men (5). Both the American Cancer Society 2010 guideline and the American Urological Society 2013 guidelines state that race should factor into decisions to screen for the early detection of prostate cancer (4,6).
Screening guidelines are inherently challenging in a disease with a long and often indolent natural history. Screening performed too early or with a PSA threshold that is too low increases overdiagnosis, whereas screening too late or with a PSA cutoff that is too high misses potentially curable cases, which can ultimately prove fatal. The risks and benefits of screening also potentially vary with age and race. Several studies have attempted to evaluate the effects of the 2012 USPSTF PSA screening recommendations on screening rates in actual practice. These studies suggested that screening rates declined modestly following the 2012 recommendation (7-9). Although these studies provide valuable information, they used survey data representing approximately 6000 men yearly, subject to survey challenges such as recall bias and social desirability bias (10). Large database analyses of PSA testing, and the subgroup analyses made possible through these large numbers, however, are limited (11).
We sought to characterize changes in the use of PSA screening in the Veterans Health Administration (VA) system before and after the 2012 USPSTF recommendation change. The national VA database allows the study of all care for more than 9 million enrolled veterans in the US’s largest single-payer system. The VA additionally allows us to explore physician and patient decisions on PSA screening without the fee-for-service incentive of private sector health care. In planned subgroup analyses, we further explored differences in PSA screening by veteran age and race because the risk and benefit ratio of PSA screening varies considerably with these characteristics. The USPSTF recommendations do not specify differential use of PSA screening by subgroups, so we hypothesized there would be little difference in screening rate changes among age and racial groups. However, Black men have a higher risk of prostate cancer incidence and men between the ages of 55 and 69 years have the highest evidence of benefit from PSA screening, so higher use of PSA screening might be justifiable within these subgroups if veterans and providers were to disregard the guidelines at times. The results of this study would be of great interest to policy makers suggesting practice guidelines and to the physicians and patients interpreting and acting on those guidelines.
Methods
Study Design
We performed a repeated cross-sectional study of male veterans eligible for PSA screening for prostate cancer. Because we were interested in trends in PSA screening rates across time, we created 10 annual cross-sections of men eligible for PSA screening, a group for each calendar year 2009 to 2018. As such, some men appear in more than 1 annual group. All data were obtained from the VA’s Corporate Data Warehouse (CDW), a national repository of VA clinical data and administrative claims.
We first identified a denominator of PSA-eligible men in each year of our study. We defined PSA-eligible men as all men aged 40-80 years who had a primary care or urology clinic visit at the VA in a given calendar year (11). Urology clinic visits were included to capture men who receive annual screening in the urology clinic, typically after referral for another urologic concern. Men were excluded from an annual group if they were diagnosed with prostate cancer at any time before their first primary care or urology clinic visit in that year (11). Primary care and urology clinic visits were identified using the VA’s primary stop codes, the VA clerical method used to identify and define the location of clinical care.
Our primary dependent variable of interest was the proportion of screening-eligible men who received at least 1 PSA test in a given year. For each annual denominator of screening-eligible men, we calculated a numerator of men who underwent PSA screening at any point during that year. PSA screening tests were identified by searching the VA inpatient and outpatient visit claims for the appropriate CPT codes (84152-84154).
Our primary independent variable of interest was time period. We defined 3 time periods based on observation of inflection points in the overall PSA screening trends in our yearly groups (Figure 1). The preguidelines period was defined as 2009-2010, the guideline adoption period as 2011-2014, and the postguideline period as 2015-2018. Our independent variables of interest were veteran age and race. We created age groups as follows: 40-54 years, 55-69 years, and 70-80 years, based on established age-related changes of normal PSA and clinical trial data suggesting optimal efficacy of PSA screening (12). To determine veteran race, we queried CDW patient demographic data for all instances of self-identified or provider-identified race. We excluded all patients who had missing data for any of our covariates.
Figure 1.
Prostate-specific antigen screening rates in the Veteran’s Affairs, by age group, 2009-2018.
Other covariates included screening year and Elixhauser comorbidity score, categorized as 0, 1-2, or 3+ comorbidities (13). A comorbidity score was calculated for each unique veteran in our study using inpatient and outpatient claims from the year before each patient’s index clinic visit. For men appearing in more than 1 annual group, a comorbidity score was calculated using the visit closest to the mid-point of the study (July 1, 2013). Marital status was also included as either married or not married, and 2010 US Census data from the Area Hospital Resource File was used to establish quartiles of both median income (wealth) and percentage of college graduates (education) for each veteran’s county of residence.
Statistical Analysis
We used a logistic mixed effect regression to test for trends in PSA screening rates, treating 2 time periods as a binary outcome and designating the patient as the random effect to control for men who appear more than once in the overall data. We also used a piecewise generalized mixed model (12) with logit link function to estimate the adjusted probability of receiving PSA screening in each subgroup defined by age group, race, and guideline period adjusted for comorbidity, VA geographic division, marital status, wealth, and education. Patient ID number was treated as a random effect variable to adjust for the potential correlation among repeated measures from the same patient. The coefficients in the piecewise generalized mixed model were estimated by the maximum likelihood estimation method (13). The probabilities of receiving PSA screening were then estimated for all subgroups based on those estimates. The final adjusted probabilities of receiving PSA screening by year were calculated as weighted averages based on the law of total probability theorem (14) where the weights were the observed relative frequency for each subgroup defined by the adjusting covariates (14-16). All analyses we performed using SAS Enterprise Guide version 7.1 (SAS Institute Inc, Cary, NC).
Results
The demographics of our yearly PSA-eligible groups were consistent across the time period of the study (Table 1). Within each yearly cross-section were approximately 2 million PSA-screening eligible veterans (range = 1.8-2.5 million). The study population was 18.9% Black and 81.1% non-Black. Veterans aged 40-54 years, 55-70 years, and 70-80 years accounted for 12%-15%, 41%-44%, and 41%-47% of the age groups, respectively. We found that the overall rate of PSA testing among eligible men in the VHA decreased from 63.3% in 2009 to 51.2% in 2018 (P < .001; Table 2).
Table 1.
Demographics of PSA-eligible veterans in the VA health system, by year, 2009-2018a
| Demographic variable | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| PSA-eligible men | 2 244 252 | 2 348 005 | 2 417 579 | 2 442 330 | 2 491 736 | 2 476 478 | 2 154 155 | 1 929 301 | 1 912 089 | 1 801 327 |
| PSA screen | ||||||||||
| Screened | 1 420 419 (63.3) |
1 468 133 (62.5) |
1 508 696 (62.4) |
1 420 649 (58.2) |
1 374 822 (55.2) |
1 330 700 (53.7) |
1 140 943 (53.0) |
992 956 (51.5) |
978 460 (51.2) |
922 768 (51.2) |
| Not screened |
823 833 (36.7) |
879 872 (37.5) |
908 883 (37.6) |
1 021 681 (41.8) |
1 116 914 (44.8) |
1 145 778 (46.3) |
1 013 212 (47.0) |
936 345 (48.5) |
933 629 (48.8) |
878 559 (48.8) |
| Race | ||||||||||
| Non-Black race |
1 826 071 (81.4) |
1 908 864 (81.3) |
1 967 802 (81.4) |
1 986 357 (81.3) |
2 024 527 (81.2) |
2 011 218 (81.2) |
1 739 290 (80.7) |
1 560 607 (80.9) |
1 546 549 (80.9) |
1 451 583 (80.6) |
| Black race |
418 181 (18.6) |
439 141 (18.7) |
449 777 (18.6) |
455 973 (18.7) |
467 209 (18.8) |
465 260 (18.8) |
414 865 (19.3) |
368 694 (19.1) |
365 540 (19.1) |
349 744 (19.4) |
| Age, y | ||||||||||
| 40-54 |
259 575 (11.6) |
285 670 (12.2) |
305 972 (12.7) |
325 548 (13.3) |
350 366 (14.1) |
370 907 (15.0) |
320 435 (14.9) |
278 797 (14.5) |
284 336 (14.9) |
276 681 (15.4) |
| 55-69 |
919 436 (41.0) |
964 583 (41.1) |
994 280 (41.1) |
1 008 961 (41.3) |
1 033 644 (41.5) |
1 027 338 (41.5) |
907 065 (42.1) |
837 292 (43.4) |
827 470 (43.3) |
783 722 (43.5) |
| 70-80 |
1 065 241 (47.5) |
1 097 752 (46.8) |
1 117 327 (46.2) |
1 107 821 (45.4) |
1 107 726 (44.5) |
1 078 233 (43.5) |
926 655 (43.0) |
813 212 (42.2) |
800 283 (41.9) |
740 924 (41.1) |
| Marriage status | ||||||||||
| Married |
1 252 216 (55.8) |
1 315 841 (56.0) |
1 365 037 (56.5) |
1 384 168 (56.7) |
1 419 296 (57.0) |
1 414 322 (57.1) |
1 203 712 (55.9) |
1 015 521 (52.6) |
1 014 657 (53.1) |
952 555 (52.9) |
| Not married |
992 036 (44.2) |
1 032 164 (44.0) |
1 052 542 (43.5) |
1 058 162 (43.3) |
1 072 440 (43.0) |
1 062 156 (42.9) |
950 443 (44.1) |
913 780 (47.4) |
897 432 (46.9) |
848 772 (47.1) |
| Comorbidities | ||||||||||
| 0 |
1 127 690 (50.2) |
1 204 584 (51.3) |
1 302 138 (53.9) |
1 364 005 (55.8) |
1 435 568 (57.6) |
1 478 397 (59.7) |
1 283 922 (59.6) |
1 156 680 (60.0) |
1 168 324 (61.1) |
1 112 923 (61.8) |
| 1-2 |
867 139 (38.6) |
894 983 (38.1) |
880 714 (36.4) |
856 599 (35.1) |
842 745 (33.8) |
800 019 (32.3) |
695 329 (32.3) |
617 758 (32.0) |
596 471 (31.2) |
555 560 (30.8) |
| 3+ |
249 423 (11.1) |
248 438 (10.6) |
234 727 (9.7) |
221 726 (9.1) |
213 423 (8.6) |
198 062 (8.0) |
174 904 (8.1) |
154 863 (8.0) |
147 294 (7.7) |
132 844 (7.4) |
| Percent of county population with at least 4 y of college | ||||||||||
| 1st Quartile |
24 200 (1.1) |
25 480 (1.1) |
26 175 (1.1) |
25 663 (1.1) |
25 334 (1.0) |
25 006 (1.0) |
22 186 (1.0) |
21 112 (1.1) |
20 872 (1.1) |
19 633 (1.1) |
| 2nd Quartile |
640 967 (28.6) |
672 717 (28.7) |
690 677 (28.6) |
694 067 (28.7) |
70 266 (2.8) |
696 667 (28.1) |
612 455 (28.4) |
572 947 (29.7) |
568 400 (29.7) |
536 009 (29.8) |
| 3rd Quartile |
871 921 (38.9) |
904 115 (38.5) |
939 695 (38.9) |
948 302 (39.2) |
969 108 (38.9) |
967 620 (39.1) |
842 621 (39.1) |
750 593 (38.9) |
743 311 (38.9) |
702 557 (39.0) |
| 4th Quartile |
707 164 (31.5) |
745 693 (31.8) |
761 032 (31.5) |
774 298 (32.0) |
1 427 028 (57.3) |
787 185 (31.8) |
676 893 (31.4) |
584 649 (30.3) |
579 506 (30.3) |
543 128 (30.2) |
| County median income | ||||||||||
| 1st Quartile |
677 831 (30.2) |
696 316 (29.7) |
709 550 (29.3) |
713 853 (29.5) |
726 028 (29.1) |
714 749 (28.9) |
497 019 (23.1) |
254 951 (13.2) |
241 922 (12.7) |
233 902 (13.0) |
| 2nd Quartile |
562 145 (25) |
588 814 (25.1) |
614 534 (25.4) |
623 316 (25.8) |
630 768 (25.3) |
622 143 (25.1) |
544 608 (25.3) |
466 297 (24.2) |
455 119 (23.8) |
429 749 (23.9) |
| 3rd Quartile |
548 063 (24.4) |
572 553 (24.4) |
586 732 (24.3) |
593 618 (24.6) |
603 848 (24.2) |
597 127 (24.1) |
569 956 (26.5) |
544 759 (28.2) |
534 478 (28.0) |
482 081 (26.8) |
| 4th Quartile |
456 213 (20.3) |
490 322 (20.9) |
506 763 (21.0) |
511 543 (21.2) |
531 092 (21.3) |
542 459 (21.9) |
542 572 (25.2) |
663 294 (34.4) |
680 570 (35.6) |
655 595 (36.4) |
PSA = prostate-specific antigen; VA = Veteran’s Affairs.
Table 2.
Multiple comparisons of unadjusted PSA screening rates before and after 2012 guidelines change
| Groups of interest | 2009 vs 2018 |
2009-2011 vs 2013-2018 |
2009-2011 vs 2013-2015 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2009 % | 2018 % | P a | 2009-2011 % | 2013-2018 % | P a | 2009-2011 % | 2013-2015 % | P a | |
| All men | |||||||||
| Overall | 63.3 | 51.2 | <.001 | 62.7 | 52.8 | <.001 | 62.7 | 54.0 | <.001 |
| Aged 40-54 y | 26.2 | 37.8 | <.001 | 27.4 | 31.0 | <.001 | 27.4 | 28.0 | <.001 |
| Aged 55-69 y | 65.2 | 58.9 | <.001 | 65.0 | 58.1 | <.001 | 65.0 | 58.4 | <.001 |
| Aged 70-80 y | 70.7 | 48.1 | <.001 | 69.9 | 55.1 | <.001 | 69.9 | 58.4 | <.001 |
| Black men | |||||||||
| Overall | 61.2 | 52.3 | <.001 | 60.6 | 52.5 | <.001 | 60.6 | 53.0 | <.001 |
| Aged 40-54 y | 33.2 | 43.2 | <.001 | 34.5 | 37.4 | <.001 | 34.5 | 34.8 | .03 |
| Aged 55-69 y | 64.4 | 57.4 | <.001 | 64.0 | 57.1 | <.001 | 64.0 | 57.4 | <.001 |
| Aged 70-80 y | 70.6 | 48.4 | <.001 | 69.4 | 55.0 | <.001 | 69.4 | 58.0 | <.001 |
| Non-Black men | |||||||||
| Overall | 63.8 | 51.0 | <.001 | 63.2 | 52.9 | <.001 | 63.2 | 54.2 | <.001 |
| Aged 40-54 y | 23.7 | 35.9 | <.001 | 24.9 | 28.7 | <.001 | 24.9 | 25.6 | <.001 |
| Aged 55-69 y | 65.4 | 59.4 | <.001 | 65.4 | 58.4 | <.001 | 65.4 | 58.7 | <.001 |
| Aged 70-80 y | 70.7 | 48.1 | <.001 | 69.9 | 55.1 | <.001 | 69.9 | 58.6 | <.001 |
Logistic mixed effect regression treating 2 time periods as a binary outcome. PSA = prostate-specific antigen.
Multivariable-adjusted screening patterns varied markedly by age group, with the highest initial rates and largest declines in screening occurring among men aged 70-80 years (70.7% in 2009 to 48.1% in 2018, P < .001) (Table 3), intermediate initial rates and a smaller decrease among men aged 55-69 years (65.2% in 2009 to 58.9% in 2018, P < .001), and the lowest absolute rates but a statistically significant increase in PSA testing among men aged 40-54 years (26.2% in 2009 to 37.8% in 2018, P < .001) (Table 3). Among the youngest age group, an initial decrease in screening was subsequently reversed, with a net increase over the 10-year study period ultimately. Unlike age group, patient race was not statistically significantly associated with screening rates (Table 3).
Table 3.
Adjusted probability of receiving PSA screening in the VA, 2009-2018a
| Year | Aged 40-54 y Black | Aged 40-54 y non-Black | Aged 55-69 y Black | Aged 55-69 y non-Black | Aged 70-80 y Black | Aged 70-80 y non-Black |
|---|---|---|---|---|---|---|
| 2009 | 0.268 | 0.265 | 0.656 | 0.652 | 0.710 | 0.707 |
| 2010 | 0.272 | 0.274 | 0.647 | 0.650 | 0.694 | 0.697 |
| 2011 | 0.275 | 0.284 | 0.637 | 0.648 | 0.677 | 0.687 |
| 2012 | 0.274 | 0.280 | 0.617 | 0.624 | 0.646 | 0.652 |
| 2013 | 0.273 | 0.276 | 0.596 | 0.600 | 0.613 | 0.617 |
| 2014 | 0.272 | 0.271 | 0.575 | 0.575 | 0.580 | 0.579 |
| 2015 | 0.295 | 0.295 | 0.576 | 0.576 | 0.554 | 0.554 |
| 2016 | 0.319 | 0.319 | 0.577 | 0.577 | 0.528 | 0.528 |
| 2017 | 0.344 | 0.344 | 0.578 | 0.578 | 0.503 | 0.503 |
| 2018 | 0.371 | 0.371 | 0.579 | 0.579 | 0.477 | 0.477 |
PSA = prostate-specific antigen; VA = Veteran’s Affairs.
We attempted to measure the effect of the 2012 USPSTF PSA screening guidelines by performing 3 before and after calculations (Table 2). We compared the first and last year of our study; the years before the guideline was issued vs the years after, with 2012, the year of publication, removed; and finally, the 3 years before and the 3 years after guideline publication with 2012 again removed. Results for all 3 comparisons were similar, showing a 9%-11% overall, unadjusted drop in PSA screening, suggesting a modest effect on screening associated with the USPSTF recommendations.
Discussion
We found a relatively modest overall decrease of approximately 12.1% in PSA testing from 2009 to 2018. Most of the decrease in PSA testing occurred between 2012 and 2015, the period immediately after the change in USPSTF PSA screening recommendations, with relatively stable testing in the preceding and subsequent years. Screening rates in the overall population remained near 50% in 2018 despite the longstanding USPSTF recommendation against screening. We found that patterns of PSA testing varied statistically significantly by patient age. The oldest men had the highest initial rates of testing and the largest decrease (−22.6%) in PSA testing. The youngest men had the lowest initial rates of testing but the greatest increase (+11.6%) in testing rates over the study period. All age groups had a statistically significant decrease in 2013-2015 following the 2012 USPSTF recommendation against PSA testing for all age groups. Black and non-Black men in all age groups experienced very similar adjusted rates of PSA testing as well as similar changes over time.
Several studies have addressed the effects of the 2012 USPSTF recommendations against prostate cancer screening, but methodological limitations in those studies necessitate additional investigation. Jemal and colleagues (8) reported in a 2015 analysis that self-reported rates of PSA testing for men aged 50 years and older decreased from 37.8% in 2010 to 30.8% in 2013 based on a survey of approximately 6000 men yearly. Drazer and colleagues (7) used the same National Health Interview Survey and reported similar findings in 2015, with additional evidence that PSA screening rates were stable among men younger than 50 years between 2010 and 2013. Both of these studies had findings similar to our finding of a 12.1% decrease in screening for our overall population. Jemal et al. (8) reported a follow-up analysis suggesting that PSA testing rates stayed stable from 2013 to 2015. Self-reported rates of testing, as used in all of these studies, are subject to statistically significant limitations, including social desirability bias and recall bias (17). The national VA database, however, includes records of all PSA testing for more than 9 million enrolled veterans, offering a more reliable analysis than those previously reported. Our VA data also included a screening eligible population of approximately 2 million men yearly, which is substantially larger than the populations in previous studies.
A primary finding of our study was that changes in PSA screening rates between 2009 and 2018 were statistically significantly different by veteran age group, with a 11.6% increase in screening rates for our youngest group (aged 40-54 years) and a 22.6% decrease in screening rates for our oldest group (aged 70-80 years). Our results differ from some results in previous studies. The study by Drazer et al. (7) cited above reported that age groups 50-59 years, 60-74 years, and older than 74 years had similar decreases in PSA testing rates (6.8%-8.4%) between 2010 and 2013. Our study, however, covered a much longer time frame, from 2009-2018, and younger men in our youngest age group. Of note, we, like Drazer and colleagues (7), also found stable to decreasing rates of PSA screening in all groups when we limited our analysis to 2010-2013, a likely initial consequence of the USPSTF recommendation. Consistent with our results, a study by Aslani et al. (18) that evaluated a similar group of young men, aged 40-49 years, in a study sample from a health-care system in Ohio also found stable PSA testing from 2008 to 2012. Although most studies have reported no difference in change of PSA testing by age group in a limited time frame around 2012, a study by Kim et al. (19) using a private insurer’s database did find a much larger decrease in PSA testing rates for men aged 75 years and older. Our study adds substantially to the literature by continuing follow-up well beyond 2013 to evaluate the longer term practice patterns after the 2012 recommendation and by including men as young as 40 years old. These essential changes in study design help illuminate more complex practice patterns that become evident over longer follow-up.
We furthermore found no statistically significant differences in rates of PSA testing between Black and non-Black men when we evaluated adjusted rates by age cohort (Table 3). Adjusted screening rates and changes over time by age group were similar for Black and non-Black men. Studies of the differential effects of prostate cancer–screening guidelines by race are very limited (20). Turini et al. (21) reported from survey data collected in 2012 that Black or African American men were more likely than White men to report having been advised to have a PSA test (odds ratio = 1.51, 95% confidence interval = 1.37 to 1.67). Misra-Hebert and colleagues, using a database of more than 160 000 men treated in the Cleveland Clinic Health System, reported that decreases in PSA screening rates were similar for Black and non-Black men between 2007 and 2014 (22). Our results are consistent with these previous studies and furthermore show that differences in PSA screening rates for different age groups were similar between Black and non-Black men. Recent studies have suggested that prostate cancer outcomes are equivalent for Black and non-Black men in the VA, and our results confirm equal rates of PSA screening (23). Although we showed no difference in PSA testing rates by race, it is not clear that this represents ideal care. Black patients are at a higher risk of incident prostate cancer at younger ages, and screening discussions should be adjusted accordingly.
Our results suggest that the USPSTF guidelines had a modest but consistent effect across age groups in the years 2013-2015 immediately after the recommendation. Practice patterns with respect to age groups over the longer term, however, were statistically significantly more complicated than the guidelines advised, with increased testing of younger men and decreased testing of older men. Adjusted analyses by race showed that Black and non-Black men were treated similarly. It seems likely that factors such as potential life years saved in younger men and potential morbidity of diagnosis and treatment in older men influence practice patterns that diverge from USPSTF recommendations. It is interesting to note that updated USPSTF guidelines released in 2018 recommend individualized shared decision making regarding PSA screening in men younger than 70 years old and recommend against PSA based screening in men aged 70 years and older (24). Our study provides evidence that practice patterns were moving toward more individualized decision making before the 2018 guideline release.
Our study has a number of strengths and limitations. The analysis benefits from the national, visit-level data reliably available in the VA, allowing capture of all PSA screening tests ordered within the system. This is the first study, to our knowledge, to explore associations of race and age with changes in PSA screening rates over an extended time period following the USPSTF recommendation. Among our limitations, the analysis was performed using administrative claims rather than through a retrospective chart review. It is possible that occasional errors exist in the data, but the VA CDW data have standardized, centralized quality control and have been used extensively for research (25). As with all administrative database studies, we have limited data to understand the causes of differential effects of the recommendation on different age groups and races. Our methodology also included the same Individuals in multiple years, so it is possible that some men have PSA values that were being followed for abnormal results. We do however, consider these repeat screening exams and excluded any patient with a diagnosis of prostate cancer.
In this analysis of PSA screening rates among screening-eligible men cared for in the VA before and after the 2012 USPSTF recommendation against screening, we found veterans of varying age experienced very different trends in PSA screening. PSA screening for younger men increased and PSA screening for older men decreased, consistent with the different risk benefit ratios for men of different ages. We found similar PSA screening for Black and non-Black men, adding to the literature suggesting that care in the VA system may minimize racial disparities in cancer screening and in medical care in general (26). This study attests to both the influence of the USPSTF to decrease screening and to the more nuanced care of individual veterans, with differences in care offered to veterans of different ages.
Funding
Funding for this study was provided by the US Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service grant CDA 16-206 (Becker), and the National Institutes of Health/NIMHD grant R01 MD012243 (Makarov, Ravenell). This study is supported by The Prostate Cancer Foundation (PCF), The John and Daria Barry Precision Oncology Center of Excellence of the VANYHHS, Edward Blank and Sharon Cosloy-Blank Family Foundation, and the Gertrude and Louis Feil Family Foundation. Dr Makarov is a Prostate Cancer Foundation Young Investigator Awardee.
Notes
Role of the funders: The sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors report no conflicts of interest.
Author contributions: DJB, TR, DVM: Scientific design and concept. DJB, DW, CW, HL, DVM: Statistical methods and analysis. DJB, TR, CW, SC, MK, DVM: Interpretation of results. DJB, TR, CW, SC, MK, DVM: Drafting & preparation of the manuscript. DJB, TR, CW, SL, SC, MK, SBZ, AF, HL, SS, JER, DVM: Supervision and critical revision.
Disclaimer: The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
Data Availability
Data are available from the Veterans Administration office of Health Services Research and Development for researchers who comply with Veterans Health Administration (VHA) policy and procedures for release of data. The VHA requires that VHA data be maintained on VHA approved devices and networks and that all persons accessing the data have IRB and VA R&D approval to do so. This includes having a direct appointment in the VHA or a WOC (without compensation) appointment if a faculty member at a collaborating university/ medical school.
References
- 1. Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001—testing for early lung cancer detection. CA Cancer J Clin. 2001;51(1):38-75; quiz 77-80. [DOI] [PubMed] [Google Scholar]
- 2. Carroll P, Coley C, McLeod D, et al. Prostate-specific antigen best practice policy—part I: early detection and diagnosis of prostate cancer. Urology. 2001;57(2):217-224. [DOI] [PubMed] [Google Scholar]
- 3. Preventive U.S. Services Task Force. Screening for prostate cancer: recommendation and rationale. Ann Intern Med. 2002;137(11):915-916. [DOI] [PubMed] [Google Scholar]
- 4. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190(2):419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer Facts & Figures for African Americans 2016-2018. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 6. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70-78. [DOI] [PubMed] [Google Scholar]
- 7. Drazer MW, Huo D, Eggener SE.. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33(22):2416-2423. [DOI] [PubMed] [Google Scholar]
- 8. Jemal A, Fedewa SA, Ma J, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA. 2015;314(19):2054-2061. [DOI] [PubMed] [Google Scholar]
- 9. Fedewa SA, Ward EM, Brawley O, et al. Recent patterns of prostate-specific antigen testing for prostate cancer screening in the United States. JAMA Intern Med. 2017;177(7):1040-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Backinger CL, Lawrence D, Swan J, et al. Using the National Health Interview Survey to understand and address the impact of tobacco in the United States: past perspectives and future considerations. Epidemiol Perspect Innov. 2008;5(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeliadt SB, Hoffman RM, Etzioni R, et al. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst. 2011;103(6):520-523. [DOI] [PubMed] [Google Scholar]
- 12. Greene KL, Albertsen PC, Babaian RJ, et al. Prostate specific antigen best practice statement: 2009 update. J Urol. 2009;182(5):2232-2241. [DOI] [PubMed] [Google Scholar]
- 13. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 14. Dean CB, Nielsen JD.. Generalized linear mixed models: a review and some extensions. Lifetime Data Anal. 2007;13(4):497-512. [DOI] [PubMed] [Google Scholar]
- 15. Owen AB. Empirical Likelihood. 1st ed.Boca Raton, FL: Chapman and Hall/CRC; 2001. [Google Scholar]
- 16. Zwillinger D, Kokoska S.. CRC Standard Probability and Statistics Tables and Formulae. Boca Raton, FL: Chapman and Hall/CRC; 2000. [Google Scholar]
- 17. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9(1):211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aslani A, Minnillo BJ, Johnson B, et al. The impact of recent screening recommendations on prostate cancer screening in a large health care system. J Urol. 2014;191(6):1737-1742. [DOI] [PubMed] [Google Scholar]
- 19. Kim SP, Karnes RJ, Gross CP, et al. Contemporary national trends of prostate cancer screening among privately insured men in the United States. Urology. 2016;97(1):111-117. [DOI] [PubMed] [Google Scholar]
- 20. Fleshner K, Carlsson SV, Roobol MJ.. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol. 2017;14(1):26-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turini G, Gjelsvik A, Golijanin D, et al. PD09-07 the role of patient race and ethnicity in predicting physician recommendation of prostate-specific antigen (PSA) testing. J Urol. 2016;195(4):e236. [Google Scholar]
- 22. Misra-Hebert AD, Hu B, Klein EA, et al. Prostate cancer screening practices in a large, integrated health system: 2007-2014. BJU Int. 2017;120(2):257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dess RT, Hartman HE, Mahal BA, et al. Association of Black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(7):975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Preventive U.S. Services Task Force. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA. 2018;319(18):1901-1913. [DOI] [PubMed] [Google Scholar]
- 25. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff. 2014;33(7):1203-1211. [DOI] [PubMed] [Google Scholar]
- 26. Dolan NC, Ferreira MR, Fitzgibbon ML, et al. Colorectal cancer screening among African-American and White male veterans. Am J Prev Med. 2005;28(5):479-482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Veterans Administration office of Health Services Research and Development for researchers who comply with Veterans Health Administration (VHA) policy and procedures for release of data. The VHA requires that VHA data be maintained on VHA approved devices and networks and that all persons accessing the data have IRB and VA R&D approval to do so. This includes having a direct appointment in the VHA or a WOC (without compensation) appointment if a faculty member at a collaborating university/ medical school.