Abstract
Background
Per- and polyfluoroalkyl substances (PFAS) are highly persistent chemicals that have been detected in the serum of over 98% of the US population. Studies among highly exposed individuals suggest an association with perfluorooctanoic acid (PFOA) exposure and kidney cancer. It remains unclear whether PFOA or other PFAS are renal carcinogens or if they influence risk of renal cell carcinoma (RCC) at concentrations observed in the general population.
Methods
We measured prediagnostic serum concentrations of PFOA and 7 additional PFAS in 324 RCC cases and 324 individually matched controls within the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Multivariable conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CIs) relating serum PFAS concentrations and RCC risk. Individual PFAS were modeled continuously (log2-transformed) and categorically, with adjustment for kidney function and additional potential confounders. All statistical tests were 2-sided.
Results
We observed a positive association with RCC risk for PFOA (doubling in serum concentration, ORcontinuous = 1.71, 95% CI = 1.23 to 2.37, P = .002) and a greater than twofold increased risk among those in the highest quartile vs the lowest (OR = 2.63, 95% CI = 1.33 to 5.20, Ptrend = .007). The association with PFOA was similar after adjustment for other PFAS (ORcontinuous = 1.68, 95% CI = 1.07 to 2.63, P = .02) and remained apparent in analyses restricted to individuals without evidence of diminished kidney function and in cases diagnosed 8 or more years after phlebotomy.
Conclusions
Our findings add substantially to the weight of evidence that PFOA is a renal carcinogen and may have important public health implications for the many individuals exposed to this ubiquitous and highly persistent chemical.
Per- and polyfluoroalkyl substances (PFAS) are a diverse class of synthetic chemicals that have been used extensively since the 1950s in a wide range of commercial and industrial applications, including nonstick cookware, textiles, and firefighting foams. PFAS are highly persistent in the environment and many can bioaccumulate in humans, with serum elimination half-lives ranging from approximately 3 to 8 years (1,2). Exposure to PFAS is widespread in the general population; serum concentrations of 4 major PFAS were all detectable in more than 98% of participants in the nationally representative US National Health and Nutrition Examination Survey (NHANES) (3). Elevated concentrations of PFAS have been observed in drinking water supplies near PFAS point sources such as industrial sites, military firefighting training areas, and wastewater treatment plants (4). In addition, studies of individuals exposed to contaminated drinking water have reported higher than background serum concentrations of certain PFAS (5–8).
The International Agency for Research on Cancer (IARC) has classified perfluorooctanoic acid (PFOA) as a possible human carcinogen (group 2B) based in part on limited epidemiologic evidence of associations with kidney cancer; the carcinogenic potential of other PFAS have not yet been evaluated (9). Higher kidney cancer incidence and mortality were observed among individuals with high PFOA exposures from employment in a PFAS-producing chemical plant or residence in the surrounding community with contaminated drinking water (10–12). However, to our knowledge, no prospective studies have assessed the relationship between PFOA and kidney cancer risk in the general population, and associations between other PFAS and risk of kidney cancer have not been evaluated. To address these research gaps, we conducted a nested case-control study within the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial to evaluate the risk of renal cell carcinoma (RCC; the most common form of kidney cancer) in relation to prediagnostic serum concentrations of PFOA and 7 other PFAS.
Methods
Study Population
The design and sample collection procedures for PLCO have been described (13,14). Briefly, PLCO is a randomized screening trial that recruited approximately 150 000 adults ages 55-74 years from study centers in 10 US cities between 1993 and 2001; participants in the screening arm provided nonfasting blood samples. The PLCO Cancer Screening Trial protocol was approved by institutional review boards of the National Cancer Institute and the individual study centers, and all participants provided written informed consent.
Among participants in the screening arm with available prediagnostic serum samples, we identified 326 incident RCC cases (International Classification of Diseases for Oncology, Second Edition C64.9) diagnosed an average of 8.8 years after phlebotomy (range=2-18 years). Controls were individually matched to cases with a 1:1 ratio on age at enrollment (55-59 years, 60-64 years, 65-69 years, or ≥70 years), sex, race and ethnicity (non-Hispanic white, non-Hispanic Black, Hispanic, Asian, or Native American), study center, and study year of blood draw. All controls were alive and free of RCC as of the diagnosis date of their corresponding matched case.
Laboratory Tests
At the Centers for Disease Control and Prevention (CDC, Atlanta, GA), using online solid phase extraction liquid chromatography isotope dilution tandem mass spectrometry as described previously (15), we quantified serum concentrations of 10 analytes: 2-N-methyl-perfluorooctane sulfonamido acetic acid, 2-N-ethyl-perfluorooctane sulfonamido acetic acid, perfluorohexane sulfonic acid (PFHxS), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), linear PFOA, sum of branched PFOA isomers, linear perfluorooctane sulfonic acid, and sum of perfluoromethylheptane sulfonic acid isomers. The limit of detection (LOD) was 0.1 µg/L for all analytes; concentrations below the LOD were assigned a value of one-half the LOD.
We report results for total PFOA and perfluorooctane sulfonic acid (PFOS), which were calculated by summing the concentrations of their respective isomers (ie, linear PFOA and sum of branched PFOA isomers for total PFOA and linear perfluorooctane sulfonic acid and sum of perfluoromethylheptane sulfonic acid isomers for total PFOS) (16). Samples from each matched case-control set were analyzed in the same analytical batch. Intra-assay coefficients of variation for individual PFAS were 7.2%-16.6%, and overall intraclass correlation coefficients were 0.92-0.97 (Supplementary Table 1, available online). Laboratory staff was blinded to the case or control status of each sample and the quality control replicates among the test samples. The CDC determined that analyzing coded specimens at the CDC laboratory did not constitute engagement in human subject research.
Given concerns that diminished kidney function may impact PFAS serum concentrations (17–20), we measured serum cystatin C and creatinine in all cases and controls and calculated the estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (21) Serum cystatin C was measured using a microbead-based assay on a Luminex system (22), and serum creatinine was measured using a clinical chemistry analyzer.
Statistical Analysis
Measurements of PFAS concentrations were missing for 2 RCC cases; we excluded those matched case-control sets, leaving a total of 324 cases and 324 matched controls for analysis. For our primary analyses, we estimated odds ratios (ORs) and 95% confidence intervals (CIs) using multivariable conditional logistic regression analysis with PFAS concentrations modeled both continuously (log2-transformed) and categorically. Category cut points were assigned based on quartiles of serum concentrations of each PFAS among controls except for PFUnDA and PFDA, for which over 25% of measurements were below the LOD (categories for PFUnDA: ≤LOD, >0.1-0.2 µg/L, >0.2 µg/L; and PFDA: ≤LOD, >0.1-0.2 µg/L, >0.2-0.3 µg/L, >0.3 µg/L). Each model implicitly controlled for matching factors and was further adjusted for eGFR (continuous), body mass index (<18.5 kg/m2, 18.5 to <25 kg/m2, 25 to <30 kg/m2, ≥30 kg/m2, or missing), smoking status (never, former, or current), history of hypertension, prior freeze-thaw cycles, and calendar year of blood draw. Wald tests for linear trend were performed by modeling the within-category median of each quartile of exposure as a continuous variable. We also conducted secondary analyses where we flexibly modeled the relationship between the log-OR and the log2-transformed PFAS concentrations using a natural spline with 3 degrees of freedom and then used a likelihood ratio test to assess model improvement over the primary model with only the linear term.
To assess the individual effects of specific PFAS, we performed further analyses adjusting for log2-transformed concentrations of PFOA, PFOS, and PFHxS. Then we evaluated the joint effects of PFOA, PFOS, and PFHxS with concentrations of each analyte categorized into tertiles based on the distributions among controls.
We performed secondary stratified analyses using unconditional logistic regression models, adjusting for individual matching factors and other covariates included in the primary analyses noted above, to estimate stratum-specific odds ratios and 95% confidence intervals for individual PFAS modeled continuously. Because cases and controls within the same matched set may have differed with respect to some of the stratifying variables, unconditional models were used to reduce the impact of missing data on the stratified analyses. Analyses were stratified by the following: age at enrollment (55-59 years, 60-64 years, ≥65 years), sex, body mass index (18.5 to <25, 25 to <30, ≥30 kg/m2), history of hypertension, smoking history (ever, never), eGFR (60-89, ≥90 mL/min/1.73 m2), samples with and without prior freeze-thaw cycles, and years from blood collection to RCC diagnosis (2 to <8, ≥8 years). Wald tests of heterogeneity were performed by including an interaction term in the model. We also conducted sensitivity analyses restricted to non-Hispanic white participants, those without evidence of diminished kidney function (ie, eGFR ≥60 mL/min/1.73 m2), and RCC cases of clear cell histology (International Classification of Diseases for Oncology, Second Edition morphology code 8310).
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Results with 2-sided P less than .05 were considered statistically significant.
Results
Based on the matched design, RCC cases and controls had the same distributions for sex, race, age at enrollment, and study center (Table 1). Cases were more likely than controls to report being obese and to have a history of hypertension at enrollment. A higher proportion of cases had diminished kidney function (eGFR < 60 mL/min/1.73 m2) compared with controls (9.0% vs 5.6%), but this difference was not statistically significant (P = .25). The overall distributions of each PFAS among cases and controls are shown in Supplementary Figure 1 (available online). Among the controls, several PFAS were moderately correlated with one another (eg, Spearman correlation coefficients of 0.62 for PFOA vs PFOS, 0.42 for PFOA vs PFHxS, and 0.45 for PFOS vs PFHxS; Supplementary Table 2, available online). In multivariable analyses, adjusted geometric mean concentrations of PFOS, PFNA, PFDA, and PFUnDA were statistically significantly elevated among African Americans relative to non-Hispanic whites (P < .01) (Supplementary Table 3, available online). These analyses also indicated PFOS, PFHxS, PFDA, and PFUnDA concentrations were statistically significantly lower among women compared with men (P < .02).
Table 1.
Selected demographic and health characteristics of renal cell carcinoma cases and controls in the PLCO Cancer Screening Trial
| Characteristic | Study participants, No. (%)a |
||
|---|---|---|---|
| Controls (n = 324) | Cases (n = 324) | P b | |
| Age,c y | |||
| 55-59 | 95 (29.3) | 95 (29.3) | — |
| 60-64 | 112 (34.6) | 112 (34.6) | |
| 65-69 | 80 (24.7) | 80 (24.7) | |
| 70+ | 37 (11.4) | 37 (11.4) | |
| Center | |||
| Colorado | 20 (6.2) | 20 (6.2) | — |
| Georgetown (Washington, DC) | 15 (4.6) | 15 (4.6) | |
| Hawaii | 9 (2.8) | 9 (2.8) | |
| Henry Ford (Michigan) | 37 (11.4) | 37 (11.4) | |
| Minnesota | 84 (25.9) | 84 (25.9) | |
| Washington University (Missouri) | 33 (10.2) | 33 (10.2) | |
| University of Pittsburgh (Pennsylvania) | 40 (12.4) | 40 (12.4 | |
| University of Utah | 27 (8.3) | 27 (8.3) | |
| Marshfield (Wisconsin) | 46 (14.2) | 46 (14.2) | |
| University of Alabama | 13 (4.0) | 13 (4.0) | |
| Sex | |||
| Male | 216 (66.7) | 216 (66.7) | — |
| Female | 108 (33.3) | 108 (33.3) | |
| Race | |||
| White, non-Hispanic | 287 (88.6) | 287 (88.6) | — |
| Black, non-Hispanic | 21 (6.5) | 21 (6.5) | |
| Other | 16 (4.9) | 16 (4.9) | |
| Body mass index,c kg/m2 | |||
| <18.5 | 3 (0.9) | 2 (0.6) | .008 |
| 18.5 to <25 | 83 (25.6) | 71 (21.9) | |
| 25 to <30 | 158 (48.8) | 135 (41.7) | |
| 30+ | 76 (23.5) | 115 (35.5) | |
| Unknown | 4 (1.2) | 1 (0.3) | |
| History of hypertensionc | |||
| Nod | 216 (66.7) | 183 (56.5) | .008 |
| Yes | 108 (33.3) | 141 (43.5) | |
| Smoking statusc | |||
| Never | 155 (47.8) | 143 (44.1) | .54 |
| Former | 134 (41.4) | 148 (45.7) | |
| Current | 35 (10.8) | 33 (10.2) | |
| Calendar yeare | |||
| 1993-1995 | 84 (25.9) | 88 (27.2) | .67 |
| 1996-1997 | 116 (35.8) | 123 (38.0) | |
| 1998-2002 | 124 (38.3) | 113 (34.9) | |
| eGFR,e mL/min/1.73 m2 | |||
| 90+ | 109 (33.6) | 106 (32.7) | .25 |
| <90-60 | 197 (60.8) | 189 (58.3) | |
| <60 | 18 (5.6) | 29 (9.0) | |
Groups may not sum to 100% due to rounding. eGFR = estimated glomerular filtration rate; PLCO = Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
χ2 test, except for body mass index (Fisher’s exact test). Not reported for matching factors (age, center, sex, and race).
Self-reported at study baseline.
Includes 1 case with missing information for history of hypertension.
At blood draw.
As shown in Table 2, we observed statistically significant positive trends in RCC risk with increasing prediagnostic concentrations of several PFAS, including PFOA (highest quartile vs lowest, OR = 2.63, 95% CI = 1.33 to 5.20, Ptrend = .007), PFOS (OR = 2.51, 95% CI = 1.28 to 4.92, Ptrend = .009), and PFHxS (OR = 2.07, 95% CI = 1.06 to 4.04, Ptrend = .04). No evidence of a gradient in RCC risk with serum concentrations of other PFAS was apparent. When PFAS concentrations were modeled continuously (per a 1-unit increase in log2-transformed concentrations), we observed that a doubling in serum PFOA concentrations was associated with an approximately 70% increase in the risk of RCC (ORcontinuous = 1.71, 95% CI = 1.23 to 2.37, P = .002); the corresponding risk estimates for PFOS and PFHxS were 1.39 (95% CI = 1.04 to 1.86, P = .03) and 1.27 (95% CI = 1.03 to 1.56, P = .02), respectively. The association with PFOA persisted after adjusting for PFOS and PFHxS concentrations (ORcontinuous = 1.68, 95% CI = 1.07 to 2.63, P = .02), whereas the estimates of risk for the other PFAS were attenuated in mutually adjusted analyses. In joint analyses, no statistically significant interactions with PFOA were observed for PFOS (P = .71) or PFHxS (P = .77) (Supplementary Figure 2, available online). Secondary analyses of log2-transformed concentrations of each PFAS modeled continuously using natural splines found no evidence of a nonlinear relationship between PFOA and RCC risk, although there was some suggestion of nonlinearity for several other PFAS (Supplementary Figure 3, available online).
Table 2.
Odds ratios and 95% confidence intervals evaluating PFAS serum concentrations and risk of renal cell carcinoma in the PLCO Cancer Screening Trial
| PFAS | Controls, No. | Cases, No. | μg/La | OR (95% CI)b | P trend c | OR (95% CI)d | P trend c |
|---|---|---|---|---|---|---|---|
| PFOA | 81 | 47 | <4.0 | 1.00 (Reference) | .007 | 1.00 (Reference) | .13 |
| 79 | 83 | ≥4.0-5.5 | 1.47 (0.77 to 2.80) | 1.41 (0.69 to 2.90) | |||
| 83 | 69 | >5.5-7.3 | 1.24 (0.64 to 2.41) | 1.12 (0.52 to 2.42) | |||
| 81 | 125 | >7.3-27.2 | 2.63 (1.33 to 5.20) | 2.19 (0.86 to 5.61) | |||
| Continuouse | 1.71 (1.23 to 2.37) | 1.68 (1.07 to 2.63) | |||||
| PFOS | 81 | 60 | ≤26.3 | 1.00 (Reference) | .009 | 1.00 (Reference) | .64 |
| 81 | 82 | >26.3-38.4 | 1.67 (0.84 to 3.30) | 1.24 (0.59 to 2.57) | |||
| 81 | 61 | >38.4-49.9 | 0.92 (0.45 to 1.88) | 0.53 (0.22 to 1.24) | |||
| 81 | 121 | >49.9-154.2 | 2.51 (1.28 to 4.92) | 1.14 (0.45 to 2.88) | |||
| Continuouse | 1.39 (1.04 to 1.86) | 0.92 (0.60 to 1.42) | |||||
| PFHxS | 88 | 75 | ≤2.2 | 1.00 (Reference) | .04 | 1.00 (Reference) | .40 |
| 83 | 74 | >2.2-3.4 | 1.41 (0.75 to 2.64) | 1.28 (0.66 to 2.51) | |||
| 76 | 88 | >3.4-5.5 | 1.14 (0.59 to 2.20) | 0.89 (0.43 to 1.85) | |||
| 77 | 87 | >5.5-37.4 | 2.07 (1.06 to 4.04) | 1.46 (0.67 to 3.18) | |||
| Continuouse | 1.27 (1.03 to 1.56) | 1.12 (0.88 to 1.43) | |||||
| PFUnDA | 166 | 161 | <LOD | 1.00 (Reference) | .09 | 1.00 (Reference) | .20 |
| 104 | 108 | ≥0.1-0.2 | 1.29 (0.71 to 2.34) | 1.15 (0.62 to 2.16) | |||
| 54 | 55 | >0.2-1.7 | 2.07 (0.90 to 4.76) | 1.83 (0.75 to 4.48) | |||
| Continuouse | 1.17 (0.93 to 1.47) | 1.14 (0.88 to 1.47) | |||||
| PFNA | 119 | 95 | ≤0.5 | 1.00 (Reference) | .08 | 1.00 (Reference) | .45 |
| 79 | 73 | >0.5-0.7 | 1.43 (0.81 to 2.51) | 1.08 (0.57 to 2.07) | |||
| 50 | 78 | >0.7-1.0 | 2.59 (1.30 to 5.15) | 2.00 (0.95 to 4.20) | |||
| 76 | 78 | >1.0-4.9 | 1.81 (0.91 to 3.61) | 1.29 (0.58 to 2.89) | |||
| Continuouse | 1.19 (0.91 to 1.55) | 1.00 (0.73 to 1.37) | |||||
| EtFOSAA | 90 | 65 | ≤0.7 | 1.00 (Reference) | .74 | 1.00 (Reference) | .63 |
| 76 | 82 | >0.7-1.2 | 1.54 (0.83 to 2.88) | 1.37 (0.72 to 2.63) | |||
| 79 | 97 | >1.2-2.4 | 1.69 (0.91 to 3.14) | 1.33 (0.69 to 2.58) | |||
| 79 | 80 | >2.4-60.4 | 1.41 (0.71 to 2.81) | 1.04 (0.49 to 2.20) | |||
| Continuouse | 1.07 (0.90 to 1.27) | 0.97 (0.79 to 1.18) | |||||
| MeFOSAA | 101 | 84 | ≤0.9 | 1.00 (Reference) | .86 | 1.00 (Reference) | .31 |
| 73 | 78 | >0.9-1.4 | 1.00 (0.53 to 1.89) | 0.77 (0.40 to 1.50) | |||
| 73 | 83 | >1.4-2.1 | 1.38 (0.73 to 2.63) | 1.00 (0.50 to 2.01) | |||
| 77 | 79 | >2.1-8.2 | 0.92 (0.48 to 1.76) | 0.65 (0.32 to 1.33) | |||
| Continuouse | 1.01 (0.80 to 1.29) | 0.86 (0.66 to 1.12) | |||||
| PFDA | 91 | 92 | <LOD | 1.00 (Reference) | .20 | 1.00 (Reference) | .61 |
| 147 | 135 | ≥0.1-0.2 | 1.01 (0.57 to 1.79) | 0.80 (0.42 to 1.51) | |||
| 34 | 40 | >0.2-0.3 | 1.47 (0.62 to 3.45) | 1.03 (0.40 to 2.64) | |||
| 52 | 57 | >0.3-2.1 | 1.70 (0.72 to 4.03) | 1.21 (0.44 to 3.31) | |||
| Continuouse | 1.19 (0.95 to 1.48) | 1.11 (0.85 to 1.44) |
Category cut points were assigned based on quartiles of serum concentrations of each PFAS among controls except for PFUnDA and PFDA, for which more than 25% of measurements were below the LOD. CI = confidence interval; EtFOSAA = 2-N-ethyl-perfluorooctane sulfonamido acetic acid; LOD = limit of detection; MeFOSAA = 2-N-methyl-perfluorooctane sulfonamido acetic acid; OR = odds ratio; PFAS = per- and polyfluoroalkyl substances; PFDA = perfluorodecanoic acid; PFHxS = perfluorohexane sulfonic acid; PFNA = perfluorononanoic acid; PFOA = perfluorooctanoic acid; PFOS = perfluorooctane sulfonic acid; PFUnDA = perfluoroundecanoic acid; PLCO = Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial.
Adjusted for body mass index (missing, <18.5, 18.5 to <25, 25 to <30, or ≥30 kg/m2), smoking status (never, former, current), history of hypertension (no, yes), estimated glomerular filtration rate (continuous), previous freeze-thaw cycle, and calendar year of blood draw (1993-1995, 1996-1997, 1998-2002).
Based on intraquartile median value.
Further adjusted for other PFAS (ie, log2-transformed concentrations of PFOA, PFOS, and PFHxS).
Continuous odds ratios (95% confidence intervals) for RCC risk in relation to a 1-unit increase in serum PFAS concentrations on the log base 2 scale, corresponding to an approximate doubling in analyte levels.
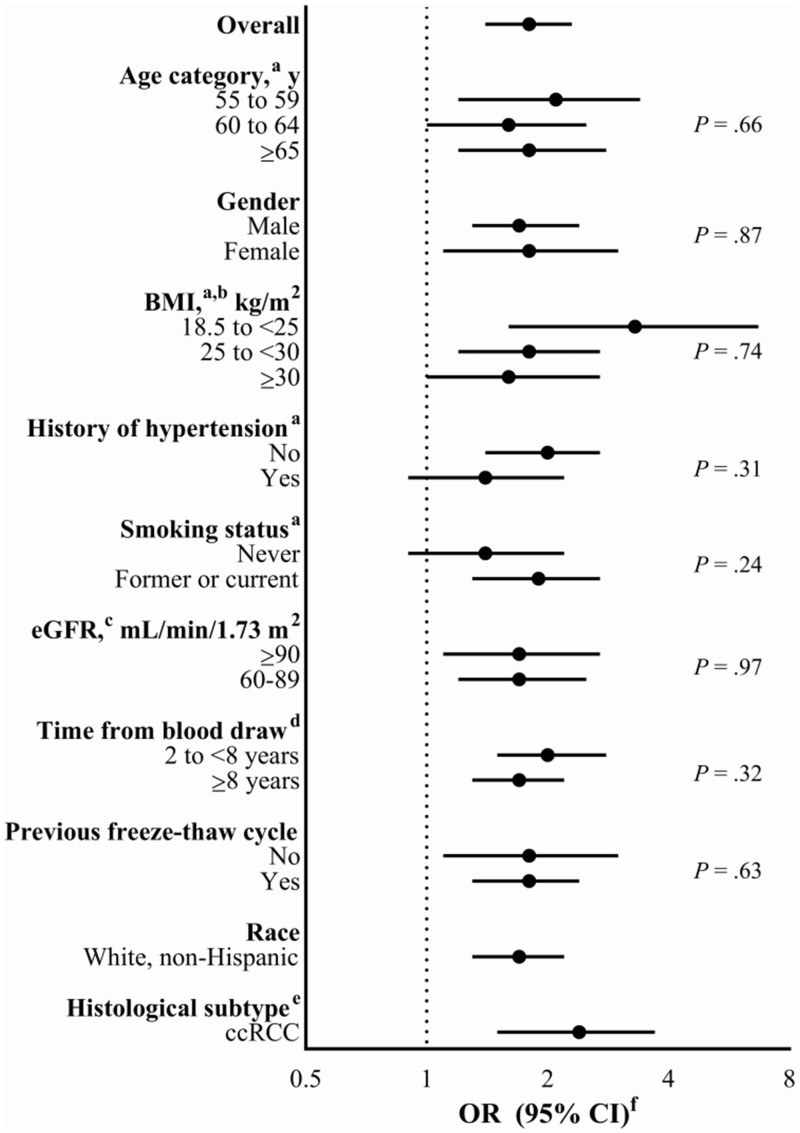
Figure 1 shows the results of stratified and sensitivity analyses further assessing the relationship between PFOA concentrations and RCC risk. Notably, our results were unchanged after excluding participants with diminished kidney function (eGFR < 60 mL/min/1.73 m2). In addition, associations were similar among participants with mild loss of kidney function (eGFR 60-89 mL/min/1.73 m2) and those with high function (eGFR ≥90 mL/min/1.73 m2). Furthermore, the association persisted among cases diagnosed 8 years or more after blood collection (OR = 1.66, 95% CI = 1.25 to 2.19), and associations were similar in analyses of samples with and without prior freeze-thaw cycles.
Figure 1.
Odds ratios (ORs) and 95% confidence intervals (CIs) evaluating serum perfluorooctanoic acid concentrations and risk of renal cell carcinoma in stratified and sensitivity analyses in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. P values represent Wald tests of heterogeneity across strata. BMI = body mass index; ccRCC = clear cell renal cell carcinoma; eGFR = estimated glomerular filtration rate. aSelf-reported at study enrollment. bBMI-specific analyses exclude individuals with BMI that is missing or less than 18.5 kg/m2. cAt blood draw. dTime from blood draw to diagnosis for cases. eInternational Classification of Diseases for Oncology, Second Edition morphology code = 8310. fContinuous odds ratios (95% confidence intervals) for RCC risk in relation to a 1-unit increase in serum PFAS concentrations on the log2 scale, or an approximate doubling in analyte levels, were estimated using unconditional multivariable logistic regression models adjusted for age at enrollment (55-59 years, 60-64 years, 65-69 years, ≥70 years), sex (male, female), race and ethnicity (white non-Hispanic, Black non-Hispanic, or other), eGFR (continuous), BMI (<18.5 kg/m2, 18.5 to <25 kg/m2, 25 to <30 kg/m2, ≥30 kg/m2, missing), history of hypertension (no or missing, yes), smoking status (never, former, current), previous freeze-thaw cycle, calendar year of blood draw (1993-1995, 1996-1997, 1998-2002), study year of blood draw (enrollment, other), and study center ([1] Minnesota or Marshfield; [2] Colorado, Hawaii, Washington University, University of Utah, or University of Alabama; [3] Georgetown, Henry Ford, or University of Pittsburgh).
We observed a stronger association with PFOA in analyses restricted to clear cell RCC (ncases = 92; OR = 2.38, 95% CI = 1.51 to 3.74). Associations were also somewhat stronger among those with normal body weight (BMI 18.5 to <25 kg/m2), those without a history of hypertension, and former or current smokers, although tests of heterogeneity were not statistically significant. We observed similar patterns in stratified and sensitivity analyses of PFOA after simultaneously adjusting for PFOS and PFHxS (Supplementary Figure 4, available online).
Discussion
In this nested case-control study of 324 cases and 324 matched controls in a general population cohort, we observed a statistically significant increased risk of RCC among participants with higher prediagnostic serum concentrations of PFOA based on models adjusted for kidney function and other potential confounding factors. This association persisted in analyses restricted to participants without evidence of diminished kidney function and among cases diagnosed 8 or more years after blood collection. When we restricted the case series to those with confirmed clear cell histology, the association with PFOA was more pronounced. We also observed associations with RCC for PFOS and PFHxS in models unadjusted for other PFAS. However, after mutual adjustment for these 3 chemicals, only the association with PFOA remained statistically significant.
To our knowledge, this is the first prospective study to investigate the associations between serum concentrations of individual PFAS and kidney cancer risk in a cohort with PFAS concentrations comparable with the general population. The distributions of serum PFAS concentrations among the controls in our study were similar to those observed among adults in the nationally representative NHANES study during the same time period. In particular, participants in the highest quartile of PFOA serum concentrations in our study (>7.3 μg/L) had concentrations comparable with the highest quartile of the distribution among US adults in NHANES in 1999-2000 (>7.0 μg/L), the earliest NHANES cycle for which such data were available (23). Notably, quantification of PFAS concentrations for the current study was performed by the same laboratory analyzing NHANES using the same analytical approach (3).
Moreover, the patterns of PFAS serum concentrations by demographic factors (eg, sex and race and ethnicity) reflect those observed in NHANES, further supporting the relevance of our results for the general US population. Individuals in the general population can be exposed to PFAS through various sources, including food, dust, and contaminated drinking water (24–26). With an estimated 6 million US residents using public water supplies with PFAS concentrations exceeding the US Environmental Protection Agency's lifetime health advisory limit (4), elucidating the carcinogenic potential of PFAS is a major public health concern.
Our results for PFOA are notable in light of suggestive but somewhat inconsistent prior findings for kidney cancer risk among those with occupational or high environmental PFOA exposure (10–12,27). In IARC’s evaluation of the carcinogenicity of PFOA in 2014 (9), this chemical was classified as possibly carcinogenic to humans (group 2B) based in part on limited evidence in humans that PFOA causes renal cancer and on limited evidence of carcinogenicity in experimental animals. The IARC evaluation noted evidence of positive associations with kidney cancer among individuals highly exposed to PFOA who were working or living near a PFAS-producing facility in the mid-Ohio Valley (10–12). In an analysis of 5791 workers from this facility, mortality from kidney cancer was elevated among those with high estimated cumulative serum PFOA concentrations (11). Two complementary studies of environmentally exposed community members in the mid-Ohio Valley also observed suggestive associations between higher estimated serum PFOA concentrations and increased kidney cancer risk (10,12). Estimates of lifetime cumulative serum PFOA concentrations in these investigations were based on modeling approaches that have been previously described in detail and validated (28,29). In contrast, another study of 4668 workers (including 4231 who were eligible for cancer follow-up) exposed to ammonium perfluorooctanoate (APFO, the ammonium salt of PFOA) at a facility in Minnesota found no evidence of an excess incidence of kidney cancer (27). However, the characterization of APFO exposure for this analysis was based on an assessment of inhalation exposure that used air-monitoring data (in APFO production areas) and expert judgment (in non-APFO production areas). It is possible that this exposure assessment approach, which did not consider other potential routes of exposure, may have resulted in greater exposure misclassification than in the mid-Ohio Valley studies, potentially obscuring an effect. Methodologic advantages of the current study relative to prior work include the direct assessment of serum PFAS concentrations in participants and prospective follow-up.
Serum concentrations of PFOA and other PFAS have been inversely associated with kidney function (ie, lower eGFR) in cross-sectional analyses among children, adolescents, and adults in the mid-Ohio Valley (17,18). Similar cross-sectional associations have been observed in NHANES (19,30), although more recent analyses suggest that this relationship may be nonlinear in the general population (20). Researchers have suggested that these inverse associations could be due to reverse causation as a result of reduced capacity to filter and excrete PFAS among those with diminished kidney function (18).
Given that lower eGFR has been linked to an increased risk of RCC (31,32), we assessed kidney function in this investigation and performed multiple sensitivity and stratified analyses to evaluate the potential for confounding and effect modification. We found that the observed association between PFOA and RCC persisted among individuals without evidence of diminished kidney function (ie, eGFR ≥60 mL/min/1.73 m2) and when restricted to individuals with high kidney function (ie, eGFR ≥90 mL/min/1.73 m2). Overall, these findings suggest that the relationship between PFOA and RCC observed in our study population is likely to be independent of potential effects related to kidney function.
An increasing number of studies are investigating the biologic plausibility and mechanisms through which PFOA may induce nephrotoxicity and possibly influence renal carcinogenesis (9,33). Although information on the distribution of PFAS in human tissues remains sparse, 1 study of 20 individuals detected PFOA in 95% of autopsy kidney samples assayed (34). This finding is consistent with evidence from previous animal studies, which suggest that the distribution of PFOA may be enriched in the kidneys, serum, and liver (35). Studies of PFAS exposure in animal models have observed evidence of renal tubular hypertrophy or hyperplasia as well as increased kidney weights (36). In particular, adverse health effects of PFOA and other PFAS in animal studies have been attributed to activation of peroxisome proliferator-activated receptor alpha (37), which may influence pathways related to oxidative stress and lipid metabolism (36) and has been implicated in RCC development (38,39).
This study has several important strengths that help advance our understanding of the relationship between exposure to PFAS and risk of kidney cancer. It is, to our knowledge, the largest investigation of PFOA exposure and RCC risk to date, the first to investigate RCC risk in relation to other PFAS beyond PFOA, and the first to prospectively examine associations with RCC using prediagnostic serum PFAS concentrations. We were able to demonstrate that the observed associations are unlikely to be attributable to reverse causation as a result of diminished kidney function among the RCC cases and were able to adjust for other potential confounding factors, including obesity and hypertension.
Several limitations of this study should also be noted. Our assessment of PFAS exposure was based on serum concentrations in samples collected from a single point in time. Nevertheless, the long serum elimination half-lives of many PFAS, including PFOA, PFOS, and PFHxS (1,2), and evidence from other population-based studies of within-individual temporal stability in PFAS concentrations using samples collected multiple years apart (40), indicate that measured concentrations likely reflect long-term exposures. Also, non-Hispanic whites largely composed our study population, limiting our ability to assess racial or ethnic differences in the relationship between PFAS concentrations and RCC risk. Consistent with findings from NHANES (3), we observed evidence of higher concentrations of certain PFAS (including PFOS) among African Americans compared with non-Hispanic whites among controls in our study. Future efforts extending this work to more diverse study populations would be informative, given that the incidence of RCC in the United States differs by race, with the highest rates among African Americans (32).
In summary, we observed a statistically significant positive exposure-response association between prediagnostic serum PFOA concentrations and subsequent risk of RCC within a population-based US prospective cohort. We also found that this association between PFOA and RCC remained after adjustment for other PFAS. These findings add substantially to the weight of evidence that PFOA is a renal carcinogen and may have important public health implications for the many individuals exposed to this ubiquitous and highly persistent chemical worldwide.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute (NCI) and by the Department of Defense through the Kidney Cancer Research Program under Award No. WH81XWH-18–1-0507. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702–5014, is the awarding and administering acquisition office. The NCI funded the PLCO Cancer Screening Trial, which was also supported by contracts from the Division of Cancer Prevention of the NCI and by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, NCI, National Institutes of Health (NIH). VS is supported by NIH grants R01 CA2229772 and U01 DK127587.
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors declare no competing financial interests.
Disclaimers: Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services. JJS and CLC contributed to this manuscript while employed at the National Cancer Institute and are currently employed at the U.S. Food and Drug Administration. This manuscript reflects the views of the authors and should not be construed to represent the U.S. Food and Drug Administration’s views or policies.
Acknowledgments: The authors thank the PLCO Cancer Screening Trial screening center investigators; Craig Williams, Matt Moore, and other staff at Information Management Services, Inc.; and, most important, the study participants for their contributions that made this study possible. We acknowledge the technical assistance of K. Kato, K. Hubbard, J. Botelho, and T. Jia (CDC, Atlanta, GA) in measuring the serum concentrations of PFAS.
Author contributions: Conceptualization: CLC, MPP, JNH; Methodology: JJS, CLC, AMC, WYH, RRJ, VSS, NDF, JNS, DTS, MPP, JNH; Formal analysis: JJS, JNS, JNH; Writing – original draft: JJS, JNH; Writing – review & editing: CLC, AMC, WYH, RRJ, VSS, NDF, JNS, DTS, MPP.
Data Availability
Requests for access to the data underlying this article should be submitted through the PLCO Cancer Data Access System (https://cdas.cancer.gov/learn/plco/instructions/? type=data).
Supplementary Material
References
- 1. Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Fletcher T, Mucs D, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018;75(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL.. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect. 2007;115(11):1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016;3(10):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurley S, Houtz E, Goldberg D, et al. Preliminary associations between the detection of perfluoroalkyl acids (PFAAs) in drinking water and serum concentrations in a sample of California women. Environ Sci Technol Lett. 2016;3(7):264–269. [Google Scholar]
- 6. Barton KE, Starling AP, Higgins CP, McDonough CA, Calafat AM, Adgate JL.. Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. Int J Hyg Environ Health. 2020;223(1):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daly ER, Chan BP, Talbot EA, et al. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int J Hyg Environ Health. 2018;221(3):569–577. [DOI] [PubMed] [Google Scholar]
- 8. Gyllenhammar I, Berger U, Sundstrom M, et al. Influence of contaminated drinking water on perfluoroalkyl acid levels in human serum--A case study from Uppsala, Sweden. Environ Res. 2015;140:673–683. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. Perfluorooctanoic acid (PFOA). 2017. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono110-01.pdf. Accessed May 20, 2020.
- 10. Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T.. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121(3):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steenland K, Woskie S.. Cohort mortality study of workers exposed to perfluorooctanoic acid. Am J Epidemiol. 2012;176(10):909–917. [DOI] [PubMed] [Google Scholar]
- 12. Barry V, Winquist A, Steenland K.. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121(11-12):1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayes RB, Reding D, Kopp W, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6):349S–355S. [DOI] [PubMed] [Google Scholar]
- 14. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6):273S–309S. [DOI] [PubMed] [Google Scholar]
- 15. Kato K, Kalathil AA, Patel AM, Ye X, Calafat AM.. Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere. 2018;209:338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Perfluoroalkyl and polyfluoroalkyl substances NHANES 2015-2016. 2018. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/PFAS_I_MET.pdf. Accessed May 20, 2020.
- 17. Watkins DJ, Josson J, Elston B, et al. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect. 2013;121(5):625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K.. A study of reverse causation: examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ Health Perspect. 2017;125(3):416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shankar A, Xiao J, Ducatman A.. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol. 2011;174(8):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jain RB, Ducatman A.. Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: implications for future research. Environ Res. 2019;169:476–482. [DOI] [PubMed] [Google Scholar]
- 21. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lagos-Arevalo P, Palijan A, Vertullo L, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol. 2015;30(4):665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. 2019. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf. Accessed January 31, 2020.
- 24. Hu XC, Tokranov AK, Liddie J, et al. Tap water contributions to plasma concentrations of poly- and perfluoroalkyl substances (PFAS) in a Nationwide Prospective Cohort of U. S. Women. Environ Health Perspect. 2019;127(6):067006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int. 2016;92-93:357–365. [DOI] [PubMed] [Google Scholar]
- 26. Haug LS, Huber S, Becher G, Thomsen C.. Characterisation of human exposure pathways to perfluorinated compounds--comparing exposure estimates with biomarkers of exposure. Environ Int. 2011;37(4):687–693. [DOI] [PubMed] [Google Scholar]
- 27. Raleigh KK, Alexander BH, Olsen GW, et al. Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup Environ Med. 2014;71(7):500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin HM, Vieira VM, Ryan PB, et al. Environmental fate and transport modeling for perfluorooctanoic acid emitted from the Washington Works Facility in West Virginia. Environ Sci Technol. 2011;45(4):1435–1442. [DOI] [PubMed] [Google Scholar]
- 29. Shin HM, Vieira VM, Ryan PB, Steenland K, Bartell SM.. Retrospective exposure estimation and predicted versus observed serum perfluorooctanoic acid concentrations for participants in the C8 Health Project. Environ Health Perspect. 2011;119(12):1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L.. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health. 2015;14(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lowrance WT, Ordonez J, Udaltsova N, Russo P, Go AS.. CKD and the risk of incident cancer. J Am Soc Nephrol. 2014;25(10):2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chow WH, Scelo G, Tarone RE.. Renal Cancer. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, eds. Schottenfeld and Fraumeni Cancer Epidemiology and Prevention, Fourth Edition. New York, NY: Oxford University Press; 2018. pp. 961–976. [Google Scholar]
- 33.National Toxicology Program. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Perfluorooctanoic Acid (CAS No. 335-67-1) Administered in Feed to Sprague Dawley (Hsd: Sprague Dawley® SD®) Rats. 2019; https://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2019/december/tr598draft.pdf. Accessed January 13, 2020. [DOI] [PMC free article] [PubMed]
- 34. Perez F, Nadal M, Navarro-Ortega A, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–362. [DOI] [PubMed] [Google Scholar]
- 35. Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J.. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–394. [DOI] [PubMed] [Google Scholar]
- 36. Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE.. Perfluorinated chemicals as emerging environmental threats to kidney health: a scoping review. Clin J Am Soc Nephrol. 2018;13(10):1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agency for Toxic Substances and Disease Registry. Toxicological profile for perfluoroalkyls. 2018; https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf. Accessed May 20, 2020. [PubMed]
- 38. Abu Aboud O, Wettersten HI, Weiss RH.. Inhibition of PPARalpha induces cell cycle arrest and apoptosis, and synergizes with glycolysis inhibition in kidney cancer cells. PLoS One. 2013;8(8):e71115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abu Aboud O, Donohoe D, Bultman S, et al. PPARalpha inhibition modulates multiple reprogrammed metabolic pathways in kidney cancer and attenuates tumor growth. Am J Physiol Cell Physiol. 2015;308(11):C890-C898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P.. Plasma concentrations of perfluoroalkyl substances and risk of type 2 diabetes: a prospective investigation among U.S. women. Environ Health Perspect. 2018;126(3):037001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to the data underlying this article should be submitted through the PLCO Cancer Data Access System (https://cdas.cancer.gov/learn/plco/instructions/? type=data).