Abstract
Background
The purpose was to examine associations between treatment and chronic health conditions with neurocognitive impairment survivors of acute lymphoblastic leukemia (ALL) treated with chemotherapy only.
Methods
This cross-sectional study included 1207 ALL survivors (54.0% female; mean age 30.6 years) and 2273 siblings (56.9% female; mean age 47.6 years), who completed the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Multivariable logistic regression compared prevalence of neurocognitive impairment between survivors and siblings by sex. Associations between neurocognitive impairment with treatment exposures and chronic conditions (graded according to Common Terminology Criteria for Adverse Events) were also examined. Statistical tests were 2-sided.
Results
Relative to same-sex siblings, male and female ALL survivors reported increased prevalence of impaired task efficiency (males: 11.7% vs 16.9%; adjusted odds ratio [OR] = 1.89, 95% confidence interval [CI] = 1.31 to 2.74; females: 12.5% vs 17.6%; OR = 1.50, 95% CI = 1.07 to 2.14), as well as impaired memory (males: 11.6% vs 19.9%, OR = 1.89, CI = 1.31 to 2.74; females: 14.78% vs 25.4%, OR = 1.96, 95% CI = 1.43 to 2.70, respectively). Among male survivors, impaired task efficiency was associated with 2-4 neurologic conditions (OR = 4.33, 95% CI = 1.76 to 10.68) and with pulmonary conditions (OR = 4.99, 95% CI = 1.51 to 16.50), and impaired memory was associated with increased cumulative dose of intrathecal methotrexate (OR = 1.68, 95% CI = 1.16 to 2.46) and with exposure to dexamethasone (OR = 2.44, 95% CI = 1.19 to 5.01). In female survivors, grade 2-4 endocrine conditions were associated with higher risk of impaired task efficiency (OR = 2.19, 95% CI = 1.20 to 3.97) and memory (OR = 2.26, 95% CI = 1.31 to 3.92).
Conclusion
Neurocognitive impairment is associated with methotrexate, dexamethasone, and chronic health conditions in a sex-specific manner, highlighting the need to investigate physiological mechanisms and monitor impact through survivorship.
Success in treating childhood acute lymphoblastic leukemia (ALL) has led to a growing number of survivors and accompanying concerns about long-term morbidities (1). The transition from central nervous system prophylaxis using cranial irradiation to intrathecal (IT) chemotherapy combined with higher doses of intravenous (IV) methotrexate (MTX) has resulted in improved neurocognitive outcomes (2). Nonetheless, impairments in discrete neurocognitive functions persist in ALL survivors treated with chemotherapy only (3). Neurocognitive impairment interferes with quality of life, academic attainment, independent living, and employment (4,5). Characterization of factors associated with risk of neurocognitive impairment in ALL survivors treated with chemotherapy only could improve long-term management and monitoring.
Increased risk of neurocognitive impairment may be associated with patient-specific factors, chemotherapy route and intensity, and chronic health conditions (6,7). Female sex has been associated with increased risk of neurocognitive impairment after treatment for ALL with cranial radiation treatment (8). Some studies in ALL survivors treated with chemotherapy only have also reported a similar vulnerability of female sex (9–11). However, other reported findings imply that males and females differ in the type of neurocognitive domains that are affected (7,12). A number of studies in ALL survivors treated with chemotherapy only have shown that younger age at diagnosis is associated with poorer neurocognitive outcomes (7,9,11,13–16), although others have not identified a statistically significant association (17–19). A number of studies in ALL survivors treated with chemotherapy only have shown that younger age at diagnosis is associated with poorer neurocognitive outcomes (7,9,11,13–16), although others have not identified a statistically significant association (17–19).
The literature on chemotherapy-related risk factors is inconsistent as well. Several studies have shown that ALL survivors treated with high-intensity chemotherapy are at increased risk of neurocognitive impairment compared with those treated on low or standard-intensity regimens (7,12,17), although others have failed to find statistically significant associations (18). Treatment-related neurocognitive impairment may be agent specific, with dexamethasone modifying memory performance (20) and MTX affecting processing speed (21), attention (22), and executive function (23). Chronic health conditions are also more common in survivors than control subjects (24) and have emerged as potential factors associated with neurocognitive function after treatment for childhood cancer (6), although it is not clear if these associations are evident in ALL survivors who have been treated with chemotherapy only.
Knowledge of risk factors of neurocognitive impairment is necessary for the development of effective surveillance and remediation strategies. The majority of relevant studies include fewer than 50 participants (3), often from a single institution, which could contribute to unreliable risk estimates and/or poor generalizability. The Childhood Cancer Survivor Study (CCSS) has followed a large multi-institutional survivor cohort over the past 3 decades, collecting detailed patient information, treatment exposures, chronic health conditions, and psychological outcomes. CCSS was recently expanded to include survivors diagnosed through 1999, the majority of whom were treated without cranial radiation. The addition of the expansion cohort offers an opportunity to assess risk factors for neurocognitive impairment in ALL survivors treated with chemotherapy only. Because sex is an important determinant in treatment duration and response, and because sex is known to modulate neurocognitive development (25), primary analyses were stratified by sex. The first aim of this study was to compare prevalence of neurocognitive impairment between survivors and same-sex sibling controls. The second aim was to determine if age at diagnosis, type and mode of chemotherapy exposures, and incidence and severity of chronic conditions modulate risk of neurocognitive impairment in male and female ALL survivors.
Methods
Study Population
The CCSS is a retrospective, multi-institutional cohort study; detailed methodology and study design have been described previously (26,27). CCSS participants included in this investigation met the following criteria: 1) diagnosis of ALL; 2) diagnosis and treatment with chemotherapy only at 1 of 32 participating institutes; 3) diagnosis between January 1, 1970, and December 31, 1999; 4) aged younger than 21 years at diagnosis; 5) survival of at least 5 years after diagnosis; 6) at least 18 years old at the follow-up assessment; and 7) no history of neurosurgical operations defined as operations involving the central nervous system. A group of ALL survivors treated with less than 30 Gy cranial radiation treatment who had not experienced relapse was included as an additional reference group for neurocognitive outcomes. Participants completed comprehensive surveys on demographics, medical history, and psychosocial outcomes. Participants were included in the analysis if they completed the neurocognitive survey as part of follow-up 2 (baseline cohort diagnosed between 1970 and 1986) or follow-up 5 (expansion cohort diagnosed between 1987 and 1999) (see Figure 1). For comparison purposes, siblings of survivors who also completed the CCSS neurocognitive questionnaires were also included from the follow-up 2 and follow-up 5 surveys.
Figure 1.
Consort diagram. ALL = acute lymphoblastic leukemia; CRT = cranial radiation treatment; NCQ = neurocognitive questionnaire
The human subjects committee at each participating institution reviewed and approved the protocol and questionnaires. Participants or parents (if participant was younger than 18 years) gave consent for participation and for the release of medical records.
Outcome Measure
Neurocognitive impairment was assessed with the CCSS Neurocognitive Questionnaire (CCSS-NCQ) (28). The NCQ is a 25-item instrument that was validated in childhood cancer survivors and siblings and includes 4 domains of neurocognitive function: task efficiency (measures attention and processing speed), emotional regulation, organization, and memory (29). Raw scores were converted to z scores based on norms, with higher scores indicating greater impairment. Neurocognitive impairment was defined as z scores of 1.28 or higher, corresponding to the 90th or higher percentile of the sibling reference (6).
Predictors
Treatment exposures included cumulative doses of intrathecal methotrexate (IT MTX; mL), intravenous MTX (IV MTX; g/m2), anthracyclines (mg/m2), and alkylating agents (mg/m2), as well as oral MTX (PO MTX; yes/no), intramuscular MTX (IM MTX; yes/no), cytarabine (yes/no), and dexamethasone (yes/no). Anthracyclines and alkylating agents represent classes of drugs that include different agents with similar effects; dose equivalencies were applied to combine these agents for analytical purposes (27).
Because MTX can be administered via several routes on the same individual, we evaluated all possible combinations of MTX administrations in the study sample. MTX groups were created empirically based on patterns of co-occurrence (IT + PO, IT + IV, IT + IV + IM, IT + IV + PO, and other combinations; see Supplementary Table 1, available online). For each MTX group, IT and IV MTX cumulative doses were centered around the median of that group. In statistical models, IT MTX was scaled per 100 mL and IV MTX was scaled per 10 g/m2 to account for their large dosage range. All other chemotherapy exposures were treated as binary indicators (ie, yes or no).
Chronic health conditions were taken from surveys about organ-based health conditions at baseline and follow-up. The chronic conditions were graded for severity by an adapted application of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.03), including asymptomatic and/or mild conditions (grade 1), moderate conditions (grade 2), severe and/or disabling conditions (grade 3), and life-threatening conditions (grade 4) (30). Specific chronic health conditions and their prevalence in the sample are listed in Supplementary Table 2 (available online). For analytical purposes, grades of impairment for each organ system (cardiovascular, neurological, pulmonary, endocrine, hearing, ocular, and gastrointestinal) were categorized into 2 levels: grade less than 2 (none, mild) and grade 2-4 (moderate, severe, life threatening).
Statistical Analysis
Demographic characteristics of the ALL survivors and siblings were summarized using descriptive statistics. Clinical characteristics of the survivors were described, including treatment exposures and doses. To describe sex-specific differences in neurocognitive impairment between ALL survivors and siblings, as well as male vs female ALL survivors, a multivariable logistic regression model adjusting for age at NCQ assessment (as cubic splines) and race and ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and others) was used, with the generalized estimating equation modification to account for potential within-family correlation. To convey the sex-specific and age-, race-, and ethnicity-adjusted differences between the survivors and siblings, model-predicted proportions of impairment were calculated that represented an “average participant” in the sample. Associations of neurocognitive impairment with treatment exposures, and then separately with chronic conditions, were evaluated in our primary analysis using multivariable logistic regression models among the ALL survivors, stratified by sex. These regression models were adjusted for age at cancer diagnosis (younger than 1, 1-4, 5-9, and 10 years and older) as well as age at NCQ assessment and race and ethnicity as above. Tests of statistical significance were 2-sided using a P value of less than .05 as the cutoff. All analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC). Figures were created using R (version 3.5.1; https://www.R-project.org).
Results
Study Sample
A total of 1207 ALL survivors treated with chemotherapy only and 2273 siblings were included in analyses. Table 1 summarizes baseline demographic and treatment characteristics of survivors and siblings. The sample included more females than males across groups (P = .05). A smaller proportion of siblings were minorities (P < .001), and siblings were older than survivors at evaluation (P < .001). A detailed description of the sample is supplied in Supplementary Table 3 (available online). Notably, after controlling for age differences, ALL survivors were less likely than siblings to live independently (75.9% vs 88.7%, respectively; P < .001).
Table 1.
Sex-stratified demographics and clinical characteristics
| Variable | Males |
Females |
||
|---|---|---|---|---|
| ALL (n = 549) No. (%) | Siblings (n = 979) No. (%) | ALL (n = 658) No. (%) | Siblings (n = 1294) No. (%) | |
| Demographics | ||||
| Race/Ethnicity | ||||
| White | 460 (81.6) | 880 (89.9) | 562 (84.0) | 1150 (88.9) |
| Black | 18 (4.0) | 8 (0.8) | 22 (3.7) | 23 (1.8) |
| Hispanic | 42 (7.6) | 31 (3.2) | 48 (7.3) | 43 (3.3) |
| Other | 26 (6.4) | 23 (2.4) | 25 (4.7) | 28 (2.2) |
| Unknown | 3 (0.5) | 37 (3.8) | 1 (0.3) | 50 (3.9) |
| Age at evaluation, mean (SD) | 30.8 (8.3) | 47.1 (11.5) | 30.5 (7.7) | 47.6 (11.2) |
| Age at diagnosis | ||||
| <1 y | 8 (0.8) | — | 14 (1.2) | — |
| 1-4 y | 284 (55.7) | — | 360 (58.1) | — |
| 5-9 y | 141 (30.0) | — | 179 (30.0) | — |
| ≥10 y | 116 (13.5) | — | 105 (10.7) | — |
| Years since diagnosis, mean (SD) | 21.7 (5.2) | — | 21.8 (5.2) | — |
| Relapse (<5 y of 1st diagnosis) | ||||
| Yes | 23 (3.5) | — | 14 (1.5) | — |
| No | 526 (96.5) | — | 644 (98.5) | — |
| Treatment | ||||
| IT MTX | ||||
| Median, mL (IQR) | 204.0 (146.0-228.0) | — | 170.0 (144.0-204.0) | — |
| Yes | 519 (96.4) | — | 640 (98.6) | — |
| IV MTX | ||||
| Median, g/m² (IQR) | 12.4 (4.3-23.6) | — | 11.9 (4.2-22.7) | — |
| Yes | 324 (62.3) | — | 364 (57.8) | — |
| PO MTX, Yes | 353 (53.9) | — | 430 (53.7) | — |
| IM MTX, Yes | 103 (24.8) | — | 116 (22.3) | — |
| Cytarabine, Yes | 385 (79.3) | — | 448 (76.7) | — |
| Anthracyclinea | ||||
| Median, mg/m² (IQR) | 72.5 (50.7-128.3) | — | 60.2 (49.0-99.7) | — |
| Yes | 322 (67.9) | — | 332 (64.8) | — |
| Alkylating agentsa | ||||
| Median, mg/m² (IQR) | 3760.8 (1666.7-8764.5) | — | 2045.5 (1000.0-7416.4) | — |
| Yes | 303 (54.7) | — | 307 (47.1) | — |
| Dexamethasone, Yes | 148 (34.8) | — | 177 (35.4) | — |
Dose equivalencies were used. All analyses, except the reported frequency counts, were weighted to account for restricted sampling of ALL survivors (diagnosed in 1987-1999) according to their age at diagnosis, which was a study-design feature for efficiency. Specifically, we used a weight of 1.21 for ALL survivors who were 0 or 11-20 years of age at diagnosis and a weight of 3.63 for survivors who were 1-10 years of age at diagnosis. These weights were based on random sampling of 28% of ALL survivors diagnosed between 1 and 10 years old () and random sampling of 83% of survivors diagnosed between 11 and 20 years (). ALL = acute lymphoblastic leukemia; IM = intramuscular; IQR = interquartile range; IT = intrathecal; IV = intravenous; MTX = methotrexate; PO = oral.
Neurocognitive Impairment
Female survivors reported a higher prevalence of impairment in emotion regulation (15.6% vs 9.9%) and memory (25.4% vs 19.9%) than did male survivors (odds ratio [OR] = 1.68, 95% confidence interval [CI] = 1.15 to 2.47, and OR = 1.37, 95% CI = 1.00 to 1.90, respectively).
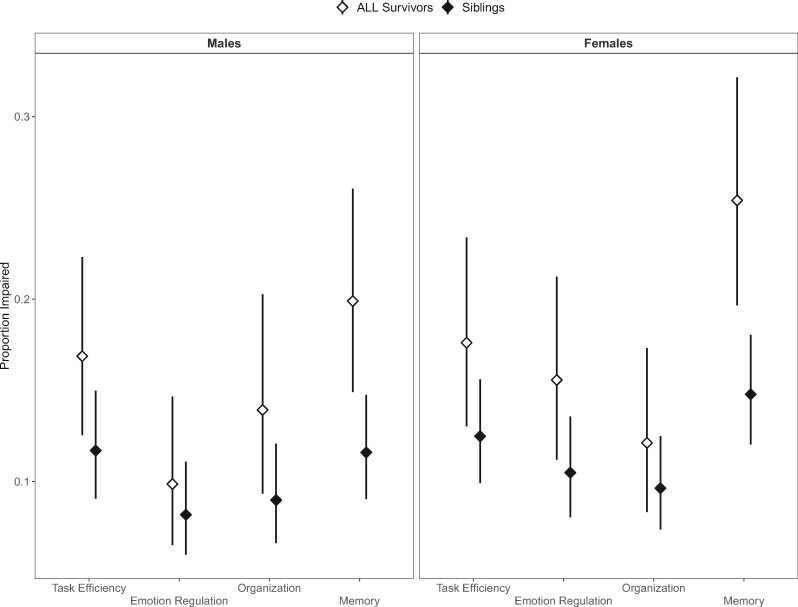
Both female and male ALL survivors treated with chemotherapy only had higher rates of neurocognitive impairments compared with same-sex siblings (Figure 2). Increased risk of impaired task efficiency among survivors relative to same-sex siblings was apparent in males (11.7% vs 16.9%, OR = 1.89, 95% CI = 1.31 to 2.74) and in females (12.5% vs 17.6%, OR = 1.50, 95% CI = 1.07 to 2.14; Figure 2). Both male and female ALL survivors reported greater impairment in memory than did siblings (11.6% vs 19.9%, OR = 1.89, 95% CI = 1.31 to 2.74; 14.78% vs 25.4%, OR = 1.96, 95% CI = 1.43 to 2.70, respectively; Figure 2). Supplementary Table 4 (available online) includes summary statistics for neurocognitive impairment comparing siblings with ALL survivors treated with chemotherapy only, as well as with a reference group of survivors treated with CRT. Age at diagnosis was not statistically significantly associated with neurocognitive impairment in either male or female survivors (Supplementary Table 5, available online).
Figure 2.
Proportion of impairment across neurocognitive domains, group, and sex. Adjusted proportions are shown on the y-axis for each of the 4 cognitive domains of the neurocognitive questionnaire listed on the x-axis. Results are shown for males (left) and females (right) and for ALL survivors treated with chemotherapy only (white triangles) and sibling (black triangles) separately. The proportions are adjusted to represent an “average” participant, who is White and aged 40 years at evaluation. Error bars represent 95% confidence limits of the adjusted proportion.
As a follow-up analysis, we explored if increased neurocognitive impairment was associated with increased risk of self-reported anxiety and depression, using the Behavioral Symptom Inventory. Impairment on the NCQ emotion regulation domain was statistically significantly associated with Behavioral Symptom Inventory depression and anxiety in male (depression: OR = 6.65, 95% CI = 2.57 to 17.21; P < .001; anxiety: OR = 7.99, 95% CI = 3.04 to 21.02; P < .001) and female ALL survivors (depression: OR = 6.12, 95% CI = 2.97 to 12.61; P < .001; anxiety: OR = 3.95, 95% CI = 1.57 to 9.92; P < .001). Impairment in NCQ-task efficiency was related to depression in male survivors (OR = 5.41, 95% CI = 2.14 to 13.71; P < .001) and with depression and anxiety in female ALL survivors (depression: OR = 2.60, 95% CI = 1.12 to 6.04; P = .03; anxiety: OR = 2.66, 95% CI = 1.20 to 5.91; P = .02). Memory impairment was not associated with depression or anxiety in either males or females.
Treatment Exposures
Neurocognitive impairment was associated with different patterns of chemotherapy exposures in male vs female survivors (Table 2). Males exposed to a higher cumulative dose of IT MTX had increased risk of memory impairment (OR = 1.68, 95% CI = 1.16 to 2.46). Males exposed to dexamethasone had increased risk of memory impairment relative to males not exposed to dexamethasone (OR = 2.44, 95% CI = 1.19 to 5.01). Males exposed to the combination of IT, IV, and IM MTX had increased risk of impaired task efficiency (OR = 3.25, 95% CI = 1.19 to 8.91) compared with males exposed to the combination of IT and PO MTX.
Table 2.
Treatment parameters associated with neurocognitive impairment
| Variable | Task efficiency | Emotional regulation | Organization | Memory |
|---|---|---|---|---|
| OR (95% CI)c | OR (95% CI)c | OR (95% CI)c | OR (95% CI)c | |
| Males | ||||
| Administration pattern | ||||
| IT + PO | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| IT + IV | 1.12 (0.45 to 2.81) | 1.20 (0.40 to 3.64) | 0.73 (0.26 to 2.08) | 1.22 (0.47 to 3.18) |
| IT + IV + IM | 3.25 (1.19 to 8.91) | 1.84 (0.47 to 7.21) | 0.52 (0.17 to 1.57) | 2.64 (0.90 to 7.77) |
| IT + IV + PO | 1.75 (0.79 to 3.87) | 1.72 (0.66 to 4.50) | 0.54 (0.19 to 1.52) | 1.77 (0.77 to 4.05) |
| Other combinations | 1.77 (0.71 to 4.42) | 1.01 (0.33 to 3.13) | 0.43 (0.13 to 1.48) | 1.94 (0.80 to 4.71) |
| IT MTX cumulative dosea | 1.04 (0.98 to 1.12) | 1.00 (0.93 to 1.08) | 1.39 (0.97 to 1.99) | 1.68 (1.16 to 2.46) |
| IV MTX cumulative doseb | 0.98 (0.94 to 1.02) | 0.97 (0.91 to 1.03) | 1.05 (1.00 to 1.09) | 1.01 (0.97 to 1.06) |
| Cytarabine | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 0.66 (0.29 to 1.49) | 0.68 (0.23 to 2.02) | 0.76 (0.30 to 1.95) | 0.46 (0.19 to 1.08) |
| Anthracycline | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.16 (0.52 to 2.58) | 1.31 (0.49 to 3.47) | 0.99 (0.36 to 2.75) | 1.21 (0.53 to 2.78) |
| Alkylating agents | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.44 (0.67 to 3.10) | 0.95 (0.36 to 2.52) | 1.47 (0.62 to 3.49) | 0.91 (0.41 to 2.02) |
| Dexamethasone | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.40 (0.69 to 2.84) | 1.48 (0.61 to 3.63) | 1.06 (0.48 to 2.35) | 2.44 (1.19 to 5.01) |
| Females | ||||
| Administration pattern | ||||
| IT + PO | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| IT + IV | 0.58 (0.23 to 1.42) | 0.63 (0.28 to 1.39) | 1.15 (0.41 to 3.28) | 1.11 (0.52 to 2.35) |
| IT + IV + IM | 1.18 (0.45 to 3.11) | 1.08 (0.47 to 2.47) | 1.74 (0.62 to 4.89) | 1.37 (0.60 to 3.14) |
| IT + IV + PO | 0.94 (0.46 to 1.93) | 1.10 (0.55 to 2.20) | 1.13 (0.48 to 2.67) | 1.75 (0.93 to 3.27) |
| Other combinations | 1.49 (0.68 to 3.29) | 1.11 (0.48 to 2.56) | 1.40 (0.47 to 4.12) | 2.07 (0.96 to 4.48) |
| IT MTX cumulative dosea | 1.23 (0.87 to 1.76) | 1.02 (0.72 to 1.44) | 1.15 (0.76 to 1.74) | 0.85 (0.60 to 1.20) |
| IV MTX cumulative doseb | 1.01 (0.97 to 1.06) | 0.97 (0.92 to 1.02) | 0.98 (0.92 to 1.05) | 0.98 (0.93 to 1.03) |
| Cytarabine | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 0.91 (0.42 to 2.02) | 0.66 (0.30 to 1.49) | 0.65 (0.26 to 1.65) | 1.17 (0.56 to 2.45) |
| Anthracycline | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 0.92 (0.46 to 1.83) | 0.71 (0.34 to 1.49) | 0.77 (0.34 to 1.75) | 1.27 (0.69 to 2.33) |
| Alkylating agents | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.31 (0.68 to 2.50) | 1.08 (0.56 to 2.09) | 1.54 (0.68 to 3.47) | 0.56 (0.32 to 0.97) |
| Dexamethasone | ||||
| No | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Yes | 1.64 (0.85 to 3.14) | 0.95 (0.50 to 1.82) | 0.82 (0.35 to 1.94) | 1.33 (0.74 to 2.37) |
Dose centered at median of corresponding group (every 100 mL). Reference values are marked with (referent) annotation. CI = confidence interval; IM = intramuscular; IT = intrathecal; IV = intravenous; MTX = methotrexate; OR = odds ratio; PO = oral.
Centered at median of corresponding group (every 10 g/m²).
Note that results were adjusted for age at diagnosis and ethnicity.
Females exposed to the combination of IT, IV, and PO MTX had increased risk of memory impairment relative to those exposed to IT and PO MTX only (OR = 1.75, 95% CI = 0.93 to 3.27; Table 2). Likewise, female survivors exposed to any other combination of MTX administration had increased risk of memory impairment compared with females exposed to IT and PO MTX only (OR = 2.07, 95% CI = 0.96 to 4.48).
Chronic Conditions
Risks for neurocognitive impairment in male and female ALL survivors were associated with presence of chronic conditions (Table 3). Impaired memory in male survivors was associated with a grade 2-4 neurological condition (OR = 4.33, 95% CI = 1.76 to 10.68). Impaired task efficiency in male survivors was associated with grade 2-4 chronic neurological condition (OR = 6.32, 95% CI = 2.25 to 17.72) or pulmonary condition (OR = 4.99, 95% CI = 1.51 to 16.50). In female survivors, an endocrine condition increased the risk of impaired task efficiency (OR = 2.19, 95% CI = 1.20 to 3.97) and impaired memory (OR = 2.26, 95% CI = 1.31 to 3.92; Table 3).
Table 3.
Associations between chronic conditions and neurocognitive impairmenta
| Variable | Grade | Task efficiency | Emotional regulation | Organization | Memory |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Males | |||||
| Cardiovascular | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 1.17 (0.55 to 2.50) | 1.17 (0.51 to 2.66) | 1.15 (0.51 to 2.60) | 0.75 (0.38 to 1.49) | |
| Neurology | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 6.32 (2.25 to 17.72) | 2.29 (0.84 to 6.21) | — | 4.33 (1.76 to 10.68) | |
| Pulmonary | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 4.99 (1.51 to 16.50) | — | — | 2.09 (0.76 to 5.72) | |
| Endocrine | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 1.54 (0.78 to 3.05) | 1.11 (0.50 to 2.49) | 1.69 (0.81 to 3.53) | 1.18 (0.61 to 2.31) | |
| Gastrointestinal | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 1.51 (0.61 to 3.73) | — | — | 1.80 (0.72 to 4.50) | |
| Females | |||||
| Cardiovascular | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 0.69 (0.33 to 1.44) | 0.81 (0.39 to 1.72) | — | 1.42 (0.73 to 2.76) | |
| Neurology | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 1.45 (0.54 to 3.89) | 1.74 (0.67 to 4.50) | — | 1.49 (0.58 to 3.83) | |
| Pulmonary | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 1.74 (0.86 to 3.54) | — | — | 0.86 (0.41 to 1.83) | |
| Endocrine | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 2.19 (1.20 to 3.97) | 1.25 (0.67 to 2.34) | 2.12 (1.04 to 4.34) | 2.26 (1.31 to 3.92) | |
| Gastrointestinal | 0-1 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 2-4 | 1.55 (0.58 to 4.19) | — | — | 1.04 (0.45 to 2.42) |
Note that chronic conditions were dropped from the model for the specific neurocognitive questionnaire outcome if there were fewer than 10 patients within each level of the condition after combining categories (denoted by “—” in the table). CI = confidence interval; OR = odds ratio.
Discussion
The present study includes a large sample of ALL survivors treated with chemotherapy only, providing a unique opportunity to systematically assess risk factors of neurocognitive impairment associated with therapeutic approaches similar to contemporary protocols. A higher proportion of female ALL survivors reported impairment in emotion regulation and memory relative to male ALL survivors, which may indicate that female sex carries an increased risk of neurocognitive impairment after treatment with chemotherapy only. Relative to siblings, both male and female ALL survivors had increased risk of impairment in task efficiency and memory. At a mean interval of 22 years from diagnosis, we did not observe statistically significant associations between neurocognitive impairment and age at diagnosis. Our findings are seemingly in contrast with other studies in ALL survivors treated with chemotherapy only (7,9,11,13–16), which may be attributed to several factors. First, the impact of age at diagnosis may be more modest in survivors treated with chemotherapy only than what has been reported in survivors treated with cranial radiation (11). Second, the relative impact of age at diagnosis may diminish as survivors grow older. The studies that reported statistically significant associations were generally conducted within 10 years of diagnosis (7,11,14–16). Notably, our results are in line with prior work on neurocognitive function among ALL survivors who were evaluated at a mean follow-up time of more than 20 years (9,31).
Methotrexate was associated with neurocognitive impairment in male and female ALL survivors, whereas dexamethasone increased risk of memory impairment in male survivors only. A sex-dependent pattern was observed for associations between neurocognitive impairment and chronic health conditions. In male survivors, neurological conditions increased risk of impairment in memory and task efficiency, and a pulmonary condition was associated with impaired task efficiency. In females, endocrine conditions were associated with increased risk of memory impairment. Focused surveillance for neurocognitive late effects provides opportunities for implementation of function-preserving interventions (32). These results highlight the need to investigate sex-related differences in physiological mechanisms and treatment effects in ALL survivors treated with chemotherapy only.
Associations between methotrexate and neurocognitive impairment could be linked with changes in phospholipids that affect white matter architecture (33). White matter development is nonlinear, and rates of change vary regionally across the brain (34). Frontal-temporal regions exhibit increases in fractional anisotropy into young adulthood, which is thought to represent continued myelination (34). Exposure to methotrexate during childhood and adolescence is likely to disrupt developing networks with associated consequences on cognitive outcomes.
The adverse effects of dexamethasone on memory impairment was limited to male survivors only. Sex hormones can modulate the effects of glucocorticoid receptors, and rodent studies have shown that the male brain responds to steroids differently than the female brain (35). Female and male rats exhibit opposite effects of chronic stress on hippocampus-dependent memory, with male rats showing impairment and female rats showing no effect or enhancement (35). The observed male-specific vulnerability to dexamethasone-associated cognitive impairment may in part be related to interactions between sex hormones and synthetic glucocorticoid exposure.
Unexpectedly, exposure to alkylating agents was associated with better memory in female ALL survivors. Whereas this appears to be in line with a study in female rats treated with cyclophosphamide (36), other studies have demonstrated negative effects of alkylating agents on cognition (37). Parsing out the contributions of chemotherapy agents on neurocognitive impairment in a clinical setting is complicated because agents are given in combination and via various routes, such that higher alkylating exposure could be a marker of lower exposure to a more toxic agent. In the present sample, increased exposure to alkylating agents was associated with decreased exposure to IT methotrexate. Isolated exposure to vincristine, cytarabine, or anthracyclines was previously shown to affect mouse brain development, suggesting that these agents pose a risk of subsequent cognitive impairment (38). Expanding the scope and depth of cognitive assessments to include functional neuroimaging and measures that evaluate discrete aspects of cognitive function may elucidate the impact of individual agents on outcomes.
Overall, prevalence of chronic conditions was similar between ALL survivors and siblings; however, the presence of certain chronic conditions increased risk of neurocognitive impairment in ALL survivors in a sex-dependent manner. Pulmonary and neurological chronic conditions were statistically significantly associated with impaired memory and task efficiency among male ALL survivors. The association between pulmonary disease and neurocognitive impairment was previously observed in a general sample of childhood cancer survivors (6) and in survivors of neuroblastoma (39). The brain consumes 20% of total body oxygen provided by the lungs, and impaired pulmonary function is associated with a higher prevalence of subclinical cerebral infarctions and white matter lesions that affect cognitive function (40). Studies in cultured astrocytes and neurons from male and female mouse embryos have demonstrated increased male sensitivity to ischemic injury (41). This phenomenon is thought to be related to estrogen, which promotes vasodilation that protects premenopausal woman from stroke (41).
Neurological chronic conditions reported by ALL survivors in the sample included stroke, epilepsy, and peripheral neuropathy. Leukoencephalopathy (LE) is a well-known acute neurological condition associated with high-dose methotrexate, and LE could have long-term implications for neurocognitive function (42). However, it is not clear if the incidence of LE is dependent on sex and if the presence of LE confers a greater risk of neurocognitive impairment among male ALL survivors relative to female survivors. Myelination, dendritic pruning, and cerebral perfusion exhibit marked developmental changes during childhood and adolescence that are modulated by sex (25), possibly resulting in differential pathophysiological responses to neurotoxic agents and neuronal injury.
Endocrine complications are prevalent in childhood cancer survivors and have been associated with exposure to alkylating agents and/or glucocorticoids (though not only dexamethasone) in survivors treated with chemotherapy only (43). In females, endocrine conditions increased risk of impaired task efficiency and memory. A common presentation of endocrine conditions in our sample is hypothyroidism, which leaves the brain with inadequate energy reserves needed to perform cognitive tasks (44). Female ALL survivors had higher incidence of hypothyroidism than did male survivors, which reflects trends in the general population. Higher prevalence of thyroid disease among females is likely due to interactions between estrogen and thyroid hormones (45). Hypothyroidism can result in reduced cerebral blood flow in regions that support memory, attention, and motor speed (46). It will be important to consider endocrine morbidity in surveillance strategies of neurocognitive impairment among female childhood ALL survivors.
The size of the current sample is a clear strength of the study; however, certain limitations must be noted. As is common in large cohort studies, we relied on self-rated cognitive impairment, which may be prone to misclassification. Refinements in study designs, such as the inclusion of performance-based measures, may identify specific impairment and could advance our understanding of neurocognitive impairment in long-term childhood ALL survivors. Another important consideration is potential participation bias, which could lead to decreased generalizability of the results. Slightly fewer siblings from minority racial and ethnic groups participated, underscoring a need for strategies that reduce potential participation disparities across groups. Additionally, neurocognitive impairment could be associated with factors that were not assessed as part of the present study. For example, previous work in ALL survivors has demonstrated associations between neurocognitive impairment and fatigue (10). Determining the full scope of risk factors of neurocognitive impairment will be important for effective screening and follow-up. Finally, prospective longitudinal studies (7,14,47,48) are essential in clarifying neurocognitive changes after treatment, particularly in the context of changes related to sex and aging.
Risk of neurocognitive impairment in ALL survivors treated with chemotherapy only differs by sex, exposures to methotrexate and/or dexamethasone, and subsequent chronic conditions. Identification of specific risk factors is complicated by potential indirect associations (eg, chronic conditions and neurocognitive impairment may be indirectly linked with treatment exposures) (6). Statistically significant associations between neurocognitive impairment and anxiety and depression were also noted. The cross-sectional nature of the present study precluded determination of causality, highlighting a need for studies aimed at understanding co-occurrence of neurocognitive performance and mental health. Understanding these complex relationships is critical for developing effective screening programs and interventions to maximize quality of life for childhood cancer survivors.
Funding
This work was supported by the National Cancer Institute (CA55727, GT Armstrong). Support to St Jude Children’s Research Hospital was also provided by the National Cancer Institute Cancer Center Support grant (CA 21765) and by the American, Lebanese, Syrian Associated Charities.
Notes
Role of the funder: The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Disclosure: The authors declare no competing interests.
Role of the authors: EvdP, BJN, KRK developed the concept and drafted the manuscript. WQ, EvdP prepared tables and figures. WQ, YY, QL, SD, WL contributed to data preparation and modeling. All other authors provided guidance on the methodology, reviewed the manuscript, and provided critical revisions. The corresponding author (EvdP) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data Availability
The Childhood Cancer Survivor Study is an NCI-funded resource (U24 CA55727) to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence. Investigators interested in potential uses of this resource are encouraged to visit http://ccss.stjude.org.
Supplementary Material
References
- 1. Hunger SP, Mullighan CG.. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–1552. [DOI] [PubMed] [Google Scholar]
- 2. Spiegler BJ, Kennedy K, Maze R, et al. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol. 2006;24(24):3858–3864. [DOI] [PubMed] [Google Scholar]
- 3. Cheung YT, Krull KR.. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: a systematic review. Neurosci Biobehav Rev. 2015;53:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirchhoff AC, Krull KR, Ness KK, et al. Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1838–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kunin-Batson A, Kadan-Lottick N, Neglia JP.. The contribution of neurocognitive functioning to quality of life after childhood acute lymphoblastic leukemia. Psychooncology. 2014;23(6):692–699. [DOI] [PubMed] [Google Scholar]
- 6. Cheung YT, Brinkman TM, Li C, et al. Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2018;110(4):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacola LM, Krull KR, Pui C-HH, et al. Longitudinal assessment of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated on a contemporary chemotherapy protocol. J Clin Oncol. 2016;34(11):1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Armstrong GT, Sklar CA, Hudson MM, Robison LL.. Long-term health status among survivors of childhood cancer: does sex matter? J Clin Oncol. 2007;25(28):4477–4489. [DOI] [PubMed] [Google Scholar]
- 9. Sherief LM, Sanad R, ElHaddad A, et al. A cross-sectional study of two chemotherapy protocols on long term neurocognitive functions in Egyptian children surviving acute lymphoblastic leukemia. Curr Pediatr Rev. 2018;14(4):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung YT, Brinkman TM, Mulrooney DA, et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2017;123(17):3410–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age- and sex-related differences. Eur J Cancer. 2003;39(3):359–365. [DOI] [PubMed] [Google Scholar]
- 12. Jain N, Brouwers P, Okcu MF, Cirino PT, Krull KR.. Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer. 2009;115(18):4238–4245. [DOI] [PubMed] [Google Scholar]
- 13. Hardy KK, Embry L, Kairalla JA, et al. Neurocognitive functioning of children treated for high-risk B-acute lymphoblastic leukemia randomly assigned to different methotrexate and corticosteroid treatment strategies: a report from the Children’s Oncology Group. J Clin Oncol. 2017;35(23):2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sleurs C, Lemiere J, Vercruysse T, et al. Intellectual development of childhood ALL patients: a multicenter longitudinal study. Psychooncology. 2017;26(4):508–514. [DOI] [PubMed] [Google Scholar]
- 15. Lofstad GE, Reinfjell T, Weider S, Diseth TH.. Neurocognitive outcome and compensating possibilities in children and adolescents treated for acute lymphoblastic leukemia with chemotherapy only. Front Psychol. 2019;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jansen NC, Kingma A, Schuitema A, et al. Post-treatment intellectual functioning in children treated for acute lymphoblastic leukaemia (ALL) with chemotherapy-only: a prospective, sibling-controlled study. Eur J Cancer. 2006;42(16):2765–2772. [DOI] [PubMed] [Google Scholar]
- 17. Conklin HM, Krull KR, Reddick WE, Pei D, Cheng C, Pui CH.. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104(18):1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halsey C, Buck G, Richards S, Vargha-Khadem F, Hill F, Gibson B.. The impact of therapy for childhood acute lymphoblastic leukaemia on intelligence quotients; results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI. J Hematol Oncol. 2011;4(1):42–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanellopoulos A, Andersson S, Zeller B, et al. Neurocognitive outcome in very long-term survivors of childhood acute lymphoblastic leukemia after treatment with chemotherapy only. Pediatr Blood Cancer. 2016;63(1):133–138. [DOI] [PubMed] [Google Scholar]
- 20. Edelmann MN, Ogg RJ, Scoggins MA, et al. Dexamethasone exposure and memory function in adult survivors of childhood acute lymphoblastic leukemia: a report from the SJLIFE cohort. Pediatr Blood Cancer. 2013;60(11):1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aukema EJ, Caan MWA, Oudhuis N, et al. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2009;74(3):837–843. [DOI] [PubMed] [Google Scholar]
- 22. Krawczuk-Rybak M, Grabowska A, Protas P, Muszynska-Roslan K, Holownia A, Braszko J.. Intellectual functioning of childhood leukemia survivors—relation to Tau protein—a marker of white matter injury. Adv Med Sci. 2012;57(2):266–272. [DOI] [PubMed] [Google Scholar]
- 23. Krull KR, Cheung YT, Liu W, et al. Chemotherapy pharmacodynamics and neuroimaging and neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2016;34(22):2644–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11(6):552–557. [DOI] [PubMed] [Google Scholar]
- 26. Robison LL, Armstrong GT, Boice JD, et al. The childhood cancer survivor study: a national cancer institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krull KR, Okcu MF, Potter B, et al. Screening for neurocognitive impairment in pediatric cancer long-term survivors. J Clin Oncol. 2008;26(25):4138–4143. [DOI] [PubMed] [Google Scholar]
- 29. Kenzik KM, Huang I-C, Brinkman TM, et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) revised: item response analysis and concurrent validity. Neuropsychology. 2015;29(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 31. Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(35):4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Landier W, Skinner R, Wallace WH, et al. Surveillance for late effects in childhood cancer survivors. J Clin Oncol. 2018;36(21):2216–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krull KR, Hockenberry MJ, Miketova P, Carey M, Moore IM.. Chemotherapy-related changes in central nervous system phospholipids and neurocognitive function in childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54(3):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lebel C, Treit S, Beaulieu C.. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019;32(4):e3778. [DOI] [PubMed] [Google Scholar]
- 35. McEwen BS, Milner TA.. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95(1-2):24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee GD, Longo DL, Wang Y, et al. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12(1):198–205. [DOI] [PubMed] [Google Scholar]
- 37. Seigers R, Fardell JE.. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci Biobehav Rev. 2011;35(3):729–741. [DOI] [PubMed] [Google Scholar]
- 38. Spencer Noakes TL, Przybycien TS, Forwell A, et al. Brain development and heart function after systemic single-agent chemotherapy in a mouse model of childhood leukemia treatment. Clin Cancer Res. 2018;24(23):6040–6052. [DOI] [PubMed] [Google Scholar]
- 39. Zheng DJ, Krull KR, Chen Y, et al. Long-term psychological and educational outcomes for survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. Cancer. 2018;124(15):3220–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lahousse L, Tiemeier H, Ikram MA, Brusselle GG.. Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir Med. 2015;109(11):1371–1380. [DOI] [PubMed] [Google Scholar]
- 41. Roy-O’Reilly M, McCullough LD.. Sex differences in stroke: the contribution of coagulation. Exp Neurol. 2014;259:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheung YT, Sabin ND, Reddick WE, et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: a longitudinal analysis. Lancet Haematol. 2016;3(10):e456–e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chemaitilly W, Cohen LE, Mostoufi-Moab S, et al. Endocrine late effects in childhood cancer survivors. J Clin Oncol. 2018;36(21):2153–2159. [DOI] [PubMed] [Google Scholar]
- 44. Bauer M, Silverman DHS, Schlagenhauf F, et al. Brain glucose metabolism in hypothyroidism: a positron emission tomography study before and after thyroid hormone replacement therapy. J Clin Endocrinol Metab. 2009;94(8):2922–2929. [DOI] [PubMed] [Google Scholar]
- 45. Grigorova M, Sherwin BB.. Thyroid hormones and cognitive functioning in healthy, euthyroid women: a correlational study. Horm Behav. 2012;61(4):617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Krausz Y, Freedman N, Lester H, et al. Regional cerebral blood flow in patients with mild hypothyroidism. J Nucl Med. 2004;45(10):1712–1715. [PubMed] [Google Scholar]
- 47. Liu W, Cheung YT, Conklin HM, et al. Evolution of neurocognitive function in long-term survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. J Cancer Surviv. 2018;12(3):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boulet-Craig A, Drouin S, Sultan S, et al. DIVERGT screening procedure predicts general cognitive functioning in adult long-term survivors of pediatric acute lymphoblastic leukemia: a PETALE study. Pediatr Blood Cancer. 2018;65(9):e27259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Childhood Cancer Survivor Study is an NCI-funded resource (U24 CA55727) to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence. Investigators interested in potential uses of this resource are encouraged to visit http://ccss.stjude.org.