Abstract
Objective
Sarcopenic obesity is associated with several negative health outcomes. However, there are only a few studies on the relationship between SO and metabolic diseases such as diabetes, hypertension, and abnormal lipid metabolism in Chinese adults. The aim of this work was to evaluate the association between SO and hypertension, diabetes, and abnormal lipid metabolism in Chinese adults, and explore the prediction of SO using relevant anthropometric indicators.
Materials and Methods
All participants underwent a questionnaire interview for the collection of demographic data. Thereafter, they underwent physical examination for the measurement of anthropometric variables, which was performed using bioelectrical impedance analysis. Biochemical measurements were determined according to standard laboratory procedures used for the evaluation of blood parameters.
Results
We included 14,926 patients aged 35–74 years old. The mean age of the participants was 56.75 ± 9.76 years old, and 39.80% of them were male. The mean body mass index (BMI) was 24.94 ± 3.40 kg/m2, and the overall prevalence of SO was 65.1%. The results showed that shorter people; people with faster heart rate; heavier weight; lower waist circumference (WC), BMI, triglyceride level, total cholesterol, and low-density lipoprotein cholesterol levels; and higher high-density lipoprotein cholesterol level are at risk for SO.
Conclusion
The prevalence of SO is high (65.1%) in Chinese adults aged 35–74 years old. The occurrence of SO is related to hypertension, diabetes, and abnormal lipid metabolism. BMI, WC, and waist-hip ratio may be predictive indicators of SO. The incidence of SO may be reduced by timely intervention and health education for persons at risk of the condition.
Keywords: sarcopenic obesity, hypertension, diabetes mellitus, abnormal lipid metabolism
Introduction
With increasing age, human hormonal levels and body activity decrease, muscle mass and muscle function decline, and abdominal obesity and visceral fat content increase, resulting in sarcopenic obesity (SO).1–3 SO, which reflects a combination of sarcopenia and obesity, is a complex systemic condition that often has a negative impact on body activities. Its pathogenesis may be related to aging or other diseases, and as such can occur in individuals of all age groups.4–6 Studies have shown that SO is closely related to falls and abnormal lipid metabolism in the elderly. The condition has a poor prognosis and leads to an increased risk of disability, cardiovascular disease, and death.7–9 The rapidly increasing morbidity and serious consequences associated with SO are recognized as critical public health risks among the aging population.10,11
Hypertension, which is another global public health problem, has become one of the important causes of death from cardiovascular disease. It is characterized by high disability and mortality rates, which reflect an enormous consumption of medical and social resources. Studies have demonstrated that SO may be related to the occurrence of hypertension, and that fat-to-muscle ratio is a significant predictor of metabolic diseases in Korean adults.12 However, there are only a few studies on the relationship between SO and metabolic diseases such as diabetes, hypertension, and abnormal lipid metabolism in Chinese adults. More studies are needed to explore the association between SO and its pathogenesis and other metabolic diseases to permit the initiation of preventive measures at the level of anthropometric variables. These anthropometric variables can be analyzed to understand the body movement and the nutrition and health status of an individual, particularly to increase the understanding of metabolic diseases. This analysis of anthropometric variables can play a timely warning role in the prevention of hypertension. Thus, the aim of the present study was to evaluate the association between SO and hypertension, diabetes, and abnormal lipid metabolism in Chinese adults, and to explore the appropriate predictive index of SO using relevant anthropometric indicators.
Materials and Methods
Study Design and Participants
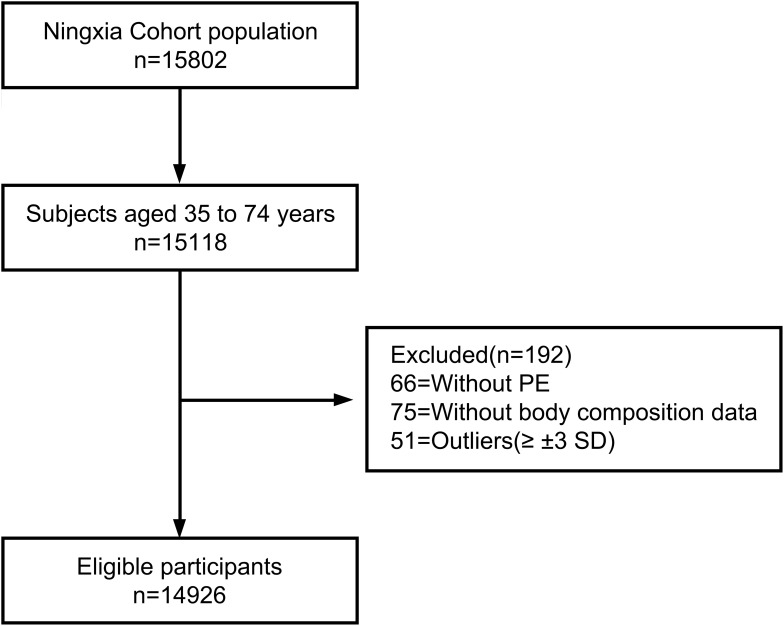
This study was the baseline of a population-based cohort study conducted at Ningxia Medical University from March 2018 to May 2019. Participants were recruited through the Ningxia cohort from the China Northwest Cohort, a prospective population study that comprised a sample of 14,926 men and women aged 35 to 74 years old. The inclusion criteria were as follows: male and female adults aged between 35 and 74 years old (born between 1943 and 1982); registered permanent residents of the selected investigation sites (those who stay at home for more than five months throughout the year); persons with no serious physical disability and who are able to communicate normally. All participants met these inclusion criteria and provided written informed consent before participating in the study. All investigations were performed in accordance with the tenets of the Declaration of Helsinki. This study was approved by the Ethical Committee of Ningxia Medical University (ID: 2018–021). The specific participant selection process is outlined in Figure 1.
Figure 1.
Respondent screening process for the participants.
Setting
All participants underwent a questionnaire interview for collection of demographic data and information on living habits, and then a physical examination for measurement of anthropometric variables. After blood samples were sent to the clinical laboratory testing department, biochemical measurements were determined according to standard laboratory procedures. All procedures were performed by trained investigators and all samples were processed by trained and experienced laboratory technicians.
Variables
Demographic Data
After signing the informed consent form, all participants completed a questionnaire to provide information on demographic data such as age, sex, marital status, smoking, and alcohol consumption. Living habits like salt intake and exercise behaviors were assessed to explore the risk factors to health.
Anthropometric Variables
The measurement of the anthropometric variables was performed by well-trained investigators. Body height (cm) was measured using a height bar, with the participant in light clothing and without shoes. Brachial artery blood pressure and pulse were measured using an electronic sphygmomanometer. Each measurement was taken twice; if the measured values were within 0.5 cm of each another, the average value was calculated and used for analysis. If the difference between the two measurements exceeded 0.5 cm, a third measurement was taken. Weight, body mass index (BMI), appendicular skeletal muscle mass (ASM) and other anthropometric variables of the participants were acquired using a bioelectrical impedance analyzer (BIA) (Inbody370 Co., Seoul, Korea) according to standard guidelines. The use of bioelectrical impedance analysis for the measurement of anthropometric variables has gained popularity.13,14 The BIA used in this study calculates the resistance of body tissues by transmission of an electrical signal sent through the hands and feet. According to the manufacturer’s instructions, participants were directed to remove extra clothing items such as shoes, coats, and sweaters, and metal accessories such as earrings, rings, and watches. They were then asked to stand on the balance scale barefoot and grasp the handles of the BIA. The examination takes nearly 30 seconds to complete; the result is displayed upon completion of the procedure.
Biochemical Variables
Blood samples were collected between 8:00 a.m. and 10:00 a.m. following an overnight fasting. The samples were biochemically analyzed using standard laboratory enzymatic methods. The enzymatic methods are optimized for blood biochemical analysis.15,16 These analyses included evaluation of plasma glucose (fasting blood glucose [FBG], mmol/L), total cholesterol (TC, mmol/L), triglyceride (TG, mmol/L), and high-density lipoprotein cholesterol (HDL-C, mmol/L) levels. The serum was centrifuged, aliquoted, and stored at −80°C.
Definitions
Sarcopenia was defined as ASM/height2 (kg/m2) according to the recommendations of the Asian Working Group for Sarcopenia, which suggests that based on BIA measurements, 7.0 kg/m2 is the cut-off value for men and 5.7 kg/m2 is the cut-off value for women.17 According to the World Health Organization, the definition of obesity is a body fat rate greater than 30% for women or greater than 25% for men. Individuals defined as having sarcopenia according to the criteria of the Asian Working Group and concurrent obesity by any of the standard definitions, were considered individuals with SO. The diagnosis of hypertension was based on the recommendations in the Chinese guidelines for the treatment of hypertension in 2005, which defined systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP) ≥90 mmHg as hypertension. The diabetes discussed in this study is type 2 diabetes, and the diagnosis defined by the American Diabetes Association in 2010, which is fasting plasma glucose ≥7.0 mmol/l of FBG, was selected. The diagnosis for dyslipidemia used in this study refers to the 2016 (revised) guidelines for the prevention and treatment of dyslipidemia in Chinese adults. Patients with hypercholesterolemia (TC ≥ 5.20 mmol/L), hypertriglyceridemia (TG ≥ 1.70 mmol/L) or low high-density lipoprotein cholesterolemia (HDL-C <1.00 mmol/L) were diagnosed with dyslipidemia.
Statistical Analyses
Data are presented as group mean ± standard deviation (SD) unless otherwise stated. All analyses were performed separately for women and men. Chi-square tests and Student’s t-tests were used to analyze the prevalence of SO for different BMI, waist circumference (WC), and hip circumference levels. Logistic regression model was used to analyze the influence of predictive variables on the classification results. A receiver operating characteristic (ROC) curve adjusted for potential confounding variables was constructed, using the point on the ROC curve closest to (0, 1) as a criterion to calculate the cut-off for assessing the risk for SO according to the anthropometric variables. All analyses were performed using SPSS statistical software version 26.0. All tests were two-tailed; p < 0.05 was considered statistically significant.
Results
Participants
A total of 14,926 persons (8978 women and 5948 men) aged 35–74 years old volunteered to participate in this study and underwent all the measurements for further analysis. The mean age of the respondents was 56.75 ± 9.76 years old and 39.80% of them were male. The mean BMI of the respondents was 24.94 ± 3.40 kg/m2, 67.3% had a primary school education or had never been to school, 15.1% were current smokers, and 24.3% had an alcohol consumption habit. Figure 1 shows the prevalence of SO according to sexual parameters. Of the included participants, 65.1% (9721/14,926) had SO according to the previously outlined definition. Most of the participants that had SO were female, and their mean age was 58.59 ± 9.59 years old. The proportion of participants who had hypertension, diabetes, and abnormal lipid metabolism was 21.1%, 5.0%, and 57.6%, respectively.
Outcome Data and Main Results
Main Characteristics of the Participants According to the Presence of Sarcopenic Obesity
The main characteristics of the female and male reference groups are outlined in Table 1. The SO and non-SO groups showed differences in sex, age, education level, weight, height, WC, HC, SBP, DBP, pulse, BMI, waist-to-hip ratio (WHR), ASM, ASMI, FBG, TG, TC, HDL-C and LDL-C (p < 0.05). In addition, current smoking status, alcohol consumption, obesity, hypertension, diabetes, and abnormal lipid metabolism were different between two groups (p < 0.05), whereas marital status, income level, and exercise habit showed no differences (p > 0.05) [Table 1].
Table 1.
Participants Characteristic According to the Presence of SO
| Variables | SO | Without SO | p value | ||||
|---|---|---|---|---|---|---|---|
| Males (n=3298) | Females (n=6423) | Total (n=9721) | Males (n=2650) | Females (n=2555) | Total (n=5205) | ||
| Demographic characteristics | |||||||
| Age (years) | 58.37±9.52 | 55.84±9.40 | 56.70±9.52 | 58.85±9.57 | 54.77±10.33 | 56.85±10.21 | 0.394 |
| 35–44 | 318 (9.6) | 807 (12.6) | 1125 (11.6) | 254 (9.2) | 470 (18.4) | 715 (13.7) | <0.001 |
| 45–54 | 857 (26.0) | 2204 (34.3) | 3061 (31.5) | 641 (24.2) | 895 (35.0) | 1536 (29.5) | |
| 55–64 | 1009 (30.6) | 1951 (30.4) | 2960 (30.4) | 819 (30.9) | 587 (23.0) | 1406 (27.1) | |
| 65–74 | 1114 (33.8) | 1461 (22.7) | 2575 (26.5) | 945 (35.7) | 603 (23.6) | 1548 (29.7) | |
| Marital status | |||||||
| Married | 3136 (95.1) | 5981 (91.7) | 9027 (92.9) | 2523 (95.2) | 2346 (91.8) | 4869 (93.5) | 0.332 |
| Widowed | 109 (3.3) | 481 (7.5) | 590 (6.1) | 84 (3.2) | 198 (7.7) | 282 (5.4) | |
| Divorce | 24 (0.7) | 22 (0.3) | 46 (0.5) | 15 (0.6) | 5 (0.2) | 20 (0.4) | |
| Unmarried | 29 (0.9) | 29 (0.5) | 58 (0.5) | 28 (1.0) | 6 (0.3) | 34 (0.7) | |
| Education | |||||||
| Not been to school | 741 (22.5) | 2704 (42.1) | 3445 (35.4) | 690 (26.0) | 1000 (391) | 1690 (32.5) | <0.001 |
| Primary school | 1242 (37.7) | 1951 (30.4) | 3193 (32.8) | 943 (35.6) | 767 (30.0) | 1710 (32.9) | |
| Junior high school | 1123 (34.0) | 1607 (25.0) | 2730 (28.2) | 882 (33.3) | 711 (27.8) | 1593 (30.6) | |
| Senior high school and above | 192 (5.8) | 161 (2.5) | 353 (3.6) | 135 (5.1) | 77 (3.0) | 212 (4.0) | |
| Income (RMB/year) | |||||||
| Less than 10,000 | 815 (24.7) | 1569 (24.4) | 2384 (24.5) | 658 (24.8) | 595 (23.3) | 1253 (24.0) | 0.114 |
| 10,000~ | 1920 (58.2) | 3811 (59.3) | 5731 (59.0) | 1620 (61.1) | 1519 (59.4) | 3139 (60.3) | |
| 50,000~ | 345 (10.5) | 558 (8.7) | 903 (9.3) | 229 (8.6) | 258 (10.1) | 487 (9.4) | |
| More than 100,000 | 218 (6.6) | 485 (7.6) | 703 (7.2) | 143 (5.4) | 183 (7.2) | 326 (6.3) | |
| Exercise(days/week) | |||||||
| ≦1 | 1888 (57.2) | 3743 (58.3) | 5631 (57.9) | 1553 (58.6) | 1485 (58.1) | 3038 (58.4) | 0.680 |
| 2~3 | 263 (8.0) | 569 (8.8) | 832 (8.6) | 199 (7.5) | 225 (8.8) | 424 (8.1) | |
| 4~5 | 337 (10.2) | 659 (10.3) | 996 (10.2) | 287 (10.8) | 266 (10.4) | 553 (10.6) | |
| 6~7 | 810 (24.6) | 1452 (22.6) | 2262 (23.3) | 611 (23.1) | 579 (22.7) | 1190 (22.9) | |
| Current Smoking (yes), n (%) | 1103 (33.4) | 120 (1.9) | 1223 (12.6) | 999 (37.7) | 28 (1.1) | 1027 (19.7) | <0.001 |
| Alcohol consumption (yes), n (%) | 1388 (42.1) | 917 (14.3) | 2305 (23.7) | 937 (35.4) | 383 (15.0) | 1320 (25.4) | 0.025 |
| Obesity, n (%) | 3298 (100.0) | 6423 (100.0) | 9721 (100.0) | 414 (15.6) | 550 (21.5) | 964 (18.5) | <0.001 |
| Hypertension, n (%) | 819 (24.8) | 1494 (23.3) | 2313 (23.8) | 439 (16.6) | 403 (15.8) | 842 (16.2) | <0.001 |
| Diabetes, n (%) | 199 (6.0) | 340 (5.3) | 539 (5.5) | 103 (3.9) | 102 (4.0) | 205 (3.9) | <0.001 |
| Abnormal lipid metabolism, n (%) | 2104 (63.8) | 3835 (59.7) | 5939 (61.1) | 1164 (43.9) | 1175 (46.0) | 2339 (44.9) | <0.001 |
| Anthropometric variables | |||||||
| Weight (kg) | 74.79±8.78 | 63.85±8.01 | 67.56±9.77 | 61.81±7.97 | 52.78±6.59 | 57.38±8.61 | <0.001 |
| Height (m) | 1.66±0.06 | 1.56±0.06 | 1.59±0.08 | 1.66±0.67 | 1.56±0.66 | 1.61±0.84 | <0.001 |
| WC (cm) | 95.03±8.23 | 89.49±7.77 | 91.37±8.35 | 80.70±6.88 | 77.35±5.89 | 79.06±6.63 | <0.001 |
| HC (cm) | 99.02±4.05 | 95.29±4.30 | 96.56±4.57 | 92.04±3.79 | 88.63±3.47 | 90.36±4.02 | <0.001 |
| SBP (mm Hg) | 139.39±18.91 | 136.69±19.33 | 137.60±19.23 | 131.77±18.90 | 130.58±19.78 | 131.19±19.34 | <0.001 |
| DBP (mm Hg) | 86.95±12.77 | 83.63±12.20 | 84.76±12.49 | 81.21±12.55 | 76.65±12.14 | 80.44±12.38 | <0.001 |
| Pulse (bpm) | 74.06±12.17 | 77.69±11.88 | 76.45±12.10 | 72.51±12.18 | 78.17±12.09 | 75.28±12.46 | <0.001 |
| BMI (kg/m2) | 27.10±2.55 | 26.26±2.86 | 26.54±2.79 | 22.29±2.20 | 21.61±2.13 | 21.95±2.20 | <0.001 |
| WHR | 0.96±0.56 | 0.94±0.05 | 0.95±0.54 | 0.88±0.05 | 0.87±0.05 | 0.87±0.50 | <0.001 |
| ASM (kg) | 22.21±2.70 | 16.44±2.17 | 19.40±3.62 | 20.67±3.21 | 15.26±2.74 | 18.02±4.03 | <0.001 |
| ASMI (kg/m2) | 8.03±0.60 | 6.74±0.60 | 7.18±0.86 | 7.43±0.75 | 6.21±0.74 | 6.83±0.96 | <0.001 |
| Biochemical variables | |||||||
| FBG (mmol/L) | 5.76±1.67 | 5.62±1.56 | 5.67±1.60 | 5.66±1.82 | 5.55±1.75 | 5.61±1.79 | 0.041 |
| TG (mmol/L) | 1.89±1.26 | 1.79±1.11 | 1.82±1.17 | 1.43±1.13 | 1.51±1.10 | 1.47±1.12 | <0.001 |
| TC (mmol/L) | 4.82±1.17 | 5.03±1.02 | 4.96±1.08 | 4.57±0.96 | 4.79±0.98 | 4.68±0.98 | <0.001 |
| HDL-C (mmol/L) | 1.26±0.34 | 1.39±0.34 | 1.35±0.35 | 1.32±0.37 | 1.41±0.33 | 1.37±0.36 | <0.001 |
| LDL-C (mmol/L) | 2.80±0.89 | 2.92±0.80 | 2.88±0.83 | 2.72±0.98 | 2.78±0.79 | 2.75±0.89 | <0.001 |
Note: Bold indicates statistical significance.
The Correlation Between Sarcopenic Obesity and Hypertension, Diabetes, and Abnormal Lipid Metabolism
Table 1 also shows that there is positive correlation between SO and hypertension, diabetes, and abnormal lipid metabolism in the entire study population (p < 0.05). The positive correlation between SO and hypertension, diabetes, and abnormal lipid metabolism showed statistically significant difference in both males and females. Logistic regression was used to analyze the influencing factors. The results shown that gender, SBP, DBP, FBG, TC, TG and HDL-C were the influencing factors for SO, and diabetes mellitus (1.251, 95% CI: 1.034–1.514), hypertension (1.157, 95% CI: 1.045–1.281) and abnormal lipid metabolism (1.327, 95% CI: 1.209–1.458) were the risk factors for SO [Table 2]. Finally, when adjustment for age, sex, education, smoking, alcohol consumption, diabetes, hypertension, abnormal lipid metabolism were added into the model, with each of the SO-influencing indicators still having significant ORs for BMI, WC, and WHR [Table 3].
Table 2.
Logistic Regression of the Influencing Factors of SO
| Variables | β | S.E. | Wald | OR (95% CI) | p value |
|---|---|---|---|---|---|
| Sex | 0.765 | 0.041 | 354.063 | 2.149 (1.984, 2.327) | <0.001 |
| Age | −0.041 | 0.023 | 3.127 | 0.960 (0.918, 1.004) | 0.077 |
| SBP | 0.010 | 0.002 | 43.901 | 1.010 (1.007, 1.013) | <0.001 |
| DBP | 0.017 | 0.002 | 56.947 | 1.017 (1.013, 1.022) | <0.001 |
| FBG | −0.030 | 0.012 | 6.173 | 0.970 (0.947, 0.994) | 0.013 |
| TC | 0.174 | 0.032 | 29.822 | 1.190 (1.118, 1.266) | <0.001 |
| TG | 0.159 | 0.023 | 48.342 | 1.172 (1.121, 1.226) | <0.001 |
| HDL-C | −0.442 | 0.066 | 44.980 | 4.643 (4.565, 0.731) | <0.001 |
| LDL-C | −0.031 | 0.031 | 0.982 | 0.970 (0.912, 1.031) | 0.322 |
| Education | 0.018 | 0.024 | 0.525 | 1.018 (0.970, 1.067) | 0.469 |
| Diabetes | 0.224 | 0.097 | 5.302 | 1.251 (1.034, 1.514) | 0.021 |
| Hypertension | 0.146 | 0.052 | 7.847 | 1.157 (1.045, 1.281) | 0.005 |
| Abnormal lipid metabolism | 0.283 | 0.048 | 35.297 | 1.327 (1.209, 1.458) | <0.001 |
| Constant | −3.704 | 0.220 | 282.254 |
Note: Bold indicates statistical significance.
Table 3.
Multivariable Association of Participants’ Characteristics with SO
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| SBP | 1.000 (0.996, 1.004) | 0.936 | 0.994 (0.990, 0.999) | 0.018 | 0.994 (0.990, 0.999) | 0.017 | 0.996 (0.991, 1.000) | 0.068 |
| DBP | 0.995 (0.989, 1.002) | 0.149 | 1.003 (0.996, 1.010) | 0.430 | 1.003 (0.996, 1.010) | 0.471 | 1.003 (0.996, 1.010) | 0.358 |
| Pulse | 1.009 (1.004, 1.014) | <0.001 | 1.001 (0.996, 1.006) | 0.578 | 1.001 (0.996, 1.006) | 0.644 | 1.001 (0.996, 1.006) | 0.598 |
| FBG | 0.961 (0.931, 0.993) | 0.016 | 0.965 (0.933, 0.999) | 0.043 | 0.966 (0.934, 1.000) | 0.048 | 0.966 (0.932, 1.002) | 0.066 |
| TG | 0.981 (0.932, 1.032) | 0.452 | 0.960 (0.910, 1.012) | 0.131 | 0.963 (0.913, 1.016) | 0.168 | 0.966 (0.913, 1.023) | 0.236 |
| TC | 1.103 (1.012, 1.202) | 0.025 | 1.113 (1.012, 1.224) | 0.027 | 1.097 (0.998, 1.207) | 0.056 | 1.085 (0.983, 1.198) | 0.105 |
| HDL-C | 1.283 (1.068, 1.541) | 0.008 | 1.068 (0.881, 1.294) | 0.505 | 1.067 (0.881, 1.293) | 0.506 | 1.067 (0.877, 1.298) | 0.516 |
| LDL-C | 1.003 (0.921, 1.091) | 0.952 | 0.955 (0.865, 1.056) | 0.371 | 0.974 (0.882, 1.075) | 0.601 | 0.979(0.888, 1.080) | 0.670 |
| WC | 0.828 (0.803, 0.854) | <0.001 | 1.076 (1.034, 1.119) | <0.001 | 1.079 (1.038, 1.122) | <0.001 | 1.079 (1.038, 1.122) | <0.001 |
| WHR | 1.338 (1.296, 1.381) | <0.001 | 1.055 (1.015, 1.096) | <0.001 | 1.055 (1.016, 1.096) | 0.006 | 1.056 (1.016, 1.097) | 0.005 |
| BMI | 2.773 (2.623, 2.933) | <0.001 | 2.060 (1.936, 2.192) | <0.001 | 2.061 (1.937, 2.193) | <0.001 | 2.070 (1.944, 2.203) | <0.001 |
Notes: Bold indicates statistical significance. Model 1 has no controlled variables; Model 2 adjusted for age, sex, education; Model 3 adjusted for Model 2 and smoking, alcohol consumption; Model 4 adjusted for Model 3 and diabetes, hypertension, abnormal lipid metabolism.
The Area Under the Curve of Different Anthropometric Variables in the Evaluation of Sarcopenic Obesity
The prevalence of SO was 55.40% in males and 71.50% in females. The area under the curve (AUC) of weight, height, WC, WHR, BMI, SBP, DBP, pulse, FBG, TG, TC, HDL-C and LDL-C for SO showed statistical difference. These indicators also showed significant difference in males; however, pulse showed no statistical difference in females.
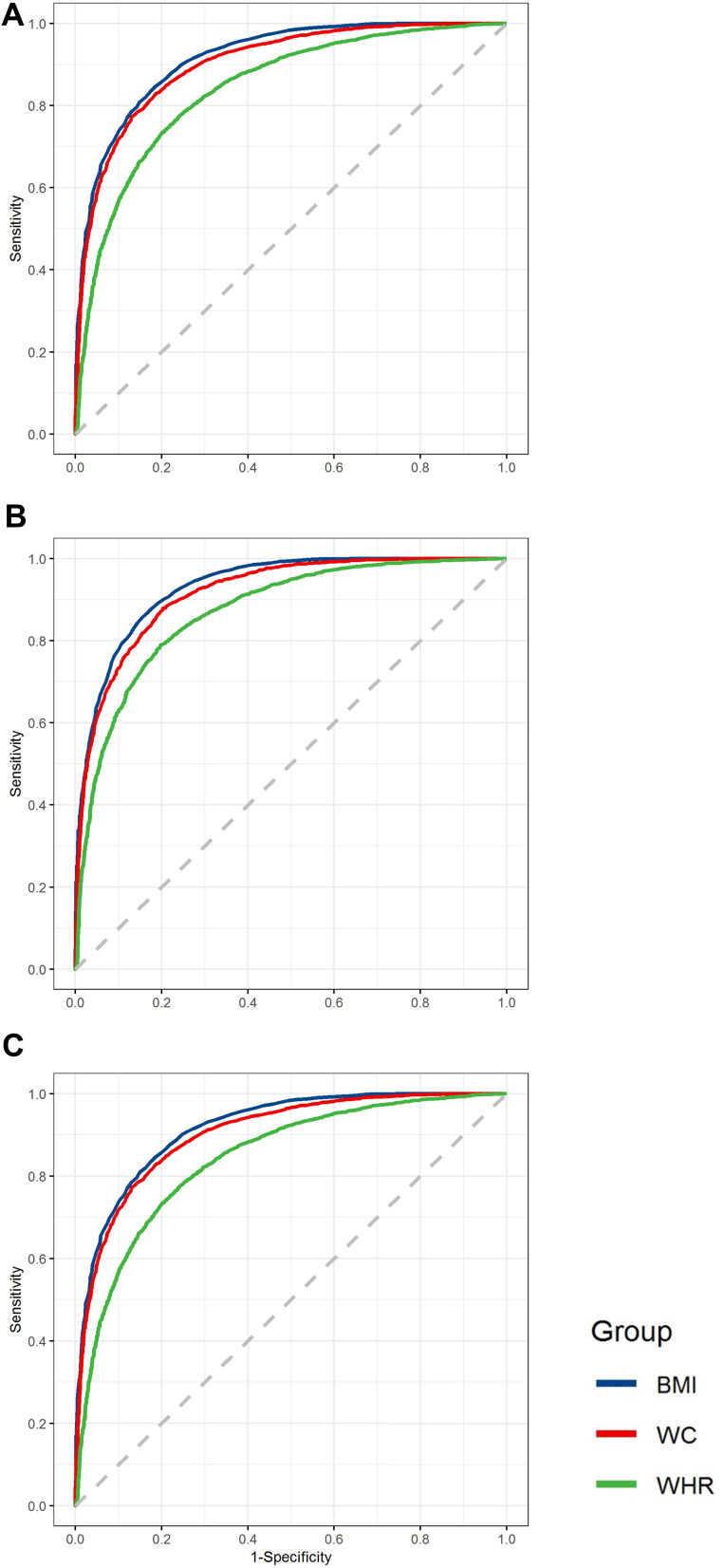
Among these indicators, BMI was the best variable for predicting SO in both males and females; the cut-off value was 24.45 for males and 23.45 for females. WC and WHR can also be predictors for SO, with 85.95 and 0.91 as the cut-off values for males and 82.25 and 0.90 as the cut-off values for females, respectively [Table 4], [Figure 2].
Table 4.
AUC of Different Anthropometric Variables Evaluating SO
| Total (n=14,926) | Male (n=5948) | Female (n=8978) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | Cut-Off | Sensitivity (%) | Specificity (%) | AUC (95% CI) | Cut-Off | Sensitivity (%) | Specificity (%) | AUC (95% CI) | Cut-Off | Sensitivity (%) | Specificity (%) | |
| Weight | 0.786 (0.778, 0.794) ** |
60.05 | 76.60 | 64.86 |
0.866
(0.857, 0.876)** |
67.15 | 79.64 | 76.83 |
0.863
(0.854, 0.872)** |
56.75 | 81.14 | 74.33 |
| Height | 0.425 (0.415, 0.436) ** |
1.62 | 32.55 | 54.85 | 0.484 (0.468, 0.500) * |
1.71 | 22.31 | 73.90 | 0.484 (0.469, 0.499) * |
1.58 | 31.70 | 62.95 |
| WC |
0.883
(0.877, 0.889) ** |
84.25 | 80.07 | 79.70 |
0.915
(0.908, 0.923) ** |
85.95 | 87.62 | 79.59 |
0.903
(0.895, 0.910)** |
82.25 | 82.96 | 81.03 |
| WHR |
0.844
(0.837, 0.851) ** |
0.91 | 76.77 | 76.62 |
0.867
(0.857, 0.876) ** |
0.91 | 78.48 | 79.63 |
0.841
(0.832, 0.851)** |
0.90 | 78.94 | 74.37 |
| BMI |
0.912
(0.907, 0.917) ** |
23.75 | 84.91 | 80.41 |
0.931
(0.925, 0.938) ** |
24.45 | 85.32 | 84.64 |
0.918
(0.911, 0.924)** |
23.45 | 84.42 | 81.97 |
| SBP | 0.604 (0.593, 0.614) ** |
131.50 | 59.44 | 55.88 | 0.624 (0.609, 0.639) ** |
135.50 | 55.97 | 63.29 | 0.599 (0.585, 0.613) ** |
123.50 | 73.67 | 41.64 |
| DBP | 0.602 (0.592, 0.613) ** |
79.50 | 66.11 | 48.96 | 0.631 (0.616, 0.646) ** |
83.50 | 58.30 | 59.92 | 0.598 (0.584, 0.612) ** |
79.50 | 63.03 | 51.92 |
| Pulse | 0.530 (0.520, 0.541) ** |
71.50 | 64.36 | 41.07 | 0.539 (0.524, 0.555) ** |
71.50 | 54.88 | 51.77 | 0.489 (0.475, 0.504) |
80.50 | 35.86 | 60.92 |
| FBG (mmol/L) |
0.530 (0.519, 0.540) ** |
5.56 | 39.89 | 64.43 | 0.536 (0.520, 0.551) ** |
5.56 | 44.51 | 61.48 | 0.539 (0.525, 0.53) ** |
5.17 | 61.81 | 44.29 |
| TG | 0.626 (0.616, 0.636) ** |
1.25 | 65.90 | 53.20 | 0.642 (0.627, 0.657) ** |
1.30 | 63.46 | 59.23 | 0.610 (0.596, 0.624) ** |
1.48 | 52.96 | 63.87 |
| TC | 0.580 (0.569, 0.590) ** |
4.60 | 63.07 | 49.14 | 0.572 (0.556, 0.587) ** |
4.54 | 59.19 | 51.86 | 0.566 (0.552, 0.581) ** |
4.67 | 63.60 | 47.20 |
| HDL-C (mmol/L) |
0.479 (0.469, 0.490) ** |
1.34 | 46.89 | 49.44 | 0.442 (0.427, 0.458) ** |
1.21 | 52.72 | 37.79 | 0.474 (0.460, 0.489) ** |
1.59 | 23.72 | 71.90 |
| LDL-C (mmol/L) |
0.550 (0.540, 0.561) ** |
2.57 | 63.21 | 44.25 | 0.534 (0.518, 0.550) ** |
2.44 | 65.55 | 39.60 | 0.551 (0.537, 0.565) ** |
2.80 | 53.05 | 54.83 |
Notes: *Indicates p-value from Pearson correlation<0.05, **indicates p-value from Pearson correlation<0.001. Bold indicates AUC>0.85.
Figure 2.
ROC curves for total participants (A), male (B) and female (C) in predicting sarcopenic obesity according to BMI, WC, WHR (GraphPad Prism 8 used to create the artwork).
Notes: ROC curves for total participants (A) in predicting sarcopenic obesity according to BMI, WC, WHR; ROC curves for males (B) in predicting sarcopenic obesity according to BMI, WC, WHR; ROC curves for females (C) in predicting sarcopenic obesity according to BMI, WC, WHR (GraphPad Prism 8 used to create the artwork).
Discussion
In this study, we investigated the associations between SO and hypertension, diabetes, and abnormal lipid metabolism. After controlling for potential confounders, we found that hypertension was significantly associated with the risk of sarcopenia. We also evaluated the prevalence of SO in Chinese adults and found that the overall prevalence of SO was 65.1%. The prevalence of SO has been reported to be higher in patients with chronic diseases such as hypertension, diabetes, abnormal lipid metabolism.18–21
The results of this study also indicate that the participants in the SO group were older than the those in the non-SO group, suggesting that increasing age may be a risk factor for the occurrence of SO.22,23 The participants in the SO group were shorter; had faster heart rate; heavier weight; lower WC, BMI, TG, TC, LDL-C, and higher HDL-C; and lower education level than those in the non-SO group. This may be related to decreased hormone levels, decreased mobility, and decreased protein intake and synthesis caused by aging. Early education and appropriate intervention in the elderly may reduce or delay the occurrence of SO.24–26
Many studies have shown that the occurrence of SO is related to cardiovascular diseases and metabolic diseases such as hypertension, diabetes, and abnormal lipid metabolism.27,28 The results of the present study suggest that hypertension, diabetes, and elevated TC are risk factors for the occurrence of SO. Loss of muscle represents the reduction of target tissues for insulin action, and leads to insulin resistance, which then leads to obesity, metabolic syndrome, hypertension, and other diseases. In both frailty and sarcopenia, there is dysfunction and degeneration of muscle and adipose tissue. Studies have shown that loss of muscle mass and strength in adults is associated with metabolic disorders and dysfunction, while insulin resistance and higher ectopic fat accumulation may play a role.29,30
Glucocorticoid receptors activate and promote progressive loss of cardiac myocyte apoptosis during heart failure. In addition, myocardial apoptosis is also present in the skeletal muscle of patients with congestive heart failure. This apoptosis exacerbates muscle atrophy, weakness, and decreased exercise tolerance, with a pathophysiological process similar to that of SO.31,32 In obese adipose tissue, adipose cells undergo hypertrophy and hyperplasia, and activate, causing the proinflammatory macrophages and other immune cells, as well as aging cells and immune cells, to release cytokines and chemokines that lead to enhanced secretion of proinflammatory myokine. Also, myokines induce inflammatory state in muscles as a result of abnormal adipose tissue functions. These proinflammatory muscle growth factors can be induced through autocrine/paracrine manner and cause muscle dysfunction, triggering and sustaining the development of the SO.33 Moreover, multiple factors in the inflammatory response are closely associated with chronic conditions including obesity,34 metabolic syndrome,35 type 2 diabetes36,37 and coronary heart disease,38 which all promote to SO. Studies have shown that inflammatory factors are associated with type 2 diabetes, and the increase in white blood cells is mainly due to neutrophil count, while the neutrophil to lymphocyte ratio (NLR) in type 2 diabetes patients is increased compared with the healthy control group. Neutrophilia can respond quickly to inflammatory stimuli and rapidly increase neutrophil counts. At the same time, interleukin levels in inflammatory conditions lead to neutrophilia, which together lead to increased NLR.36,39 This study was mainly focused on rural areas, where the prevalence of diabetes and SO are both relatively low.
At present, the association between hypertension and SO is mentioned in only a few reports.40 The findings of the present study showed that hypertension has a significant association with SO. In this study, the DBP and SBP of the SO group were higher than those of the non-SO group, suggesting that blood pressure may be a predictive indicator of SO. However, this finding needs to be confirmed by further investigation. SO is often accompanied by functional impairment and physical disability, which results in a decrease in anti-inflammatory factors (also known as myokines) produced by muscle contractions. The relative deficiency of anti-inflammatory factors in SO may increase the risk of cardiovascular and metabolic diseases in individuals.
However, there are still some disputes regarding the evaluation criteria for SO, and the forecast indicators in the proposed criteria are not the same.41,42 Some studies indicate that anthropometric data such as WC and BMI are important indicators for predicting SO.43,44 The results of the present study suggest that BMI is the best indicator for predicting the occurrence of SO; WC and WHR were also found to be suitable predictors for SO. Since these measures are both anthropometric indicators and can be conveniently measured and calculated during general physical examination, we recommend that these indexes may be used as predictive indicators for SO.
Limitations
The present study had some limitations. First, we only used data acquired using the BIA to measure sarcopenia; however, the diagnosis of the condition may include stride speed and grip at the same time. Second, the data for this study were the baseline of a cohort study, which precluded our ability to identify cause-effect association, particularly in the association between SO and other diseases. Despite these limitations, we investigated the prevalence of SO in 35–74-year-old Chinese adults, and identified some factors associated with SO. Our findings will provide some evidence and basis for subsequent research on SO.
Conclusion
This study showed that prevalence of SO is high (65.1%) in Chinese adults aged 35–74 years old. The results also indicate that the occurrence of SO is related to hypertension, diabetes, and abnormal lipid metabolism. BMI, WC, and WHR were found to be possible predictive indicators of SO.
Acknowledgments
We would like to thank all the subjects, doctors, nurses, and technicians who participated in the study.
Funding Statement
This work was supported by Natural Science Foundation of Ningxia (2018AAC03089), National Key Research and Development Project Ningxia cohort from China Northwest Cohort (2017YFC0907204) and National Natural Science Foundation of China (12061058). The sponsors (Ting Yin, Yu-Hong Zhang and Yu Zhao) all conducted the field investigation and reviewed the manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declared no conflicts of interest in this work.
References
- 1.Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31(6):1054–1060. doi: 10.3904/kjim.2016.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenholm S. Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K. Association of osteosarcopenic obesity and its components: osteoporosis, sarcopenia and obesity with insulin resistance. J Bone Miner Metab. 2020. doi: 10.1007/s00774-020-01104-2 [DOI] [PubMed] [Google Scholar]
- 4.Abete I, Konieczna J, Zulet MA, et al. Association of lifestyle factors and inflammation with sarcopenic obesity: data from the PREDIMED-Plus trial. J Cachexia Sarcopenia Muscle. 2019;10:974–984. doi: 10.1002/jcsm.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Im J-S, Choi CH, et al. The association between red blood cell distribution width and sarcopenia in U.S. adults. Sci Rep. 2018;8(1):11484. doi: 10.1038/s41598-018-29855-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasaei N, Kashavarz SA, Yekaninejad MS, Mirzaei K. The association between sarcopenic obesity (SO) and major dietary patterns in overweight and obese adult women. Diabetes Metab Syndr. 2019;9(4):2519–2524. doi: 10.1016/j.dsx.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 7.Atkins JL, Whincup PH, Morris RW, et al. Sarcopenic obesity and risk of cardiovascular disease and mortality: a population-based cohort study of older men. J Am Geriatr Soc. 2014;62(2):253–260. doi: 10.1111/jgs.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellanti F, Romano AD, Lo Buglio A, et al. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas. 2018;109:6–12. doi: 10.1016/j.maturitas.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 9.Petermann-Rocha F, Yang S, Gray SR, et al. Sarcopenic obesity and its association with respiratory disease incidence and mortality. Clin Nutr. 2020;39(11):3461–3466. doi: 10.1016/j.clnu.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 10.Barazzoni R, Bischoff S, Boirie Y, et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018;11(4):294–305. doi: 10.1159/000490361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marty E, Liu Y, Samuel A, Or O, Lane J. A review of sarcopenia: enhancing awareness of an increasingly prevalent disease. Bone. 2017;105:276–286. doi: 10.1016/j.bone.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 12.Seo Y-G, Song HJ, Song YR. Fat-to-muscle ratio as a predictor of insulin resistance and metabolic syndrome in Korean adults. J Cachexia Sarcopenia Muscle. 2020;11(3):710–725. doi: 10.1002/jcsm.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosy-Westphal A, Danielzik S, Dörhöfer R-P, et al. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. J Parenter Enteral Nutr. 2006;30(4):309–316. doi: 10.1177/0148607106030004309 [DOI] [PubMed] [Google Scholar]
- 14.Sullivan PA, Still CD, Jamieson ST, et al. Evaluation of multi-frequency bioelectrical impedance analysis for the assessment of body composition in individuals with obesity. Obes Sci Pract. 2019;5(2):141–147. doi: 10.1002/osp4.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefani S, Ngatidjan S, Paotiana M, et al. Dietary quality of predominantly traditional diets is associated with blood glucose profiles, but not with total fecal bifidobacterium in Indonesian women. PLoS One. 2018;13(12):e0208815. doi: 10.1371/journal.pone.0208815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S-Z, Wu M, Chen K-J, et al. Hawthorn extract alleviates atherosclerosis through regulating inflammation and apoptosis related factors: an experimental study. Chin J Integr Med. 2019;25(2):108–115. doi: 10.1007/s11655-018-3020-4 [DOI] [PubMed] [Google Scholar]
- 17.Chen L-K, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307 e302. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 18.Billingsley H, Rodriguez-Miguelez P, Del Buono MG, et al. Lifestyle interventions with a focus on nutritional strategies to increase cardiorespiratory fitness in chronic obstructive pulmonary disease, heart failure, obesity, sarcopenia, and frailty. Nutrients. 2019;11(12):2849. doi: 10.3390/nu11122849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koliaki C, Liatis S, Dalamaga M, Kokkinos A. Sarcopenic obesity: epidemiologic evidence, pathophysiology, and therapeutic perspectives. Curr Obes Rep. 2019;8:458–471. doi: 10.1007/s13679-019-00359-9 [DOI] [PubMed] [Google Scholar]
- 20.Kong HH, Won CW, Kim W. Effect of sarcopenic obesity on deterioration of physical function in the elderly. Arch Gerontol Geriatr. 2020;89:104065. doi: 10.1016/j.archger.2020.104065 [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro Santos V, Dias Correa B, De Souza Pereira CG, Alberto Gobbo L. Physical activity decreases the risk of sarcopenia and sarcopenic obesity in older adults with the incidence of clinical factors: 24-month prospective study. Exp Aging Res. 2020;46:166–177. doi: 10.1080/0361073X.2020.1716156 [DOI] [PubMed] [Google Scholar]
- 22.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi: 10.1038/ejcn.2014.117 [DOI] [PubMed] [Google Scholar]
- 23.Molero J, Moizé V, Flores L, et al. The impact of age on the prevalence of sarcopenic obesity in bariatric surgery candidates. Obes Surg. 2020;30(6):2158–2164. doi: 10.1007/s11695-019-04198-4 [DOI] [PubMed] [Google Scholar]
- 24.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537. doi: 10.1038/s41574-018-0062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 26.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue—link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11(2):90–100. doi: 10.1038/nrendo.2014.185 [DOI] [PubMed] [Google Scholar]
- 27.Fukuda T, Bouchi R, Takeuchi T, et al. Sarcopenic obesity assessed using dual energy X-ray absorptiometry (DXA) can predict cardiovascular disease in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol. 2018;17(1):55. doi: 10.1186/s12933-018-0700-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116(7):1171–1178. doi: 10.1002/jcb.25077 [DOI] [PubMed] [Google Scholar]
- 29.Buch A, Carmeli E, Boker LK, et al. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age — an overview. Exp Gerontol. 2016;76:25–32. doi: 10.1016/j.exger.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 30.Bilgin S, Aktas G, Kurtkulagi O, Atak BM, Duman TT. Edmonton frail score is associated with diabetic control in elderly type 2 diabetic subjects. J Diabetes Metab Disord. 2020;19:511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han P, Yu H, Ma Y, et al. The increased risk of sarcopenia in patients with cardiovascular risk factors in suburb-dwelling older Chinese using the AWGS definition. Sci Rep. 2017;7(1):9592. doi: 10.1038/s41598-017-08488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton LA, McMurdo ME, Struthers AD. Mineralocorticoid antagonism: a novel way to treat sarcopenia and physical impairment in older people? Clin Endocrinol. 2011;75:725–729. doi: 10.1111/j.1365-2265.2011.04148.x [DOI] [PubMed] [Google Scholar]
- 33.Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. J Ageing Res Rev. 2016;35. [DOI] [PubMed] [Google Scholar]
- 34.Aktas G, Kocak MZ, Duman TT, et al. Mean Platelet Volume (MPV) as an inflammatory marker in type 2 diabetes mellitus and obesity. Bali Med J. 2018;7(3). doi: 10.15562/bmj.v7i3.806 [DOI] [Google Scholar]
- 35.Kocak MZ, Aktas G, Erkus E, et al. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras. 2019;65(1):9–15. doi: 10.1590/1806-9282.65.1.9 [DOI] [PubMed] [Google Scholar]
- 36.Duman TT, Aktas G, Atak BM, et al. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr Health Sci. 2019;19(1):1602–1606. doi: 10.4314/ahs.v19i1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilgin S, Aktas G, Zahid Kocak M, et al. Association between novel inflammatory markers derived from hemogram indices and metabolic parameters in type 2 diabetic men. Aging Male. 2019:1–5. [DOI] [PubMed] [Google Scholar]
- 38.Sincer I, Gunes Y, Mansiroglu AK, Aktas G. Differential value of eosinophil count in acute coronary syndrome among elderly patients. Aging Male. 2019;1–4. doi: 10.1080/13685538.2019.1643310 [DOI] [PubMed] [Google Scholar]
- 39.Atak B, Aktas G, Duman TT, Erkus E, Kocak MZ, Savli H. Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Rev Assoc Med Bras. 2019;65(1):38–42. doi: 10.1590/1806-9282.65.1.38 [DOI] [PubMed] [Google Scholar]
- 40.Atkins JL, Wannamathee SG. Sarcopenic obesity in ageing: cardiovascular outcomes and mortality. Br J Nutr. 2020;1–26. doi: 10.1017/S0007114520002172 [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Kim T-H, Jeong C-W, et al. Development of quantification software for evaluating body composition contents and its clinical application in sarcopenic obesity. Sci Rep. 2020;10(1):10452. doi: 10.1038/s41598-020-67461-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim WS, Lim JP, Chew J, Tan AWK. Calf circumference as a case-finding tool for sarcopenia: influence of obesity on diagnostic performance. J Am Med Dir Assoc. 2020;21(9):1359–1361. doi: 10.1016/j.jamda.2020.03.033 [DOI] [PubMed] [Google Scholar]
- 43.Liao Q, Zheng Z, Xiu S, Chan P. Waist circumference is a better predictor of risk for frailty than BMI in the community-dwelling elderly in Beijing. Aging Clin Exp Res. 2018;30(11):1319–1325. doi: 10.1007/s40520-018-0933-x [DOI] [PubMed] [Google Scholar]
- 44.Chung W, Park JH, Chung HS, et al. Utility of the Z-score of log-transformed A Body Shape Index (LBSIZ) in the assessment for sarcopenic obesity and cardiovascular disease risk in the United States. Sci Rep. 2019;9(1):9292. doi: 10.1038/s41598-019-45717-8 [DOI] [PMC free article] [PubMed] [Google Scholar]