Abstract
Background
Smoking cessation is a key intervention for all smokers with chronic obstructive pulmonary disease (COPD). Poor treatment adherence is a challenge in clinical practice that might contribute to the lower efficacy of medication (eg, oral drug). However, it is unclear what factors will influence adherence among smokers with COPD.
Methods
This study was based on an open-label randomized controlled trial (RCT) of varenicline and bupropion for smoking cessation among patients with COPD in China. The medication was given for 12 weeks, and visits and assessments were conducted at weeks 0, 1, 2, 4, 6, 9, 12, and 24. We assessed whether the adherence to smoking cessation treatment affects the smoking cessation efficacy and evaluated predictors of adherence.
Results
A total of 136 participants were recruited from February 2019 to June 2020, and analyzed using the intention-to-treat (ITT) method. In this study, 48.5% (66/136) of the total participants had good adherence to smoking cessation, and good adherence significantly improved the efficacy of smoking cessation (OR=9.60, 95% CI 4.02–22.96, P < 0.001). After adjusting for age, gender, nationality, education, and marital status, we found older age, higher education level, having more previous quitting attempts, stronger self-efficacy and preparation in quitting smoking, recognizing hazards of smoking, longer duration of COPD, and higher St. George’s Respiratory Questionnaire (SGRQ) scores were relevant to good adherence (P < 0.05).
Conclusion
To our best knowledge, this is the first study to evaluate adherence to smoking cessation treatment among patients with COPD in China. Our study found that good adherence to smoking cessation treatment significantly improved the smoking cessation efficacy, and predictors of adherence were evaluated. We call on the medical community to pay attention to the adherence to smoking cessation among patients with COPD.
Keywords: COPD, smoking cessation, adherence, China
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterized by persistent airflow limitations and respiratory symptoms.1 COPD is a worldwide public health challenge because of its high prevalence and disability, and it remains the third leading cause of death worldwide in 2016.2
The economic and social burden of COPD in China is heavier than that in developed countries, with COPD rapidly becoming a leading cause of mortality in China.3,4 It is estimated that the overall prevalence of COPD in China is 8.6%, accounting for 99.9 million in 2018.4
The 2021 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guideline points out that the risk for developing COPD results from an interaction between genetics and many environmental factors, and smoking is the most common environmental factor. For example, a higher prevalence of respiratory symptoms and mortality rates of COPD are seen among smokers than non-smokers.1
Smoking cessation is a key evidence-based intervention for all smokers with COPD, which can slow the accelerated decline in lung function and reduce the risk of development of COPD.1 To date, the treatment for smoking cessation mainly includes psychological intervention, behavioral support, pharmacotherapy, etc.5 Among them, pharmacotherapy can significantly improve the success rate of smoking cessation.6 Nevertheless, despite different interventions for smoking cessation, the chance of smokers with COPD definitively quitting smoking was still low in the real world.7
European Society for Patient Adherence, Compliance and Persistence defined medication/treatment adherence as “the process by which patients take their medication/treatment as prescribed”.8 Poor treatment adherence is a challenge in clinical practice that might reduce the efficacy of medication (eg, oral drug).8 Previous studies founded that 20% of the smokers who receive pharmacotherapies for smoking cessation never take medication as prescribed,9,10 and smokers often use them in fewer doses and for less time than health professionals suggest. For instance, good adherence to smoking cessation treatment occurs among 50% or fewer users of nicotine replacement therapy.11
Previous studies related to smoking cessation treatment adherence mostly focused on the general population. However, most studies did not distinguish different kinds of smokers, although there is some evidence that smoking-related features differ between smokers with COPD and smokers without COPD. For example, smokers with COPD might have more difficulty quitting smoking because of their longer duration of smoking, and lower self-efficacy.12 This suggests that smokers maybe are not a homogeneous population, and therefore it is important to make the intervention most suitable for smokers with COPD.13
Moreover, predictors of adherence to smoking cessation treatment may vary based on the population being assessed. Individuals with chronic diseases (eg, COPD) may have more difficulties as the use of smoking cessation medication further complicates medication self-management, and they tend to face a greater burden of smoking-related morbidity compared with the general population.11,14 Therefore, identified predictors of adherence maybe not be generalized to patients with chronic diseases, and this highlights the need for more studies to evaluate predictors of adherence for these diseases.
There are a few studies about adherence to smoking cessation treatment among patients with chronic diseases.11,15 However, to the best of our knowledge, it is unclear what factors will influence adherence to smoking cessation among smokers with COPD.
Therefore, this study aimed to assess whether the adherence to smoking cessation treatment affects the smoking cessation efficacy, and evaluate predictors of adherence to smoking cessation treatment among patients with COPD in China.
Method
Study Design
This study was an exploratory analysis of data from an ongoing open-label randomized controlled trial (RCT) of varenicline and bupropion for smoking cessation among patients with COPD in China. This study was approved by Institutional Review Boards at China–Japan Friendship Hospital (No. 2018–108-K77) and registered in the Chinese Clinical Trial Registry (No. CTR1900021400, URL: http://www.chictr.org.cn).
This study was carried out at the China–Japan Friendship hospital in Beijing, China. Eligible participants were recruited via a trial site, a hotline of smoking cessation, advertisements in the community from February 2019 to June 2020.
All participants signed informed consent forms, received compensation for transportation, and all medication was distributed to participants free of charge.
Participants
Participants were included if they voluntarily participated in this trial and signed the informed consent form; they were diagnosed as COPD;1 they were diagnosed as tobacco dependence;16 they reported smoking for more than 5 years and smoking an average of more than 10 cigarettes per day during the previous year; exhaled carbon monoxide (ECO) ≥10ppm; they were required to be age 18–85.
COPD was diagnosed as the post-bronchodilator ratio of forced expiratory volume in 1s and forced vital capacity (FEV1/FVC) less than 0.70, according to the GOLD guideline.1
Tobacco dependence was diagnosed if a minimum of three of the following six were met: 1) craving or a strong desire to use tobacco; 2) there is an unsuccessful effort to control the use of tobacco; 3) tobacco withdrawal after abrupt cessation or reduction of tobacco use; 4) tolerance, defined as the need for markedly increased amounts of tobacco to achieve the desired effect; 5) important social, or recreational activities or hobbies are given up or reduced because of tobacco use; and 6) tobacco use is continued despite recognizing the hazards of smoking.16
Participants were excluded if they had severe cardiovascular diseases (eg, acute myocardial infarction) or cerebrovascular diseases (eg, stroke); had neuropsychiatric disorders (eg, seizure and anorexia), had severe impairment of liver and kidney function (eg, renal failure), were pregnant or lactating women, had use of bupropion or varenicline within the last 30 days, or were allergic to them.
Randomization and Blindness
First, randomization was stratified by high/low nicotine metabolic rates; second, the allocation was assigned in a ratio of 1:1 in blocks of four patients (2/treatment/block) to ensure approximate balance. Third, a biostatistician, independent of the study used Proc Plan in SAS version 9.4 (SAS Institute) to generate a table of random digit to randomly assign the numbers to the two groups. (the number of the random seed is 87,654,321).
To ensure random concealment, the group information assigned to each participant was put in a sealed, and opaque envelope. At the same time, the people who generated and saved the random allocation plan and the researchers who determined the selected participant were ensured to be different people.
Because of the different medication packaging, only statisticians were blinded to medication allocation.
Intervention
If eligible and willing to enroll, participants received 12 weeks of medication, and they were required to set a target quit date within 2 weeks after medication. Participants were required to make eight outpatient visits to China-Japan Friendship Hospital through 24 weeks. Face-to-face visits and assessments were conducted at weeks 0, 1, 2, 4, 6, 9, 12, and 24 after initiation of treatment. Participants received a counseling session for more than 60 minutes when they began medication at week 0, and they also received up to 10 min of counseling at weeks 1, 2, 4, 6, 9, 12, and 24.
The participants received the varenicline (purchased from Pfizer, Illertissen, Germany) 0.5 mg once per day for the first 3 days; 0.5 mg twice per day for the next 4 days; 1 mg twice per day from day 8.
The participants received the bupropion (purchased from Venturepharm, Hainan, China) one 150 mg tablet per day.
This study was conducted according to the China Clinical Guideline for Tobacco Cessation.17
Measures
Baseline Characteristics
A questionnaire completed by participants at baseline provided measures of participant characteristics, including:
Demographics gender, age, nationality, education, marital status, etc.
Tobacco-related characteristics the number of cigarettes per day; duration of smoking; the Fagerstrom Test for Nicotine Dependence (FTND), which included six items, and the total score of 0–3, 4–6, and 7–10 meant mild, moderate, and severe tobacco dependence, respectively;18 previous quitting attempts; the Visual Analogue Scale was used to assess the self-efficacy and preparation in quitting smoking, and the score of 1–3, 4–6, 7–10 meant the level weak, medium, strong, respectively;19 whether recognizing the hazards of smoking, etc.
COPD-related features the duration of COPD; the GOLD stage I-IV, which were the classification of airflow limitation severity. FEV1≥80% predicted value (GOLD stage I), 50% predicted value ≤FEV1<80% predicted value (stage II), 30% predicted value ≤FEV1<50% (stage III), FEV1<30% predicted value (stage IV) means mild, moderate, severe and very severe airflow limitation, respectively;1 St. George’s Respiratory Questionnaire (SGRQ), which consisted of 50 questions divided into three subscales: symptom (8 questions), activity (16 questions), and impact (26 questions). All the questions had an attributed weight, and the total score was obtained from the sum of the three categories.20
Adherence to Smoking Cessation Treatment
Good adherence to smoking cessation treatment was defined as taking >80% of medication across 12 weeks and face-to-face visits more than 5; otherwise, it was considered to have poor adherence. Pill count data were collected using a timeline follow-back method (TLFB)21 through self-report and by collecting used pill blister-packages to confirm the accuracy of self-reports.
Smoking Abstinence
ECO was used to determine abstinence among participants who received smoking cessation treatment, with 10ppm used as a cut-point for abstinence.17 ECO was collected in-person at weeks 0, 1, 2, 4, 6, 9, 12, and 24.
FTND Score
FTND score was also collected at weeks 1, 2, 4, 6, 9, 12, and 24.
Statistical Analysis
SPSS 26.0 software was used for all data statistics. Categorical measures were indicated with frequency and percentage, and continuous measures were expressed with Means ± Standard Deviation (X±SD). For comparison of baseline characteristics between the two groups, the t-test was used for comparison which met Gaussian distribution and homogeneity of variance, and Wilcoxon Rank Sum Test was used for comparisons that did not meet homogeneity of variance. The chi-square test was used for categorical comparison.
Intention-to-treat (ITT) analysis was used, and the participants who were lost were recognized to be smokers. Logistic regression analysis was used to analyze the predictors of smoking cessation efficacy and adherence. The results were indicated as the Odds Ratio (OR) value and 95% Confidence Interval (CI). P < 0.05 was statistically significant.
Results
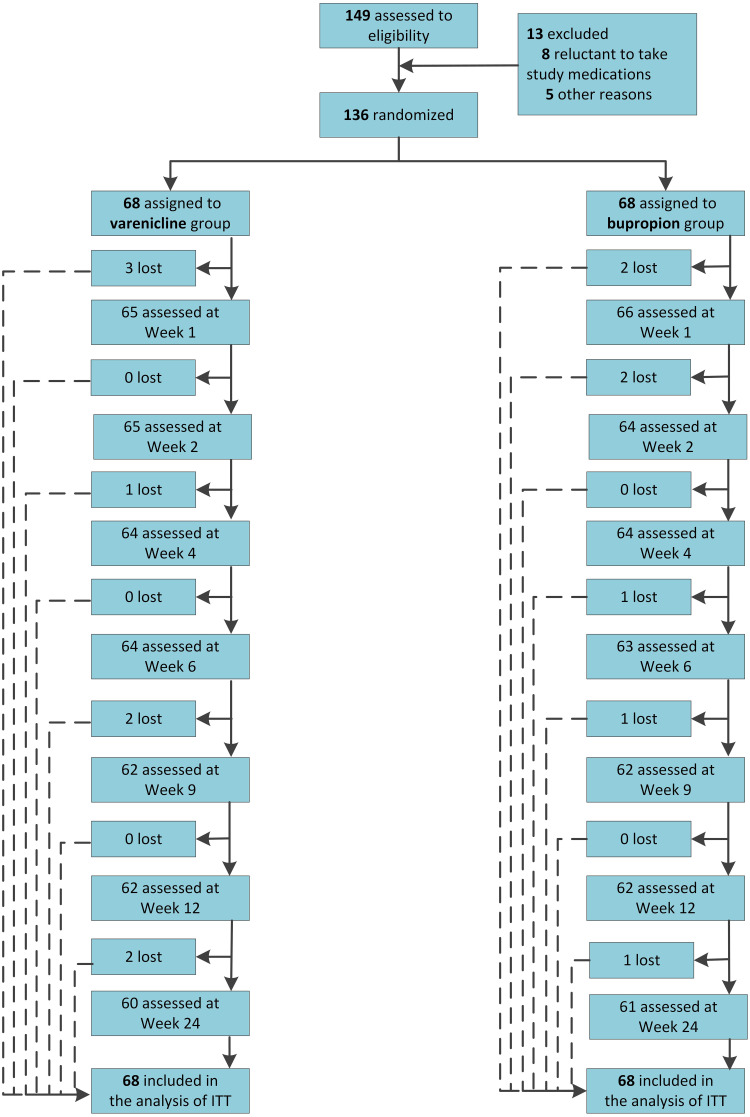
A total of 149 participants were assessed to be eligible, of which 13 did not meet the inclusion and exclusion criteria, and were excluded, and 136 participants were eventually recruited and analyzed. Eight and seven participants were lost in the varenicline and bupropion group, respectively (Figure 1).
Figure 1.
Flowchart of participants.
Abbreviation: ITT, intention to treatment.
Of these, 97.1% (132/136) were male, 2.9% (4/136) were female, and the total mean age (SD) was 62.16 (7.43) years; FTND score was 4.65 (2.45); cigarettes per day was 19.21 (10.67); duration of smoking was 42.00 (8.61) years; the percentage of COPD by GOLD stages I, II, III and IV was 75.7% (103/136), 19.1% (26/136), 5.1% (7/136) and 0% (0/136), respectively. There was no significant difference in baseline characteristics between the two groups (P > 0.05) (Table 1).
Table 1.
Characteristics of Participants
| Characteristics | Varenicline (n=68) | Bupropion (n=68) | Total | P value |
|---|---|---|---|---|
| Gender | 0.31 | |||
| Male | 67 (98.5%) | 65 (95.6%) | 132 (97.1%) | |
| Female | 1 (1.5%) | 3 (4.4%) | 4 (2.9%) | |
| Age, y | 0.69 | |||
| ≤55 | 10 (14.7%) | 11 (16.2%) | 21 (15.4%) | |
| 56–60 | 14 (20.6%) | 11 (16.2%) | 25 (18.4%) | |
| 61–65 | 24 (35.3%) | 19 (27.9%) | 43 (31.6%) | |
| 66–70 | 16 (23.5%) | 20 (29.4%) | 36 (26.5%) | |
| >70 | 4 (5.9%) | 7 (10.3%) | 11 (8.1%) | |
| Nationality | 0.17 | |||
| Han | 67 (98.5%) | 64 (94.1%) | 131 (96.3%) | |
| Non-Han | 1 (1.5%) | 4 (5.9%) | 5 (3.7%) | |
| Education | 0.67 | |||
| Primary school and below | 8 (11.8%) | 5 (7.4%) | 13 (9.6%) | |
| Middle school | 40 (58.8%) | 43 (63.2%) | 83 (61.0%) | |
| College and above | 20 (29.4%) | 20 (29.4%) | 40 (29.4%) | |
| Marital status | 0.30 | |||
| Married | 67 (98.5%) | 64 (94.1%) | 131 (96.3%) | |
| Divorced, widowed, and separated | 1 (1.5%) | 2 (2.9%) | 3 (2.2%) | |
| Unmarried | 0 (0.0%) | 2 (2.9%) | 2 (1.5%) | |
| Cigarettes per day | 0.25 | |||
| ≤10 | 14 (20.6%) | 20 (29.4%) | 34 (25.0%) | |
| 11–20 | 38 (55.9%) | 40 (58.8%) | 78 (57.4%) | |
| 21–30 | 8 (11.8%) | 3 (4.4%) | 11 (8.1%) | |
| >30 | 8 (11.8%) | 5(7.4%) | 13 (9.6%) | |
| Duration of smoking, y | 0.17 | |||
| ≤30 | 5 (7.4%) | 10 (14.7%) | 15 (11.0%) | |
| 31–40 | 24 (35.3%) | 22 (32.4%) | 46 (33.8%) | |
| 41–50 | 36 (52.9%) | 28 (41.2%) | 64 (47.1%) | |
| >50 | 3 (4.4%) | 8 (11.8%) | 11 (8.1%) | |
| FTND score | 0.29 | |||
| 0–3 | 21 (30.9%) | 29 (42.6%) | 50 (36.8%) | |
| 4–6 | 23 (33.8%) | 22 (32.4%) | 45 (33.1%) | |
| 7–10 | 24 (35.3%) | 17 (25.0%) | 41 (30.1%) | |
| Previous quitting attempts | 0.90 | |||
| None | 23 (33.8%) | 22 (32.4%) | 45 (33.1%) | |
| 1 | 15 (22.1%) | 18 (26.5%) | 33 (24.3%) | |
| 2–3 | 23 (33.8%) | 20 (29.4%) | 43 (31.6%) | |
| ≥4 | 7 (10.3%) | 8 (11.8%) | 15 (11.0%) | |
| Self-efficacy in quitting smoking | 0.17 | |||
| Weak | 15 (22.1%) | 11 (16.2%) | 26 (19.1%) | |
| Medium | 23 (33.8%) | 16 (23.5%) | 39 (28.7%) | |
| Strong | 30 (44.1%) | 41 (60.3%) | 71 (52.2%) | |
| Preparation in quitting smoking | 0.32 | |||
| Weak | 14 (20.6%) | 17 (25.0%) | 31 (22.8%) | |
| Medium | 17 (25.0%) | 10 (14.7%) | 27 (19.9%) | |
| Strong | 37 (54.4%) | 41 (60.3%) | 78 (57.4%) | |
| Whether recognizing hazards of smoking | 0.41 | |||
| Yes | 13 (19.1%) | 17 (25.0%) | 30 (22.1%) | |
| No | 55 (80.9%) | 51 (75.0%) | 106 (77.9%) | |
| Duration of COPD, y | 0.09 | |||
| <1 | 55 (80.9%) | 50 (73.5%) | 105 (77.2%) | |
| 1–4 | 4 (5.9%) | 12 (17.6%) | 16 (11.8%) | |
| >4 | 9 (13.2%) | 6 (8.8%) | 15 (11.0%) | |
| GOLD stage | 0.68 | |||
| Stage I | 50 (73.5%) | 53 (77.9%) | 103 (75.7%) | |
| Stage II | 14 (20.6%) | 12 (17.6%) | 26 (19.1%) | |
| Stage III | 4 (5.9%) | 3 (4.4%) | 7 (5.1%) | |
| Stage IV | 0 (0%) | 0 (0%) | 0 (0%) | |
| SGRQ | ||||
| Symptom score | 28.95 (23.02) | 24.49 (18.34) | 26.71 (20.83) | 0.22 |
| Activity score | 12.20 (14.57) | 11.54 (17.62) | 11.87 (16.11) | 0.81 |
| Impact score | 12.26 (13.68) | 10.49 (14.95) | 11.37 (14.30) | 0.48 |
| Total score | 15.37 (14.11) | 13.46 (14.23) | 14.42 (14.15) | 0.41 |
Abbreviations: FTND, Fagerstrom Test for Nicotine Dependence; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SGRQ, St. George’s Respiratory Questionnaire.
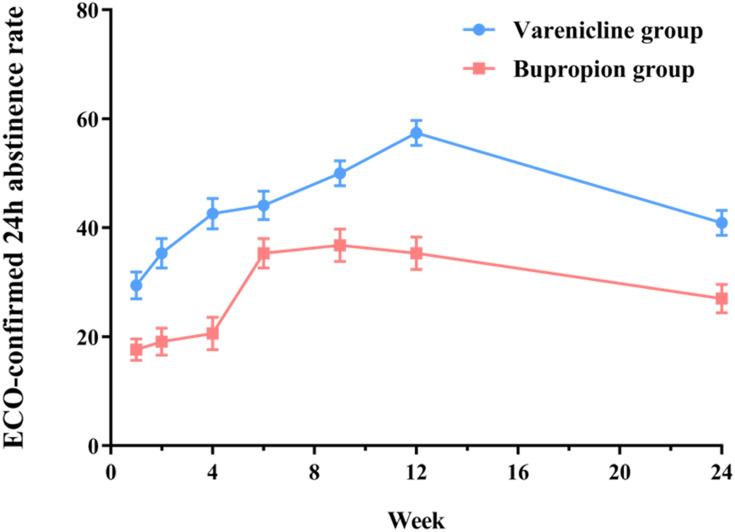
The abstinence rate at week 12 was significantly higher in the varenicline group (57.4%) than in the bupropion group (35.3%) (OR=2.62, 95% CI1.25–5.52, P < 0.05, the OR was adjusted for gender, age, nationality, education, and marital status). The comparison of abstinence rates at other time points is shown in Figure 2.
Figure 2.
Comparison of abstinence rate between varenicline and bupropion at different time points.
Abbreviation: ECO, exhaled carbon monoxide.
After adjusting for age and gender (Model 1), the logistic regression analysis of smoking cessation efficacy showed that the FTND score, the number of cigarettes per day, adherence to smoking cessation treatment, and smoking cessation medication were correlated with the efficacy of smoking cessation (P < 0.05). The same results were obtained after adjusting for age, gender, nationality, education, and marital status (Model 2) (Table S1, as shown in the Appendix).
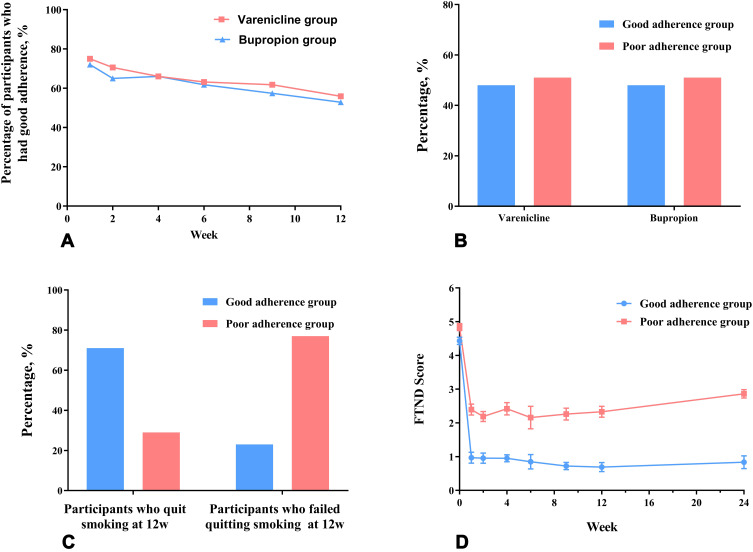
Because good adherence to smoking cessation treatment significantly improved the efficacy of smoking cessation (OR=9.60, 95% CI 4.02–22.96, P < 0.001), we further compared the differences in treatment adherence between the two groups and the predictors of adherence. The good adherence group (66/136) and poor adherence group (70/136) accounted for 48.5% and 51.5% of the total participants, respectively. In both groups, good adherence decreased as treatment time extended (Figure 3A), and rates of good adherence were similar in the varenicline and bupropion groups (Figure 3B). Overall, 71% of participants with good adherence had quit smoking vs 23% of those with poor adherence (Figure 3C). At each visit, the FTND score of participants with good adherence was lower than that with poor adherence (Figure 3D).
Figure 3.
Adherence to smoking cessation treatment and its relationship with medication use and the efficacy of smoking cessation.
Notes: (A) The overall treatment adherence; (B) the relationship between adherence to smoking cessation treatment and medication use; (C) the relationship between adherence and smoking cessation efficacy; (D) FTND Score change.
Abbreviation: FTND, Fagerstrom Test for Nicotine Dependence.
After adjusting for age and gender (Model 1), the logistic regression analysis of adherence showed that older age, higher education level, having more attempts to quit, stronger self-efficacy and preparation, recognizing the hazards of smoking, longer duration of COPD, and higher SGRQ symptom, impact, and total score were relevant to good adherence (P < 0.05). Besides higher SGRQ activity scores related to good adherence, the same results were obtained after adjusting for age, gender, nationality, education, and marital status (Model 2) (Table 2).
Table 2.
Logistic Regression Analyses of Participants’ Characteristics to Predict Adherence
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| (OR, 95% CI) | P value | (OR, 95% CI) | P value | |
| Gender | ||||
| Male | Ref | Ref | ||
| Female | 1.28 (0.16–10.25) | 0.815 | 1.80 (0.15–21.40) | 0.643 |
| Age, y | ||||
| <56 | Ref | Ref | ||
| 56–60 | 1.96 (0.57–6.75) | 0.284 | 1.65 (0.45–5.98) | 0.448 |
| 61–65 | 2.39 (0.78–7.36) | 0.129 | 1.81 (0.56–5.89) | 0.325 |
| 66–70 | 2.80 (0.88–8.88) | 0.081 | 2.56 (0.76–8.67) | 0.130 |
| >70 | 11.24 (1.85–68.24) | 0.009 | 9.93 (1.47–67.29) | 0.019 |
| Nationality | ||||
| Han | Ref | Ref | ||
| Non-Han | 0.00 (0.00–0.00) | 0.999 | 0.00 (0.00–0.00) | 0.999 |
| Education | ||||
| Primary school and below | Ref | Ref | ||
| Middle school | 2.14 (0.52–8.81) | 0.294 | 1.98 (0.48–8.24) | 0.347 |
| College and above | 5.52 (1.22–24.98) | 0.027 | 5.31 (1.17–24.20) | 0.031 |
| Marital status | ||||
| Married | Ref | Ref | ||
| Divorced, widowed, separated | 1.64 (0.09–29.73) | 0.740 | 2.17 (0.12–38.56) | 0.597 |
| Unmarried | 0.46 (0.04–5.31) | 0.531 | 0.64 (0.05–7.61) | 0.722 |
| Cigarettes per day | ||||
| ≤10 | Ref | Ref | ||
| 11–20 | 1.01 (0.43–2.38) | 0.982 | 1.01 (0.41–2.50) | 0.989 |
| 21–30 | 1.34 (0.32–5.56) | 0.692 | 1.95 (0.40–9.57) | 0.410 |
| >30 | 0.56 (0.14–2.27) | 0.413 | 0.75 (0.17–3.25) | 0.697 |
| Duration of smoking, y | ||||
| ≤30 | Ref | Ref | ||
| 31–40 | 1.62 (0.42–6.37) | 0.486 | 1.66(0.40–6.84) | 0.486 |
| 41–50 | 1.33 (0.34–5.23) | 0.688 | 1.39(0.33–5.90) | 0.656 |
| >50 | 0.73(0.10–5.18) | 0.753 | 1.15(0.14–9.53) | 0.896 |
| FTND score | ||||
| 0–3 | Ref | Ref | ||
| 4–6 | 1.26 (0.54–2.94) | 0.591 | 1.40 (0.57–3.43) | 0.467 |
| 7–10 | 0.53 (0.22–1.30) | 0.166 | 0.60 (0.23–1.56) | 0.294 |
| Previous quitting attempts | ||||
| None | Ref | Ref | ||
| 1 | 0.69 (0.26–1.81) | 0.448 | 0.77 (0.28–2.14) | 0.621 |
| 2–3 | 0.82 (0.34–1.97) | 0.650 | 0.65 (0.25–1.69) | 0.374 |
| ≥4 | 5.46 (1.28–23.30) | 0.022 | 5.17 (1.05–25.50) | 0.044 |
| Self-efficacy in quitting smoking | ||||
| Weak | Ref | Ref | ||
| Medium | 1.45 (0.48–4.38) | 0.506 | 1.45 (0.47–4.51) | 0.519 |
| Strong | 3.61 (1.34–9.72) | 0.011 | 2.85 (1.02–7.95) | 0.046 |
| Preparation to quitting smoking | ||||
| Weak | Ref | Ref | ||
| Medium | 1.92 (0.60–6.12) | 0.268 | 2.37 (0.70–8.00) | 0.164 |
| Strong | 3.93 (1.52–10.17) | 0.005 | 4.03 (1.45–11.19) | 0.008 |
| Whether recognizing hazards of smoking | ||||
| Yes | Ref | Ref | ||
| No | 0.39 (0.16–0.96) | 0.040 | 0.37 (0.14–0.95) | 0.039 |
| Duration of COPD, y | ||||
| <1 | Ref | Ref | ||
| 1–4 | 1.53 (0.47–5.03) | 0.483 | 1.61 (0.44–5.95) | 0.475 |
| >4 | 5.26 (1.37–20.17) | 0.015 | 7.09 (1.77–28.37) | 0.006 |
| SGRQ | ||||
| Symptom score | 1.02 (1.00–1.04) | 0.022 | 1.03 (1.01–1.05) | 0.012 |
| Activity score | 1.02 (1.00–1.05) | 0.074 | 1.03 (1.00–1.05) | 0.038 |
| Impact score | 1.04 (1.01–1.08) | 0.008 | 1.05 (1.02–1.08) | 0.004 |
| Total score | 1.04 (1.01–1.07) | 0.008 | 1.05 (1.02–1.08) | 0.003 |
| Medication | ||||
| Bupropion | Ref | Ref | ||
| Varenicline | 1.07 (0.53–2.17) | 0.844 | 1.27 (0.60–2.71) | 0.537 |
Notes: Model 1 was adjusted for gender and age; Model 2 was adjusted for gender, age, nationality, education, and marital status.
Abbreviations: FTND, Fagerstrom Test for Nicotine Dependence; COPD, chronic obstructive pulmonary disease; SGRQ, St. George’s Respiratory Questionnaire.
Discussion
To the best of our knowledge, this was the first study to assess adherence to smoking cessation treatment among patients with COPD in China, and our study found that good adherence to smoking cessation treatment will increase smoking cessation efficacy and predictors of adherence were evaluated.
Adherence has been assessed by many measures, but none can be considered as a gold standard. Different measures have been used to describe the adherence, ranging from the proportion of medication consumed, dichotomized to evaluate a cutoff (ie, 80% of pills used) to questionnaires such as the Adherence Starts with Knowledge questionnaire.12 We applied measures with an 80% cutoff, which is widely used to varenicline, bupropion and oral medication for chronic diseases.3,22,23
To date, the proportion of good adherence to smoking cessation treatment varied in different studies.24 For example, an RCT among the American adolescent population found that 74.24% of participants had good adherence to bupropion.25 Nevertheless, in an observational study of the Dutch population, good adherence was found in only 14.3% of the participants using varenicline.26 Our study found that only about half of the COPD patients in China had good adherence to smoking cessation treatment, which was similar to the results of RCTs among AIDS patients (56%)3 and cancer patients (56%)27 in the United States. The variation is likely due to the differences in the definition of adherence, interventions, adjunctive supports, and population selection.
Our study founded that the abstinence rate of varenicline at the end of treatment was significantly higher than bupropion, which was consistent with the results of previous studies.6,28 Importantly, good adherence to smoking cessation treatment among patients with COPD in China significantly improved the smoking cessation efficacy, which was consistent with studies in the other population.15,29
At the same time, our study identified the predictors of adherence. The preventable factors include self-efficacy and preparation in quitting smoking and cognition of smoking hazards, and the non-preventable factors included age, education, and previous quitting attempts, which was consistent with the results of studies in the general population.12 For example, older age is a known predictor of good adherence to smoking cessation treatment, which was consistent with the results of previous studies.30,31 Older people especially those older than 70, usually had good adherence to smoking cessation treatment. This might be due to older people having more chronic diseases, more experience with medications, deeper recognition of smoking hazards and more previous quitting attempts.
Importantly, we found that a longer duration of COPD and higher SGRQ score were relevant to good adherence to smoking cessation treatment. Previous studies32–34 found the relationship between COPD-related features and the efficacy of smoking cessation. For example, the study by Tøttenborg et al found that the higher GOLD stage, the better the efficacy of smoking cessation.33 With the extension of the duration of COPD, respiratory symptoms were aggravated, activities were gradually restricted, and the recognition of the smoking hazards was deeper. Therefore, deterioration of health could increase the adherence to smoking cessation treatment to improve the efficacy of smoking cessation.
However, our study did not find a correlation between adherence to smoking cessation treatment and tobacco dependence, which was consistent with the result of the study by Okuyemi et al.35 Studies by Vaz et al36 and Balmford et al37 found that a positive correlation between adherence to smoking cessation treatment and tobacco dependence, while the study by Hood et al38 found a negative correlation. The inconsistency of results might be due to the differences in the interventions and population selection.
This study has important clinical implications. Considering that good adherence significantly increased the efficacy of smoking cessation, we call on the medical community to pay attention to the adherence to smoking cessation treatment among patients with COPD. Healthcare providers play a very important role in helping patients to quit smoking,39 and they should provide adequate education to patients on the importance of adherence to smoking cessation treatment. What’s more, health professionals can improve adherence to smoking cessation treatment among patients with COPD via preventable characteristics such as improving self-efficacy in quitting smoking and deepening cognition of smoking hazards. It appears that this will be particularly important for individuals with characteristics (eg, younger age and lower education level), which were associated with poor adherence.
This study has several strengths, including being the first study to assess adherence to smoking cessation among patients with COPD in China, stringent procedure design, assessment of medication use at multiple time points, ECO-confirmed abstinence rates, as well as assessments of multiple variables that could affect adherence to smoking cessation. Nevertheless, our study also has limitations. First, compared with biological measures such as blood levels of medication metabolites, self-report may not be ideal. However, most studies utilized self-report as the primary measure of adherence because self-report is convenient and easy to administer.11 Second, most participants in our study were patients with moderate to mild COPD, so the results may not be directly extrapolated to patients with more severe COPD. Third, varenicline and bupropion provided free to participants, and the results could have been different if participants paid for them because cost-prohibitive prices were identified as barriers to adherence.40 Last, although we have considered many predictors, other potential genetic factors such as gene-phenotype of cytochrome P450 2A641 were not assessed.
Conclusion
To our best knowledge, this is the first study to evaluate adherence to smoking cessation among patients with COPD in China. Our study found that only about half of COPD smokers had good adherence to smoking cessation treatment. Good adherence to smoking cessation treatment significantly improved the smoking cessation efficacy, and predictors of adherence were evaluated. We call on the medical community to pay attention to the adherence to smoking cessation treatment among patients with COPD.
Acknowledgment
We thank all the patients for their participation in this study.
Funding Statement
This study was supported by the Capital Health Development Research Project in China (Grant No. 2018-2-4066), the National Natural Science Foundation of China (Grant No. 81720108001) and the National Key R&D Program of China (Grant No. 2017YFC1309400).
Abbreviations
COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; RCT, randomized controlled trial; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; SGRQ, St. George’s Respiratory Questionnaire; ECO, Exhaled carbon monoxide; FTND, Fagerstrom Test for Nicotine Dependence; OR, odds ratio; CI, confidence interval.
Data Sharing Statement
The data analyzed in the current study are not publicly available but may be available from the corresponding author Dr Xiao upon reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.The GOLD Science Commitee. Global strategy for the diagnosis, management, and prevention of chronic pulmonary disease (2021 REPORT); 2020. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.0-16Nov20_WMV.pdf. Accessed November18, 2020.
- 2.World Health Organization. The top 10 causes of death; 2018. Available from: http://www.who.int/en/newsroom/fact-sheets/detail/the-top-10-causes-of-death. Accessed November28, 2020.
- 3.Shelley D, Tseng TY, Gonzalez M, et al. Correlates of adherence to varenicline among HIV+ smokers. Nicotine Tob Res. 2015;17(8):968–974. doi: 10.1093/ntr/ntv068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 5.Stead LF, Koilpillai P, Fanshawe TR, et al. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. doi: 10.1002/14651858.CD008286.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Eerd EA, van der Meer RM, van Schayck OC, et al. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2016;8:CD010744. doi: 10.1002/14651858.CD010744.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solberg LI, Parker ED, Foldes SS, et al. Disparities in tobacco cessation medication orders and fills among special populations. Nicotine Tob Res. 2010;12(2):144–151. doi: 10.1093/ntr/ntp187 [DOI] [PubMed] [Google Scholar]
- 10.Zeng F, Chen CI, Mastey V, et al. Effects of copayment on initiation of smoking cessation pharmacotherapy: an analysis of varenicline reversed claims. Clin Ther. 2011;33(2):225–234. doi: 10.1016/j.clinthera.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Pacek LR, McClernon FJ, Bosworth HB. Adherence to pharmacological smoking cessation interventions: a literature review and synthesis of correlates and barriers. Nicotine Tob Res. 2018;20(10):1163–1172. doi: 10.1093/ntr/ntx210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Eerd EA, van Rossem CR, Spigt MG, et al. Do we need tailored smoking cessation interventions for smokers with COPD? A comparative study of smokers with and without COPD regarding factors associated with tobacco smoking. Respiration. 2015;90(3):211–219. doi: 10.1159/000398816 [DOI] [PubMed] [Google Scholar]
- 13.Borrelli B, Hayes RB, Dunsiger S, et al. Risk perception and smoking behavior in medically ill smokers: a prospective study. Addiction. 2010;105(6):1100–1108. doi: 10.1111/j.1360-0443.2010.02900.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helleberg M, Afzal S, Kronborg G, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56(5):727–734. doi: 10.1093/cid/cis933 [DOI] [PubMed] [Google Scholar]
- 15.Crawford G, Weisbrot J, Bastian J, et al. Predictors of varenicline adherence among cancer patients treated for tobacco dependence and its association with smoking cessation. Nicotine Tob Res. 2019;21(8):1135–1139. doi: 10.1093/ntr/nty133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines; 1992.
- 17.China National Health and Family Planning Commission. China clinical guidelines for tobacco cessation; 2015.
- 18.Heatherton TF, Kozlowski LT, Frecker RC, et al. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 19.Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behav Res Methods. 2018;50(4):1694–1715. doi: 10.3758/s13428-018-1041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones P. St. George’s respiratory questionnaire manual. 2009.
- 21.Hjorthoj CR, Hjorthoj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances–systematic review and meta-analysis. Addict Behav. 2012;37(3):225–233. doi: 10.1016/j.addbeh.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 22.DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 23.Browning KK, Wewers ME, Ferketich AK, et al. Adherence to tobacco dependence treatment among HIV-infected smokers. AIDS Behav. 2016;20(3):608–621. doi: 10.1007/s10461-015-1059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moulding HD, Friedman DP, Curtis M, et al. Revisiting anaplastic astrocytomas I: an expansive growth pattern is associated with a better prognosis. J Magn Reson Imaging. 2008;28(6):1311–1321. doi: 10.1002/jmri.21593 [DOI] [PubMed] [Google Scholar]
- 25.Leischow SJ, Muramoto ML, Matthews E, et al. Adolescent smoking cessation with bupropion: the role of adherence. Nicotine Tob Res. 2016;18(5):1202–1205. doi: 10.1093/ntr/ntv179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Boven JF, Vemer P. Higher adherence during reimbursement of pharmacological smoking cessation treatments. Nicotine Tob Res. 2016;18(1):56–63. doi: 10.1093/ntr/ntv064 [DOI] [PubMed] [Google Scholar]
- 27.Carroll AJ, Veluz-Wilkins AK, Blazekovic S, et al. Cancer-related disease factors and smoking cessation treatment: analysis of an ongoing clinical trial. Psychooncology. 2018;27(2):471–476. doi: 10.1002/pon.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzales D, Jorenby DE, Brandon TH, et al. Immediate versus delayed quitting and rates of relapse among smokers treated successfully with varenicline, bupropion SR or placebo. Addiction. 2010;105(11):2002–2013. doi: 10.1111/j.1360-0443.2010.03058.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandi SM, Eisenberg MJ, Joseph L, et al. Cessation treatment adherence and smoking abstinence in patients after acute myocardial infarction. Am Heart J. 2016;173:35–40. doi: 10.1016/j.ahj.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 30.Catz SL, Jack LM, McClure JB, et al. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob Res. 2011;13(5):361–368. doi: 10.1093/ntr/ntr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hays JT, Leischow SJ, Lawrence D, et al. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine Tob Res. 2010;12(6):574–581. doi: 10.1093/ntr/ntq047 [DOI] [PubMed] [Google Scholar]
- 32.Pezzuto A, Carico E. Effectiveness of smoking cessation in smokers with COPD and nocturnal oxygen desaturation: functional analysis. Clin Respir J. 2020;14(1):29–34. doi: 10.1111/crj.13096 [DOI] [PubMed] [Google Scholar]
- 33.Tøttenborg SS, Thomsen RW, Johnsen SP, et al. Determinants of smoking cessation in patients with COPD treated in the outpatient setting. Chest. 2016;150(3):554–562. doi: 10.1016/j.chest.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 34.Kupiainen H, Kinnula VL, Lindqvist A, et al. Successful smoking cessation in COPD: association with comorbidities and mortality. Pulm Med. 2012;2012:725024. doi: 10.1155/2012/725024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuyemi KS, Zheng H, Guo H, et al. Predictors of adherence to nicotine gum and counseling among African-American light smokers. J Gen Intern Med. 2010;25(9):969–976. doi: 10.1007/s11606-010-1386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaz LR, Aveyard P, Cooper S, et al. The association between treatment adherence to nicotine patches and smoking cessation in pregnancy: a secondary analysis of a randomized controlled trial. Nicotine Tob Res. 2016;18(10):1952–1959. doi: 10.1093/ntr/ntw080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balmford J, Borland R, Hammond D, et al. Adherence to and reasons for premature discontinuation from stop-smoking medications: data from the ITC Four-Country Survey. Nicotine Tob Res. 2011;13(2):94–102. doi: 10.1093/ntr/ntq215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hood NE, Ferketich AK, Paskett ED, et al. Treatment adherence in a lay health adviser intervention to treat tobacco dependence. Health Educ Res. 2013;28(1):72–82. doi: 10.1093/her/cys081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis JA, Senft N, Chen H, et al. Evidence-based smoking cessation treatment: a comparison by healthcare system. BMC Health Serv Res. 2021;21(1):33. doi: 10.1186/s12913-020-06016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yingst JM, Veldheer S, Hrabovsky S, et al. Reasons for non-adherence to nicotine patch therapy during the first month of a quit attempt. Int J Clin Pract. 2015;69(8):883–888. doi: 10.1111/ijcp.12644 [DOI] [PubMed] [Google Scholar]
- 41.Pezzuto A, Lionetto L, Ricci A, et al. Inter-individual variation in CYP2A6 activity and chronic obstructive pulmonary disease in smokers: perspectives for an early predictive marker. Biochim Biophys Acta Mol Basis Dis. 2021;1867(1):165990. doi: 10.1016/j.bbadis.2020.165990 [DOI] [PubMed] [Google Scholar]