OBJECTIVES:
Management of constipation is still challenging in childhood. The pharmacological effect of XiaojiDaozhi Decoction, a prescription of Chinese Herbal Medicine (CHM), has been well described for the treatment of food and Qi stagnation which account for childhood constipation. However, the efficacy and safety of XiaojiDaozhi Decoction in childhood constipation remains unclear.
METHODS:
A randomized, double-blind, and placebo-controlled trial was conducted to evaluate the efficacy and safety of XiaojiDaozhi Decoction in childhood constipation. Two hundred children were recruited and randomly allocated to the CHM or placebo group to receive their respective interventions. The duration of treatment was 8 weeks, with a 12-week follow-up. Main outcome measures were complete spontaneous bowel movements and satisfaction with bowel function. Safety and adverse effects were evaluated by blood laboratory measurements.
RESULTS:
At the end of follow-up, the response rates of CHM and placebo were 62% and 31%, respectively (χ2 = 19.315, P < 0.01). At the end of treatment, recurrence was found in 7 cases (10.14%) in CHM and 11 cases (26.19%) in placebo (χ2 = 4.947, P < 0.05). In the main outcome measures, 56 patients (56%) in the CHM group and 25 patients (25%) in the placebo group were satisfied with their bowel movements (χ2 = 19.940, P < 0.05). Increased complete spontaneous bowel movements ≥3 per week from baseline were found in 40 patients (40%) who received CHM and 19 patients (19%) who received placebo (χ2 = 10.602, P < 0.05). No serious adverse effects were found in any of the recruited cases.
DISCUSSION:
CHM XiaojiDaozhi Decoction is a safe and effective method for the treatment of childhood constipation.
INTRODUCTION
Constipation is the most common complaint in childhood, affecting an estimated 20% of children globally (1). The treatment strategies of childhood constipation consist of diet control, behavioral intervention, and oral laxatives (2,3). Given its higher success rate and fewer side effects, the laxative PEG3350 has been considered the first choice (4). However, the effectiveness of PEG3350 laxative does not last, or it does not work after long-term use (5). Therefore, additional treatment interventions are necessary. With an unsatisfactory response to current treatments, many patients seek help from Chinese Herbal Medicine (CHM) (6).
For thousands of years, CHM has been the main medical method in China (7,8). Traditional CHM theory holds that the transit of the digestive system depends on Qi. Although the cause of constipation originates from the colon and intestine, it is closely related to the function of the spleen and stomach. Functional weakness in the spleen and stomach leads to food and Qi stagnation. Food stagnation slows gastrointestinal motility, and Qi stagnation disturbs the tone of the stomach and descending movement of the intestine. Food and Qi stagnation further cause heat accumulation in the large intestine, and the pathological heat causes constipation by drying the intestine (7–9). In children, both spleen and stomach functions are very vulnerable because they are still in growth and development. The substantial function has not yet been fully established, and functional weakness of the spleen and stomach is in fact a physiological characteristic. On the other hand, childhood constipation is generally considered as a continuation of infant constipation because most of the childhood constipation has been reported to appear at the onset of babies and infants, although the pathophysiological basis is multifactorial (10). In babies and infants, solid feeding is introduced and the eating behavior coverts from swallowing to chewing. Because breastfeeding contributes to acceptance of a novel dietary and solid feeding introduction, it has been recommended in many guidelines and practices (11–13). However, many mothers would rather formula milk than breast milk; an improper formula-fed duration might increase the risk of eating disorders (12,13). The quantity, quality, and time of food introduction can all place a heavy burden on the baby's digestive system causing food and Qi stagnation (14). Therefore, a physiological weakness of the spleen and stomach in children and a long duration of improper feeding style from the onset of babies and infants are the main sources of childhood constipation; the principles of treatment must pay attention to these pathophysiological bases accordingly.
In the documented traditional medicine dictionary (Pi Wei Lun), the prescription XiaojiDaozhi Decoction has been well described. The prescription consists of 12 kinds of CHMs. Through the combined action of these CHMs, XiaojiDaozhi Decoction can wake up gastric and Qi function, eliminate food stagnation, and remove pathological heat accumulation. In the modern medicine, XiaojiDaozhi Decoction has been proven to contain components beneficial to gastrointestinal peristalsis. Pharmacological studies have shown that Fructus Aurantii can alleviate small intestinal spasms (15). Accumulated solid or accumulated shell can increase the rhythm of gastrointestinal contraction (16). Rhubarb mainly contains onion-brewed derivatives, such as emodin formic acid glucoside, rhein-8-glucoside, and a variety of sennosides (17). Cannabis seeds were found to interact with alkaline intestinal liquid to produce fatty acids in the intestine, which stimulated the intestinal wall and enhanced intestinal peristalsis (18). Consequently, the effects of XiaojiDaozhi Decoction may be related to the promotion of bowel motility and digestive secretion, and it can be used in the treatment of constipation.
Concerning the pathophysiological basis of childhood constipation and the pharmacological effects, XiaojiDaozhi Decoction was considered for the treatment of childhood constipation, holding the principle of promoting Qi movement, relieving Qi stagnation, and removing pathological heat accumulation (19). However, there is still a lack of evidence-based support for the treatment of childhood constipation by XiaojiDaozhi Decoction. Therefore, we designed a randomized, controlled, double-blind clinical trial to confirm the efficacy and safety of XiaojiDaozhi Decoction in the treatment of childhood constipation.
METHODS
Patients and study design
This randomized, placebo-controlled, double-blind trial was conducted in accordance with the amended Declaration of Helsinki. The Ethics Committee of China Medical University (2018 PS427K) approved the protocol, and written informed consent was obtained from all parents. The study protocol was registered prospectively with https://clinicaltrials.gov/(NCT 03186079). Patients were aged 4–14 years and met the inclusion criteria from June 2017 to August 2018 at the Constipation Clinic of Shengjing Hospital. A total of 268 age-eligible participants were recruited, of which 200 (74.63%) were randomly enrolled (CHM group, n = 100; placebo group, n = 100).
Eligibility and exclusion criteria
The eligibility criteria were (1) 4–14 years old; (2) meeting the Roman IV criteria for childhood constipation (20); (3) being able to tolerate the odor of CHM; and (4) signing informed consent. The exclusion criteria included (1) congenital and/or acquired intestinal diseases, such as congenital or severe secondary megacolon, intestinal stenosis, polyps, Crohn's disease, tuberculosis, inflammation, and tumors; (2) anorectal diseases, such as anal atresia, fistula, abscess, and tumor; (3) neurological diseases—such as brain and spinal cord diseases—genetic metabolic diseases, psychosocial and behavioral diseases, and other systemic diseases; and (4) refusal to participate in the study.
Protocol
All participants were randomly assigned to receive 8 weeks of treatment, followed by a 12-week follow-up period. Four visits were arranged for each participant. Participants were required to maintain daily fiber intake and defecation training. For each visit, participants were required to record defecation details, improvement of related symptoms, self-assessment of their symptoms, and any adverse reactions daily. General health assessments for participants were arranged at the beginning and end of the trial, such as blood cell analysis, blood tests for liver and kidney function, and blood lead measurements. The symptoms before treatment, 8 weeks after treatment, and 12 weeks after follow-up were recorded, and the improvement of symptoms after 12 weeks of follow-up was comprehensively evaluated for the efficacy and safety of XiaojiDaozhi Decoction. All records were examined by the research assistant, and the presence of any gastroenterological symptoms was specifically queried by the investigator. At the last visit, researchers and patients assessed whether they had taken XiaojiDaozhi Decoction or placebo. In particular, patients were asked to rate the appearance, color, taste, and efficacy of the drug to show whether these factors were important to the conclusion that they were taking XiaojiDaozhi Decoction or placebo.
Intervention
All participants were randomly divided into 2 groups, the CHM group and the placebo group. Both groups were given basic treatment (defecation training and adjusting diet), fiber intake (20 g/d), and the medication of their respective groups. Defecation training refers to going to the toilet under the supervision of a parent or guardian at a regular time. In the CHM group, participants were given a mixture of 12 herbs, including Raphanus sativus L., Areca catechu L., Fructus aurantll immaturus, Citrus aurantium L., Crataegus pinnatifida, Magnolia officinalis Rehd, Cannabis sativa L., Atractylodes macrocephala Koidz., Semen armeniacae amarum, Paeonia lactiflora Pall, Radix et rhizoma rhei, and Honey mel. The dosage, proportion, and pharmacological effects of these components are shown in Table 1 (21). The placebo group received a placebo designed to match the CHM group based on appearance, weight, color, taste, and odor, including 5% drug ingredients and 95% dextrin. Each group was given medication twice daily, with fasting, before a meal for 8 weeks.
Table 1.
Composition and roles of CHM
| Composition | Percent (%) | Dose (g/kg) | Maximum dosage(g) | Pharmacological effects |
| Raphanus sativus L. | 12.5 | 0.75 | 20 | CHM: eliminating food stagnation and flatulence. Pharmacology: facilitating intestinal motility and restraining gastric emptying, hypotensive and antimicrobial effect. |
| Areca catechu L. | 8.33 | 0.5 | 15 | CHM: relieving food stagnation and flatulence, driving Qi and liquid downward. Pharmacology: stimulating gastrointestinal cholinergic receptor, vermifuge effect, hypotensive effect, and antimicrobial effect. |
| Fructus aurantll immaturus | 6.67 | 0.4 | 12 | CHM: pushing Qi downward and relieving food stagnation and flatulence. Pharmacology: two-way effect on gastrointestinal smooth muscle and stimulating effect on cardiovascular and uterine smooth muscle. |
| Citrus aurantium L. | 8.33 | 0.5 | 15 | CHM: promoting circulation of Qi and relieving food stagnation and flatulence. Pharmacology: two-way effect on gastrointestinal smooth muscle and stimulating effect on cardiovascular and uterine smooth muscle. |
| Crataegus pinnatifida | 6.67 | 0.4 | 12 | CHM: eliminating food stagnation and coordinating the function of Pi. Pharmacology: stimulating gastrointestinal smooth muscle and helping digestion, cardiotonic, hypotensive and hypolipidemic effect, and immunopotentiator effect. |
| Magnolia officinalis Rehd | 6.67 | 0.4 | 12 | CHM: promoting Qi downward, relieving flatulence, and eliminating dampness. Pharmacology: two-way effect on gastrointestinal smooth muscle, antiulcerative and antimicrobial effect. |
| Cannabis sativa L. | 8.33 | 0.5 | 15 | CHM: moistening the bowel and softening the stools. Pharmacology: purgative and hypotensive effects. |
| Atractylodes macrocephala Koidz. | 21.66 | 1.3 | 36 | CHM: coordinating the function of Pi, enriching Qi, and moistening the bowel Pharmacology: two-way effect on gastrointestinal smooth muscle, antiulcerative and antimicrobial, cardiotonic, and hypotensive and hypolipidemic effect. |
| Semen armeniacae amarum | 6.67 | 0.4 | 12 | CHM: guiding Qi downward, moistening the bowel, and purging the stools. Pharmacology: purgative, antitussive and antiasthmatic effect, and antineoplastic and antimutagenic effect. |
| Paeonia lactiflora Pall | 6.67 | 0.4 | 12 | CHM: nourishing Yin and harmonizing homeostasis. Pharmacology: analgesic, anti-inflammatory, and immunologic effect. |
| Radix et rhizoma rhei | 5.0 | 0.3 | 9 | CHM: draining heat and purging the stools. Pharmacology: stimulating smooth muscle, purgative and antimicrobial effect, antineoplastic effect, and immunosuppressive effect, and choleretic effect. |
| Honey mel | 2.5 | 0.15 | 4 | CHM: harmonizing homeostasis, moistening the bowel, and purging the stools. Pharmacology: providing energy, purgative and immunologic effect. |
CHM, Chinese Herbal Medicine.
Random assignment and masking
According to the sequence generated by Random Allocation Software (version 1.0.0), the grouping was randomized in a ratio of 1:1, which was performed by a nonrecruited researcher. The masking process was double-blind, and the appearance of the placebo was the same as that of the CHM group. Blinding of CHM and placebo subjects was maintained throughout the study. Patients, researchers, evaluators, and sponsors did not know which patients received which treatments.
Outcomes
The measures included main outcomes and secondary outcomes. In the main outcomes, improvement of complete spontaneous bowel movements (sub/wk) from baseline and satisfied with bowel function were used. Complete spontaneous bowel movements (CSBMs) referred to the frequency of defecation without drugs or other auxiliary methods. Satisfaction with bowel function was collected from the parents and defined as whether they were satisfied with bowel function after the treatment (yes or no). The secondary outcomes included bowel movements, large diameter or scybalous stools, painful or hard bowel movements, excessive volitional stool retention, and encopresis. A full remission was defined as 2 main outcomes along with most or all secondary outcomes recovered; improvement refers to 1 main outcome with at least 1 secondary outcome recovered, and an invalid responder was defined as both the main outcomes not recovered. An effective rate and a recurrence rate were used to describe the global symptom assessment.
The safety of CHM was assessed by reporting symptoms, including abdominal pain, diarrhea, vomiting, and jaundice. Laboratory assessments were also performed for objective evaluation.
Sample size
Based on the preliminary experiment, we assumed a response rate of 65% in the CHM group and 37% in the placebo group; 62 children per treatment group were deemed sufficient to achieve 90% power in detecting a treatment difference based on a 2-sided χ2 test at a significance level of 0.05. Concerning a 25% dropout rate, a total of 166 patients (83 per arm) were needed to be recruited.
Statistical analysis
Intent-to-treat analysis was performed, and outcomes at the last follow-up were considered the final outcomes in all the participants even if they were dropped. All data were expressed by counting and percentage (classification measure). The χ2 test was used for counting data, and the rank-sum test was used for ranking data. Statistical software SPSS (SPSS, Chicago, IL) was used for statistical analysis. A P < 0.05 was considered significant. All tests are reported as double-sided tests.
RESULTS
Demographics
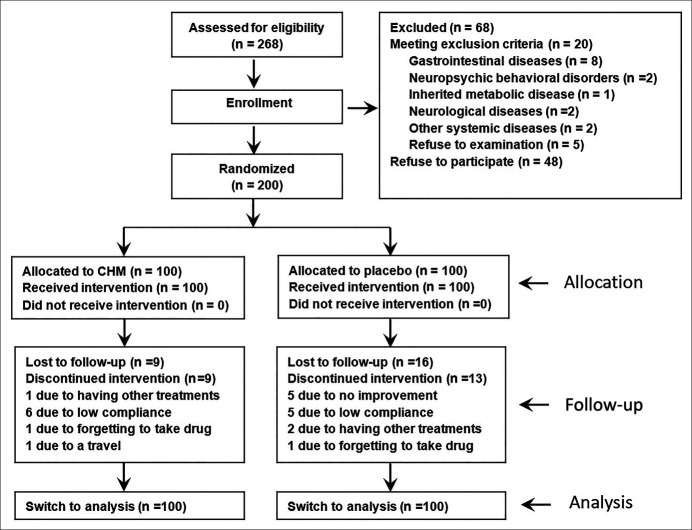
A total of 268 age-eligible participants were recruited, of which 200 were randomly enrolled (CHM group, n = 100; placebo group, n = 100). Figure 1 shows the participant flow chart. Overall, 153 subjects completed the trial, 82 in the CHM group (82%) and 71 in the placebo group (71%). Sixty-eight patients were excluded because they met the exclusion criteria (n = 20), including gastrointestinal diseases (n = 8), neuropsychic behavioral disorders (n = 2), inherited metabolic disease (n = 1), neurological diseases (n = 2), and other systemic diseases (n = 2) or refused to participate (n = 48). The demographic data and baseline variables are shown in Table 2.
Figure 1.
The consort flow diagram. CHM, Chinese Herbal Medicine.
Table 2.
The demographic and baseline data
| Placebo (n = 100) | CHM (n = 100) | |
| Gender | ||
| Male | 48 (48%) | 51 (51%) |
| Female | 52 (52%) | 49 (49%) |
| Age (yr) | 6.61 ± 2.79 | 6.24 ± 2.20 |
| Duration (mo) | 6.78 ± 3.10 | 6.51 ± 2.80 |
| Weight (kg) | 24.87 ± 9.22 | 23.76 ± 7.77 |
| Bowel movements per wk | 2.99 ± 1.47 | 2.74 ± 1.27 |
| Large diameter or scybalous stools | 67 (67%) | 61 (61%) |
| Painful or hard bowel movements | 56 (56%) | 55 (55%) |
| Excessive volitional stool retention | 26 (26%) | 27 (27%) |
| Encopresis | 21 (21%) | 23 (23%) |
CHM, Chinese Herbal Medicine.
Global symptom assessment
At the end of treatment, the response rate was 69% in the CHM group and 42% in the placebo group (χ2 = 14.759, P < 0.01). At the end of follow-up, the response rates of CHM and placebo were 62% and 31%, respectively (χ2 = 19.315, P < 0.01). Seven patients in CHM and 11 patients in placebo received constipation symptoms, and the recurrence rates were 10.14% and 26.19%, respectively (χ2 = 4.947, P < 0.05). (Table 3).
Table 3.
Global symptom assessment
| Placebo (n = 100) | CHM (n = 100) | χ2 and P value | |
| At the end of treatment | |||
| Effective | 42 (42%) | 69 (69%) | |
| Invalid | 58 (58%) | 31 (31%) | χ2 = 14.759, P < 0.05 |
| At the end of follow-up | |||
| Effective | 31 (31%) | 62 (62%) | |
| Invalid | 69 (69%) | 38 (38%) | χ2 = 19.315, P < 0.05 |
CHM, Chinese Herbal Medicine.
Main outcome assessment
At the end of the treatment, 67 patients in the CHM group and 38 in the placebo group were satisfied with bowel function (χ2 = 16.862, P < 0.05); however, at the end of the follow-up, 56 patients in the CHM group and 25 patients in the placebo group were satisfied (χ2 = 19.940, P < 0.05). At the end of the treatment, there were 46 cases with increased CSBMs ≥3 per week from baseline in the CHM group and 26 cases in the placebo group (χ2 = 8.681, P < 0.05). In addition, 40 patients in the CHM group and 19 patients in the placebo group were found at the end of the follow-up (χ2 = 10.602, P < 0.05). (Table 4).
Table 4.
Primary outcome assessment
| Placebo (n = 100) | CHM (n = 100) | χ2 and P value | |
| Satisfied with bowel function | |||
| Baseline, week 0 | |||
| At the end of treatment | 38(38%) | 67(67%) | χ2 = 16.862, P < 0.05 |
| At the end of follow-up | 25(25%) | 56(56%) | χ2 = 19.940, P < 0.05 |
| Increased CSBMs ≥3 per wk from baseline | |||
| Baseline, week 0 | |||
| At the end of treatment | 26(26%) | 46(46%) | χ2 = 8.681, P < 0.05 |
| At the end of follow-up | 19(19%) | 40(40%) | χ2 = 10.602, P < 0.05 |
CHM, Chinese Herbal Medicine.
Secondary outcome assessment
The frequency of defecation was 5.19 ± 1.57 times in the CHM group and 4.02 ± 1.92 times in the placebo group at the end of the treatment and 5.00 ± 1.54 times and 3.76 ± 1.83 times in these 2 groups at the end of the follow-up (P < 0.05). Other secondary outcomes including large diameter or scybalous stools, painful or hard bowel movements, excess volatile stool retention, and encopresis. These were evaluated at both the end of the treatment and the follow-up, and all statistical differences were significant (All P < 0.05, Table 5).
Table 5.
Secondary outcome assessment
| Placebo (n = 100) | CHM (n = 100) | χ2 and P value | |
| Bowel movements per wk | |||
| Baseline, week 0 | 2.99 ± 1.47 | 2.74 ± 1.27 | |
| At the end of treatment | 4.02 ± 1.92 | 5.19 ± 1.57 | P < 0.05 |
| At the end of follow-up | 3.76 ± 1.83 | 5.00 ± 1.54 | P < 0.05 |
| Large diameter or scybalous stools | |||
| Baseline, week 0 | 67 (67%) | 61 (61%) | |
| At the end of treatment | 54 (54%) | 36 (36%) | χ2 = 7.124, P < 0.05 |
| At the end of follow-up | 58 (58%) | 41 (41%) | χ2 = 6.825, P < 0.05 |
| Painful or hard bowel movements | |||
| Baseline, week 0 | 56 (56%) | 55 (55%) | |
| At the end of treatment | 47 (47%) | 32 (32%) | χ2 = 8.965, P < 0.05 |
| At the end of follow-up | 50 (50%) | 35 (35%) | χ2 = 10.177, P < 0.05 |
| Excessive volitional stool retention | |||
| Baseline, week 0 | 26 (26%) | 27 (27%) | |
| At the end of treatment | 20 (20%) | 13 (7.32%) | χ2 = 4.668, P < 0.05 |
| At the end of follow-up | 21 (21%) | 15 (8.54%) | χ2 = 3.865, P < 0.05 |
| Encopresis | |||
| Baseline, week 0 | 21( 21%) | 23 (23%) | |
| At the end of treatment | 15 (15%) | 6 (6%) | χ2 = 9.046, P < 0.05 |
| At the end of follow-up | 16 (16%) | 8 (8%) | χ2 = 7.591, P < 0.05 |
CHM, Chinese Herbal Medicine.
Success of blinding
Among the 153 participants who finished the protocol, 48 (31.37%) correctly guessed the group they were assigned to, 17 (11.11%) participants were in the CHM group, and 31 (20.26%) were in the placebo group, while the investigators correctly guessed 69 (45.10%) of them, 8 (5.23%) in the CHM group and 61 (39.87%) in the placebo group. Of the 31 participants who received placebo and correctly guessed, 24 (77.41%) were based on therapeutic effect, 4 (12.9%) were based on taste, and 3 (9.67%) were based on outside view.
Safety and adverse reactions
During the treatment, vomiting was found in 8 patients, 5 in the CHM group and 3 in the control group. Abdominal distention was noted in 4 patients in the CHM group. All the symptoms were relieved after temporary withdrawal of the drug. There were 2 cases of mild diarrhea observed in the CHM group, which recovered after reducing the oral dose. Abdominal pain occurred in 2 patients, which improved by itself without any treatment. Cold and fever were found in 6 patients, and they were considered irrelevant to the drug. Jaundice was not observed in any participants. Adverse reactions were not observed in any participants at follow-up. Routine blood tests, liver function, renal function, and blood lead tests were performed in all 153 participants, and they were all within the normal range.
DISCUSSION
The management of childhood constipation remains challenging for paediatricians. To the best of our knowledge, this is the first time that the treatment principle of childhood constipation has been proposed by relieving food stagnation, promoting Qi movement, and removing pathological heat accumulation. The CHM XiaojiDaozhi Decoction has just pharmacological effects of this nature. Therefore, we designed a randomized, double-blind, and placebo-controlled to evaluate its safety and efficacy (22). In this trial, fiber intake and defecation training were used as the basic treatment. The results showed the basic treatment achieved a certain effect, which was consistent with the results of previous studies (23,24). However, after the combination of CHM and placebo, the 2 groups demonstrated statistical difference, indicating that CHM XiaojiDaozhi Decoction could effectively relieve constipation symptoms in childhood. Furthermore, recurrence rate was 26.19% in the patients receiving only basic treatment, compared with 10.14% in the CHM group. The recurrence rate of CHM is significantly lower than that of conventional basic treatment. Although there was no comparison with other drugs such as polyethylene glycol (PEG) designed in this trial, the response and recurrence rate of CHM are still desirable, according to data reported in documented literatures. The response rate of PEG has been reported to be 73%–77%, which is similar to that of CHM (25). However, the recurrence rate was as high as 40%, significantly higher than in CHM leaving many patients not satisfied (26). Therefore, XiaojiDaozhi Decoction is an effective method with a lower recurrence rate for comparison with PEG. It can be used as optional alternative for PEG in the treatment of childhood constipation.
Although the effect of XiaojiDaozhi Decoction has been verified, the potential mechanism remains uncertain because the drug is composed of 12 herbal components. In animal experiments, XiaojiDaozhi Decoction has been proven to contain components beneficial to gastrointestinal peristalsis, and therefore, the effects of XiaojiDaozhi Decoction may be related to the promotion of bowel motility and digestive secretion. In addition to the roles in bowel motility, traditional CHMs can also play roles by interacting with intestinal flora and their metabolites. It has been demonstrated that CHMs can promote the proliferation and colonization of intestinal flora, while intestinal flora and their metabolites can promote the decomposition of CHM, but the exact mechanism needs to be confirmed by further basic experiments (27,28).
In the present trial, the design of the placebo group was successful. The placebo used a mixture of 5% of the drug ingredients and dextrin to ensure that the placebo was identical to the CHM group in appearance, color, odor, and texture. Feedback from follow-up showed that 11.11% of patients and 20.26% of respondents in the CHM group and placebo group guessed correctly, respectively. Of the patients and respondents who guessed correctly, 75.0% were based on efficacy, not on placebo traits. Approximately 68.63% of the subjects did not know whether they were taking CHM or placebo, which proved that the blinding process of the study was successful. Because the placebo component contains 5% of the drug ingredients, the effectiveness of the placebo has also been fully evaluated. In the preliminary experiment, placebo effectiveness was 8%. It was confirmed that 5% of the drug ingredients did not affect the results of the placebo group. The experimental data obtained in the placebo group were reliable.
During the trial, 5 patients relieved by CHM received constipation symptoms after an antibiotic use. As a result, we assumed that antibiotic use may serve as a potential of constipation in children. Antibiotics might destroy the newly established beneficial intestinal flora, leading to an alteration of gastrointestinal motility in these patients (29). The efficacy of probiotics in the treatment of constipation has been reported, despite the function of probiotics alone is still controversial (30,31). In addition to antibiotic use, another potential of constipation recurrence is food intolerance (32,33). Food intolerance can cause bacterial overgrowth in the small intestine, affecting the mucosal barrier, intestinal motility, and absorption (34). In our cases, 4 patients regained constipation after eating milk and eggs, and the symptoms were significantly relieved after stopping these foods, indicating that constipation was tentatively related to food intolerance. Although great potentials of antibiotic use and food intolerance have been observed in constipation recurrence, there is still not sufficient evidence for a formal consensus established because of the limit number in this study; big data should be performed to confirm this conception. Nevertheless, the use of antibiotics and food allergens contact are still recommended to avoid during the treatment of constipation to reduce the potential of symptom recurrence.
In terms of safety, the greatest concerns about the application of CHM are liver and kidney damage and excessive heavy metals (35), but in this study, none of these problems were observed. In fact, such side effects were equally common in laxatives (36). Moreover, all the symptoms are negligible; they can be resolved after drug withdrawal or adjustment and do not require additional medical intervention, indicating XiaojiDaozhi Decoction had good tolerance and safety.
There are some limitations in the conception and design of the present trial. Subjective evaluation was used to check symptoms, but some younger children could not express themselves accurately. This could result in errors in the questionnaires because of the subjective judgement of their guardians. In addition, because of the bitterness of traditional CHM, children may refuse to take the medicine or some parents may stop using the medicine if they perceive they may be in the placebo group, leading to a higher rate of lost interviews. Despite these drawbacks, CHM is still a promising and optional method for the treatment of childhood constipation.
CHM XiaojiDaozhi Decoction is a safe and efficient method for the treatment of childhood constipation.
CONFLICTS OF INTEREST
Guarantor of the article: Shu-Cheng Zhang, MD.
Specific author contributions: S.C.Z. and L.Q. conceptualized and designed the study and reviewed and revised the manuscript. L.J.W., H.L.Z., Y.W., and Y.C. collected the data. L.J.W. performed the initial analyses and drafted the initial manuscript. L.Q. supervised data collection and critically reviewed the manuscript for important intellectual content. The study was sponsored by Shengjing Hospital.
Financial support: This study was supported by the National Natural Science Foundation of China (No. 81570465, 30700917) and the General Funding Project from Department of Education of Liaoning Province (No. JC2019014).
Potential competing interests: None to report.
Data sharing statement: All data collected for the study, including individual participant data and a data dictionary defining each field in the set, will be made available to others.
Clinical trial registration: https://clinicaltrials.gov/ (NCT 03186079). Efficacy of Chinese Herbal Medicine in the Treatment of Childhood Constipation: A Randomized, Double Blind and Placebo-Controlled Trial.
Study Highlights.
WHAT IS KNOWN
✓ Traditional Chinese Herbal Medicine (CHM) theory holds that childhood constipation derives from food and Qi stagnation and further pathological heat accumulation in the large intestine.
✓ A physiological weakness of the spleen and stomach and a long duration of improper feeding style from the onset of babies and infants in children may account for the stagnation.
✓ The pharmacological effect of XiaojiDaozhi Decoction, a prescription of CHM, has been well described in the traditional medicine dictionary (Pi Wei Lun) for the treatment of food and Qi stagnation. However, there is still a lack of evidence-based support for the treatment of childhood constipation by XiaojiDaozhi Decoction.
WHAT IS NEW HERE
✓ To the best of our knowledge, this is the first time that the treatment principle of childhood constipation has been proposed by relieving food stagnation, promoting Qi movement, and removing pathological heat accumulation.
✓ The CHM XiaojiDaozhi Decoction has pharmacological effects of this nature. In the present randomized clinical trial, CHM XiaojiDaozhi Decoction was verified as a safe and effective method for the treatment of childhood constipation.
Footnotes
Luo-Jia Wang has equal rights with the first author Lei Qiao, and Hai-Lan Zhang has equal rights with the corresponding author Shu-Cheng Zhang.
Contributor Information
Lei Qiao, Email: 18940255527@189.cn.
Luo-Jia Wang, Email: wangluojia1004@qq.com.
Yang Wang, Email: wangyang01@cmu.edu.cn.
Ying Chen, Email: 18940257148@163.com.
Hai-Lan Zhang, Email: hailanzhang2008@sina.com.
REFERENCES
- 1.Mota DM, Barros AJ, Santos I, et al. Characteristics of intestinal habits in children younger than 4 years: Detecting constipation. J Pediatr Gastroenterol Nutr 2012;55:451–6. [DOI] [PubMed] [Google Scholar]
- 2.van Dijk M, de Vries GJ, Last BF, et al. Parental child-rearing attitudes are associated with functional constipation in childhood. Arch Dis Child 2015;100:329–33. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE, Pemberton JH, Locke GR, III. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koppen IJN, Broekaert IJ, Wilschanski M, et al. Role of polyethylene glycol in the treatment of functional constipation in children. J Pediatr Gastroenterol Nutr 2017;65:361–3. [DOI] [PubMed] [Google Scholar]
- 5.Koppen IJN, van Wassenaer EA, Barendsen RW, et al. Adherence to polyethylene glycol treatment in children with functional constipation is associated with parental illness perceptions, satisfaction with treatment, and perceived treatment convenience. J Pediatr 2018;199:132–9 e1. [DOI] [PubMed] [Google Scholar]
- 6.Zhong LLD, Cheng CW, Kun W, et al. Efficacy of MaZiRenWan, a Chinese herbal medicine, in patients with functional constipation in a randomized controlled trial. Clin Gastroenterol Hepatol 2019;17:1303–10 e18. [DOI] [PubMed] [Google Scholar]
- 7.Teschke R, Wolff A, Frenzel C, et al. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J Gastroenterol 2015;21:4466–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobos G, Tao I. The model of western integrative medicine: The role of Chinese medicine. Chin J Integr Med 2011;17:11–20. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CW, Bian ZX, Zhu LX, et al. Efficacy of a Chinese herbal proprietary medicine (Hemp Seed Pill) for functional constipation. Am J Gastroenterol 2011;106:120–9. [DOI] [PubMed] [Google Scholar]
- 10.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults. Gastroenterology 2011;140:S687–S688. [DOI] [PubMed] [Google Scholar]
- 11.Hausner H, Nicklaus S, Issanchou S, et al. Breastfeeding facilitates acceptance of a novel dietary flavour compound. Clin Nutr 2010;29:141–8. [DOI] [PubMed] [Google Scholar]
- 12.Nicklaus S. The role of dietary experience in the development of eating behavior during the first years of life. Ann Nutr Metab 2017;70:241–5. [DOI] [PubMed] [Google Scholar]
- 13.Hetherington MM, Cecil JE, Jackson DM, et al. Feeding infants and young children. From guidelines to practice. Appetite 2011;57:791–5. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AL, Bentley ME. The critical period of infant feeding for the development of early disparities in obesity. Soc Sci Med 2013;97:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan W, Li Y, Wang Y, et al. Anti-coagulative and gastrointestinal motility regulative activities of Fructus Aurantii Immaturus and its effective fractions. Biomed Pharmacother 2017;90:244–52. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Bai X, Zhu X, et al. The effects of Fructus Aurantii extract on the 5-hydroxytryptamine and vasoactive intestinal peptide contents of the rat gastrointestinal tract. Pharm Biol 2014;52:581–5. [DOI] [PubMed] [Google Scholar]
- 17.Chen JQ, Li DW, Chen YY, et al. Elucidating dosage-effect relationship of different efficacy of rhubarb in constipation model rats by factor analysis. J Ethnopharmacol 2019;238:111868. [DOI] [PubMed] [Google Scholar]
- 18.Huang T, Zhao L, Lin CY, et al. Chinese herbal medicine (MaZiRenWan) improves bowel movement in functional constipation through down-regulating oleamide. Front Pharmacol 2019;10:1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medicine CAoC. Guideline on design and evaluation of clinical trials for Chinese medicine in common pediatric diseases. Funct Constipation 2020;43:173–8. [Google Scholar]
- 20.Hyams JS, Di Lorenzo C, Saps M, et al. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016;150:1456. [Google Scholar]
- 21.Commission CP. Pharmacopoeia of the People's Republic of China. People’s Medical Pub House, Beijing, 2015. [Google Scholar]
- 22.Cheng CW, Bian ZX, Wu TX. Systematic review of Chinese herbal medicine for functional constipation. World J Gastroenterol 2009;15:4886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korczak R, Kamil A, Fleige L, et al. Dietary fiber and digestive health in children. Nutr Rev 2017;75:241–59. [DOI] [PubMed] [Google Scholar]
- 24.van Dijk M, Bongers ME, de Vries GJ, et al. Behavioral therapy for childhood constipation: A randomized, controlled trial. Pediatrics 2008;121:e1334–41. [DOI] [PubMed] [Google Scholar]
- 25.Nurko S, Youssef NN, Sabri M, et al. PEG3350 in the treatment of childhood constipation: A multicenter, double-blinded, placebo-controlled trial. J Pediatr 2008;153:254–61. 261.e1. [DOI] [PubMed] [Google Scholar]
- 26.Pijpers MA, Bongers ME, Benninga MA, et al. Functional constipation in children: A systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr 2010;50:256–68. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Liu T, Zhou Q, et al. Roles of Chinese medicine and gut microbiota in chronic constipation. Evid Based Complement Alternat Med 2019;2019:9372563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng WW, Ao H, Peng C, et al. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res 2019;142:176–91. [DOI] [PubMed] [Google Scholar]
- 29.Block JP, Bailey LC, Gillman MW, et al. Early antibiotic exposure and weight outcomes in young children. Pediatrics 2018;142:e20180290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo M, Giugliano FP, Quitadamo P, et al. Efficacy of a mixture of probiotic agents as complementary therapy for chronic functional constipation in childhood. Ital J Pediatr 2017;43:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimidi E, Christodoulides S, Scott SM, et al. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv Nutr 2017;8:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta M, Beg M. Fructose intolerance: Cause or cure of chronic functional constipation. Glob Pediatr Health 2018;5:2333794X18761460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilder-Smith CH, Materna A, Wermelinger C, et al. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther 2013;37:1074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan Z, Yan J, Wen H, et al. Feeding intolerance alters the gut microbiota of preterm infants. PLoS One 2019;14:e0210609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu SH, Chuang WC, Lam W, et al. Safety surveillance of traditional Chinese medicine: Current and future. Drug Saf 2015;38:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarzebicka D, Sieczkowska-Golub J, Kierkus J, et al. PEG 3350 versus lactulose for treatment of functional constipation in children: Randomized study. J Pediatr Gastroenterol Nutr 2019;68:318–24. [DOI] [PubMed] [Google Scholar]