Abstract
Chronic infections and the subsequent immune response have recently been shown to be risk factors for cognitive decline and Alzheimer disease and related dementias (ADRD). While some studies have shown an association between cytomegalovirus (CMV), a chronic and highly prevalent infection, and cognition and/or ADRD, these studies have been limited by nonrepresentative and small samples. Using 2016 data on 5,617 adults aged 65 years or more from the Health and Retirement Study, we investigated the cross-sectional associations of both CMV serostatus and immunoglobulin G (IgG) antibody response with cognitive function using linear regression models adjusting for age, sex, race/ethnicity, and educational attainment. We further investigated potential effect-measure modification by educational attainment. Overall, both CMV seropositivity and higher IgG antibody response were associated with lower cognitive function, though the relationship was not statistically significant in adjusted models. Among participants with less than a high school diploma, CMV seropositivity and being in the first tertile of IgG response, relative to seronegative persons, were associated with lower scores on the Telephone Interview for Cognitive Status (−0.56 points (95% confidence interval: −1.63, 0.52) and −0.89 points (95% confidence interval: −2.07, 0.29), respectively), and the relationship was attenuated among those with higher education. Our results suggest that CMV may be a risk factor for cognitive impairment, particularly among persons with fewer educational resources.
Keywords: Alzheimer disease and related dementias, cognitive decline, cytomegalovirus, educational attainment, immune function
Abbreviations
- ADRD
Alzheimer disease and related dementias
- APOE
apolipoprotein E gene
- CI
confidence interval
- CMV
cytomegalovirus
- HRS
Health and Retirement Study
- IgG
immunoglobulin G
- TICS
Telephone Interview for Cognitive Status
Cognitive impairment and Alzheimer disease and related dementias (ADRD) present a unique health-care challenge for the United States (1, 2). The estimated number of prevalent ADRD cases grew to over 5.5 million in 2019 (3) and is expected to reach almost 14 million by 2050 (4). Long-term care spending for persons with ADRD was estimated to be $162.7 billion in 2016 alone (5). There are several known risk factors for ADRD, including chronological age, possession of the apolipoprotein E gene (APOE) ε4 allele, sex, and education (6–8). However, the search for novel risk factors and early indicators of ADRD continues, and there are compelling data supporting immune-system alterations as an important risk factor for the onset of ADRD.
Recent evidence has implicated chronic infection in the etiology of both cognitive decline and ADRD (9, 10). Immune response to infection, both seropositivity and immunoglobulin G (IgG) level, and resulting elevations in inflammation have been implicated in the accumulation of amyloid β, which is a key pathological feature of ADRD and is associated with other neurodegenerative processes such as microglial senescence (9, 11, 12). The resulting shifts in the T-cell compartment toward aged T-cell phenotypes (i.e., lower CD4+:CD8+ and effector memory:naive T-cell ratios) may also play a role (13). Several studies have implicated infections in ADRD, and the role of immune system response to cytomegalovirus (CMV), a highly prevalent, chronic infection, has been investigated in several studies (13–29). In particular, several studies have demonstrated a negative association between CMV seroprevalence or serointensity (i.e., IgG level) and cognitive function, cognitive decline, and/or ADRD (14–24). Sample sizes in these studies ranged from 44 to 1,625, drawing from a range of populations in the United States (14–23) and 1 from Finland with 7,112 participants (24). At the same time, a few investigators have reported results that were not in the hypothesized direction or did not reach statistical significance (13, 24–29). These latter studies included 100–1,308 participants from largely clinical cohorts, though 1 was a community-based sample (i.e., the Northern Manhattan Study). Methodological variation such as differences in the sample composition (e.g., by age, sex, and education) and national representativeness of existing studies, different kinds of assessments of CMV infection (i.e., seropositivity, continuous IgG levels, CMV-specific CD8+ cells, etc.), and/or the inclusion of differing cognitive domains in the studies may account for the inconsistent findings. In the current study, we tested the CMV-cognition association in a nationally representative sample of older adults in the United States, providing better generalizability of the findings in comparison with prior research.
There is growing recognition that both social and biological factors work together to influence ADRD and cognitive decline (30–32). CMV is a highly prevalent virus (50.4% of the US population aged 6–49 years (33) and higher prevalence among older populations) but is strongly socially patterned, whereby socially disadvantaged populations carry a greater burden (34–39). Therefore, educational attainment may confound the association between CMV and cognition. On the other hand, educational attainment reflects different levels of resource availability throughout the life course—for example, through access to health care and knowledge of healthy behaviors—and therefore may shape a person’s ability to minimize the effects of CMV on cognitive decline (40). In addition, educational attainment may also reflect one’s level of cognitive reserve, and thus resiliency of the human brain against the adverse effects of CMV. This could then protect an individual from the changes in brain pathology that lead to cognitive decline and act as an effect-measure modifier of the relationship between CMV and cognition (41).
Cognitive reserve, or the capacity of the brain to cope with damage differentially, is thought of in one of 2 ways: either as a passive process in which there is a threshold past which neurodegeneration is expressed clinically or as an active process in which the brain is constantly working to compensate for degeneration. The passive models conceptualize this as brain size, number of synapses, etc. (i.e., the “hardware” of the brain) being the “reserve” and there being a “critical threshold” at which functional deficits manifest themselves clinically (41, 42). Thus, theoretically, the greater a reserve someone starts with, the more degradation he/she can sustain before reaching clinical impairment. The active models, on the other hand, conceptualize reserve as “software” that allows someone to process information and tasks more efficiently, and therefore the same amount of degradation in brain physiology may not manifest in the same clinical symptoms (41). This ultimately means that even if 2 individuals sustain the same amount of brain pathology, one with more cognitive reserve (i.e., a larger amount of hardware, or a greater ability to recruit brain networks efficiently) will present fewer clinical cognitive deficits (or none) because of his/her reserve. In practice, cognitive reserve is often proxied by educational attainment (43). In theory, educational attainment could operate through both the hardware and software mechanisms; higher educational attainment during brain development could result in more neurons, synapses, etc., for the remainder of the life course, while higher educational attainment could also provide the skills necessary for a person to compensate for physiological changes through behaviors and executive functioning.
In this study, we used data from a large US-representative sample of adults aged 65 years or more to test the associations of CMV serostatus and IgG response with cognitive function and to test whether educational attainment modifies the association between CMV and cognitive function. We hypothesized that CMV is associated with lower cognitive function and that based on the theory of cognitive reserve, higher educational attainment modifies the relationship between CMV and an individual’s cognitive decline.
METHODS
Study population
Data for this analysis came from the Health and Retirement Study (HRS), the largest ongoing nationally representative longitudinal survey of older adults in the United States, conducted at the University of Michigan (Ann Arbor, Michigan). The HRS began in 1992 and included over 22,000 adults over the age of 50 years (i.e., >50 years) at baseline. Follow-up occurred, and continues to occur, every 2 years. The survey design and methods of the HRS have been described previously (44–46). Data collection consisted of face-to-face baseline interviews and primarily telephone interviews for follow-up waves until 2006, when half of the sample (alternated at each subsequent wave) was randomly assigned to undergo face-to-face interviews for measurement of additional physical and biological variables (47). Our analysis utilized existing demographic, social, and cognitive data from the 2016 core interview, with CMV data obtained from the 2016 Venous Blood Study. Of the 9,934 participants who consented to the venous blood draw and were tested (consent rate = 78.5%; completion rate = 65%), our analysis included 5,617 persons who 1) were at least 65 years of age at baseline and 2) had full covariate and cognitive data (48). Web Figure 1 (available at doi.org/10.1093/aje/kwaa238) depicts the sample derivation.
Measures
Cytomegalovirus.
CMV seropositivity was based on the presence of IgG antibodies targeted against CMV in blood serum. Measurement was carried out using the Roche e411 immunoassay analyzer (Roche Diagnostics Corporation, Indianapolis, Indiana). The lower limit of detection was 0.015 antibody units per milliliter (U/mL). Seroprevalence was reported in the study as nonreactive (<0.50 U/mL), borderline (0.50–0.99 U/mL), or reactive (≥1.00 U/mL), and for this study “borderline” was considered seropositive. In order to investigate the association between immune response to CMV and our outcomes, we also categorized seropositive individuals into tertiles of CMV IgG antibody level and compared persons in each tertile of CMV IgG antibody level with seronegative persons.
Global cognition.
Global cognition is a summary measure based on several cognitive tests including word recall and mental status items. Questions included in this score were asked over the telephone by interviewers as part of the Telephone Interview for Cognitive Status (TICS) and were answered by the study participants. This variable has a possible score range of 0–35 and includes measures of immediate recall (0–10), delayed recall (0–10), serial 7’s (0–5), backwards counting from 20 (0–2), object naming (scissors and cactus; 0–2), President naming (0–1), Vice President naming (0–1), and date naming (month, day, year, and day of the week; 0–4) encompassing the cognitive domains of verbal memory, orientation, executive functioning, and attention. TICS score was treated as a continuous variable in this analysis.
Covariates.
Data on sociodemographic characteristics, including educational attainment, sex, age, existing comorbidity, and race/ethnicity, were collected in the 2016 core interview. These data are self-reported during interviews conducted by trained study staff, either in the participant’s home or via telephone, depending on whether the participant received a face-to-face interview in that study year. Educational attainment was categorized as less than a high school diploma, a high school diploma, some college, or a college degree or more. Race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Hispanic, or other, and sex was self-reported as either male or female. We also constructed a comorbidity index, which is a sum of indicators for whether a physician has diagnosed the respondent with high blood pressure, diabetes, cancer, lung disease, heart disease, stroke, and/or arthritis. This was modeled after the RAND HRS comorbidity index (RAND Corporation, Santa Monica, California), with the exception of psychiatric problems, which were excluded because of missing data.
Statistical analyses
All statistical analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). Descriptive statistics were compiled to characterize the study population overall and by CMV seropositivity. We used linear regression models to estimate the relationships of CMV serostatus and IgG antibody response with TICS score in the study population. Fully adjusted models included age (grand mean–centered, quadratic term), sex, race/ethnicity, and educational attainment. This minimally sufficient adjustment set was determined a priori through directed acyclic graph analysis (49). The directed acyclic graph included other variables, such as wealth and marital status, whose pathways were blocked by the inclusion of the aforementioned variables. Analyses adjusting for comorbid conditions, which may be confounders or mediators, were performed as sensitivity analyses. We further investigated potential effect-measure modification of the relationship between CMV and TICS score by educational attainment by using stratified regression models and testing for interaction between CMV and education.
RESULTS
Sample characteristics
The 5,617 adults who met eligibility criteria and were included in our analysis were 57.6% female and were a mean of 75 years of age at the time of data collection in 2016, with an age range of 65–107 years. The prevalence of CMV was 73.2% in the overall population, 77.1% percent in females, and 67.9% in males. Overall, 80.0% of the population described themselves as non-Hispanic White, though a smaller proportion of non-Hispanic Whites was CMV-seropositive (68.7%) compared with non-Hispanic Blacks and persons of other races/ethnicities (91.4% and 91.4%, respectively). Ninety percent of those with less than a high school diploma were CMV-seropositive, compared with 77.3%, 69.0%, and 59.1% of those with a high school diploma, some college, and a college degree or above, respectively. Sixty-three percent of the study population was married or partnered, and 22.5% were widowed. The distribution of smoking status overall included 44.8% categorized as never smokers, 46.9% as former smokers, and 8.3% as current smokers. A total of 75.5% of never smokers were CMV-seropositive, as were 70.3% of former smokers and 77.6% of current smokers. The median TICS score was 2 points lower among CMV-seropositive persons than among CMV-seronegative persons. The demographic and social characteristics of our study population are shown in Table 1.
Table 1.
Baseline Sociodemographic Characteristics of Participants Eligible for Inclusion in a Study of Cross-Sectional Associations of Cytomegalovirus Serostatus and Immunoglobulin G Antibody Response With Cognitive Function, Health and Retirement Study, 2016
| Cytomegalovirus Serostatus | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total (n = 5,617) | Positive (n = 4,113) | Negative (n = 1,504) | |||
| No. | % | No. | Row % | No. | Row % | |
| Age, yearsa | 75 (65–107) | 75 (65–107) | 73 (65–100) | |||
| Sex | ||||||
| Female | 3,234 | 57.6 | 2,495 | 77.1 | 739 | 22.9 |
| Male | 2,383 | 42.4 | 1,618 | 67.9 | 765 | 32.1 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 4,492 | 80.0 | 3,085 | 68.7 | 1,407 | 31.3 |
| Non-Hispanic Black | 823 | 14.7 | 752 | 91.4 | 71 | 8.6 |
| Other | 302 | 5.4 | 276 | 91.4 | 26 | 8.6 |
| Education | ||||||
| Less than high school | 982 | 17.5 | 888 | 90.4 | 94 | 9.6 |
| High school | 1,938 | 34.5 | 1,498 | 77.3 | 440 | 22.7 |
| Some college | 1,344 | 23.9 | 927 | 66.0 | 417 | 31.0 |
| College or above | 1,353 | 24.1 | 800 | 59.1 | 553 | 40.9 |
| Marital status | ||||||
| Married/partnered | 3,511 | 63.1 | 2,463 | 70.2 | 1,048 | 29.8 |
| Separated/divorced | 645 | 11.6 | 498 | 77.2 | 147 | 22.8 |
| Widowed | 1,253 | 22.5 | 996 | 79.5 | 257 | 20.5 |
| Never married | 159 | 2.9 | 116 | 73.0 | 43 | 27.0 |
| Missing data | 49 | 0.9 | 40 | 81.6 | 9 | 18.4 |
| Smoking status | ||||||
| Never smoker | 2,478 | 44.8 | 1,870 | 75.5 | 608 | 24.5 |
| Former smoker | 2,591 | 46.9 | 1,821 | 70.3 | 770 | 29.7 |
| Current smoker | 459 | 8.3 | 356 | 77.6 | 103 | 22.4 |
| Missing data | 89 | 1.6 | 66 | 74.2 | 23 | 25.8 |
| Comorbidity indexb | 0.33 (0.0) | 0.34 (0.0) | 0.32 (0.0) | |||
| TICS scorec,d | 21.9 (0.09) | 21.2 (0.11) | 23.2 (0.15) | |||
Abbreviation: TICS, Telephone Interview for Cognitive Status.
a Values are expressed as mean (range).
b Values are expressed as mean (standard error).
c Values are expressed as median (standard error).
d Possible scores on the TICS range from 0 to 35.
CMV and cognition
Table 2 shows the association between CMV serostatus and TICS score before and after covariate adjustment. In the age-adjusted model, we found that CMV seropositivity was significantly associated with a lower mean TICS score, though this association was largely attenuated after adjustment for confounding variables. The adjusted estimate for a CMV-seropositive participant compared with a CMV seronegative participant was attenuated to −0.19 (95% confidence interval (CI): −0.45, 0.08) points on the TICS scale as compared with the age-adjusted estimate of −1.69 (95% CI: −1.98, −1.40) TICS points.
Table 2.
Linear Model Estimates of the Association Between Cytomegalovirus Seropositivity and Cognitive Function (n = 5,617), Health and Retirement Study, 2016
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Estimate a | 95% CI | Estimate a | 95% CI | |
| Intercept | 23.82 | 23.53, 24.11 | 19.94 | 19.54, 20.34 |
| CMV serostatus | ||||
| Seropositive | −1.76 | −2.05, −1.47 | −0.19 | −0.45, 0.08 |
| Seronegative | 0.00 | Referent | 0.00 | Referent |
| Age | −0.17 | −0.19, −0.14 | −0.18 | −0.20, −0.16 |
| Age2 | −0.01 | −0.01, 0.00 | −0.01 | −0.01, 0.00 |
| Sex | ||||
| Male | −0.61 | −0.87, −0.36 | −0.85 | −1.08, −0.62 |
| Female | 0.00 | Referent | 0.00 | Referent |
| Educational attainment | ||||
| Less than high school | 0.00 | Referent | ||
| High school | 3.12 | 2.77, 3.46 | ||
| Some college | 4.06 | 3.69, 4.43 | ||
| College or above | 5.83 | 5.45, 6.21 | ||
| Race/ethnicity | ||||
| Non-Hispanic White | 0.00 | Referent | ||
| Non-Hispanic Black | −2.94 | −3.28, −2.61 | ||
| Hispanic | −1.56 | −1.97, −1.16 | ||
| Other | −2.16 | −2.90, −1.43 | ||
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; TICS, Telephone Interview for Cognitive Status.
a Difference in TICS score.
Table 3 shows β estimates and 95% confidence intervals from 2 models for the association between CMV IgG antibody response tertile and TICS score in comparison with persons who were seronegative. We saw similar results with CMV tertiles as we did when we investigated simple dichotomous CMV seropositivity. In the age-adjusted model, higher CMV tertiles were significantly associated with lower TICS scores in comparison with seronegative persons, with the size of the association increasing as CMV IgG response increased. Comparing persons in the highest tertile of CMV IgG response (tertile 3) with seronegative persons, in the fully adjusted model, the association was attenuated but remained in the expected direction, with those in highest tertile of IgG response having a TICS score 0.22 (95% CI: −0.55, 0.11) points lower than those who were seronegative. Tertile 2 showed similar results, with a smaller association for those in tertile 1 compared with seronegative persons.
Table 3.
Linear Model Estimates of the Association Between Tertiles of Cytomegalovirus Immunoglobulin G Response and Cognitive Function (n = 5,617), Health and Retirement Study, 2016
| Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Estimate a | 95% CI | Estimate a | 95% CI | |
| Intercept | 23.50 | 23.24, 23.76 | 19.95 | 19.55, 20.35 |
| CMV IgG response | ||||
| Tertile 3 | −1.98 | −1.73, −1.02 | −0.22 | −0.55, 0.11 |
| Tertile 2 | −1.71 | −2.07, −1.36 | −0.26 | −0.58, 0.06 |
| Tertile 1 | −1.37 | −2.34, −1.63 | −0.09 | −0.41, 0.23 |
| Seronegative | 0.00 | Referent | 0.00 | Referent |
| Age | −0.17 | −0.19, −0.15 | −0.18 | −0.20, −0.16 |
| Age2 | −0.01 | −0.01, 0.00 | −0.01 | −0.01, 0.00 |
| Sex | ||||
| Male | −0.86 | −1.09, −0.63 | ||
| Female | 0.00 | Referent | ||
| Educational attainment | ||||
| Less than high school | 0.00 | Referent | ||
| High school | 3.11 | 2.77, 3.46 | ||
| Some college | 4.05 | 3.68, 4.42 | ||
| College or above | 5.82 | 5.44, 6.21 | ||
| Race/ethnicity | ||||
| Non-Hispanic White | 0.00 | Referent | ||
| Non-Hispanic Black | −2.94 | −3.28, −2.60 | ||
| Hispanic | −1.56 | −1.96, −1.15 | ||
| Other | −2.17 | −2.90, −1.43 | ||
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; IgG, immunoglobulin G; TICS, Telephone Interview for Cognitive Status.
a Difference in TICS score.
Effect-measure modification by educational attainment
Tables 4 and 5 show the association between CMV and cognition across strata of educational attainment. We see that the association between CMV seropositivity and TICS score was larger, though less precise, among persons with lower educational attainment than among those with higher levels of educational attainment. In fact, among participants with less than a high school diploma, those who were seropositive for CMV had a TICS score 0.56 (95% CI: −1.63, 0.52) points lower than that of those who were seronegative (Table 4). When investigating the association between tertiles of CMV IgG antibodies and TICS score, we observed similar modification by educational attainment, such that among persons with less than a high school diploma, those in the first tertile of CMV IgG response had a TICS score 0.89 (95% CI: −2.07, 0.29) points lower than that of seronegative persons (Table 5). Those in the second and third tertiles of CMV IgG response had TICS scores 0.45 (95% CI: −1.53, 0.77) and 0.38 (95% CI: −1.53, 0.77) points lower, respectively. These associations were larger than the associations we saw in each other educational stratum. We also fitted a linear model with interaction terms between educational attainment (modeled as dummy variables) and CMV measurement to assess the joint statistical significance of interaction. The terms for interaction with CMV seropositivity and CMV tertile were not statistically significant (P = 0.73 and P = 0.45, respectively). The differences in TICS score for both CMV seropositivity and high CMV antibody levels were equivalent to approximately 3- and 5-year differences in cognitive function among persons with less than a high school diploma, respectively, based on age norms for the TICS scale. Figures 1 and 2 show these age-normed associations with TICS score stratified by educational attainment.
Table 4.
Linear Model Estimates of the Association Between Cytomegalovirus Seropositivity and Cognitive Function, by Educational Attainment (n = 5,617), Health and Retirement Study, 2016
| Educational Attainment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Less Than High School | High School Diploma | Some College | College or Above | |||||
| Estimate a | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
Interaction
P Value |
|
| Intercept | 20.22 | 19.17, 21.27 | 23.12 | 22.66, 23.58 | 23.82 | 23.32, 24.32 | 25.76 | 25.34, 26.18 | |
| CMV serostatus | 0.733 | ||||||||
| Seropositive | −0.56 | −1.63, 0.52 | −0.02 | −0.49, 0.44 | −0.20 | −0.70, 0.31 | −0.31 | −0.73, 0.12 | |
| Seronegative | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | |
| Age | −0.17 | −0.22, −0.11 | −0.17 | −0.20, −0.13 | −0.19 | −0.23, −0.15 | −0.19 | −0.23, −0.15 | 0.739 |
| Age2 | −0.01 | −0.01, 0.00 | −0.01 | −0.01, 0.00 | 0.00 | −0.01, 0.00 | −0.01 | −0.01, −0.01 | 0.403 |
| Sex | 0.038 | ||||||||
| Male | −0.29 | −0.97, 0.32 | −1.24 | −1.63, −0.85 | −0.78 | −1.25, −0.31 | −0.68 | −1.09, −0.27 | |
| Female | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | |
| Race/ethnicity | 0.292 | ||||||||
| Non-Hispanic White | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | |
| Non-Hispanic Black | −3.09 | −3.91, −2.28 | −3.30 | −3.86, −2.73 | −2.94 | −3.62, −2.27 | −1.95 | −2.71, −1.20 | |
| Hispanic | −1.86 | −2.62, −1.10 | −1.56 | −2.31, −0.80 | −1.13 | −2.06, −0.20 | −1.12 | −2.39, 0.15 | |
| Other | −2.96 | −4.96, −0.96 | −2.67 | −3.98, −1.35 | −1.91 | −3.45, −0.36 | −1.28 | −2.52, −0.05 | |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; TICS, Telephone Interview for Cognitive Status.
a Difference in TICS score.
Table 5.
Linear Model Estimates of the Association Between Tertiles of Cytomegalovirus Immunoglobulin G Response and Cognitive Function, by Educational Attainment (n = 5,617), Health and Retirement Study, 2016
| Educational Attainment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Less Than High School | High School Diploma | Some College | College or Above | |||||
| Estimate a | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
Interaction
P Value |
|
| Intercept | 20.19 | 19.15, 21.24 | 23.14 | 22.68, 23.60 | 23.82 | 23.32, 24.32 | 25.77 | 25.35, 26.19 | |
| CMV IgG response | 0.445 | ||||||||
| Tertile 3 | −0.38 | −1.53, 0.77 | −0.26 | −0.82, 0.29 | −0.21 | −0.85, 0.44 | −0.17 | −0.79, 0.44 | |
| Tertile 2 | −0.45 | −1.60, 0.71 | −0.05 | −0.61, 0.50 | −0.27 | −0.92, 0.39 | −0.66 | −1.20, −0.11 | |
| Tertile 1 | −0.89 | −2.07, 0.29 | 0.22 | −0.33, 0.77 | −0.13 | −0.74, 0.49 | −0.05 | −0.59, 0.49 | |
| Seronegative | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | |
| Age | −0.17 | −0.22, −0.11 | −0.17 | −0.20, −0.13 | −0.19 | −0.23, −0.15 | −0.19 | −0.23, −0.15 | 0.751 |
| Age2 | −0.01 | −0.01, 0.00 | −0.01 | −0.01, 0.00 | 0.00 | −0.01, 0.00 | −0.01 | −0.01, −0.01 | 0.383 |
| Sex | 0.018 | ||||||||
| Male | −0.24 | −0.86, 0.39 | −1.29 | −1.68, −0.90 | −0.78 | −1.25, −0.31 | −0.69 | −1.10, −0.28 | |
| Female | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | |
| Race/ethnicity | 0.383 | ||||||||
| Non-Hispanic White | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | 0.00 | Referent | |
| Non-Hispanic Black | −3.12 | −3.94, −2.30 | −3.28 | −3.84, −2.71 | −2.94 | −3.62, −2.26 | −1.95 | −2.70, −1.19 | |
| Hispanic | −1.86 | −2.61, −1.10 | −1.52 | −2.28, −0.77 | −1.12 | −2.05, −0.19 | −1.11 | −2.37, 0.16 | |
| Other | −2.92 | −4.92, −0.93 | −2.65 | −3.96, −1.34 | −1.91 | −3.46, −0.36 | −1.34 | −2.58, −0.11 | |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; IgG, immunoglobulin G; TICS, Telephone Interview for Cognitive Status.
a Difference in TICS score.
Figure 1.
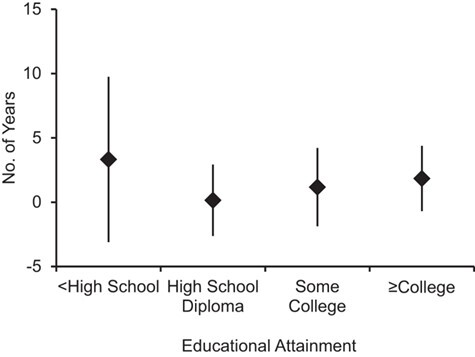
Estimated additional years of cognitive aging associated with cytomegalovirus (CMV) seropositivity as compared with being CMV-seronegative, by educational attainment, Health and Retirement Study, 2016. The figure shows modification of the association between CMV serostatus and cognitive function by education; persons with less than a high school diploma had a larger but less precise association with CMV seropositivity than those with higher educational attainment. Estimates were based on the study-sample–specific average change in Telephone Interview for Cognitive Status score per year of age. Data were obtained from the Health and Retirement Study 2016 Core (Early, Version 1.0) Release and the 2016 Venous Blood Study (Early, Version 1.0) Release. Bars, 95% confidence intervals.
Figure 2.
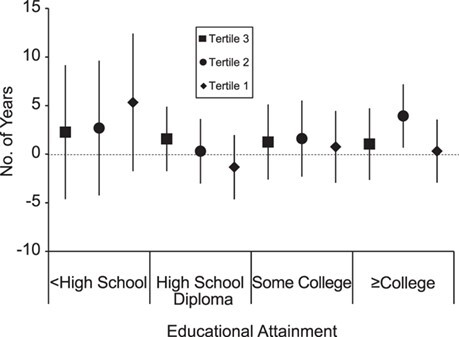
Estimated additional years of cognitive aging associated with tertiles of cytomegalovirus (CMV) immunoglobulin G (IgG) response as compared with being CMV-seronegative, by educational attainment, Health and Retirement Study, 2016. The figure shows potential modification of the association between CMV IgG tertiles and cognitive function by education; persons with less than a high school diploma had a somewhat larger but less precise association with CMV IgG response than those with higher educational attainment. Estimates were based on the study-sample–specific average change in Telephone Interview for Cognitive Status score per year of age. Data were obtained from the Health and Retirement Study 2016 Core (Early, Version 1.0) Release and the 2016 Venous Blood Study (Early, Version 1.0) Release. Bars, 95% confidence intervals.
Because comorbid conditions may be both confounders (since they may weaken the immune system and exacerbate immune-system difficulty in controlling the infection) and mediators of the relationship between CMV antibody level and cognition, we conducted a sensitivity analysis that added the comorbidity index to each regression model. While the index itself was statistically significantly associated with TICS score, the addition of this index to the model did not change any of the estimates meaningfully. These results are presented in Web Tables 1–4.
DISCUSSION
We found that overall, CMV seropositivity and higher IgG response were both associated with worse cognitive function in a US population-based survey of adults over the age of 65 years, though associations were not statistically significant in the fully adjusted models. While the magnitude of the association between CMV serostatus and cognition among participants with less than a high school diploma was slightly larger than that in other strata of educational attainment (0.56 TICS points, 95% CI: −1.63, 0.52), there was no statistically significant interaction between CMV serostatus and educational attainment. Similarly, persons with less than a high school diploma who were in the first tertile of CMV antibody response scored 0.89 (95% CI: −2.07, 0.29) points lower on the TICS, whereas associations were smaller across other levels of educational attainment. The differences for both CMV seropositivity and high CMV antibody levels in the lowest educational group were equivalent to approximately 4- and 5-year differences in cognition, respectively, based on age norms for the TICS scale.
These findings are consistent with the previous literature finding a negative relationship between CMV and cognition, despite the lack of statistical significance. Previous research has found CMV seropositivity and IgG levels to be associated with global cognition, specific domains of cognition, or ADRD (14–22, 50). However, several studies have also found results that were in the expected direction but not statistically significant (13, 24–29), and all of the previous research has taken place in specialized populations. Therefore, these results add to prior research by showing that CMV may play a role in cognitive decline and ADRD at the population level in a population-based sample, though that role is likely to be one of many components contributing to cognitive decline.
There are several mechanisms through which CMV may be associated with ADRD and cognitive impairment. As the human brain ages, the presence of misfolded proteins, free radicals, and epigenetic changes increases (51, 52) and microglia become senescent, leading to an elevation in the production of proinflammatory cytokines, impaired phagocytosis, reduced motility, and a reversal of the demyelination of axons, which is a common change in neurodegenerative diseases (12, 52, 53). The presence of misfolded proteins (such as amyloid β) elevates production of proinflammatory cytokines in microglia (9, 51, 52, 54) but may additionally inhibit the ability of microglia to secrete antiinflammatory cytokines (52, 55, 56). While advanced peripheral immunosenescence could itself be integral in the pathogenesis of dementia, it would require accompanying perturbations in the blood-brain barrier to allow movement of immune molecules into the central nervous system. Leakage in the blood-brain barrier has been observed early on in cases of Alzheimer disease (9, 57) and allows the passage of peripheral immune cells, microbes, and inflammatory mediators into the central nervous system (12). While the blood-brain barrier naturally deteriorates with age (9), environmental stimuli, and possibly chronic infections, can also accelerate this process. Furthermore, CMV has been found in the brain tissue of patients with vascular dementia (17), indicating that CMV itself may more directly impact neurodegeneration.
This research further added to that literature by investigating modification of the CMV-cognition relationship by educational attainment. We found indications that the association between CMV and cognition may be more pronounced among persons with the lowest educational attainment, supporting the cognitive reserve theory. However, because CMV is acquired early in life, it may contribute to cognitive impairment early on. In that case, educational attainment may act as a mediator of the relationship between CMV and cognition, and adjustment for education would bias our results toward the null. Our findings are consistent with those of earlier studies regarding the buffering of biological risk for poor cognition by educational attainment and add to them by showing that the adverse effects of a biological insult such as CMV, specifically, may also be ameliorated by higher educational attainment. While further studies are needed to fully elucidate the mechanisms and effects of the buffering impacts of education against the adverse effects of CMV, particularly immune system control of CMV later in life, these findings support the cognitive reserve theory that educational returns in the form of cognitive reserve may preserve brain function in the presence of biological assaults on the central nervous system and cells in the brain (41). As we discussed above, a growing body of research indicates that educational attainment may buffer the detrimental impact of biological risk factors, such as having 1 or 2 copies of APOE-ε4, on cognitive impairment and dementia (58–60). However, further studies are needed to fully elucidate the timing, mechanisms, and effects of the buffering impacts of education and the biological mechanisms by which this protective mechanism works in human populations.
Our study had several strengths, foremost of which was the availability of both biomarker and cognitive data in a large, population-based cohort. We were able to include over 5,600 participants in our analysis and operationalize CMV in 2 ways: serostatus and strength of IgG response. Furthermore, we were able to assess effect-measure modification of this relationship by an indicator of cognitive reserve: educational attainment. However, our study did have limitations. While the HRS is a large population-based study, the participants who consented to participate in the Venous Blood Study may have been healthier than the overall cohort, which may have biased measures of association away from the null. Additionally, educational attainment categorized by degree will likely have variation within each category, as well as between categories. Finally, this study was cross-sectional, and therefore we cannot infer causality. Future studies which assess the effect of CMV on cognitive impairment over time are needed.
Together with previous research carried out in a range of populations, our results suggest that CMV infection may be negatively associated with cognitive function. If CMV is a causal factor in the neurodegenerative process, our results could provide new microbial avenues such as prevention of infection for the delay and prevention of ADRD and could lead to a higher-quality end-of-life period for many people. However, because CMV is often acquired early in life and many people do not know that they are infected, prevention of CMV infection has been a low priority in the biomedical research community, and effective prevention tools are probably far off in the future. The first step toward prevention would likely be spreading awareness of CMV’s potentially adverse health effects in healthy individuals. Furthermore, understanding the role of educational attainment as a potential effect-measure modifier of this relationship provides further evidence that education may act as a buffer against biological triggers of cognitive impairment. Understanding the mechanisms through which these social and microbial factors may interact to affect cognitive decline and onset of dementia will move the field forward and will allow us to design targeted interventions to reduce disparities in dementia and overall incidence of dementia in the US population.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States (Rebecca C. Stebbins, Grace A. Noppert, Allison E. Aiello); Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States (Grace A. Noppert, Yang Claire Yang, Allison E. Aiello); Department of Sociology, College of Arts and Sciences, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, United States (Yang Claire Yang); Leverhulme Centre for Demographic Science, Department of Sociology, Nuffield College, Social Sciences Division, University of Oxford, Oxford, United Kingdom (Jennifer B. Dowd); and Department of Epidemiology, Joseph J. Zilber School of Public Health, University of Wisconsin-Milwaukee, Milwaukee, Wisconsin, United States (Amanda Simanek). R.C.S. is now affiliated with the Carolina Population Center, University of North Carolina at Chapel Hill.
This work was supported by the National Institute on Aging (grant R01AG057800). G.A.N. was supported by the National Institute on Aging (grant K99AG062749).
We thank the members of the Aiello Research Group and the Health and Retirement Study staff at the University of Michigan for their assistance with data management and their work on the study.
Conflict of interest: none declared.
REFERENCES
- 1. Centers for Disease Control and Prevention . Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003.52(6):101–104, 106. [PubMed] [Google Scholar]
- 2. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institute on Aging . Alzheimer’s disease fact sheet. https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet. Reviewed May 22, 2019. Accessed August 17, 2018.
- 4. Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 Census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mongan E. National long-term care spending hits all-time high at $163 billion. https://www.mcknights.com/news/national-long-term-care-spending-hits-all-time-high-at-163-billion/article/712421/. Published December 7, 2017. Accessed November 25, 2018.
- 6. Hersi M, Irvine B, Gupta P, et al. Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. Neurotoxicology. 2017;61:143–187. [DOI] [PubMed] [Google Scholar]
- 7. Alzheimer’s Association . Causes and risk factors for Alzheimer’s disease. https://www.alz.org/alzheimers-dementia/what-is-alzheimers/risk-factors. Accessed November 25, 2018.
- 8. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1-2):125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. VanItallie TB. Alzheimer’s disease: innate immunity gone awry? Metabolism. 2017;69S:S41–S49. [DOI] [PubMed] [Google Scholar]
- 10. Jones L, Holmans PA, Hamshere ML, et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One. 2010;5(11):e13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fakhoury M. Role of immunity and inflammation in the pathophysiology of neurodegenerative diseases. Neurodegener Dis. 2015;15(2):63–69. [DOI] [PubMed] [Google Scholar]
- 13. Wikby A, Ferguson F, Forsey R, et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60(5):556–565. [DOI] [PubMed] [Google Scholar]
- 14. Aiello AE, Haan M, Blythe L, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54(7):1046–1054. [DOI] [PubMed] [Google Scholar]
- 15. Barnes LL, Capuano AW, Aiello AE, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis. 2015;211(2):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katan M, Moon YP, Paik MC, et al. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology. 2013;80(13):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin WR, Wozniak MA, Wilcock GK, et al. Cytomegalovirus is present in a very high proportion of brains from vascular dementia patients. Neurobiol Dis. 2002;9(1):82–87. [DOI] [PubMed] [Google Scholar]
- 18. Nimgaonkar VL, Yolken RH, Wang T, et al. Temporal cognitive decline associated with exposure to infectious agents in a population-based, aging cohort. Alzheimer Dis Assoc Disord. 2016;30(3):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strandberg TE, Pitkala KH, Linnavuori K, et al. Cognitive impairment and infectious burden in the elderly. Arch Gerontol Geriatr Suppl. 2004;(9):419–423. [DOI] [PubMed] [Google Scholar]
- 20. Strandberg TE, Pitkala KH, Linnavuori K, et al. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34(9):2126–2131. [DOI] [PubMed] [Google Scholar]
- 21. Wright CB, Gardener H, Dong C, et al. Infectious burden and cognitive decline in the Northern Manhattan Study. J Am Geriatr Soc. 2015;63(8):1540–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carbone I, Lazzarotto T, Ianni M, et al. Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging. 2014;35(1):122–129. [DOI] [PubMed] [Google Scholar]
- 23. Tarter KD, Simanek AM, Dowd JB, et al. Persistent viral pathogens and cognitive impairment across the life course in the Third National Health and Nutrition Examination Survey. J Infect Dis. 2014;209(6):837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torniainen-Holm M, Suvisaari J, Lindgren M, et al. Association of cytomegalovirus and Epstein-Barr virus with cognitive functioning and risk of dementia in the general population: 11-year follow-up study. Brain Behav Immun. 2018;69:480–485. [DOI] [PubMed] [Google Scholar]
- 25. Lövheim H, Olsson J, Weidung B, et al. Interaction between cytomegalovirus and herpes simplex virus type 1 associated with the risk of Alzheimer’s disease development. J Alzheimers Dis. 2018;61(3):939–945. [DOI] [PubMed] [Google Scholar]
- 26. Lurain NS, Hanson BA, Martinson J, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013;208(4):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Westman G, Lidehall A-K, Magnusson P, et al. Decreased proportion of cytomegalovirus specific CD8 T-cells but no signs of general immunosenescence in Alzheimer’s disease. PLoS One. 2013;8(10):e77921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yolken RH, Torrey EF, Lieberman JA, et al. Serological evidence of exposure to herpes simplex virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res. 2011;128(1–3):61–65. [DOI] [PubMed] [Google Scholar]
- 29. Bartlett DB, Firth CM, Phillips AC, et al. The age-related increase in low-grade systemic inflammation (inflammaging) is not driven by cytomegalovirus infection. Aging Cell. 2012;11(5):912–915. [DOI] [PubMed] [Google Scholar]
- 30. Lenehan ME, Summers MJ, Saunders NJ, et al. Relationship between education and age-related cognitive decline: a review of recent research. Psychogeriatrics. 2015;15(2):154–162. [DOI] [PubMed] [Google Scholar]
- 31. Bruandet A, Richard F, Bombois S, et al. Cognitive decline and survival in Alzheimer’s disease according to education level. Dement Geriatr Cogn Disord. 2008;25(1):74–80. [DOI] [PubMed] [Google Scholar]
- 32. Tschanz JT, Pfister R, Wanzek J, et al. Stressful life events and cognitive decline in late life: moderation by education and age. The Cache County Study. Int J Geriatr Psychiatry. 2013;28(8):821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin Infect Dis. 2010;50(11):1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dowd JB, Aiello A. Socioeconomic differentials in immune response in the US. Epidemiology. 2009;20(6):902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dowd JB, Haan MN, Blythe L, et al. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2008;167(1):112–120. [DOI] [PubMed] [Google Scholar]
- 36. Dowd JB, Palermo TM, Aiello AE. Family poverty is associated with cytomegalovirus antibody titers in U.S. children. Health Psychol. 2012;31(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meier HCS, Haan MN, de Leon CFM, et al. Early life socioeconomic position and immune response to persistent infections among elderly Latinos. Soc Sci Med. 2016;166:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simanek AM, Dowd JB, Aiello AE. Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. Int J Epidemiol. 2008;38(3):775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stebbins RC, Noppert GA, Aiello AE, et al. Persistent socioeconomic and racial and ethnic disparities in pathogen burden in the United States, 1999–2014. Epidemiol Infect. 2019;147:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;(spec no.;80–94. [PubMed] [Google Scholar]
- 41. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- 42. Satz P, Morgenstern H, Miller EN, et al. Low education as a possible risk factor for cognitive abnormalities in HIV-1: findings from the Multicenter AIDS Cohort Study (MACS). J Acquir Immune Defic Syndr. 1993;6(5):503–511. [PubMed] [Google Scholar]
- 43. Harrison SL, Sajjad A, Bramer WM, et al. Exploring strategies to operationalize cognitive reserve: a systematic review of reviews. J Clin Exp Neuropsychol. 2015;37(3):253–264. [DOI] [PubMed] [Google Scholar]
- 44. Heeringa SG, Connor JH. Technical Description of the Health and Retirement Survey Sample Design. Ann Arbor, MI: University of Michigan; 1995. [Google Scholar]
- 45. Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30(suppl):S7–S56. [Google Scholar]
- 46. Survey Research Center, Institute for Social Research, Univeristy of Michigan . Sample Sizes and Response Rates. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2017. https://hrs.isr.umich.edu/sites/default/files/biblio/ResponseRates_2017.pdf. Accessed November 25, 2018. [Google Scholar]
- 47. Sonnega A, Faul JD, Ofstedal MB, et al. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crimmins E, Faul J, Thyagarajan B, et al. Venous Blood Collection and Assay Protocol in the 2016 Health and Retirement Study 2016 Venous Blood Study (VBS). Ann Arbor, MI: University of Michigan; 2017. https://hrs.isr.umich.edu/sites/default/files/biblio/2016%20VBS%20data%20ver%207_2.pdf. Accessed November 25, 2018. [Google Scholar]
- 49. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 50. Kawasaki M, Arai Y, Takayama M, et al. Carotid atherosclerosis, cytomegalovirus infection, and cognitive decline in the very old: a community-based prospective cohort study. Age (Dordr). 2016;38(2):Article 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–224. [DOI] [PubMed] [Google Scholar]
- 52. Rawji KS, Mishra MK, Michaels NJ, et al. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016;139(3):653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miron VE, Boyd A, Zhao J-W, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flanary BE, Sammons NW, Nguyen C, et al. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10(1):61–74. [DOI] [PubMed] [Google Scholar]
- 55. Doyle KP, Cekanaviciute E, Mamer LE, et al. TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:Article 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohen M, Matcovitch O, David E, et al. Chronic exposure to TGFβ1 regulates myeloid cell inflammatory response in an IRF7-dependent manner. EMBO J. 2014;33(24):2906–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. [DOI] [PubMed] [Google Scholar]
- 58. Dekhtyar S, Marseglia A, Xu W, et al. Genetic risk of dementia mitigated by cognitive reserve: a cohort study. Ann Neurol. 2019;86(1):68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Glaser R. Stress-associated immune dysregulation and its importance for human health: a personal history of psychoneuroimmunology. Brain Behav Immun. 2005;19(1):3–11. [DOI] [PubMed] [Google Scholar]
- 60. Vonk JMJ, Rentería MA, Medina VM, et al. Education moderates the relation between APOE ɛ4 and memory in nondemented non-Hispanic black older adults. J Alzheimers Dis. 2019;72(2):495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


