SUMMARY
The APOE ε4 allele remains the strongest genetic risk factor for sporadic Alzheimer’s disease and the APOE ε2 allele the strongest genetic protective factor after multiple large scale genome-wide association studies and genome-wide association meta-analyses. However, no therapies directed at APOE are currently available. Although initial studies causally linked APOE with amyloid-β peptide aggregation and clearance, over the past 5 years our understanding of APOE pathogenesis has expanded beyond amyloid-β peptide-centric mechanisms to tau neurofibrillary degeneration, microglia and astrocyte responses, and blood-brain barrier disruption. Because all these pathological processes can potentially contribute to cognitive impairment, it is important to use this body of knowledge to develop therapies directed at APOE. Several therapeutic approaches have been successful in mouse models expressing human APOE alleles, including increasing or reducing APOE levels, enhancing its lipidation, blocking the interactions between APOE and amyloid-β peptide, and genetically switching APOE4 to APOE3 or APOE2 isoforms, but translation to human clinical trials has proven challenging.
Keywords: Alzheimer’s disease, apolipoprotein E, amyloid beta peptide, tau, microglia, astrocytes, blood-brain barrier, drug development
INTRODUCTION
Even after multiple large-scale genome-wide association studies (GWAS) and GWAS meta-analyses1, the ε4 allele of the APOE gene (compared to the most common ε3 allele) continues to be the strongest genetic risk factor associated with sporadic Alzheimer’s disease since its discovery in 1993. Moreover, the relatively rare APOE ε2 allele remains by far the strongest genetic protective factor against sporadic Alzheimer’s disease (Panel 1), emphasising the importance of APOE’s role in Alzheimer’s disease pathogenesis. Because Alzheimer’s disease is defined by the accumulation of two hallmark pathological protein aggregates: amyloid-β peptide (Aβ) plaques and neurofibrillary tangles containing hyperphosphorylated tau, one postulate is that APOE affects these lesions. Although solid evidence supports this view, emerging advances are changing our understanding of APOE involvement in Alzheimer’s disease. First, new genetic modifiers and the APOE local ancestry (i.e., the population-specific genetic variation in the APOE region) have been associated with a differential APOE ε4-linked increased risk of Alzheimer’s disease. Second, although APOE modification of Alzheimer’s disease risk has been long attributed to its effects on Aβ, systematic neuropathological examination of large autopsy cohorts has suggested that the APOE genotype also correlates with the presence and severity of other proteinopathies, pointing to new causal links. Third, technological advances in the past decade —including mouse models genetically engineered to express human APOE alleles; virally-mediated gene transfer; proteomics and transcriptomics; patient-derived human-induced pluripotent stem cells; plasma, CSF, PET and MRI biomarkers— have implicated APOE in other aspects of Alzheimer’s disease pathophysiology, such as tau-induced neurodegeneration, microglial and astrocyte reactions (including neuroinflammation), and blood-brain barrier disruption. Lastly, although no APOE-based therapy is yet available, several APOE-directed therapeutic approaches have been shown to be effective in mouse models and hold promise for translation to human clinical trials. In this Review, we discuss the advances made in genetics, pathophysiology, and therapeutic approaches related to APOE and Alzheimer’s disease.
GENETIC DISCOVERIES RELATED TO APOE
Over the past 3 years, human genetic studies have suggested risk modifiers that mitigate or increase APOE ε4-associated Alzheimer’s disease risk, and identified haplotypes with heterogeneous effects. Understanding the risk variation in APOE ε4 carriers has the potential to shed further light on APOE pathobiology and mechanisms of resilience and resistance to Alzheimer’s disease, which could have therapeutic value.
APOE ε2 homozygosity
In an analysis2 of a US cohort with approximately 5,000 neuropathologically confirmed Alzheimer’s disease and control subjects, APOE ε2 homozygosity was associated with much lower odds of Alzheimer’s disease than was APOE ε3 homozygosity (odds ratio [OR] 0.13 [95% CI 0.05–0.36]), and the APOE ε2/ε3 genotype (0.39 [0.30–0.50]). The contrast of APOE ε2 homozygosity versus APOE ε4 homozygosity was even greater (0.004 [0.001–0.014]), and APOE ε2 was also associated with milder Alzheimer’s disease neuropathological changes (i.e., less widespread Aβ plaques and neurofibrillary tangles) in this autopsy cohort. However, these exceptionally low Alzheimer’s disease ORs in APOE ε2 homozygotes were not found in the larger clinically defined but neuropathologically unconfirmed group (23,857 individuals; 10,430 with probable Alzheimer’s disease and 13,426 cognitively unimpaired), suggesting a stronger protection against Alzheimer’s disease neuropathology.
APOE Christchurch mutation
A single case report3 described an approximately 70-year-old Colombian woman who, despite carrying a fully penetrant autosomal dominant E280A mutation in PSEN1, which is linked to familial Alzheimer’s disease, and abundant fibrillary Aβ deposits in her PET scan, remained cognitively healthy well beyond her expected year of symptom onset (age 44 years). After whole exome sequencing, it was concluded that a rare homozygous APOE ε3 Christchurch (R136S) mutation conferred her resilience to Alzheimer’s disease. Mechanistically, the APOE3 R136S mutation appears to inhibit Aβ oligomerization, disrupt APOE binding to low-density lipoprotein receptor, and interfere with APOE affinity for heparan sulfate proteoglycans, which are involved in toxic tau uptake by neurons, perhaps explaining the lower than average radioligand uptake observed in her tau PET scan3.
Other genetic modifiers
A meta-analysis of 22 studies has revealed that KLOTHO-VS heterozygosity —a polymorphism previously associated with longevity— might attenuate the increased Alzheimer’s disease risk associated with the APOE ε4 allele, because APOE ε4 carriers older than 60 years with KLOTHO-VS heterozygosity had a reduced Alzheimer’s disease risk (OR 0.75 [95% CI 0.67–0.84]; p=7.4×10−7), reduced risk of conversion from mild cognitive impairment to dementia (hazard ratio [HR] 0.64 [95% CI 0.44–0.94]; p=0.02), higher CSF Aβ levels, and lower Aβ PET burden; the results were significant specifically in the group of individuals aged 60–80 years4. A whole genome sequencing on a mainland Chinese cohort identified nine potential causal variants in two genes located in the vicinity of the APOE, PVRL2 and APOC15, which increased the risk of developing Alzheimer’s disease independently of the APOE ε4 allele. The risk haplotypes associated with these variants correlated with some Alzheimer’s disease endophenotypes such as worse cognition, more severe hippocampal atrophy, lower plasma Aβ levels, and higher brain APOE mRNA levels. Another analysis of whole genome sequencing data stratified by APOE genotype identified three genes significantly associated with Alzheimer’s disease in APOE ε4 carriers only: OR8G5 (p=4.67×10−7), IGHV3–7 (p=9.75×10−16), and SLC24A3 (p=2.67×10−12)6. Conversely, a systematic review investigating the genetic basis of resilience to Alzheimer’s disease among APOE ε4 homozygotes revealed that CASP7 (encoding caspase 7) rs10553596 and SERPINA3 (encoding α1-antichymotrypsin) rs4934-A/A polymorphisms possibly reduce Alzheimer’s disease risk7.
Influence of race in APOE-linked Alzheimer’s disease risk
An interaction between race and the APOE genotype on Alzheimer’s disease risk has long been known, with African American and Hispanic APOE ε4 carriers having lower risk than white APOE ε4 carriers, and Asian (i.e., Japanese) carriers having the highest ORs.8–10 Studies have found that the local ancestry of APOE (i.e., the population-specific genetic variation within the APOE region), rather than global ancestry (i.e., the population-specific genetic variation in the entire genome) or environmental factors, explains these inter-racial differences in Alzheimer’s disease risk. Specifically, an African local ancestry region surrounding APOE underlies the smaller APOE ε4 allele effect on Alzheimer’s disease risk observed in African American and Caribbean Hispanic (from Puerto Rico) populations11,12. Another study of 809 individuals identified a potentially protective African ancestral haplotype within APOE defined by the rs769449 SNP13, but this was not been confirmed in a larger (7,997 individuals) study14.
NEW PATHOLOGICAL CORRELATES OF APOE GENOTYPE
The classic post-mortem neuropathological correlates of the APOE genotype are a higher Aβ plaque burden and more severe cerebral amyloid angiopathy in APOE ε4 carriers, and a lower Aβ plaque burden in APOE ε2 carriers, relative to APOE ε3 homozygotes15. These differential effects of APOE alleles have been confirmed by Aβ PET imaging across preclinical and clinical stages of Alzheimer’s disease (i.e., mild cognitive impairment and mild-to-moderate dementia)16,17. The APOE ε4 allele has also been associated with more severe tau pathology as defined by Braak neurofibrillary tangle stages2,18, and the APOE ε2 allele with a lower Braak neurofibrillary tangle stages15, independently of their effects on Aβ plaques. Cross-sectional data on tau PET imaging examining APOE effects on tau radioligand uptake after controlling for Aβ radioligand uptake are conflicting19,20, but longitudinal combined tau and Aβ PET studies will elucidate this important question.
The APOE genotype can also impact the finding of comorbid brain pathologies at autopsy. On one hand, APOE ε4 partly drives (together with aging) the presence of Aβ plaques and neurofibrillary tangles in individuals with other primary neuropathological diagnoses such as amyotrophic lateral sclerosis, primary tauopathies, and Lewy body diseases21. On the other hand, in individuals with Alzheimer’s disease the APOE ε4 allele appears to to correlate with the presence and severity of TDP-43 pathology18,22, Lewy body diseases23, and possibly cerebrovascular disease24, independently of its effects on Aβ plaques and neurofibrillary tangles. Lastly, APOE could be a genetic risk factor for neurodegenerative diseases other than Alzheimer’s disease. Indeed, APOE ε4 has been associated with Lewy body diseases, independently of the Aβ plaque and neurofibrillary tangle burdens25,26 (but see also27). Of note, APOE ε4 has been associated with an earlier age of symptom onset in patients with MAPT-linked or autopsy-proven frontotemporal lobar degeneration-tau independently of its effects on Aβ plaque burden28, and with more severe neurodegeneration at post-mortem examination in primary tauopathies29. Paradoxically, APOE ε2 might increase the risk of progressive supranuclear palsy30, but results are conflicting23. The validation and expansion of PET imaging and CSF biomarkers for other neurodegenerative diseases will help confirm these correlations between APOE genotype and non-Alzheimer’s pathologies.
APOE PATHOPHYSIOLOGICAL MECHANISMS
Although traditionally the APOE ε4 allele was represented as a trigger of Aβ accumulation at the top of the sporadic Alzheimer’s disease amyloid cascade, numerous new data show that the APOE alleles have differential downstream effects in many other pathophysiological processes beyond Aβ metabolism (Figure).
Figure. Multifaceted effects of APOE in the brain and potential strategies to decrease APOE4 and increase APOE2 levels.
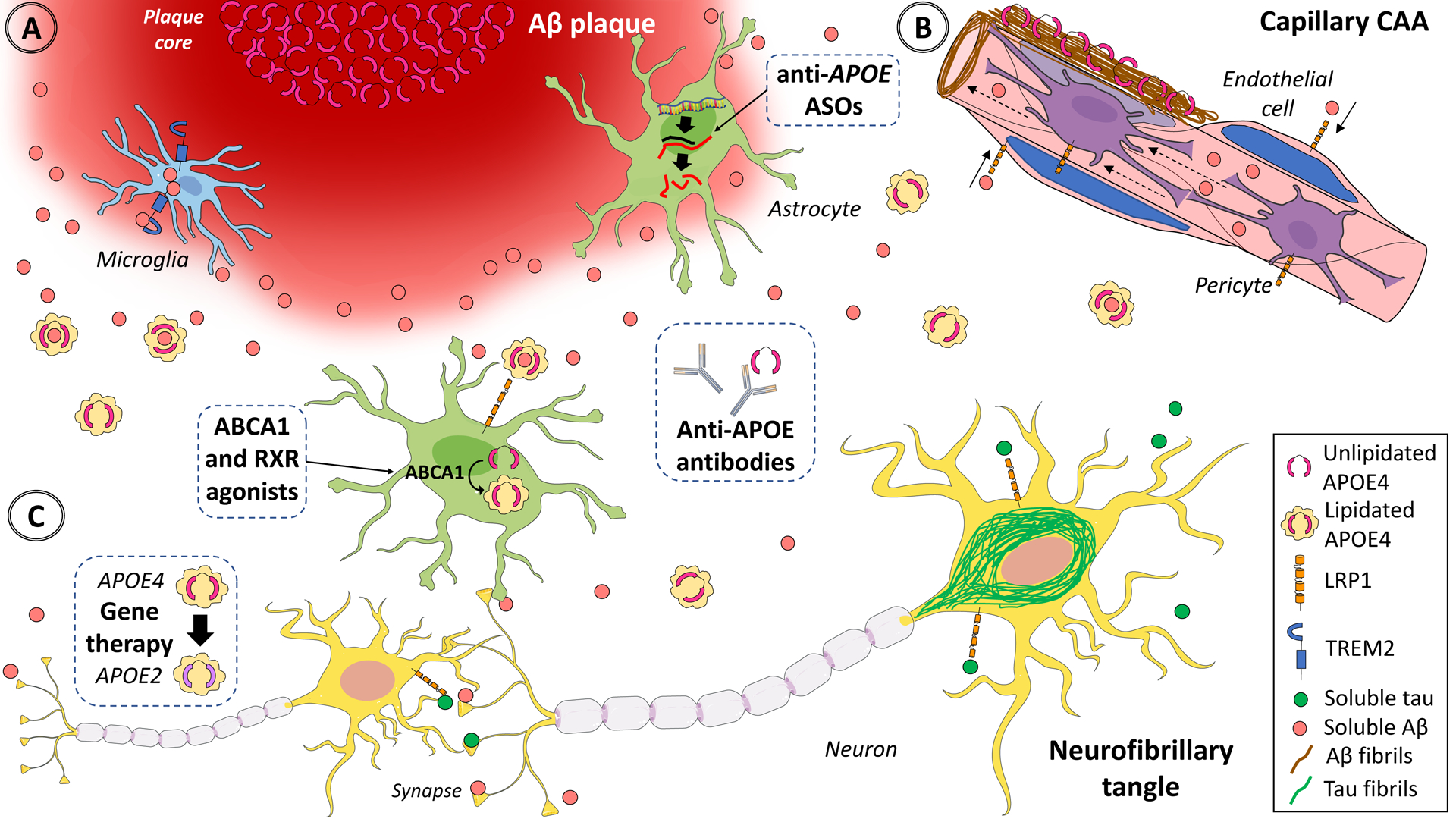
In the healthy brain, APOE is expressed and secreted predominantly by astrocytes, and to a lesser extent by microglia. Most brain APOE is lipidated by the ATP-binding cassettes A1 (ABCA1) and G1 (ABCG1) and lipidated APOE is internalized via APOE receptors such low-density lipoprotein receptor-related protein 1 (LRP1), which is expressed in astrocytes, neurons, vascular smooth muscle cells, endothelial cells, and pericytes. In the Alzheimer’s disease brain, astrocytes and microglia react to (A) dense-core Aβ plaques63, (B) cerebral amyloid angiopathy-laden arteries and capillaries, and (C) neurofibrillary tangles, activating transcriptional programmes that include APOE mRNA up-regulation in microglia35,37 and down-regulation in astrocytes35,36 and lead to altered lipid metabolism (not shown). APOE directly interacts with both soluble and fibrillar Aβ. Relative to APOE3 and APOE2, APOE4 promotes Aβ seeding and aggregation in oligomers and fibrils46–48 and reduces its clearance from the interstitial fluid45, potentially leading to Aβ deposition as dense-core (Thioflavin-S positive) amyloid plaques and cerebral amyloid angiopathy together with APOE39. This evidence suggests that decreasing APOE (especially APOE4) expression or blocking the effects of APOE4 or enhancing the effects APOE2 would be beneficial (dashed boxes). Experimental approaches to achieving these outcomes include lowering APOE4 levels with isoform-specific antisense oligonucleotides56 or antibodies89–91, which could also target lipid-poor APOE associated with plaques91. Alternatively, APOE4 could be switched to APOE3 or APOE265, or APOE2 could be added79,99, with gene therapy. Last, APOE lipidation could be enhanced with RXR82–84 and ABCA1 or ABCG185 agonists to improve APOE4 receptor-mediated internalization and lower Aβ in the interstitial fluid. Dashed boxes illustrate the most promising therapeutic approaches. ASO=antisense oligonucleotides. Aβ=amyloid-β peptide. CAA=cerebral amyloid angiopathy. TREM2=triggering receptor expressed in myeloid cells 2. RXR=retinoid X receptor. Adapted from Servier Medical Art by Servier (https://smart.servier.com/category/medical-specialties/neurology/), which is licensed under a Creative Commons Attribution 3.0 Unported License (CC BY 3.0).
Cellular sources of APOE in brains with and without Alzheimer’s disease
Brain and peripheral pools of APOE are independent from each other because liver transplantation changes APOE isoforms towards the donor’s in the recipient’s blood but not CSF31, and depleting APOE from hepatocytes —its main source cell type in the periphery— does not affect brain APOE (or Aβ) levels in mice32. Understanding the cell types expressing APOE in the brain is relevant because APOE is a secreted glycoprotein and could have autocrine effects on the secreting cell, but also paracrine effects on neighbouring cells. Although astrocytes are the main source of APOE in the normal brain, in the Alzheimer’s disease brain reactive astrocytes around Aβ plaques were reported to be devoid of APOE, whereas Aβ plaque-associated microglia express high levels of APOE33 (Figure). Single nuclei RNA-sequencing studies in human Alzheimer’s disease and control brains have confirmed a down-regulation of APOE expression in reactive astrocytes35,36 and an up-regulation in activated microglia35–37. Neuropathological studies also reported APOE staining in pyramidal neurons in neurodegenerating areas such as the hippocampus, but rare colocalisation between APOE and tangles, suggesting little direct interaction between APOE and tau34. The presence of APOE in pyramidal neurons suggested the internalization of APOE lipoparticles from the interstitial space through the APOE receptor LRP1 (Panel 1, Figure), which is highly expressed in neurons among other cell types. Expression of APOE has also been shown in vascular cells from the human brain, specifically pericytes38.
Effects on Aβ
The disparate impact of APOE isoforms on Alzheimer’s disease risk was attributed to a differential effect — deleterious for APOE4 and protective for APOE2, with respect to APOE3)— on both Aβ plaque burden and cerebral amyloid angiopathy severity15. These well-established autopsy neuropathological correlates of APOE alleles and the early observation that compact (dense-core, fibrillar, Thioflavin-S-positive), but not diffuse (amorphous, Thioflavin-S-negative), Aβ plaques contain APOE39, supported the idea that APOE interacts with Aβ and promotes its aggregation and deposition in insoluble fibrillar deposits (Figure). Indeed, genetic deletion and haploinsufficiency of APOE reduces dense-core Aβ plaque burden in various mouse models of cerebral β-amyloidosis40–42. Of note, APOE deficiency inhibits diffuse Aβ deposits in some of these models40 but increases them in others42, further reinforcing the requirement of APOE for plaque compaction. When these Aβ-plaque depositing mice were crossed with APOE targeted replacement mice expressing human APOE alleles in place of the murine Apoe coding sequence (knock-in mice; Panel 1), APOE4 knock-in mice consistently exhibited higher Aβ plaque burden than did APOE3 knock-in mice, and these APOE3 knock-in mice had higher Aβ plaque burden than did APOE2 knock-in mice43–45, thus recapitulating the allele-specific differences observed in human post-mortem autopsy and Aβ PET studies.
In-vitro and in-vivo studies have shown that, relative to APOE2 and APOE3, APOE4 promotes the seeding of Aβ peptide into Aβ oligomers, protofibrils, and fibrils46–48, but also inhibits Aβ clearance from the brain prolonging its half-life in the interstitial fluid45,48 and inhibiting its enzymatic degradation49. The intimate mechanism underlying these APOE isoform-driven differences in Aβ metabolism remains debated. On one hand, it has been proposed that APOE and Aβ direct interaction in the brain extracellular space might be negligible in physiological conditions (i.e., Aβ monomers and lipidated APOE), but that both APOE and Aβ can compete for the same receptors, namely LRP150, which is involved in Aβ clearance by neurons51, astrocytes52, endothelial cells, vascular smooth muscle cells53, and pericytes54. On the other hand, there is evidence supporting a direct interaction between APOE and oligomeric and fibrillar Aβ. First, APOE colocalises with synaptotoxic Aβ oligomers at the synapses in the vicinity of Aβ plaques and leads to synapse loss in an isoform-dependent manner (APOE4 more than APOE3)55. Second, in-vivo experiments in which APOE expression by astrocytes was conditionally deleted, or APOE expression globally silenced with antisense oligonucleotides at different stages of Aβ deposition, have shown that APOE influences Aβ plaque burden mainly during the seeding phase of Aβ aggregation, but has a lesser effect during the exponential growth phase (i.e., when fibrillar Aβ deposits are already formed)48,56. Third, a subtle difference in tertiary conformation across APOE isoforms (i.e., closer N-terminus and C-terminus in APOE4 versus APOE3 and more open in APOE2) could affect both the affinity of the Aβ and APOE interaction (higher for APOE4 vs APOE3 and APOE2) and the APOE propensity to enzymatic cleavage in its hinge region between the N-terminus and the C-terminus, rendering presumably toxic C-terminal fragments (also higher for APOE4 vs APOE3 and APOE2)57–59.
Effects on tau
Unlike Aβ, there is little overlap between APOE-immunoreactive neurons and neurons that have neurofibrillary tangles34. No direct interaction between APOE (primarily secreted) and the microtubule-associated protein tau (primarily intraneuronal and axonal) has been shown in vivo30. However, studies in transgenic Apoe knockout or APOE knock-in mice overexpressing the P301S mutant of tau have shown that the human APOE isoforms do affect tau downstream pathology29,60. Specifically, APOE4 promotes tau-induced neurodegeneration and atrophy compared to APOE3, whereas APOE2 is protective with respect to these outcomes29. The mechanism underlying these effects is indirect, mediated by APOE effects on microglia, rather than a direct interaction between APOE and tau; transcriptome profiling and cytokine measures indicate that APOE4 microglia is primed towards a proinflammatory phenotype compared to APOE3, whereas APOE2 exhibits a more homeostatic phenotype29,60. Of note, LRP1 has been recently shown to be a receptor for tau uptake by neurons, and APOE affects the ability of tau to bind LRP1 (Figure), although in vitro all APOE isoforms reduced tau uptake to a similar extent61. Additionally, knocking down neuronal LRP1 reduced neuronal tau spreading in mice, but some astrocytes took up the human tau61. Whether different receptors have a role in tau uptake into different cell types remains unknown. Moreover, since LRP1 is a recycling receptor delivered into endosomal/lysosomal compartments, it remains unclear how tau escapes to the cytoplasm to interact with endogenous tau in neurons or to accumulate as glial fibrillary tangles in astrocytes, but it is plausible that APOE affects tau intracellular trafficking in an isoform-dependent manner62.
Effects on glia
Astrocytes and microglia are known to react to plaques, neurofibrillary tangles, and neurodegeneration. Although quantitation of reactive (GFAP+) astrocytes and activated (IBA1+, CD68+) microglia per Aβ plaque in post-mortem sections of the temporal neocortex has shown no difference between APOE ε4 carriers and non-carriers63, transcriptomic studies have reported that APOE influences glia reactions. Microglia from APOE4 knock-in mice is primed towards a proinflammatory response compared with those from APOE3 knock-in mice64 and APOE ε4 microglia derived from human-induced pluripotent stem cells exhibit a proinflammatory gene expression programme and impaired Aβ phagocytosis relative to APOE ε3 microglia65. These APOE-mediated differential effects on microglia phenotype appear to be at least partially mediated by the triggering receptor expressed on myeloid cells 2 (TREM2), which is another receptor for both Aβ and APOE expressed by microglia66. Loss of function mutations in TREM2 (e.g., R47H, R62H) have been associated with a 2–3 times increased risk of developing Alzheimer’s disease and with less compact Aβ plaques that have more neuritic dystrophies, less coverage by microglia, and less APOE content67. The Aβ plaque features of Alzheimer’s disease mouse models deficient in TREM2 or APOE are phenocopies42,67, suggesting that APOE and TREM2 are both involved in chemotaxis of microglia towards plaques and that plaque-associated microglia has a neuroprotective role minimising neuritic dystrophies. The transcriptomic changes associated with the conversion from homeostatic to Alzheimer’s disease microglia require both APOE and TREM2 because genetic deletion of either in Alzheimer’s disease transgenic mice precludes such transition68,69, and TREM2 loss of function mutations partially abrogate the microglia transcriptomic changes observed in the brains of patients with Alzheimer’s disease37. Regarding APOE effects on astrocytes, APOE ε4 astrocytes derived from human-induced pluripotent stem cells exhibit impaired cholesterol metabolism and Aβ phagocytosis65, reduced neurotrophic support70, and impaired synaptic pruning71, relative to APOE ε3 and APOE ε2 astrocytes.
Effects on blood brain barrier
Another area of growing interest is the effects of APOE ε4 allele on the blood-brain barrier. Traditionally, APOE ε4 had been associated with a more severe cerebral amyloid angiopathy15 (Figure), resulting in a higher risk of lobar intracerebral haemorrhage, but also focal subarachnoid haemorrhage and cortical superficial siderosis, cortical microinfarcts, and white matter ischemic changes. APOE ε4 effects on the blood-brain barrier were also shown in the first randomised clinical trials with anti-Aβ monoclonal antibodies, which reported a higher incidence of MRI findings (brain oedema, microbleeds, and cortical superficial siderosis) in the treatment versus placebo groups —collectively termed amyloid-related imaging abnormalities. Only occasionally symptomatic (e.g., headaches, confusion, and seizures), these amyloid-related imaging abnormalities are indicative of an increased blood-brain barrier permeability presumably caused by the antibody-mediated Aβ efflux from the brain parenchyma into the bloodstream72. Because amyloid-related imaging abnormalities are twice as probable in APOE ε4 carriers, their occurrence has been attributed to a more severe pre-existing cerebral amyloid angiopathy in APOE ε4 carriers vs APOE ε3 homozygotes72. Supporting this interpretation, immunotherapy with an anti-Aβ monoclonal antibody is associated with higher numbers of cerebral microbleeds in APPswePSEN1dE9 × APOE4 knock-in mice versus APOE3 knock-in and APOE2 knock-in mice73. A post-mortem quantitative neuropathological study on individuals who participated in a phase 2 anti-Aβ active immunotherapy trial (NCT00021723) also showed that Aβ plaque clearance is associated with a redistribution of Aβ and APOE from plaques to vessels, and more severe cerebral amyloid-angiopathy-related vasculopathic changes74. Pericytes, which are another cellular source of APOE, are gaining attention for their implication in cerebral amyloid angiopathy pathogenesis (Figure). Pericyte loss resulted in increased cerebral amyloid angiopathy and Aβ plaques in a mouse model of Aβ deposition75. Pericytes take up Aβ via LRP1 in an APOE isoform-dependent manner, with APOE4 interfering with this uptake compared to APOE354. Human APOE ε4 pericytes express higher levels of APOE mRNA and protein than APOE ε3 pericytes, resulting in increased Aβ vascular accumulation38. Pericytes exposed to Aβ oligomers constrict capillaries via endothelin-1 receptor ETA activation, leading to reduced blood flow76.
APOE4 can also increase blood-brain barrier permeability with respect to APOE3 in an Aβ-independent manner, as shown in APOE4 versus APOE3 knock-in mice77 and confirmed with dynamic contrast-enhanced MRI in the medial temporal lobe of cognitively healthy (clinical dementia rating score 0) and mildly impaired (clinical dementia rating score 0.5) APOE ε4 carriers versus APOE ε3 homozygotes24. The underlying proposed mechanisms include the activation of cyclophilin A, resulting in increased levels of MMP9 and pericyte injury78, and disruption of the capillary basement membrane (i.e., collagen IV)77.
APOE-BASED THERAPEUTIC OPPORTUNITIES
Experimental in-vivo studies in Alzheimer’s disease mouse models that have a human APOE knock-in background have suggested promising approaches to ameliorate phenotypes related to Alzheimer’s disease (Table 1). However, there are only a few APOE-directed clinical trials completed or underway (Table 2), highlighting a lag in therapeutic translation for this target.
Table 1.
APOE-directed therapeutic approaches tested in Alzheimer’s disease mouse models.
| Rationale | Treatment | Route | Mouse model | Age | Duration | Results | Comments / Limitations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Increasing APOE levels | Bexarotene | p.o. | APPswe/PSEN1dE9 | 6 & 11 mo | 3, 7, 14 & 90 d | Increased APOE, ABCA1, ABCG1, and HDL; reduced s/i-Aβ and Aβ plaques; improved memory |
Subsequent mouse studies by other groups yielded mixed results on the efficacy of bexarotene on Aβ phenotypes | Cramer et al. 201282 |
| APPPS1–21 | 7–8 mo | 20 d | Increased APOE, ABCA1, ABCG1, and HDL; reduced s/i-Aβ and Aβ plaques; improved memory |
|||||
| Tg2576 | 12–14 mo | 3 & 9 d | Improved social and olfaction/circuit connectivity | |||||
| CS-6253 | i.p. | APOE3 knock-in & APOE4-knock-in | 2.5 mo | 6 wks | APOE4 knock-in only: reduced Aβ and p-tau; increased ABCA1, APOE lipidation, APOER2, VGLUT1, and memory | ABCA1 agonist derived from APOE C-terminus, target engagement shown by CS-6253+ astrocytes in IHC | Boehm-Cagan et al. 201485 | |
| Probucol | p.o. | Wt male rats | 26 mo | 30 d | Increased hipp APOE, Apoe mRNA, HMGCoAR, LRP, and SNAP25; = chol, LDLR, and SYP; reduced GFAP | Aged Wt rats used as a model of normal cognitive aging | Champagne et al. 200387 | |
| i.p. | Wt mice, i.c.v. aggregated Aβ1–40 | 90 d | 2 wks | Reduced plasma chol; improved memory and synapses | Acute i.c.v. injection of Aβ in Wt mice does not model well the AD scenario of chronic Aβ deposition | Santos et al. 201286 | ||
| APOE mimetics | COG1410 | s.c. | SwDI-APP/NOS2−/− | 9 mo | 3 mo | Reduced Aβ plaques, p-tau, and Il6 mRNA; improved memory | SwDI-APP mice lacking NOS2 exhibit endogenous tau pathology and neuron loss besides Aβ plaque | Vitek et al. 201294 |
| COG112 | i.p. | AICD Tg (FeCγ25 line) | 1 mo | 3 mo | Reduced p-tau and CD45+/IBA1+ mg; improved neurogenesis | APP intracellular domain (AICD)-overexpressing mice exhibit microglial activation but no Aβ plaques | Ghosal et al. 201395 | |
| CN-105 | s.c. | APPPS1–21 × APOE4 knock-in | 14–18 wks | 40 d | Reduced s-Aβ and Aβ plaques; improved memory | Greater benefits in young mice (e.g. improved fear conditioning but not spatial memory in older mice) | Krishnamurthy et al. 202093 | |
| 25–28 wks | 40 d | = s-Aβ; slightly improved memory | ||||||
| Blocking APOE-Aβ interaction | HJ6.3 anti-APOE Ab | i.p. | APPswe/PSEN1dE9 | 4 mo | 14 wks | Reduced s/i-Aβ, Aβ plaques, IFN-γ, and IL-1α; increased CD45+ mg |
Preventative use prior to Aβ plaques very effective, microglial activity modulation suggested | Kim et al. 201289 |
| 7 mo | 21 wks | Reduced s/i-Aβ, Aβ plaques, brain APOE, CD45+ mg; increased plasma Aβ; improved memory and connectivity; = CAA, plasma APOE, and plasma chol |
Therapeutic use after Aβ plaque deposition also effective due to inhibition of plaque formation and growth plus removal of existing plaques | Liao et al. 201490 | ||||
| HAE-4 anti-APOE Ab | i.c.v. | APPPS1–21 × APOE4 knock-in | 2 mo | 6 wks | i.c.v.: reduced Aβ plaques; = i-Aβ | Additionally, AAV2/8-mediated expression of HAE-4 and HAE-1 anti-APOE Abs via i.c.v. injection at birth reduced Aβ plaque and i-Aβ at age 3.5-mo in an Fc-dependent manner, implicating FcγR1 in microglia | Liao et al. 201891 | |
| i.p.: reduced i-Aβ and Aβ plaques; = brain s/i-APOE, plasma APOE, and plasma Aβ | ||||||||
| Aβ12–28P | i.p. | APPswe/PSEN1dE9 × APOE2 knock-in APPswe/PSEN1dE9 × APOE4 knock-in | 6 mo | 4 mo | Reduced s/i-Aβ, s/i-APOE, Aβ plaques, DNs, and serum chol in both mice; improved memory (only E4 mice); = serum APOE | Greater cognitive benefit in E4 mice because only vehicle-treated APPswe/PSEN1dE9 × APOE4 knock-in mice (but not APPswe/PSEN1dE9 × APOE2 knock-in mice) exhibited impaired cognition | Pankiewicz et al. 201492 | |
| Silencing APOE | Anti-APOE ASOs | i.p. |
APPPS1–21 × APOE3 knock-in APPPS1–21 × APOE4 knock-in |
Birth (P0) | 16 wks | Reduced s/i-Aβ, Aβ plaques, DNs, and s-APOE; = i-APOE |
Reduced Aβ plaque burden when treated at birth but no change or increased when treated at 6 wks suggests preventative rather than therapeutic use | Hyunh et al. 201756 |
| i.c.v. | 6 wks | 10 wks | Reduced s-APOE and DNs; = s/i-Aβ, i-APOE, and CD45+ mg; = or increased Aβ plaques |
|||||
| Switching APOE4 to APOE2 |
AAV4-APOE2/3/4 | i.vent. | APPswe/PSEN1dE9 | 7 mo | 2 mo & 5 mo |
AAV4-APOE2 vs E3: reduced s/i Aβ, Aβ plaques, and DNs; = plasma Aβ; increased synapses | AAV4-mediated expression of APOE2 mainly in choroid plexus and ependymal cells via i.vent. injection can improve Aβ measures in aged mice after Aβ plaque deposition | Hudry et al. 201399 |
| AAV4-APOE4 vs E3: increased s/i Aβ, Aβ plaques, DNs, and plasma Aβ; reduced synapses | ||||||||
| i.vent. | Tg2576 | 16–18 mo | 3 mo | AAV4-APOE2 vs E3: reduced ISF Aβ and o-Aβ; = i-Aβ | ||||
| AAV4-APOE4 vs E3: increased ISF Aβ, o- Aβ and i-Aβ | ||||||||
| AAV8-GFAP-APOE2/3/4 | i.c.v. |
APOE3 knock-in APOE4 knock-in |
Birth (P2) | 3 mo | AAV8-GFAP-APOE2 vs E3: increased APOE and APOE lipidation; reduced ms Aβ40 (trend in APOE4 knock-in only); = APP-FL, APP-CTFs, ABCA1, ABCG1, Apoe mRNA | Results suggest that APOE4 is less lipidated and stable than APOE2, and that exogenous expression of APOE4 in APOE4 carriers could be deleterious by increasing endogenous Aβ, whereas expression of APOE2 in APOE4 carriers would have opposing beneficial effects | Hu et al. 201579 | |
| AAV8-GFAP-APOE4 vs E3: increased ms Aβ40 (APOE4 knock-in only); reduced APOE lipidation; = APP-FL, APP-CTFs, ABCA1, ABCG1, APOE and Apoe mRNA |
Abbreviations (excepting those shown in the main text): Ab = antibody; AICD = amyloid-β precursor protein (APP) intracellular domain; APP = amyloid-β precursor protein; APP-CTFs = APP C-terminal fragments; APP-FL = APP full length; APPPS1–21 = Swedish APP mutation (K670N/M671L) and presenilin-1 L166P mutation; APPswe = Swedish APP mutation (K670N/M671L); APPswePSEN1dE9 = Swedish APP mutation (K670N/M671L) and presenilin-1 exon 9 deletion; chol = cholesterol; d = days; DNs = dystrophic neurites; Fc = constant fraction of immunoglobulin/antibody; FcγR1 = Fc gamma receptor-1; GFAP = glial fibrillary acidic protein; HMGCoAR = 3-hydroxy-3-methylglutaryl coenzyme A reductase; HDL = high density lipoprotein; i = insoluble; i.c.v. = intracerebroventricular; IBA1 = ionized calcium binding adapter molecule-1; IFN-γ = interferon-γ; IL-1α = interleukin-1α; ll6 = interleukin-6 mRNA; i.p. = intraperitoneal; i.vent. = intraventricular; LRP1 = low density lipoprotein related protein-1; mg = microglia; mo = months; mRNA= messenger RNA; ms = mouse; NOS2 = nitric oxide synthetase 2; o = oligomeric; p.o. = per os (orally); p-tau = phospho-tau; s = soluble; s.c. = subcutaneous; s/i = soluble and insoluble; SNAP25 = synaptosome associated protein-25; SwDI-APP = Swedish (K670N/M671L), Dutch (E693Q) and Iowa (D694N) APP mutation; SYP = synaptophysin; Tg2576 = Transgenic Swedish (K670N/M671L) APP mutation overexpressing mice; VGLUT1 = vesicular glutamate transporter-1; wks = weeks; Wt = wild-type.
Table 2.
Early development of APOE-directed therapeutic approaches in human clinical trials.
| Drug | Rationale | ID | Status | Design | Phase | Subjects | Dose/route/ duration |
Primary outcome | Secondary outcomes | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bexarotene | Increase APOE levels | NCT02061878 | Completed | Randomised, double-blind, placebo-controlled | 1b | Healthy young (aged 21–49) APOE ε3/ε3 volunteers | 225 mg BID PO × 5 days | Newly generated Aβ in CSF (SILK) | Fractional clearance rate of Aβ from CNS (SILK) | Poor bexarotene brain penetration (not detectable in >95% CSF samples), 25% increase in CSF APOE levels, marginally significant increase in newly synthesized APOE, no change in Aβ synthesis/clearance or levels |
Ghosal et al. 201683 |
| Bexarotene | Increase APOE levels | NCT01782742 | Completed | Randomized double blind placebo-controlled | 2 | Moderate AD (MMSE 10–20) with positive baseline amyloid PET scan | 150 mg BID PO × 4 weeks | Aβ burden in amyloid PET imaging | Cognition (MMSE, ADAS-Cog, CDR), behavior (NPI), ADL, serum Aβ40/42 | Significant reduction in Aβ burden only in APOE ε4 non-carriers which correlated with increased serum Aβ42 levels, increased triglyceride plasma level in bexarotene group, no efficacy in clinical outcomes | Cummings et al. 201684 |
| Probucol | Increase APOE levels | NCT02707458 | Completed | Open label, dose finding | 1 & 2 | Cognitively intact at risk of AD by family history | Initial 600 mg QD PO, then individualized, 1 year of follow-up | Plasma probucol and CSF and plasma APOE levels | N.A. | N.A. | n.a. |
| CN-105 | APOE mimetic | NCT03802396 | Recruiting | Randomized double blind placebo-controlled | 2 | ≥60 year-old undergoing major surgery | 0.1 mg/kg vs 0.5 mg/kg vs 1 mg/kg IV Q6H × 4 days and 6 weeks of follow-up | Safety | CSF cytokine levels, change in cognition, post-operative delirium | N.A. | n.a. |
| Gene therapy (AAVrh.10hPOE2 vector) | Switch APOE4 to APOE2 | NCT03634007 | Recruiting | Open label, dose ranging | 1 | Symptomatic (any stage), APOE ε4/ε4, positive CSF biomarkers or amyloid PET scan | 8×1010 GC/kg vs 2.5×1011 GC/kg vs 8×1011 GC/kg, single intracisternal, injection and 2 years of follow-up | Safety | Maximum dose tolerated | N.A. | n.a. |
AAV = adeno-associated virus; AD = Alzheimer’s disease; ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive subscale; ADL = Activities of Daily Living; BID = bis in die (twice daily); CSF = cerebrospinal fluid; GC = genome copies; IV = intravenously; MMSE = Mini Mental State Examination; N.A. = not available. NPI = neuropsychiatric inventory; PET = positron emission tomography; PO = per os (orally); Q6H = quaque sexta hora (every 6 hours); QD = quaque die (once daily); SILK = stable isotope labelling kinetics.
Increasing APOE levels and its lipidation
Because brain APOE4 is less lipidated and stable than APOE3 and APOE257,79, increasing brain APOE levels and lipidation has been proposed as a therapeutic approach. Genetic deletion of ABCA1 results in poor APOE lipidation and increased Aβ plaque burden80, whereas ABCA1 overexpression reduces Aβ deposition81. ABCA1 and ABCG1 expression is induced by the stimulation of the retinoid X receptor. Bexarotene is a US Food and Drug Administration approved retinoid X receptor agonist for use in cutaneous T-cell lymphoma and was reported to cause a rapid reduction of Aβ plaque burden and restoration of cognitive functioning in Alzheimer’s disease mouse models by inducing ABCA1 and ABCG1 expression, enhancing APOE lipidation, and increasing APOE levels (Table 1)82. This result was, at least partly, replicated by some investigators but not others, and led to examine bexarotene for Alzheimer’s disease in human clinical trials. A phase 1b proof-of-mechanism trial in young (21–49 years) volunteers revealed poor penetration of bexarotene in the CNS according to its plasma versus CSF levels. Bexarotene was able to increase CSF APOE levels by 25% although it had no effects on CSF Aβ levels as measured by stable isotope labelling kinetics (Table 2)83. In a proof of concept, double-blind, placebo-controlled clinical trial in 20 patients with moderate Alzheimer’s disease (Mini Mental State Examination range was 10–20; Table 2), bexarotene 150 mg twice daily for 4 weeks was associated with a significant reduction in Aβ PET burden and a proportional increase in serum Aβ42 levels but, contrary to the prediction, only in APOE ε4 non-carriers84.
A non-toxic small peptide derived from the C-terminus of APOE, CS-6253, has been shown to increase ABCA1 levels and, subsequently, APOE lipidation without changing brain APOE levels, in APOE4 but not APOE3 knock-in mice. These effects correlated with a reduction of hippocampal Aβ and phosphorylated tau and improved learning and memory in APOE4 knock-in mice85.
Probucol, the now abandoned non-statin lipid-lowering drug, has been shown to counteract hippocampal synaptic loss and cognitive impairment in Aβ-injected wild-type mice86, increase APOE and LRP1 levels in the hippocampus of aged rats87 and, while results from a phase 1/2 clinical trial (NCT02707458) are awaited, might also increase CSF APOE levels in humans (Table 2)88.
Blocking APOE and Aβ interaction
Another therapeutic strategy is to interfere with the APOE and Aβ interaction, because this is thought to stabilise toxic oligomeric and fibrillar Aβ species existing within and around Aβ plaques46,55,47,48. This strategy has been achieved in Alzheimer’s disease mouse models with both monoclonal anti-APOE antibodies and small molecules that act as Aβ mimetics.
Chronic intraperitoneal administration of an anti-APOE monoclonal antibody (HJ6.3) to APPswePSEN1dE9 mice led to a statistically significant reduction of insoluble Aβ levels and Aβ plaque burden, and APOE levels in the brain, which correlated with improved learning and memory and higher cortical network connectivity in the resting state. While the Aβ plaque reduction was larger when administered before plaque deposition, in older mice with substantial plaque deposition this antibody appears to prevent the formation of new plaques and clear the smallest previously existing plaques by binding APOE within them. Of note, systemic treatment with this anti-APOE antibody increased plasma Aβ levels, but did not have systemic (i.e., unchanged plasma cholesterol and APOE levels) or local (i.e., cerebral amyloid angiopathy did not worsen) adverse side effects89,90. Another anti-APOE monoclonal antibody specific for non-lipidated APOE (HAE-4) reduced the plaque burden in APPPS1–21 × APOE4 knock-in mice through microglia-mediated clearance, without affecting the levels of plasma APOE, which is mostly lipidated91. Therefore, anti-APOE immunotherapy has promise for testing in future trials.
Aβ12–28P, a small peptide corresponding to the APOE-binding motif within Aβ except for a Val18Prol substitution, reduced soluble and insoluble Aβ levels and Aβ plaque burden in APPswePSEN1dE9 × APOE3 knock-in and APPswePSEN1dE9 × APOE4 knock-in mice and improved memory deficits in APPswePSEN1dE9 × APOE4 knock-in mice. Moreover, Aβ12–28P reduced soluble and insoluble APOE levels and the deposition of APOE into Aβ plaques. Of note, this improvement was not due to an active immunisation effect, because these mice did not generate antibodies against this Aβ fragment92. Aβ12–28P efficacy remains to be tested in clinical trials.
APOE mimetics
Another approach is to use APOE N-terminal fragments including its receptor-binding motif, so called APOE mimetics. Chronic subcutaneous administration of CN-105, a pentapeptide corresponding to the receptor binding face of APOE, reduced both soluble Aβ and Aβ plaque burden and improved cognition in APP1–21 × APOE4 knock-in mice before plaque deposition, but not after this93. CN-105 is being tested to prevent delirium after major surgery in a phase 2 clinical trial (NCT03802396; Table 2). APOE mimetics spanning the APOE receptor binding motif such as COG1410 (12 amino acids) and COG112 (34 amino acids) have been shown to ameliorate Aβ levels and Aβ plaque burden, tau hyperphosphorylation, and neuroinflammation in various Alzheimer’s disease mouse models94,95, but have not been tested in human clinical trials.
Lowering APOE levels
Lowering brain APOE levels has also been proposed as a therapy because Apoe genetic deletion or haploinsufficiency reduces Aβ deposition in mouse models of cerebral β-amyloidosis40–42 and rescues neurodegeneration induced by tau in tauopathy mouse models29. Additionally, null mutations in the APOE gene do not seem to have adverse effects on cognition in humans, although they are associated with familial dyslipoproteinemia (also known as type III hyperlipoproteinemia)96. One way of reducing brain APOE levels is increasing the expression of its receptors. Over-expression of LDLR reduced Aβ plaque deposition97 due to increased efflux of Aβ from the brain through the blood-brain barrier98. A more specific approach is to silence APOE expression with specific antisense oligonucleotides. In APPPS1–21 × APOE3 knock-in mice and APPPS1–21 × APOE4 knock-in mice, a reduction in soluble APOE levels by half with anti-APOE antisense oligonucleotides resulted in lower soluble and insoluble Aβ levels and lower total and dense-core plaque burden when administered intracerebroventricularly at birth, but did not change much these Aβ measures when applied at the onset of Aβ plaque deposition (i.e., 6 weeks in this mouse model)56. However, both treatments resulted in fewer plaque-associated dystrophic neurites, suggesting less neuronal toxicity of existing plaques and some beneficial effect of APOE reduction on microglia and astrocyte responses to plaques, and lending support for testing in patients with Alzheimer’s disease in clinical trials.
Genetic switch of APOE isoforms
Gene therapy has become a reality in several diseases, including neurodegenerative diseases such as spinal muscular atrophy. The application of CRISPR-Cas9 editing technology to switch APOE alleles has been successful in a dish with neurons and glial cells derived from human-induced pluripotent stem cells65, but remains to be shown in APOE knock-in mice. However, the application of gene therapy to express APOE ε2 and increase APOE2 levels in APOE ε4 carriers (or even APOE ε3 homozygotes) has become feasible and the first phase 1 clinical trial with this approach has been initiated (NCT03634007; Table 2). In mice, intraventricular transfer of human APOE alleles with an adeno-associated virus type-4 leads to sustained expression of human APOE in the choroid plexus and ependymal cell lining, that diffuses to the brain parenchyma reaching a concentration of 10% of mouse endogenous APOE99. Adeno-associated virus type-4-mediated delivery and expression of APOE ε2 in 7-month-old APPswePSEN1dE9 mice (i.e., after Aβ plaque deposition) resulted in reduced soluble and insoluble Aβ levels and enhanced plaque clearance, whereas delivery of APOE ε4 had the opposite effects. Plasma Aβ40 concentrations were decreased in the APOE ε3 and APOE ε4 treated mice versus APOE ε2 treated mice, suggesting a reduced efflux from brain to plasma through the blood-brain barrier, relative to APOE ε2 treated mice. Monitoring of Aβ plaque growth by in-vivo multiphoton microscopy in living mice showed a significant effect on plaque growth rate, slower in APOE ε2 treated mice and faster in APOE ε4 treated mice. Moreover, APOE ε2 ameliorated plaque-associated dystrophic neurites and synapse loss, which were more severe in APOE ε4 treated mice. Similarly, intracerebroventricular AAV8-mediated astrocyte-specific expression of human APOE ε2 in APOE4 knock-in mice from birth increased APOE lipidation and decreased endogenous murine Aβ, whereas APOE ε4 delivery had opposite deleterious effects79.
CONCLUSIONS AND FUTURE DIRECTIONS
New insights in genetic modifiers, neuropathological and gene expression correlates, and pathophysiological mechanisms in different brain cell types are broadening our understanding of the implications of APOE in Alzheimer’s disease and offering previously unforeseeable opportunities for therapeutic and preventative interventions. Because of this mounting evidence, strategies to lower APOE4 levels and to increase APOE2 levels in the brain hold the greatest promise (Table 2). Against this remarkable momentum, there remains a paucity in translation of APOE-based therapies to human clinical trials, especially when compared with the expedited cases of anti-Aβ and anti-tau immunotherapies. What are the hurdles slowing APOE-based drug development programmes down? First, further development of small molecules that reliably change APOE4 conformation to APOE3 or APOE2 has proven to be difficult because the variable degree of lipidation of APOE might influence its tertiary conformation. Second, the new data implicating a variety of non-Aβ and non-tau targets in APOE pathophysiology raises new questions, such as determining what the best downstream therapeutic target should be and monitoring the consequences of target engagement. Third, there are some unique problems related to APOE separate peripheral (liver generated) and CNS pools and its inability to cross the blood-brain barrier. This means that affecting CNS APOE (levels, isoforms, or interactions) will require drugs with adequate blood-brain barrier penetration. Moreover, potential systemic off-target adverse effects of some of these approaches should be carefully considered: rare APOE ε2 homozygotes and Christchurch mutant carriers, and even more rare individuals with homozygous APOE null mutations, suffer from type III hyperlipoproteinemia3,96,100, resulting in accelerated atherosclerosis; in fact, APOE2 knock-in and Apoe knockout mice are widely used to model atherosclerosis. Therefore, gene therapies to lower APOE levels or switch APOE4 to APOE2 should probably be targeted specifically to the CNS (i.e., via direct injection or with viral capsids that penetrate the blood-brain barrier and appropriate promoters), which poses its own challenges.
Notwithstanding all these barriers, the risk and protective profiles of APOE genotype in human populations across the globe reinforce the robustness of the effects of subtle variations in this gene, and encourage the field to redouble its efforts at further understanding the pathophysiology of APOE effects in Alzheimer’s disease (Panel 2), and attempts at translating that knowledge into therapeutics.
Panel 1: Apolipoprotein E basic facts.
Two single nucleotide polymorphisms (SNPs) —rs429358 and rs7412— define the three alleles of APOE, located in chromosome 19q13.2: ε2, ε3, and ε4. Relative to the most common APOE ε3/ε3 genotype (reference group), possessing one APOE ε4 allele increases the risk of developing Alzheimer’s disease by approximately 3.7 times and being homozygous for the APOE ε4 allele increases the risk up to 12 times, whereas carrying a single APOE ε2 allele reduces the risk by approximately 40%, and being homozygous for APOE ε2 reduces the risk even further2,39.
Besides Alzheimer’s disease risk, the APOE genotype mainly affects the age of onset of cognitive impairment, with APOE ε4 carriers having an earlier age of onset and APOE ε2 carriers a later age of onset than APOE ε3 homozygotes. By contrast, the effect of APOE genotype on the rate of cognitive decline after symptom onset remains controversial, with allele differences typically considered not clinically relevant15.
APOE is a 299-amino acid (MW 34 kDa) secreted glycoprotein that binds cholesterol and phospholipids through the C-terminus domain and to its receptors through the N-terminus domain57.
The three APOE isoforms differ in two amino acid residues at positions 112 (Cys in APOE2 and APOE3, and Arg in APOE4) and 158 (Cys in APOE2, and Arg in APOE3 and APOE4), and these polymorphisms cause significant differences across APOE isoforms in both lipid binding properties (i.e., APOE4 is hypolipidated compared to APOE3 and APOE257,79) and receptor affinities.
APOE transports lipids packed into HDL-like particles in the brain, or LDL particles in the peripheral blood. APOE main receptors in the brain are the LRP1, the LDL receptor, the very LDL receptor, and the apolipoprotein E receptor 2, all of which are also Aβ receptors39,51–53,98.
Lipidation of brain APOE is mediated by ATP-binding cassette transporters A1 and G180,81,85.
APOE directly interacts with amyloid-β peptide46,47,50,57,59, but there is no solid in-vivo evidence of a direct interaction between APOE and tau30.
Mouse models to study the effects of APOE isoforms on amyloid-β peptide and tau include mice deficient in APOE (Apoe knockout) and mice genetically engineered to replace the mouse Apoe with each of the human APOE alleles (APOE-targeted replacement or knock-in), crossed with either mice overexpressing one or more familial Alzheimer’s disease-linked APP mutations —with or without one or PSEN1 mutations (e.g., APPV717F 43,45, APPswePSEN1dE942,99, 5xFAD44, APPPS1–2142,56)— or mice overexpressing frontotemporal lobar degeneration-tau-linked MAPT mutations (e.g., MAPTP301S)29,60.
Panel 2: Areas of uncertainty and research priorities.
Possible differential independent effects of APOE alleles on Alzheimer’s disease progression through preclinical and clinical stages with longitudinal multimodal imaging, CSF, and plasma or serum biomarkers.
Influence of genetic modifiers of APOE-linked Alzheimer’s disease risk, including the intimate mechanisms of local ancestry, interaction with longevity genetic polymorphisms such as KLOTHO-VS heterozygosity, and APOE mutations such as R136S (Christchurch), as plausible substrates of resistance or resilience to Alzheimer’s disease.
Possible Aβ-independent mechanisms of APOE on tau seeding and propagation through neuronal circuits.
Influence of APOE genotype on other neurodegenerative proteinopathies, such as primary tauopathies (e.g., Pick’s disease, progressive supranuclear palsy, corticobasal degeneration), TDP-43 proteinopathies (amyotrophic lateral sclerosis, frontotemporal lobar degeneration-TDP-43), and α-synucleinopathies (Parkinson’s disease, dementia with Lewy bodies, multiple system atrophy), as well as on other neurological disorders in which the blood-brain barrier or the immune system play a substantial role.
Autocrine versus paracrine effects of APOE on each brain cell type (astrocytes, microglia, neurons, oligodendrocytes, vascular smooth muscle cells, endothelial cells, and pericytes).
In-vivo applications of gene therapy, including genetic editing of APOE alleles with CRISPR-Cas9 technology and strategies for viral vector delivery to specific brain cell types.
Search strategy and selection criteria
We searched PubMed articles in English published between Jan 1, 1993 and May 15, 2020 using the search terms “APOE AND Alzheimer’s disease”, “APOE AND blood-brain barrier”, “APOE AND Lewy body disease”, “APOE AND alpha-synuclein”, “APOE AND TAR DNA-binding protein 43”, “APOE AND tau”, “APOE AND microglia”, “APOE AND astrocytes”, “APOE AND TREM2”, “APOE AND immunotherapy”, and “APOE AND gene therapy”. Only human, mouse model, and human-induced pluripotent stem cell studies were reviewed. In-vitro studies using recombinant APOE and synthetic or recombinant Aβ or tau species and in-cellulo studies using cultured cell lines or primary neuron, astrocyte, or microglial cultures were excluded. The final reference list was generated on the basis of relevance and originality with regards to the topics covered in this Review.
ACKNOWLEDGEMENTS
We want to thank Ayush Noori, Neurology Department, Massachusetts General Hospital, Boston, MA, USA for his invaluable help preparing the figure. The funder of the study had no role in data interpretation or writing of the report.
DECLARATION OF INTERESTS
AS-P is funded by the US National Institute on Aging (K08AG064039) and the Alzheimer’s Association (AACF-17-524184).
SD is funded by the US National Institute on Aging (P30AG062421).
BTH receives research funds from AbbVie and F Prime; received consultant or science advisory boards honoraria from Arvinas, Biogen, Cell Signaling Technology, US Department of Justice, Dewpoint Therapeutics, and Novartis; has a family member who works for Novartis and they own Novartis stocks; serves on the Board of Dewpoint Therapeutics and owns stock; and is funded by the US National Institute on Aging (1R01AG047644-01) and the US National Institute of Neurological Disorders and Stroke (U01NS111671-01).
Footnotes
The published journal article can be found at The Lancet Neurology website: https://www.thelancet.com/journals/laneur/home
REFERENCES
- 1.Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet 2019; 51: 414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nat Commun 2020; 11: 667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arboleda-Velasquez JF, Lopera F, O’Hare M, et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat Med 2019; 25: 1680–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belloy ME, Napolioni V, Han SS, Le Guen Y, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative. Association of Klotho-VS Heterozygosity With Risk of Alzheimer Disease in Individuals Who Carry APOE4. JAMA Neurol 2020; published online April 13. DOI: 10.1001/jamaneurol.2020.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X, Chen Y, Mok KY, et al. Non-coding variability at the APOE locus contributes to the Alzheimer’s risk. Nat Commun 2019; 10: 3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, Jun GR, Zhang X, et al. Analysis of Whole-Exome Sequencing Data for Alzheimer Disease Stratified by APOE Genotype. JAMA Neurol 2019; published online June 10. DOI: 10.1001/jamaneurol.2019.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huq AJ, Fransquet P, Laws SM, et al. Genetic resilience to Alzheimer’s disease in APOE ε4 homozygotes: A systematic review. Alzheimers Dement J Alzheimers Assoc 2019; 15: 1612–23. [DOI] [PubMed] [Google Scholar]
- 8.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997; 278: 1349–56. [PubMed] [Google Scholar]
- 9.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 1998; 279: 751–5. [DOI] [PubMed] [Google Scholar]
- 10.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol 2003; 60: 185–9. [DOI] [PubMed] [Google Scholar]
- 11.Rajabli F, Feliciano BE, Celis K, et al. Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet 2018; 14: e1007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blue EE, Horimoto ARVR, Mukherjee S, Wijsman EM, Thornton TA. Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimers Dement J Alzheimers Assoc 2019; 15: 1524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babenko VN, Afonnikov DA, Ignatieva EV, Klimov AV, Gusev FE, Rogaev EI. Haplotype analysis of APOE intragenic SNPs. BMC Neurosci 2018; 19: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mezlini Aziz M., Magdamo Colin, Merrill Emily, et al. Characterizing Clinical and Neuropathological Traits of APOE Haplotypes in African Americans and Europeans. J Alzheimers Dis JAD 2020; 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano-Pozo A, Qian J, Monsell SE, Betensky RA, Hyman BT. APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann Neurol 2015; 77: 917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015; 313: 1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015; 313: 1939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wennberg AM, Tosakulwong N, Lesnick TG, et al. Association of Apolipoprotein E ε4 With Transactive Response DNA-Binding Protein 43. JAMA Neurol 2018; 75: 1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanan VK, Castillo AM, Knopman DS, et al. Association of Apolipoprotein E ɛ4, Educational Level, and Sex With Tau Deposition and Tau-Mediated Metabolic Dysfunction in Older Adults. JAMA Netw Open 2019; 2: e1913909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therriault J, Benedet AL, Pascoal TA, et al. Association of Apolipoprotein E ε4 With Medial Temporal Tau Independent of Amyloid-β. JAMA Neurol 2020; 77: 470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain J Neurol 2018; 141: 2181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H-S, Yu L, White CC, et al. Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE ε4 haplotype status: a community-based cohort study. Lancet Neurol 2018; 17: 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabir MS, Blauwendraat C, Ahmed S, et al. Assessment of APOE in atypical parkinsonism syndromes. Neurobiol Dis 2019; 127: 142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020; 581: 71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuang D, Leverenz JB, Lopez OL, et al. APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 2013; 70: 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickson DW, Heckman MG, Murray ME, et al. APOE ε4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 2018; 91: e1182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prokopenko I, Miyakawa G, Zheng B, et al. Alzheimer’s disease pathology explains association between dementia with Lewy bodies and APOE-ε4/TOMM40 long poly-T repeat allele variants. Alzheimers Dement N Y N 2019; 5: 814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koriath C, Lashley T, Taylor W, et al. ApoE4 lowers age at onset in patients with frontotemporal dementia and tauopathy independent of amyloid-β copathology. Alzheimers Dement Amst Neth 2019; 11: 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y, Yamada K, Liddelow SA, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017; 549: 523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao N, Liu C-C, Van Ingelgom AJ, et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat Commun 2018; 9: 4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linton MF, Gish R, Hubl ST, et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest 1991; 88: 270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huynh T-PV, Wang C, Tran AC, et al. Lack of hepatic apoE does not influence early Aβ deposition: observations from a new APOE knock-in model. Mol Neurodegener 2019; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchihara T, Duyckaerts C, He Y, et al. ApoE immunoreactivity and microglial cells in Alzheimer’s disease brain. Neurosci Lett 1995; 195: 5–8. [DOI] [PubMed] [Google Scholar]
- 34.Mathys H, Davila-Velderrain J, Peng Z, et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 2019; 570: 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grubman A, Chew G, Ouyang JF, et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat Neurosci 2019; 22: 2087–97. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Song WM, Andhey PS, et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat Med 2020; 26: 131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han SH, Hulette C, Saunders AM, et al. Apolipoprotein E is present in hippocampal neurons without neurofibrillary tangles in Alzheimer’s disease and in age-matched controls. Exp Neurol 1994; 128: 13–26. [DOI] [PubMed] [Google Scholar]
- 38.Blanchard JW, Bula M, Davila-Velderrain J, et al. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med 2020; 26: 952–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 1993; 11: 575–80. [DOI] [PubMed] [Google Scholar]
- 40.Holtzman DM, Fagan AM, Mackey B, et al. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol 2000; 47: 739–47. [PubMed] [Google Scholar]
- 41.Kim J, Jiang H, Park S, et al. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-β amyloidosis. J Neurosci Off J Soc Neurosci 2011; 31: 18007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulrich JD, Ulland TK, Mahan TE, et al. ApoE facilitates the microglial response to amyloid plaque pathology. J Exp Med 2018; 215: 1047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 2000; 97: 2892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youmans KL, Tai LM, Nwabuisi-Heath E, et al. APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem 2012; 287: 41774–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med 2011; 3: 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto T, Serrano-Pozo A, Hori Y, et al. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J Neurosci Off J Soc Neurosci 2012; 32: 15181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hori Y, Hashimoto T, Nomoto H, Hyman BT, Iwatsubo T. Role of Apolipoprotein E in β-Amyloidogenesis: ISOFORM-SPECIFIC EFFECTS ON PROTOFIBRIL TO FIBRIL CONVERSION OF Aβ IN VITRO AND BRAIN Aβ DEPOSITION IN VIVO. J Biol Chem 2015; 290: 15163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu C-C, Zhao N, Fu Y, et al. ApoE4 Accelerates Early Seeding of Amyloid Pathology. Neuron 2017; 96: 1024–1032.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deane R, Sagare A, Hamm K, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008; 118: 4002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verghese PB, Castellano JM, Garai K, et al. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci U S A 2013; 110: E1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanekiyo T, Cirrito JR, Liu C-C, et al. Neuronal clearance of amyloid-β by endocytic receptor LRP1. J Neurosci Off J Soc Neurosci 2013; 33: 19276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C-C, Hu J, Zhao N, et al. Astrocytic LRP1 Mediates Brain Aβ Clearance and Impacts Amyloid Deposition. J Neurosci Off J Soc Neurosci 2017; 37: 4023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanekiyo T, Liu C-C, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-β. J Neurosci Off J Soc Neurosci 2012; 32: 16458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma Q, Zhao Z, Sagare AP, et al. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol Neurodegener 2018; 13: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koffie RM, Hashimoto T, Tai H-C, et al. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-β. Brain J Neurol 2012; 135: 2155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huynh T-PV, Liao F, Francis CM, et al. Age-Dependent Effects of apoE Reduction Using Antisense Oligonucleotides in a Model of β-amyloidosis. Neuron 2017; 96: 1013–1023.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones PB, Adams KW, Rozkalne A, et al. Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-β in human Alzheimer brain. PloS One 2011; 6: e14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kara E, Marks JD, Fan Z, et al. Isoform- and cell type-specific structure of apolipoprotein E lipoparticles as revealed by a novel Forster resonance energy transfer assay. J Biol Chem 2017; 292: 14720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kara E, Marks JD, Roe AD, et al. A flow cytometry-based in vitro assay reveals that formation of apolipoprotein E (ApoE)-amyloid beta complexes depends on ApoE isoform and cell type. J Biol Chem 2018; 293: 13247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi Y, Manis M, Long J, et al. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J Exp Med 2019; 216: 2546–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rauch JN, Luna G, Guzman E, et al. LRP1 is a master regulator of tau uptake and spread. Nature 2020; 580: 381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xian X, Pohlkamp T, Durakoglugil MS, et al. Reversal of ApoE4-induced recycling block as a novel prevention approach for Alzheimer’s disease. eLife 2018; 7. DOI: 10.7554/eLife.40048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serrano-Pozo A, Betensky RA, Frosch MP, Hyman BT. Plaque-Associated Local Toxicity Increases over the Clinical Course of Alzheimer Disease. Am J Pathol 2016; 186: 375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao N, Ren Y, Yamazaki Y, et al. Alzheimer’s Risk Factors Age, APOE Genotype, and Sex Drive Distinct Molecular Pathways. Neuron 2020; published online March 18. DOI: 10.1016/j.neuron.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin Y-T, Seo J, Gao F, et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018; 98: 1141–1154.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atagi Y, Liu C-C, Painter MM, et al. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). J Biol Chem 2015; 290: 26043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parhizkar S, Arzberger T, Brendel M, et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci 2019; 22: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keren-Shaul H, Spinrad A, Weiner A, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017; 169: 1276–1290.e17. [DOI] [PubMed] [Google Scholar]
- 69.Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017; 47: 566–581.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao J, Davis MD, Martens YA, et al. APOE ε4/ε4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum Mol Genet 2017; 26: 2690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung W-S, Verghese PB, Chakraborty C, et al. Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A 2016; 113: 10186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 2012; 11: 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pankiewicz JE, Baquero-Buitrago J, Sanchez S, et al. APOE Genotype Differentially Modulates Effects of Anti-Aβ, Passive Immunization in APP Transgenic Mice. Mol Neurodegener 2017; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakai K, Boche D, Carare R, et al. Aβ immunotherapy for Alzheimer’s disease: effects on apoE and cerebral vasculopathy. Acta Neuropathol (Berl) 2014; 128: 777–89. [DOI] [PubMed] [Google Scholar]
- 75.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Nortley R, Korte N, Izquierdo P, et al. Amyloid β oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 2019; 365. DOI: 10.1126/science.aav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamazaki Y, Shinohara M, Yamazaki A, et al. ApoE (Apolipoprotein E) in Brain Pericytes Regulates Endothelial Function in an Isoform-Dependent Manner by Modulating Basement Membrane Components. Arterioscler Thromb Vasc Biol 2020; 40: 128–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu J, Liu C-C, Chen X-F, Zhang Y-W, Xu H, Bu G. Opposing effects of viral mediated brain expression of apolipoprotein E2 (apoE2) and apoE4 on apoE lipidation and Aβ metabolism in apoE4-targeted replacement mice. Mol Neurodegener 2015; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wahrle SE, Jiang H, Parsadanian M, et al. Deletion of Abca1 increases Abeta deposition in the PDAPP transgenic mouse model of Alzheimer disease. J Biol Chem 2005; 280: 43236–42. [DOI] [PubMed] [Google Scholar]
- 81.Wahrle SE, Jiang H, Parsadanian M, et al. Overexpression of ABCA1 reduces amyloid deposition in the PDAPP mouse model of Alzheimer disease. J Clin Invest 2008; 118: 671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cramer PE, Cirrito JR, Wesson DW, et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 2012; 335: 1503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghosal K, Haag M, Verghese PB, et al. A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimers Dement N Y N 2016; 2: 110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cummings JL, Zhong K, Kinney JW, et al. Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer’s disease. Alzheimers Res Ther 2016; 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boehm-Cagan A, Bar R, Liraz O, Bielicki JK, Johansson JO, Michaelson DM. ABCA1 Agonist Reverses the ApoE4-Driven Cognitive and Brain Pathologies. J Alzheimers Dis JAD 2016; 54: 1219–33. [DOI] [PubMed] [Google Scholar]
- 86.Santos DB, Peres KC, Ribeiro RP, et al. Probucol, a lipid-lowering drug, prevents cognitive and hippocampal synaptic impairments induced by amyloid β peptide in mice. Exp Neurol 2012; 233: 767–75. [DOI] [PubMed] [Google Scholar]
- 87.Champagne D, Pearson D, Dea D, Rochford J, Poirier J. The cholesterol-lowering drug probucol increases apolipoprotein E production in the hippocampus of aged rats: implications for Alzheimer’s disease. Neuroscience 2003; 121: 99–110. [DOI] [PubMed] [Google Scholar]
- 88.Poirier J, Miron J, Picard C, et al. Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer’s disease. Neurobiol Aging 2014; 35 Suppl 2: S3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J, Eltorai AEM, Jiang H, et al. Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Aβ amyloidosis. J Exp Med 2012; 209: 2149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liao F, Hori Y, Hudry E, et al. Anti-ApoE antibody given after plaque onset decreases Aβ accumulation and improves brain function in a mouse model of Aβ amyloidosis. J Neurosci Off J Soc Neurosci 2014; 34: 7281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liao F, Li A, Xiong M, et al. Targeting of nonlipidated, aggregated apoE with antibodies inhibits amyloid accumulation. J Clin Invest 2018; 128: 2144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pankiewicz JE, Guridi M, Kim J, et al. Blocking the apoE/Aβ interaction ameliorates Aβ-related pathology in APOE ε2 and ε4 targeted replacement Alzheimer model mice. Acta Neuropathol Commun 2014; 2: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krishnamurthy K, Cantillana V, Wang H, et al. ApoE mimetic improves pathology and memory in a model of Alzheimer’s disease. Brain Res 2020; 1733: 146685. [DOI] [PubMed] [Google Scholar]
- 94.Vitek MP, Christensen DJ, Wilcock D, et al. APOE-mimetic peptides reduce behavioral deficits, plaques and tangles in Alzheimer’s disease transgenics. Neurodegener Dis 2012; 10: 122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghosal K, Stathopoulos A, Thomas D, Phenis D, Vitek MP, Pimplikar SW. The apolipoprotein-E-mimetic COG112 protects amyloid precursor protein intracellular domain-overexpressing animals from Alzheimer’s disease-like pathological features. Neurodegener Dis 2013; 12: 51–8. [DOI] [PubMed] [Google Scholar]
- 96.Mak ACY, Pullinger CR, Tang LF, et al. Effects of the absence of apolipoprotein e on lipoproteins, neurocognitive function, and retinal function. JAMA Neurol 2014; 71: 1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim J, Castellano JM, Jiang H, et al. Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron 2009; 64: 632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castellano JM, Deane R, Gottesdiener AJ, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Aβ clearance in a mouse model of β-amyloidosis. Proc Natl Acad Sci U S A 2012; 109: 15502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hudry E, Dashkoff J, Roe AD, et al. Gene transfer of human Apoe isoforms results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci Transl Med 2013; 5: 212ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pocovi M, Cenarro A, Civeira F, et al. Incomplete dominance of type III hyperlipoproteinemia is associated with the rare apolipoprotein E2 (Arg136-->Ser) variant in multigenerational pedigree studies. Atherosclerosis 1996; 122: 33–46. [DOI] [PubMed] [Google Scholar]


