Abstract
Background and purpose
Post COVID-19 seizures are relatively rare. The aim of the present study was to estimate the frequency of acute symptomatic seizures among patients with COVID-19 and to discuss possible pathophysiological mechanisms.
Material and methods
Out of 439 cases with COVID-19 that were admitted to Assiut and Aswan University hospitals during the period from 1 June to 10 August 2020, 19 patients (4.3 %) presented with acute symptomatic seizures. Each patient underwent computed tomography (CT) or magnetic resonance imaging (MRI) of the brain and conventional electroencephalography (EEG). Laboratory investigations included: blood gases, complete blood picture, serum D-Dimer, Ferritin, C-reactive protein, renal and liver functions, and coagulation profile.
Results
Of the 19 patients, 3 had new onset seizures without underlying pathology (0.68 % out of the total 439 patients); 2 others (0.46 %) had previously diagnosed controlled epilepsy with breakthrough seizures. The majority of cases (14 patients, 3.19 %) had primary pathology that could explain the occurrence of seizures: 5 suffered a post COVID-19 stroke (3 ischemic and 2 hemorrhagic stroke); 6 patients had COVID-related encephalitis; 2 patients were old ischemic stroke patients; 1 patient had a brain tumor and developed seizures post COVID-19.
Conclusion
acute symptomatic seizure is not a rare complication of post COVID-19 infection. Both new onset seizures and seizures secondary to primary brain insult (post COVID encephalitis or recent stroke) were observed.
Keywords: COVID-19, Seizure, Cerebrovascular stroke, Epilepsy, CNS, Encephalitis
1. Introduction
In addition to systemic and respiratory symptoms, it has recently been reported that 36.4 % (78/214) of patients with COVID-19 develop neurological symptoms (Mao et al., 2020), of which anosmia and headache are the most common whereas seizures and stroke are relatively rare (Whittaker et al., 2020). The proposed mechanisms of CNS involvement could be attributed to retrograde movement from the olfactory nerve, entry into CNS through circulating lymphocytes or entry through permeable blood brain barrier (BBB) (Moriguchi et al., 2020; Ye et al., 2020).
Given that patients with COVID-19 may have hypoxia, multiorgan failure, and severe metabolic and electrolyte derangements, it is plausible to expect clinical or subclinical acute symptomatic seizures to occur in some cases (Emami et al., 2020; Nalleballe et al., 2020; Romero-Sánchez et al., 2020). Only a few studies had been conducted to investigate the underlying mechanism of seizures associated COVID-19 (Emami et al., 2020; Hepburn et al., 2020; Nikbakht et al., 2020; Sohal and Mansur, 2020).
The aim of the present study is to estimate the frequency of seizures among Egyptian patients with COVID-19 and to discuss possible pathophysiological mechanisms.
2. Material and methods
This was an observational, retrospective cohort study sampled from all patients with suspected COVID-19 who were sufficiently ill to be admitted during the period of 1 June to 10 August 2020 to the largest two University hospitals in Upper Egypt (Assiut University Hospitals and Aswan University hospital). Evidence of SARS-CoV-2 infection was defined as: 1- Cases with definite COVID-19 if patients came with clinical symptoms of infection and PCR of respiratory samples (eg, nasal or throat swab) was positive. 2- Cases with probable COVID-19 if clinical symptoms and chest CT was consistent with COVID-19 plus one or 2 laboratory investigations were positive (lymphopenia, high serum ferretin and D-Dimer Level) but PCR was negative or unavailable. Unfortunately it was not possible to sample CSF in these situations.
All patients who had “seizure” as a presenting manifestation of their illness were recruited and included in the study. The data of clinical manifestations of COVID-19 and the associated comorbidities were recorded. A chest CT was taken to demonstrate the ground‑glass opacities of the lungs with consolidation and laboratory investigations including blood gases, complete blood picture, serum D-Dimer, Ferritin, C-reactive protein, renal, liver functions, coagulation profile. CT and/or MRI brain scan and EEG were performed in each patient.
2.1. The primary outcome
-
1
Estimation of the frequency of seizures either primary or secondary.
-
2
Determination of the type of seizures associated with COVID-19, complications with serial seizures or status epilepticus and correlation with laboratory and imaging studies.
2.2. Consent
Written informed consent was obtained from conscious patients or from first degree relatives of the others. The Local Ethical Committee of the Faculty of Medicine, Assiut and Aswan University/Egypt approved the study.
2.3. Statistical analysis
Data was coded, tabulated and recorded on a PC using the Statistical package for Social Science (SPSS 25). Number and percent or means ± standard deviation (SD) were used to represent data. The level of significance was set at P < 0.05.
3. Results
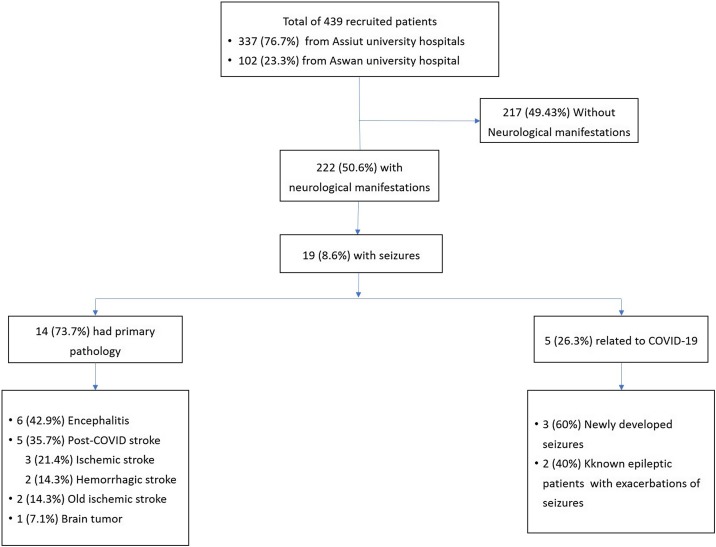
Among 439 confirmed or probable COVID-19 patients (337 (76.7 %) recruited from Assiut university hospitals, and 102 (23.3 %) from Aswan university hospital, 19 (4.3 %) presented with seizures. The median age was 47 [IQR; 35–65] years; 7 (36.8 %) were males and 12 (63.2 %) were females.
Only 3 patients presented with new onset seizures without underlying pathology (0.68 %), another 2 patients had previously diagnosed, controlled epilepsy with breakthrough seizures (0.46 % of the total sample of patients). The other 14 patients had primary pathology that could account for the seizures (3.19 %). Five patients had a recent post COVID-19 stroke (ischemic or hemorrhagic stroke); 6 patients had COVID-19 related encephalitis; 2 patients had a previous stroke before developing COVID-19, and 1 patient had an old brain tumor and developed new onset seizures post COVID-19. See Fig. 1 flow chart. Details of all cases are presented below and in the Tables.
Fig. 1.
The flowchart of all confirmed or probable COVID-19 patients (439) presented with seizures (19 patients).
3.1. Post-COVID-19: new onset seizure without underlying pathology
Three patients developed new onset seizures post COVID-19. All had fever, fatigue, dyspnea and gastrointestinal symptoms (GIT) for 3–5 days before developing generalized tonic-clonic convulsions. One patient had DM, the second had DM, hypertension (HTN) and a history of hepatic disease and the third patient had no comorbid disorders. Two patients had a positive PCR for COVID-19 and showed ground-glass opacity in CT chest; the remaining patient had a normal CT chest. One patient had anemia, thrombocytopenia, one had lymphopenia and the third one had normal laboratory data (Table 1 ).
Table 1.
Post-COVID-19: new onset seizures without underlying pathology and in previously diagnosed, controlled epilepsy with breakthrough seizures.
| Patient Number | Age (years)s) | Constitutional symptoms and duration of it | Type of seizures | CT Chest | PCR | Laboratory data | Comorbidity disorders |
|---|---|---|---|---|---|---|---|
| 1 | Mid 50 s | Fever, fatigue, diarrhea and confusion. 3 days | Generalized seizures (GTCC) | Not available | Positive | Normal | DM |
| 2 | late 40s | Fever, fatigue, dyspnea, abdominal pain and confusion. 5 days | Generalized seizures (GTCC) | Bilateral ground-glass opacity. | Negative | Normal | No |
| 3 | Mid 80s | Fever, cough and DCL. 3 days | Generalized seizures (myoclonus) | Normal | Positive | Anemia, thrombocytopenia | HTN, DM, Hepatic. |
| 4 | late 10s | Fever, fatigue, cough, dyspnea and confusion 4 days | Generalized seizures (GTCC) complicated by serial seizures | Bilateral ground-glass opacity. | Positive | Raised liver enzymes, low PO2, anemia, leukocytosis, lymphopenia. | Epilepsy |
| 5 | late 60s | Fever, for 2 days | Generalized seizures (GTCC) | Bilateral ground-glass opacity. | Positive | Low PO2, anemia and lymphopenia. | Epilepsy, HTN and DM. |
PCR; polymerase reaction; GTCC; generalized tonic clonic seizures, HTN; hypertension, DM; diabetes Mellitus; DCL; disturbed level of consciousness; CT; computer tomography.
3.2. Post COVID-19: previously diagnosed, controlled epilepsy with breakthrough seizures
Two patients had a history of previously diagnosed, controlled epilepsy and had been seizure-free for up to 2 years prior to infection but had a recurrence of their seizures post COVID-19. One of them had fever, fatigue, cough, dyspnea and confusion for 4 days followed by serial seizures. The other one had fever for only 2 days before developing generalized seizures. Details of PCR and CT chest and laboratory data are illustrated in Table 1.
3.3. Post-COVID-19: acute stroke (ischemic and hemorrhagic stroke as documented by CT/MRI brain) presenting with seizure onset
Three patients had a recent ischemic stroke with seizure onset after COVID-19 infection. All had fever, cough and sputum; one had additional dyspnea as constitutional symptoms for 3–7 days. Onset of seizure was coincident with stroke in all cases. One patient had generalized seizures and the other 2 had focal seizures with secondary generalization. Two patients had HTN and cardiovascular disease (CVD); one of them also had renal impairment. The third patient had raised liver enzymes. All 3 patients had a negative PCR for COVID-19 but they had bilateral ground-glass opacities on chest CT. All patients had raised D-dimer, anemia and leukocytosis; 2 of them had lymphopenia.
Of the two patients who had acute cerebral hemorrhage, one had fever, myalgia, cough and headache for 2 days followed by repeated focal seizures and disturbed consciousness with and left hemiplegia. The other patient had fever, headache, cough, dyspnea and confusion for 3 days before having generalized seizures and left hemiplegia. Other details are illustrated in Table 2 .
Table 2.
Post-COVID-19: acute stroke (ischemic or hemorrhagic) associated with seizure onset.
| Patient Number | Age (years) | Constitutional symptoms and duration (days) of it | Type of seizures | CT Chest | PCR | Laboratory data | Comorbid disorders |
|---|---|---|---|---|---|---|---|
| Symptoms and signs of stroke (Type of stroke) | |||||||
| 6 | mid 50s | Fever, cough, sputum. 7days |
Focal seizures with secondary generalization. | Bilateral ground-glass opacity. | Negative | Hypoalbuminemia, raised liver enzymes, increased PT, decreased PC, raised D dimer, anemia, leukocytosis and lymphopenia. | No |
| DCL, recent left hemiplegia and seizures. (Ischemic stroke) | |||||||
| 7 | late 60s | Fever, cough and sputum. 5 days | Generalized seizures (GTCC) |
Bilateral ground-glass opacity. | Negative | Raised PH (alkalosis), low PO2, raised D dimer, anemia, leukocytosis and lymphocytosis. | HTN, CVD and old left hemiplegia. |
| Double stroke, recent right hemiplegia,mixed aphasia and seizures. (Ischemic stroke) | |||||||
| 8 | early 70s | Fever, cough, sputum and dyspnea. 3 days | Focal seizures with secondary generalization. | Bilateral ground-glass opacity. | Negative | Hypoalbuminemia, raised blood urea and creatinine, low PO2 and PCO2, raised D dimer, anemia, leukocytosis and lymphopenia. | HTN, CVD and renal impairment. |
| DCL, recurrent left hemiplegia and seizures. (Ischemic stroke) | |||||||
| 9 | mid 30s | Fever, myalgia and cough. 2 days. |
Focal seizures | Bilateral ground-glass opacity. | Positive | Raised PH (alkalosis), low PO2 and PCO2 and leukocytosis. | Asthmatic. |
| Headache and neck stiffness, DCL, left hemiplegia and seizures. (Hemorrhagic stroke) | |||||||
| 10 | Early 40s | Fever, headache, cough, dyspnea. 3 days | Generalized seizures (GTCC) |
Not available | Positive | Raised creatinine, leukocytosis, neutrophilia and lymphopenia. | No |
PCR; polymerase reaction; GTCC; generalized tonic clonic seizures, HTN; hypertension, DM; diabetes Mellitus; DCL; disturbed level of consciousness; CT; computer tomography; CVD; cerebrovascular disease; PCO2; partial pressure oxygen; PCO2; partial pressure carbon dioxide; PT; prothrombine time; PC; prothrombine concentration.
3.4. Post-COVID-19 encephalitis with seizure onset
Six patients developed seizures in the context of COVID-19 related encephalitis. All of them had fever and 5 of them had headache and cough; these constitutional symptoms lasted between 2–6 days before seizures developed. One patient had visual hallucinations and another one had meningeal signs. All 6 patients had generalized seizures and 2 of them developed status epilepticus; one of these had serial seizures. All were transferred to ICU. MRI brain scans with contrast showed a combination of diffuse cerebral oedema, leptomeningeal enhancement with T2 and FLAIR hyper-intensities in the frontal lobes and/or bilateral medial temporal lobes and thalami. One patient had a comorbid pineal body tumor and hydrocephalus. EEG showed diffuse slowing of the background activity with bilateral periodic sharp waves and epileptiform discharges. One patient was 4 months pregnant. Other details are illustrated in Table 3 .
Table 3.
Post-COVID-19 encephalitis with seizure onset.
| Patient Number | Age (years) | Constitutional symptoms and duration in days and symptoms of encephalitis | Type of seizures | CT Chest | PCR | Laboratory data | Comorbid disorders |
|---|---|---|---|---|---|---|---|
| 11 | late 10s | Fever, headache, cough, dyspnea and tachycardia for 6 days followed by DCL seizures | Generalized seizures (GTCC) Complicated by status epileptics. |
Bilateral ground-glass opacity. | Positive | Hypoalbuminemia, increased PT, raised PH (alkalosis), low PCO2, anemia, neutrophilia, lymphopenia. | No |
| 12 | early 20s | Fever, headache, nausea, cough, dyspnea and tachycardia for 4 days followed by DCL, and meningeal signs, and seizures | Generalized seizures (GTCC) Complicated by status epilepticus |
Bilateral ground-glass opacity. | Positive | Raised liver enzymes, increased PT, increased blood urea and creatinine, raised PH (alkalosis), low PO2 and PCO2, anemia, neutrophilia and lymphopenia. | Pineal body tumor and hydrocephalus. |
| 13 | mid 40s | Fever, fatigue, bone pain, cough, dyspnea, nausea for 4 days followed by confusion seizures | Generalized seizures (GTCC) | Bilateral ground-glass opacity. | Positive | Increased creatinine, leukocytosis, neutrophilia and lymphopenia. | No |
| 14 | mid 60s | Fever for 2 days followed by confusion and seizures | Generalized seizures (GTCC) |
Bilateral ground-glass opacity. | Positive | Hypoalbuminemia, raised liver enzymes, increased PT, increased blood urea and creatinine, low PO2, anemic, leukocytosis, neutrophilia and lymphopenia. | No |
| 15 | early 40s | Fever, cough, visual for 5 days followed by hallucination and behavioral changes and seizures | Generalized seizures (GTCC) |
Normal | Positive | Hypoalbuminemia, raised AST, raised PH (alkalosis), low PO2 and PCO2, anemia, neutrophilia, lymphopenia and thrombocytopenia. | Pregnancy (± 4 months) |
| 16 | early 20s | Fever, headache, cough, dyspnea for 6 days followed by confusion and seizures | Generalized seizures (GTCC) Complicated by serial seizures |
Bilateral ground-glass opacity. | Positive | Hypoalbuminemia, low PO2, anemia, leukocytosis, lymphopenia | Epilepsy |
PCR; polymerase reaction; GTCC; generalized tonic clonic seizures, HTN; hypertension, DM; diabetes Mellitus; DCL; disturbed level of consciousness; CT; computer tomography; CVD; cerebrovascular disease; PCO2; partial pressure oxygen; PCO2; partial pressure carbon dioxide; PT; prothrombine time; PC; prothrombine concentration.
3.5. Post-COVID-19: seizures in patients with old neurological disorders (2 ischemic stroke/ one brain tumor)
Two patients had a history of old ischemic stroke confirmed by CT brain. One patient had fever, cough and dyspnea as constitutional symptoms for 4 days while the other had no constitutional symptoms. One patient presented with focal seizures with secondary generalization and the other presented with generalized seizures complicated by serial seizures. The remaining patient had an old parafalcine meningioma as documented by brain MRI. After admission to hospital she had disturbed consciousness for 1 day and then developed frequent generalized seizures complicated by status epilepticus. PCR, comorbidities, CT chest and laboratory data are illustrated in Table 4 .
Table 4.
Post-COVID-19 seizure in old neurological disorders (2 ischemic stroke patients + one had brain tumor “meningioma”).
| Patient Number | Age (years) | Constitutional symptoms and duration of it | Type of seizures | CT Chest | PCR | Abnormal Laboratory data | Comorbid disorders |
|---|---|---|---|---|---|---|---|
| 17 | early 50s | Fever, cough and dyspnea for 4 days followed by DCL and seizures |
Focal seizures with secondary generalization. | Bilateral ground-glass opacity. | Negative | Raised AST, low PO2, anemia, neutropenia and lymphocytosis | HTN, DM and old CVD. |
| 18 | early 40s | No constitutional symptoms. 0 day | Generalized seizures (GTCC) complicated by serial seizures. |
Bilateral ground-glass opacity. | Positive | Low PH (acidosis) and lymphocytosis. | Tramadol dependenceand old CVD. |
| 19 | early 50s | Fever for one day followed by DCL and seizures | Generalized seizures (GTCC) Complicated by status epilepticus. | Bilateral ground-glass opacity. | Negative | Leucocytosis. | Brain tumor |
PCR; polymerase reaction; GTCC; generalized tonic clonic seizures, HTN; hypertension, DM; diabetes Mellitus; DCL; disturbed level of consciousness; CT; computer tomography; CVD; cerebrovascular disease; PCO2; partial pressure oxygen; PCO2; partial pressure carbon dioxide; PT; prothrombine time; PC; prothrombine concentration.
4. Discussion
Severe acute respiratory syndrome Coronavirus-2 (SARS)-CoV-2 was first reported in Wuhan, China and officially named COVID-19 by the WHO on February 2020. The primary target cells for SARS-CoV-2 are the epithelial cells of the respiratory and gastrointestinal tract, which contain angiotensin converting enzyme 2 (ACE2), which is the portal of the virus to enter the cell (Li et al., 2020b). The ACE2 is expressed in the brain, particularly in the brain stem and in the regions responsible for regulation of cardiovascular function; expression of ACE2 has been found in both neurons and glia (Gowrisankar and Clark, 2016). In present study we found 19 (4.3 %) patients who presented with seizures mostly after constitutional symptoms of COVID-19, which was higher than other studies(Romero-Sánchez et al., 2020). In a study from Spain, during March 2020, of 841 patients hospitalized with confirmed COVID-19, seizures occurred in 6 (0.7 %) patients, 2 of which followed intracranial hemorrhages; 1 patient had encephalitis and 1 patient was previously diagnosed with epilepsy (Romero-Sánchez et al., 2020). In Wuhan, China, from January to February 2020, of 214 patients with confirmed COVID-19, seizures occurred in only 1 (0.5 %) patient. In the USA, from January to June 2020, of 40,469 patients with confirmed COVID-19, only 258 (0.6 %) patients had seizures (Nalleballe et al., 2020). All these cases were newly-developed seizures "without underlying pathology" or occurred in known epileptic patients, or were secondary to primary brain injury (ischemic or hemorrhagic stroke) post COVID-19.
In the present study; only 3 patients (0.68 %) developed new onset seizures without underlying pathology. It could be contributed to direct entry of the virus to the CNS via olfactory nerve or the affected sensory or motor neurons lead to release of pro-inflammatory cytokines (TNF-α, IL-6, IL-1B), nitric oxide, prostaglandin E2, and free radicals, and causes chronic inflammation neural hyper-excitability, seizure, and death (Huang et al., 2020a; Tufan and Avanoğlu Güler, 2020). SARS-CoV-2 triggers a systemic inflammatory storm (peripherally) with a massive release of cytokines, chemokines, and other inflammation signals which causes a significant disruption of the blood brain Barrier (BBB) leading to neuroinflammation, neuronal apoptosis and seizures (Steardo et al., 2020). The accumulation of inflammatory markers also may lead to local cortical irritation that precipitates seizures (Hepburn et al., 2020). High levels of glutamate released from ischemic or hypoxic cells into the extracellular spaces may activate AMPA and NMDA receptors leading to neuronal apoptosis or death (Prentice et al., 2015). Decreased GABA receptors may also lead to hyper-excitability of neural networks and seizure (Galanopoulou, 2008). In the present study the 2 (0.46 %) patients who previously had well-controlled epilepsy and experienced breakthrough seizures. Seizures exacerbation in controlled epileptic patients could be attributed to factors such as fever and hypoxemia which may trigger the seizures and sometimes introduce complications such as serial seizures or status. Stress also might be an independent precipitant for triggering seizures in some epileptic patients (Huang et al., 2020b).
On the other hand the incidence of seizure following acute stroke is rare, in the present study 5 patients had post-COVID-19 acute stroke (3 ischemic and 2 hemorrhagic) associated with seizure onset. The Incidence of acute ischemic stroke was approximately 5% in hospitalized patients with severe disease in Wuhan, China (Li et al., 2020a). COVID-19 leads to hypoxemia and excessive secretion of inflammatory cytokines, which contributes to the occurrence of ischemic stroke (Zhai et al., 2020) and increases the incidence of seizure (Hepburn et al., 2020). The incidence of seizures following spontaneous intracerebral hemorrhage reportedly ranges from 2.8 to 18.7% (Woo et al., 2012). Seizures after hemorrhagic strokes are thought to be due to irritation by products of blood metabolism. The exact pathophysiology is unclear, but an associated ischemic area secondary to hemorrhage is thought to play a part. Hemosiderin deposits are thought to cause irritability after a hemorrhagic stroke (Silverman et al., 2002). About 11.5 % of stroke patients are at risk of developing post‐stroke seizures within five years (Myint et al., 2006). The occurrence of seizure in old stroke patients could occur due to changes in neuronal excitability and gliotic scarring (Myint et al., 2006). In present study, 2 patients had a history of old stroke and newly-developed seizures following COVID-19. The occurrence of seizure in old stroke patients in the present study may be unrelated to COVID-19 infection, but one patient had fever and hypoxemia and the other was dependent on tramadol and stopped suddenly while in the hospital which could potentially explain seizure occurrence.
The occurrence of seizures associated encephalitis or brain tumors are common. The incidence of encephalitis related to SARS-CoV-2 is debated, with only case reports available (Moriguchi et al., 2020; Pilotto et al., 2020; Wong et al., 2020) but this also emphasis the neuroinvasive potential of the virus. In our study, 6 (1.37 %) patients developed seizures in the context of encephalitis with COVID-19 infection. Two of them developed status epilepticus and one developed serial seizures; one patient had neck stiffness suggesting meningioencephalitis and another one developed behavioral changes and visual hallucinations associated with disturbance of consciousness. The clinical manifestation, EEG findings and brain imaging supported diagnosis of encephalitis. An immunologic response induced by SARS-CoV-2 may cause inflammatory injury and edema and may lead to alterations in consciousness and seizures (Wu et al., 2020). Only 1 patient with old parafalcine meningioma as documented by MRI brain developed seizures post COVID-19. About 20–40 % of all brain tumor patients develop seizures (Glantz et al., 2000). Seizures exacerbation in patient with brain tumor could be attributed to fever and hypoxemia of COVID-19 which may trigger the seizures.
5. Conclusion
seizure is not a rare complication of post covid-19 infection. We observed both newly-developed seizures and seizures secondary to primary brain insult (post COVID-19 encephalitis or post COVID acute stroke as well as old stroke). Central and peripheral accumulation of cytokines, fever, and hypoxia could contribute to the occurrence of seizures.
References
- Emami A., Fadakar N., Akbari A., Lotfi M., Farazdaghi M., Javanmardi F., Rezaei T., Asadi-Pooya A.A. 2020. Seizure in Patients With COVID-19; pp. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou A.S. GABA(A) receptors in normal development and seizures: friends or foes? Curr. Neuropharmacol. 2008;6:1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz M.J., Cole B.F., Forsyth P.A., Recht L.D., Wen P.Y., Chamberlain M.C., Grossman S.A., Cairncross J.G. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- Gowrisankar Y.V., Clark M.A. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J. Neurochem. 2016;138:74–85. doi: 10.1111/jnc.13641. [DOI] [PubMed] [Google Scholar]
- Hepburn M., Mullaguri N., George P., Hantus S., Punia V., Bhimraj A., Newey C.R. Acute symptomatic seizures in critically Ill patients with COVID-19: is there an association? Neurocrit. Care. 2020:1–5. doi: 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Wu C., Jia Y., Li G., Zhu Z., Lu K., Yang Y., Wang F., Zhu S. 2020. COVID-19 Outbreak: The Impact of Stress on Seizures in Patients With Epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., Wang D. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc. Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H., Kojin H., Ito M., Onishi H., Shimizu T., Sasaki Y., Enomoto N., Ishihara H., Furuya S., Yamamoto T., Shimada S. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint P.K., Staufenberg E.F., Sabanathan K. Post-stroke seizure and post-stroke epilepsy. Postgrad. Med. J. 2006;82:568–572. doi: 10.1136/pgmj.2005.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalleballe K., Reddy Onteddu S., Sharma R., Dandu V., Brown A., Jasti M., Yadala S., Veerapaneni K., Siddamreddy S., Avula A., Kapoor N., Mudassar K., Kovvuru S. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020;88:71–74. doi: 10.1016/j.bbi.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikbakht F., Mohammadkhanizadeh A., Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A., Odolini S., Masciocchi S., Comelli A., Volonghi I., Gazzina S., Nocivelli S., Pezzini A., Focà E. 2020. Steroid-Responsive Encephalitis in Coronavirus Disease 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice H., Modi J.P., Wu J.Y. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/964518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., González E., Redondo-Peñas I., Perona-Moratalla A.B., Del Valle-Pérez J.A., Gracia-Gil J., Rojas-Bartolomé L., Feria-Vilar I., Monteagudo M., Palao M., Palazón-García E., Alcahut-Rodríguez C., Sopelana-Garay D., Moreno Y. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman I.E., Restrepo L., Mathews G.C. Poststroke seizures. Arch. Neurol. 2002;59:195–201. doi: 10.1001/archneur.59.2.195. [DOI] [PubMed] [Google Scholar]
- Sohal S., Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steardo L., Steardo L., Jr., Zorec R. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. (Oxf) 2020;229 doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan A., Avanoğlu Güler A. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker A., Anson M., Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol. Scand. 2020;142:14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P.F., Craik S., Newman P., Makan A., Srinivasan K., Crawford E., Dev D., Moudgil H., Ahmad N. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin. Med. Lond. (Lond) 2020;20:293–294. doi: 10.7861/clinmed.2020-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K.M., Yang S.Y., Cho K.T. Seizures after spontaneous intracerebral hemorrhage. J. Korean Neurosurg. Soc. 2012;52:312–319. doi: 10.3340/jkns.2012.52.4.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Ren Y., Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav. Immun. 2020;88:945–946. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P., Ding Y., Li Y. The impact of COVID-19 on ischemic stroke. Diagn Pathol. 2020;15:78. doi: 10.1186/s13000-020-00994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]