Abstract
Real-time RT-PCR remains a gold standard in the detection of various viral diseases. In the coronavirus 2019 pandemic, multiple RT-PCR–based tests were developed to screen for viral infection. As an emergency response to increasing testing demand, we established a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR diagnostics platform for which we compared different commercial and in-house RT-PCR protocols. Four commercial, one customized, and one in-house RT-PCR protocols were evaluated with 92 SARS-CoV-2–positive and 92 SARS-CoV-2–negative samples. Furthermore, economical and practical characteristics of these protocols were compared. In addition, a highly sensitive digital droplet PCR (ddPCR) method was developed, and application of RT-PCR and ddPCR methods on SARS-CoV-2 environmental samples was examined. Very low limits of detection (1 or 2 viral copies/μL), high sensitivities (93.6% to 97.8%), and high specificities (98.7% to 100%) for the tested RT-PCR protocols were found. Furthermore, the feasibility of downscaling two of the commercial protocols, which could optimize testing capacity, was demonstrated. Tested commercial and customized RT-PCR detection kits show very good and comparable sensitivity and specificity, and the kits could be further optimized for use on SARS-CoV-2 viral samples derived from human and surface swabbed samples.
On March 11, 2020, the World Health Organization (WHO) (Geneva, Switzerland) declared a pandemic because of the quick spread of a respiratory disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). With cases increasing in multiple countries and high transmissibility of SARS-CoV-2, eradication is rather unrealistic in the short term.1 In Switzerland, the second wave of SARS-CoV-2 is predicted to be slower than the first one but with a higher case fatality rate.2 The same situation was reported by the WHO for Spanish influenza for which the second and third waves of the infection claimed more lives and the pandemic lasted for almost 2 years and resulted in at least 50 million deaths worldwide [Centers for Disease Control and Prevention (CDC), https://www.cdc.gov/flu/pandemic-resources/1918-commemoration/three-waves.htm, last accessed September 7, 2020]. Another important factor contributing to the rapid spread of the coronavirus disease 2019 (COVID-19) pandemic is an unusually high number of asymptomatic spreaders.3 , 4 Therefore, continuous testing and reliable detection of the virus are essential parts of controlling the spread of SARS-CoV-2 (WHO, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/strategies-and-plans, last accessed September 7, 2020).
In March 2020, an in-house platform for SARS-CoV-2 diagnostics was initiated as part of an emergency response to an increasing demand for test capacity in a routine microbiology laboratory at University Hospital in Zurich, Switzerland. Currently, the gold standard for the detection and diagnosis of SARS-CoV-2 infection is based on the real-time RT-PCR. The overall goal was to provide in-house SARS-CoV-2 diagnosis to all patients and personnel to ensure the safe and efficient continuation of the health care work within the hospital and the protection of high-risk patients. The aims of this study were to i) evaluate four commercially available, one customized, and one in-house RT-PCR test by comparing the limit of detection (LoD), sensitivity using a panel of SARS-CoV-2 confirmed cases, and specificity using a group of non–COVID-19 respiratory samples; ii) examine the feasibility of down-scaling two commercial protocols to optimize the testing capacity; iii) develop a droplet digital PCR (ddPCR) assay to increase test sensitivity and provide more accurate quantitation of viral RNA; and iv) examine applicability of two validated RT-PCR protocols as well as of a ddPCR protocol on SARS-CoV-2 environmental samples.
Materials and Methods
Clinical Samples
Patient samples were collected by nasopharyngeal and/or oropharyngeal swabs (CM-FS913, iClean, San Ramon, CA) at the University Hospital Zurich and at ADMed Laboratory in La Chaux-de-Fonds, Switzerland (Copan Diagnostics, Brescia, Italy). The non–COVID-19 samples (other respiratory disease samples) were provided by ADMed Laboratory and were selected after having been tested on the Respiratory Panel FilmArray on Biofire (bioMérieux, Marcy-l'Étoile, France). Household samples were collected by swabbing of the different surfaces in a quarantined household of a SARS-CoV-2–positive patient. All swabs were stored in a viral transport medium (CDC, https://www.cdc.gov/coronavirus/2019-ncov/downloads/Viral-Transport-Medium.pdf, Accessed March 20, 2020) or Eswab (Copan Diagnostics, Murrieta, CA) at 4°C for a maximum of 48 hours or stored at −80°C until further analyses. All household swabbing participants provided informed consent for the study, and both the assay establishment and household studies were approved by the Cantonal Ethics Committee (BASEC-Nr-2020-00660 and BASEC-Nr-2020-00659, respectively).
RNA Extraction
Viral RNA was extracted as previously described5 using a magnetic bead–based (SpeedBeads, GE Healthcare, Darmstadt, Germany) extraction kit for the KingFisher instrument (MagMax, Thermo Fisher Scientific, Waltham, MA).
Detection of SARS-CoV-2 by RT-PCR Protocols
Four commercially available, one customized (Pasteur Institute, Paris, France), and in-house optimized RT-PCR protocols (Table 1 )6 , 7 were compared. Primer probes design, reaction mix, and thermal cycling conditions are given in Table 2, Table 3, Table 4 respectively. All RT-PCR protocols were run according to manufacturer instructions on a QuantStudio 5 DX real-time PCR system (catalog number A36324, Thermo Fisher Scientific), and data were analyzed with the Design and Analysis Software DA version 2.4 (Thermo Fisher Scientific) except for the Euroimmun protocol, which was run on LightCycler 480 II (RocheDiagnostics, Basel, Switzerland). Fast cycling mode was used, and a comparative Ct analysis method was used.
Table 1.
Description of Real-Time RT-PCR Assays Compared in the Study
| RT-PCR protocol | Abbreviated name | RT-PCR kit/primer and probes | Mastermix used in this study | Positive control |
|---|---|---|---|---|
| CDC 2019-Novel Coronavirus Real-Time RT-PCR Diagnostic Panel (for in vitro diagnostics) | CDC | 2019-nCoVEUA-01 Diagnostic Panel Box, catalog number 10006606, IDT, Newark, NJ | TaMan, Fast Virus 1-step Maste Mix, 4444436, 10 mL, Applied Biosystems/Thermo Fisher Scientific, Waltham, MA | 2019-nCoV_N_Positive Control, catalog number 10006625, IDT |
| Applied Biosystems TaqMan 2019-nCoV Assay Kit version 1 | TF-SinglePlex | TaqMan 2019-nCoV Assay Kit v1, catalog number A47532, Applied Biosystems/Thermo Fisher Scientific | TaMan, Fast Virus 1-step Maste Mix, catalog number 4444436, 10 mL, Applied Biosystems/Thermo Fisher Scientific | 2019-nCoV Control version 1, catalog number A47533, Applied Biosystems/Thermo Fisher Scientific |
| Applied Biosystems Multiplex TaqMan 2019-nCoV Assay Kit version 2 (research use only) | TF-MultiPlex | TaqPath COVID-19 Combo Kit, catalog number A47813/A47814, Applied Biosystems/Thermo Fisher Scientific | TaqPath1-Step Multiplex Master Mix (No ROX) (4×), catalog number A28523, Applied Biosystems/Thermo Fisher Scientific | Positive Control (TaqPath COVID-19 Control Kit), catalog number A47816, Applied Biosystems/Thermo Fisher Scientific |
| EURORealTime SARS-CoV-2 (for research use only) | Euroimmun | Catalog number MP 2606-0425 | Provided with the kit | Provided with the kit |
| Real-time RT-PCR assays for the detection of SARS-CoV-2, Pasteur Institute, Paris, France |
Pasteur Institute Protocol Paris (WHO) | https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf, last accessed November 12, 20206; ordered from Microsynth (Balgach, Switzerland) | Invitrogen Superscript III Platinum One-Step quantitative RT-PCR system, catalog number 11732-088 | Available on request from the Pasteur Institute |
| In-house customized RT-PCR protocol | Oncobit | https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html, last accessed September 7, 20207; ordered from Microsynth | TaqPath 1-Step Multiplex Master Mix (no ROX), catalog number A28521, Thermo Fisher Scientific | SARS-CoV-2 Positive Run Control, catalog number COV019CE, Bio-Rad, Luxembourg, Luxembourg |
CDC, Centers for Disease Control and Prevention; nCoV, novel coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Table 2.
Oligonucleotide Sequences of Primers and Probes of Oncobit Real-Time RT-PCR and Digital Droplet PCR Protocols
| Primer/probe name | Sequence |
|---|---|
| N2 forward primer | 5′-TTACAAACATTGGCCGCAAA-3′ |
| N2 reverse primer | 5′-GCGCGACATTCCGAAGAA-3′ |
| N2 probe (FAM) | 5′-ACAATTTGCCCCCAGCGCTTCA-3′ |
| ORF1ab forward primer | 5′-CCCTGTGGGTTTTACACTTAA-3′ |
| ORF1ab reverse primer | 5′-ACGATTGTGCATCAGCTGA-3′ |
| ORF1ab probe (Cy5) | 5′-CCGTCTGCGGTATGTGGAAAGGTTATGG-3′ |
| RNaseP forward primer | 5′-AGATTTGGACCTGCGAGCG-3′ |
| RNaseP reverse primer | 5′-GAGCGGCTGTCTCCACAAGT-3′ |
| RNaseP probe (HEX) | 5′-TTCTGACCTGAAGGCTCTGCGCG-3′ |
Table 3.
Reaction Mix for Oncobit Real-Time RT-PCR Protocol
| Reagent | Volume per reaction, μL |
|---|---|
| TaqPath 1-Step Multiplex Master Mix (no ROX) (catalog number A28521, Thermo Fisher Scientific, Waltham, MA), 4× | 5 |
| N2 probe (FAM) (100 μmol/L) | 0.05 |
| ORF1ab probe (Cy5) (100 μmol/L) | 0.05 |
| RNaseP probe (HEX) (100 μmol/L) | 0.05 |
| N2 forward primer (100 μmol/L) | 0.06 |
| N2 reverse primer (100 μmol/L) | 0.06 |
| ORF1ab forward primer (100 μmol/L) | 0.06 |
| ORF1ab reverse primer (100 μmol/L) | 0.06 |
| RNaseP forward primer (100 μmol/L) | 0.03 |
| RNaseP reverse primer (100 μmol/L) | 0.03 |
| Nuclease-free water | 4.55 |
| Total | 20.0 |
Table 4.
Thermal Cycling Conditions for Oncobit Real-Time RT-PCR Protocol
| Stage | Step | Temperature, °C | Time |
|---|---|---|---|
| Hold | Uracil-DNA glycosylase incubation | 25 | 2 minutes |
| Hold | Reverse transcription | 53 | 10 minutes |
| Hold | Activation | 95 | 2 minutes |
| Cycling (40 cycles) | Denaturation | 95 | 3 seconds |
| Anneal/extension | 60 | 30 seconds |
For the CDC protocol, an RT-PCR result was defined as inconclusive if only the N1 gene (±N3 gene) was positive or if only the N2 gene (±N3 gene) was positive. For the TF-MultiPlex (Thermo Fisher Scientific), TF-SinglePlex (Thermo Fisher Scientific), and Oncobit protocols, an RT-PCR result was considered inconclusive if only one of the two or three of the viral genes was positive. Inconclusive results were not repeated. The Euroimmun protocol (Luebeck, Germany) does not have the inconclusive category.
Detection of SARS-CoV-2 by ddPCR
The ddPCR protocol for SARS-CoV-2 detection targets two viral genomic regions of the SARS-CoV-2 gene (ORF1ab and N2) and uses the human RNase P gene as an in-process control. The following probes for the three genes were used: ORF1ab (FAM and HEX), N2 (FAM), and RNase P (HEX) (Table 2). Briefly, 20 μL of reaction mix (containing 1-Step RT-ddPCR Advanced Kit for Probes Mastermix; Bio-Rad, Luxembourg, Luxembourg) was combined with 10 μL of RNA sample for a final reaction volume of 30 μL. The final concentrations were 90 nmol/L for primers (ORF1ab, N2, RNaseP), 19.5 nmol/L for RdRP probes, 30 nmol/L for the N2 probe, and 40 nmol/L for the RNase P probe. The SARS-CoV-2 Positive Run Control (catalog number COV019CE, Bio-Rad) was used as positive control. ddPCR was run according to the program listed in Table 5 using QX200 Droplet Digital PCR System (Bio-Rad). The swabbing household samples from a laptop, newspaper, or door handle as well as the nontemplate control were tested in two independent runs.
Table 5.
Thermal Cycling Conditions for Oncobit Digital Droplet PCR Protocol
| Stage | Temperature, °C | Time |
|---|---|---|
| Hold | 50 | 60 minutes |
| Hold | 95 | 10 minutes |
| Cycling (55 cycles) | 95 | 30 seconds |
| 59 | 1 minute | |
| Hold | 98 | 10 minutes |
| Hold | 4 | 1 minute |
LoD, Sensitivity, and Specificity Calculation
The LoD of four published SARS-CoV-2 detection protocols (CDC, TF-MultiPlex, TF-SinglePlex, and Euroimmun) was determined using a dilution of an external quality assessment quantitative test sample (Instand, https://www.instand-ev.de/en/news/detail/news/neuartiges-coronavirus-sars-cov-2-2019-ncov-im-vorgezogenen-instand-ringversuch-virusgenom-nachw/?tx_news_pi1%5Bcontroller%5D=News&tx_news_pi1%5Baction%5D=detail&cHash=f91865b86af167390788c7f404b16e7e, last accessed November 12, 2020). Linear regression was used to determine the line of best fit for the relationship between Ct and viral copies. A Ct value of 40 was set as the minimum amount of viral copies detected by RT-PCR. LoD for Oncobit ddPCR protocol was determined using a dilution of the SARS-CoV-2 Positive Run Control (catalog number COV019CE, Bio-Rad).
For sensitivity and specificity value calculations of each assay, the results of RT-PCR obtained from the ADMed Laboratory were used as the gold standard reference. The sensitivity was defined with the formula TP/(TP + FN), whereas specificity was defined as TN/(TP + FP), where TP indicates true positive, FP indicates false positive, TN indicates true negative, and FN indicates false negative. If the result of tested assays matched the reference, it was labeled concordant. If the result from the tested assays did not match the reference, it was labeled discordant. Inconclusive results were excluded from sensitivity and specificity calculations.
SARS-CoV-2 Infectivity Assay
The viral infectivity assay was performed as previously described8, 9, 10 with slight modifications. Briefly, 5 × 104 Vero E6 cells (catalog number CCL-81, ATCC, Manassas, VA) were seeded on 96-well flat bottom cell culture plates in 200 μL of high glucose Dulbecco’s modified Eagle’s medium supplemented with l-glutamate, sodium pyruvate, nonessential amino acids, HEPES, 5% fetal cow serum, and Normocin (catalog number ant-nr-1, InvivoGen, Toulouse, France). After 24 hours of incubation (37°C, 5% CO2), the medium was removed, and 100 μL of a virus test solution or the positive SARS-CoV-2 control (provided by Prof. Volker Thiel, Inst. Virology & Immunology, University of Berne, Switzerland) was added in twofold serial dilutions to the cells. The plates were incubated for 48 hours at 37°C. The cells were then fixed with 10% formaldehyde solution for 15 minutes at room temperature, rinsed with phosphate-buffered saline, and stained with 1% crystal violet stain solution (catalog number 252532.1211, Pan Reac AppliChem, Darmstadt, Germany) for 15 minutes at room temperature. The staining solution was removed, the cells were rinsed twice with phosphate-buffered saline, and the plates dried at room temperature before assessment for viral plaques.
Results
Description and Comparison of SARS-CoV-2 RT-PCR Detection Protocols
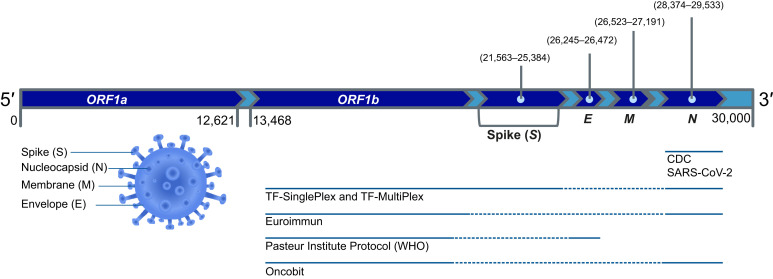
The six RT-PCR protocols compared in this study use the same principle of isolating viral RNA from the nasopharyngeal and/or oropharyngeal swabs or bronchial fluid and running a 1-step RT reaction followed by real-time amplification of two or three SARS-CoV-2 target genes (Figure 1 ). Summary and comparison of all tested RT-PCR protocols is given in Table 6 . All protocols have internal controls, nontemplate controls, and positive controls. In TF-MultiPlex, the phage MS2 is added as the internal control that serves as both RNA isolation and reaction control. All other protocols except for Euroimmun (where the type of interncal control is not indicated) use a widely accepted reaction control RNAseP to ensure that RNA isolation worked and RT-PCR reaction was not inhibited. The protocol design is single plex, double plex, or multiplex. Euroimmun protocol stands out with its design, with two target probes coupled to the same reporter color FAM. The viral RNA input is 5 to 10 μL. Because of unspecific E-gene amplification (Supplemental Table S1), the protocol developed by Pasteur Institute was not used further in this comparative study.
Figure 1.
Summary of different severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time RT-PCR detection protocols. SARS-CoV-2 genome structure and coverage by different protocols are shown. Continuous line indicates relative gene coverage by the detection protocol. The Euroimmun and TF-MultiPlex, protocols were for research use only. CDC, Centers for Disease Control and Prevention; WHO, World Health Organization.
Table 6.
Comparative Overview of Six Real Time RT-PCR Protocols
| Characteristic | CDC SARS-CoV-2 | TF-SinglePlex | TF-MultiPlex | Euroimmun | Pasteur Institute Protocol (WHO) | Oncobit RT-PCR |
|---|---|---|---|---|---|---|
| Targets genes (dyes) |
N1 (FAM) N2 (FAM) N3 (FAM) RNAseP (FAM) |
ORF1ab (FAM) N (VIC) S (ABY) RNAseP (JUN) |
ORF1ab (FAM) N (VIC) S (ABY) MS2 (JUN) |
ORF1ab and N (SARS-CoV-2, FAM) IC (VIC) |
RdRp_IP2 (FAM) RdRp_IP4 (HEX) E gene (FAM) RNAseP (HEX) |
ORF1ab (HEX) N2 (FAM) RNAseP (Cy5) |
| Targets per well | 4/4 | 4/3 | 4/1 | 3/1 | 4/2 | 3/1 |
| Sample volume per well, μL | 5 | 5 | 5 | 10 | 5 | 10 |
| Total reaction volume per well, μL | 20 | 25 | 25 | 20 | 30 | 20 |
| Design | SinglePlex | DoublePlex | MultiPlex | MultiPlex | DoublePlex | MultiPlex |
| Costs, CHF | 11.60 | 42 | 16.50 | 19 | 8 | 5 |
| Mean reaction time, minutes | 70 | 60 | 70 | 70–75 | 105 | 55 |
CDC, Centers for disease control and prevention; IC, internal control; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
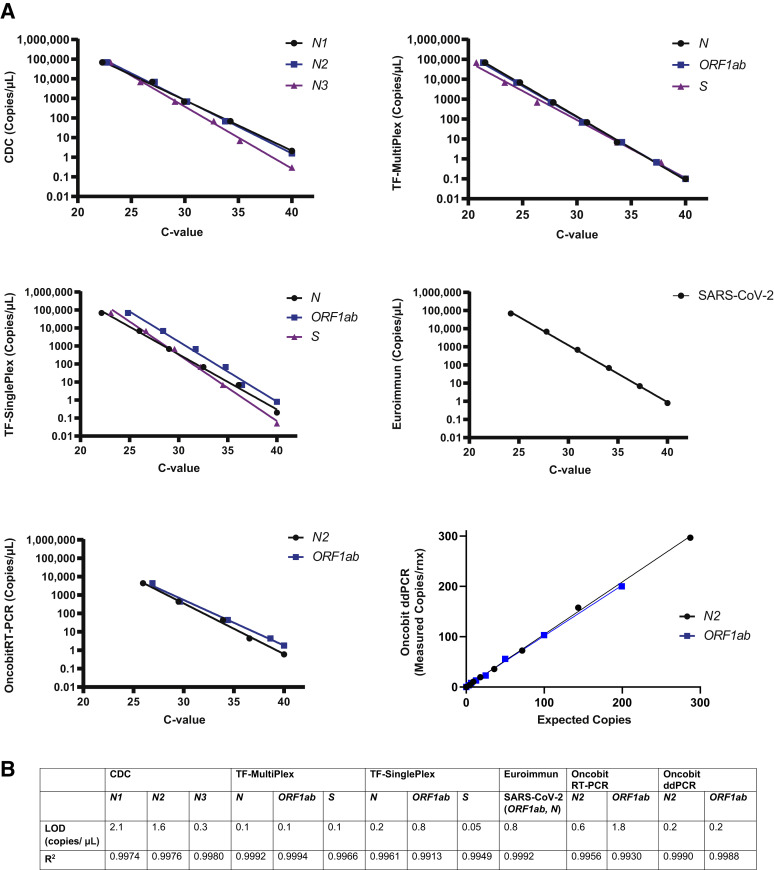
LoD of Real-Time RT-PCR and ddPCR SARS-CoV-2 Detection Protocols
With a Ct value cut-off of 40, the five RT-PCR SARS-CoV-2 detection protocols (CDC, TF-MultiPlex, TF-SinglePlex, Euroimmun, and Oncobit) as well as the Oncobit ddPCR protocol had an LoD between 1 and 2 viral copies/μL (Figure 2 , A and B). Values <1 copy/μL indicate high sensitivity of the tested protocol (Figure 2, A and B).
Figure 2.
Limit of detection (LoD) of real-time RT-PCR and digital droplet PCR (ddPCR) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection protocols. A: LoD (viral copies per microliter) of different target genes of Centers for Disease Control and Prevention (CDC), TF-SinglePlex, TF-MultiPlex, Euroimmun, Oncobit RT-PCR, and Oncobit ddPCR SARS-CoV-2 detection protocols. B: Calculated R2 values of SARS-CoV-2 detection protocols.
Specificity and Sensitivity of Real-Time RT-PCR SARS-CoV-2 Detection Protocols
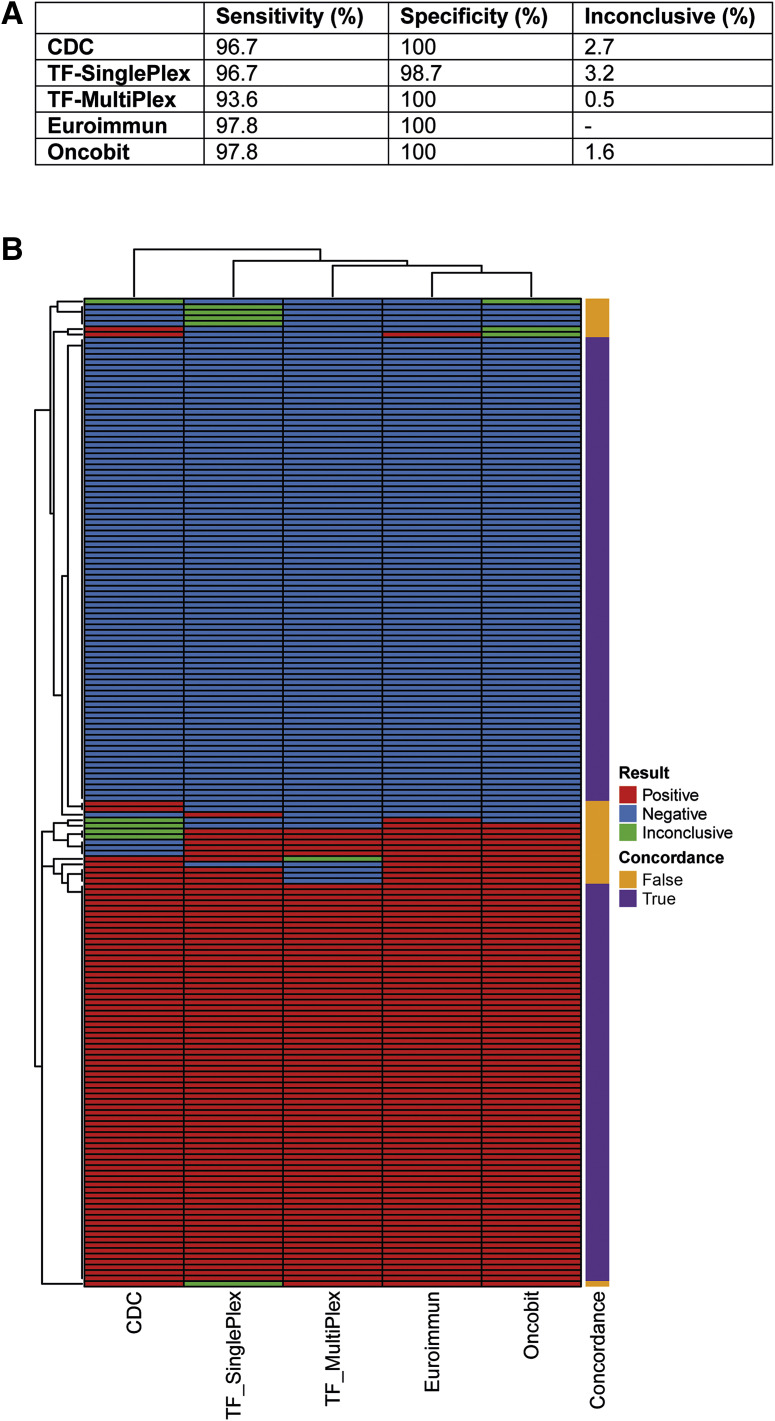
For the sensitivity and specificity of the SARS-CoV-2 detection protocols (CDC, TF-SinglePlex, TF-MultiPlex, Euroimmun, and Oncobit), a cohort of 92 SARS-CoV-2–positive samples and 92 SARS-CoV-2–negative samples was used that were provided by ADMed Laboratory. A comparison to SARS-CoV-2–positive results showed similar sensitivity of all tested protocols, with a 93.6% sensivity for TF-SinglePlex and 96.7% to 97.8% sensitivity for the other protocols (Figure 3 A). In the specificity cohort, 22 samples had a confirmed diagnosis of other respiratory diseases (Supplemental Table S2), and 70 samples tested negative for all listed respiratory diseases, including SARS-CoV-2. All protocols, except TF-SinglePlex, had no cross-reactivity (Figure 3A), including samples that tested positive for four other types of coronaviruses (Supplemental Table S2). The specificity was thus 100% for all protocols except for TF-SinglePlex, which had a specificity of 98.7% (Figure 3A).
Figure 3.
Specificity and sensitivity of real-time RT-PCR severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection protocols. A: Performance calculation (sensitivity/specificity) as well as calculation of percentage of inconclusive results of five real-time RT-PCR detection protocols [Centers for Disease Control and Prevention (CDC), TF-SinglePlex, TF-MultiPlex, Euroimmun, and Oncobit]. The Euroimmun RT-PCR detection protocol does not have the inconclusive category; inconclusive for Euroimmun equals an invalid result. B: Heatmap summarizing concordance of five real-time RT-PCR detection protocols (CDC, TF-SinglePlex, TF-MultiPlex, Euroimmun, and Oncobit) for both sensitivity (bottom) and specificity (top) sample cohorts.
Inconclusive results were found in 0.5% to 3.2% of these 184 samples, with TF-MultiPlex and Oncobit providing the most accuracy (Figure 3A). Comparing RT-PCR results (positive, negative, or inconclusive) of all 184 samples, the overall nonconcordance between all the protocols was 14.7% (Figure 3B).
Optimization of Testing Capacity
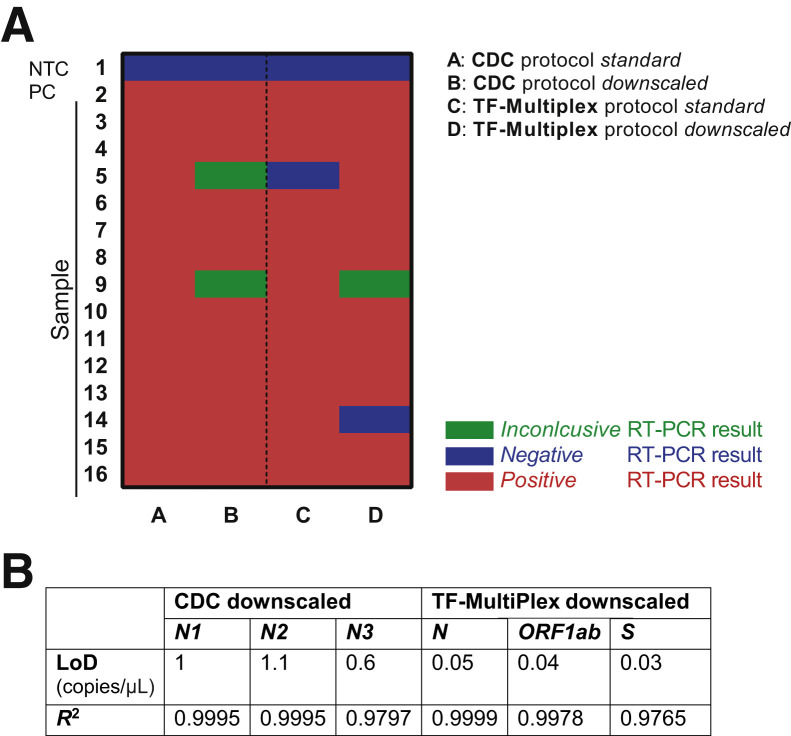
To optimize testing capacity, recommended reaction volumes in commercial protocols were downscaled, and published primer/probe sequences were customized to have an in-house developed protocol (Oncobit). Using a previously confirmed SARS-CoV-2–positive cohort of 14 samples, the CDC and TF-MultiPlex protocols were compared with recommended reaction volume and reaction volumes reduced by 50%. RNA sample input was always the same. The Oncobit protocol was the cheapest (Table 6), had the shortest RT-PCR reaction time requirement (Table 6), and had the most reliable access to consumables (Microsynth, Balgach, Switzerland). The specificity and sensitivity of the Oncobit protocol were comparable with other commercial SARS-CoV-2 RT-PCR detection kits (Figure 3, A and B).
A downscaled CDC protocol showed two (14.3%) inconclusive results, a standard TF-MultiPlex protocol showed one (7.1%) false-negative result, and a downscaled TF-MultiPlex protocol revealed one (7.1%) false-negative as well as one (7.1%) inconclusive result (Figure 4 A). Furthermore, LoD of downscaled CDC and TF-MultiPlex protocols showed a sensitivity of 1 copy/μL with a Ct value cut-off of 40 (Figure 4B).
Figure 4.
Downscaling of the Centers for Disease Control and Prevention (CDC) and TF-MultiPlex protocols. A: Heatmap summarizing results of standard and downscaled protocol (CDC and TF-MultiPlex). For the CDC protocol, a RT-PCR result was defined inconclusive if RT-PCR was positive for only N1 (±N3) or for only N2 (±N3). For TF-MultiPlex a RT-PCR result was considered inconclusive if only one of the viral genes was positive. B: Limit of detection (LoD) (copies per microliter) and R2 values of downscaled protocols (CDC and TF-MultiPlex). NTC, nontemplate control; PC, positive control.
Application of SARS-CoV-2 Detection Protocols on Swabbed Surfaces
Having compared and established the RT-PCR protocols for SARS-CoV-2 diagnostics, the possibility of application of the RT-PCR and ddPCR protocol for SARS-CoV-2 detection on environmental samples was examined. Swabs of different surfaces from a SARS-CoV-2 quarantined household were collected and analyzed by two validated RT-PCR protocols. In addition, an in-house ddPCR protocol was developed to accurately detect and quantify virus.
On the day of household surface swabbing (April 25, 2020) of the SARS-CoV-2–positive family, only patient 2 was swabbed again and tested positive but reported no symptoms (Supplemental Figure S1A). The pharyngeal swab as well as the swabbed surface samples were collected on the same day and tested with three different SARS-CoV-2 detection protocols (CDC, TF-MultiPlex, and ddPCR). The pharyngeal swab tested positive (cycle thresholds >30) on three different protocols. The laptop keyboard and two more swabbed surface (the door handle and newspaper) samples had positive and inconclusive results, respectively (Supplemental Table S3), whereas no infectivity for any of the samples was detected (Supplemental Figure S1B).
Discussion
Real-time RT-PCR remains the most sensitive method for early detection of SARS-CoV-2. We report a comparison of LoD, specificity, sensitivity, economic, and practical advantages of four commercial SARS-CoV-2 detection kits as well as one optimized in-house RT-PCR SARS-CoV-2 protocol. A study comparing RT-PCR with rapid fluorescence immunochromatographic assay–based SARS-CoV-2 nucleocapsid protein antigen detection method showed that sensitivity of the rapid method was only approximately 75.6%11; therefore, RT-PCR remains a more sensitive detection method for SARS-CoV-2. Most of the reported multiplatform comparison studies on real-time RT-PCR SARS-CoV-2 detection performed the benchmarking only on a limited number of samples and tested only commercial detection kits,12 , 13 and some studies limited the comparison only to sensitivity assessment.14
In this study, a low LoD and high sensitivity for four commercial SARS-CoV-2 RT-PCR detection protocols were observed by using standard quantitative test samples and a cohort of 92 SARS-CoV-2–positive samples, respectively. Furthermore, specificity of those protocols was tested and confirmed with 92 samples that had confirmed SARS-CoV-2–negative result or were collected in prepandemic times from patients presenting with respiratory symptoms (Supplemental Table S2).
In addition, downscaling of two commercial protocols that were chosen for the diagnostic routine (CDC and TF-MultiPlex) could be an option to save resources. This downscaling is especially important in times when a high demand for SARS-CoV-2 testing causes supply chain problems as occurred at the beginning of the pandemic in Europe. As an alternative strategy to optimize costs and increase testing capacity, an in-house protocol was developed in collaboration with the diagnostics company Oncobit by adapting previously published primer sequences for multiplex analysis. The customized Oncobit protocol was the least costly and fastest protocol when compared with other commercial RT-PCR protocols tested in this study.
To expand the application of RT-PCR–based detection protocols, a testing of swabbed surfaces from a SAR-CoV-2 quarantined household was performed. Results showed that RT-PCR protocols detected the viral genetic material on the laptop keyboard, and this result was confirmed by a more sensitive ddPCR method. Two more surfaces showed inconclusive results (a newspaper and a door handle, with viral copies detectable by ddPCR, however below the LoD) (Supplemental Table S3). Nasopharyngeal swab taken on the same day tested positive; however, infectivity assay for all samples showed negative results. These findings demonstrate the possibility of applying the RT-PCR–based protocols on nonpatient samples that could be of use for larger environmental studies. Summarizing the comparative study, we found that most commercial and customized RT-PCR–based detection protocols are highly effective at detecting viral presence in classic nasopharyngeal and/or oropharyngeal swabs, and because of its high sensitivity, RT-PCR–based detection protocols can be applied to the testing of environmental samples.
Acknowledgments
We thank Gaetana Restivo for help with obtaining ethical clearance for the study, and Jan Kaesler, Mirka Schmid, Muriel Traexler, Melanie Maudrich, and the entire Dermatology biobank team of University Hospital Zurich for big help with running the experiments and technical help. We also thank prof. Volker Thiel, Institute of Virology and Immunology, University of Berne, Switzerland for providing positive SARS-CoV-2 control for infectivity assay.
Footnotes
Supported by University Hospital Zurich Innovation Fund grant INOV00093 (M.P.L.) and Innosuisse grant 46938.1 INNO-LS for the development of the digital droplet PCR assay.
A.T. and C.I.S. contributed equally to this work.
P.P.B. and M.P.L. contributed equally to this work as senior authors.
Disclosures: M.P.L. is a founder and shareholder of Oncobit, which partially funded the establishment of the novel real-time RT-PCR and digital droplet PCR assays.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2021.04.009.
Author Contributions
The idea of the study was conceived by M.P.L., P.P.B., A.T., C.I.S., A.Dz., and P.F.C. Experiments were designed by M.P.L., P.P.B., A.T., C.I.S., E.B., A.Dz., and P.J. The experiment were conducted A.T., C.I.S., E.B., A.Dz., P.J., and A.D. Data were analyzed by C.I.S., P.F.C., A.T., A.Dz., E.B., and P.J. The study was supervised by M.P.L. and P.P.B. The manuscript was primarily written by A.T. and C.I.S. All authors edited the final manuscript.
Supplemental Data
Patients Ct values and symptom progression as well as infectivity examination of swabbed patient and surface samples. A: The patient's Ct values and symptom progression. Mean of Ct values of the N1, N2, and N3 viral genes are shown (Centers for Disease Control and Prevention protocol). Patient 1 was a 42-year-old woman; patient 2, 42-year-old man; patient 3, 6-year-old boy; and patients 4, 4-year-old boy. The Ct values at the time of diagnosis for patient 1 are missing because the patient was tested in a different laboratory. On April 7, 2020, the patient experienced shortness of breath, which lasted for 3 days. On April 7, 2020, the 42-year-old man (father, patient 2) tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with symptoms resolving after 10 days. As the symptoms gradually resolved, we observed increasing Ct values in both patients. On April 12, the two children (patients 3 and 4) also tested positive for SARS-CoV-2 but remained asymptomatic at all times. Family members in the household were swabbed on the April 25, with patients being asymptomatic for at least 2 days. B: An infectivity assay using Vero E6 cells found no plaque formation for any of the samples that tested positive by real-time RT-PCR (patient's throat, laptop keyboard, newspaper, and toilet rim).
References
- 1.Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., Storgaard M., Al Khalili S., Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balabdaoui F., Mohr D. Age-stratified discrete compartment model of the COVID-19 epidemic with application to Switzerland. Sci Rep. 2020;10:21306. doi: 10.1038/s41598-020-77420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi J., Lin Y., Zhong R., Jiang G., Verma V., Shi H., Li J., Tong X., Li Y., Hu D., Liang W., Han G., He J. Prevalence and clinical characterization of cancer patients with asymptomatic SARS-CoV-2 infection history. J Infect. 2020;81:e22–e24. doi: 10.1016/j.jinf.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichhoff O.M., Bellini E., Lienhard R., Stark W.J., Bechtold P., Grass R.N., Bosshard P.P., Levesque M.P. Comparison of RNA extraction methods for the detection of SARS-CoV-2 by RT-PCR. medRxiv. 2020 doi: 10.1101/2020.08.13.20172494. [DOI] [Google Scholar]
- 6.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brunink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong L., Zhou J., Niu C., Wang Q., Pan Y., Sheng S., Wang X., Zhang Y., Yang J., Liu M., Zhao Y., Zhang X., Zhu T., Peng T., Xie J., Gao Y., Wang D., Dai X., Fang X. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta. 2021;224:121726. doi: 10.1016/j.talanta.2020.121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki-Utsubo C., Chen M., Hotta H. Virucidal and neutralizing activity tests for antiviral substances and antibodies. Bio-protocol. 2018;8:e2855. doi: 10.21769/BioProtoc.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu F., Wang A., Liu M., Wang Q., Chen J., Xia S., Ling Y., Zhang Y., Xun J., Lu L., Jiang S., Lu H., Wen Y., Huang J. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 doi: 10.1101/2020.03.30.20047365. [DOI] [Google Scholar]
- 10.Chu H., Chan J.F., Yuen T.T., Shuai H., Yuan S., Wang Y., Hu B., Yip C.C., Tsang J.O., Huang X., Chai Y., Yang D., Hou Y., Chik K.K., Zhang X., Fung A.Y., Tsoi H.W., Cai J.P., Chan W.M., Ip J.D., Chu A.W., Zhou J., Lung D.C., Kok K.H., To K.K., Tsang O.T., Chan K.H., Yuen K.Y. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diao B., Wen K., Zhang J., Chen J., Han C., Chen Y., Wang S., Deng G., Zhou H., Wu Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin Microbiol Infect. 2021;27:289.e1–289.e4. doi: 10.1016/j.cmi.2020.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igloi Z., Leven M., Abdel-Karem Abou-Nouar Z., Weller B., Matheeussen V., Coppens J., Koopmans M., Molenkamp R. Comparison of commercial realtime reverse transcription PCR assays for the detection of SARS-CoV-2. J Clin Virol. 2020;129:104510. doi: 10.1016/j.jcv.2020.104510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Kasteren P.B., van der Veer B., van den Brink S., Wijsman L., de Jonge J., van den Brandt A., Molenkamp R., Reusken C., Meijer A. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol. 2020;128:104412. doi: 10.1016/j.jcv.2020.104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhen W., Manji R., Smith E., Berry G.J. Comparison of four molecular in vitro diagnostic assays for the detection of SARS-CoV-2 in nasopharyngeal specimens. J Clin Microbiol. 2020;58:e00743-20. doi: 10.1128/JCM.00743-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients Ct values and symptom progression as well as infectivity examination of swabbed patient and surface samples. A: The patient's Ct values and symptom progression. Mean of Ct values of the N1, N2, and N3 viral genes are shown (Centers for Disease Control and Prevention protocol). Patient 1 was a 42-year-old woman; patient 2, 42-year-old man; patient 3, 6-year-old boy; and patients 4, 4-year-old boy. The Ct values at the time of diagnosis for patient 1 are missing because the patient was tested in a different laboratory. On April 7, 2020, the patient experienced shortness of breath, which lasted for 3 days. On April 7, 2020, the 42-year-old man (father, patient 2) tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with symptoms resolving after 10 days. As the symptoms gradually resolved, we observed increasing Ct values in both patients. On April 12, the two children (patients 3 and 4) also tested positive for SARS-CoV-2 but remained asymptomatic at all times. Family members in the household were swabbed on the April 25, with patients being asymptomatic for at least 2 days. B: An infectivity assay using Vero E6 cells found no plaque formation for any of the samples that tested positive by real-time RT-PCR (patient's throat, laptop keyboard, newspaper, and toilet rim).