Abstract
Therapeutic approaches to COVID-19 treatment require appropriate inhibitors to target crucial proteins of SARS-CoV-2 replication machinery. It’s been approximately 12 months since the pandemic started, yet no known specific drugs are available. However, research progresses with time in terms of high throughput virtual screening (HTVS) and rational design of repurposed, novel synthetic and natural products discovery by understanding the viral life cycle, immuno-pathological and clinical outcomes in patients based on host’s nutritional, metabolic, and lifestyle status. Further, complementary and alternative medicine (CAM) approaches have also improved resiliency and immune responses. In this article, we summarize all the therapeutic antiviral strategies for COVID-19 drug discovery including computer aided virtual screening, repurposed drugs, immunomodulators, vaccines, plasma therapy, various adjunct therapies, and phage technology to unravel insightful mechanistic pathways of targeting SARS-CoV-2 and host’s intrinsic, innate immunity at multiple checkpoints that aid in the containment of the disease.
Keywords: COVID-19, Immunopathology, Therapeutics, Antiviral, Immunomodulators, Polyherbal
1. Introduction
Recent outbreak of SARS-CoV-2 has infected vast population across the globe by now. With millions of deaths and yet the COVID-19 pandemic is growing exponentially without any specific prophylaxis or treatment modalities. Parallelly, the worldwide scientific community has been trying to explore and target the key proteins of SARS-CoV-2 mutant’s to identify key therapeutic molecules. Subsequently, a lot of data has been reported in terms of review articles that describe hosts responses, clinical features, proposed treatment interventions and their mechanism of action (MOA) associated with SARS-CoV-2 infection or COVID-19 [1], [2], [3], [4], [5], [6], [7]. Nevertheless, research on SARS-CoV-2 and COVID-19 is progressing with time in terms of designing novel drugs, natural product based drug discovery, immuno-pathological and clinical outcomes based on host nutritional, metabolic and lifestyle status, role of herbo-mineral formulations in innate/adaptive immunity modulation to contain the disease. Hence, it is necessary to review the current data for critical therapeutic options and therefore, is the highlights of this article.
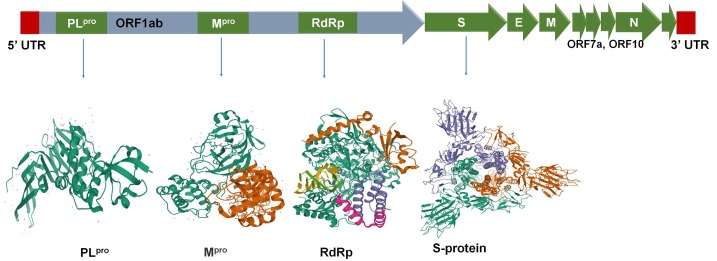
SARS-CoV-2 is a single stranded positive-sense RNA virus of ~ 30 kb in size [8] with a diameter of approximately 125 nm [9] which manipulates the host cell environment [10] depending on host’s nutritional, metabolic and lifestyle status [11], [12], [13] to alter the host gene expression and immune responses [14] leading to long incubation period [15] and a large number of asymptomatic [16] but transmissible infection preventing effective containment and mitigation of the disease [17]. SARS-CoV-2 has four structural proteins namely; the homo-trimer spike (S) protein, the membrane (M) protein, the envelope (E) protein, and the nucleocapsid (N) protein [Fig. 1 ]. The S protein mediates host cell attachment whereas M protein aids in envelope formation and viral entry [8], [18], [19]. The other lifecycle processes such as viral assembly, budding; envelope formation and release are facilitated through E protein. The N protein plays a pivotal role in viral transcription and assembly. The neutralizing antibodies/plasma therapy provides protective active immunity through competitively binding with viral S protein whereas N, M and E proteins do not respond significantly to neutralizing antibodies. Further, there are non-structural proteins (nsps) namely; main protease or serine-type protease (Mpro or CLpro), RNA dependent RNA polymerase (RdRp), Papain-like proteases (PLpro) encoded by ORF1a and ORF1b [Fig. 1] and forms replicase–transcriptase complex (RTC) to initiate RNA synthesis for replication and transcription of the sub-genomic RNAs [20], [21], [22], [23]. Hence, our focus to use structure based-drug designing to identify potential antivirals or natural drugs should leverage on these crucial target proteins of the SARS-CoV-2 replication machinery. Nevertheless, coronaviruses (CoVs) have exceptional mutation [9] and spillover [24] efficiency which makes the drug discovery process difficult, tedious and time consuming.
Fig. 1.
SARS-CoV-2 target proteins important for viral replication and conventional therapeutic approaches: PLpro, Mpro, S-protein and RdRp.
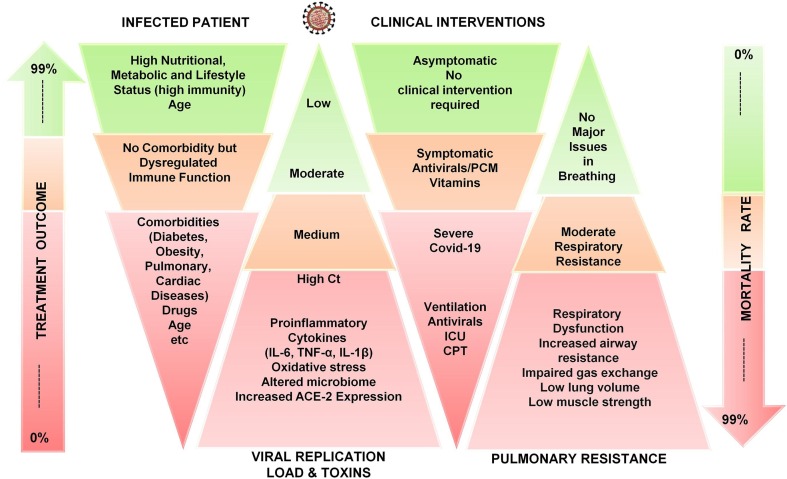
After suspected SARS-CoV-2 infection, the prediction of prognosis, infectiousness and viral load estimation through cycle threshold (Ct) values via real-time polymerase chain reaction (RT-PCR) help in patient management decisions [25]. However, the viral loads of asymptomatic or mildly symptomatic patients with COVID-19 individuals are comparable to those in symptomatic patients with severe and persistent positive upper respiratory RT-PCR results [26]. Being asymptomatic, mildly symptomatic and severely symptomatic depends on various factors including host’s nutritional, metabolic (microbiota), age, comorbidities and lifestyle status which either modulates the immune system to generate antibodies against the virus or being disproportionate generates cytokine storm [Fig. 2 ]. The longer the immune system takes to generate antibodies, the more is the production of proinflammatory cytokines [12], [13]. Patients with obesity [27], metabolic diseases (such as diabetes) [28], autoimmune diseases (rheumatoid arthritis [29]), dermatological diseases [30], neuronal diseases (such as Parkinson’s [31] and Alzheimer’s disease [32]) or cardiac [33] and pulmonary [34] diseases have low immune strength and are more susceptible to severe COVID-19 leading to dual immuno-pathophysiological inflammation through viremia and metabolic toxins. In comparison, the subjects with high nutritional, metabolic and good lifestyle status remain not only asymptomatic [Fig. 2] but recover even without the need of any medical assistance and supplementary medications [35]. Hence, the lifestyle improvement should be immensely promoted as part of healthcare system to improve the resiliency and immunity of population for coming future. Therefore, the present article covers host’s clinical responses and immuno-pathophysiology among asymptomatic, symptomatic and comorbid symptomatic subjects including all the therapeutic approaches for prophylaxis, treatment, and cure of COVID-19 including antivirals, repurposed drugs, CAM approaches, vaccines, and adjuvant therapies with their brief mechanistic pathways to exclusively target SARS-CoV-2 at various checkpoints that aid in the containment of the disease.
Fig. 2.
Consequences of SARS-CoV-2 infection and COVID-19 in high nutritional, metabolic, lifestyle, dysregulated immune system and comorbidity status.
2. Immuno-pathophysiology of SARS-CoV-2 infection
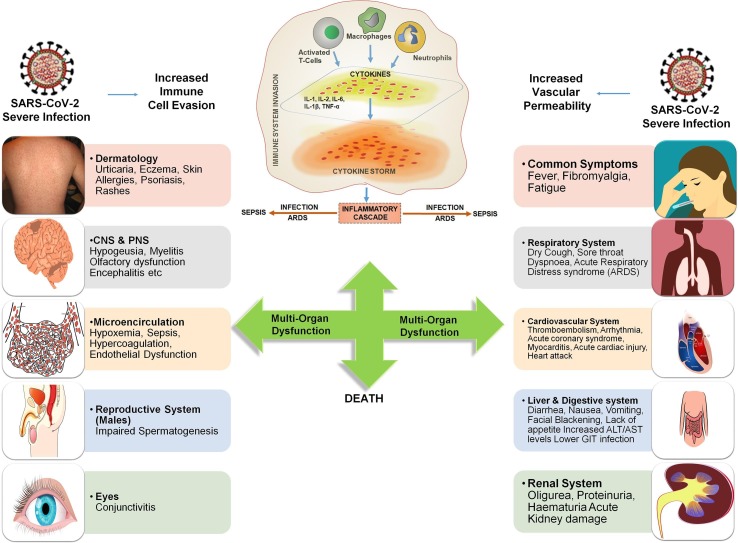
Acute hyperinflammatory “cytokine storm” after SARS-CoV-2 infection may lead to several severe clinical manifestations in COVID-19 patients including acute respiratory distress syndrome (ARDS), thromboembolic diseases, cardiac and gastrointestinal issues, acute kidney injury, encephalitis, vasculitis (Kawasaki-like syndrome in children), sepsis, multi-organ dysfunction and death [Fig. 3 ]. Hence, to design and develop the therapeutics for multiple complexities of COVID-19, it is utmost necessary to study the immuno-pathogenesis of cytokine storm and its clinical manifestations.
Fig. 3.
Representation of Immuno-pathophysiology and associated problems of severe SARS-CoV-2 infection in multiple organs leading to inflammatory cascade, “Cytokine Storm”, multi-organ failure and death.
2.1. Cytokine storm and its clinical manifestation
Destruction of lung cells due to SARS-CoV-2 infection triggers macrophages, neutrophils, monocytes, adaptive T and B cell immune responses. Among healthy subjects with robust immune system, this process itself overcomes the infection. However, among subjects with dysfunctional immune system or comorbidity, viral infection and its repetitive replication in pulmonary alveolar epithelial cells [36] causes programmed cell death (pyroptosis) [37] with associated vascular permeability releasing cytokine IL-1β. Further, these epithelial cells and macrophages use variety of pattern-recognition receptors (PRRs) to detect released pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). This recognition further enhances the secretion of pro-inflammatory cytokines and chemokines such as Interleukins (IL-2, IL-6, IL-8, IL-17), IFNγ, monocyte chemotactic protein 1 (MCP1) and Interferon inducible protein-10 (IP-10) [38], [39]. Simultaneously, these cytokines and chemokines further attract immune cells notably monocytes and T lymphocytes at the infected site causing pulmonary infiltration of lymphocytes into the airways leading to lymphopenia [40], [41], [42]. Severe COVID-19 patients generally exhibit lymphopenia and a marked reduction in CD4+ T, CD8+ T, NK, and B cell number on admission. Further, as compared to mild cases, the severe COVID-19 cases showed a significant decrease in CD8+ T cells. In addition, the memory helper T cells such as CD3+, CD4+, CD45RO+ are also decreased in severe cases compared with mild cases. However, lymphopenia was observed in a few mild and pregnant cases [39], [40], [41], [43], [44], [45], [46], [47], [48], [49], [50], [51]. Subsequent production of other inflammatory cytokines including IL-7, IL-10, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF), macrophage inflammation protein-1α (MIP-1α), and tumor necrosis factor-α (TNF-α) leads to “cytokine storm” along with series of adverse reactions and toxins production [46] in human body [Fig. 3]. In comparison, non-severe patients have significantly lower cytokine and toxins production [39], [40], [52], [53]. Severe patients with lymphopenia and cytokine storm are also prone to microbial infection which further promotes the disease progression and severity leading to viral sepsis and inflammation-induced lung injury. Further, the disease progresses to acute respiratory distress syndrome (ARDS), respiratory failure, shock, gastrointestinal (GIT) issues, multiple organ failure [Fig. 3], and potentially death [54]. Hence, a safe “multicomponent- multitarget -multichannel therapeutic approach” along with respiratory and physical exercises has to be considered primarily to avoid the disease progression towards severity.
3. Potential therapeutic approaches
3.1. Antiviral approaches
3.1.1. Entry/Fusion inhibitors and peptides
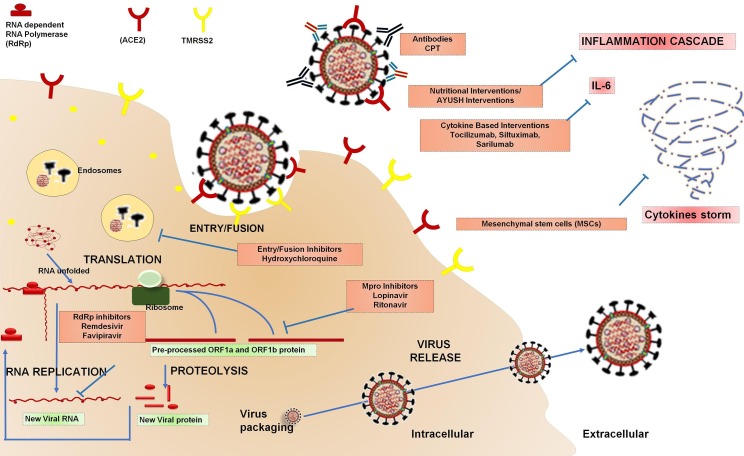
Initially, SARS-CoV-2 Spike (S) glycoprotein utilizes its receptor binding domain (RDB) to recognise, interact and attach itself with host cell receptor angiotensin converting enzyme 2 (ACE2). S-protein has two extracellular subunits S1 and S2 [55]. The RBD of S1 subunit after binding to ACE2 gets cleaved at the interface of S1 and S2 by host cell derived transmembrane proteases serine 2 (TMPRSS2) enzyme, cathepsin L, and furin. Further, the fusion protein (FP) of S2 subunit triggers viral fusion process [56]. Therefore, S-protein becomes the principle target for vaccines and therapeutic drugs to inhibit viral entry [21] (Fig. 4. ).
Fig. 4.
SARS-CoV-2 Life Cycle and Therapeutic Approaches: Antiviral approaches (Entry/Fusion Inhibitors, Mpro inhibitors, PLpro inhibitors, and RdRp inhibitors); Immunomodulation approaches (Micronutrients and Nutritional Intervention, Immunomodulatory Steroids, Cytokine Based Interventions, Convalescent Plasma Therapy, Vaccines).
Structural understanding of the RBD-ACE2 interface [57] is a crucial step for inhibitor design. Besides peptides, monoclonal antibodies (mAb) and small molecule inhibitors are still the preferred intervention modality in terms of cost, dosage, stability, pharmacokinetics and logistics. To identify small molecule inhibitors against RBD, virtual insilico screening of 1582 FDA-approved drugs was carried out which showed that Simeprevir and Lumacaftor bind RDB with high affinity and prevent ACE2 interaction. Further, virtual screening and in vitro studies of the same drugs suggested that Lumacaftor and Simeprevir are also SARS-CoV-2 Mpro inhibitors showcasing the concept of multi-target drugs that inhibit several proteins simultaneously [58]. Similarly, few natural products are screened against RBD of SARS-CoV-2 were found effective in inhibiting the interaction of spike glycoprotein with its receptor ACE2. Further, few molecules such as Nimbin, Curcumin, Withaferin A, Mangiferin, Piperine, Thebaine, Andrographolide, and Berberine were found effective in inhibiting the interaction of spike glycoprotein with its receptor ACE2 [59]. Nevertheless, few other molecules such as Eufoliatorin, Amarogentin, Caesalpinins, α-Amyrin, Kutkin, β-Sitosterol, and Belladonnine [60] showed the high affinity towards both the S-protein RBD and ACE2. ACE2 is a functional receptor required for SARS-CoV-2 attachment and internalization. In this context, Chloroquine, an antimalarial repurposed drug, was reported to block SARS-CoV-2 virus infection, with an IC50 value of 1.13 μM and a CC50 > 100 μM in Vero E6 cells. Chloroquine is believed to inhibit terminal glycosylation of ACE2 along with increased endosomal pH required for fusion leading to reduced affinity of SARS-CoV-2 to ACE2. Apart from its antiviral activity, chloroquine is also shown to synergistically enhance its antiviral effect through immunomodulation [61]. Another analogue of chloroquine, namely, Hydroxychloroquine exhibited much safer and better in vitro results than chloroquine [62]. Nevertheless, these repurposed drugs are also reported to cause ventricular arrhythmias, QT prolongation, and other cardiac-related toxicities in severely ill patients [63]. Regardless of the availability of ACE2 inhibitors, its inhibition is not a viable therapeutic approach as it plays important physiological roles including lung injury protective role in ARDS [64] and its attenuation may aggravate oxidative inflammatory responses [65].
Clinically approved TMPRSS2 inhibitors are safe and effective drugs considered to contribute in the containment of the disease by inhibiting host cell entry. Few TMPRSS2 inhibitors such as Camostat, Nafamostat and Aprotinin have shown to effectively decrease the rate of infection and replication of the virus in Calu-3 lung cell lines. Camostat is an FDA approved drug for the treatment of pancreatitis and was found effective in reducing airway virus replication by inhibiting S-protein initiated fusion. Similarly, Nafamostat, an FDA approved anticoagulant drug in Japan for continuous renal replacement, was recently reported to show 15 folds higher inhibitory potency than Camostat with 50% effective concentration [EC50] in the low-nanomolar range against SARS-CoV-2 fusion [66], [67], [68]. In comparison, Gabexate mesylate is least active in inhibiting SARS-CoV-2 S-driven host cell entry [69]. The suitability of these TMPRSS2 inhibitors including Bicalutamide to block TMPRSS2 for treatment of COVID-19 is currently being evaluated under clinical trial [70], [71], [72]. Further, in silico approaches using homology modelling, docking and ADME/T (absorption, distribution, metabolism, excretion, toxicity) studies for the identification of high affinity interaction and potent antagonists of TMPRSS2 have been reported. The study revealed that, six amino acid residues are essential which act as an active site of TMPRSS2 where three residues His296, Asp345, Ser441 present at the catalytic site and three residues Asp435, Ser460, Gly462 present at the substrate binding site. The results unravelled various natural and synthetic molecules including columbin, meloxicam, proanthocyanidin A2, ganodermanontriol, myricetin, jatrorrhizine and baicalein and should be proceeded for wet-lab evaluations [73], [74].
Further, various studies have also demonstrated that low endosomal pH environment activates pH sensitive proteases such as cathepsins L. Hence, few potent cathepsin L inhibitors, namely, MDL28170, EST, dec-RVKR-CMK, 5705213, K11777, oxocarbazate, and SSAA09E1 has been reported. However, due to concern over their unwanted side effects, FDA approved drugs that exhibit cathepsin L inhibitory activity including antimicrobials, immunomodulators, antimalarials, anti-tuberculous, anti-HIV, antioxidant, etc were considered to be repurposed. Nevertheless, these drugs have their own unwanted side effects in patients [75].
Additionally, an abelson non-receptor tyrosine kinase (Abl) promotes cathepsin L secretion which indicate that drugs inhibiting Abl tyrosine kinases might indirectly serve as cathepsin secretion inhibitors and inhibit entry/fusion of SARS-CoV-2 [76]. Subsequently, imatinib, has been shown to inhibit SARS-CoV-2 in an in vitro study [77]. Similarly, several kinase inhibitors as anti-inflammatory immunomodulators for cytokine suppression are proposed as potential therapeutic approach to contain COVID-19 [78].
Apart from these host-based, cell surface and endosomal proteases inhibitors, fusion inhibition is an attractive strategy to block viral entry through inhibition of a heptad repeat region HR1 of S-protein [79]. EK1 (optimized analogue of OC43-HR2 peptide) was found to be highly potent (IC50 = 0.19 μM) in shutting down S-protein mediated cell–cell fusion through hydrophobic interactions for SARS-CoV-2. EK1 was further optimized to EK1C4 which showed higher effectiveness than EK1 against SARS-CoV-2 S protein-mediated membrane fusion [80], [81].
3.1.2. Papain-like protease (PLpro) inhibitors
Inflammatory signalling pathways directed by distinct ubiquitin signals which are regulated by complex mechanisms in human cells. Viral proteases generally regulate innate immune pathways through antagonising ubiquitin and interferon-stimulated gene 15 (ISG15) from proteins involved in human’s antiviral immune response [82]. However, SARS-CoV-2 PLpro, despite having high amino acid sequence in ubiquitin domain, has lost both interferon-antagonising and deubiquitinase activities [83]. Nevertheless, SARS-CoV-2 encoded PLpro harbors two other active domains; a labile Zn-binding domain (Cys189–X–X–Cys192–Xn–Cys224–X–Cys226) and a classic catalytic cysteine cleavage domain (Cys111–His272–Asp286) which play vital role in viral replication. Hence, PLpro represent a promising target for design of PLpro inhibitors to retard viral replication either by selective ejection of Zn(II) ion from the labile Zn domain and/or by blocking the cysteine residue at the catalytic domain. However, no approved therapies targeting SARS-CoV-2 PLpro are available in the market yet [84]. Several compounds such as ribavirin, valganciclovir, ritonavir, fostamatinib, chloramphenicol, chlorphenesin, levodropropizine, phenformin, including natural products such as platycodin D, baicalin, rosemarinic acid, cryptotanshinone, tanshinone IIa, quercetin, etc have been reported to have high binding affinity with PLpro suggesting for their repurposed clinical evaluation and potential usefulness of these compounds in the treatment of SARS-CoV-2 infection [85], [86], [87]. Similarly, cyanobacterial metabolites namely; cryptophycin 1, cryptophycin 52, and deoxycylindrospermopsin [88] and fungi metabolites namely; norquinadoline A and scedapin C [89] have also been identified as potential inhibitors of PLpro. However, few of these drugs are not suitable for oral administration due to pharmacokinetic restrictions and few have their respective pharmacological actions on physiological functions [87]. Nevertheless, pharmacokinetic restrictions may be resolved using nano-encapsulation approach.
3.1.3. The 3C-like proteinase (Mpro) inhibitors
SARS-CoV-2 RNA encodes for two large polyproteins, pp1a and pp1ab, which are inactive until the viral chymotrypsin-like cysteine protease enzyme (3CL Mpro or Nsp5) cleaves them into 12 non-structural proteins (Nsp4-Nsp16) including RdRp (Nsp12) and helicase (Nsp13). Inhibition of Mpro would prevent the virus from replication. Hence, making it an attractive drug target for SARS-CoV-2 [90], [91]. In the absence of targeted therapeutic drugs, the only option for identification and discovery of lead compounds is through the application of computer aided structure-based high-throughput virtual screening (HTVS) of approved or clinical candidates. Therefore, utilising the same HTVS route, a mechanism-based inhibitor (N3) was identified and crystal structure of Mpro complexed with N3 was determined. Further, HTVS was carried out using 10,000 known compounds including approved drugs, natural products and drug candidates in clinical trials. The primary hits were seven compounds namely; Ebselen, Disulfiram, Tideglusib, Carmofur, Shikonin, PX-12 and TDZD-8 having IC50 ranging from 0.67 to 21.4 μM. Ebselen demonstrated the strongest inhibition of Mpro activity with an IC50 of 0.67 μM [92]. Ebselen is an organoselenium compound with anti-oxidant, anti-inflammatory, and cytoprotective properties. It was first introduced as an enzyme mimetic catalysing the glutathione peroxidase reaction [93]. Ebselen interacts with thiol groups forming selenosulfide with cysteine as its basic pleiotropic effect on numerous proteins [94] including Mpro [92]. Further, Ebselen attenuates overproduction of ROS, cytokines and neutrophil infiltration to counteract pulmonary and vascular inflammation [95]. Moreover, Ebselen has also been reported for its activity to inhibit release of IL-6 under prolonged hypoxia [96]. Further, its protective efficacy in microbial or chemical stimuli induced liver dysfunction (as seen in severe cases of COVID-19) has also been reported [97], [98], [99]. Hence, Ebselen looks logical and beneficial therapeutic candidate to be used in COVID-19 patients after clinical evaluation.
A natural product, Baiclein, is a non-covalent, non-peptidomimetic inhibitor of SARS-CoV-2 Mpro with inhibition potential of IC50 = 0.94 μM. Further, it showed potent dose-dependent inhibition (EC50 = 1.69 μM) of viral replication in SARS-CoV-2 infected Vero E6 cell assay. Unlike Ebselen, Baicalein and its derivatives disrupts both substrate recognition and stabilization of proteolytic reaction by blocking the substrates from approaching the catalytic site instead of covalently binding with cysteine [100], [101]. To date, several potential SARS-CoV-2 Mpro inhibitors have been reported from compound library screening, rational design, and natural products including ketoamide analogues, peptidomimetics, N-substituted isatin compounds, organo-mercuric compounds and several repurposed approved drugs and drug candidates with diverse chemical structure [91], [92], [102], [103], [104], [105], [106], [107].
Maraviroc is a U.S. FDA approved drug for the treatment of HIV-1. Chemically, it is azabicyclic and inhibits cell entry by blocking the interaction of HIV-1 glycoprotein 120 and chemokine receptor 5, on human CD4-presenting cells. [108]. Recent, in silico screening suggested that Maraviroc is a potential inhibitor of Mpro [109] and currently the drug is being evaluated in clinical trials (NCT04441385, NCT04435522, and NCT04475991) for COVID-19 treatment. Similarly, Glecaprevir is a Hepatitis C virus (HCV) NS3/4A protease inhibitor that targets the viral RNA replication [110]. Glecaprevir has also been evaluated in silico for its Mpro inhibition [109] which suggested that Glecaprevir is a highly potential inhibitor of SARS-CoV-2 Mpro. Likewise, Lopinavir, a small peptidomimetic antiretroviral aspartate protease inhibitor, was assumed to inhibit the Mpro of the SARS-CoV-2 as well, based on invitro (EC50 = 26.63 µM) [111] and in silico results [112], [113]. However, a recent research on Lopinavir and Ritonavir using purified Mpro revealed that these repurposed drugs have no activity against SARS-CoV-2 Mpro. This explains why these drugs consistently failed in COVID-19 clinical trials [114], [115].
Analogously, Darunavir/Cobicistat is a fixed-dose combination of 800 mg of the HIV protease inhibitor Darunavir and 150 mg Cobicistat, a CYP3A4 inhibitor, which is indicated in combination with other antiretroviral agents for the treatment of HIV infection. Darunavir showed no antiviral activity against SARS-CoV-2 at clinically relevant concentrations (EC50 > 100 μM). Whereas, remdesivir, used as a positive control, demonstrated potent antiviral activity (EC50 = 0.38 μM) [116]. GC376, a dipeptidyl bisulfite broad-spectrum inhibitor of picornavirus-like supercluster, is a potent inhibitor for the SARS-CoV-2 Mpro with IC50 of 26.4 ± 1.1 nM and inhibits viral replication with EC50 of 0.91 ± 0.03 μM. However, expedite clinical research on GC376 or its designed analogues for treatment of COVID-19 is required [117].
In comparison, repurposed drugs, Atazanavir and Danoprevir have shown promising results against COVID-19. Atazanavir is an FDA approved antiretroviral drug that competitively inhibits the HIV-1 aspartate protease. However, recently, Atazanavir has been reported to inhibit SARS-CoV-2 replication through Mpro inhibition with EC50 = 2 ± 0.12 µM in Vero and human pulmonary epithelial cell lines. In combination with ritonavir the activity was found to be much potent with EC50 = 0.5 ± 0.08 µM. The drugs in combination also impaired the virus-induced enhancement of proinflammatory cytokine production such as IL-6 and TNF-α [118]. As per recent clinical study results under review, Danoprevir (a macrocyclic peptidomimetic drug) in combination with Ritonavir alleviated the symptoms in COVID-19 patients and accelerated their recovery in 4–12 days [119]. Hence, these combinations may be considered based on clinical conditions of COVID-19 patients under treatment.
3.1.4. RNA dependent RNA polymerase (RdRp) inhibitors
Coronavirus replication and transcription is mediated through a multisubunit complex of viral nonstructural proteins (nsps) including the core component, nsp12 (RdRp), and accessory cofactors, nsp7 and nsp8, that increase RdRp template binding and processivity [120]. Hence, RdRp is a crucial target for inhibition of viral replication and is also known to be inhibited by a class of antivirals called as “nucleotide analogs” such as remdesivir [120].
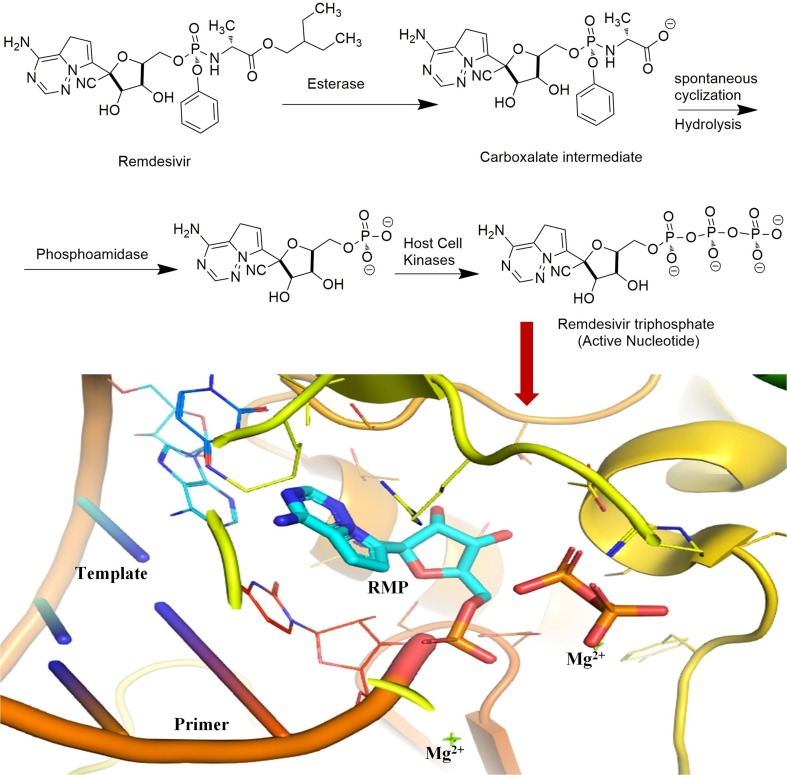
Remdesivir (GS-5734) is an adenosine monophosphate antiviral prodrug which gets metabolized to its pharmacologically active nucleoside triphosphate metabolite. The triphosphate metabolite acts as a competitive inhibitor of RdRp [Fig. 5 ] which leads to termination of chain elongation and ceases the viral RNA replication process [121]. Remdesivir was observed to be active in vitro against Vero E6 cells infected with SARS-CoV-2 with an EC50 0.77 µM [61]. Currently, remdesivir alone or in combination with other drugs such as tocilizumab (NCT04409262), merimepodib (NCT04410354), or baricitinib (NCT04401579) is being evaluated as a treatment for COVID-19 patients [122]. Food and Drug Administration (FDA) has approved remdesivir for the treatment of COVID-19 in hospitalized adult and pediatric patients (aged ≥ 12 years and weighing ≥ 40 kg) with severe disease. However, the clinical data for the use of remdesivir in mild to moderate COVID-19 patients is insufficient. Nevertheless, FDA has also warned against the concomitant use of remdesivir and chloroquine or HCQ as these drugs may decrease the antiviral activity of remdesivir [123].
Fig. 5.
Chemical structure and schematic representation of remdesivir, metabolic bioactivation and its bioactive triphosphate form inhibiting the viral RdRp. The close pymol view of the RdRp active site (pdb 7bv2), showing the covalently bound RMP, pyrophosphate, and magnesium ions.
In contrast, a pyrazine-carboxamide derivative, favipiravir is currently in use against mild to moderate COVID-19 infections in various countries including China, Italy, Japan, Russia, Ukraine, Uzbekistan, Moldova, Kazakhstan, Saudi Arabia, UAE, Turkey, Bangladesh, Egypt and India. Favipiravir is a broad-spectrum antiviral drug which gets converted to its potentially active form favipiravir-ribose-5′-triphosphate, in host-infected cells and selectively inhibits RdRp of RNA viruses [124]. A nonrandomized, open-label study with non-severe COVID-19 patients (n = 80) showed that favipiravir (1600 mg orally on the first day, then 600 mg orally twice daily for 13 days) in combination with IFN-α was significantly better as compared to lopinavir/ritonavir and IFN-α in terms of disease progression and viral clearance [125]. Further, in an open-label, prospective, randomized, multicenter clinical trial with moderate COVID-19 patients (n = 236), favipiravir had a higher 7 day’s clinical recovery rate with reduced incidence of fever and cough. Favipiravir (1600 mg orally twice daily on the first day, then 600 mg orally twice daily for 7–10 days) had a recovery rate of 71.43% as compared to control group (umifenovir 200 mg three times daily for 7–10 days) with recovery rate of 55.86% [126]. However, it is contraindicated in women with known or suspected pregnancy.
Further, an FDA approved guanosine nucleoside analogue, Ribavirin, is a broad-spectrum systemic antiviral prodrug for chronic hepatitis C virus [127] and viral hemorrhagic fever [128]. It blocks the replication of virus after getting metabolized to its triphosphate nucleotide active form (Fig. 5). Ribavirin has also been accounted for its property to inhibit host’s inosine monophosphate dehydrogenase by ribavirin monophosphate which leads to decreased intracellular Guanosine triphosphate (GTP) and inhibition of viral protein synthesis [129]. Although various clinical trials for ribavirin alone or in combination are in progress, however, one open-label randomized control study (NCT04276688) revealed that early triple antiviral (ribavirin, lopinavir/ritonavir and IFN-β-1b) therapy is safe and effective then lopinavir/ritonavir alone in treating mild to moderate COVID-19 patients [130]. Likewise, clevudine (NCT04347915) is a thymidine nucleoside prodrug that requires phosphorylation to form the corresponding active nucleotide triphosphate [131]. Furthermore, a prophylactic combination of emtricitabine (cytosine nucleoside analogue) and tenofovir alafenamide (adenine based acyclic nucleotide analogue) against SARS-CoV-2 infection is being evaluated in a large randomized, double-blind, controlled clinical trial (NCT04405271) for health care workers exposed to COVID-19 patients [132]. One of the important treatment measures is the FDA approved drug ivermectin which has been proposed to inhibit the importin (IMP) α/β receptor, responsible for transmitting viral proteins into the host cell nucleus. The drug has been found to be effective as a preventive and therapeutics in various clinical settings. Regular use of ivermectin has been shown to reduce the risk of contracting COVID-19.
Nevertheless, HTVS and in silico (docking) studies have also been performed in search of novel RdRp inhibitors of SARS-CoV-2. One such study recently revealed that biologically active alkaloids of Argemone mexicana viz protopine, allocryptopine and (±) 6-acetonyldihydrochelerythrine could be the potential RdRp inhibitors of SARS-CoV-2 [133]. Protopine was observed to be the most potential inhibitor ligand.
3.2. Immunomodulation approaches
3.2.1. Micronutrients and nutritional intervention
To combat SARS-CoV-2 infection, apart from physical barriers, host’s immune defence system comprises of innate immune responses (cellular and biochemical responses), inflammatory responses and adaptive immune responses (antigen presentation and cell-mediated immunity) which functions in a step wise process and has been explained in detail elsewhere [134]. In the course of battle between host immune system and SARS-CoV-2, nutritional micronutrients such as vitamins (Vitamin A, D, E, B12, B6, Folic acid and vitamin C), minerals (Zinc, Iron, Copper, Selenium and Magnesium) and microbiota (probiotic bacteria) have synergistic and homeostasis roles to play based on their complementary mode of action [135], [136].
Vitamin A has immunoregulatory effects and aids in differentiation, proliferation and functional integrity of innate immune cells such as natural killer (NK) cells regulate the activity of phagocytes such as macrophages, neutrophils for microbial, phagocytic and oxidative burst activity during inflammatory responses [135], [137], [138]. Further, it helps in the development and differentiation of CD4+ T helper cells and bring about anti-inflammatory responses by downregulating the production of IL-12, TNF- α and interferon-γ. Vitamin A is also required for normal functioning of B cells and antibody responses [137], [138], [139], [140], [141]. Nevertheless, in silico pharmacology analysis and assays also indicated that vitamin A works against SARS-CoV-2 via enrichment of immunoreaction, inhibition of inflammatory reaction, and biological processes related to ROS. Further, it is also indicated that vitamin A has seven core targets including MAPK1, IL10, EGFR, ICAM1, MAPK14, CAT, and PRKCB against COVID-19 suggesting that vitamin A may act as a potent treatment option for COVID-19 [142].
Similarly, vitamin D also plays a crucial role in immune-modulation during viral infections. Vitamin D production in human body takes place through subcutaneous sunlight exposure or is made available through external dietary sources such as dairy products, fish liver oil or cholecalciferol pills. After absorption, vitamin D binds to intracellular nuclear vitamin D receptors (VDRs) and subsequently dimerise either with themselves or retinoid X receptors (RXRs). Further, the dimer translocates to nucleus and engage vitamin D receptor element (VDRE) which regulate various host genes such as beta defensin and cathelicidin [143], [144]. These genes cleave the viral membrane and are involved in the activation of phagocytes respectively [145]. Vitamin D also regulates suppression of adaptive immune responses during viral infection via inhibiting T-cell proliferation (type I) resulting in reduced pro-inflammatory cytokines [145], [146]. Further, it inhibits nuclear factor kappa-light-chain-enhancer of activated B cells (NFκβ) pathway [147] and also diverts the development of inflammatory T-helper (type 17) cells into anti-inflammatory regulatory T-cells (T-reg cell) which reduces the expression of pro-inflammatory cytokines such as IL-1, IL-6, IL-10, IL-12, IL-17, IL-23 and TNF-α [145], [146], [148]. Vitamin D has also been reported to downregulate the expression of ACE2 receptors [149]. In addition to immunomodulation, vitamin D has also been predicted in silico to inhibit SARS-CoV-2 endoribonuclease Nsp15. The results suggested that vitamin D have the highest potency with strongest interaction in terms of lowest binding energy, lowest RMSD, and lowest inhibition intensity Ki than the standard (remdesivir, chloroquin, and HCQ) compounds selected for the study [150].
During COVID-19 pandemic, 20% population in Northern Europe, 30–60% in Western, Southern and Eastern Europe and up to 80% in Middle East countries are vitamin D deficient either due to poor diet or limited sunlight exposure [151]. Recently, an observational study revealed that an inverse correlation exists between the mean level of vitamin D and SARS-CoV-2 infection (r = − 0.43, p = 0.02) and mortality (r = − 0.42, p = 0.02) rate, concluding, the association of vitamin D with SARS-CoV-2 infection and COVID-19 related mortality [152]. Further, a retrospective, observational analysis of 191,779 patients showed that the SARS-CoV-2 positivity rate was higher in the 39,190 patients with deficient vitamin D values of < 20 ng/mL than in the 27,870 patients with values 30–34 ng/mL and 12,321 patients with values ≥ 55 ng/mL. This study suggested to explore the role of vitamin D supplementation in reducing the risk of COVID-19 disease [153]. Further, a systemic meta-analysis report suggested that vitamin D supplementation may prevent acute respiratory tract infections [154], and, therefore, adequate levels of vitamin D in host is utmost important during SARS-CoV-2 infection and COVID-19.
Parallelly, Vitamin C maintains the intracellular redox homeostasis to protect the integrity of epithelial barriers. It also aids in the proliferation, functions, and migration of phagocytes (neutrophils, macrophages), and lymphocytes to promote phagocytosis and antibodies generation respectively. Further, it modulates cytokines production and bring about anti-inflammatory responses [155], [156]. Vitamin C has a good safety profile as a therapeutic agent among wide range of clinical cases and therefore, vitamin C supplementation may favourably impact patients with severe COVID-19 disease by; reducing inflammation and pathogen virulence, optimising immune defence and reducing tissue injury [157].
It is well documented that the deficiency of zinc is associated with immune dysfunction [158], growth retardation, hypogonadism [159], cognitive impairment [160] and various other nonviral/viral human diseases [161], [162]. Further, various specialized reviews about the use of zinc as intervention, supplementation and its role in elderly immunity during COVID-19 crisis have been published [163], [164], [165]. Zn being well tolerated with its antioxidant, anti-inflammatory, immunomodulatory, and antiviral activities, Zn supplementation can be safely recommended in COVID-19 [163]. Likewise, other nutritional micronutrients including vitamins, minerals and microbiota are crucial for proper structure and functioning of numerous proteins, physiological processes, signalling pathways associated with normal functioning and modulation of immune system. Sever deficiency of these micronutrients, especially during SARS-CoV-2 infection, may contribute to cytokine storm, comorbidity and mortality [166]. Hence, nutritional interventions and supplementation of these micronutrients is highly recommended.
3.2.2. Immunomodulatory Steroids
A preliminary report by researchers announcing that synthetic glucocorticoid dexamethasone (6 mg once daily) is a potential treatment option, to reduce mortality in severely ill patients, filled the scientific community with excitement. The study trial reported that, after initiation of immunosuppressive dexamethasone therapy, the mortality rate of patients who were on ventilator support was dropped by one-third, whereas, the mortality rate was reduced by one-fifth among patients who were on oxygen support without ventilation. However, patients who were not receiving any respiratory support did not see any positive outcome [167], [168]. In most infected people, mild or moderate symptomatic disease is successfully resolved through a coordinated antiviral immune response. Whereas, an unregulated cell death and tissue damage in severely ill comorbid COVID-19 patients reaches a threshold at which alarmins (such as heat shock proteins, ATP, uric acid, HMGB1, IL-1α and IL-33) or damage-associated molecular patterns (DAMPs) are released which results in added systemic production of hyperinflammatory cytokines and chemokines (‘cytokine storm’) leading to vascular leakage and uncontrolled feedforward inflammatory loop which manifests ARDS, sepsis, organ failure and, eventually, death. It is in this phase of disease where the immunomodulatory effects of glucocorticoids are beneficial by breaking the inflammatory feedforward loop [168], [169].
3.2.3. Cytokine based interventions
3.2.3.1. Interferons
Having few encouraging results of interferons (IFNs) treatment over SARS and MERS, it was worthwhile to validate the impact of interferons (Type I-III) treatment on SARS-CoV-2. In early 2020, a report claimed that type I IFN-α or IFN-β at a concentration of 50 international units (IU) per mL reduced viral titers by 3.4 log or over 4 log, respectively, in Vero cells. Further they reported that the EC50 of type I IFN-α and IFN-β treatment was 1.35 IU/ml and 0.76 IU/ml, respectively, in Vero cells. These results suggest that SARS-CoV-2 is more sensitive to IFNs treatment as compared to SARS or MERS-CoV [170]. Nevertheless, a study indicated that IFNs upregulate the expression of host ACE2 (entry receptor for SARS-CoV-2) [171] raising a question on IFNs possibility to exacerbate COVID-19. However, a recent comparison between the antiviral- and ACE2-inducing properties of IFNs (type I, II and III) in human lung cell line and primary human bronchial epithelial cells suggested that IFNs antiviral actions counterbalance the increased expression of ACE2 and restrict SARS-CoV-2 [172]. Further, two other separate studies also reported that interferons type I (IFN-α) and type III (IFN-λ) inhibit SARS-CoV-2 replication in dose dependent manner. However, type III (IFN-λ) generated a weaker but long-lasting antiviral response [173], [174]. Several clinical trials of type-I IFN or pegylated interferon alfa-2b are being conducted, either alone (NCT04293887, NCT04320238, ChiCTR2000029989) or in combination (NCT04254874, NCT04276688, NCT04273763, NCT04315948, NCT04350684, NCT04350281, NCT04343768, NCT04350671, NCT04379518) [122].
3.2.3.2. Interleukin inhibitors
Interleukin-6 (IL-6) is a major factor responsible for cytokine storm and ARDS in COVID-19. Hence, several anti-IL-6 receptor monoclonal antibodies (tocilizumab, sarilumab) or anti-IL-6 monoclonal antibodies (siltuximab, clazakizumab, sirukumab) [175] are currently under clinical trials (NCT04306705, NCT04322773, NCT04320615, NCT04324073, NCT04315298, NCT04315480, NCT04321993, NCT04335071, NCT04317092, NCT04348500, NCT04329650) are under way to evaluate the safety and efficacy of IL-6 inhibitors [122]. Sarilumab is a recombinant humanized anti-IL-6 receptor monoclonal antibody approved by FDA for patients with rheumatoid arthritis. The efficacy and safety of sarilumab 400 mg IV and sarilumab 200 mg IV versus placebo was evaluated in hospitalized patients with COVID-19 in an adaptive Phase 2 and 3, randomized (2:2:1), double-blind, placebo-controlled trial (NCT04315298). However, the trial findings do not support a clinical benefit of sarilumab for severe COVID-19 [176]. In contrast, a clinical trial using tocilizumab on 30 selected adult patients hospitalized with COVID-19 suggested that tocilizumab significantly reduced mechanical ventilation requirement (OR: 0.42; p = 0.025) and risk of subsequent ICU admission (OR = 0.17; p = 0.001). Further, no moderate or severe adverse events attributable to tocilizumab were reported. The overall mortality rate was 11% [176]. Thus, inhibition of cytokine IL-6 axis using tocilizumab in severe COVID-19 patients appears promising therapy. However, the clinical data of using anti-IL-6 monoclonal antibody (siltuximab) in patients with COVID-19 is limited and unpublished [176].
Interleukin-1β (IL-1β), a proinflammatory cytokine, along with its natural interleukin-1 receptor antagonist (IL-1Ra) has been observed in patients with COVID-19 induced ARDS and pneumonia [177]. Further, elevated IL-1β is also central to macrophage activation syndrome (MAS) and hemophagocytic lymphohistiocytosis (HLH) [178], [179]. Anakinra (recombinant IL-1Ra) and canakinumab (monoclonal antibody targeting IL-1 β) has proved their effectiveness in treating MAS and HLH through continuous intravenous infusion (IVF) [179], [180]. However, one patient had progressive multisystem organ failure despite anakinra at 2400 mg daily [180], [181]. Therefore, being potential drugs to treat cytokine storm syndrome, the two drugs are presently under clinical trials (NCT04341584, NCT04339712, NCT04330638, NCT04348448, NCT04324021) for severe COVID19 [122].
3.2.4. Convalescent plasma therapy (CPT)
The ongoing viral pandemic has resulted in scaling up of pooled plasma from recovered patients to unprecedented levels. Compared to the historical usage of CPT, modern blood screening, banking and pathogen inactivation technologies have now added an extra layer of safety to its usage. Nevertheless, CPT is also associated with certain known and theoretical risk factors including transfusion-related acute lung injury (TRALI), antibody-dependent enhancement (ADE) and anaphylactic immunological responses. Our group recently published a detailed review on various aspects of CPT including current technologies and the shortcomings related to the collection, manufacture, pathogen inactivation, and banking of convalescent plasma, with a specific focus on their plausible applications, benefits, and risks in the COVID-19 pandemic [182].
3.2.5. Vaccines
The nature of protective immune responses against COVID-19 has not been completely understood. Further, it is also not clear that which strategy of vaccine development will get through the expectation of maximum protection. Hence, it is imperative to develop vaccines through diverse platforms and variant formulation technologies such as nucleic acid (DNA/RNA) vaccines, live attenuated virus vaccines, recombinant viral vector vaccines, inactivated virus vaccines, protein subunit vaccines, conjugated vaccines, toxoid vaccines [183]. Various review articles regarding COVID-19 vaccine development have already been published [183], [184], [185], [186], [187], [188], [189], [190] pertaining to immunological principles [183], experimental and clinical data obtained from recent SARS-CoV-2 vaccines trials [184], advanced manufacturing [185], vaccine-associated immune enhanced disease [186], non-viral vaccine development technologies [187], clinical efficacy [188], advantages and disadvantages of various vaccine technologies [189], vaccinomics (effectiveness) and adversomics (adverse effects) of vaccine candidates [190]. Nevertheless, there are concerns regarding virus evolution and resistance [191]. Although, vaccine resistance rarely emerges since vaccines tend to work prophylactically to induce immune responses against multiple targets of pathogen, consequently, to generate less variation for vaccine resistance [192]. However, RBD surface often have distinct antibodies escape mutations [193] overtime. Hence, a detailed review covering all the aspects of vaccine design and development using recombinant technology has recently been published by our group [194].
3.3. Miscellaneous adjuvant therapy approaches
Human tissues being sensitive to oxygen may undergo irreversible damage due to ischemia and tissue hypoxia, which is quite evident in COVID-19, leading to systemic, multi-organ failure among patients [195]. Thus, apart from the main stream antiviral and immunomodulatory interventions, various adjuvant therapy approaches, based on patient’s clinical and/or comorbid condition, are required during and after COVID-19. These adjuvant therapies may include; statins [196], antihypertensives [197], anti-diabetics [198], anti-thrombotics and platelets in thromboembolism and coagulopathy [199], [200], iron depletion therapy [201], hyperbaric oxygen/ozone therapy [202], mesenchymal stem cell therapy [203], etc for the management of clinical and/or comorbid conditions.
3.4. Complementary and integrative health approaches
National Center for Complementary and Integrative Health (NCCIH), formerly known as the National Center for Complementary and Alternative Medicine, in NIH, USA is the lead agency for scientific research on the diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine (CM) [204]. Complementary and integrative health approaches comprise of natural products practices such as dietary supplements, herbs, botanicals and probiotics [204]. In India, these practices are classified under AYUSH (Ayurveda, Yoga, Unani, Siddha, and Homeopathy) [4], [205] whereas in China its Traditional Chinese Medicine (TCM) [206]. Since ancient times, Indian medicinal herbs and TCM have been in use as a treatment and preventive strategy for several diseases including respiratory viral infections [4], [205], [206]. These antioxidant medicinal herbs not only have the capability to modulate immune system with downregulation of proinflammatory cytokines but also have antiviral properties to directly inhibit viral proteins and viral replication machinery [4], [207], [208]. Further, these herbs are generally administered in the form of polyherbal formulations [209] (Ayurveda/TCM) or herbo-mineral formulations [210] (Siddha) or homeopathic dilutions [211]. The polyherbal formulations have multiple components in micromolar concentration which work together in a synergistic manner [212] to suppress the production of nitric oxide, prostaglandin E2, IL-6, IL1β, phosphorylations of mitogen-activated protein (MAP) kinases, including extracellular signal-regulated kinase (ERK) [213]. Further, polyherbal formulations inhibit the production of inflammatory cytokines and macrophage activation to treat upper respiratory tract infections via down-regulating the activation of NF-κB signaling pathway, demonstrating their multicomponent- multitarget -multichannel molecular mechanisms [213]. Hence, these therapies should be considered as an adjuvant therapy while treating COVID-19.
4. Emerging future strategies for SARS-CoV-2
The debris of dying and virally infected cells in COVID-19 acts as a substrate for secondary bacterial infections which leads to additional inflammatory responses, vascular leakage, sepsis and death [214]. Bacteriophages are viruses which selectively attach specific bacterial species but are otherwise harmless to human cells. They hijack the bacteria’s biological machinery to replicate and attack other neighboring bacterial colonies. Further, the modified bacteriophages could quickly manufacture specific antibodies against SARS-CoV-2 using “phage display technique”. Hence, bacteriophages could be the potential game changer in the trajectory of COVID-19 [215] by not only reducing the probability of secondary bacterial infection and sepsis but also by giving an extra time to the patient’s adaptive immune responses to produce their own specific antibodies against SARS-CoV-2 [215].
Further, immune function is a highly energetically-expensive process and requires energy-dependent processes for activation, migration of cells, antigen processing, and phagocytosis [216]. In the process of activation of innate immunity, the body temperature rises (induction of fever) at a cost of > 10% increase in metabolic rate per 1 ◦C [217]. Finally, the adaptive immunity intensifies energy metabolism for the production of virus specific T- and B- lymphocytes [218]. Hence, progressive energy depletion leads to dysfunctional immune system and, ultimately, to cell death. Therefore, understanding the bioenergetics view of COVID-19 immunopathology, photomagnetic catalysis of ATP synthesis, regenerative photobiomodulation and the ultrasonic acceleration of cell restructuring has been proposed [219], [220]. Furthermore, a coherent application of multiple biophysical radiances (coMra) in enhancing the energy-matter-information kinetics synergistically which may improve immune functions to accelerate recovery has also been proposed [220].
Finally, viral surveillance through stand-off biosensors to detect and classify viruses would be required for future pandemics. Laser-induced fluorescence-light detection and ranging (LIF-LiDAR) is a versatile tool that has been explored for detection of bacteriophage on artificially contaminated biological surfaces and aerosol particles. Considering the increasing applications of LIF-LiDAR to potentially detect and classify pathogens, the research over prospects and challenges of LIF-LiDAR technology has already been initiated [221].
5. Conclusions and future perspectives
The SARS-CoV-2 outbreak as a pandemic has lasted for almost a year now and it is likely that the infection will remain in humans until clinically approved vaccines are made available through out the world. Nevertheless, mutation and cross species jump among coronaviruses remain the matter of concern and needs to be kept under scientific surveillance for future pandemics. Currently, the best way to prevent SARS-CoV-2 infection, indeed, are personal preventive measures such as social distancing, washing hands regularly, and wearing face mask. However, high nutritional, metabolic and lifestyle status has a major role to play in activating host’s innate and adaptive immune system and keeping the disease asymptomatically mild while fighting infection. Whereas, low values with comorbidities may lead to severely symptomatic ARDS, sepsis, multi-organ failure and death even after treatment with FDA approved antivirals, immunomodulators and adjuvant therapies. Although a number of repurposed drugs are currently in use, however, specific antivirals against SARS-CoV-2 replication machinery are desperately needed. Parallelly, complementary and integrative health approaches including physical exercises, botanicals and poly-herbo-mineral formulations such as Ayurveda, Siddha and TCM have evolved as a prophylactic, adjuvant and treatment options demonstrating their multicomponent- multitarget -multichannel molecular mechanisms and network pharmacology. Pharma giants with modern scientific and pharmaceutical technology should take up these evidence based complementary and alternative indigenous medicines to discover, develop and bring deserving candidates into mainstream CM.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the Director, Department of Health & Family Welfare, Government of Sikkim, India; the Principal, Government Pharmacy College, Sajong, India; and the Vice Chancellor, King George’s Medical University (KGMU), Lucknow, India for the support and encouragement for this work.
References
- 1.Burki T. Understanding variants of SARS-CoV-2. Lancet (London, England) 2021;397(10273):462. doi: 10.1016/S0140-6736(21)00298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall M.W., Joshi I., Leal L., Ooi E.E. Immune modulation in COVID-19: strategic considerations for personalized therapeutic intervention. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benani A., Ben Mkaddem S. Mechanisms underlying potential therapeutic approaches for COVID-19. Front. Immunol. 2020;11:1841. doi: 10.3389/fimmu.2020.01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P., Cho S.G., Kumar N.S., Subramaniam M.D. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acúrcio R.C., Carreira B., Yeini E., Tiram G., Liubomirski Y., Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15(8):630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asrani P., Hassan M.I. SARS-CoV-2 mediated lung inflammatory responses in host: targeting the cytokine storm for therapeutic interventions. Mol. Cell Biochem. 2021;476(2):675–687. doi: 10.1007/s11010-020-03935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Horani R.A., Kar S. Potential anti-SARS-CoV-2 therapeutics that target the post-entry stages of the viral life cycle: a comprehensive review. Viruses. 2020;12(10):1092. doi: 10.3390/v12101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The Architecture of SARS-CoV-2 transcriptome. Cell. 2020;181(4):914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chand G.B., Banerjee A., Azad G.K. Identification of novel mutations in RNA-dependent RNA polymerases of SARS-CoV-2 and their implications on its protein structure. PeerJ. 2020;8 doi: 10.7717/peerj.9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouhaddou M., Memon D., Meyer B., White K.M., Rezelj V.V., Correa Marrero M., Polacco B.J., Melnyk J.E., Ulferts S., Kaake R.M., Batra J., Richards A.L., Stevenson E., Gordon D.E., Rojc A., Obernier K., Fabius J.M., Soucheray M., Miorin L., Moreno E., Koh C., Tran Q.D., Hardy A., Robinot R., Vallet T., Nilsson-Payant B.E., Hernandez-Armenta C., Dunham A., Weigang S., Knerr J., Modak M., Quintero D., Zhou Y., Dugourd A., Valdeolivas A., Patil T., Li Q., Hüttenhain R., Cakir M., Muralidharan M., Kim M., Jang G., Tutuncuoglu B., Hiatt J., Guo J.Z., Xu J., Bouhaddou S., Mathy C.J.P., Gaulton A., Manners E.J., Félix E., Shi Y., Goff M., Lim J.K., McBride T., O'Neal M.C., Cai Y., Chang J.C.J., Broadhurst D.J., Klippsten S., De W.E., Leach A.R., Kortemme T., Shoichet B., Ott M., Saez-Rodriguez J., tenOever B.R., Mullins R.D., Fischer E.R., Kochs G., Grosse R., García-Sastre A., Vignuzzi M., Johnson J.R., Shokat K.M., Swaney D.L., Beltrao P., Krogan N.J. The global phosphorylation landscape of SARS-CoV-2 infection. Cell. 2020;182(3):685–712.e19. doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burridge J., Bradfield J., Jaffee A., Broadley I., Ray S. Metabolic health and COVID-19: a call for greater medical nutrition education. Lancet Diabetes Endocrinol. 2020;8(8):665–666. doi: 10.1016/S2213-8587(20)30220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R. Silverio, D.C. Gonçalves, M.F. Andrade, M. Seelaender, Coronavirus Disease 2019 (COVID-19) and nutritional status: the missing link? Adv. Nutr. (2020) nmaa125. Advance online publication. 10.1093/advances/nmaa125. [DOI] [PMC free article] [PubMed]
- 13.Wood T.R., Jóhannsson G.F. Metabolic health and lifestyle medicine should be a cornerstone of future pandemic preparedness. Lifestyle Med. 2020;1 doi: 10.1002/lim2.2. [DOI] [Google Scholar]
- 14.Srivastava R., Daulatabad S.V., Srivastava M., Janga S.C. Role of SARS-CoV-2 in altering the RNA-binding protein and miRNA-directed post-transcriptional regulatory networks in humans. Int. J. Mol. Sci. 2020;21(19):7090. doi: 10.3390/ijms21197090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann. Intern. Med. 2020;173(5):362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson N.M., Norton A., Young F.P., Collins D.W. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020;75(8):1086–1095. doi: 10.1111/anae.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan M., Wu N.C., Zhu X., Lee C.D., So R., Lv H., Mok C., Wilson I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368(6491):630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang S., Yang M., Hong Z., Zhang L., Huang Z., Chen X., He S., Zhou Z., Zhou Z., Chen Q., Yan Y., Zhang C., Shan H., Chen S. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin B. 2020;10(7):1228–1238. doi: 10.1016/j.apsb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hemert M.J., van den Worm S.H., Knoops K., Mommaas A.M., Gorbalenya A.E., Snijder E.J. SARS-coronavirus replication/transcription complexes are membrane-protected and need a host factor for activity in vitro. PLoS Pathog. 2008;4(5) doi: 10.1371/journal.ppat.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori M., Capasso C., Carta F., Donald W.A., Supuran C.T. A deadly spillover: SARS-CoV-2 outbreak. Expert Opin. Ther. Pat. 2020;30(7):481–485. doi: 10.1080/13543776.2020.1760838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao S.N., Manissero D., Steele V.R., Pareja J. Correction to: a systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect. Dis. Ther. 2020;9(3):587. doi: 10.1007/s40121-020-00328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ra S.H., Lim J.S., Kim G.U., Kim M.J., Jung J., Kim S.H. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. 2021;76(1):61–63. doi: 10.1136/thoraxjnl-2020-215042. [DOI] [PubMed] [Google Scholar]
- 27.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai S., Sun W., Li M., Dong L. A complex COVID-19 case with rheumatoid arthritis treated with tocilizumab. Clin. Rheumatol. 2020;39(9):2797–2802. doi: 10.1007/s10067-020-05234-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutlu Ö., Metin A. Relative changes in the pattern of diseases presenting in dermatology outpatient clinic in the era of the COVID-19 pandemic. Dermatol. Ther. 2020;33(6) doi: 10.1111/dth.14096. [DOI] [PubMed] [Google Scholar]
- 31.Elbeddini A., To A., Tayefehchamani Y., Wen C. Potential impact and challenges associated with Parkinson’s disease patient care amidst the COVID-19 global pandemic. J. Clin. Mov. Disord. 2020;7:7. doi: 10.1186/s40734-020-00089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heneka M.T., Golenbock D., Latz E., Morgan D., Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res. Ther. 2020;12(1):69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salehi S., Reddy S., Gholamrezanezhad A. Long-term Pulmonary Consequences of Coronavirus Disease 2019 (COVID-19): what we know and what to expect. J. Thorac Imaging. 2020;35(4):W87–W89. doi: 10.1097/RTI.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 35.Gao Z., Xu Y., Sun C., Wang X., Guo Y., Qiu S., Ma K. A Systematic Review of Asymptomatic Infections with COVID-19. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.05.001. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narasaraju T. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;173(4):323–324. doi: 10.7326/L20-0894. [DOI] [PubMed] [Google Scholar]
- 37.Yap J., Moriyama M., Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J. Immunol. 2020;205(2):307–312. doi: 10.4049/jimmunol.2000513’. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang B., Zhou X., Qiu Y., Song Y., Feng F., Feng J., Song Q., Jia Q., Wang J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE. 2020;15(7):e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J., Jiang H. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 45.Tan M., Liu Y., Zhou R., Deng X., Li F., Liang K., Shi Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261–268. doi: 10.1111/imm.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., Dai J., Sun Q., Zhao F., Qu J., Yan F. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J., Zhang W., Wang Y., Bao S., Li Y., Wu C., Liu H., Liu D., Shao J., Peng X., Yang Y., Liu Z., Xiang Y., Zhang F., Silva R.M., Pinkerton K.E., Shen K., Xiao H., Xu S., Wong G.W.K., Chinese Pediatric Novel Coronavirus Study Team SARS-CoV-2 infection in children. N. Engl. J. Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., Li S.B., Wang H.Y., Zhang S., Gao H.N., Sheng J.F., Cai H.L., Qiu Y.Q., Li L.J. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D., Xu W., Zhang C., Yu J., Jiang B., Cao H., Li L. Clinical characteristics of imported cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 2020;71(15):706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L., Gou J., Gao J., Huang L., Zhu Z., Ji S., Liu H., Xing L., Yao M., Zhang Y. Immune characteristics of severe and critical COVID-19 patients. Signal Transduct. Target Ther. 2020;5(1):179. doi: 10.1038/s41392-020-00296-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofmann H., Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12(10):466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trezza A., Iovinelli D., Santucci A., Prischi F., Spiga O. An integrated drug repurposing strategy for the rapid identification of potential SARS-CoV-2 viral inhibitors. Sci. Rep. 2020;10(1):13866. doi: 10.1038/s41598-020-70863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maurya V.K., Kumar S., Prasad A.K., Bhatt M., Saxena S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. Virusdisease. 2020;31(2):179–193. doi: 10.1007/s13337-020-00598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maurya V.K., Kumar S., Bhatt M., Saxena S.K. Antiviral activity of traditional medicinal plants from Ayurveda against SARS-CoV-2 infection. J. Biomol. Struct. Dyn. 2020:1–17. doi: 10.1080/07391102.2020.1832577. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71(15):732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44(2):173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 64.Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008;93(5):543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodrigues Prestes T.R., Rocha N.P., Miranda A.S., Teixeira A.L., Simoes-E-Silva A.C. The anti-inflammatory potential of ACE2/Angiotensin-(1–7)/Mas Receptor axis: evidence from basic and clinical research. Curr. Drug Targets. 2017;18(11):1301–1313. doi: 10.2174/1389450117666160727142401. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K., Matsuda Z., Kawaguchi Y., Kawaoka Y., Inoue J.I. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12(6):629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bestle D., Heindl M.R., Limburg H., Van Lam van T., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C., Klenk H.D., Garten W., Steinmetzer T., Böttcher-Friebertshäuser E. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance. 2020;3(9):e202000786. doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bojkova D., Bechtel M., McLaughlin K.M., McGreig J.E., Klann K., Bellinghausen C., Rohde G., Jonigk D., Braubach P., Ciesek S., Münch C., Wass M.N., Michaelis M., Cinatl J., Jr Aprotinin inhibits SARS-CoV-2 replication. Cells. 2020;9(11):2377. doi: 10.3390/cells9112377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 2020;64(6):e00754–20. doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camostat mesilate treating patients with hospitalized patients with COVID-19 (RECOVER). NCT04470544. NIH U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04470544 (Accessed on 10th Nov 2020).
- 71.Clinical Efficacy of Nafamostat Mesylate for COVID-19 Pneumonia. NCT04418128. NIH U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04509999 (Accessed on 10th Nov 2020).
- 72.Bicalutamide to Block TMPRSS2 in Males With COVID-19 Infection. NCT04509999. NIH U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04509999 (Accessed on 10th Nov 2020).
- 73.Idris M.O., Yekeen A.A., Alakanse O.S., Durojaye O.A. Computer-aided screening for potential TMPRSS2 inhibitors: a combination of pharmacophore modeling, molecular docking and molecular dynamics simulation approaches. J. Biomol. Struct. Dyn. 2020:1–19. doi: 10.1080/07391102.2020.1792346. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pooja M., Reddy G.J., Hema K., Dodoala S., Koganti B. Unravelling high-affinity binding compounds towards transmembrane protease serine 2 enzyme in treating SARS-CoV-2 infection using molecular modelling and docking studies. Eur. J. Pharmacol. 2021;890 doi: 10.1016/j.ejphar.2020.173688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu T., Luo S., Libby P., Shi G.P. Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020;213 doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tripathi R., Fiore L.S., Richards D.L., Yang Y., Liu J., Wang C., Plattner R. Abl and Arg mediate cysteine cathepsin secretion to facilitate melanoma invasion and metastasis. Sci. Signal. 2018;11(518):eaao0422. doi: 10.1126/scisignal.aao0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weston S., Coleman C.M., Haupt R., Logue J., Matthews K., Li Y., Reyes H.M., Weiss S.R., Frieman M.B. Broad anti-coronavirus activity of food and drug administration-approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J. Virol. 2020;94(21):e01218–20. doi: 10.1128/JVI.01218-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weisberg E., Parent A., Yang P.L., Sattler M., Liu Q., Liu Q., Wang J., Meng C., Buhrlage S.J., Gray N., Griffin J.D. Repurposing of kinase inhibitors for treatment of COVID-19. Pharm. Res. 2020;37(9):167. doi: 10.1007/s11095-020-02851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.K., Wang Q., Du L., Tan W., Wilson I.A., Jiang S., Yang B., Lu L. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019;5(4):eaav4580. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol. Immunol. 2020;17(7):765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klemm T., Ebert G., Calleja D.J., Allison C.C., Richardson L.W., Bernardini J.P., Lu B.G., Kuchel N.W., Grohmann C., Shibata Y., Gan Z.Y., Cooney J.P., Doerflinger M., Au A.E., Blackmore T.R., van der Heden van Noort G.J., Geurink P.P., Ovaa H., Newman J., Riboldi-Tunnicliffe A., Czabotar P.E., Mitchell J.P., Feltham R., Lechtenberg B.C., Lowes K.N., Dewson G., Pellegrini M., Lessene G., Komander D. Mechanism and inhibition of the papain-like protease, PLpro, of SARS-CoV-2. EMBO J. 2020;39(18):e106275. doi: 10.15252/embj.2020106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuen C.K., Lam J.Y., Wong W.M., Mak L.F., Wang X., Chu H., Cai J.P., Jin D.Y., To K.K., Chan J.F., Yuen K.Y., Kok K.H. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg. Microbes Infect. 2020;9(1):1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maiti B.K. Can papain-like protease inhibitors halt SARS-CoV-2 replication? ACS Pharmacol. Transl. Sci. 2020;3(5):1017–1019. doi: 10.1021/acsptsci.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang D.H., Wu K.L., Zhang X., Deng S.Q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kandeel M., Abdelrahman A., Oh-Hashi K., Ibrahim A., Venugopala K.N., Morsy M.A., Ibrahim M. Repurposing of FDA-approved antivirals, antibiotics, anthelmintics, antioxidants, and cell protectives against SARS-CoV-2 papain-like protease. J. Biomol. Struct. Dyn. 2020:1–8. doi: 10.1080/07391102.2020.1784291. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Naidoo D., Roy A., Kar P., Mutanda T., Anandraj A. Cyanobacterial metabolites as promising drug leads against the Mpro and PLpro of SARS-CoV-2: an in silico analysis. J. Biomol. Struct. Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1794972. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Quimque M., Notarte K., Fernandez R., Mendoza M., Liman R., Lim J., Pilapil L., Ong J., Pastrana A.M., Khan A., Wei D.Q., Macabeo A. Virtual screening-driven drug discovery of SARS-CoV2 enzyme inhibitors targeting viral attachment, replication, post-translational modification and host immunity evasion infection mechanisms. J. Biomol. Struct. Dyn. 2020:1–18. doi: 10.1080/07391102.2020.1776639. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rut W., Groborz K., Zhang L., Sun X., Zmudzinski M., Pawlik B., Wang X., Jochmans D., Neyts J., Młynarski W., Hilgenfeld R., Drag M. SARS-CoV-2 Mpro inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2021;17(2):222–228. doi: 10.1038/s41589-020-00689-z. [DOI] [PubMed] [Google Scholar]
- 91.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368(6489):409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582(7811):289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 93.Müller A., Cadenas E., Graf P., Sies H. A novel biologically active seleno-organic compound–I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen) Biochem. Pharmacol. 1984;33(20):3235–3239. doi: 10.1016/0006-2952(84)90083-2. [DOI] [PubMed] [Google Scholar]
- 94.Carroll L., Gardiner K., Ignasiak M., Holmehave J., Shimodaira S., Breitenbach T., Iwaoka M., Ogilby P.R., Pattison D.I., Davies M.J. Interaction kinetics of selenium-containing compounds with oxidants. Free Radic. Biol. Med. 2020;155:58–68. doi: 10.1016/j.freeradbiomed.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 95.Sies H., Parnham M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020;156:107–112. doi: 10.1016/j.freeradbiomed.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ali M.H., Schlidt S.A., Chandel N.S., Hynes K.L., Schumacker P.T., Gewertz B.L. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am. J. Physiol. 1999;277(5):L1057–L1065. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- 97.Feng G., Zheng K.I., Yan Q.Q., Rios R.S., Targher G., Byrne C.D., Poucke S.V., Liu W.Y., Zheng M.H. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J. Clin. Transl. Hepatol. 2020;8(1):18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kono H., Arteel G.E., Rusyn I., Sies H., Thurman R.G. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic. Biol. Med. 2001;30(4):403–411. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- 99.Koyanagi T., Nakamuta M., Enjoji M., Iwamoto H., Motomura K., Sakai H., Nawata H. The selenoorganic compound ebselen suppresses liver injury induced by Propionibacterium acnes and lipopolysaccharide in rats. Int. J. Mol. Med. 2001;7(3):321–327. doi: 10.3892/ijmm.7.3.321. [DOI] [PubMed] [Google Scholar]
- 100.Liu H., Ye F., Sun Q., Liang H., Li C., Li S., Lu R., Huang B., Tan W., Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzyme Inhib. Med. Chem. 2021;36(1):497–503. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su H., Yao S., Zhao W., Li M., Liu J., Shang W., Xie H., Ke C., Gao M., Yu K., Liu H., Shen J., Tang W., Zhang L., Zuo J., Jiang H., Bai F., Wu Y., Ye Y., Xu Y. Discovery of Baicalin and Baicalein as Novel, Natural Product Inhibitors of SARS-CoV-2 3CL Protease. bioRxiv. 2020 doi: 10.1101/2020.04.13.038687. [DOI] [Google Scholar]
- 102.Dai W., Zhang B., Jiang X.M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368(6497):1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Q., Kang C. Progress in developing inhibitors of SARS-CoV-2 3C-like protease. Microorganisms. 2020;8(8):1250. doi: 10.3390/microorganisms8081250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu P., Liu H., Sun Q., Liang H., Li C., Deng X., Liu Y., Lai L. Potent inhibitors of SARS-CoV-2 3C-like protease derived from N-substituted isatin compounds. Eur. J. Med. Chem. 2020;206 doi: 10.1016/j.ejmech.2020.112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coelho C., Gallo G., Campos C.B., Hardy L., Würtele M. Biochemical screening for SARS-CoV-2 main protease inhibitors. PLoS ONE. 2020;15(10) doi: 10.1371/journal.pone.0240079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kneller D.W., Galanie S., Phillips G., O'Neill H.M., Coates L., Kovalevsky A. Malleability of the SARS-CoV-2 3CL Mpro active-site cavity facilitates binding of clinical antivirals. Structure. 2020;28(12):1313–1320.e3. doi: 10.1016/j.str.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cherrak S.A., Merzouk H., Mokhtari-Soulimane N. Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: a molecular docking and simulation studies. PLoS ONE. 2020;15(10) doi: 10.1371/journal.pone.0240653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacArthur R.D., Novak R.M. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin. Infect. Dis. 2008;47(2):236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- 109.Shamsi A., Mohammad T., Anwar S., AlAjmi M.F., Hussain A., Rehman M.T., Islam A., Hassan M.I. Glecaprevir and Maraviroc are high-affinity inhibitors of SARS-CoV-2 main protease: possible implication in COVID-19 therapy. Biosci Rep. 2020;40(6) doi: 10.1042/BSR20201256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.National Center for Biotechnology Information, “PubChem Compound Summary for CID 66828839, Glecaprevir” PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/66828839 (Accessed 12 Nov., 2020).
- 111.Choy K.T., Wong A.Y., Kaewpreedee P., Sia S.F., Chen D., Hui K., Chu D., Chan M., Cheung P.P., Huang X., Peiris M., Yen H.L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Q., Zhao Y., Chen X., Hong A. Virtual screening of approved clinic drugs with main protease (3CLpro) reveals potential inhibitory effects on SARS-CoV-2. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1817786. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics. 2020;47(2):119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jang M., Park Y.I., Park R., Cha Y.E., Namkoong S., Lee J.I. Lopinavir-ritonavir is not an effective inhibitor of the main protease activity of SARS-CoV-2 in vitro. bioRxiv. 2020 doi: 10.1101/2020.09.16.299800. [DOI] [Google Scholar]
- 115.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M., Woodfall B., Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hung H.C., Ke Y.Y., Huang S.Y., Huang P.N., Kung Y.A., Chang T.Y., Yen K.J., Peng T.T., Chang S.E., Huang C.T., Tsai Y.R., Wu S.H., Lee S.J., Lin J.H., Liu B.S., Sung W.C., Shih S.R., Chen C.T., Hsu J.T. Discovery of M protease inhibitors encoded by SARS-CoV-2. Antimicrob. Agents Chemother. 2020;64(9):e00872–20. doi: 10.1128/AAC.00872-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fintelman-Rodrigues N., Sacramento C.Q., Ribeiro Lima C., Souza da Silva F., Ferreira A.C., Mattos M., de Freitas C.S., Cardoso V., Soares S., da Silva Gomes Dias, Temerozo J.R., Miranda M.D., Matos A.R., Bozza F.A., Carels N., Alves C.R., Siqueira M.M., Bozza P.T., Souza T. Atazanavir, alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob. Agents Chemother. 2020;64(10):e00825–20. doi: 10.1128/AAC.00825-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen H., Zhang Z., Wang L., Huang Z., Gong F., Li X., Chen Y., Wu J.J. First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients. Medicine (Baltimore) 2020;99(48) doi: 10.1097/MD.0000000000023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.C., Tian G., Jiang H.W., Tao S.C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., Hall M.D. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.NIH U.S. National Library of Medicine (Accessed on 10th Nov 2020) https://clinicaltrials.gov.
- 123.NIH U.S. National Library of Medicine. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/remdesivir/ (Accessed on 10th Nov 2020).
- 124.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020;76(4):370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing, China) 2020;6(10):1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prakash A., Singh H., Kaur H., Semwal A., Sarma P., Bhattacharyya A., Dhibar D.P., Medhi B. Systematic review and meta-analysis of effectiveness and safety of favipiravir in the management of novel coronavirus (COVID-19) patients. Indian J. Pharmacol. 2020;52(5):414–421. doi: 10.4103/ijp.ijp_998_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dienstag J.L., McHutchison J.G. American Gastroenterological Association medical position statement on the management of hepatitis C. Gastroenterology. 2006;130(1):225–230. doi: 10.1053/j.gastro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 128.Ergönül Ö., Keske Ş., Çeldir M.G., Kara İ.A., Pshenichnaya N., Abuova G., Blumberg L., Gönen M. Systematic review and meta-analysis of postexposure prophylaxis for Crimean-Congo Hemorrhagic Fever Virus among healthcare workers. Emerg. Infect. Dis. 2018;24(9):1642–1648. doi: 10.3201/eid2409.171709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Te H.S., Randall G., Jensen D.M. Mechanism of action of ribavirin in the treatment of chronic hepatitis C. Gastroenterol Hepatol (N Y). 2007;3(3):218–225. [PMC free article] [PubMed] [Google Scholar]
- 130.Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment. NCT04276688. NIH U.S. National Library of Medicine https://clinicaltrials.gov/ct2/show/NCT04276688 (Accessed on 10th Nov 2020).
- 131.The Phase 2 Study to Evaluate the Safety and Efficacy of Clevudine in Patients With Moderate COVID-19. NCT04347915. NIH U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04347915 (Accessed on 10th Nov 2020).
- 132.TAF/FTC for Pre-exposure Prophylaxis of COVID-19 in Healthcare Workers (CoviPrep Study). NCT04405271. NIH U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04405271 (Accessed on 10th Nov 2020).
- 133.Pandeya K.B., Ganeshpurkar A., Mishra M.K. Natural RNA dependent RNA polymerase inhibitors: molecular docking studies of some biologically active alkaloids of Argemone mexicana. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paces J., Strizova Z., Smrz D., Cerny J. COVID-19 and the immune system. Physiol. Res. 2020;69(3):379–388. doi: 10.33549/physiolres.934492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stephensen C.B. Vitamin A, infection, and immune function. Annu. Rev. Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 138.Reifen R. Vitamin A as an anti-inflammatory agent. Proc. Nutr. Soc. 2002;61(3):397–400. doi: 10.1079/PNS2002172. [DOI] [PubMed] [Google Scholar]
- 139.Chew B.P., Park J.S. Carotenoid action on the immune response. J. Nutr. 2004;134(1):257S–261S. doi: 10.1093/jn/134.1.257S. [DOI] [PubMed] [Google Scholar]
- 140.Ross A.C., Chen Q., Ma Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam. Horm. 2011;86:103–126. doi: 10.1016/B978-0-12-386960-9.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bono M.R., Tejon G., Flores-Santibañez F., Fernandez D., Rosemblatt M., Sauma D. Retinoic acid as a modulator of T cell immunity. Nutrients. 2016;8(6):349. doi: 10.3390/nu8060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li R., Wu K., Li Y., Liang X., Tse W., Yang L., Lai K.P. Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging (Albany NY) 2020;12(15):15784–15796. doi: 10.18632/aging.103888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Azrielant S., Shoenfeld Y. Vitamin D and the immune system. Isr Med. Assoc. J. 2017;19(8):510–511. [PubMed] [Google Scholar]
- 147.Chen Y., Zhang J., Ge X., Du J., Deb D.K., Li Y.C. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J. Biol. Chem. 2013;288(27):19450–19458. doi: 10.1074/jbc.M113.467670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Olszowiec-Chlebna M., Koniarek-Maniecka A., Brzozowska A., Błauż A., Rychlik B., Stelmach I. Vitamin D inhibits pro-inflammatory cytokines in the airways of cystic fibrosis patients infected by Pseudomonas aeruginosa- pilot study. Ital. J. Pediatr. 2019;45(1):41. doi: 10.1186/s13052-019-0634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Malek M.A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: implications for a potential treatment for COVID-19. Rev Med Virol. 2020;30(5) doi: 10.1002/rmv.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.M.H. Shalayel, G.M. Al-Mazaideh, S.H. Aladaileh, F.K. Al-Swailmi, M.G. Al-Thiabat, Vitamin D is a potential inhibitor of COVID-19: in silico molecular docking to the binding site of SARS-CoV-2 endoribonuclease Nsp15. Pak. J. Pharm. Sci. 33 (2020) 2179-2186. 10.36721/PJPS.2020.33.5.REG.2179-2186.1. [PubMed]
- 151.Lips P., Cashman K.D., Lamberg-Allardt C., Bischoff-Ferrari H.A., Obermayer-Pietsch B., Bianchi M.L., Stepan J., El-Hajj Fuleihan G., Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019;180(4):P23–P54. doi: 10.1530/EJE-18-0736. [DOI] [PubMed] [Google Scholar]
- 152.Padhi S., Suvankar S., Panda V.K., Pati A., Panda A.K. Lower levels of vitamin D are associated with SARS-CoV-2 infection and mortality in the Indian population: an observational study. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE. 2020;15(9) doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Jr., Stelmach I., Kumar G.T., Urashima M., Camargo C.A., Jr Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cerullo G., Negro M., Parimbelli M., Pecoraro M., Perna S., Liguori G., Rondanelli M., Cena H., D’Antona G. The long history of vitamin C: from prevention of the common cold to potential aid in the treatment of COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.574029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 157.Hoang B.X., Shaw G., Fang W., Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J. Glob. Antimicrob. Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fukada T., Hojyo S., Hara T., Takagishi T. Revisiting the old and learning the new of zinc in immunity. Nat. Immunol. 2019;20(3):248–250. doi: 10.1038/s41590-019-0319-z. [DOI] [PubMed] [Google Scholar]
- 159.A.S. Prasad, A. Miale Jr, Z. Farid, H.H. Sandstead, A.R. Schulert, Clinical and experimental. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. 1963. J. Lab. Clin. Med. 116 (5) (1990) 737–749. [PubMed]
- 160.Sensi S.L., Granzotto A., Siotto M., Squitti R. Copper and zinc dysregulation in Alzheimer’s disease. Trends Pharmacol. Sci. 2018;39(12):1049–1063. doi: 10.1016/j.tips.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 161.Gumulec J., Masarik M., Krizkova S., Adam V., Hubalek J., Hrabeta J., Eckschlager T., Stiborova M., Kizek R. Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 2011;18(33):5041–5051. doi: 10.2174/092986711797636126. [DOI] [PubMed] [Google Scholar]
- 162.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K., Sahoo S., Goswami K., Sharma P., Prasad R. Zinc and COVID-19: basis of current clinical trials. Biol. Trace Elem. Res. 2020:1–11. doi: 10.1007/s12011-020-02437-9. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.de Almeida Brasiel P.G. The key role of zinc in elderly immunity: a possible approach in the COVID-19 crisis. Clin. Nutr. ESPEN. 2020;38:65–66. doi: 10.1016/j.clnesp.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carr A.C. Micronutrient status of COVID-19 patients: a critical consideration. Crit. Care. 2020;24(1):349. doi: 10.1186/s13054-020-03085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.RECOVERY Collaborative Group, P. Horby, W.S. Lim, J.R. Emberson, M. Mafham, J.L. Bell, L. Linsell, N. Staplin, C. Brightling, A. Ustianowski, E. Elmahi, B. Prudon, C. Green, T. Felton, D. Chadwick, K. Rege, C. Fegan, L.C. Chappell, S.N. Faust, T. Jaki, K. Jeffery, A. Montgomery, K. Rowan, E. Juszczak, J.K. Baillie, R. Haynes, M.J. Landray, Dexamethasone in hospitalized patients with Covid-19 - preliminary report, N. Engl. J. Med. (2020) NEJMoa2021436. 10.1056/NEJMoa2021436. Advance online publication.
- 168.Cain D.W., Cidlowski J.A. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat. Rev. Immunol. 2020;20(10):587–588. doi: 10.1038/s41577-020-00421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang D., Han Z., Oppenheim J.J. Alarmins and immunity. Immunol. Rev. 2017;280(1):41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ziegler C., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., Feldman J., Muus C., Wadsworth M.H., 2nd, Kazer S.W., Hughes T.K., Doran B., Gatter G.J., Vukovic M., Taliaferro F., Mead B.E., Guo Z., Wang J.P., Gras D., Plaisant M., Ansari M., Angelidis I., Adler H., Sucre J.M.S., Taylor C.J., Lin B., Waghray A., Mitsialis V., Dwyer D.F., Buchheit K.M., Boyce J.A., Barrett N.A., Laidlaw T.M., Carroll S.L., Colonna L., Tkachev V., Peterson C.W., Yu A., Zheng H.B., Gideon H.P., Winchell C.G., Lin P.L., Bingle C.D., Snapper S.B., Kropski J.A., Theis F.J., Schiller H.B., Zaragosi L.E., Barbry P., Leslie A., Kiem H.P., Flynn J.L., Fortune S.M., Berger B., Finberg R.W., Kean L.S., Garber M., Schmidt A.G., Lingwood D., Shalek A.K., Ordovas-Montanes J., HCA Lung Biological Network SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Busnadiego I., Fernbach S., Pohl M.O., Karakus U., Huber M., Trkola A., Stertz S., Hale B.G. Antiviral activity of type I, II, and III interferons counterbalances ACE2 inducibility and restricts SARS-CoV-2. mBio. 2020;11(5):e01928–20. doi: 10.1128/mBio.01928-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Felgenhauer U., Schoen A., Gad H.H., Hartmann R., Schaubmar A.R., Failing K., Drosten C., Weber F. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 2020;295(41):13958–13964. doi: 10.1074/jbc.AC120.013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vanderheiden A., Ralfs P., Chirkova T., Upadhyay A.A., Zimmerman M.G., Bedoya S., Aoued H., Tharp G.M., Pellegrini K.L., Manfredi C., Sorscher E., Mainou B., Lobby J.L., Kohlmeier J.E., Lowen A.C., Shi P.Y., Menachery V.D., Anderson L.J., Grakoui A., Bosinger S.E., Suthar M.S. Type I and Type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J. Virol. 2020;94(19):e00985–20. doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Interleukin-6 Inhibitors. COVID-19 Treatment guidelines (nih.gov) (Accessed on 10th Nov 2020).
- 177.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wampler T.L., Muskardin Intravenous anakinra for macrophage activation syndrome may hold lessons for treatment of cytokine storm in the setting of Coronavirus Disease 2019. ACR Open Rheumatol. 2020;2(5):283–285. doi: 10.1002/acr2.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Monteagudo L.A., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020;2(5):276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., Walzer T., François B., Sève P. Should we stimulate or suppress immune responses in COVID-19?Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gupta A., Karki R., Dandu H.R., Dhama K., Bhatt M.L., Saxena S.K. COVID-19: benefits and risks of passive immunotherapeutics. Hum. Vaccin. Immunother. 2020;16(12):2963–2972. doi: 10.1080/21645515.2020.1808410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dong Y., Dai T., Wei Y., Zhang L., Zheng M., Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct. Target Ther. 2020;5(1):237. doi: 10.1038/s41392-020-00352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., Wirth D.M., Chen A., Sack M., Pokorski J.K., Steinmetz N.F. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 186.Haynes B.F., Corey L., Fernandes P., Gilbert P.B., Hotez P.J., Rao S., Santos M.R., Schuitemaker H., Watson M., Arvin A. Prospects for a safe COVID-19 vaccine. Sci. Transl. Med. 2020;12(568):eabe0948. doi: 10.1126/scitranslmed.abe0948. [DOI] [PubMed] [Google Scholar]
- 187.Brisse M., Vrba S.M., Kirk N., Liang Y., Ly H. Emerging concepts and technologies in vaccine development. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hodgson S.H., Mansatta K., Mallett G., Harris V., Emary K., Pollard A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21(2):e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brown S., Brown T., Cederna P.S., Rohrich R.J. The race for a COVID-19 vaccine: current trials, novel technologies, and future directions. Plast. Reconstr. Surg. Glob. Open. 2020;8(10) doi: 10.1097/GOX.0000000000003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Omersel J., Karas Kuželički N. Vaccinomics and adversomics in the era of precision medicine: a review based on HBV, MMR, HPV, and COVID-19 vaccines. J. Clin. Med. 2020;9(11):3561. doi: 10.3390/jcm9113561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kennedy D.A., Read A.F. Monitor for COVID-19 vaccine resistance evolution during clinical trials. PLoS Biol. 2020;18(11) doi: 10.1371/journal.pbio.3001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kennedy D.A., Read A.F. Why does drug resistance readily evolve but vaccine resistance does not? Proc. Biol. Sci. 2017;284(1851):20162562. doi: 10.1098/rspb.2016.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Greaney A.J., Starr T.N., Gilchuk P., Zost S.J., Binshtein E., Loes A.N., Hilton S.K., Huddleston J., Eguia R., Crawford K., Dingens A.S., Nargi R.S., Sutton R.E., Suryadevara N., Rothlauf P.W., Liu Z., Whelan S., Carnahan R.H., Crowe J.E., Jr, Bloom J.D. Complete mapping of mutations to the SARS-CoV-2 spike receptor-binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44–57.e9. doi: 10.1016/j.chom.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yadav T., Srivastava N., Mishra G., Dhama K., Kumar S., Puri B., Saxena S.K. Recombinant vaccines for COVID-19. Hum Vaccin Immunother. 2020;16(12):2905–2912. doi: 10.1080/21645515.2020.1820808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Williams T.M., Davis R.W. Physiological resiliency in diving mammals: insights on hypoxia protection using the Krogh principle to understand COVID-19 symptoms. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021;253 doi: 10.1016/j.cbpa.2020.110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Minz M.M., Bansal M., Kasliwal R.R. Statins and SARS-CoV-2 disease: current concepts and possible benefits. Diabetes Metab. Syndr. 2020;14(6):2063–2067. doi: 10.1016/j.dsx.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang Y., Chen B., Li Y., Zhang L., Wang Y., Yang S., Xiao X., Qin Q. The use of renin-angiotensin-aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID-19 patients: a systematic review and meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.26625. Advance online publication. [DOI] [PubMed] [Google Scholar]
- 198.Chen X., Guo H., Qiu L., Zhang C., Deng Q., Leng Q. Immunomodulatory and antiviral activity of metformin and its potential implications in treating Coronavirus Disease 2019 and lung injury. Front Immunol. 2020;11:2056. doi: 10.3389/fimmu.2020.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Costanzo L., Palumbo F.P., Ardita G., Antignani P.L., Arosio E., Failla G., Italian Society for Vascular Investigation and the Italian Society of Vascular Medicine Coagulopathy, thromboembolic complications, and the use of heparin in COVID-19 pneumonia. J. Vasc. Surg. Venous Lymphat. Disord. 2020;8(5):711–716. doi: 10.1016/j.jvsv.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wool G.D., Miller J.L. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88(1):15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perricone C., Bartoloni E., Bursi R., Cafaro G., Guidelli G.M., Shoenfeld Y., Gerli R. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol. Res. 2020;68(4):213–224. doi: 10.1007/s12026-020-09145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.K. Thibodeaux, M. Speyrer, A. Raza, R. Yaakov, T.E. Serena, Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: a retrospective case series. J. Wound Care. 29 (Sup5a) (2020) S4–S8. 10.12968/jowc.2020.29.Sup5a.S4. [DOI] [PubMed]
- 203.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev. Rep. 2020;16(3):427–433. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.National Center for Complementary and Integrative Health. What are complementary and integrative health approaches? NCCIH’s Funding Priorities and Research Focus | NCCIH (nih.gov) (Accessed on 20th Nov 2020).
- 205.AYUSH | National Health Portal of India (nhp.gov.in) (Accessed on 20th Nov 2020).
- 206.Huang S.T., Lai H.C., Lin Y.C., Huang W.T., Hung H.H., Ou S.C., Lin H.J., Hung M.C. Principles and treatment strategies for the use of Chinese herbal medicine in patients at different stages of coronavirus infection. Am. J. Cancer Res. 2020;10(7):2010–2031. [PMC free article] [PubMed] [Google Scholar]
- 207.Nugraha R.V., Ridwansyah H., Ghozali M., Khairani A.F., Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: A review of their mechanisms, pros and cons. Evid. Based Complement Alternat. Med. 2020;2020:2560645. doi: 10.1155/2020/2560645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of ayurveda and yoga for COVID-19 prophylaxis. J. Altern. Complement Med. 2020;26(5):360–364. doi: 10.1089/acm.2020.0129. [DOI] [PubMed] [Google Scholar]
- 209.Gautam S., Gautam A., Chhetri S., Bhattarai U. Immunity against COVID-19: potential role of Ayush Kwath. JJ Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.08.003. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.GUIDELINES for SIDDHA PRACTITIONERS for COVID 19. Ministry of AYUSH. siddhaGuidlines_v4a.cdr (ayush.gov.in) (Accessed on 20th Nov 2020).
- 211.GUIDELINES for HOMOEOPATHIC PRACTITIONERS for COVID 19. Ministry of AYUSH. https://www.ayush.gov.in/docs/homeopathy-guidelines.pdf (Accessed on 20th Nov 2020).
- 212.Deng Y., Xie J., Luo Z., Li S.P., Zhao J. Synergistic immunomodulatory effect of complex polysaccharides from seven herbs and their major active fractions. Int. J. Biol. Macromol. 2020;165(Pt A):530–541. doi: 10.1016/j.ijbiomac.2020.09.199. [DOI] [PubMed] [Google Scholar]
- 213.Ma Y.M., Zhang X.Z., Su Z.Z., Li N., Cao L., Ding G., Wang Z.Z., Xiao W. Insight into the molecular mechanism of a herbal injection by integrating network pharmacology and in vitro. J. Ethnopharmacol. 2015;173:91–99. doi: 10.1016/j.jep.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 214.Shi Z., Gewirtz A.T. Together forever: bacterial-viral interactions in infection and immunity. Viruses. 2018;10(3):122. doi: 10.3390/v10030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wojewodzic M.W. Bacteriophages could be a potential game changer in the trajectory of coronavirus disease (COVID-19) Phage: Therapy Appl. Res. 2020;60–65 doi: 10.1089/phage.2020.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Straub R.H. The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat. Rev. Rheumatol. 2017;13(12):743–751. doi: 10.1038/nrrheum.2017.172. [DOI] [PubMed] [Google Scholar]
- 217.Kluger M.J. Phylogeny of fever. Fed Proc. 1979;38:30–34. [PubMed] [Google Scholar]
- 218.Maciver N.J., Jacobs S.R., Wieman H.L., Wofford J.A., Coloff J.L., Rathmell J.C. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc Biol. 2008;84(4):949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Soheilifar S., Fathi H., Naghdi N. Photobiomodulation therapy as a high potential treatment modality for COVID-19. Lasers Med Sci. 2020:1–4. doi: 10.1007/s10103-020-03206-9. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Surazakov A., Klassen A., Gizinger O. The bioenergetics of COVID-19 immunopathology and the therapeutic potential of biophysical radiances. J. Photochem. Photobiol. B. 2020;213 doi: 10.1016/j.jphotobiol.2020.112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Owoicho O., Olwal C.O., Quaye O. Potential of laser-induced fluorescence-light detection and ranging for future stand-off virus surveillance. Microb. Biotechnol. 2020 doi: 10.1111/1751-7915.13698. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]