Abstract
Nonalcoholic fatty liver disease (NAFLD) is a rising global public health concern due to its prevalence. Danning Tablets (DNt), a composite prescription of Chinese herbal medicine, shows significant curative effects on NAFLD in clinical application. This study aimed to decipher the bioactive substances and potential mechanisms of action of DNt in the treatment of NAFLD, applying an integrated network pharmacology approach. First, the bioactive compounds of DNt were screened based on their pharmacokinetic properties, and the corresponding drug targets were predicted. Then, the NAFLD-related targets were collected. The overlapping targets between the putative targets of DNt and NAFLD-related targets were identified as the potential therapeutic targets of DNt against NAFLD. Subsequently, the networks were constructed and analyzed, and the key bioactive compounds and targets were screened out depending on their importance in the networks. Functional enrichment analysis was carried out to elucidate the potential mechanisms of DNt acting on NAFLD. Finally, a molecular docking simulation was implemented to assess the potential binding affinity between the key targets and the bioactive compounds. As a result, 43 bioactive compounds of DNt and 69 putative targets were identified. Based on the network analysis, we found seven key bioactive compounds (quercetin, ß-sitosterol, luteolin, kaempferol, supraene, curcumenolactone C, and stigmasterol) of DNt might treat NAFLD via intervening IL6, MAPK8, VEGFA, CASP3, ALB, APP, MYC, PPARG, and RELA. The functional enrichment analysis revealed that DNt might affect NAFLD by modulating the signaling pathways involved in lipid metabolism, inflammation, oxidation, insulin resistance (IR), atherosclerosis, and apoptosis. Furthermore, most key bioactive compounds might bind firmly with the key targets. This study predicted the multicomponent, multitarget, and multipathway mechanisms of DNt in the treatment of NAFLD from a holistic perspective. DNt could be a promising agent for NAFLD, but further experimental verifications are still needed.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is presently the most common liver disease, affecting a quarter of the adult population worldwide [1]. NAFLD, hallmarked by lipid accumulation in hepatocytes without significant alcohol intake, encompasses simple hepatic steatosis and nonalcoholic steatohepatitis (NASH), along with cirrhosis and even liver cancer developed by NASH [1]. NASH takes up 25 percent of NAFLD, and more than 1/3 of NASH would die from end-stage liver disease, making it the main reason for liver transplantation [2, 3]. Even though the pathogenesis of NAFLD has not been fully elucidated, insulin resistance (IR), inflammation, and oxidative stress are acknowledged as the essential contributors to the onset and progression of the disease [4, 5]. Currently, insulin sensitizers, antioxidants, lipid-lowering drugs, and lifestyle modifications are the mainly recommended treatment for NAFLD. However, there is no officially approved medication for NAFLD at present, and less than half of the patients can achieve the goal of weight loss [6, 7]. As an important part of complementary and alternative treatments, herbal medicine is a promising candidate for the treatment strategies of chronic liver diseases [8].
Danning Tablet (DNt) is a composite Chinese patent medicine, which consists of seven Chinese herbs: Rhei Radix et Rhizome (Dahuang, RRR), Polygoni Cuspidati Rhizoma et Radix (Huzhang, PCRR), Citri Reticulatae Pericarpium Viride (Qingpi, CRPV), Imperatae Rhizoma (Baimaogen, IR), Citrus Reticulatae Pericarpium (Chenpi, CRP), Curcumae Radix (Yujin, CR), and Crataegi Fructus (Shanzha, CF). DNt has been widely used to treat hepatobiliary diseases, such as cholelithiasis, chronic cholecystitis, and NAFLD for more than ten years in China, and it had obtained a product license by Canadian Natural Medicines and Over-The-Counter Medicines Agency in 2017. A multicenter clinical trial showed that DNt could improve clinical symptoms, serum ALT (alanine aminotransferase) level, and blood lipids of NAFLD patients without significant adverse events [9]. Another two clinical studies reported that DNt could reduce tumor necrosis factor α (TNF-α) and malondialdehyde, increase superoxide dismutase in serum, and attenuate IR significantly [10, 11]. The experimental studies demonstrated that DNt might alleviate hyperlipidemia, liver function damage, and fatty degeneration and punctiform necrosis of liver tissue in animals with NAFLD [12, 13]. It was also reported that the lipid modulation effects of DNt might be associated with the induction of peroxisomal proliferator-activated receptor α (PPARα) and cholesterol 7-hydroxylase (CYP7A1) in rat liver cells [13]. Besides, DNt could exert hepatoprotective effects by regulating oxidative stress, hepatic transporters, hepatic metabolic enzymes, and inflammatory signal pathways [14–16]. However, the essential compounds and the underlying mechanisms of DNt in the treatment of NAFLD have not been elucidated.
Chinese herbal medicine serves as a multicomponent, multitarget, and multipathway therapy, which makes it unique in treating complex diseases, but causes difficulty in mechanism elucidation meanwhile. As an approach integrated systems biology, bioinformatics, and polypharmacology, network pharmacology is a powerful tool to investigate complex diseases and uncover the complex interactions among drugs, targets, and diseases [13–17]. Molecular docking is a widely used in silico structure-based method to predict the interactions between molecules and biological targets, which enables the virtual screening of amounts of compounds in a limited time [18].
In this study, a network pharmacology approach was applied to explore the potential mechanisms of DNt against NAFLD. First of all, the bioactive compounds of DNt were screened according to their pharmacokinetic properties, and the corresponding targets of the bioactive compounds were predicted. Then, NAFLD-related targets were collected, the overlapping targets of DNt and NAFLD were regarded as the potential therapeutic targets of DNt against NAFLD. Furthermore, two interactive networks were built to screen out the key bioactive compounds and the key therapeutic targets. After that, functional enrichment analysis was conducted to expound the anti-NAFLD mechanisms of DNt. At last, the binding potentiality between the key target proteins and the bioactive compounds was evaluated through molecular docking. A flowchart of this study is described in Figure 1.
Figure 1.

A flowchart of this study.
2. Materials and Methods
2.1. Screening of the Bioactive Compounds of DNt
The information of the compounds of the seven herbs in DNt was collected from Traditional Chinese Medicine Systems Pharmacology Database [19] (TCMSP, http://tcmspw.com/index.php), Bioinformatics Analysis Tool for Molecular Mechanisms of Traditional Chinese Medicine Database (BATMAN-TCM, http://bionet.ncpsb.org/batman-tcm/), Natural Product Activity & Species Source Database (NPASS, http://bidd2.nus.edu.sg/NPASS/), and Traditional Chinese Medicine Information Database [20] (TCM-ID, http://bidd.nus.edu.sg/group/TCMsite/Default.aspx).
Oral bioavailability (OB) is the percentage of an orally administered dose of medicine that reaches the circulatory system; compounds with a higher OB value signify a better capability of being oral drugs [21]. Intestinal epithelial permeability (IEP) measures the intestinal absorption of drugs, which is partly responsible for the bioavailability of oral drugs [22]. Drug-likeness (DL) is a qualitative concept estimating the druggability of compounds, which assesses the compounds' capability of modulating targets [23]. Based on the TCMSP database, OB ≧ 30%, IEP ≧ -0.4, and DL ≧ 0.18 were set as the pharmacokinetic criteria to screen out the bioactive compounds of DNt [24]. The molecule structures of all the bioactive compounds were identified using the PubChem database [25] (https://pubchem.ncbi.nlm.nih.gov/).
2.2. Putative Targets of DNt
The putative targets corresponding to the bioactive compounds of DNt were collected from three databases: (1) TCMSP database provides the related targets for the certain compounds; (2) Swiss Target Prediction [26] platform (http://www.swisstargetpredic- tion.ch) predicts targets that have the actives displaying similarity with the query molecule. The species is limited to “Homo sapiens,” and only the results with a probability value of >0.7 were collected. (3) PharmMapper [27] platform (http://lilab-ecust.cn/pharmmapper/submitfile.html) predicts targets via a pharmacophore mapping approach; the targets with the setting of “human protein targets only” and a fit score of >3.5 were obtained. After eliminating duplicates, all the predicted targets were converted into Uniprot IDs using UniProtKB database (https://www.uniprot.org/) for the subsequent study.
2.3. Therapeutic Targets of DNt against NAFLD
Four disease databases, MalaCards [28] (https://www.malacards.org), DisGeNET [29] (http://www.disgenet.org/), Comparative Toxicogenomics Database [30] (CTD, http://ctdbase.org), and Online Mendelian Inheritance in Man® (OMIM) [31] database (http://www.omim.org/) were used to collect the NAFLD-related targets. The search terms used were “non-alcoholic fatty liver disease” or “NAFLD”.
Based on the preceding steps, two sets of targets had been prepared: the putative targets of DNt and the NAFLD-related targets. Venn diagram package in R was used to screen the overlapping targets, which were identified as the potential therapeutic targets of DNt against NAFLD.
2.4. Network Construction and Analysis
Two networks were constructed in this study: (1) a bioactive compound-therapeutic target network was built to show the interactions of the bioactive compounds and the therapeutic targets of DNt; (2) a protein-protein interaction (PPI) network was built to explore the interactions of the therapeutic targets of DNt against NAFLD. The PPI analysis was conducted using Search Tool for the Retrieval of Interacting Genes/Proteins platform [32] (STRING, https://string-db.org/), and the criteria were limited in “Homo sapiens” and a high confidence score of 0.7.
The networks were constructed by Cytoscape software version 3.7.2 [33], and the NetworkAnalyzer plug-in was used to analyze the nodes' topological parameters in the network. Two topological parameters, degree and closeness centrality, were calculated to measure the importance of the nodes. A degree value is defined as the number of edges linked to a node. A closeness centrality value is defined as the average of the shortest path length from a node to all other nodes, indicating the closeness of a node to the others in the network [34]. The key compounds and targets were screened out according to their network topological property, which was defined as two times greater than the median degree value and one time greater than the median closeness centrality value of all nodes in the network [35].
2.5. Functional Enrichment Analysis
To elucidate the biological mechanisms of therapeutic targets of DNt acting on NAFLD on a systematic level, the ClueGO 2.5.4 plug-in of Cytoscape was used to perform Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway functional enrichment analysis. The minimum gene counts enriched in each GO or KEGG term was set as three, and the terms with p values < 0.05 were considered statistically significant. The “ggplot2” package in R software (version 4.0.3, the R Foundation for Statistical Computing, Vienna, Austria) was used to visualize the GO and KEGG terms.
2.6. Molecular Docking Simulation
To evaluate the binding potential of the key bioactive compounds of DNt to key target proteins, molecular docking simulation was performed using GEMDOCK software (version 2.1, Hsinchu, Taiwan) [34]. The empirical scoring function of GEMDOCK is as follows: Fitness = van der Waal energy (vdW) + hydrogen bonding energy (Hbond) + electro statistic energy (Elec). A fitness value was used to estimate the binding affinity of a protein-ligand complex. A lower fitness value indicates a stabler binding between a protein and a ligand. The fitness value of the corresponding protein–original ligand was used as a comparison. And the generic evolutionary method parameters were set as population size = 200, generations = 70, and number of solutions = 2.
All 3D crystal structures of the target protein-ligand complexes were retrieved from Protein Data Bank [35] (PDB, http://www.rcsb.org/pdb/). All 3D molecular structures of the compounds were retrieved from ZINC15 database [36] (http://zinc.docking.org).
3. Results
3.1. Bioactive Compounds and Putative Targets of DNt
A total of 1303 compounds of DNt were collected from four databases, including 239 compounds in RRR, 188 compounds in PCRR, 54 compounds in CRPV, 64 compounds in IR, 348 compounds in CRP, 315 compounds in CR, and 95 compounds in CF. After excluding duplicates, we finally screened out 43 bioactive compounds with eligible OB, IEP, and DL properties (shown in Supplementary Table 1). What is more, 219 diverse putative targets of these bioactive compounds were obtained from the three target prediction databases.
3.2. Bioactive Compound-Therapeutic Target Network
We collected a total of 779 NAFLD-related targets from the four databases after the removal of duplicates. And 69 overlapping targets between the putative targets of DNt and NAFLD-related targets were recognized as the therapeutic targets of DNt in the treatment of NAFLD (Figure 2(a) and Supplementary Table 2).
Figure 2.
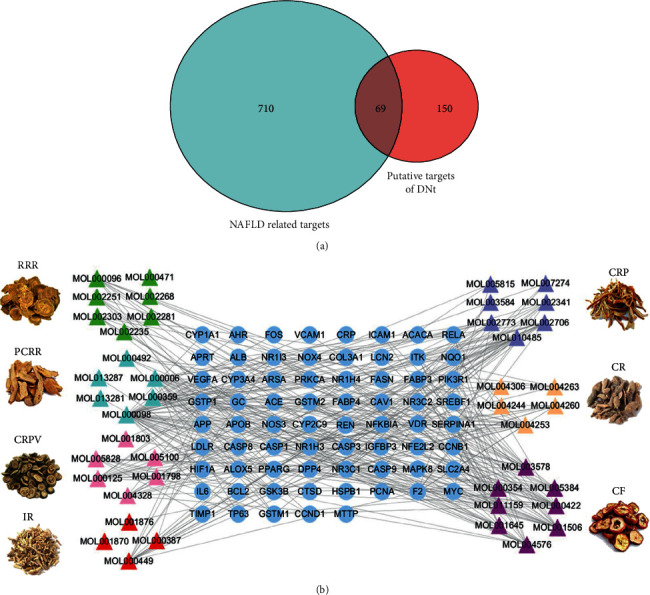
The therapeutic targets of DNt against NAFLD and the bioactive compound-therapeutic target network. (a) The Venn diagram of the overlapping targets between the putative targets of DNt and NAFLD-related targets. (b) The bioactive compound-therapeutic target network of DNt against NAFLD. Triangle nodes represent the bioactive compounds of DNt; circular nodes represent the therapeutic targets; edges represent the relations between compounds and targets. RRR, Rhei Radix et Rhizome; PCRR, Polygoni Cuspidati Rhizoma et Radix; CRPV, Citri Reticulatae Pericarpium Viride; IR, Imperatae Rhizoma; CRP, Citrus Reticulatae Pericarpium; CR, Curcumae Radix; CF, Crataegi Fructus.
The bioactive compound-therapeutic target network (Figure 2(b)) consisted of 112 nodes (43 bioactive compounds and 69 therapeutic targets) and 356 edges, manifesting a multicompound and multitarget mode of action. Seven bioactive compounds with the highest degree values, quercetin (degree = 41), luteolin (degree = 20), ß-sitosterol (degree = 18), curcumenolactone C (degree = 17), supraene (degree = 17), kaempferol (degree = 18), and stigmasterol (degree = 16) were recognized as the key bioactive compounds of DNt against NAFLD, owing to that they were twofold higher than the median degree value of 7 and onefold higher than the median closeness centrality value of 0.37 (Table 1). Higher degree values indicate that these bioactive compounds interact with more therapeutic targets, and higher closeness centrality values indicate their more central position in the network. Additionally, six of the bioactive compounds were from CF, three of them were from PCRR, manifesting the contributions of CF and PCRR in DNt.
Table 1.
The information of the key bioactive compounds of DNt.
| TCMSP ID | Name | Molecular formula | Structural formula | OB (%) | IEP | DL | Source | Degree value | Closeness centrality value |
|---|---|---|---|---|---|---|---|---|---|
| MOL000098 | Quercetin | C15H10O7 |

|
46.43 | 0.05 | 0.28 | PCRR, CF | 41 | 0.53 |
| MOL000006 | Luteolin | C15H10O6 |

|
36.16 | 0.19 | 0.25 | PCRR, CF | 20 | 0.43 |
| MOL000359 | β-sitosterol | C29H50O |

|
36.91 | 1.32 | 0.75 | PCRR, IR, CRP, RRR, CR, CF | 18 | 0.43 |
| MOL004253 | Curcumenolactone C | C15H20O4 |

|
39.7 | 0.16 | 0.19 | CR | 17 | 0.43 |
| MOL001506 | Supraene | C30H50 |
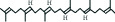
|
33.55 | 2.08 | 0.42 | CF | 17 | 0.41 |
| MOL000422 | Kaempferol | C15H10O6 |

|
41.88 | 0.26 | 0.24 | CF | 17 | 0.40 |
| MOL000449 | Stigmasterol | C29H48O |

|
43.83 | 1.44 | 0.76 | IR, CF | 16 | 0.42 |
OB, oral bioavailability; IEP, intestinal epithelial permeability; DL, drug-likeness.
3.3. Functional Enrichment Analysis
The functional enrichment analysis was performed for the 69 therapeutic targets. In total, 359 GO terms were identified, including 35 molecule function (MF) terms, three cellular component (CC) terms, and 320 biological process (BP) terms. For BP, the therapeutic targets were mainly responsible for the responses to oxidative stress, xenobiotic stimulus, inflammation, vitamin D, and the regulation of lipid, apoptosis, and foam cell differentiation. For MF, the therapeutic targets mainly participated in the binding of protease, fatty acid, steroid, oxygen, glutathione, and the activation of the transcription factor, vitamin D 24-hydroxylase, and cysteine-type endopeptidase involved in apoptosis. For CC, the potential targets were mainly distributed in the endoplasmic reticulum (ER), death-inducing signaling complex, and platelet. The most significantly enriched 15 GO terms are visualized in Figure 3(a)–3(c).
Figure 3.

Functional enrichment analysis of the therapeutic targets of DNt against NAFLD. (a) The top 15 GO-BP terms; (b) all the GO-CC terms; (c) the top 15 GO-MF terms. (d) The KEGG pathway enrichment conducted by ClueGO: nodes represent KEGG terms, the node's size represents the enriched significance, and the node's color reflects the enriched classification. (e) The top 15 KEGG pathway terms. Note: the bubble size represents the count of therapeutic targets enriched in a certain pathway; the associated genes (%) mean the percentage of target genes to the background genes of a certain pathway. GO, Gene Ontology; BP, biological process; CC, cellular component; MF, molecule function; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Sixty-one KEGG pathway terms were enriched (Figure 3(d)); among them, signaling pathways of apoptosis, advanced glycation end products (AGE) and receptor for AGE (RAGE), and fluid shear stress and atherosclerosis had the highest proportion of the target genes. The top 15 most significantly enriched terms are shown in Figure 3(e).
3.4. PPI Network of the Therapeutic Targets
Because two targets were excluded for the lack of any interaction with other proteins, the PPI network consisted of 235 interactions among 67 therapeutic targets (Figure 4). The median degree value and median closeness centrality value of the PPI network were 6 and 0.31, respectively. Nine proteins, interleukin-6 (IL6), mitogen-activated protein kinase 8 (MAPK8), vascular endothelial growth factor A (VEGFA), caspase-3 (CASP3), Myc proto-oncogene protein (MYC), serum albumin (ALB), amyloid-beta A4 protein (APP), peroxisome proliferator-activated receptor gamma (PPARG), and transcription factor p65 (RELA) met the screening criteria of key nodes in this network, demonstrating their central roles in the pathological processes of NAFLD. These targets acted as irreplaceable mediums to establish the connections between other targets in the development of NAFLD, so they were identified as the key targets of DNt treating NAFLD. Table 2 lists the detailed information of the key therapeutic targets.
Figure 4.
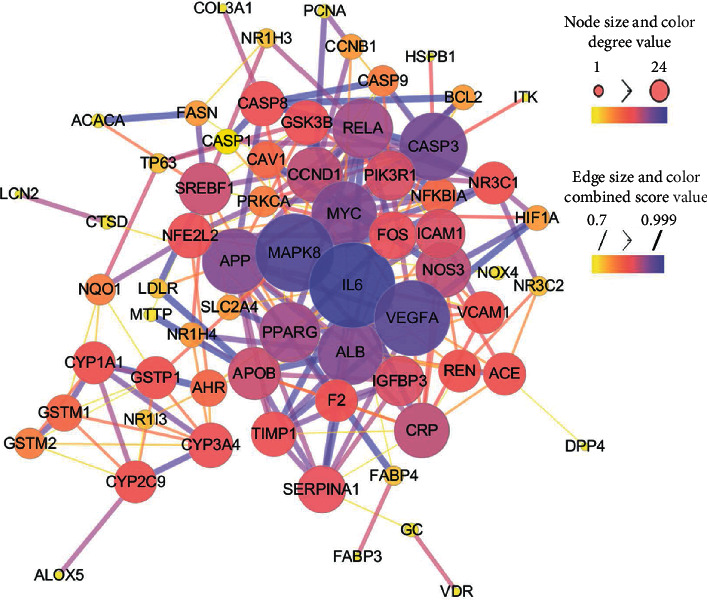
The PPI network of the therapeutic targets of DNt against NAFLD. The size and color of a node are proportional to the degree value, the size and color of an edge are proportional to the combined score. Smaller sizes and brighter colors signify lower degree values.
Table 2.
The key therapeutic targets of DNt in the treatment of NAFLD.
| No. | Target name | Gene symbol | Uniprot ID | Degree value | Closeness centrality value |
|---|---|---|---|---|---|
| 1 | Interleukin-6 | IL6 | P05231 | 24 | 0.55 |
| 2 | Mitogen-activated protein kinase 8 | MAPK8 | P45983 | 21 | 0.53 |
| 3 | Vascular endothelial growth factor A | VEGFA | P15692 | 20 | 0.52 |
| 4 | Caspase-3 | CASP3 | P42574 | 17 | 0.50 |
| 5 | Myc proto-oncogene protein | MYC | P01106 | 16 | 0.49 |
| 6 | Amyloid-beta A4 protein | APP | P05067 | 16 | 0.50 |
| 7 | Serum albumin | ALB | P02768 | 16 | 0.48 |
| 8 | Peroxisome proliferator-activated receptor gamma | PPARG | P37231 | 14 | 0.47 |
| 9 | Transcription factor p65 | RELA | Q04206 | 14 | 0.47 |
3.5. Molecular Docking Simulation
Nine key target proteins, IL6, MAPK8, VEGFA, CASP3, MYC, APP, ALB, PPARG, and RELA, were docked with seven key bioactive compounds, quercetin, luteolin, ß-sitosterol, curcumenolactone C, supraene, kaempferol, and stigmasterol. The results found that 30 target protein–bioactive compound complexes (47.62% of all) might bind firmer than the corresponding protein–original ligand complexes, indicating that numerous key bioactive compounds might bind closely with the key therapeutic targets to exercise regulatory effects. Among all, the RELA-quercetin complex had the best fitness value of −113.07 Kcal/mol, followed by ALB-stigmasterol (−111.65 Kcal/mol) and RELA-luteolin (−110.22 Kcal/mol). Table 3 shows the results of docking, and the docking model of every certain key target and the corresponding best-binding bioactive compound is shown in Figure 5.
Table 3.
The results of molecular docking simulation.
| Protein (PDB ID) | Ligand | Fitness (Kcal/mol) | Protein (PDB ID) | Ligand | Fitness (Kcal/mol) |
|---|---|---|---|---|---|
| IL6 (5FUC) | NAG∗ | −82.47 | MAPK8 (2GMX) | 877∗ | −105.32 |
| Quercetin | −96.46 | Stigmasterol | −103.52 | ||
| Kaempferol | −94.28 | β-Sitosterol | −102.45 | ||
| Supraene | −93.65 | Supraene | −98.66 | ||
| Luteolin | −86.82 | Luteolin | −90.16 | ||
| Stigmasterol | −83.16 | Curcumenolactone C | −88.64 | ||
| Curcumenolactone C | −78.64 | Quercetin | −80.01 | ||
| β-Sitosterol | −78.38 | Kaempferol | −78.28 | ||
|
| |||||
| VEGFA (6D3O) | XCP∗ | −74.42 | CSP3 (3GJR) | DZE∗ | −64.24 |
| Quercetin | −81.67 | Luteolin | −109.76 | ||
| Kaempferol | −81.48 | Quercetin | −101.75 | ||
| Curcumenolactone C | −65.03 | β-Sitosterol | −93.91 | ||
| Supraene | −62.96 | Kaempferol | −92.97 | ||
| Luteolin | −62.85 | Stigmasterol | −90.54 | ||
| Stigmasterol | −53.81 | Supraene | −85.62 | ||
| β-Sitosterol | −51.30 | Curcumenolactone C | −82.71 | ||
|
| |||||
| MYC (5I4Z) | GOL∗ | v42.74 | APP (3OVJ) | ORA∗ | −89.52 |
| Supraene | −102.18 | Stigmasterol | −82.77 | ||
| β-Sitosterol | −89.16 | β-Sitosterol | −80.19 | ||
| Stigmasterol | −85.25 | Supraene | −76.58 | ||
| Luteolin | −83.55 | Quercetin | −69.45 | ||
| Quercetin | −78.06 | Luteolin | −68.01 | ||
| Kaempferol | −73.66 | Kaempferol | −66.05 | ||
| Curcumenolactone C | −71.46 | Curcumenolactone C | −59.21 | ||
|
| |||||
| ALB (1E7A) | PFL∗ | −69.57 | PPARG (2ATH) | 3EA∗ | −97.19 |
| Stigmasterol | −111.65 | Luteolin | −99.33 | ||
| Luteolin | −107.49 | Stigmasterol | −97.79 | ||
| β-Sitosterol | −100.86 | β-Sitosterol | −95.81 | ||
| Curcumenolactone C | −93.57 | Supraene | −93.44 | ||
| Supraene | −90.49 | Kaempferol | −93.44 | ||
| Quercetin | −88.94 | Quercetin | −91.09 | ||
| Kaempferol | −86.36 | Curcumenolactone C | −83.09 | ||
|
| |||||
| RELA (3QXY) | SAM∗ | −142.70 | RELA (3QXY) | Kaempferol | −107.02 |
| Quercetin | −113.07 | β-Sitosterol | −95.78 | ||
| Luteolin | −110.22 | Stigmasterol | −94.77 | ||
| Supraene | −107.85 | Curcumenolactone C | −83.78 | ||
∗NAG, 877, XCP, DZE, GOL, ORA, PFL, 3EA, and SAM are the names of original ligands for corresponding targets in the PDB database.
Figure 5.

Molecular docking models of the key target proteins with the key bioactive compounds. (a) IL6-quercetin, (b) MAPK8-stigmasterol, (c) VEGFA-quercetin, (d) CSP3-luteolin, (e) MYC- supraene, (f) APP-stigmasterol, (g) ALB-stigmasterol, (h) PPARG-luteolin, and (i) RELA-quercetin. The original ligands are labeled in green color, and the predicted poses of the bioactive compounds are labeled in pink color.
4. Discussion
In the present study, the multicompound, multitarget, multipathway mechanisms of action of DNt against NAFLD were clarified applying the network pharmacology approach.
First of all, the bioactive compounds and the putative targets of DNt were identified, and the overlapping targets between DNt and NAFLD were recognized as the therapeutic targets of DNt against NAFLD (Figure 2(a)). Quercetin, ß-sitosterol, luteolin, kaempferol, supraene, curcumenolactone C, and stigmasterol were selected as the key bioactive compounds of DNt treating NAFLD, based on their contributions in the bioactive compound-therapeutic target network (Figure 2(b) and Table 1). Quercetin, luteolin, and kaempferol are all members of the flavone family, which are widely found in vegetables, fruits, and herbs. The flavones display extensive pharmacological activities, including anti-inflammatory, antioxidative, antimicrobial, antiapoptotic, hepatoprotective, and anticancer [37]. Quercetin could ameliorate NAFLD progression by mitigating oxidative stress, inflammation, and lipid metabolic disorders [38]. It could also modulate the intestinal probiotics and sequentially regulate gut-liver axis activation in NAFLD [39]. Luteolin could relieve diet-induced obesity and its metabolic complications, such as adiposopathy, hepatic steatosis, and IR, by regulating PPARG [40] and Toll-like receptor signaling pathways [41]. Luteolin and kaempferol could both abrogate lipid accumulation induced by the activation of liver X receptor- (LXR-) sterol regulatory element-binding protein 1c (SREBP-1c) pathway to mitigate hyperlipemia, NAFLD, and other components of metabolic syndrome (MetS) [42–44].
Supraene, also known as squalene, is a triterpenoid performing anticancer, antioxidant, and detoxicant bioactivities. Supraene had anticholesterolemia and antiatherosclerotic effects through the transactivation of LXR but without the alteration of SREBP-1c expression [45]. ß-sitosterol and stigmasterol are two kinds of the most occurring phytosterols, which can decrease the absorption of dietary lipids and bile acids and attenuate hepatic lipid accumulation and weight gain [46, 47]. Curcumenolactone is a carabrane-type sesquiterpene lactones. A previous study showed curcumenolactones A, B, C could protect the liver from D-galactosamine-induced acute injury in mice [48]. To conclude, three flavonoids (quercetin, luteolin, and kaempferol), three natural lipids (β-sitosterol, supraene, stigmasterol), and one sesquiterpene lactone undertake the pharmacology basis of the anti-NAFLD effects of DNt, which were consistent with the pathogenesis of promotion of NAFLD.
When it comes to herbs, CF and PCRR stood out for their major contributions of providing more bioactive compounds. In a systematic review, CF was the most frequently used herb in the treatment of NAFLD [49], the extract of which could significantly enhance the activities of antioxidant enzymes, lipid metabolism enzymes, and fatty acid oxidation-related enzymes in the liver of high-fat diet-fed mice [50]. The PCRR extract could inhibit cell proliferation, cell migration, and vessel formation in endothelial cells by suppressing VEGF-induced activations of c-Jun N-terminal kinase (JNK) and reactive oxygen species (ROS) [51]. As for the other component herbs, the citrus flavonoids isolated from CRP might exert hepatoprotective and anti-inflammatory effects by suppressing nuclear factor-κB (NF-κB) and MAPK signaling pathways in rats with NAFLD [52]. RRR was reported to show liver-protective function [53]; the extract of IR showed antioxidation ability in vitro [54]; the combined treatment with CR and Glycyrrhizae Radix et Rhizoma could inhibit body weight gain, lipid metabolic disturbances, serum liver enzymes, and hepatic steatosis in rats [55].
Then, the functional enrichment analysis of the therapeutic targets demonstrated the mechanisms of DNt against NAFLD might be focused on apoptosis-associated pathways, MetS-associated pathways (e.g., AGE-RAGE, IR, and atherosclerosis pathways), inflammation-associated pathways (e.g., IL-17, TNF, hypoxia-inducible factor-1 (HIF-1), and Toll-like pathways), and cancer-associated pathways (e.g., VEGF and p53 pathways) (Figure 3). As components of MetS, NAFLD and atherosclerosis share various pathophysiological mechanisms, including inflammation, oxidative stress, unbalanced coagulation-fibrinolysis, chronic intermittent hypoxia, altered adipokine profile, and unfavorable lipidosis [56]. AGEs accelerate the production of RAGEs and the subsequent AGE-RAGE interactions contribute to hepatic fat accumulation, giving rise to inflammation, IR, fibrosis, and other complications of NAFLD [57].
After that, IL6, MAPK8, VEGFA, CASP3, MYC, APP, ALB, PPARG, and RELA were identified as the key targets of DNt against NAFLD, due to their high degree and closeness centrality values (Figure 4). These targets interacted broadly and closely with other targets, indicating their central roles in the disease network. IL-6 has been acknowledged as a central factor in liver inflammation. The activation of IL-6 signaling pathway can induce the production of acute-phase proteins, such as fibrinogen and haptoglobin, leading to hepatitis syndrome, cholestasis subtype, fibrosis, and worse [58]. The activation of MAPK8, also named JNK1, participates in fat-induced apoptosis and IR, and the latter would propel NAFLD to more advanced stages [59]. VEGFA is a sentinel regulator of angiogenesis. It is widely believed that angiogenesis is correlated with NAFLD and fibrogenic progression [60]. For example, serum VEGFA and VEGFR1 levels were significantly elevated in NAFLD patients, and the borderline was even higher in NASH patients [61].
Among the key targets, CASP3 is well-known for its irreplaceable role in the apoptosis process, which can promote inflammation, IR, fibrosis, and cirrhosis in turn [62]. RELA is one protein of the NF-κB family. Saturated fatty acids can activate NF-κB and MYC signaling pathways, followed by increased proinflammatory cytokines, such as TNF-α and IL-6, resulting in the development of IR and NAFLD [63, 64]. APP is the precursor protein of amyloid-β, which is a consequence of IR and is widely known as a key pathological hallmark of Alzheimer's disease [65]. Moreover, APP can also promote IR in the liver in turn [66]. ALB acts as a key carrier in plasma, possessing the ability of binding with free fatty acids, inflammatory mediators, ROS, lipopolysaccharide, and bacterial antigens, which enable it to perform a vast series of regulatory activity in inflammation, antioxidation, and endothelial function [67, 68]. PPARG is one isotype of PPARs, which is pivotal for the lipogenesis in hepatocytes. Therefore, PPARG is regarded as a characteristic of NAFLD [69]. Contradictorily, PPARG also possesses anti-inflammatory properties, especially in macrophages [70], which might limit hepatic stellate cell proliferation and subsequent fibrosis [71]. Thus, it is undoubted that PPARG plays a critical role in NAFLD, but its multiple actions are still controversial. To sum up, it was speculated that DNt might perform integrative functions of lipid mediation, anti-inflammation, antioxidant, anti-IR, antiapoptosis, and antiendothelial/fibrosis on NAFLD through key targets in associated signaling pathways.
In the end, the molecular docking (Table 3 and Figure 5) showed that about half of the key bioactive compounds might bind firmly to the key target proteins, which provided the possibility of compound-target combination and pharmacological activities realization. The molecular docking simulation should strengthen the ratiocinations in this study, to some extent, but experimental validations are still needed.
5. Conclusions
This study applied a network pharmacology approach to elucidate the underlying mechanisms of DNt against NAFLD in a holistic manner. Seven bioactive compounds of DNt, quercetin, ß-sitosterol, luteolin, kaempferol, supraene, curcumenolactone C, and stigmasterol, might exert the crucial pharmacological effects. Moreover, IL6, MAPK8, VEGFA, CASP3, MYC, APP, ALB, PPARG, and RELA might be the key targets regulated by those bioactive compounds. Besides, signaling pathways of apoptosis, inflammation, oxidant stress, IR, and lipid metabolism should be mainly involved. This study may inspire insights into novel therapeutic strategies of NAFLD and provide references for future researches. Nevertheless, further experiments are still required to validate the findings of this study.
Data Availability
All the data used to support the findings of this study are included in the article or could be accessed in the open online databases mentioned in the Methods section.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Supplementary Materials
Supplementary Table 1: information of all the bioactive compounds of DNt. Supplementary Table 2: the potential targets of DNt in the treatment of NAFLD
References
- 1.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Diehl A. M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. New England Journal of Medicine. 2017;377(21):2063–2072. doi: 10.1056/nejmra1503519. [DOI] [PubMed] [Google Scholar]
- 3.Schuster S., Cabrera D., Arrese M., Feldstein A. E. Triggering and resolution of inflammation in NASH. Nature Reviews Gastroenterology & Hepatology. 2018;15(6):349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 4.Rvinen Y. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes & Endocrinology. 2014;2(11):901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 5.Byrne C. D., Targher G. NAFLD: a multisystem disease. Journal of Hepatology. 2015;62(1):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Rinella M. E. Nonalcoholic fatty liver disease. JAMA. 2015;313(22):2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 7.Musso G., Gambino R., Cassader M., Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y., Sugimoto K., Inui H., et al. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World Journal of Gastroenterology. 2015;21(13):3777–3785. doi: 10.3748/wjg.v21.i13.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J. G. Evaluating the efficacy and safety of Danning Pian in the short-term treatment of patients with non-alcoholic fatty liver disease: a multicenter clinical trial. Hepatobiliary & Pancreatic Diseases International: HBPD INT. 2004;3(3):375–380. [PubMed] [Google Scholar]
- 10.Wang Y., Qi H. Clinical study on Danning Tablets combined with tolynicate and naphthylacetic acid in treatment of non-alcoholic fatty liver. Drugs&Clinic (In Chinese) 2019;34(1):88–92. [Google Scholar]
- 11.Du W., Ye Q., Li J., et al. Changes of blood liver function index and TNF-α levels before and after treatment of patients with nonalcoholic fatty liver disease with combination therapy of DanNing tablets and simvastatin. Journal of Practical Hepatology (In Chinese) 2017;20(4):490–491. [Google Scholar]
- 12.Zhang J., Wang X., Jiang N., et al. Therapeutic eff ect of Danning tablets on nonalcoholic fatty liver disease in rats. World Chinese Journal of Digestology (In Chinese) 2016;24(18):2875–2880. doi: 10.11569/wcjd.v24.i27.3860. [DOI] [Google Scholar]
- 13.Liu R., Chen Z., Xu R., et al. The protective effect of Danning tablet on experimental fatty liver. Pharmaceutical Care and Research. 2007;7(3):202–205. in Chinese. [Google Scholar]
- 14.Yang Y., Zhu P., Zhang J., Zhang X., Fang B. Effects of Danning tablets on expression of PPAR α and CYP7A1 in rats with nonalcoholic fatty liver disease. Chinese Journal of New Drugs and Clinical Remedies (In Chinese) 2007;26(10):721–726. [Google Scholar]
- 15.Ding L., Zhang B., Zhan C., et al. Danning tablets attenuates alpha-naphthylisothiocyanate- induced cholestasis by modulating the expression of transporters and metabolic enzymes. BMC Complementary and Alternative Medicine. 2014;14(17):p. 249. doi: 10.1186/1472-6882-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu L., Zhang F., Zhan C., et al. The protective effect of Danning tablet against hepatic sinusoidal obstruction syndrome induced by Gynura Rhizoma. Acta Pharmaceutica Sinica (In Chinese) 2019;54(3):494–501. [Google Scholar]
- 17.Ding L.-L., Zhang B.-F., Dou W., Yang L., Zhan C.-S., Wang Z.-T. Protective effect of Danning tablet on acute livery injury with cholestasis induced by α-naphthylisothiocyanate in rats. Journal of Ethnopharmacology. 2012;140(2):222–229. doi: 10.1016/j.jep.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 18.Pinzi L., Rastelli G. Molecular docking: shifting paradigms in drug discovery. International Journal of Molecular Sciences. 2019;20(18):p. 4331. doi: 10.3390/ijms20184331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(16):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L., Xie D., Yu Y., et al. Tcmid 2.0: a comprehensive resource for TCM. Nucleic Acids Research. 2018;46(D1):D1117–D1120. doi: 10.1093/nar/gkx1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X., Zhang W., Huang C., et al. A novel chemometric method for the prediction of human oral bioavailability. International Journal of Molecular Sciences. 2012;13(6):6964–6982. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn T. J., Vohra S. N. Simultaneous determination of intestinal permeability and potential drug interactions of complex mixtures using Caco-2 cells and high-resolution mass spectrometry: studies with Rauwolfia serpentina extract. Chemico-Biological Interactions. 2018;290(25):37–43. doi: 10.1016/j.cbi.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Casas A. I., Hassan A. A., Larsen S. J., et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proceedings of the National Academy of Sciences. 2019;116(14):7129–7136. doi: 10.1073/pnas.1820799116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao W., Xu X., Wang X., et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. Journal of Ethnopharmacology. 2013;145(1):1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Kim S., Chen J., Cheng T., et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Research. 2019;47(D1):D1102–D1109. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daina A., Michielin O., Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Research. 2019;47(W1):W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X., Shen Y., Wang S., et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Research. 2017;45(W1):W356–W360. doi: 10.1093/nar/gkx374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rappaport N., Fishilevich S., Nudel R., et al. Rational confederation of genes and diseases: NGS interpretation via GeneCards, MalaCards and VarElect. Biomedical Engineering Online. 2017;16(S1):p. 72. doi: 10.1186/s12938-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinero J., Ramirez-Anguita J. M., Sauch-Pitarch J., et al. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Research. 2019;48(D1):D845–D855. doi: 10.1093/nar/gkz1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis A. P., Grondin C. J., Johnson R. J., et al. The comparative Toxicogenomics database: update 2019. Nucleic Acids Research. 2019;47(D1):D948–D954. doi: 10.1093/nar/gky868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamosh A., Scott A. F., Amberger J. S., Bocchini C. A, McKusick V. A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Research. 2005;33(1):D514–D517. doi: 10.1093/nar/gki033<remarks>Doi_inserted_from_PubMed</remarks>. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szklarczyk D., Morris J. H., Cook H., et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Research. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon P., Markiel A., Ozier O., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J.-M., Chen C.-C. GEMDOCK: a generic evolutionary method for molecular docking. Proteins: Structure, Function, and Bioinformatics. 2004;55(2):288–304. doi: 10.1002/prot.20035. [DOI] [PubMed] [Google Scholar]
- 35.Berman H. M., Westbrook J. D., Feng Z., Gilliland G., Bourne P. E. The protein Data Bank. Nucleic Acids Research. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterling T., Irwin J. J. Zinc 15 - ligand discovery for everyone. Journal of Chemical Information and Modeling. 2015;55(11):2324–2337. doi: 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisonero-Vaquero S., Martínez-Ferreras Á., García-Mediavilla M. V., et al. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Molecular Nutrition & Food Research. 2015;59(5):879–893. doi: 10.1002/mnfr.201400913. [DOI] [PubMed] [Google Scholar]
- 38.Tamura M., Nakagawa H., Hori S., Suzuki T., Hirayama K. Plasma quercetin metabolites are affected by intestinal microbiota of human microbiota-associated mice fed with a quercetin-containing diet. Journal of Clinical Biochemistry and Nutrition. 2019;65(3):232–239. doi: 10.3164/jcbn.19-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porras D., Nistal E., Martínez-Flórez S., et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radical Biology and Medicine. 2017;102:188–202. doi: 10.1016/j.freeradbiomed.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 40.Kwon E.-Y., Jung U. J., Park T., Yun J. W., Choi M.-S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes. 2015;64(5):1658–1669. doi: 10.2337/db14-0631. [DOI] [PubMed] [Google Scholar]
- 41.Kwon E. Y., Choi M. S. Luteolin targets the toll-like receptor signaling pathway in prevention of hepatic and adipocyte fibrosis and insulin resistance in diet-induced obese mice. Nutrients. 2018;10(10):p. 1415. doi: 10.3390/nu10101415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoang M.-H., Jia Y., Mok B., Jun H.-j., Hwang K.-Y., Lee S.-J. Kaempferol ameliorates symptoms of metabolic syndrome by regulating activities of liver X receptor-β. The Journal of Nutritional Biochemistry. 2015;26(8):868–875. doi: 10.1016/j.jnutbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Francisco V., Figueirinha A., Costa G., et al. The flavone luteolin inhibits liver X receptor activation. Journal of Natural Products. 2016;79(5):1423–1428. doi: 10.1021/acs.jnatprod.6b00146. [DOI] [PubMed] [Google Scholar]
- 44.Yin Y., Gao L., Lin H., et al. Luteolin improves non-alcoholic fatty liver disease in db/db mice by inhibition of liver X receptor activation to down-regulate expression of sterol regulatory element binding protein 1c. Biochemical and Biophysical Research Communications. 2017;482(4):720–726. doi: 10.1016/j.bbrc.2016.11.101. [DOI] [PubMed] [Google Scholar]
- 45.Hien H. T. M., Ha N. C., Thom L. T., Hong D. D. Squalene promotes cholesterol homeostasis in macrophage and hepatocyte cells via activation of liver X receptor (LXR) α and β. Biotechnology Letters. 2017;39(8):1101–1107. doi: 10.1007/s10529-017-2345-y. [DOI] [PubMed] [Google Scholar]
- 46.Feng S., Gan L., Yang C. S., et al. Effects of stigmasterol and β-sitosterol on nonalcoholic fatty liver disease in a mouse model: a lipidomic analysis. Journal of Agricultural and Food Chemistry. 2018;66(13):3417–3425. doi: 10.1021/acs.jafc.7b06146. [DOI] [PubMed] [Google Scholar]
- 47.Feng S., Dai Z., Liu A. B., et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2018;1863(10):1274–1284. doi: 10.1016/j.bbalip.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda H., Morikawa T., Ninomiya K., Yoshikawa M. Hepatoprotective constituents from zedoariae rhizoma: absolute stereostructures of three new carabrane-type sesquiterpenes, curcumenolactones A, B, and C. Bioorganic & Medicinal Chemistry. 2001;9(4):909–916. doi: 10.1016/s0968-0896(00)00306-0. [DOI] [PubMed] [Google Scholar]
- 49.Shi K.-Q., Fan Y.-C., Liu W.-Y., Li L.-F., Chen Y.-P., Zheng M.-H. Traditional Chinese medicines benefit to nonalcoholic fatty liver disease: a systematic review and meta-analysis. Molecular Biology Reports. 2012;39(10):9715–9722. doi: 10.1007/s11033-012-1836-0. [DOI] [PubMed] [Google Scholar]
- 50.Li T.-p., Zhu R.-g., Dong Y.-p., Liu Y.-h., Li S.-h., Chen G. Effects of pectin pentaoligosaccharide from hawthorn (crataegus pinnatifida bunge. Var. Major) on the activity and mRNA levels of enzymes involved in fatty acid oxidation in the liver of mice fed a high-fat diet. Journal of Agricultural and Food Chemistry. 2013;61(31):7599–7605. doi: 10.1021/jf400283w. [DOI] [PubMed] [Google Scholar]
- 51.Hu W.-H., Chan G. K.-L., Lou J.-S., et al. The extract of Polygoni Cuspidati Rhizoma et Radix suppresses the vascular endothelial growth factor-induced angiogenesis. Phytomedicine. 2018;42:135–143. doi: 10.1016/j.phymed.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 52.Jiang J., Yan L., Shi Z., Wang L., Shan L., Efferth T. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-κB and MAPKs. Phytomedicine. 2019;64(11):p. 153082. doi: 10.1016/j.phymed.2019.153082. [DOI] [PubMed] [Google Scholar]
- 53.Yang F., Xu Y., Xiong A., et al. Evaluation of the protective effect of Rhei Radix et Rhizoma against α-naphthylisothiocyanate induced liver injury based on metabolic profile of bile acids. Journal of Ethnopharmacology. 2012;144(3):599–604. doi: 10.1016/j.jep.2012.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X. R., Wang J. H., Jiang B., Shang J., Zhao C. Q. A study of extraction process and in vitro antioxidant activity of total phenols from Rhizoma Imperatae. African Journal of Traditional, Complementary, and Alternative Medicines. 2013;10(4):175–178. doi: 10.4314/ajtcam.v10i4.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park I., Ryuk J., Lee H., Go H., Ko B. In vitro and in vivo effects of ethanol extract combined with Curcumae Radix and Glycyrrhizae Radix et Rhizoma on menopausal metabolic disturbances. International Journal of Clinical and Experimental Medicine. 2015;8(9):15076–15086. [PMC free article] [PubMed] [Google Scholar]
- 56.Asadipooya K., Lankarani K. B., Raj R., et al. RAGE is a potential cause of onset and progression of nonalcoholic fatty liver disease. International Journal of Endocrinology. 2019;2019:11. doi: 10.1155/2019/2151302.2151302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miltonprabu S., Tomczyk M., Skalicka-Woźniak K., et al. Hepatoprotective effect of quercetin: from chemistry to medicine. Food and Chemical Toxicology. 2017;108(Pt B):365–374. doi: 10.1016/j.fct.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 58.Ezure T., Sakamoto T., Tsuji H., et al. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. The American Journal of Pathology. 2000;156(5):1627–1639. doi: 10.1016/s0002-9440(10)65034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreira D. M. S., Castro R. E., Machado M. V., et al. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia. 2011;54(7):1788–1798. doi: 10.1007/s00125-011-2130-8. [DOI] [PubMed] [Google Scholar]
- 60.Coulon S., Francque S., Colle I., et al. Evaluation of inflammatory and angiogenic factors in patients with non-alcoholic fatty liver disease. Cytokine. 2012;59(2):442–449. doi: 10.1016/j.cyto.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Lonardo A., Nascimbeni F., Mantovani A., Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? Journal of Hepatology. 2018;68(2):335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death & Disease. 2014;5(1):p. e996. doi: 10.1038/cddis.2013.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Z., Yu R., Xiong Y., et al. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids in Health and Disease. 2017;16(1) doi: 10.1186/s12944-017-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu T., Zhou Y., Ko K. S., et al. Interactions between Myc and mediators of inflammation in chronic liver diseases. Mediators of Inflammation. 2015;2015 doi: 10.1155/2015/276850.276850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verdile G., Keane K. N., Cruzat V. F., et al. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators of Inflammation. 2015;2015:17. doi: 10.1155/2015/105828.105828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y., Zhou B., Deng B., et al. Amyloid- induces hepatic insulin resistance in vivo via JAK2. Diabetes. 2013;62(4):1159–1166. doi: 10.2337/db12-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arroyo V., García-Martinez R., Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. Journal of Hepatology. 2014;61(2):396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 68.Bocca C., Novo E., Miglietta A., Parola M. Angiogenesis and fibrogenesis in chronic liver diseases. Cellular and Molecular Gastroenterology and Hepatology. 2015;1(5):477–488. doi: 10.1016/j.jcmgh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skat-Rørdam J., Højland Ipsen D., Lykkesfeldt J. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic & Clinical Pharmacology & Toxicology. 2019;124(5):528–537. doi: 10.1111/bcpt.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villanueva C. J., Tontonoz P. Licensing PPARγ to work in macrophages. Immunity. 2010;33(5):647–649. doi: 10.1016/j.immuni.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z., Xu J.-P., Zheng Y.-C., et al. Peroxisome proliferator-activated receptor gamma inhibits hepatic fibrosis in rats. Hepatobiliary & Pancreatic Diseases International. 2011;10(1):64–71. doi: 10.1016/s1499-3872(11)60009-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: information of all the bioactive compounds of DNt. Supplementary Table 2: the potential targets of DNt in the treatment of NAFLD
Data Availability Statement
All the data used to support the findings of this study are included in the article or could be accessed in the open online databases mentioned in the Methods section.


