Abstract
Introduction
Studies have proved that exposure of adults to phthalates might be related to cardiometabolic risk factors and changes in markers of oxidative stress. Such studies conducted on school-age children and adolescents are limited and fail to assess the simultaneous effect of phthalates on these risk factors and oxidative stress markers. Therefore, it was attempted to identify the relationship of urinary phthalate metabolites with cardiometabolic risk factors and oxidative stress markers in children and adolescents. Methods. In this cross-sectional study, 108 children and adolescents, living in Isfahan industrial city of Iran, were examined. Urine samples taken from the participants were analyzed for mono-butyl phthalate (MBP), mono-benzyl phthalate (MBzP), mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-exohexyl) phthalate (MEOHP), and mono-methyl phthalate (MMP).
Results
Results showed that, among phthalate metabolites, MBP had the highest concentration, followed by MBzP, MEOHP, MEHHP, MEHP, and MMP. Concentrations of these metabolites had a significant relationship with some of the cardiometabolic risk factors including systolic blood pressure (SBP), fasting blood sugar (FBS), and triglycerides (TG) (p < 0.05). Furthermore, the crude and adjusted linear regression models indicated the significant association of phthalate metabolites with superoxide dismutase (SOD), malondialdehyde (MDA), and homeostasis model assessment of insulin resistance (HOMA-IR) (p < 0.05).
Conclusion
Although urinary phthalate concentrations could not exactly reflect the long-term exposure level in the studied age groups, the consumption of phthalate-free products during childhood and adolescent development shall be assumed helpful in maintaining a healthy lifestyle. To confirm these findings and develop effective intervention strategies, it would be necessary to perform longitudinal studies on diverse population.
1. Introduction
Changes in diet and lifestyle have increased the incidence and prevalence of the health disorders associated with emerging micropollutants [1]. Phthalic acid esters (phthalates), as priority pollutants and endocrine disruptive compounds (EDCs), are the synthetic chemicals widely used as plasticizers in children's toys, hygiene and cosmetics products, medical equipment, food packaging and processing, building materials, and floorings [2–5]. Due to the widespread use, phthalates can increase the chance of infertility, primary ovarian insufficiency, and other abnormalities of the female reproductive system [2, 6–9]. They can also lead to disorders of hormonal signaling pathways of the thyroid, immune, and metabolic systems [10–12]. The US Environmental Protection Agency (EPA) classified di-(2-ethylhexyl) phthalate (DEHP) and benzylbutyl phthalate (BzBP) as potential human carcinogens, respectively [10]. Exposure to phthalates may occur via oral ingestion, inspiration, and dermal contact [13–16]. Based on molecular weight, phthalates may be classified into two groups including low-molecular-weight phthalates (with carbon chains of four or less) and high-molecular-weight phthalates (with carbon chains more than four). The major sources of human exposure to low and high molecular weight phthalates are personal care products and diet, respectively [10]. In a human body, phthalates are quickly converted into their monoesters excreted in the urine either freely or conjugated as glucuronides. Therefore, human exposure to phthalates is measured by urinary phthalate metabolites [17–19]. Exposure to phthalates can influence the cardiometabolic risk factors (obesity, hypertension, blood sugar level, high triglyceride levels, and low- and high-density lipoprotein levels) [20]. Moreover, a direct relationship has been observed between phthalate exposure and increase of oxidative stress markers [21]. Many studies have provided strong evidence on the relationship between phthalate exposure and higher risk of insulin resistance syndrome [21–27]. The relationship between phthalate concentration and insulin resistance syndrome can be evaluated through homeostasis model assessment of insulin resistance (HOMA-IR), which is known as an alternative useful technique [21, 28–30]. Higher HOMA-IR concentrations have been observed among the elderly participants exposed to DEHP metabolites [31]. Oxidative stress, known as an imbalance between the amount of free oxidative radicals and antioxidants, can adversely alter lipid peroxidation, protein oxidation, and DNA oxidation [21, 32]. Moreover, inappropriate production of other oxidative stress markers, such as malondialdehyde (MDA) and superoxide dismutase (SOD), can occur under exposure to DEHP concentration [33]. Previous studies showed a positive correlation between phthalates exposure and MDA concentration [34–36]. Both DEHP and monobutyl phthalate (MnBP) exposures were involved in increase of oxidative stress markers and fatty acid oxidation [37].
Since cardiometabolic and oxidative stress markers, such as SOD, MDA, and HOMA-IR, are related to phthalates exposure, it is assumed that children and adolescents are more susceptible to phthalates exposure. To the best of the author's knowledge, no study yet has been conducted on the simultaneous effect of phthalates on cardiometabolic and oxidative stress markers. Therefore, the purpose of this study is to identify the relationships of phthalates with cardiometabolic risk factors and oxidative stress markers among children and adolescents.
2. Materials and Methods
A cross-sectional study was conducted among 108 children and adolescents aged 6–18 years living in Isfahan industrial city, Iran. It was approved by the Research and Ethics Committee of Isfahan University of Medical Sciences (project code: 394984). The participants gave an oral assent and their parents signed a form for informed consent. Demographic characteristics of the participants were collected using a validated questionnaire. To reduce the effect of recall bias, the questionnaires were completed jointly by parents and their children. Accordingly, physical examinations, including measurements of weight, height, waist circumference, diastolic blood pressure (DBP), and systolic blood pressure (SBP), were conducted using the standards protocols [38]. Moreover, the body mass index (BMI) was calculated as wt (kg) divided by height squared (m2). Other cardiometabolic risk factors, such as fasting blood sugar (FBS), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and total cholesterol (TC), were determined by standard kits (Pars Azmoun, Tehran) and automatic analyzers [38]. The abnormality levels of the measured clinical parameters were TC ≥ 200 mg/dL; TG ≥ 100 mg/dL for children below nine years old and TG ≥ 130 mg/dL for children aged 10–18 years; HDL-C < 40 mg/dL; LDL-C ≥ 130 mg/dL; FBS ≥ 100 mg/dL. Serum MDA and SOD were measured by Elisa Kits. Insulin resistance (IR) was calculated according to (1) [39].
| (1) |
The abnormality level for HOMA-IR is > 2.5.
The standard stock solutions of phthalates (MEHP, MMP, MBzP, MBP, MEOHP, and MEHHP) were prepared by adding methanol, poured into glass bottles to avoid contamination, and stored at a temperature of less than 0°C. Concentrations of 0.001, 0.005, 0.01, 0.05, 0.1, and 0.5 μg/mL of the aforementioned metabolites were used to draw the calibration curve. Urine samples were thawed at room temperature before analysis. Afterwards, 5 ml of the urine samples was poured into a glass test tube made of borosilicate, and 20 μL of β-glucuronidase enzyme was added to digest the samples and remove the compounds from the glucuronide state. The samples were incubated in a shaker (37°C for 18 h). After removing the samples from the shaker incubator, they were diluted and their pH was adjusted to 2 by 10% sulfuric acid. Different amounts of acetonitrile (as a disperser) and chlorobenzene (as an extractant) were used to extract the phthalate metabolites by dispersive liquid-liquid microextraction (DLLME) method. After several trials and errors, the best extraction was obtained using 750 μL of acetonitrile and 80 μL of chlorobenzene. Chlorobenzene and acetonitrile were rapidly injected into the samples to form a cloudy solution. The samples were then placed in a centrifuge (at 6000 rpm for 5 m). The precipitate at the bottom of the tube (20 μL) was extracted by a syringe and dried by nitrogen gas. After adding N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) derivative to the obtained substance, it was injected into a gas chromatography/mass spectrometry (GC/MS) system [38]. A gas chromatography (GC) device (Agilent, model: 7890A) equipped with a mass selective detector (MSD) (Agilent, model: 5975C) made by Agilent technology was used to measure the amount of urinary phthalate metabolites, and ChemStation software under Windows XP was used to operate the gas chromatography/mass selective detector (GC/MSD) device. Moreover, DB5-MS column produced by Agilent company (with a length of 60 m, column diameter of 25 mm, and internal film of 25µ) and helium (grade 5.5) as a carrier gas with a flow rate of 1 mL/min were applied. The injection port (injector) at 270°C was used as a split with a ratio of 2 : 1 to inject the sample. The oven temperature started with 100°C (initial temperature) and remained at 100°C for 3 m. Then, it reached 300°C with a slope of 20°C/min and was kept at the same temperature for 7 m. The ion source temperature and transfer line temperature (transfer line) were set at 230°C and 290°C, respectively. Finally, the MSD was set to selected ion monitoring (SIM) mode. The ions used for SIM, retention time, and time window for the studied metabolites are presented in Table 1.
Table 1.
Chemical structure, retention time, selected ions, and time window for the analyzed analytes (MEHP, MMP, MBzP, MBP, MEOHP, and MEHHP).
| Metabolites | The chemical structure | Time separation (minutes) | Selected ions | Time window |
|---|---|---|---|---|
| MEHP |
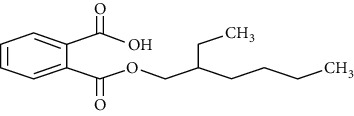
|
14.7 | 149, 221, 239 | 14–15.1 |
| MEOHP |
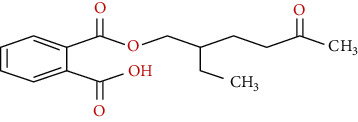
|
15.8 | 108, 127, 149, 221, 239 | 15.6–15.95 |
| MEHHP |
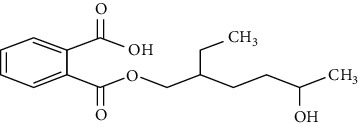
|
16.05 | 117, 147, 221, 265, 295 | 15.95–20 |
| MMP |
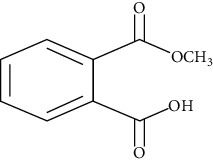
|
11.8 | 89, 237, 163 | 11.3–12 |
| MBP |
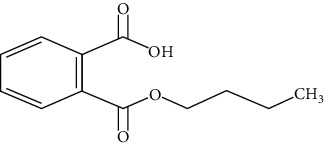
|
13.2 | 223, 149, 163 | 12–13.8 |
| MBzP |
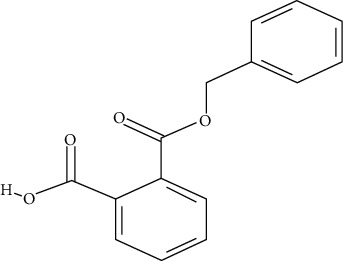
|
15.4 | 91, 179, 222 | 14.9–15.6 |
2.1. Method Validation
Recovery at three concentrations of low, medium, and high was evaluated using (2). Limit of detection (LOD) and limit of quantitation (LOQ) for each phthalate monoester were calculated using 3S0 and 10S0, respectively. S0 is the value of the standard deviation obtained through blank analysis (in this study: 15 blank analyses). In this study, all values below the LOD were replaced by one-half of the LOD [38].
| (2) |
where R is recovery; C is measurement concentration; Cref is reference concentration.
Relative standard deviation (RSD) is a standard deviation of a set of data divided by the mean of the data and can be calculated by
| (3) |
where S is standard deviation and Xave is the mean.
2.2. Statistical Analysis
Statistical analyses were performed using SPSS software (version 25.0, IBM, USA) and Graph-Pad Prism® 8.0 software (USA). An independent sample t-test was used to compare the means of cardiometabolic risk factors (age, obesity, waist, SBP, DBP, FBS, LDL-C, HDL-C, TC, and TG) and oxidative stress markers (SOD, MDA, and HOMA-IR). Linear regression was also used to estimate the relationships of phthalate concentrations with cardiometabolic risk factors, three tertiles of oxidative stress markers, and exposure sources. In the adjusted model, the relationships between the phthalate metabolites and oxidative stress markers were adjusted based on gender, age, BMI, SBP, DBP, FBS, TC, and TG variables. However, due to the high correlation of these variables with other variables and probability of multicollinearity error occurrence, waist, HDL-C, and LDL-C were excluded from the analysis. It should be noted that p-values < 0.05 were considered as statistically significant.
3. Results
3.1. Validation of Results
Validation of results indicated the high accuracy of the method (Table 2). Recovery was evaluated at three concentrations: low, medium, and high. According to Table 2, recoveries of MEHP, MEOHP, MEHHP, MMP, MBP, and MBzP metabolites in three different concentrations were 55–68, 90–96, 69–109, 86–77, 93–83, and 102–93, respectively. The LOD and LOQ calculated for each monoester are presented in Table 2 [40].
Table 2.
Validation results of the method to identify the studied metabolites.
| Metabolites | LOD | LOQ | Recovery | RSD (%) | R 2 | ||
|---|---|---|---|---|---|---|---|
| Low concentration (1 µ/L) | Medium concentration (10 µ/L) | High concentration (100 µ/L) | |||||
| MEHP | 0.024 | 0.05 | 90 | 55 | 69 | 13.2 | 0.997 |
| MEOHP | 0.23 | 0.48 | 80 | 68 | 96 | 8.5 | 0.997 |
| MEHHP | 0.088 | 0.18 | 69 | 109 | 105 | 6.2 | 0.995 |
| MMP | 0.03 | 0.1 | 77 | 79 | 86 | 5.6 | 0.993 |
| MBP | 0.36 | 1.2 | 83 | 89 | 93 | 7 | 0.997 |
| MBzP | 0.54 | 1.8 | 94 | 93 | 102 | 6.9 | 0.991 |
3.2. Characteristics of the Studied Population
Among the participants' demographic characteristics, including age group, parents' education level and employment status, physical activity, shower times per week, toy materials preference (plastic or metals), cosmetic consumption, plastic packaging use, and bottled beverage use, only mothers' employment status (p = 0.034) and toy materials preference (p = 0.008) had significant differences among children and adolescents (Table 3).
Table 3.
Participant's demographic and behavioral characteristics.
| Girl | Boy | Total | p-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Age groups | ||||
| 6–11 years | 28 (49.1) | 17 (33.3) | 45 (41.66) | 0.098 |
| 12–18 years | 29 (50.9) | 34 (66.7) | 63 (58.34) | |
| Fathers' education level | ||||
| Illiterate | 4 (7) | 2 (3.9) | 6 (5.55) | 0.338 |
| No college education | 44 (77.2) | 38 (74.5) | 82 (75.9) | |
| College education | 9 (15.8) | 11 (21.6) | 20 (18.55) | |
| Mothers' education level | ||||
| Illiterate | 1 (1.8) | 1 (2) | 2 (1.85) | 0.249 |
| No college education | 46 (80.7) | 38 (70.6) | 84 (77.77) | |
| College education | 10 (17.5) | 14 (27.5) | 24 (20.38) | |
| Fathers' job | ||||
| Unemployed | 10 (17.5) | 6 (11.8) | 16 (14.81) | 0.585 |
| Employed | 10 (17.5) | 10 (19.6) | 20 (18.51) | |
| Self-employment | 37 (64.9) | 35 (68.6) | 72 (66.68) | |
| Mothers' job | ||||
| Unemployed | 46 (80.7) | 48 (94.1) | 94 (87) | 0.034 |
| Employed | 7 (12.3) | 3 (5.9) | 10 (9.25) | |
| Self-employment | 4 (7) | — | 4 (3.75) | |
| Physical activity | ||||
| Low | 29 (50.9) | 23 (45.1) | 52 (48.14) | 0.638 |
| Moderate | 19 (33.3) | 20 (39.2) | 39 (36.11) | |
| High | 9 (15.8) | 8 (15.7) | 17 (15.75) | |
| Shower times per week | ||||
| 2 times | 35 (61.4) | 25 (49) | 60 (55.5) | 0.306 |
| 3 times | 12 (21.1) | 17 (33.3) | 29 (26.85) | |
| >4 times | 10 (17.5) | 9 (17.6) | 19 (18) | |
| Toy materials | ||||
| Plastic | 51 (89.5) | 35 (68.6) | 86 (79.62) | 0.008 |
| Metallic | 6 (10.5) | 16 (31.4) | 22 (20.38) | |
| Cosmetic consumption | ||||
| Yes | 51 (89.5) | 46 (90.2) | 97 (89.81)) | 0.902 |
| No | 6 (10.5) | 5 (9.8) | 11 (10.9) | |
| Plastic packing use | ||||
| Yes | 36 (63.2) | 28 (54.9) | 64 (59.25) | 0.386 |
| No | 21 (36.8) | 23 (45.1) | 44 (40.75) | |
| Bottled beverage use | ||||
| Low | 45 (80.7) | 38 (74.5) | 83 (76.85) | 0.415 |
| Moderate | 11 (19.3) | 12 (23.5) | 23 (21.29) | |
| High | 0 | 1 (20) | 1 (1.86) |
3.3. Clinical and Physical Examinations
Table 4 shows the participants' clinical and physical examination results based on mean and standard deviation (SD). Overall, the participants, with a mean (SD) age of 11.63 (2.31) years, were composed of 57 girls (57.9%) and 51 boys (42.1%). According to BMI, 49 (54%), 40 (37%), and 19 (17.6%) of the participants were considered normal, overweight, and obese, respectively. The participants' mean (SD) waist circumference was 85.57 (12.07) cm, with the boys and the girls having the mean (SD) waist circumferences of 89.29 (10.64) cm and 82.24 (12.38) cm, respectively. SBP and DBP for the whole participants were 11.28 (1.28) and 6.85 (0.69) mmHg, respectively. As shown in Table 4, the means (SD) of TC, HDL-C, LDL-C, and TG in the participants were 171.33 (33.08), 48.38 (12), 102.85 (25.76), and 108.63 (60.34) mg/dL, respectively. Among the participants, 17.6% had abnormal TC, 64.8% had abnormal triglycerides, 20.37% had abnormal HDL-C, 13.88% had abnormal LDL-C, 89% had normal FBS, and 11% had impaired fasting glucose (IFG). Moreover, the means (SD) of SOD, MDA, and HOMA-IR in the participants were 152 (69.96), 21.95 (9.23), and 2.66 (1.86), respectively, and 37% of the children and adolescents had abnormal HOMA-IR. The mean (SD) concentrations of MMP, MBP, MEHP, MBzP, MEOHP, and MEHHP were 63.09 (25.96), 260.46 (158.26), 133.09 (133.46), 249.51 (186.88), 249.16 (142.47), and 210.37 (138.80) μg/L, respectively. Among the metabolites of phthalates, MBP had the highest concentration, followed by MBzP, MEOHP, MEHHP, MEHP, and MMP.
Table 4.
Clinical and physical examinations result of the participants.
| All subjects (n = 108) | Girls (n = 57) | Boys (n = 51) | ||
|---|---|---|---|---|
| BMI; n (%) | Normal | 49 (45.4) | 32 (56.1) | 17 (33.3) |
| Overweight | 40 (37) | 18 (31.6) | 22 (43.1) | |
| Obese | 19 (17.6) | 7 (12.3) | 12 (23.5) | |
| Age; mean (SD) | 11.63 (2.31) | 11.39 (2.45) | 11.91 (2.13) | |
| Waist; mean (SD) | 85.57 (12.07) | 82.24 (12.38) | 89.29 (10.64) | |
| SBP; mean (SD) | 11.28 (1.28) | 10.94 (1.27) | 11.65 (1.20) | |
| DBP; mean (SD) | 6.85 (0.69) | 6.82 (0.71) | 6.88 (0.68) | |
| FBS; mean (SD) | 89.78 (8.28) | 88.33 (8.84) | 91.39 (7.34) | |
| TC; mean (SD) | 171.33 (33.08) | 164.95 (29.78) | 178.47 (35.35) | |
| HDL-C; mean (SD) | 48.38 (12) | 46.85 (10.11) | 50.09 (13.71) | |
| LDL-C; mean (SD) | 102.85 (25.76) | 98.24 (23.87) | 108 (27.02) | |
| TG; mean (SD) | 108.63 (60.34) | 109 (56.37) | 108.22 (65.07) | |
| SOD; mean (SD) | 152 (69.96) | 150.88 (63) | 153.5 (76.84) | |
| MDA; mean (SD) | 21.95 (9.23) | 22.22 (9.3) | 21.67 (9.27) | |
| HOMA-IR, mean (SD) | 2.66 (1.86) | 2.68 (1.93) | 2.22 (1.77) | |
| MMP; mean (SD) | 63.09 (25.96) | 63.10 (24.86) | 63.07 (27.38) | |
| MBP; mean (SD) | 260.46 (158.26) | 252.40 (166.3) | 269.47 (149.89) | |
| MEHP; mean (SD) | 133.09 (133.46) | 120.04 (120.71) | 147.67 (146.25) | |
| MBzP; mean (SD) | 249.51 (186.88) | 225.68 (171.15) | 276.15 (201.39) | |
| MEOHP; mean (SD) | 249.16 (142.74) | 228.33 (137.76) | 272.44 (145.95) | |
| MEHHP; mean (SD) | 210.37 (138.80) | 193.48 (146.54) | 229.25 (128.39) |
3.4. Phthalates and Cardiometabolic Risk Factors
Figure 1 shows the relationships between each phthalate metabolite and cardiometabolic risk factors. Statistically, the significant relationships were observed among the phthalate metabolites, obesity status, and waist circumference (p < 0.05) (Figures 1(a) and 1(b)). A positive relationship was observed between SBP and MBP [β: 0.299, CI: (0.001, 0.004), p = 0.002], SBP and MEHP (β = 0.218, CI: (0.001, 0.001), p = 0.023] and SBP and MBZP [β: 0.293, CI: (0.001, 0.003), p = 0.002]. Likewise, there was a significant relationship between FBS and MEHHP [β: 0.205, CI: (0.001, 0.023), p = 0.034] (Figure 1(c)). However, an insignificant relationship was found among the phthalate metabolites, DBP, TC, HDL-C, and LDL-C (p > 0.05) (Figures 1(d)–1(h)). Serum TG was related to the MBZP concentration [β: 0.217, CI: (0.009, 0.131), p = 0.024] (Figure 1(i)).
Figure 1.
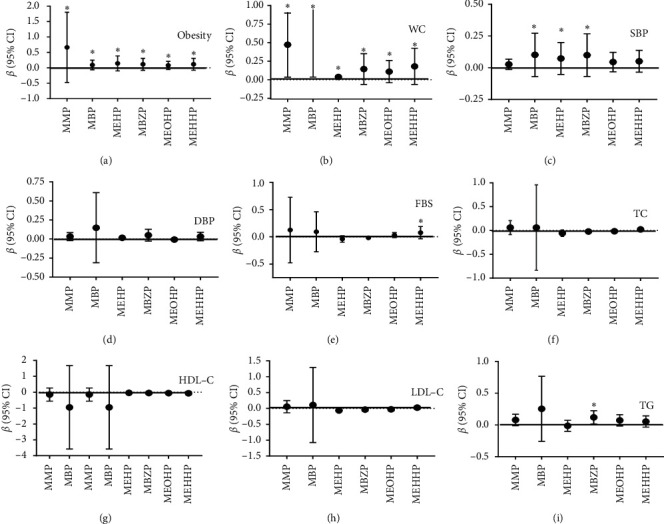
The association between urinary phthalates and cardiometabolic risk factors ((a) obesity status, (b) WC, (c) SBP, (d) DBP, (e) FBS, (f) TC, (g) HDL-C, (h) LDL-C, (i) TG) in children and adolescents. ∗p < 0.05.
3.5. Phthalate Metabolites and Oxidative Stress Markers (SOD, MDA, and HOMA-IR)
The relationships between the phthalate metabolites and tertiles of oxidative stress markers (SOD, MDA, and HOMA-IR) have been presented in Table 5. In the middle tertile, the crude and adjusted models showed that one-unit increase in MEOHP concentration led to 0.03 decrease (p = 0.040) and 0.044 decrease (p = 0.015) in SOD concentration, respectively. In the highest tertile, in the adjusted model, one-unit increase in MEOHP concentration led to 0.282 decrease in SOD concentration. Moreover, the 2nd and the 3rd tertiles of the crude and adjusted models revealed that all the phthalate metabolites, except MEHP, had a negative relationship with MDA (p < 0.05). In the crude model, one-unit increase in MMP, MBP, MBzP, MEOHP, and MEHHP led to 0.006–0.029 decrease in MDA concentration (p < 0.05). However, this decrease was 0.005–0.028 in the adjusted model (p < 0.05). In an analysis stratified by HOMA-IR in the highest tertile, MMP in the crude model (p = 0.049) and MBP in the adjusted model (p = 0.046) had negative relationships with HOMA-IR.
Table 5.
The association between exposure to urinary phthalates and tertiles of oxidative stress markers in children and adolescent.
| Tertile 1 | Tertile 2 | Tertile 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |||||||
| β (S.E) | p | β (S.E) | p | β (S.E) | p | β (S.E) | p | β (S.E) | p | β (S.E) | p | |
| SOD | <116 | 116–162 | >162 | |||||||||
| MMP | −0.060 (0.075) | 0.428 | −0.017 (0.075) | 0.825 | −0.098 (0.097) | 0.311 | −0.047 (0.125) | 0.712 | 0.307 (0.505) | 0.544 | 0.463 (0.638) | 0.469 |
| MBP | 0.004 (0.011) | 0.717 | 0.018 (0.014) | 0.189 | 0.003 (0.015) | 0.828 | 0.009 (0.022) | 0.691 | 0.037 (0.089) | 0.677 | −0.016 (0.121) | 0.897 |
| MEHP | −0.008 (0.019) | 0.699 | −0.023 (0.023) | 0.333 | −0.010 (0.018) | 0.592 | −0.037 (0.026) | 0.156 | 0.051 (0.090) | 0.576 | −0.001 (0.116) | 0.993 |
| MBZP | 0.002 (0.012) | 0.894 | 0.019 (0.015) | 0.216 | 0.006 (0.013) | 0.633 | 0.002 (0.017) | 0.905 | 0.030 (0.074) | 0.690 | −0.027 (0.101) | 0.790 |
| MEOHP | −0.003 (0.014) | 0.820 | 0.001 (0.014) | 0.946 | −0.030 (0.014) | 0.040 | −0.044 (0.015) | 0.004 | −0.167 (0.100) | 0.097 | −0.282 (0.095) | 0.003 |
| MEHHP | −0.011 (0.014) | 0.445 | −0.014 (0.015) | 0.355 | −0.011 (0.016) | 0.500 | −0.007 (0.020) | 0.744 | −0.056 (0.118) | 0.636 | −0.303 (0.157) | 0.055 |
| MDA | <16 | 16–24 | >24 | |||||||||
| MMP | −0.006 (0.012) | 0.640 | 0.002 (0.012) | 0.867 | −0.029 (0.014) | 0.043 | −0.028 (0.014) | 0.042 | 0.133 (0.068) | 0.053 | 0.033 (0.090) | 0.716 |
| MBP | −0.002 (0.002) | 0.313 | −0.001 (0.002) | 0.534 | −0.006 (0.003) | 0.015 | −0.008 (0.003) | 0.003 | 0.001 (0.012) | 0.968 | −0.020 (0.010) | 0.061 |
| MEHP | −0.002 (0.003) | 0.594 | −0.004 (0.005) | 0.452 | −0.002 (0.003) | 0.421 | −0.002 (0.003) | 0.507 | 0.023 (0.012) | 0.057 | −0.003 (0.012) | 0.798 |
| MBZP | −0.001 (0.002) | 0.465 | −0.003 (0.002) | 0.151 | −0.004 (0.002) | 0.135 | −0.007 (0.003) | 0.030 | −0.019 (0.009) | 0.036 | −0.025 (0.010) | 0.017 |
| MEOHP | −0.001 (0.002) | 0.667 | −0.001 (0.002) | 0.585 | −0.004 (0.002) | 0.124 | −0.005 (0.003) | 0.044 | −0.008 (0.013) | 0.556 | −0.031 (0.010) | 0.003 |
| MEHHP | 0.001 (0.002) | 0.960 | 0.001 (0.002) | 0.627 | −0.006 (0.002) | 0.023 | −0.013 (0.003) | <0.001 | 0.005 (0.015) | 0.713 | −0.027 (0.013) | 0.053 |
| HOMA-IR | <1.55 | 1.55–2.66 | >2.66 | |||||||||
| MMP | −0.001 (0.003) | 0.636 | 0.002 (0.003) | 0.385 | 0.001 (0.002) | 0.792 | 0.004 (0.002) | 0.060 | −0.021 (0.010) | 0.049 | −0.021 (0.012) | 0.085 |
| MBP | <0.001 (0.001) | 0.327 | <0.001 (0.001) | 0.163 | <0.001 (0.001) | 0.940 | <0.001 (0.001) | 0.446 | −0.002 (0.002) | 0.241 | −0.005 (0.002) | 0.046 |
| MEHP | <0.001 (0.001) | 0.435 | <0.001 (0.001) | 0.749 | <0.001 (0.001) | 0.860 | <0.001 (0.001) | 0.764 | −0.001 (0.002) | 0.606 | −0.002 (0.003) | 0.521 |
| MBZP | <0.001 (0.001) | 0.208 | <0.001 (0.001) | 0.381 | <0.001 (0.001) | 0.331 | <0.001 (0.001) | 0.119 | <0.001 (0.001) | 0.803 | −0.001 (0.002) | 0.682 |
| MEOHP | −0.001 (0.001) | 0.055 | <0.001 (0.001) | 0.812 | <0.001 (0.001) | 0.725 | <0.001 (0.001) | 0.427 | −0.003 (0.002) | 0.241 | −0.004 (0.002) | 0.077 |
| MEHHP | <0.001 (0.001) | 0.733 | <0.001 (0.001) | 0.892 | <0.001 (0.001) | 0.323 | <0.001 (0.001) | 0.870 | −0.001 (0.002) | 0.700 | −0.001 (0.003) | 0.795 |
The linear regression results were adjusted by gender, age, BMI, SBP, DBP, FBS, TC, and TG variables.
3.6. Phthalate Concentrations and Health Behaviors
Table 6 indicates that MEOHP has inverse (β = -63.44, p = 0.031) and positive (β = 79.15, p = 0.01) relationships with moderate physical activity and shower times per week, respectively.
Table 6.
Association between phthalate concentrations with health behaviors based on multiple linear regression.
| Health behaviors | MMP | MBP | MEHP | MBZP | MEOHP | MEHHP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (S.E) | p | B (S.E) | p | β (S.E) | p | β (S.E) | p | β (S.E) | p | β (S.E) | p | |
| Physical activity | ||||||||||||
| Low (n = 52) | Ref. | |||||||||||
| Moderate (n = 39) | −6.291 (5.422) | 0.246 | −43.93 (33.09) | 0.184 | −27.25 (27.91) | 0.329 | −27.65 (39.25) | 0.481 | −63.44 (29.46) | 0.031 | −22.82 (29.10) | 0.433 |
| High (n = 17) | 2.924 (7.151) | 0.683 | −12.66 (43.64) | 0.772 | −42.87 (36.81) | 0.244 | 15.20 (51.77) | 0.769 | −34.69 (38.86) | 0.372 | −37.00 (38.38) | 0.335 |
| Shower times per week | ||||||||||||
| 2 times (n = 60) | Ref. | |||||||||||
| 3 times (n = 29) | 8.378 (5.779) | 0.147 | 10.94 (35.60) | 0.759 | 22.44 (29.90) | 0.453 | 12.42 (41.98) | 0.767 | 79.15 (30.75) | 0.010 | 36.79 (30.98) | 0.235 |
| >4 times (19) | 6.268 (6.727) | 0.351 | −2.401 (41.44) | 0.954 | −15.38 (34.80) | 0.659 | −24.12 (48.86) | 0.622 | −36.14 (35.80) | 0.313 | −10.39 (36.06) | 0.773 |
| Toy materials | ||||||||||||
| Plastic (n = 86) | Ref. | |||||||||||
| Metallic (n = 22) | 7.304 (6.133) | 0.234 | 55.31 (37.25) | 0.138 | −0.241 (31.73) | 0.994 | 39.28 (44.28) | 0.375 | 70.43 (33.26) | 0.054 | 50.92 (32.64) | 0.119 |
| Cosmetic consumption | ||||||||||||
| Yes (n = 97) | Ref. | |||||||||||
| No (n = 11) | −3.173 (8.214) | 0.699 | −22.71 (50.06) | 0.650 | −46.47 (42.02) | 0.269 | −63.98 (58.85) | 0.277 | −3.079 (45.20) | 0.946 | 31.71 (43.84) | 0.469 |
| Special drug | ||||||||||||
| Yes (n = 19) | Ref. | |||||||||||
| No (n = 89) | 2.149 (6.526) | 0.742 | 27.24 (39.72) | 0.493 | −21.51 (33.50) | 0.521 | 37.23 (46.87) | 0.427 | 10.59 (35.89) | 0.768 | −4.965 (34.91) | 0.887 |
| Plastic packing use | ||||||||||||
| Yes (n = 64) | Ref. | |||||||||||
| No (n = 44) | −2.396 (5.054) | 0.635 | 6.996 (30.84) | 0.821 | 0.756 (26.01) | 0.977 | 12.24 (36.40) | 0.737 | −17.61 (27.77) | 0.526 | 40.21 (26.77) | 0.133 |
| Bottled beverage use | ||||||||||||
| Low (n = 84) | Ref. | |||||||||||
| Moderate (n = 23) | 8.722 (5.971) | 0.144 | 6.663 (37.05) | 0.858 | 13.68 (31.23) | 0.661 | 27.55 (43.44) | 0.526 | 6.500 (33.32) | 0.845 | 37.31 (32.23) | 0.247 |
| High (n = 1) | — | — | — | — | — | — | — | — | — | — | — | — |
4. Discussion
The evaluation of the demographic variables revealed that mothers' employment status and toy materials preference had significant differences among boys and girls (p < 0.05). Demographic variables are the most important predictors for health and illness of girls and boys in childhood and adolescence [41]. For instance, age and gender are important variables for exposure to the endocrine-disrupting chemicals (EDCs) in the environment. Buser et al. reported a significant positive relationship between high molecular weight (HMW) phthalates and male gender. Their results showed an increased odd of being obese among the adults exposed to HMW phthalate metabolites [42]. Won et al. observed that age and gender had significant relationships with urinary phthalate concentrations. They found that 6–11-year-old children were more susceptible to phthalate concentrations, which might lead to neurobehavioral development [41]. In the current study, we found no relationship among concentrations of phthalate metabolites, age, and gender. This could be due to the younger age of the participants we studied. The simple linear regression analyses showed that MMP, MBP, MEHP, MBZP, MEOHP, and MEHHP metabolites had significant positive relationships with measures of generalized and abdominal obesity. In fact, adipose tissue or body fat acts as an endocrine organ and can be the prime target for endocrine disruptions. Under exposure to EDCs, such as phthalates, this organ can improperly secrete numerous chemical signals or might have an inappropriate response to them [43].
Our findings were in agreement with the results obtained by Wang et al., who reported a positive relationship among urinary phthalate concentrations, waist circumference, and BMI in the Chinese schoolchildren. This relationship remained significant after making adjustments for age and gender [44]. Likewise, Teitelbaum et al. identified a positive relationship between urinary monoethyl phthalate (MEP) concentration and BMI among overweight children [45]. In contrast, Boas et al. observed that some phthalate metabolites, such as MEP, MEOHP, and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), had an inverse correlation with BMI in the Danish children aged four to nine years [46]. In an experimental study, Zhou et al. reported that, compared with control, weight gain in rats was significantly increased after exposure to DEHP [47].
The present study indicated the relationships of all phthalates with obesity. It also showed that MBP and MBzP metabolites were associated with SBP but not DBP. Moreover, MBZP and MEHHP were associated with elevated TG and FBS, respectively. Baralic et al. demonstrated that a mixture of low doses of DEHP, DBP, and BPA could induce significant changes in weight gain, appetite rate, lipid profile, glucose level, and hormonal concentrations in various glands [48]. Blood pressure (BP) has been extensively studied for phthalate implications [49]. For example, it has been found that exposures to DEHP and MEP metabolites were associated with lower SBP (but not DBP) in the girls aged four to seven years [50]. Su et al. showed that BMI, DBP, BP, diabetes mellitus, FBS, and TG were associated with increased levels of MEHP among young people, which is in agreement with our findings [51]. Another study demonstrated that phthalate compounds had positive relationships with BP, but not TG or HDL, in children and adolescents [52]. According to Poursafa et al., obesity, elevated BP, hyperglycemia, and dyslipidemia are among the leading risk factors for noncommunicable diseases (NCDs), whose origins can be traced back in childhood [53].
We found that, among phthalates, MEOHP had a negative relationship with SOD levels (p < 0.05), and MMP and MBP metabolites had negative relationships with HOMA-IR of children and adolescents (p < 0.05). Previous studies revealed that, after exposure to phthalate metabolites, MDA content was increased in the infants and children undergoing cyclic parenteral nutrition [34]. However, in the current study, the relationships between MDA and all phthalates, except MEHP, were negatively significant. This might be attributed to two factors: (1) high antioxidants capacity of adolescents and children due to probable use of dietary supplements, such as alpha-lipoic acid (ALA), for weight loss or other treatment purposes, and (2) stimulation of body protective defense responses to the oxidative stress neutralization under exposure to phthalate compounds. It has been already proved that ALA administration to the phthalate-treated mice could significantly reduce MDA concentrations compared with phthalate group [54]. Yang et al. also found that 6-gingerol, as an antioxidant, could prevent the oxidative stress induced by MEHP [55]. We found that physical activity and shower times per week had a significant relationship with urinary concentration of MEOHP metabolite. Since phthalates are used in the personal care products, shower times per week were considered as a source of exposure to phthalates among children and adolescents. Moreover, systemic absorption of VOCs was partially increased in young children during the physical activities (as a source of exposure). Respiratory rates and cardiac outputs observed in infants and young children were relatively high and favored the uptake of inhaled VOCs [56].
The main limitation of the current study stems from the cross-sectional nature of the data, which impeded the study of the cause-effects of variables. Moreover, we failed to examine the pubertal status of the participants, which might affect the variables studied. However, novelty in choosing the pediatric age group, determining various metabolites of phthalates, and considering the simultaneous effect of phthalates on cardiometabolic risk factors and oxidative stress are the main strengths of this study.
5. Conclusion
The results indicated the relationships of all the studied phthalate metabolites with generalized and abdominal obesity. They asserted the necessity of considering the exposure to phthalates as an emerging risk factor for obesity among children and adolescents. We also detected a significant relationship between SBP and MBP, MEHP and MBzP metabolites; FBS and MEHHP metabolite; TG and MBzP metabolite. Moreover, most of the phthalates had a significant relationship with SOD, MDA, and HOMA-IR. Due to the hydrophobic feature of phthalate and wide range of ages in children and adolescents, urinary phthalate concentrations could not exactly reflect the long-term exposure level and severity of cardiometabolic diseases. However, still it seemed more cautious to consume phthalate-free products during childhood and adolescent development in order to maintain a healthy lifestyle. However, longitudinal studies on diverse population seem necessary to confirm these findings and develop the effective interventions strategies.
Acknowledgments
The authors wish to thank the Vice Chancellery of Research of Isfahan University of Medical Sciences (IUMS), Iran, for the financial support (ethics code: IR.MUI.REC.1394.3.984).
Contributor Information
Mohammad Mehdi Amin, Email: mohammadmehdia@gmail.com.
Afsane Chavoshani, Email: chavoshani.afsane@yahoo.com.
Data Availability
The data used in this paper are available at https://pubmed.ncbi.nlm.nih.gov/30092535/ (Phthalates Implications in the Cardiovascular System).
Ethical Approval
All the authors declared to have read and abided by the statement of ethical standards for manuscripts submitted to the Obesity Research & Clinical Practice.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Amin M. M., Tabatabaeian M., Chavoshani A., et al. Paraben content in adjacent normal-malignant breast tissues from women with breast cancer. Biomedical and Environmetal Sciences. 2019;32(12):893–904. doi: 10.3967/bes2019.112. [DOI] [PubMed] [Google Scholar]
- 2.Rowdhwal S. S. S., Chen J. Toxic effects of di-2-ethylhexyl phthalate: an overview. Biomed Research International. 2018;2018:10. doi: 10.1155/2018/1750368.1750368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casas M., Valvi D., Ballesteros-Gomez A., et al. Exposure to bisphenol A and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-Sabadell cohort. Environmental Health Perspectives. 2016;124(4):521–528. doi: 10.1289/ehp.1409190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Net S., Delmont A., Sempéré R., Paluselli A., Ouddane B. Reliable quantification of phthalates in environmental matrices (air, water, sludge, sediment and soil): a review. Science of the Total Environment. 2015;515-516:162–180. doi: 10.1016/j.scitotenv.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 5.González-Sálamo J., Socas-Rodríguez B., Hernández-Borges J., Rodríguez-Delgado M. Á. Determination of phthalic acid esters in water samples using core-shell poly (dopamine) magnetic nanoparticles and gas chromatography tandem mass spectrometry. Journal of Chromatography A. 2017;1530:35–44. doi: 10.1016/j.chroma.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Kay V. R., Chambers C., Foster W. G. Reproductive and developmental effects of phthalate diesters in females. Critical Reviews in Toxicology. 2013;43(3):200–219. doi: 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karaconji I. B., Jurica S. A., Lasic D., Jurica K. Facts about phthalate toxicity in humans and their occurrence in alcoholic beverages. Archives of Industrial Hygiene and Toxicology. 2017;68(2):81–92. doi: 10.1515/aiht-2017-68-2951. [DOI] [PubMed] [Google Scholar]
- 8.Ambe K., Sakakibara Y., Sakabe A., Makino H., Ochibe T., Tohkin M. Comparison of the developmental/reproductive toxicity and hepatotoxicity of phthalate esters in rats using an open toxicity data source. The Journal of Toxicological Sciences. 2019;44(4):245–255. doi: 10.2131/jts.44.245. [DOI] [PubMed] [Google Scholar]
- 9.Hart L. B., Beckingham B., Wells R. S., et al. Urinary phthalate metabolites in common bottlenose dolphins (Tursiops truncatus) from Sarasota bay, FL, USA. GeoHealth. 2018;2(10):313–326. doi: 10.1029/2018gh000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Zhu H., Kannan K. A review of biomonitoring of phthalate exposures. Toxics. 2019;7(2):p. 21. doi: 10.3390/toxics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shehata A., Mohamed Z., El-Haleem M., Samak M. Effects of exposure to plasticizers di-(2-ethylhexyl) phthalate and trioctyltrimellitate on the histological structure of adult male albino rats’ liver. Journal of Clinical Toxicology. 2013;3(4):p. 9. doi: 10.4172/2161-0495.1000169. [DOI] [Google Scholar]
- 12.Wei Z., Song L., Wei J., et al. Maternal exposure to di-(2-ethylhexyl) phthalate alters kidney development through the renin-angiotensin system in offspring. Toxicology Letters. 2012;212(2):212–221. doi: 10.1016/j.toxlet.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 13.Beko G., Weschler C. J., Langer S., Callesen M., T oftum J., Clausen G. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062442.e62442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fierens T., Vanermen G., Van Holderbeke M., De Henauw S., Sioen I. Effect of cooking at home on the levels of eight phthalates in foods. Food and Chemical Toxicology. 2012;50(12):4428–4435. doi: 10.1016/j.fct.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y., Kannan K. Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environmental SciencesTechnology. 2012;45(8):3788–3794. doi: 10.1021/es2002106. [DOI] [PubMed] [Google Scholar]
- 16.Tran T. M., Kannan K. Occurrence of phthalate diesters in particulate and vapor phases in indoor air and implications for human exposure in Albany, New York, USA. Archives of Environmental Contamination and Toxicology. 2015;68(3):489–499. doi: 10.1007/s00244-015-0140-0. [DOI] [PubMed] [Google Scholar]
- 17.Cutanda F., Koch H. M., Esteban M., Sánchez J., Angerer J., Castaño A. Urinary levels of eight phthalate metabolites and bisphenol A in mother-child pairs from two Spanish locations. International Journal of Hygiene and Environmental Health. 2015;218(1):47–57. doi: 10.1016/j.ijheh.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y., Wu Q., Kannan K. Phthalate metabolites in urine from China, and implications for human exposures. Environment International. 2011;37(5):893–898. doi: 10.1016/j.envint.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Chen G., Christie P., Zhang M., Luo Y., Teng Y. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Science of the Total Environment. 2015;523:129–137. doi: 10.1016/j.scitotenv.2015.02.101. [DOI] [PubMed] [Google Scholar]
- 20.Golestanzadeh M., Riahi R., Kelishadi R. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: a systematic review and meta-analysis. Environmental Science and Pollution Research. 2019;26(35):35670–35686. doi: 10.1007/s11356-019-06589-7. [DOI] [PubMed] [Google Scholar]
- 21.Kataria A., Levine D., Wertenteil S., et al. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatric Research. 2017;81(6):857–864. doi: 10.1038/pr.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S.-Y., Hwang J.-S., Sung F.-C., et al. Mono-2-ethylhexyl phthalate associated with insulin resistance and lower testosterone levels in a young population. Environmental Pollution. 2017;225:112–117. doi: 10.1016/j.envpol.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q., Cornelis M. C., Townsend M. K., et al. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the nurses’ health study (NHS) and NHSII cohorts. Environmental Health Perspectives. 2014;122(6):616–623. doi: 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoshtari-Yeganeh B., Zarean M., Mansourian M., et al. Systematic review and meta-analysis on the association between phthalates exposure and insulin resistance. Environmental Science and Pollution Research. 2019;26(10):9435–9442. doi: 10.1007/s11356-019-04373-1. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y., Li J., Garcia J. M., et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0087430.e87430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James-Todd T., Stahlhut R., Meeker J. D., et al. Urinary phthalate metabolite concentrations and diabetes among women in the national health and nutrition examination survey (NHANES) 2001–2008. Environmental Health Perspectives. 2012;120(9):1307–1313. doi: 10.1289/ehp.1104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svensson K., Hernández-Ramírez R. U., Burguete-García A., et al. Phthalate exposure associated with self-reported diabetes among Mexican women. Environmental Research. 2011;111(6):792–796. doi: 10.1016/j.envres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radke E. G., Galizia A., Thayer K. A., Cooper G. S. Phthalate exposure and metabolic effects: a systematic review of the human epidemiological evidence. Environment International. 2019;132:p. 9. doi: 10.1016/j.envint.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dales R. E., Kauri L. M., Cakmak S. The associations between phthalate exposure and insulin resistance, β-cell function and blood glucose control in a population-based sample. Science of the Total Environment. 2018;612:1287–1292. doi: 10.1016/j.scitotenv.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Huang P.-C., Tsai C.-H., Chen C.-C., et al. Intellectual evaluation of children exposed to phthalate-tainted products after the 2011 Taiwan phthalate episode. Environmental Research. 2017;156:158–166. doi: 10.1016/j.envres.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Kim J. H., Park H. Y., Bae S., Lim Y. H., Hong Y. C. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071392.e71392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halliwell B. Antioxidants and human disease: a general introduction. Nutrition Reviews. 1997;55(1 Pt 2):S44–S52. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 33.Tang X., Tong K., Zhu L., et al. Di-2-ethylhexyl phthalate induced oxidative damage involving FasL-associated apoptotic pathway in mouse spermatogenic GC-2spd cells. Molecular & Cellular Toxicology. 2016;12(4):381–389. doi: 10.1007/s13273-016-0042-x. [DOI] [Google Scholar]
- 34.Kambia N., Dine T., Gressier B., et al. Correlation between exposure to phthalates and concentrations of malondialdehyde in infants and children undergoing cyclic parenteral nutrition. Journal of Parenteral and Enteral Nutrition. 2011;35(3):395–401. doi: 10.1177/0148607110381769. [DOI] [PubMed] [Google Scholar]
- 35.hang L. Z., Dong L., Ren L., et al. Concentration and source identification of polycyclic aromatic hydrocarbons and phthalic acid esters in the surface water of the Yangtze River Delta, China. Journal of Environmental Sciences. 2012;24(2):335–342. doi: 10.1016/s1001-0742(11)60782-1. [DOI] [PubMed] [Google Scholar]
- 36.Song P., Gao J., Li X., et al. Phthalate induced oxidative stress and DNA damage in earthworms (Eisenia fetida) Environment International. 2019;129:10–17. doi: 10.1016/j.envint.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Liu L., Wang X., Huang Q., Tian M., Shen H. Low-level environmental phthalate exposure associates with urine metabolome alteration in a Chinese male cohort. Environmental Science and Technology. 2016;50(11):5953–5960. doi: 10.1021/acs.est.6b00034. [DOI] [PubMed] [Google Scholar]
- 38.Amin M. M., Ebrahimpour K., Parastar S., et al. Association of urinary concentrations of phthalate metabolites with cardiometabolic risk factors and obesity in children and adolescents. Chemosphere. 2018;211:547–556. doi: 10.1016/j.chemosphere.2018.07.172. [DOI] [PubMed] [Google Scholar]
- 39.Amin M. M., Rafiei N., Poursafa P., et al. Association of benzene exposure with insulin resistance, SOD, and MDA as markers of oxidative stress in children and adolescents. Environmental Science and Pollution Research. 2018;25(34):34046–34052. doi: 10.1007/s11356-018-3354-7. [DOI] [PubMed] [Google Scholar]
- 40.Amin M. M., Ebrahimpour K., Parastar S., Shoshtari-Yeganeh B., Hashemi M. Method development of di-(2-ethylhexyl) phthalate metabolites detection by dispersive liquid-liquid microextraction gas chromatography-mass spectrometry from urine. International. Journal of Environmental Health Engineering. 2018;7(1) [Google Scholar]
- 41.Won E. K., Kim Y., Ha M., et al. Association of current phthalate exposure with neurobehavioral development in a national sample. International Journal of Hygiene and Environmental Health. 2016;19(4-5):364–371. doi: 10.1016/j.ijheh.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Buser M. C., Murray H. E., Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. International Journal of Hygiene and Environmental Health. 2014;217(6):687–694. doi: 10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regnier S. M., Sargis R. M. Adipocytes under assault: environmental disruption of adipose physiology. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2014;1842(3):520–533. doi: 10.1016/j.bbadis.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H., Zhou Y., Tang C., et al. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056800.e56800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teitelbaum S. L., Mervish N., Moshier E. L., et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environmental Research. 2012;112:186–193. doi: 10.1016/j.envres.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boas M., Frederiksen H., Feldt-Rasmussen U., et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environmental Health Perspectives. 2010;118(10):1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L., Chen H., Xu Q., et al. The effect of di-2-ethylhexyl phthalate on inflammation and lipid metabolic disorder in rats. Ecotoxicology and Environmental Safety. 2019;170:391–398. doi: 10.1016/j.ecoenv.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Baralic K., Buha djordjevic A., Zivancevic K., et al. Toxic effects of the mixture of phthalates and bisphenol A— subacute oral toxicity study in wistar rats. International Journal of Environmental Research and Public Health. 2020;17(3):p. 24. doi: 10.3390/ijerph17030746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao Y., Chena D. Y., Yind J. W., et al. Phthalate exposure linked to high blood pressure in Chinese children. Environment International. 2020;143(8) doi: 10.1016/j.envint.2020.105958. [DOI] [PubMed] [Google Scholar]
- 50.Mariana M., Cairrao E. Phthalates implications in the cardiovascular system. Journal of Cardiovascular and Development Disease. 2020;7(3):p. 19. doi: 10.3390/jcdd7030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su T.-C., Hwang J.-S., Torng P.-L., Wu C., Lin C.-Y., Sung F.-C. Phthalate exposure increases subclinical atherosclerosis in young population. Environmental Pollution. 2019;250:586–593. doi: 10.1016/j.envpol.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Trasande L., Attina T. M. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension. 2015;66(2):301–308. doi: 10.1161/hypertensionaha.115.05603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poursafa P., Dadvand P., Amin M. M., et al. Association of polycyclic aromatic hydrocarbons with cardiometabolic risk factors and obesity in children. Environment International. 2018;118:203–210. doi: 10.1016/j.envint.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 54.Goudarzi M., Haghi Karamallah M., Malayeri A., Kalantar M., Mansouri E., Kalantar H. Protective effect of alpha-lipoic acid on di-(2-ethylhexyl) phthalate-induced testicular toxicity in mice. Environmental Science and Pollution Research. 2020;27(12):13670–13678. doi: 10.1007/s11356-020-07817-1. [DOI] [PubMed] [Google Scholar]
- 55.Yang G., Gao X., Jiang L., et al. 6-gingerol prevents mehp-induced DNA damage in human umbilical vein endothelia cells. Human & Experimental Toxicology. 2017;36(11):1177–1185. doi: 10.1177/0960327116681650. [DOI] [PubMed] [Google Scholar]
- 56.National Research Council. Contaminated Water Supplies at Camp Lejeune: Assessing Potential Health Effects: National Research Council (US) Committee on Contaminated Drinking Water at Camp Lejeune. Washington, DC, USA: National Academies Press; 2009. Systemic exposures to volatile organic compounds and factors influencing susceptibility to their effects. https://www.ncbi.nlm.nih.gov/books/NBK215288/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this paper are available at https://pubmed.ncbi.nlm.nih.gov/30092535/ (Phthalates Implications in the Cardiovascular System).


