Multiple Mtcep mutants generated using a modified CRISPR/Cas9 system reveal the redundant roles of MtCEP1, 2, and 12 in controlling lateral root and nodule number in Medicago truncatula.
Keywords: CEP peptides, CRISPR/Cas9, lateral root, Medicago truncatula, multigene editing, symbiotic nodulation
Abstract
The multimember CEP (C-terminally Encoded Peptide) gene family is a complex group that is involved in various physiological activities in plants. Previous studies demonstrated that MtCEP1 and MtCEP7 control lateral root formation or nodulation, but these studies were based only on gain of function or artificial miRNA (amiRNA)/RNAi approaches, never knockout mutants. Moreover, an efficient multigene editing toolkit is not currently available for Medicago truncatula. Our quantitative reverse transcription–PCR data showed that MtCEP1, 2, 4, 5, 6, 7, 8, 9, 12, and 13 were up-regulated under nitrogen starvation conditions and that MtCEP1, 2, 7, 9, and 12 were induced by rhizobial inoculation. Treatment with synthetic MtCEP peptides of MtCEP1, 2, 4, 5, 6, 8, and 12 repressed lateral root emergence and promoted nodulation in the R108 wild type but not in the cra2 mutant. We optimized CRISPR/Cas9 [clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9] genome editing system for M. truncatula, and thus created single mutants of MtCEP1, 2, 4, 6, and 12 and the double mutants Mtcep1/2C and Mtcep5/8C; however, these mutants did not exhibit significant differences from R108. Furthermore, a triple mutant Mtcep1/2/12C and a quintuple mutant Mtcep1/2/5/8/12C were generated and exhibited more lateral roots and fewer nodules than R108. Overall, MtCEP1, 2, and 12 were confirmed to be redundantly important in the control of lateral root number and nodulation. Moreover, the CRISPR/Cas9-based multigene editing protocol provides an additional tool for research on the model legume M. truncatula, which is highly efficient at multigene mutant generation.
Introduction
The root architecture system, which provides structural support and participates in the perception, absorption, allocation, and transport of nutrients, is essential for the survival of the whole plant (Motte et al., 2019). The root system consists of primary and lateral roots, and its developmental responses are plastic to the heterogeneous soil environment, which enables immobile plants to adapt to the changing natural environment. Some specialized root-derived organs, such as nodules, where symbiosis is established between symbiotic bacteria and their host plants, also take part in the developmental process of the root system. This intricate system relies on various molecules that function in the coordination of different intercellular events (Takahashi et al., 2019).
Small peptide hormones play vital roles in both growth-regulating and developmental processes and responding to environmental stress in the root system (Delay et al., 2013b; Matsubayashi, 2014; Okamoto et al., 2016). C-terminally encoded peptides (CEPs) are small, secreted peptides containing 15 highly conserved amino acids in the mature state, which participate in root development and other multiple physiological activities, such as symbiotic nitrogen fixation and nitrate uptake (Ohyama et al., 2008; Imin et al., 2013; Tabata et al., 2014). These peptides are derived from non-functional pre-propeptides, processed by proteolytic cleavage and the addition of post-translational modifications (PTMs), and then transferred to the apoplast, stem xylem, or possibly the endoplasmic reticulum (ER), where they function as ligands with their corresponding receptors to trigger downstream responses (Okamoto et al., 2016; Patel et al., 2018; Zhu et al., 2020). Genes from the CEP family are present across seed plants and have evolved via duplication and variation, which has led to the diversification of CEP-associated regulatory networks (Delay et al., 2013a; Ogilvie et al., 2014).
Previous studies on the CEP biology of non-legume species have usually used Arabidopsis as the research model and have mainly focused on regulation of the root architecture system and nitrogen demand signalling (Tabata et al., 2014; Okamoto et al., 2016; Taleski et al., 2018). Fifteen CEP genes have been identified in the Arabidopsis genome (Roberts et al., 2013), and the first identified gene was CEP1, which was identified using an in silico approach; this peptide repressed both primary and lateral root growth upon its overexpression or external application (Ohyama et al., 2008). CEP receptors (CEPRs) have been subsequently characterized; these receptors perceive root-derived CEPs induced by nitrogen deficiency and trigger shoot to root nitrogen-demand reactions (Tabata et al., 2014). Despite systematic regulation, CEP5 functions locally with CEPR1 in controlling lateral root initiation (Roberts et al., 2016), and CEPs inhibit primary and lateral root growth in a CEPR1-dependent manner (Chapman et al., 2019; Delay et al., 2019).
An important property of legumes is their nodulation and nitrogen fixation capability, and the functions of CEPs in legumes warrant further investigation and discussion (Kereszt et al., 2018). In the legume model plant Medicago truncatula Jemalong A17 Mt4.0 and Mt3.5v5 genome assemblies, 17 CEP genes have been identified by bioinformatic analysis (de Bang et al., 2017); 13 of these MtCEP genes belong to group I and share similar CEP domains containing 15 amino acids, and the other four genes encode group II CEP domains that are unusual compared with group I CEPs, particularly in their N-terminal residues. MtCEP1 encodes two CEP domains, modulates lateral root development, and enhances nodulation (Imin et al., 2013). In addition, MtCEP7 has been demonstrated to enhance nodulation antagonistically to a CLE peptide systemic pathway that inhibits nodulation (Gautrat et al., 2020; Laffont et al., 2020). Using a modified isolation and characterization protocol, the presence of endogenous MtCEP1 peptides with distinct PTMs was validated, and the application of chemically synthesized peptides with different PTM patterns affected lateral root formation to varying degrees (Mohd-Radzman et al., 2015). In addition, mass spectrum (MS) and bioinformatics approaches have been used to identify more PTM diversity among MtCEP1, 2, 5, and 8 in vivo (Patel et al., 2018). COMPACT ROOT ARCHITECTURE 2 (CRA2) is referred to as the homologue of CEPR1 in M. truncatula and the candidate receptor for MtCEPs, but there is no biochemical proof that CEPs directly bind to CRA2 (Huault et al., 2014; Mohd-Radzman et al., 2015; Laffont et al., 2019; Zhu et al., 2020). A cra2 mutant exhibits a particular phenotype in its belowground parts, and this phenotype is characterized by an increase in lateral roots that is not affected by the nitrogen or sucrose concentration and significantly inhibited nodulation under symbiotic conditions (Huault et al., 2014). In addition, the cra2 mutant is unresponsive to MtCEP1 treatment, which is suggestive of a ligand–receptor relationship (Mohd-Radzman et al., 2016; Zhu et al., 2020). However, few studies based on knockout mutants have identified the contribution of other MtCEP genes to root architecture and nodulation. The lack of Mtcep mutants has hindered further progress in this area.
The CRISPR/Cas 9 [clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9] system is the most versatile genome editing tool developed over the last 30 years and has been widely used for single or multiple target(s) editing in soybean, wheat, cotton, maize, rice, etc. (Peng et al., 2016; Zhang et al., 2020). For the legume model plant M. truncatula, CRISPR/Cas9 techniques have been broadly applied for single-target mutagenesis (Meng et al., 2017). Recently, a new multiplex genome editing system in tetraploid alfalfa (Medicago sativa) based on a polycistronic tRNA–gRNA approach was created by Wolabu et al. (2020), but still uses the U6 promoter cloned from Arabidopsis and focuses on multiple sites of single gene editing. However, multigene editing remains difficult to perform, because few native MtU6 promoters, but not MtU6-1p, have been confirmed to be valid for adequately driving small guide RNA (sgRNA) cassette expression in M. truncatula, which is necessary for multigene targeting in this species.
In this study, we identified 12 group I and four group II MtCEP genes in R108, and, herein we discuss the expression patterns of the group I CEP genes, and their functions during root development and nodulation. Quantitative reverse transcription–PCR (RT–qPCR) analysis showed that root-expressed MtCEP1, 2, 4, 5, 6, 7, 8, 9, 12, and 13 were up-regulated under nitrogen starvation conditions and that MtCEP1, 2, 7, 9, and 12 were induced by rhizobial inoculation. In R108, the synthetic MtCEP1, 2, 4, 5, 6, 8, and 12 peptides reduced lateral root number and increased nodule number, whereas a cra2 mutant was insensitive to these treatments. We modified the CRISPR/Cas9 toolbox (Xing et al., 2014) for M. truncatula to generate the single-mutant lines Mtcep1C (C indicates that the mutant was created by CRISPR/Cas9), Mtcep2C, Mtcep4C, Mtcep6C, and Mtcep12C, and the double-mutant lines Mtcep1/2C and Mtcep5/8C, and these lines exhibited no visible changes in their phenotypes compared with that of the wild type (WT). We then knocked out MtCEP1, 2, and 12 in both the WT and Mtcep5/8C backgrounds to obtain triple and quintuple Mtcep mutants, and found that these mutants exhibited higher numbers of lateral roots and fewer nodules, but did not recapitulate the cra2 mutant phenotype. These results indicated that MtCEP1, 2, and 12 are redundantly important for the coordination of lateral root formation and symbiotic nodulation, and the optimization of a CRISPR/Cas9 toolkit for multiplex genome editing could accelerate research on the functions of multigene families in M. truncatula.
Materials and methods
Plant materials and growth conditions
The M. truncatula R108 ecotype, which served as the WT in this study, was used as the genetic background for Agrobacterium-mediated transformation. The cra2 (NF0903) mutant was obtained from the Tnt1 insertion mutant library at the Noble Research Institute (Zhu et al., 2020).
Seed sterilization was based on the procedure described in Duan et al. (2017). Briefly, the seeds were immersed in H2SO4 (98%, w/w) for 8 min, washed with ice-cold water three times, treated with 0.5% (v/v) NaClO for 12 min, and washed five times with sterile deionized water on a clean bench. After pre-treatment, the seeds were laid on 0.8% water agar medium and stored at 4 °C in darkness for 3 d. The seeds were then germinated at 25 °C under dark conditions for 12 h.
Plants were grown in a greenhouse at a temperature of 22 °C, a 16 h light/8 h dark photoperiod, a light intensity of 100–150 μmol s–1 m–2, and 60–70% humidity. In addition, plants were grown in a soil/perlite/vermiculite (1:2:5, v/v/v) mixture for seed production and in a perlite/vermiculite (2:5, v/v) mixture (imparts little resistance for root system penetration) saturated with nitrogen-depleted Fåhraeus liquid medium for nodulation. For root system architecture assessment, the matrix was removed with running water before the nodules and lateral roots were counted and the root length was measured and photographed.
Bacterial materials and cultivation conditions for nodulation
Sinorhizobium meliloti strain 1021 (Sm1021) was used for symbiosis-associated experiments, and rhizobia cultured overnight were resuspended in sterile water (for inoculation on plates) or nitrogen-depleted Fåhraeus liquid medium (for inoculation in pots) at OD600=0.05 for inoculation. Seedlings grown aseptically on square plates for 7 d with Fåhraeus medium without nitrogen (13 cm×13 cm) were flooded with 20 ml of suspension for 1.5 h (each plate) (in contrast, 10 ml was used in Gonzalez-Rizzo et al., 2006), and seedlings grown in pots in the greenhouse were subjected to injection with 10 ml (per plant) of suspension into the matrix (Gonzalez-Rizzo et al., 2006).
Effects of synthetic MtCEP peptides on root growth and nodulation
MtCEP peptides for external application were synthesized by GL Biochem (Shanghai, China) Ltd. MtCEP1, 2, 4, 5, 6, 8, and 12 were synthesized with hydroxylation at Pro4 and Pro11. When several conserved CEP domains existed in a precursor gene, the first of the CEP domains was selected for synthesis.
For the root growth analysis, each group of R108 and cra2 mutants was grown on the same 1/2 Murashige and Skoog (MS, without sucrose, pH 5.8) solid medium (1.5% agar, w/w) supplemented with each synthetic MtCEP peptide at a concentration of 1 μM for 10 d, and the lateral roots were then counted for phenotyping.
For the nodulation analysis, the R108 and cra2 mutants were first grown on nitrogen-depleted Fåhraeus solid medium covered with seed germination paper (Anchor Paper, www.anchorpaper.com) for 6 d and then transferred together with the germination paper to nitrogen-depleted Fåhraeus medium supplemented with 1 μM synthetic MtCEP peptide for an additional day. Inoculation with Sm1021 suspended in sterile water containing 1 μM synthetic MtCEP peptide (to maintain the concentration of CEP peptides in the germination paper) was performed at 7 d post-germination, and at 14 days post-inoculation (dpi) the nodules were counted for phenotype analysis.
RT–qPCR assays of the expression of MtCEP genes in response to nitrogen starvation or rhizobial inoculation
The samples collected for RT–qPCR for the MtCEP expression analysis in R108 under nitrogen starvation conditions, or in response to rhizobial inoculation, were aseptically grown on plates. After germination, the seedlings were first laid on Fåhraeus solid medium with 10 mM NO3− for 5 d, transferred to N-depleted Fåhraeus solid medium, and collected at 2 h, 12 h, 24 h, and 7 d after transition by dissecting the aboveground and belowground parts. For the nodulation analysis, the remaining seedlings were inoculated with Sm1021 after nitrogen starvation for 7 d; mixtures of roots and nodules from seven plants collected at 12 hours post-inoculation (hpi), 1 dpi, 3 dpi, 5 dpi, and 7 dpi acted as respective sample pools. For the expression of MtCEP4, 5, 6, 7, 8, 9, 11, and 13 in R108, Mtcep1/2/12C, and Mtcep1/2/5/8/12C, these seedlings were grown in liquid medium without nitrogen for 7 d (Zhu et al. 2020), and then the roots harvested from five plants were treated as one pool. Three independent pools of these tissues were used as three biological repetitions. TRIzol reagent (Invitrogen), trichloromethane, isopropanol, and ethyl alcohol (75%, v/v) were utilized for RNA extraction from the roots and shoots with overnight isopropanol precipitation (–20 °C), DNase (Promega, cat# M610A) was used to digest the DNA, and then the RNA was purified again with overnight isopropanol precipitation (–20 °C). Electrophoresis (1.2% agarose, v/v) was used to identify the RNA integrity, and a NanoDrop™ 2000 spectrophotometer was used to quantify the RNA concentration. Then, Moloney murine leukaemia virus (M-MLV) reverse transcriptase (Promega, cat# M1701) was used to synthesize first-strand cDNA from 4.0 μg of total RNA. RT–qPCR was carried out on a CFX-96 real-time system (Bio-Rad) with SYBR Premix Ex-Taq (TaKaRa, cat# RR420A). Gene expression was calculated using the 2–ΔΔCT method. The expression data were normalized based on two reference genes MtACTIN11 and MtRBP1 (Laffont et al., 2020). The primers used to test the expression levels of each MtCEP gene by RT–qPCR are listed in Supplementary Table S1.
Constructs for GUS reporter lines and GUS staining
The promoter regions of MtCEP1 (2.81 kb), 2 (2.25 kb), 4 (2.75 kb), 5 (2.87 kb), 6 (2.8 kb), 8 (2.91 kb), and 12 (2.77 kb) were cloned and ligated into pCAMBIA_1381 to drive the expression of the GUS (β-glucuronidase) gene. The primers for the constructs are listed in Supplementary Table S1. These constructs were transformed into R108 by Agrobacterium tumefaciens strain EHA105, which resulted in the generation of stable transgenic lines. Genomic DNA from the transgenic lines was extracted using the cetyltrimethylammonium bromide (CTAB) method (Springer, 2010), and the exogenous fragments from the plasmids inserted during the transformation were identified by PCR.
For GUS staining, the transgenic GUS reporter lines were grown in pots with nitrogen-depleted Fåhraeus medium for 7 d and then inoculated with Sm1021, and samples were collected at 0, 5, and 14 dpi (Fig. 2). For the GUS expression patterns at the early stage of rhizobial inoculation, the seedlings were grown in plates with Fåhraeus medium (without nitrogen) for 7 d and then inoculated with rhizobia, and GUS staining was carried out at 0, 1, and 2 dpi (Supplementary Fig. S4). Based on the method from Cervera (2005), the harvested material was immersed in staining buffer [50 mM phosphate buffer (pH 7.0), 1 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc), 5 mM potassium ferricyanide, and 5 mM potassium ferrocyanide], treated with a vacuum (>0.09 MPa) for 30 min, and stained at 37 °C for 6 h. The superfluous dye and chlorophyll were removed several times with 75% ethanol (6 h per wash), and the tissues were subsequently immersed in transparent liquid [4% Arabic gum (w/w), 60% trichloroacetic aldehyde (w/w), 3% glycerine (w/w)], and then imaged using an Olympus microscope (BX51) with an interference device.
Fig. 2.
Spatial expression patterns of MtCEP genes. The 2.2–3 kb promoters of MtCEP1, MtCEP2, MtCEP4, MtCEP5, MtCEP6, MtCEP8, and MtCEP12 were cloned into pCAMBIA_1381 to drive the expression of GUS, and the fused vectors were then stably transferred to R108. The stable transgenic plants ProMtCEP1:GUS, ProMtCEP2:GUS, ProMtCEP4:GUS, ProMtCEP5:GUS, ProMtCEP6:GUS, ProMtCEP8:GUS, and ProMtCEP12:GUS were germinated and grown in a soil/vermiculite mixture with Fåhraeus medium without nitrogen and inoculated with rhizobia 7 d later. The GUS staining images of the first single leaf (A), first compound leaves and two cotyledons (B), emerging lateral roots (C), primary root tips (D), and nodule primordium (E) at 5 dpi and the nodules (F) at 14 dpi are shown. The scale bar represents 1 mm in (A) and (B), and 200 µm in (C–F).
Optimization of the CRISPR/Cas9 genome editing protocol
We first cloned the MtU6-1 promoter from A17 genomic DNA using the primer pair MtU6-1-F/R, and the MtU6-3/5/6 promoter from R108 genomic DNA using the primer pairs MtU6-3-F/R, MtU6-5-F/R, and MtU6-6-F/R. For modification of the sgRNA cassette, we amplified the sgRNA scaffold and AtU6-26 terminator from the original pCBC plasmid (Xing et al., 2014) using the primer pairs pCBC-F and pCBC-MtU6-1/3/5/6-MR, conjoined this fragment with the MtU6-1/3/5/6 promoter by overlapping PCR with the primer pairs pCBC-F and MtU6-1/3/5/6-R, and ligated the final long fragments into the pLB vector (TIANGEN, cat# VT206), which resulted in p1/3/5/6CBC.
We then amplified fragments of the MtU6-3/6 promoter with the HindIII restriction enzyme cleavage site at the 5′ end and overlapping sequences of SpR at the 3′ end with the primer pairs HindIII-MtU6-3/6-F and MtU6-3/6-SpR-R, and fragments of the SpR+sgRNA scaffold+AtU6-26 terminator from the original pHSE401 plasmid using the primer pairs MtU6-3/6-SpR-F and pHSE401-R. These two components were conjoined by overlapping PCR with the primer pairs HindIII-MtU6-3/6-F and pHSE401-R, and digested with HindIII. Additionally, pHSE401 was digested with HindIII, and the long fragment with the Cas9 cassette and selectable marker Hyg cassette was retained. Fragments of the MtU6-3/6 promoter+SpR+sgRNA scaffold+AtU6-26 terminator were then inserted into digested pHSE401 to produce the binary vector pHSE3/6-401. All the primers used in this section are presented in Supplementary Table S1.
Mutant generation using CRISPR/Cas9-based approaches
Based on Xing et al. (2014), sgRNA cassettes targeting MtCEP genes for the generation of single or double mutants were amplified from modified versions of p1/3/6CBC (~5 ng) by the primer pairs Target1-401-BsF/Target1-F0/Target2-1CBC-R0/Target2-BsR, Target1-401-BsF/Target1-F0/Target2-3CBC-R0/Target2-BsR, or Target1-401-BsF/Target1-F0/Target2-6CBC-R0/Target2-BsR, in which F0/R0 was diluted to 1 μM and BsF/BsR was diluted to 10 μM, and ligated into pHSE401 by the Golden Gate reaction [2 μl of pHSE401 (~200 ng μl–1), 8 μl of sgRNA cassette fragment (~100 ng μl–1), 1 μl of BsaI (NEB, cat#R0535), 1 μl of T4 DNA ligase (NEB, cat#M0202T), 1.5 μl of 10× T4 DNA Ligase Buffer (NEB), and 1.5 μl of 10× BSA, 37 °C for 5 h, 50 °C for 5 min and 80 °C for 10 min]. The ligation products were transformed into Escherichia coli with resistance to kanamycin and the plasmids were extracted and confirmed by sequencing. The constructs were separately transformed into R108 with resistance to hygromycin by A. tumefaciens strain EHA105, and the regenerated transgenic lines were identified by PCR. The specific mutations were confirmed by amplifying the genomic fragments flanking the target sites, ligating them into intellectual vectors, and then sequencing randomly selected positive clones.
The construct targeting MtCEP1/2/12 was based on the procedure described in Xing et al. (2014). Briefly, sgRNA cassettes were cloned from p1CBC and p6CBC with the addition of the corresponding target site by the primer pairs Target1-401-BsF/Target1-F0/1CBC-R0 and Target2-6CBC-BsF/Target2-F0/Target3-6CBC-R0/Target3-BsR, which resulted in p1CBC-MtCEP1-Target and p6CBC-MtCEP2-Target/MtCEP12-Target, respectively. The two fragments were simultaneously ligated into the binary vector pHSE401 by the Golden Gate reaction in a single round as described above. The construct was transformed into R108 and Mtcep5/8 separately by EHA105 to generate Mtcep1/2/12C and Mtcep1/2/5/8/12C. The regenerated seedlings were tested by PCR using primer pairs for fragments of transferred T-DNA and genomic DNA flanking the target sites in MtCEP1/2/12, and the mutation types were identified by sequencing. The target sites selected for STENOFOLIA (STF) and MtCEP editing, and the corresponding primers are listed in Supplementary Table S2.
Nodulation and root system architecture phenotype assays
Phenotype assays were conducted in a greenhouse. Seedlings of the mutants and the control group were planted in a matrix with nitrogen-depleted Fåhraeus liquid medium for 7 d and then inoculated with Sm1021. Various metrics of the root system architecture, including root lengths and lateral root number, were measured at 14 dpi, and the lateral root densities were calculated as the lateral root number divided by the root length, whereas the nodules were counted at 14 and 21 dpi.
Results
Different expression levels of MtCEP genes induced by nitrogen starvation or rhizobial inoculation
The CEP genes identified from the Jemalong A17 Mt4.0 and Mt3.5v5 genome assemblies can be divided into two groups depending on the conservation of amino acid sequences of their CEP domain: group I CEPs and group II CEPs (de Bang et al., 2017). However, in the M. truncatula R108 genome and Jemalong A17 Mt5.0 genome assembly, 16 MtCEP genes, but not MtCEP3, were identified by comparison with the genomic database and by a transcriptomic assay, and MtCEP10 encoded three identical CEP domains in R108. In addition, we discovered that these CEP precursor genes and their CEP domains were conserved between R108 and A17 (Supplementary Table S3). The top 12 MtCEP genes with high sequence similarity in their CEP domain belonged to group I, whereas the MtCEP14–MtCEP17 genes, whose CEP domains contained unusual motifs compared with those of group I CEPs, were classified as group II (Supplementary Fig. S1A, B; Supplementary Table S3). Due to little evidence on the genetic functions of the group II CEPs and their association with group I functions, group II genes were excluded in this work. We conducted both in vitro and in vivo experiments to determine the functions of the group I MtCEP genes.
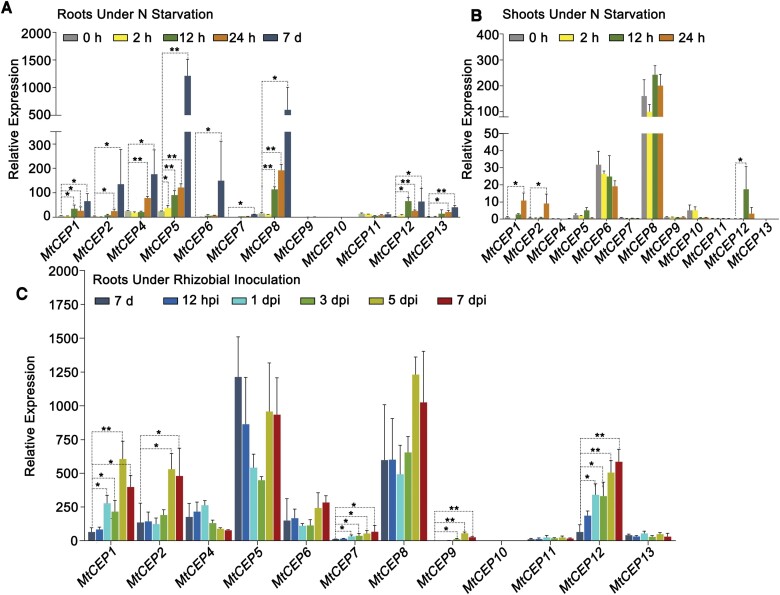
To investigate whether MtCEP genes were induced by low-nitrogen conditions, we laid germinated R108 seedlings on Fåhraeus medium plates with 10 mM NO3− for 5 d and then transferred them to Fåhraeus medium without nitrogen. Roots and shoots were collected separately for RT–qPCR at 2, 12, and 24 h, and 7 d after starvation treatment. The relative expression levels of MtCEP1, 2, 4, 5, 6, 7, 8, 9, 12, and 13 in roots increased to varying degrees, and, among these, MtCEP5 and MtCEP8 exhibited the strongest expression, and MtCEP7 and MtCEP9 showed very low expression levels (Fig. 1A; Supplementary Fig. S2A). Although mild elevations in the relative expression levels of MtCEP1, 2, and 12 were found in shoots, these CEP genes showed poor expression in shoots, with the exception of MtCEP8 (Fig. 1B).
Fig. 1.
Relative expression levels of the group I MtCEP genes under nitrogen starvation conditions or in response to rhizobial inoculation. The germinated R108 seedlings were grown on Fåhraeus medium plates with 10 mM NO3− for 5 d and then transferred to Fåhraeus medium without nitrogen, and the (A) roots and (B) shoots were harvested separately after 0, 2, 12, and 24 h, and 7 d. The remaining 12-day-old plants were inoculated with rhizobia; (C) the whole roots contained the primordium or nodules at 12 h post-inoculation (hpi) and 1, 3, 5, and 7 days post-inoculation (dpi). MtACTIN11 and MtRBP1 were used as the reference genes. The expression of MtCEP1 in shoots at 0 h was normalized to 1, and the same scale on the y-axis was used in (A), (B), and (C). The data were derived from three independent pools of samples and are presented as the means± SDs (n=3). Significant differences were determined with a paired two-tailed Student’s t-test (*P<0.05, **P<0.01).
To test whether MtCEP genes were induced by rhizobial inoculation, R108 seedlings subjected to nitrogen starvation for 7 d were inoculated with Sm1021 on plates. Root samples were collected at 12 hpi, and 1, 3, 5, and 7 dpi for RT–qPCR. After inoculation, the relative expression levels of MtCEP1, 7, 9, and 12 were up-regulated at an early stage (1 and 3 dpi), and that of MtCEP2 was up-regulated at a later stage (5 and 7 dpi). However, the expression levels of MtCEP7 and MtCEP9 were much lower than those of MtCEP1, 2, and 12 (Fig. 1C; Supplementary Fig. S2B). Additionally, our previous RNA-seq data on roots and shoots of R108 inoculated with rhizobia for 5 d (Zhu et al., 2020) revealed that MtCEP1, 2, 4, 5, 6, 8, and 12 presented higher expression in roots among the 12 MtCEP genes (Supplementary Fig. S3). We then focused on these seven MtCEP genes that were induced by nitrogen starvation or rhizobial inoculation.
Spatial expression patterns of MtCEP genes
To further investigate the spatial expression patterns of the candidate MtCEP genes, we cloned the 2.2–3 kb promoters of MtCEP1, 2, 4, 5, 6, 8, and 12 and fused them with the GUS A coding region in the backbone of pCAMBIA_1381. We generated stable transgenic GUS reporter lines in the R108 background, and these were termed ProMtCEP1:GUS, ProMtCEP2:GUS, ProMtCEP4:GUS, ProMtCEP5:GUS, ProMtCEP6:GUS, ProMtCEP8:GUS, and ProMtCEP12:GUS. Seedlings of each line were grown in a mixture of vermiculite and perlite filled with liquid Fåhraeus medium without nitrogen for 7 d and then inoculated with rhizobia. GUS staining showed that these selected MtCEP genes exhibited diverse expression patterns. Although all of the genes were expressed in roots, differences were found between each of these MtCEP genes. For example, all seven MtCEP genes showed obvious staining in the stele of the primary root tip, whereas the staining signals of ProMtCEP2:GUS, ProMtCEP4:GUS, and ProMtCEP12:GUS were additionally reflected in the root cap (Fig. 2D). All seven MtCEP genes were expressed in the stele of the lateral root primordium, but MtCEP1, 2, 4, 5, and 6 also showed diffuse staining in the periphery (Fig. 2C). During nodulation, MtCEP1, 2, 4, 5, 6, 8, and 12 were first expressed in the vascular tissue, located in the base of the nodule primordium, and then expressed in vascular bundles surrounding the established nodule (Fig. 2E, F). In addition, unlike MtCEP7 expressed in the root epidermis and infected cells during the early stage of rhizobial inoculation (Laffont et al., 2020), all seven MtCEP genes were expressed in the vascular tissues of roots and showed no signals in the epidermis at 1 and 2 dpi (Supplementary Fig. S4). Staining in shoots was also observed. These results showed that MtCEP1, 2, 5, 6, 8, and 12 presented staining signals in cotyledons, whereas in true leaves, MtCEP1, 6, 8, and 12 exhibited clearly detectable GUS signalling in the first single leaf, and all these genes were expressed in the first compound leaves (Fig. 2A, B). In summary, these selected MtCEP genes presented redundant spatial expression patterns.
External application of chemically synthesized MtCEP peptides led to fewer lateral roots and more nodules
To test the effects of different MtCEP treatments on nodulation or lateral root number, we chemically synthesized peptides of the seven candidate MtCEP genes that exhibited up-regulated expression under nitrogen starvation conditions or in response to rhizobial inoculation. According to MS analysis of CEP peptides by Tabata et al. (2014) and Patel et al. (2018), CEP peptides have variant derivatives with different hydroxylation patterns at proline residues 4, 7, and 11. For example, the MtCEP1a CEP domain with hydroxylation at Pro4 and Pro11 was the most biologically active variant of MtCEP1 (Patel et al., 2018). Based on this information, MtCEP1, 2, 4, 5, 6, 8, and 12 were synthesized with hydroxylation at Pro4 and Pro11.
To determine the effects of the MtCEPs on lateral root development, the R108 WT and cra2 mutant lines were grown on the same 1/2 MS medium supplemented with or without each MtCEP at a concentration of 1 μM, and the lateral root number was counted 10 d after germination. All the groups of R108 treated with MtCEPs had fewer lateral roots than the control, but no significant difference was found among the cra2 mutant groups. This result suggested that these artificial MtCEPs decreased lateral root number (Fig. 3A, B).
Fig. 3.
The external application of chemically synthesized MtCEPs led to fewer lateral roots and promoted nodulation. (A and B) Effects of chemically synthesized MtCEPs on lateral root development. Germinated R108 and cra2-8 plants were grown in plates containing solidified 1/2 strength MS medium (without sucrose) with or without (control) 1 μM CEPs for 10 d. Representative images of root growth (A) and quantification of the lateral root number (B). Similar results were obtained in three independent experiments. The data are presented as the means ±SDs from one representative experiment, and significant differences were determined by ANOVA with Duncan’s post-hoc test, as indicated by letters (P<0.05, n=18). (C and D) Effect of chemically synthesized MtCEPs on symbiotic nodulation. The germinated R108 and cra2-8 plants were grown in plates with Fåhraeus medium without nitrogen for 6 d and then transferred to new plates with Fåhraeus medium without nitrogen containing 0 (control) or 1 μM CEPs. One day later, these plants were inoculated with rhizobia. Representative images of R108 and cra2-8 plants (C) and quantification of the nodule number at 14 dpi (D). Similar results were obtained in three independent experiments. The data are presented as the means ±SDs from one representative experiment, and significant differences were determined by ANOVA with Duncan’s post-hoc test, as indicated by letters (P<0.05, n=18). The scale bar in (C) represents 1 mm.
For the nodulation assay, R108 and cra2 mutant lines were first planted on Fåhraeus medium without nitrogen or CEP addition, and 6 d later, the plants were transferred to nitrogen-free Fåhraeus medium containing 1 μM CEP. One day later, all the groups were inoculated with Sm1021, and the nodules were counted 2 weeks after inoculation. R108 treated with MtCEP1, 2, 4, 5, 6, 8, and 12 had more nodules than the control, and all cra2 mutants subjected to CEP addition or treated with only water had far fewer nodules than WT plants (Fig. 3C, D). This result suggested that these seven MtCEPs contribute to the nodulation process.
Optimization of the CRISPR/Cas9 genome editing protocol
Although CRISPR/Cas9 technology has been utilized for targeted mutagenesis in M. truncatula, the present system is not sufficient for multigene editing (Meng et al., 2017; Wolabu et al., 2020). We first tested the original CRISPR/Cas9 toolkit for Arabidopsis (Xing et al., 2014) with the target gene STF in M. truncatula R108, as the knockout mutation exhibits apparently visible phenotype changes in young seedlings (Tadege et al., 2011). A total of 271 transgenic seedlings were generated by Agrobacterium-mediated transformation in the R108 genetic background, and ~8% (22/271) of the seedlings showed spindly leaves. Sequencing analysis showed that all the mutations (deletions or insertions) (22/22) occurred at the first target site driven by AtU6-26p, but none (0/22) of the mutations occurred at the second target site driven by AtU6-29p, which indicated the existence of some adapted modifications of this toolkit for a new species (Supplementary Fig. S5).
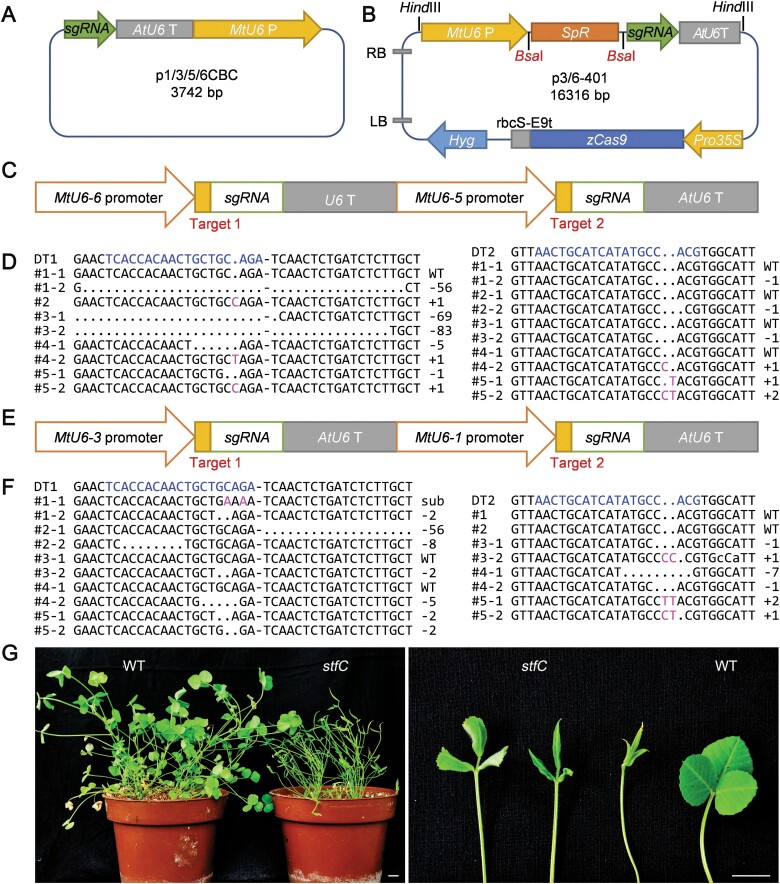
We therefore cloned the MtU6-1 promoter from the A17 ecotype, which is reportedly efficient, and another three MtU6 promoters from the R108 ecotype (Supplementary Table S4), designated MtU6-3p, MtU6-5p, and MtU6-6p. The original AtU6-26p in pHSE401 was replaced by MtU6-3p and MtU6-6p to yield p3401 and p6401 (Fig. 4A), respectively, whereas AtU6-29p in pCBC was substituted with MtU6-1p, MtU6-3p, MtU6-5p, and MtU6-6p to obtain p1CBC, p3CBC, p5CBC, and p6CBC, respectively (Fig. 4B). The p3401-1/5/6CBC and p6401-1/3/5CBC pairs can simultaneously target at most four sites in the genome of M. truncatula (Fig. 4A, B).
Fig. 4.
Multiple-target mutagenesis of M. truncatula using the modified CRISPR/Cas9 toolbox. (A) Structure model of p1/3/5/6CBC based on the pLB backbone. The original gRNA cassette was cloned from pCBC, and MtU6-1/3/5/6p replaced the original AtU6-29p. The whole fragment was ligated into the commercial blunt-ended vector pLB. (B) Structure model of optimized p3/6-401 based on the pHSE401 backbone. MtU6-3p and MtU6-6p replaced the original AtU6-26p without changing the basic vector backbone. Zea mays codon-modified zCas9 was driven by the 2×35S promoter. The hygromycin resistance gene was used as a selectable marker for plant transformation, and the kanamycin resistance gene was used for plasmid propagation. (C–F) Illustration of the modified sgRNA cassettes in p6401-5CBC-STF (C) and p3401-1CBC-STF (E), and mutagenesis at target 1 and target 2 of STF generated using the p6401-5CBC-STF (D) and p3401-1CBC-STF (F) CRISPR/Cas9 systems. The blue letters represent the target sites, and the magenta letters represent the inserted or mismatched sites. Sub, substitution. (G) Phenotype of stf mutants transferred to soil and grown for 1 month. The scale bar represents 1 cm. The ‘–’ in (D) and (F) represents a sequence of 17 bp. P, promoter; T, terminator.
The modified system for single- or double-site editing was similarly tested by targeting STF in M. truncatula. The new target sites were fused into constructs to yield p3401-1CBC-STF and p6401-5CBC-STF. Transgenic lines were generated by stable transformation, and 40% (24/60) of the seedlings transformed with p3401-1CBC-STF and 43% (19/44) of the seedlings transformed with p6401-5CBC-STF displayed spindle-like leaves as early as the regeneration stage. To determine the exact mutations of each target, we randomly selected five transgenic lines with an stf-like phenotype from each transformation event, and a sequence analysis showed that mutations occurred at both target sites, which confirmed the efficiency of each native MtU6 promoter. The analysis of the transgenic lines regenerated by the transformation of p6401-5CBC-STF revealed that the first target sites driven by the MtU6-6 promoter were all mutated; among these, #2–5 were biallelic or homozygous mutations at target 1, whereas #1 was heterozygous with a WT allele (Fig. 4C, D). Although the second target sites driven by the MtU6-5 promoter were all also edited, four out of five plants retained a WT allele (Fig. 4D). The analysis of the transgenic lines derived from the transformation event of p3401-1CBC-STF showed that #1–4 at target 1 driven by the MtU6-3 promoter were heterozygous and that #5 at target 1 was biallelic. The second targets driven by the MtU6-1 promoter, which has been previously reported, were edited only in three transgenic lines (Fig. 4E, F). These results suggested that the four MtU6 promoters with validated efficiency in STF editing might be used for multitarget editing.
Selected single or double mutations of MtCEP genes had no significant influence on root development or nodulation
Due to the difficulty in discerning functional differences among MtCEP peptides based on largely overlapping spatial expression patterns, and on the results of the treatment with chemically synthesized MtCEP peptides, it is necessary to construct stable mutant lines for further exploration. Based on the optimized CRISPR/Cas9 system and Agrobacterium-mediated transformation in R108, several single-mutant lines Mtcep1C, Mtcep2C, Mtcep4C, Mtcep6C, and Mtcep12C were generated, and two double-mutant combinations Mtcep1/2C (initially, the triple mutant of MtCEP1, 2, and 12 induced by both rhizobial inoculation and nitrogen starvation was planned, but the vector construction failed at the initial stage; therefore, Mtcep1/2C was created) and Mtcep5/8C (the double mutant was used instead of a single mutant for the preliminary functional analysis of MtCEP5 and 8, which have similar expression patterns) were selected for generation. Sequencing of four or five transgenic seedlings for each construct showed that the frequency of the homozygous and biallelic mutants for all the targets (except target 1 of MtCEP12 driven by AtU6-26p and target 2 of MtCEP4 or MtCEP6 driven by AtU6-29p) was >60% (Fig. 5).
Fig. 5.
Generation of single or double mutants of MtCEP genes. Construction of sgRNA cassettes containing one or two target sites and the corresponding promoter for the single or double mutation and representative mutant sequences of the target site for single mutations of MtCEP1 (A), MtCEP2 (B), MtCEP4 (C), MtCEP6 (D), and MtCEP12 (E), and double mutations of MtCEP1/2 (F) and MtCEP5/8 (G). The magenta letters represent target 1, and the blue letters represent target 2. T1 and T2 represent target 1 and target 2, respectively.
We then attempted to determine whether any of these single or double mutants had an effect on lateral root development or nodulation. The mutants were planted in a greenhouse for 7 d of nitrogen starvation. The seedlings were then inoculated with Sm1021 and grown for an additional 2 weeks. Several metrics relevant to common root development and nodulation processes were measured. No significant differences in the root length, lateral root number, lateral root density, or nodule number per plant were found between any of these single or double mutants and WT plants, and no other abnormal developmental phenotypes were observed (Supplementary Fig. S6, S7). Because overexpression of MtCEP1 or chemical treatments inhibited lateral roots and promoted nodulation (Imin et al., 2013), and based on the extensive overlapping patterns of MtCEP genes, all these results suggested that the functions of MtCEP genes in root development and nodulation might be highly redundant.
Triple and quintuple mutations of MtCEP genes led to more lateral roots and fewer nodules
To determine the degree of redundancy among the members of the MtCEP gene family, we subsequently utilized CRISPR/Cas9 technology and simultaneously targeted MtCEP1, MtCEP2, and MtCEP12. We generated triple and quintuple mutants through Agrobacterium-mediated transformation in the WT and the already available Mtcep5/8C double mutant genetic backgrounds, respectively (Fig. 6A, B). The specific mutations in each target were confirmed by PCR and sequencing, and the results showed that the frequencies of homozygous and biallelic mutations in MtCEP1, MtCEP2, and MtCEP12 were 52% (12/23), 59% (13/22), and 48% (10/21), respectively (Fig. 6C, D). Additionally, RT–qPCR indicated that the expression levels of MtCEP4, 5, 6, 7, 8, 9, 10, and 11 were not significantly different among R108, Mtcep1/2/12C, and Mtcep1/2/5/8/12C (Supplementary Fig. S8). These results suggested that mutations of the five MtCEP genes showed no feedback effects on the expression of the other MtCEP genes.
Fig. 6.
Generation of triple and quintuple mutants of MtCEP genes. (A) Construction of sgRNA cassettes containing triple target sites for MtCEP1, MtCEP2, and MtCEP12. For the triple mutant Mtcep1/2/12C, the CRISPR/Cas9 platform was transformed into R108, and for the quintuple mutant Mtcep1/2/5/8/12C, this vector was transformed into Mtcep5/8C. Representative mutant sequences of the target sites for the triple mutant Mtcep1/2/12C (B) and the quintuple mutant Mtcep1/2/5/8/12C (C). The magenta letters represent target 1, the cyan letters represent target 2, and the blue letters represent target 3. T1, T2, and T3 represent target 1, target 2, and target 3, respectively.
For the root and nodule phenotypic analysis, Mtcep1/2/12C, Mtcep1/2/5/8/12C, R108, and the cra2 mutant were subjected to nitrogen starvation for 7 d and then to rhizobial inoculation in a greenhouse. At 14 dpi, we measured the root length of each line and counted the lateral root number per plant. Although the root length and lateral root number of the triple and quintuple mutants were slightly but not significantly changed compared with those of the WT plants, the lateral root densities of the multigene mutants were higher than those of R108 (Fig. 7A–D), which suggested that lateral root densities were promoted in Mtcep1/2/12C and Mtcep1/2/5/8/12C. With respect to nodulation, the nodule number counted at 14 and 21 dpi in the triple and quintuple Mtcep mutants was lower than that in R108, but the lateral root and nodulation phenotype of Mtcep1/2/12C and Mtcep1/2/5/8/12C did not reach the abnormal degree of the cra2 mutant phenotype (Fig. 7E, F). In addition, the nodule numbers of Mtcep1/2/12C and Mtcep1/2/5/8/12C were not significantly different from each other. These results suggested that at least MtCEP1, MtCEP2, and MtCEP12 act redundantly in controlling lateral root and nodule number, whereas MtCEP5 and MtCEP8 have no major role.
Fig. 7.
MtCEP1, 2, and 12 play important roles in regulating lateral root development and symbiotic root nodulation. Root growth and nodulation phenotypes of R108, Mtcep1/2/12C, and Mtcep1/2/5/8/12C inoculated with Sm1021 after 1 week of growth in a perlite/vermiculite mixture with nitrogen-free Fåhraeus medium. Representative images of whole plants at 14 dpi (A) and quantification of the root length (B), lateral root number (C), and lateral root density (D). The scale bar represents 2.5 cm. Representative images of the nodulation phenotype at 14 and 21 dpi (E) and quantification of the nodule number at 14 and 21 dpi (F). Similar results were obtained in three independent experiments; the data are presented as the means ±SDs from one representative experiment, and significant differences were determined by ANOVA with a post-hoc LSD test, as indicated by letters (P<0.05, n>15). The scale bar represents 1 mm.
Discussion
The CEP peptide and its putative receptor CRA2 are thought to be the central regulatory components involved in balancing root architecture and nodulation under nitrogen-limited conditions (Imin et al., 2013; Mohd-Radzman et al., 2016; Patel et al., 2018; Laffont et al., 2020; Zhu et al., 2020). Although an external addition assay of MtCEPs was conducted by Imin et al. (2013) (MtCEP1), Patel et al. (2018) (MtCEP1, 2, 5, and 8), and Laffont et al. (2020) (MtCEP7), we conducted an external application experiment using seven group I MtCEPs that were chemically synthesized with presumed PTMs. The functional analysis suggested that these synthetic peptides had biological activities in lateral root inhibition and promoting nodulation (Fig. 3). However, these results could not distinguish the specific functions of these MtCEPs because of their highly conserved amino acid sequences. Furthermore, the PTM patterns of synthetic peptides may not represent the native circumstances. Recently, in vivo data for Medicago or soybean revealed another hydroxylated proline combination (Mohd-Radzman et al., 2015; Okamoto et al., 2015; Patel et al., 2018). Additionally, Patel et al. (2018) showed that MtCEP2 hydroxylated at Pro4 and Pro11 did not exert a positive effect on nodulation. However, in contrast, we observed a promoting effect of MtCEP2–MtCEP4, 11-hydroxylated peptides, and this difference may be explained by the different Medicago ecotypes used, the treatment pattern (e.g. duration of CEP treatment/pre-treatment), or other experimental details (e.g. inoculation method). Previous studies have revealed that cra2 could not respond to MtCEP1 and MtCEP7 peptides (Mohd-Radzman et al., 2016; Laffont et al., 2020). Consistent with that, the cra2 mutant could not respond to any of the tested MtCEPs in our study (Fig. 3), and this finding suggests a CEP–CRA2 ligand–receptor relationship.
A few studies have performed high-throughput gene expression profiling at different stages of nodulation and in response to the elimination or limitation of macronutrient elements (Larrainzar et al., 2015; de Bang et al., 2017). In this study, the MtCEP gene expression patterns were validated in a low-throughput manner by RT–qPCR and the GUS reporter system (Figs 1, 2). However, there were some discrepancies between the GUS staining and RT–qPCR results. For example, RT–qPCR analysis showed that the relative expression level of MtCEP6 was obviously higher than that of MtCEP1 in shoots, regardless of the nitrogen conditions, but GUS staining revealed that the patterns in the aboveground parts of the GUS reporter lines driven by MtCEP6 and MtCEP1 were similar (Figs 1B, 2). The different growth conditions might have led to the differences. The samples collected for RT–qPCR were grown on plates with 10 mM NO3– for 5 d and then transferred to medium without nitrogen, whereas the transgenic lines for GUS staining were grown in a matrix without nitrogen in a greenhouse for 7 d, so that excess nitrate stored in shoots and cotyledons would provide a nitrogen source for plant growth, which might delay the response to low-nitrogen conditions in shoots and roots in the RT–qPCR assay. Additionally, the cloned promoters might not represent in vivo expression adequately. Furthermore, the clearly detectable GUS signalling of MtCEP1, 6, and 12 suggested these MtCEP genes may play some roles in shoot development regulation; as in Arabidopsis, AtCEP5 expression in the shoot apical meristem and cotyledon is involved in aboveground growth regulation (Roberts et al., 2013). Imin et al. (2013) performed a detailed analysis of the expression pattern of MtCEP1 in transgenic roots expressing ProMtCEP1:GUS generated by hairy root transformation and found that staining was diminished in developing nodules and even absent in mature nodules (14 dpi). However, these phenomena were not observed in this study, in which stably inherited transgenic lines (14 dpi) were used as the experimental materials (Fig. 2F). The different genotypes used and the 3–4 week transformation process before rhizobial inoculation of hairy roots may cause the different expression patterns.
Tnt1 insertion mutants have been utilized extensively for the study of gene function in M. truncatula; however, when multiple mutants are required for genetic functional analyses, especially when genes are clustered in the same genomic region, as several CEP genes are (e.g. MtCEP1, 2, and 8 are clustered on chromosome 8), the CRISPR strategy is faster and more convenient than crossing Tnt1 mutants. However, the existing CRISPR/Cas9 technology was utilized only for single-target mutagenesis in M. truncatula (Meng et al., 2017). Based on the CRISPR/Cas9 toolkit for Arabidopsis, we cloned the MtU6-1, 3, 5, and 6 promoters from M. truncatula and optimized the original CRISPR/Cas9 system with these four promoters (Fig. 4A, B). The modified CRISPR/Cas9 system was proven to be very efficient in STF editing and in the generation of single or double MtCEP mutants (Figs 5, 6). Moreover, the frequency of homozygous and biallelic mutations for each target was >48% in MtCEP1/2/12 triple-target editing (Supplementary Table S5).
To date, to the best of our knowledge, a null-allele Mtcep mutant has not been reported, which leads to a gap in knowledge regarding its function. Although RNAi-mediated or miRNA-induced gene silencing was applied to generate knockdown mutants targeting MtCEP1/2/5/11, the efficiency and specificity of both methods seemed limited (Imin et al., 2013). The transgenic roots with declining expression levels of MtCEP1 and MtCEP2 had more lateral roots but an equivalent number of nodules (Imin et al., 2013). However, in this study, the Mtcep1/2C double mutants generated using CRISPR/Cas9 technology showed no obvious difference in the phenotypes regarding either lateral root number or nodulation (Supplementary Figs S6, S7). The differences between the two studies are possibly due to the RNAi construct targeting other CEP genes (e.g. CEP12) that were not analysed by RT–qPCR. This finding again highlighted the necessity of specific knockout mutants for gene function studies.
Although these CEP genes targeted by CRISPR/Cas9 were selected based on their expression patterns and intensity, this combination of targets might not be the optimal solution because the cra2 mutant presented a more remarkably extreme phenotype than the triple or quintuple Mtcep mutants (Fig. 7). A recent study indicated that MtCEP7 could be induced by rhizobia, and the silencing of MtCEP7 by RNAi or by an amiRNA significantly reduced the nodule number (Laffont et al., 2020). However, MtCEP7 and MtCEP9, which could actively respond to rhizobial inoculation, as demonstrated by our RT–qPCR data (Fig. 1), were excluded in the combinations of targets for CRISPR/Cas9 due to their low expression abundance. In addition, only the Mtcep1/2C double mutant was assayed, so additional combinations of mutants were needed to verify whether MtCEP1/12 or MtCEP2/12 might play more important roles, and whether any other genes played an additional role to that of MtCEP1/2/12 in the nodulation process. Together, these results provide a promising method for creating multiple mutants of MtCEP genes, and these mutants expand our knowledge of CEP biology and improve our understanding of the complexity underlying the regulation of root system architecture in legumes.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Representative scheme of the precursor protein and sequence alignment of CEP domains of the group I CEPs in R108.
Fig. S2. Fold change in the relative expression of group I MtCEP genes under nitrogen starvation conditions or in response to rhizobial inoculation.
Fig. S3. Expression of MtCEP genes in roots and shoots of R108 determined by RNA-seq.
Fig. S4. Spatial expression patterns of MtCEP1, 2, 4, 5, 6, 8, and 12 in roots inoculated with rhizobia for 0, 1, or 2 d.
Fig. S5. Mutation types at the first CRISPR/Cas9 editing target site of the STF locus.
Fig. S6. Single or double mutations of MtCEP genes had no significant influence on root development.
Fig. S7. Single or double mutations of MtCEP genes had no significant influence on nodulation.
Fig. S8. Relative expression levels of MtCEP4, 5, 6, 7, 8, 9, 10, 11, and 13 in Mtcep1/2/12C and Mtcep1/2/5/8/12C under nitrogen starvation conditions.
Table S1. List of primers used in this study.
Table S2. The target sites selected for STF and MtCEP editing, and the corresponding primers used in our study.
Table S3. The sequences of the precursor proteins of the 16 MtCEPs identified from the R108 and Jemalong A17 Mt5.0 genome assemblies.
Table S4. Sequence of the three MtU6 promoters.
Table S5. Mutation types of the CRISPR/Cas9 editing target sites of MtCEP1, 2, and 12 in Mtcep1/2/12C and Mtcep1/2/5/8/12C.
Acknowledgements
We thank Qijun Chen (College of Biological Science, China Agriculture University, China) for providing the CRISPR/Cas9 toolkit. This work was funded by the National Natural Science Foundation of China (NFSC) (grant nos 32070272, 31772658), the China Postdoctoral Science Foundation (grant no. 2019M660878), and the project for Extramural Scientists of the State Key Laboratory of Agrobiotechnology (project ID: 2020SKLAB6-16).
Author contributions
TW designed the research. FZ and QY performed the main experiments and the data analyses, with help from HC. FZ, JD, TW, and QY wrote the manuscript.
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary data published online. The multigene editing toolkit, and the stable mutants and transgenic GUS reporter lines used in this study are available from the corresponding author Tao Wang upon request.
References
- Cervera M. 2005. Histochemical and fluorometric assays for uidA (GUS) gene detection. Methods in Molecular Biology 286, 203–214. [DOI] [PubMed] [Google Scholar]
- Chapman K, Taleski M, Ogilvie HA, Imin N, Djordjevic MA. 2019. CEP–CEPR1 signalling inhibits the sucrose-dependent enhancement of lateral root growth. Journal of Experimental Botany 70, 3955–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bang TC, Lundquist PK, Dai X, et al. 2017. Genome-wide identification of Medicago peptides involved in macronutrient responses and nodulation. Plant Physiology 175, 1669–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay C, Chapman K, Taleski M, Wang Y, Tyagi S, Xiong Y, Imin N, Djordjevic MA. 2019. CEP3 levels affect starvation-related growth responses of the primary root. Journal of Experimental Botany 70, 4763–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay C, Imin N, Djordjevic MA. 2013a. CEP genes regulate root and shoot development in response to environmental cues and are specific to seed plants. Journal of Experimental Botany 64, 5383–5394. [DOI] [PubMed] [Google Scholar]
- Delay C, Imin N, Djordjevic MA. 2013b. Regulation of Arabidopsis root development by small signaling peptides. Frontiers in Plant Science 4, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M, Zhang R, Zhu F, Zhang Z, Gou L, Wen J, Dong J, Wang T. 2017. A lipid-anchored NAC transcription factor is translocated into the nucleus and activates glyoxalase I expression during drought stress. The Plant Cell 29, 1748–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautrat P, Laffont C, Frugier F. 2020. Compact root architecture 2 promotes root competence for nodulation through the miR2111 systemic effector. Current Biology 30, 1339–1345. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S, Crespi M, Frugier F. 2006. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. The Plant Cell 18, 2680–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huault E, Laffont C, Wen J, Mysore KS, Ratet P, Duc G, Frugier F. 2014. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. PLoS Genetics 10, e1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N, Mohd-Radzman NA, Ogilvie HA, Djordjevic MA. 2013. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. Journal of Experimental Botany 64, 5395–5409. [DOI] [PubMed] [Google Scholar]
- Kereszt A, Mergaert P, Montiel J, Endre G, Kondorosi É. 2018. Impact of plant peptides on symbiotic nodule development and functioning. Frontiers in Plant Science 9, 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont C, Huault E, Gautrat P, Endre G, Kalo P, Bourion V, Duc G, Frugier F. 2019. Independent regulation of symbiotic nodulation by the SUNN negative and CRA2 positive systemic pathways. Plant Physiology 180, 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont C, Ivanovici A, Gautrat P, Brault M, Djordjevic MA, Frugier F. 2020. The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nature Communications 11, 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrainzar E, Riely BK, Kim SC, et al. 2015. Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between nodulation factor and ethylene signals. Plant Physiology 169, 233–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi Y. 2014. Posttranslationally modified small-peptide signals in plants. Annual Review of Plant Biology 65, 385–413. [DOI] [PubMed] [Google Scholar]
- Meng Y, Hou Y, Wang H, Ji R, Liu B, Wen J, Niu L, Lin H. 2017. Targeted mutagenesis by CRISPR/Cas9 system in the model legume Medicago truncatula. Plant Cell Reports 36, 371–374. [DOI] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Binos S, Truong TT, Imin N, Mariani M, Djordjevic MA. 2015. Novel MtCEP1 peptides produced in vivo differentially regulate root development in Medicago truncatula. Journal of Experimental Botany 66, 5289–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Radzman NA, Laffont C, Ivanovici A, Patel N, Reid D, Stougaard J, Frugier F, Imin N, Djordjevic MA. 2016. Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiology 171, 2536–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte H, Vanneste S, Beeckman T. 2019. Molecular and environmental regulation of root development. Annual Review of Plant Biology 70, 465–488. [DOI] [PubMed] [Google Scholar]
- Ogilvie HA, Imin N, Djordjevic MA. 2014. Diversification of the C-TERMINALLY ENCODED PEPTIDE (CEP) gene family in angiosperms, and evolution of plant-family specific CEP genes. BMC Genomics 15, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Ogawa M, Matsubayashi Y. 2008. Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. The Plant Journal 55, 152–160. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Suzuki T, Kawaguchi M, Higashiyama T, Matsubayashi Y. 2015. A comprehensive strategy for identifying long-distance mobile peptides in xylem sap. The Plant Journal 84, 611–620. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Tabata R, Matsubayashi Y. 2016. Long-distance peptide signaling essential for nutrient homeostasis in plants. Current Opinion in Plant Biology 34, 35–40. [DOI] [PubMed] [Google Scholar]
- Patel N, Mohd-Radzman NA, Corcilius L, et al. 2018. Diverse peptide hormones affecting root growth identified in the Medicago truncatula secreted peptidome. Molecular & Cellular Proteomics 17, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, Lin G, Li J. 2016. Potential pitfalls of CRISPR/Cas9-mediated genome editing. The FEBS Journal 283, 1218–1231. [DOI] [PubMed] [Google Scholar]
- Roberts I, Smith S, De Rybel B, Van Den Broeke J, Smet W, De Cokere S, Mispelaere M, De Smet I, Beeckman T. 2013. The CEP family in land plants: evolutionary analyses, expression studies, and role in Arabidopsis shoot development. Journal of Experimental Botany 64, 5371–5381. [DOI] [PubMed] [Google Scholar]
- Roberts I, Smith S, Stes E, et al. 2016. CEP5 and XIP1/CEPR1 regulate lateral root initiation in Arabidopsis. Journal of Experimental Botany 67, 4889–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer NM. 2010. Isolation of plant DNA for PCR and genotyping using organic extraction and CTAB. Cold Spring Harbor Protocols 2010, pdb.prot5515. [DOI] [PubMed] [Google Scholar]
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y. 2014. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346, 343–346. [DOI] [PubMed] [Google Scholar]
- Tadege M, Lin H, Bedair M, et al. 2011. STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris. The Plant Cell 23, 2125–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Hanada K, Kondo T, Shinozaki K. 2019. Hormone-like peptides and small coding genes in plant stress signaling and development. Current Opinion in Plant Biology 51, 88–95. [DOI] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA. 2018. CEP peptide hormones: key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. Journal of Experimental Botany 69, 1829–1836. [DOI] [PubMed] [Google Scholar]
- Wolabu TW, Cong L, Park JJ, et al. 2020. Development of a highly efficient multiplex genome editing system in outcrossing tetraploid alfalfa (Medicago sativa). Frontiers in Plant Science 11, 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, Zhang HY, Han CY, Liu B, Wang XC, Chen QJ. 2014. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biology 14, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hussain A, Manghwar H, Xie K, Xie S, Zhao S, Larkin RM, Qing P, Jin S, Ding F. 2020. Genome editing with the CRISPR–Cas system: an art, ethics and global regulatory perspective. Plant Biotechnology Journal 18, 1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Deng J, Chen H, et al. 2020. A CEP peptide receptor-like kinase regulates auxin biosynthesis and ethylene signaling to coordinate root growth and symbiotic nodulation in Medicago truncatula. The Plant Cell 32, 2855–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its supplementary data published online. The multigene editing toolkit, and the stable mutants and transgenic GUS reporter lines used in this study are available from the corresponding author Tao Wang upon request.