Abstract
Biotic and abiotic cues can trigger priming in plants, which enables plants to respond to subsequent challenge with stronger and/or faster responses. It is well known that herbivory activates defense-related responses in systemic leaves. However, little is known about whether insect feeding activates priming in systemic leaves. To determine whether and how herbivory induces priming in maize systemic leaves, a combination of insect bioassays, phytohormone and defense metabolite quantification, and genetic and transcriptome analyses were performed. Actual and simulated Mythimna separata herbivory in maize local leaves primed the systemic leaves for enhanced accumulation of jasmonic acid and benzoxazinoids and increased resistance to M. separata. Activation of priming in maize systemic leaves depends on both the duration of simulated herbivory and perception of M. separata oral secretions in the local leaves, and genetic analysis indicated that jasmonic acid and benzoxazinoids mediate the primed defenses in systemic leaves. Consistently, in response to simulated herbivory, the primed systemic leaves exhibited a large number of genes that were uniquely regulated or showed further up- or down-regulation compared with the non-primed systemic leaves. This study provides new insight into the regulation and ecological function of priming in maize.
Keywords: Benzoxazinoids, insect resistance, jasmonic acid, maize, Mythimna separata, priming, transcriptome
Mythimna separata herbivory primes systemic leaves, and this priming-endowed resistance requires perception of insect oral secretions, the plant hormone jasmonic acid, and the defensive metabolites benzoxazinoids.
Introduction
Plants are sessile organisms that are often challenged by various adverse environmental factors, including insect attack. They have evolved sophisticated defense mechanisms to fend off insect herbivores. Different plants are able to synthesize structurally diverse secondary metabolites, many of which are toxic, repellent, or anti-digestive for insects (Mithofer and Boland, 2012). Constitutive defenses are physical as well as chemical defense traits that are always present regardless of herbivory. Defenses are costly. Thus, plants often depend on inducible defenses to fight against insects, as they are displayed or enhanced only after herbivore attack, allowing plants to reserve energy and resources for growth and development under insect-free conditions (Erb and Reymond, 2019).
Maize (Zea mays) is one of the most important food crops and is cultivated around the globe with production of more than 1.14 billion tons in 2018 (http://www.fao.org/faostat/en/#data/QC/visualize). Maize often suffers from insect attack, such as from the chewing insects Ostrinia nubilalis and Spodoptera frugiperda and the piercing-sucking insect Rhopalosiphum maidis, resulting in large yield losses (Carena and Glogoza, 2004; Burtet et al., 2017; Hassan et al., 2018). The phytohormone jasmonic acid (JA) plays a central role in regulating defense of plants, including maize, against insects (Howe and Jander, 2008; Qi et al., 2018; Erb and Reymond, 2019). Mechanical wounding rapidly induced highly increased JA in maize, and applying the oral secretions (OS) of the insect Mythimna separata to fresh mechanical wounds (to simulate insect feeding) induced levels of JA almost 2-fold higher than from mechanical wounding, indicating that maize is able to perceive certain elicitors in insect OS and deploy JA-dependent defenses (Qi et al., 2016). The LOX8 gene encodes a 13-lipoxygenase enzyme that is involved in JA biosynthesis in maize; beet armyworm (Spodoptera exigua) caterpillars fed on the lox8/tasselseed1 maize mutants showed better growth compared with those fed on the wild-type (WT) plants (Tzin et al., 2017). Maize mutants lacking OPR7 and OPR8, which encode two 12-oxo-phytodienoate reductases, have remarkably reduced JA content, and beet armyworms consumed more tissues on the opr7 opr8 mutant plants and grew larger than on the WT maize plants (Yan et al., 2012). In addition to phytohormone signaling, large-scale transcriptomic and metabolic rearrangements are critical for maize deployment of defenses. RNA-seq analysis indicated that maize responds to simulated M. separata feeding with transcriptional regulation of a large number of genes (Qi et al., 2016). Tzin et al., (2015) found that the aphid R. maidis feeding on maize induced the strongest transcriptomic and metabolomic changes in the first few hours; however, after 4 d, both the transcriptomes and metabolomes of the aphid-infested maize became more similar to those of the non-aphid-infested maize. The maize inbred lines B73 and Mo17 are relatively susceptible and resistant to aphids, respectively, and these maize lines exhibited distinct transcriptional responses before and after the feeding from the aphid Rhopalosiphum padi (Song et al., 2017).
Benzoxazinoids (Bxs) are secondary metabolites with the 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one skeleton that are only found in some species of cereals, including maize (Wouters et al., 2014, 2016). The Maize B73 genome encodes three indole-3-glycerolphosphate synthase enzymes, which catalyse the conversion of 1-(2-carboxyphenylamino)-l-deoxyribulose-5-phosphate to indole-3-glycerolphosphate (Wisecaver et al., 2017; Richter et al., 2021). A series of enzymes BX1 to BX9 and indole-3-glycerol phosphate lyase catalyse the conversion of indole-3-glycerolphosphate into 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one glucoside (DIMBOA-Glc) (Frey et al., 1997, 2009; Niemeyer, 2009). Recently, two enzymes, a 2-oxoglutarate-dependent dioxygenase (BX13) and an O-methyltransferase (BX14) in the Bx biosynthesis pathway were identified (Handrick et al., 2016). BX13 catalyses the conversion of DIMBOA-Glc into 2,4,7-trihydroxy-8-methoxy-1,4-benzoxazin-3-one glucoside (TRIMBOA-Glc) and BX14 converts 2,4-dihydroxy-7,8-dimethoxy-1,4-benzoxazin-3-one glucoside (DIM2BOA-Glc) to 2-hydroxy-4,7,8-trimethoxy-1,4-benzoxazin-3-one glucoside (HDM2BOA-Glc) (Handrick et al., 2016). Most of the maize Bxs are induced by insect feeding. DIMBOA-Glc and its methylation product HDMBOA-Glc accumulate in response to Leucania separata (rice armyworm) herbivory and the increase of these metabolites is associated with elevated resistance to S. exigua (Oikawa et al., 2004; Tzin et al., 2017). Mythimna separata, Diabrotica virgifera virgifera, and Spodoptera littoralis feeding all increased the levels of 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) in the damaged leaves of maize (Erb et al., 2009; Maag et al., 2016; Qi et al., 2016). Spodoptera littoralis and S. exigua larvae show decreased growth when fed on an artificial diet containing DIMBOA (Rostas, 2007; Glauser et al., 2011). The defensive functions of Bxs were also demonstrated by genetic studies. Compared with WT maize, maize mutant igps1 has reduced resistance to S. exigua (Richter et al., 2021), and the bx1 and bx2 mutants are also susceptible to the aphid R. maidis and S. exigua (Tzin et al., 2015, 2017).
Studies in various species have indicated that plants respond to wounding or herbivory not only in the damaged leaves (local leaves) but also in the other undamaged leaves and even in roots (Heil and Ton, 2008; Howe et al., 2018). In addition to the local insect-damaged leaves, maize systemic leaves also have strong defense-related responses. Wounding increased the expression levels of allene oxide synthase (AOS), transcription factor MYC7, and ribosome inactivating protein only at the treatment site, whereas N-linolenoyl-glutamine, an elicitor in the OS of S. exigua, strongly induced the expression of these genes throughout the whole maize leaf (Engelberth et al., 2007, 2012). Simulated M. separata herbivory elicited accumulation of JA and Bxs as well as large transcriptomic changes in systemic unwounded maize leaves (Malook et al., 2019).
After receiving stimuli such as pathogens, insects, chemical cues, or abiotic stresses some plants may enter a primed physiological state (this process is named priming), allowing plants to mount a faster and/or stronger defense response to subsequent challenges (Mauch-Mani et al., 2017). Treating tomato (Solanum lycopersicum) seeds with JA or β-aminobutyric acid primed the tomato plants for increased defenses against Manduca sexta, Myzus persicae, and Tetranychus urticae, and the herbivore resistance was associated with increased expression levels of allene oxide synthase 2, proteinase inhibitor II and the pathogenesis-related gene PR1b1 (Worrall et al., 2012). Oviposition also induces priming in some plant species. For example, compared with oviposition-unexperienced Nicotiana attenuata plants, those oviposited by the S. exigua showed higher resistance to S. exigua larvae, as these insects had elevated mortality, retarded development, and inflicted less feeding damage on plants (Bandoly et al., 2015). Beet armyworm (S. exigua) larvae exhibited reduced growth on M. sexta-oviposited N. attenuata plants, and similarly, S. exigua-oviposited plants had elevated resistance to M. sexta (Bandoly et al., 2016). Herbivore-induced plant volatiles (HIPVs) can also function as priming agents. Maize seedlings previously exposed to green leaf volatiles from the neighboring maize plants had greater levels of JA and sesquiterpenes upon wounding or simulated S. exigua feeding than did the control non-exposed maize seedlings (Engelberth et al., 2004). Erb et al. (2015) demonstrated that maize seedlings treateted with the OS from S. littoralis emitted indole, and indole exposure primed the production of phytohormones, green leaf volatiles, and mono- and homoterpenes in systemic leaves, and furthermore, herbivory-induced indole enhanced the induction of defensive volatiles in the neighbouring maize plants. Diabrotica virgifera virgifera infestation on roots of maize seedlings increased the DIMBOA contents in leaves, and the leaves were primed for accumulation of chlorogenic acid after subsequent infestation by S. littoralis (Erb et al., 2009).
After initial insect feeding, the same insects may subsequently migrate to other leaves, and the systemic leaves could also be attacked by other insects of the same or different species. In this study, we investigated whether maize systemic leaves can be primed for enhanced resistance to insects and what the underlying mechanisms are. We show that insect feeding on maize seedlings primed the systemic leaves for enhanced insect resistance. Genetic and biochemical analysis indicated that JA signaling and Bx accumulation are required for the primed defenses. Moreover, the transcriptome indicated large transcriptional changes in these systemic leaves.
Materials and methods
Plant growth and oral secretion collection
The seeds of maize inbred lines A188, B73, W22, and the mutants bx2::Ds and lox8/tasselseed1 were germinated in 12-cm-diameter plastic pots filled with commercial potting soil and vermiculite (about 7:1 ratio) under natural light conditions (about 12–14 h day length) in a greenhouse (25±4 °C day, 20±4 °C night). Approximately 15-day-old plants, when the third leaves were fully expanded from the whorl (V3 stage), were used for pretreatment. KeYun Pests (https://shop101732681.taobao.com) provided the eggs of M. separata. Mythimna separata larvae were reared on maize until the third to fifth instar for the collection of OS. Storkbill forceps were used to gently squeeze the caterpillars to provoke regurgitation, and OS were collected on ice with a pipette and immediately centrifuged to obtain supernatant, which was divided into small aliquots before being stored at −80 °C.
Plant treatments, sample collection, and herbivore bioassays
To study the effects of mechanical wounding- and simulated M. separata herbivory-induced priming, the third leaves of maize plants (V3 stage) were pretreated with 10 μl of water or M. separata OS at a row of puncture wounds generated by rolling a fabric pattern wheel along the midvein (W+W and W+OS pretreatment, respectively), and these treatments were repeated once a day for another 3 d, unless otherwise indicated; plants without any pretreatments were used as controls. After resting for 3, 7, or 12 d, both control and pretreated plants were treated with W+OS on the fourth leaves by immediately applying 40 μl of M. separata OS to four rows of wounds generated by a pattern wheel. Leaf samples were collected 6 or 48 h post-treatment on leaf 4, immediately frozen in liquid nitrogen, and stored at −80 °C until further analysis. All experiments were repeated twice or thrice to ensure data reproducibility except the RNA-seq. The number of replicates for each experiment varied and is indicated in the respective figure caption.
To examine the priming effect of actual M. separata feeding, the third maize leaves were infested with M. separata larvae (one larva/plant; first instar), and after 4 d of feeding, the insects were removed (pretreatment group). For the control group, no insects were infested on any plants. After another 7 d, the fourth leaves of plants from both control and pretreatment group were infested with M. separata larvae (two neonates/plant). The insect masses were recorded after 48 h of feeding. Each group contained 25 replicate maize plants.
To examine the effect of priming on maize resistance to insect herbivory, maize third leaves were pretreated by W+OS for consecutively 4 d (each day 10 μl of M. separata OS was applied to one row of wounds generated by a pattern wheel); in the control group, no pretreatment was done. After 3, 7, or 12 d, for each maize plant of both control and pretreatment groups, M. separata larvae (two neonates/plant) were enclosed in a clip cage fixed on the fourth leaves and allowed to feed for 48 h before insect masses were recorded. Each group contained 25 replicate maize plants.
Quantification of jasmonic acid and jasmonic acid–isoleucine conjugate
JA and jasmonic acid–isoleucine conjugate (JA-Ile) were measured according to the method described previously (Wu et al., 2007). In short, 150 mg of frozen leaf powder was extracted with ice-cold ethyl acetate spiked with 20 ng D6-JA and 5 ng 13C6-JA-Ile. After centrifugation at 13 000 g for 10 min at 25 °C, supernatants were transferred to fresh 2-ml microfuge tubes. Each pellet was re-extracted with 0.5 ml of ethyl acetate and centrifuged, and the supernatants from each sample were combined. The supernatants were evaporated to dryness on a vacuum concentrator (Eppendorf). The residues were resuspended in 0.5 ml of 70% methanol (v/v) and centrifuged to clarify phases. Following centrifugation, the supernatants were pipetted into glass vials and then analysed by HPLC-MS/MS (LCMS-8040 system, Shimadzu).
Quantification of benzoxazinoids
Approximately 100 mg of frozen leaf powder was suspended with methanol–H20 (50:50, v/v; containing 0.5% formic acid) in 2 ml microfuge tubes and vortexed vigorously for 10 min. Samples were centrifuged at 13 000 g for 15 min and 450 μl of the supernatants was transferred to glass vials for analysis on an HPLC-MS/MS system (LCMS-8040, Shimadzu) according to Gao et al. (2019).
RNA-seq and data analysis
Total RNA was extracted from ground leaf samples using TRIzol reagent (Thermo Fisher Scientific), and the RNA quality, purity, and concentrations were determined using a spectrophotometer (Nano-Drop 2000c, Thermo Fisher Scientific). Sequencing was performed at 5 G depth on a HiSeq2500-PE125 platform (Illumina) and the resulting sequences were trimmed based on quality scores and mapped to the maize A188 reference genome sequence V1. We used HISAT2 (Pertea et al., 2016) to map the transcripts and DESeq2 (Love et al., 2014) to identify differentially regulated genes (DEGs), and genes whose expression levels were at least 2-fold changed with adjusted P-values less than 0.05 were selected as DEGs for further analysis. Gene ontology (GO) analysis was done with the updated platform agriGO v2.0 (Tian et al., 2017). Venn diagrams were created using a web-based tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Statistical analysis
Data of herbivore bioassay were analysed with Student’s t-test. Analyses on the contents of Bxs and phytohormones were performed using one-way analysis of variance (ANOVA) and significance was determined by post hoc test (P<0.05). Two-way ANOVA was performed to analyse the effect of time of post-pretreatment resting and priming on Bx contents, and time of resting (3, 7, and 12 d) and priming (pretreatment or not of W+OS on third leaves) were treated as two independent variables. Student’s t-test and one-way and two-way ANOVA were performed using SPSS Statistics for Mac OS (IBM Corp., USA; Version 26.0). Principal component analysis was conducted and plotted using the plotPCA in the DESeq2 of the R package (Love et al., 2014). A violin plot was made using the ggplot2 package in R software (Wickham, 2016).
Results
Actual and simulated M. separata herbivory, but not mechanical wounding, prime maize systemic leaves for increased resistance to M. separata
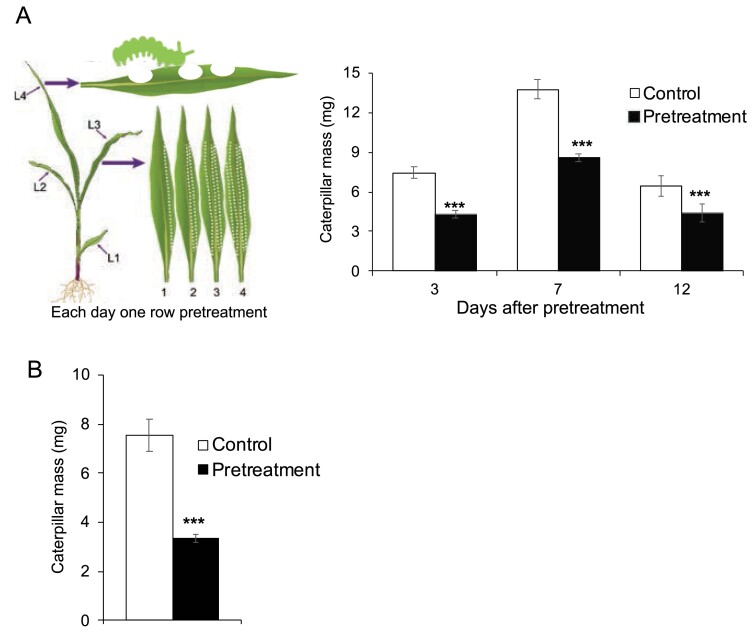
Mythimna separata is one of the major insect pests of maize in Asia. First, we sought to determine whether M. separata insect herbivory on maize primes systemic leaves for enhanced resistance. The third leaf of each maize seedling (line A188) was treated with simulated M. separata herbivory, by applying the OS of M. separata to a row of fresh wounds generated by rolling a pattern wheel along the midvein (wounding plus OS, W+OS). This mode of simulated herbivory was done on four consecutive days on the same local leaves (Fig. 1A). Three, seven, and 12 d (resting times) after the last W+OS treatment, M. separata larvae (1 d old and reared on rice seedlings since hatching) were infested on the fourth leaves of these pretreated maize plants. The control maize plants, which were not pretreated, were similarly infested with M. separata larvae. The insects were allowed to feed for 48 h before their masses were recorded. Caterpillars fed on the pretreated plants, which had 3, 7, and 12 d of resting, gained only 58, 62, and 69% of the masses of those fed on the control plants (Fig. 1A).
Fig. 1.
Simulated and actual M. separata herbivory primes maize systemic leaves for enhanced resistance. (A) M. separata growth on simulated herbivory-pretreated maize. The third leaves (L3) of maize (A188) seedlings were treated with W+OS (one row a day) for four consecutive days (pretreatment group), while in the control group, L3 were untreated. The plants were rested for another 3, 7, or 12 d before M. separata larvae were infested on the fourth leaves (L4) (two neonates/plant). The masses of insects after 48 h of feeding were recorded. (B) M. separata growth on actual herbivory-pretreated maize. Maize (A188) L3 were infested with M. separata larvae (one caterpillar/plant), and after 4 d of feeding, the insects were removed (pretreatment group). In the control group, L3 were untreated. After another 7 d of resting, the L4 were infested with M. separata larvae (two neonates/plant), and the insect masses were recorded after 48 h of feeding. Data are mean ±SE; Student’s t-test; ***P<0.001; n=25.
We also confirmed that the caterpillars were 55% smaller on fourth leaves of maize plants whose third leaves were pretreated with actual M. separata feeding for 4 d and thereafter rested 7 d, than on the control plants (Fig. 1B). Similar results were also found for the inbred lines B73 and W22 (see Supplementary Fig. S1A): the masses of insects on fourth leaves of B73 and W22, which were pretreated with simulated M. separata feeding on third leaves, were 25 and 23% smaller than those on the respective control plants.
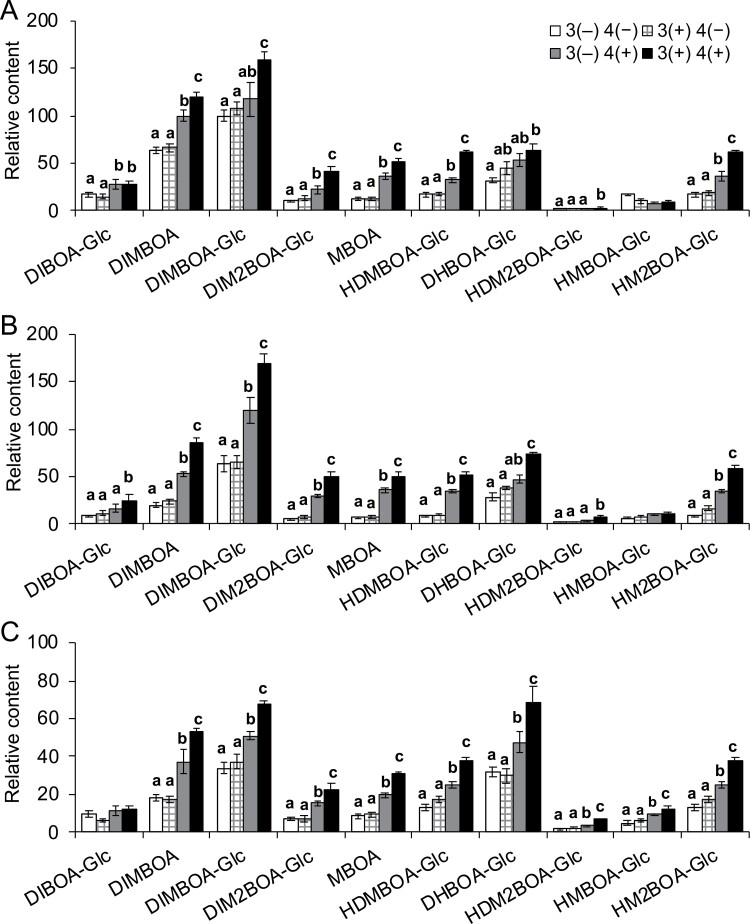
Bxs are the major anti-insect metabolites in maize (Ahmad et al., 2011; Meihls et al., 2013). Thus, next we investigated whether simulated M. separata herbivory-induced priming in systemic maize leaves is associated with increased contents of Bxs. Third leaves were untreated (for simplicity, named 3(−)) or pretreated with W+OS on four consecutive days (named 3(+)), and after 3, 7, and 12 d of resting, these seedlings’ fourth leaves were untreated (named 4(−)) or treated with W+OS (named 4(+)) to induce Bx accumulation, and these leaves were harvested in another 48 h. In the 3(+) 4(−) and 3(−) 4(−) plants, no differences in Bx contents were found between these two groups, regardless of the resting times (Fig. 2). Thus, W+OS pretreatment on third leaves did not affect the basal levels of Bxs in the fourth leaves. However, the fourth leaves of 3(+) 4(+) plants contained 1.2- to 1.9-fold more Bxs (DIMBOA, DIMBOA-Glc, DIM2BOA-Glc, MBOA, HDMBOA-Glc, and HM2BOA-Glc) than did the fourth leaves of 3(−) 4(+) plants, which experienced 3 d of resting (Fig. 2A). Similar results were obtained from plants that had experienced 7 and 12 d of resting (Fig. 2B, C). Notably, the seedlings rested for 7 d showed the strongest relative up-regulation of the W+OS-induced Bxs in fourth leaves, compared with those rested for 3 and 12 d (Fig. 2). For instance, W+OS-induced DIMBOA-Glc in the primed plants (3(+) 4(+)) was 34.8, 40.3, and 31.8% more than in the fourth leaves of the non-primed plants (3(−) 4(+)), after 3, 7, and 12 d of resting, respectively. Next, the Bx contents were analysed with two-way ANOVA, in which the resting times (3, 7, and 12 d) and priming/pretreatment (3(+) 4(+) versus 3(−) 4(+)) were considered to be two independent variables. We found that the resting time had a strong impact on levels of Bxs, and for each specific resting time, priming/pretreatment exhibited a significant effect in promoting Bx accumulation (Supplementary Table S1). Thus, both actual and simulated M. separata feeding primed the maize seedlings for enhanced herbivore defense, and the herbivory-induced priming in the systemic leaves was associated with increased accumulation of Bxs.
Fig. 2.
Simulated M. separata herbivory primes systemic defenses for at least 12 d. Maize (A188) leaves (third leaves) were pretreated with W+OS for four consecutive days or kept untreated (3(+) and 3(−), respectively). After resting 3 (A), 7 (B), or 12 (C) days, the fourth leaves were treated with W+OS or untreated (indicated as 4(+) and 4(−), respectively). After 48 h, the contents of Bxs in the fourth leaves were analysed. Data are means ±SE (n=5). Different lowercase letters indicate significant differences within same compounds determined by one-way ANOVA (P<0.05; post hoc tests).
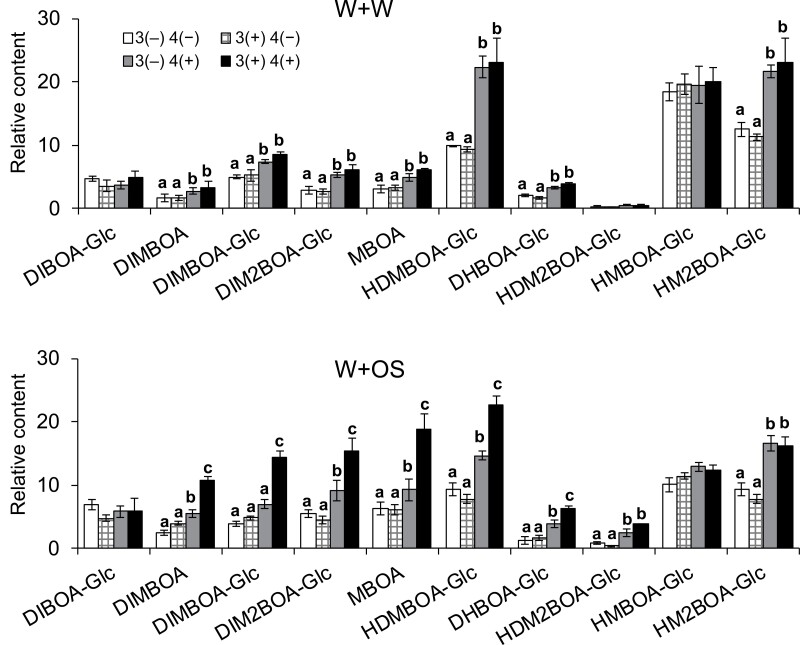
Many insect OS contain various elicitors, such as fatty acid–amino acid conjugates (FACs), and these elicitors can be recognized by plants, inducing insect-specific resistance responses (Wu and Baldwin, 2010). Next, we sought to determine whether insect OS are required for priming the defense of the systemic leaves. In the pretreatment group, the third leaves of maize seedlings were wounded with a pattern wheel, and water was applied to wounds (wounding plus water, W+W). This was done once a day and consecutively for 4 d (similarly to what was done in Fig. 1A). After the plants rested for 7 d, M. separata larvae were released to the fourth leaves and their masses were recorded after 2 d of feeding. In the control group, the masses of M. separata infested on maize fourth leaves were similarly recorded, except that these maize seedlings were not pretreated. No difference was found between the mass gains of the insects grown on the maize of the pretreatment group and the control group (Supplementary Fig. S1B), indicating that recognition of OS by maize is required for the priming of insect resistance in the systemic leaves. Given that insect feeding- or W+OS-induced priming of systemic leaves was associated with increased Bx contents in the systemic fourth leaves, we expected that mechanical wounding treatment on third leaves may not be able to prime the fourth leaves for enhanced Bx responses to herbivory. The third leaves of maize seedlings were treated with W+W, W+OS, or untreated (control group) once a day for 4 d, and after a resting time of 7 d, W+OS treatment was applied to all the fourth leaves; after another 48 h, these fourth leaves were harvested for determination of Bxs. Compared with those in the control group, the fourth leaves of the maize seedlings from the W+OS pretreatment group again showed enhanced Bx accumulation in response to subsequent W+OS treatment. However, priming was not observed for the W+W pretreatment, as the fourth leaves of the W+W pretreatment group and the control group had similar levels of Bxs after W+OS induction (Fig. 3A, B). These findings indicate that perception of the M. separata OS in the local maize leaves is required for priming the systemic leaves for enhanced resistance to insects.
Fig. 3.
Perception of M. separata OS is required for priming of maize systemic leaves. The third maize (A188) leaves were untreated or pre-treated with W+W or W+OS for four consecutive days (indicated as 3(−) and 3(+), respectively). After 7 d of resting, the fourth leaves were untreated or treated with W+OS (indicated as 4(−) and 4(+), respectively). After another 2 d, the contents of Bxs were analysed in the fourth leaves. Data are means ±SE (n=5). Different lowercase letters indicate significant differences within same compounds determined by one-way ANOVA (P<0.05; post hoc tests).
Duration of simulated herbivory but not extent of damage is required for priming the systemic leaves
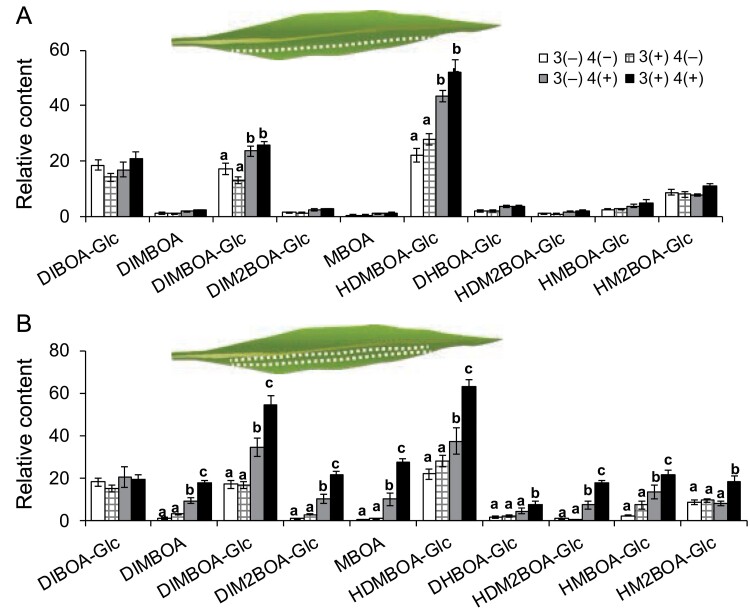
Four rolls of W+OS pretreatment primed the systemic fourth leaves, and this led us to ask whether certain damage areas or times of damaging are needed to elicit the priming effect on the fourth leaves. Thus, we generated one roll of wounds in the third leaves of maize seedlings with a pattern wheel and the OS of M. separata were immediately applied to wounds (named W+OS once). In another group, maize seedlings were similarly pretreated once a day in two consecutive days (named W+OS twice). After a resting time of 7 d, we treated the fourth leaves of both control (not pretreated at all) and these pre-induced plants with W+OS, and these fourth leaves were harvested in another 2 d for determination of Bx contents. We found that W+OS once did not affect the basal and the subsequently W+OS-induced Bx accumulation in fourth leaves (Fig. 4A); however, W+OS twice highly increased the subsequent W+OS-induced Bxs in fourth leaves, compared with those in the control plants (Fig. 4B).
Fig. 4.
At least two times of simulated M. separata treatment are needed to induce priming in maize systemic leaves. Maize (A188) third leaves were untreated or pretreated with W+OS once (A) or once a day on two consecutive days (B) as illustrated, and after 7 d of resting, the fourth leaves were kept untreated or treated with W+OS and harvested after 2 d for quantification of Bxs (3 and 4 depict leaf positions, (+) and (–), respectively, indicate treatment and no treatment. Data are means ±SE (n=5). Different lowercase letters indicate significant differences within same compounds determined by one-way ANOVA (P<0.05; post hoc tests).
In order to rule out the possibility that W+OS once was not strong enough to induce priming, in another group of maize seedlings, we generated four rolls of wounds on the third leaves without any time intervals and OS were immediately applied to wounds. After 7 d of resting, fourth leaves were treated with W+OS. However, there were no changes of Bx levels in fourth leaves between the pretreated (3(+) 4(+)) and non-pretreated (3(−) 4(+)) plants except for HDMBOA-Glc (Supplementary Fig. S2). Our findings suggest that herbivore feeding duration but not the extent of damaged area plays an important role in activating priming in the systemic leaves.
Jasmonic acid and benzoxazinoids are required for herbivore defense priming
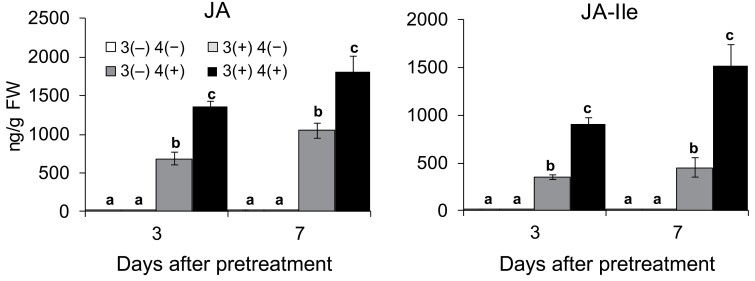
Phytohormones, especially JA, play important roles in regulating defensive metabolites (De Geyter et al., 2012; Howe et al., 2018). Therefore, we speculated that simulated herbivory on third leaves could prime the fourth leaves for enhanced JA response to subsequent W+OS treatment. Maize seedlings (third leaves) were pretreated with W+OS for consecutive 4 d. Three or seven days after pretreatment, the systemic fourth leaves of the plants from the pretreated group and control group were treated with W+OS and samples were harvested after 1 h. It was found that the levels of JA in the fourth leaves were 50% and 43% higher in the pretreatment group than in the control group, after 3 and 7 d of resting, respectively (Fig. 5). Similar results were found for the actual functional jasmonate, jasmonic acid–isoleucine conjugate (JA-Ile) (Fig. 5).
Fig. 5.
Simulated M. separata herbivory primes systemic leaves for increased JA and JA-Ile. Maize (A188) third leaves were untreated or pretreated with W+OS for four consecutive days as shown in Fig. 1A (3(−) and 3(+), respectively). After resting for 3 and 7 d, the fourth leaves were untreated or treated with W+OS (4(−) and 4(+), respectively) and after another 1 h, samples of fourth leaves were collected for analysing JA and JA-Ile contents (means ±SE, n=7). Different lowercase letters indicate significant differences within each time point determined by one-way ANOVA (P<0.05; post hoc tests).
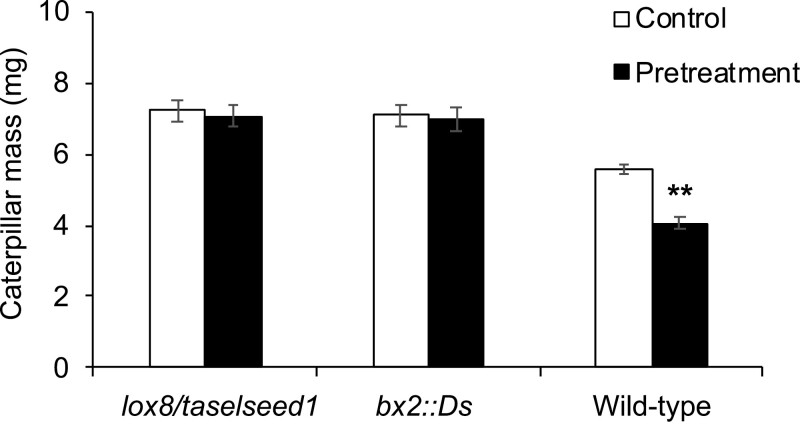
To further investigate whether the primed defenses in the the systemic leaves depend on the JA signaling and defensive Bx metabolites, we employed the lox8/tasselseed1 mutant, which is impaired in JA biosynthesis, and the bx2::Ds mutant, which lacks Bxs. For the WT maize line W22, which is the genetic background of these two mutants, and the lox8/tasselseed1 and bx2::Ds mutant, we pretreated the third leaves with 4 d of W+OS or untreated (controls) and after 7 d of resting, the fourth leaves were infested with M. separata insects for 48 h. On the pretreated W22, M. separata grew 27% smaller than the insects grown on the non-pretreated control W22 group (Fig. 6). Importantly, on the lox8/tasselseed1 and bx2::Ds mutant plants, the caterpillars exhibited very similar masses on the control and pretreated plants (Fig. 6). Notably, insects grown on lox8/tasselseed1 and bx2::Ds were always larger than those on the W22 plants, under the pretreatment or control conditions (Fig. 6), confirming the important role of JA signaling and Bx defensive metabolites. We confirmed that the lox8/tasselseed1 and bx2 mutant plants accumulated very little Bxs (Supplementary Fig. S3A, B), and W+OS pretreatment on third leaves did not have a priming effect on the subsequent W+OS-induced Bxs in fourth leaves (Supplementary Fig. S3).
Fig. 6.
Priming-enhanced maize resistance to M. separata is JA- and Bx-dependent. The third leave of wild-type maize (W22) and mutants bx2::Ds and lox8/taselseed1 were untreated (control) or pretreated with W+OS for consecutively 4 d (pretreatment), as shown in Fig. 1A. After 7 d of resting, the fourth leaves were infested with M. separata larvae and their masses were recorded 2 d after infestation. Data are mean ±SE; Student’s t-test; **P<0.001; n=25.
Thus, W+OS-induced priming in fourth leaves against subsequent insect attack requires JA signaling and Bx accumulation.
Priming enhances transcriptional regulation of various defense-related genes in systemic leaves in response to W+OS
To gain insight into the underlying molecular mechanism of defense priming, we performed a global gene expression analysis on the maize seedlings. The W+OS-pretreated and untreated seedlings (on the third leaves) were allowed to rest for 7 d, and their fourth leaves were treated with W+OS (3(+) 4(+) and 3(−) 4(+), respectively) or untreated (3(+) 4(−) and 3(−) 4(−), respectively). After 6 h, the fourth leaves of these four groups were sampled for RNA-seq analysis.
Principle component analysis (PCA) indicated little variations among the three biological replicates (Supplementary Fig. S4), and the first component PC1 accounted for 90% of the variations among samples and clearly clustered the samples of the 3(−) 4(−), 3(+) 4(−), 3(−) 4(+), and 3(−) 4(+) groups. Compared with the 3(−) 4(+) samples, samples of the 3(+) 4(+) group clustered furthest from the untreated control, 3(−) 4(−), indicating that pretreatment followed by subsequent W+OS treatment in fourth leaves induced the greatest changes in the systemic fourth leaves (Supplementary Fig. S4).
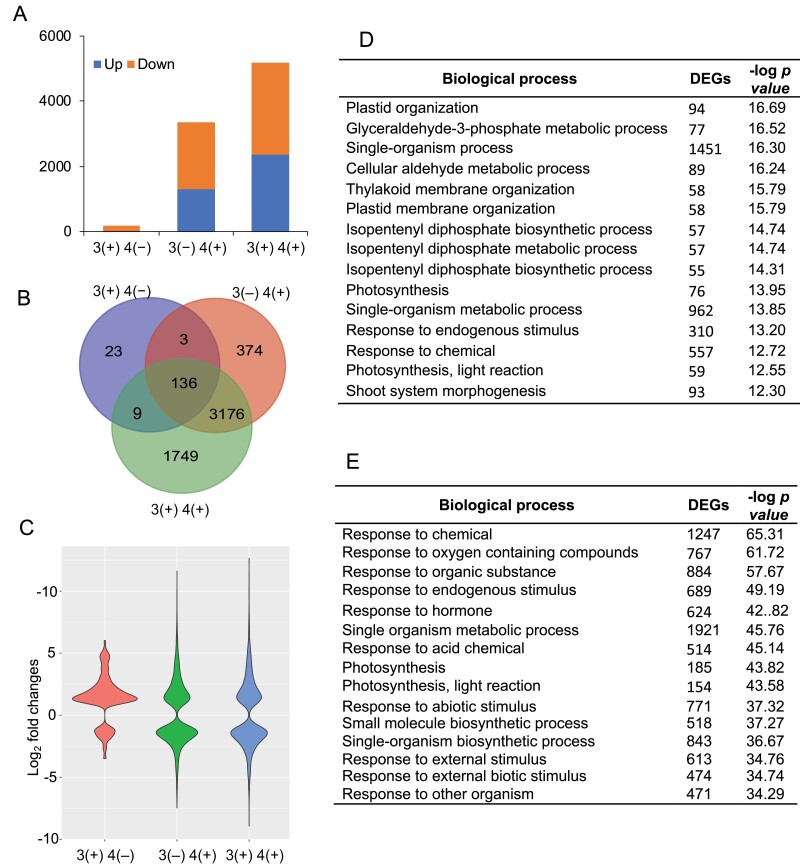
Using the transcriptome data from the fourth leaves of the 3(−) 4(−) (control) group as the reference, DEGs were inferred from all the other groups. In total, 5470 DEGs were identified (Supplementary Table S2). The fourth leaves of the 3(+) 4(−) group, which were only pretreated with W+OS, had only 171 DEGs (35 up- and 136 down-regulated). After W+OS treatment, the primed fourth leaves (3(+) 4(+)) showed, respectively, 2322 and 2748 up- and down-regulated DEGs, while in the non-primed group (3(−) 4(+)) there were 1678 up- and 2011 down-regulated genes in the fourth leaves (Supplementary Table S2; Fig. 7A). Venn diagram analysis indicated that the fourth leaves of the 3(−) 4(+) and 3(+) 4(+) groups have 3312 common DEGs (Supplementary Table S3; Fig. 7B); among the common genes, 2542 genes (76.7%) exhibited at least 10% further increased (2276 genes) or decreased (266 genes) transcript levels in the 3(+) 4(+) than in the 3(−) 4(+) group and 463 genes (14%) were found to have at least 50% further increased (456 genes) or decreased (7 genes) levels in the 3(+) 4(+) than in the 3(−) 4(+) group. Importantly, in the 3(+) 4(+) group 1749 genes were specifically up- or down-regulated, while in the non-primed 3(−) 4(+) group there were only 374 specifically regulated genes (Supplementary Table S3; Fig. 7B). Consistently, violin plot analysis showed that many genes in the 3(+) 4(+) samples were more strongly regulated than in the 3(−) 4(+) samples (Fig. 7C). These data suggest that pretreatment on third leaves enabled the fourth leaves to respond with transcriptional changes of a large number of genes, including many unique genes.
Fig. 7.
Primed maize leaves exhibit large transcriptional changes in response to simulated herbivory. Maize (A188) third leaves were untreated or pretreated with W+OS for four consecutive days as shown in Fig. 1A (3(−) and 3(+), respectively). After 7 d of resting, the fourth leaves were untreated or treated with W+OS (4(−) and 4(+), respectively) and after another 6 h, samples of fourth leaves were collected for RNA-seq analysis. (A) Numbers of up- and down-regulated genes in 3(+) 4(−), 3 (–) 4(+), and 3(+) 4(+), compared with 3(−) 4(−) samples. (B) Venn diagram depicting the specifically and commonly regulated DEGs. (C) Violin plot depicting the quantitative expression of all DEGs in 3(+) 4(−), 3(−) 4(+), and 3(+) 4(+) samples. (D, E) Enriched GO terms (biological process) from the DEGs unique for the 3(+) 4(+) group (D) and from the genes that are common for both 3(−) 4(+) and 3(+) 4(+) groups but are further up- or down-regulated in the 3(+) 4(+) group (E).
Next, gene ontology (GO) analysis was used to gain insight into the function of priming-related DEGs. The enriched biological processes from the uniquely regulated genes in the fourth leaves of the 3(+) 4(+) group included ‘plastid organization’, ‘glyceraldehyde-3-phosphate metabolic process’, ‘single-organism process’, and ‘cellular aldehyde metabolic process’ (Supplementary Table S4; Fig. 7D). Furthermore, GO terms enriched from the genes that were common in the 3(−) 4(+) and 3(+) 4(+) groups and were further promoted/suppressed in the 3(+) 4(+) group included ‘response to chemical’, ‘response to oxygen containing compounds’, ‘response to organic substance’, and ‘response to endogenous stimulus’ (Fig. 7E). The unique DEGs regulated in the 3(−) 4(+) group were not enriched in any GO terms.
Given that the accumulation of JA and Bxs was clearly regulated by priming, we specifically inspected the expression levels of JA and BX biosynthetic genes in different groups. Strikingly, among JA biosynthetic genes and catabolic genes, only LOX3 and bngle1371, a JA biosynthetic and a catabolic gene, respectively, were slightly but significantly increased in the fourth leaves of the 3(+) 4(+) group compared with the 3(−) 4(+) group (Supplementary Fig. S5). Similarly, among the 14 BX biosynthetic genes, only four, BX10, BX11, BX13, and BX14, were found to show priming-enhanced expression levels (i.e. greater levels in the fourth leaves of the 3(+) 4(+) group than in the 3(−) 4(+) group), while the rest of the BX genes seemed not to be involved in priming (Supplementary Fig. S6).
Discussion
In this study, we investigated the herbivory-induced priming of defense responses in the systemic leaves of maize seedlings. Our results demonstrate that actual or simulated M. separata herbivory induced priming in the unattacked systemic leaves, resulting in stronger and faster defenses upon subsequent caterpillar attack. The primed state could persist for at least 12 d in the systemic leaves, and the increased defense induced by herbivory on the systemic leaves was dependent on the JA pathway and Bx biosynthesis.
Previous studies have documented defense priming against insect herbivory, and the priming was induced by HIPVs, oviposition, β-amino-butyric acid, or cytokinin (Engelberth et al., 2004; Conrath et al., 2006; Frost et al., 2007; Dervinis et al., 2010). For example, indole primed the neighboring maize plants for enhanced release of herbivory-induced defense volatiles as well as increased expression of early defense signaling genes (Erb et al., 2015; Ye et al., 2019). Similarly, HIPVs released from M. separata-infested maize increased the resistance of downwind maize to insect herbivory, and the primed maize exhibited highly increased transcript levels of Bowman–Birk type trypsin inhibitor, compared with the maize plants receiving volatiles from the untreated maize (Ali et al., 2013). However, HIPV-induced defense priming seems to be species-specific. For example, exposure of the wild tobacco N. attenuata to volatiles released from simulated M. sexta feeding-induced N. attenuata did not result in different profiles of secondary metabolites or JA from those exposed to volatile from untreated controls (Paschold et al., 2006). In addition to HIPVs, the vasculature also conveys herbivory-induced systemic signals, which likely play essential roles in priming the defense of systemic tissues. Erb et al. (2008) showed that belowground herbivory by D. v. virgifera induced resistance to the caterpillar S. littoralis in maize leaves, and these leaves exhibited priming for elevated chlorogenic acid after subsequent S. littoralis feeding. Previously, it was found that simulated herbivory in maize had a strong effect on responses of systemic leaves, including increased JA levels and accumulation of Bxs (Malook et al., 2019). In our experiments, simulated herbivory-induced accumulation of Bxs in the systemic leaves could be ruled out, as quantification of Bxs indicated that the 3(−) 4(−) and 3(+) 4(−) leaves had the same concentrations of Bxs (Fig. 2); that is the enhanced defense in the fourth leaves was due to priming, but not because pretreatment on third leaves increased the contents of Bxs in fourth leaves (Fig. 2). A188, B73, and W22 all showed priming responses (Supplementary Fig. S1), suggesting that priming is a general trait of the maize defense system. However, Maag et al. (2016) used S. littoralis caterpillars to feed on local maize leaves for 24 h and 48 h, but they did not find any priming effect in the systemic leaves. The discrepancy between our study and Maag et al. (2016) may be due to different insect species: the generalist S. littoralis may be able to suppress systemic priming, while the specialist M. separata cannot. Another possibility is that S. littoralis insects used by Maag et al. (2016) were too small to make enough damage to induce priming in the systemic leaves, as we found that there was no systemic priming if simulated herbivory was performed by applying M. separata OS to only one row of mechanical wounds (Fig. 4).
We show that systemic defense priming is dependent on perception of certain elicitors in the OS of M. separata (Fig. 3). Previously it has been shown that M. separata OS are rich in several types of FACs (Qi et al., 2016), which are known to be potent elicitors in various insect OS (Wu and Baldwin, 2010). Similarly, in N. attenuata, simulated M. sexta herbivory (applying OS of M. sexta to wounds) but not mechanical wounding, activated salicylic acid-induced protein kinase and JA biosynthesis in systemic leaves (Hettenhausen et al., 2014). Thus, it is conceivable that maize perception of FACs in the M. separata OS is required for inducing the mobile priming agents. Even though the nature of mobile priming agents that promote systemic leaves into a primed state remains unclear, it is likely that these agents are a part of the herbivory-induced mobile systemic signals, which are possibly associated with Ca2+, reactive oxygen species, and ion channels (Hilleary and Gilroy, 2018; Kumari et al., 2019). It would be interesting to study whether maize mutants impaired in these signaling pathways have phenotypes of compromised defense priming in the systemic leaves.
In this study, we show that applying M. separata OS to one or even four rows of wounds in third leaves at once did not prime the systemic fourth leaves; in contrast, applying M. separata OS to one row of wounds once a day on two consecutive days successfully induced priming (Fig. 4). This was consistent with the findings that Arabidopsis that suffered from repetitive heat, cold, or salt stress was primed to have increased resistance to Pst DC3000, while long-term exposure to heat, cold, or salt did not prime the plants (Singh et al., 2014). Repeated treatment of simulated Manduca sexta herbivory on the wild tobacco N. attenuata plants elicited more rapid JA bursts and discrete increase in basal levels of JA and JA-Ile (Stork et al., 2009). Similarly, Baldwin and Schmelz (1996) showed that prior elicitation of the Nicotiana sylvestris plants twice with methyl jasmonate primed the plants for more rapid accumulation of nicotine upon a third treatment with methyl jasmonate, compared with naïve plants or plants that were pretreated only once. Although priming enables plants to gain higher fitness than non-primed ones in the presence of stresses, priming likely incurs costs (van Hulten et al., 2006); thus, being able to sense insect feeding, which includes repetitive wounding and sensing insect-specific elicitors, is necessary for minimizing the costs. It is unclear how repetitive W+OS primes maize systemic leaves.
We analysed the effect of priming on transcriptome changes (Fig. 7). The primed 3(+) 4(+) group exhibited many specifically induced/repressed genes compared with the non-primed 3(−) 4(+) (Fig. 7B). Detailed inspection of the transcriptome data indicated that (i) 34.5% of the total DEGs in the 3(+) 4(+) group were specifically regulated by priming; namely, these genes were regulated only if third leaves were pretreated with W+OS; (ii) 374 unique genes (10.1% of all DEGs in the 3(−) 4(+) group) were no longer regulated, when third leaves were pretreated, in other words, if fourth leaves were primed; and (iii) 68.9% (2542 genes) of all DEGs in the 3(−) 4(+) group showed further up- or down-regulation (at least 10%), if third leaves were pretreated. We hypothesize that in addition to the contribution of the 1749 specifically regulated genes in the fourth leaves of the 3(+) 4(+) group, the 2542 genes that were further induced/suppressed by priming could also play a role in priming-induced defense responses. How priming enables the systemic fourth leaves to mount strong defenses against M. separata, including strongly altered transcriptome and enhanced accumulation of defensive Bxs, remains unclear. Epigenetic changes have been detected in stress-treated plants (Jaskiewicz et al., 2011; Luna et al., 2012). Stresses, such as phosphate starvation or pathogen infection, lead to genome-wide methylome reconfigurations, which are often associated with transcriptome changes (Dowen et al., 2012; Secco et al., 2015; Yong-Villalobos et al., 2015). In our maize–M. separata interaction system, the involvement of maize epigenetic changes after actual/simulated herbivory seems to be very likely.
The primed resistance was abolished in the JA biosynthesis lox8 mutants and Bx biosynthesis bx2 mutants (Supplementary Fig. S3). Therefore, it is very likely that certain priming-associated responses are upstream of JA biosynthesis, as indicated by the finding that primed plants had much higher concentrations of W+OS-induced JA than did the non-primed plants. A similar finding was that touching Arabidopsis leaves repetitively increased the resistance of these leaves to the fungus Botrytis cinerea and cabbage looper (Trichoplusia ni), while touch-induced priming was not detected in the JA-deficient aos mutants (Goodspeed et al., 2012). Pretreatment by simulated herbivory strongly primed maize for enhanced levels of JA (Fig. 5) and Bxs (Fig. 2), but detailed inspection of the JA biosynthetic and catabolic genes and Bx biosynthetic genes indicated that only a few genes fitted into the transcriptional pattern of priming (Supplementary Figs S5, S6). Thus, it is possible that in addition to transcriptional regulation, post-transcriptional regulation of JA and Bx biosynthetic and catabolic genes may play an important role in priming. Given that we did not examine the expression levels of JA and Bx biosynthetic genes at multiple times (only at 6 h), there is another possibility, that priming affects the expression of JA and Bx biosynthetic genes at earlier or later times than at 6 h. The mechanism of defense priming deserves further study.
Taken together, in this study we show that maize is able to sense repetitively treated simulated herbivory and deploy priming in the systemic leaves, thus enabling these leaves to respond to subsequent insect feeding with highly increased JA and defensive Bx metabolites. Our analysis reveals that priming promotes transcriptional activation in the systemic leaves. These results provide new insight into priming, a process that is important for maize defense against insects. Further studies on the systemic and priming-associated signals will further uncover the mechanism underlying priming, which could facilitate breeding of maize lines with enhanced resistance to insects.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Simulated M. separata herbivory-induced priming is conserved in different maize lines and requires perception of OS.
Fig. S2. More than one time of simulated M. separata treatment is required to induce priming in maize systemic leaves.
Fig. S3. Bx2 and lox8/taselseed1 mutants do not exhibit priming-induced elevation of Bxs.
Fig. S4. Overview of RNA-seq data from maize fourth leaves after different treatments.
Fig. S5. JA biosynthesis and catabolism genes in 3(−) 4(+) and 3(+) 4(+) groups do not have different transcript levels.
Fig. S6. Most Bx biosynthesis genes in 3(−) 4(+) and 3(+) 4(+) groups do not have different transcript levels.
Table S1. ANOVA table from two-way ANOVA considering resting time and priming as independent variables.
Table S2. All DEGs in maize systemic leaves of 3(+) 4(−), 3(−) 4(+), and 3(+) 4(+) groups.
Table S3. Common and specific DEGs in maize systemic fourth leaves of 3(+) 4(−), 3(−) 4(+), and 3(+) 4(+) groups.
Table S4. Gene ontology enrichment from the specifically and commonly regulated DEGs in maize systemic fourth leaves of 3(+) 4(−), 3(−) 4(+), and 3(+) 4(+) groups.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31772179, 31770301, U1502263), the CAS Youth Innovation Promotion Association (2018426). S.U.M. was supported by the CAS-TWAS President’s Fellowship Program for International Ph.D. Students.
Author contributions
JW designed the research. SUM performed experiments. YX, JQ, JL, and LW analysed the data and made illustrations. JW and SUM wrote the manuscript.
Conflict of interest
Authors declare that we have no conflicts of interest to disclose.
Data availability
The raw data were deposited in the National Genomics Data Center (https://bigd.big.ac.cn) and available under the BioProject ID (PRJCA003876).
References
- Ahmad S, Van Hulten M, Martin J, Pieterse CM, Van Wees SC, Ton J. 2011. Genetic dissection of basal defence responsiveness in accessions of Arabidopsis thaliana. Plant, Cell & Environment 34, 1191–1206. [DOI] [PubMed] [Google Scholar]
- Ali M, Sugimoto K, Ramadan A, Arimura G. 2013. Memory of plant communications for priming anti-herbivore responses. Scientific Reports 3, 1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin IT, Schmelz EA. 1996. Immunological ‘memory’ in the induced accumulation of nicotine in wild tobacco. Ecology 77, 236–246. [Google Scholar]
- Bandoly M, Grichnik R, Hilker M, Steppuhn A. 2016. Priming of anti-herbivore defence in Nicotiana attenuata by insect oviposition: herbivore-specific effects. Plant, Cell & Environment 39, 848–859. [DOI] [PubMed] [Google Scholar]
- Bandoly M, Hilker M, Steppuhn A. 2015. Oviposition by Spodoptera exigua on Nicotiana attenuata primes induced plant defence against larval herbivory. The Plant Journal 83, 661–672. [DOI] [PubMed] [Google Scholar]
- Burtet LM, Bernardi O, Melo AA, Pes MP, Strahl TT, Guedes JV. 2017. Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Management Science 73, 2569–2577. [DOI] [PubMed] [Google Scholar]
- Carena MJ, Glogoza P. 2004. Resistance of maize to the corn leaf aphid: a review. Maydica 49, 241–254. [Google Scholar]
- Conrath U, Beckers GJM, Flors V, et al. 2006. Priming: getting ready for battle. Molecular Plant-Microbe Interactions 19, 1062–1071. [DOI] [PubMed] [Google Scholar]
- De Geyter N, Gholami A, Goormachtig S, Goossens A. 2012. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends in Plant Science 17, 349–359. [DOI] [PubMed] [Google Scholar]
- Dervinis C, Frost CJ, Lawrence SD, Novak NG, Davis JM. 2010. Cytokinin primes plant responses to wounding and reduces insect performance. Journal of Plant Growth Regulation 29, 289–296. [Google Scholar]
- Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR. 2012. Widespread dynamic DNA methylation in response to biotic stress. Proceedings of the National Academy of Sciences, USA 109, E2183–E2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. 2004. Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences, USA 101, 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Contreras CF, Viswanathan S. 2012. Transcriptional analysis of distant signaling induced by insect elicitors and mechanical wounding in Zea mays. PLoS One 7, e34855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelberth J, Seidl-Adams I, Schultz JC, Tumlinson JH. 2007. Insect elicitors and exposure to green leafy volatiles differentially upregulate major octadecanoids and transcripts of 12-oxo phytodienoic acid reductases in Zea mays. Molecular Plant-Microbe Interactions 20, 707–716. [DOI] [PubMed] [Google Scholar]
- Erb M, Flors V, Karlen D, de Lange E, Planchamp C, D’Alessandro M, Turlings TCJ, Ton J. 2009. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. The Plant Journal 59, 292–302. [DOI] [PubMed] [Google Scholar]
- Erb M, Reymond P. 2019. Molecular interactions between plants and insect herbivores. Annual Review Plant of Biology 70, 4.1–4.31. [DOI] [PubMed] [Google Scholar]
- Erb M, Ton J, Degenhardt J, Turlings TC. 2008. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiology 146, 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Veyrat N, Robert CA, Xu H, Frey M, Ton J, Turlings TC. 2015. Indole is an essential herbivore-induced volatile priming signal in maize. Nature Communications 6, 6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M, Chomet P, Glawischnig E, et al. 1997. Analysis of a chemical plant defense mechanism in grasses. Science 277, 696–699. [DOI] [PubMed] [Google Scholar]
- Frey M, Schullehner K, Dick R, Fiesselmann A, Gierl A. 2009. Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 70, 1645–1651. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Appel HM, Carlson JE, De Moraes CM, Mescher MC, Schultz JC. 2007. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecology Letters 10, 490–498. [DOI] [PubMed] [Google Scholar]
- Gao L, Shen G, Zhang L, Qi J, Zhang C, Ma C, Li J, Wang L, Malook SU, Wu J. 2019. An efficient system composed of maize protoplast transfection and HPLC-MS for studying the biosynthesis and regulation of maize benzoxazinoids. Plant Methods 15, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Marti G, Villard N, Doyen GA, Wolfender JL, Turlings TC, Erb M. 2011. Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. The Plant Journal 68, 901–911. [DOI] [PubMed] [Google Scholar]
- Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF. 2012. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proceedings of the National Academy of Sciences, USA 109, 4674–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handrick V, Robert CA, Ahern KR, et al. 2016. Biosynthesis of 8-O-methylated benzoxazinoid defense compounds in maize. The Plant Cell 28, 1682–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Y, Abbas N, Li Y, Zhang Y. 2018. Selection for resistance, life history traits and the biochemical mechanism of resistance to thiamethoxam in the maize armyworm, Mythimna separata (Lepidoptera: Noctuidae). Phytoparasitica 46, 627–634. [Google Scholar]
- Heil M, Ton J. 2008. Long-distance signalling in plant defence. Trends in Plant Science 13, 264–272. [DOI] [PubMed] [Google Scholar]
- Hettenhausen C, Heinrich M, Baldwin IT, Wu J. 2014. Fatty acid-amino acid conjugates are essential for systemic activation of salicylic acid-induced protein kinase and accumulation of jasmonic acid in Nicotiana attenuata. BMC Plant Biology 14, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleary R, Gilroy S. 2018. Systemic signaling in response to wounding and pathogens. Current Opinion in Plant Biology 43, 57–62. [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. 2008. Plant immunity to insect herbivores. Annual Review of Plant Biology 59, 41–66. [DOI] [PubMed] [Google Scholar]
- Howe GA, Major IT, Koo AJ. 2018. Modularity in jasmonate signaling for multistress resilience. Annual Review of Plant Biology 69, 387–415. [DOI] [PubMed] [Google Scholar]
- Jaskiewicz M, Conrath U, Peterhänsel C. 2011. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Reports 12, 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Chetelat A, Nguyen CT, Farmer EE. 2019. Arabidopsis H+-ATPase AHA1 controls slow wave potential duration and wound-response jasmonate pathway activation. Proceedings of the National Academy of Sciences, USA 116, 20226–20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Bruce TJ, Roberts MR, Flors V, Ton J. 2012. Next-generation systemic acquired resistance. Plant Physiology 158, 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maag D, Kohler A, Robert CA, Frey M, Wolfender JL, Turlings TC, Glauser G, Erb M. 2016. Highly localized and persistent induction of Bx1-dependent herbivore resistance factors in maize. The Plant Journal 88, 976–991. [DOI] [PubMed] [Google Scholar]
- Malook Su, Qi J, Hettenhausen C, Xu Y, Zhang C, Zhang J, Lu C, Li J, Wang L, Wu J. 2019. The oriental armyworm (Mythimna separata) feeding induces systemic defence responses within and between maize leaves. Philosophical Transactions of the Royal Society. Series B, Biological Sciences 374, 20180307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch-Mani B, Baccelli I, Luna E, Flors V. 2017. Defense priming: an adaptive part of induced resistance. Annual Review of Plant Biology 68, 485–512. [DOI] [PubMed] [Google Scholar]
- Meihls LN, Handrick V, Glauser G, et al. 2013. Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. The Plant Cell 25, 2341–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithofer A, Boland W. 2012. Plant defense against herbivores: chemical aspects. Annual Review of Plant Biology 63, 431–450. [DOI] [PubMed] [Google Scholar]
- Niemeyer HM. 2009. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: key defense chemicals of cereals. Journal of Agricultural and Food Chemistry 57, 1677–1696. [DOI] [PubMed] [Google Scholar]
- Oikawa A, Ishihara A, Tanaka C, Mori N, Tsuda M, Iwamura H. 2004. Accumulation of HDMBOA-Glc is induced by biotic stresses prior to the release of MBOA in maize leaves. Phytochemistry 65, 2995–3001. [DOI] [PubMed] [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT. 2006. Using ‘mute’ plants to translate volatile signals. The Plant Journal 45, 275–291. [DOI] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. 2016. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nature Protocols 11, 1650–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Malook SU, Shen G, Gao L, Zhang C, Li J, Zhang J, Wang L, Wu J. 2018. Current understanding of maize and rice defense against insect herbivores. Plant Diversity 40, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Sun G, Wang L, et al. 2016. Oral secretions from Mythimna separata insects specifically induce defence responses in maize as revealed by high-dimensional biological data. Plant, Cell & Environment 39, 1749–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Powell AF, Mirzaei M, Wang LJ, Movahed N, Miller JK, Piñeros MA, Jander G. 2021. Indole-3-glycerolphosphate synthase, a branchpoint for the biosynthesis of tryptophan, indole, and benzoxazinoids in maize. The Plant Journal (in press), doi: 10.1111/tpj.15163. [DOI] [PubMed] [Google Scholar]
- Rostas M. 2007. The effects of 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one on two species of Spodoptera and the growth of Setosphaeria turcica in vitro. Journal of Pest Science 80, 35–41. [Google Scholar]
- Secco D, Wang C, Shou H, Schultz MD, Chiarenza S, Nussaume L, Ecker JR, Whelan J, Lister R. 2015. Stress induced gene expression drives transient DNA methylation changes at adjacent repetitive elements. eLife 4, e09343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Yekondi S, Chen PW, Tsai CH, Yu CW, Wu K, Zimmerli L. 2014. Environmental history modulates Arabidopsis pattern-triggered immunity in a HISTONE ACETYLTRANSFERASE1-dependent manner. The Plant Cell 26, 2676–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Liu H, Zhuang H, Zhao C, Xu Y, Wu S, Qi J, Li J, Hettenhausen C, Wu J. 2017. Transcriptomics and alternative splicing analyses reveal large differences between maize lines b73 and mo17 in response to aphid Rhopalosiphum padi infestation. Frontiers in Plant Science 8, 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork W, Diezel C, Halitschke R, Gális I, Baldwin IT. 2009. An ecological analysis of the herbivory-elicited JA burst and its metabolism: plant memory processes and predictions of the moving target model. PLoS One 4, e4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z. 2017. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Research 45, W122–W129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzin V, Fernandez-Pozo N, Richter A, et al. 2015. Dynamic maize responses to aphid feeding are revealed by a time series of transcriptomic and metabolomic assays. Plant Physiology 169, 1727–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzin V, Hojo Y, Strickler SR, et al. 2017. Rapid defense responses in maize leaves induced by Spodoptera exigua caterpillar feeding. Journal of Experimental Botany 68, 4709–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CM, Ton J. 2006. Costs and benefits of priming for defense in Arabidopsis. Proceedings of the National Academy of Sciences, USA 103, 5602–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. [Google Scholar]
- Wisecaver JH, Borowsky AT, Tzin V, Jander G, Kliebenstein DJ, Rokas A. 2017. A global coexpression network approach for connecting genes to specialized metabolic pathways in plants. The Plant Cell 29, 944–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall D, Holroyd GH, Moore JP, Glowacz M, Croft P, Taylor JE, Paul ND, Roberts MR. 2012. Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytologist 193, 770–778. [DOI] [PubMed] [Google Scholar]
- Wouters FC, Blanchette B, Gershenzon J, Vassao DG. 2016. Plant defense and herbivore counter-defense: benzoxazinoids and insect herbivores. Phytochemistry Reviews 15, 1127–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters FC, Reichelt M, Glauser G, Bauer E, Erb M, Gershenzon J, Vassão DG. 2014. Reglucosylation of the benzoxazinoid DIMBOA with inversion of stereochemical configuration is a detoxification strategy in lepidopteran herbivores. Angewandte Chemie. International Edition 53, 11320–11324. [DOI] [PubMed] [Google Scholar]
- Wu J, Baldwin IT. 2010. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics 44, 1–24. [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT. 2007. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. The Plant Cell 19, 1096–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Christensen S, Isakeit T, Engelberth J, Meeley R, Hayward A, Emery RJ, Kolomiets MV. 2012. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. The Plant Cell 24, 1420–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Glauser G, Lou Y, Erb M, Hu L. 2019. Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in rice. The Plant Cell 31, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Villalobos L, González-Morales SI, Wrobel K, Gutiérrez-Alanis D, Cervantes-Peréz SA, Hayano-Kanashiro C, Oropeza-Aburto A, Cruz-Ramírez A, Martínez O, Herrera-Estrella L. 2015. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proceedings of the National Academy of Sciences, USA 112, E7293–E7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data were deposited in the National Genomics Data Center (https://bigd.big.ac.cn) and available under the BioProject ID (PRJCA003876).