Abstract
Objective:
To determine the association between novel lifestyle factors on risk of rheumatoid arthritis-associated interstitial lung disease (RA-ILD), define the threshold at which smoking increases RA-ILD risk, and calculate the degree to which known lifestyle and clinical factors predict RA-ILD.
Methods:
This nested case-control study matched incident RA-ILD cases to RA non-ILD controls on age, sex, RA duration, rheumatoid factor, and time from exposure assessment to RA-ILD. Exposures included education, body mass index (BMI), smoking, anti-cyclic citrullinated peptide, race, joint erosions, rheumatoid nodules, C-reactive protein (CRP), disease activity score, functional status, disease-modifying anti-rheumatic drug use, and glucocorticoid use. Odds ratios (OR) for each exposure on risk of RA-ILD were obtained from logistic regression models. Area under the curve (AUC) was calculated based all lifestyle and clinical exposures.
Results:
We identified 84 incident RA-ILD cases and 233 matched controls. After adjustment, obesity, high-positive CRP (≥10 mg/L), and poor functional status (MDHAQ ≥1) were associated with increased risk of RA-ILD (OR 2.42, 95% confidence interval [CI] 1.11–5.24 vs. normal BMI; OR 2.61, 95% CI 1.21–5.64 vs. CRP <3mg/L; OR 3.10, 95% CI 1.32–7.26 vs. MDHAQ <0.2). Smoking 30 pack-years or more was strongly associated with risk of RA-ILD compared to nonsmokers (OR 6.06, 95% CI 2.72–13.5). Together, lifestyle and clinical risk factors for RA-ILD had an AUC of 0.79 (95% CI 0.73–0.85).
Conclusion:
Obesity, CRP, functional status, and extensive smoking may be novel risk factors for RA-ILD, useful for RA-ILD risk assessment and prevention. The overall ability to predict RA-ILD remains modest.
Keywords: rheumatoid arthritis, interstitial lung disease, smoking, central obesity
INTRODUCTION
Approximately 5–10% of patients with rheumatoid arthritis (RA) have clinically significant RA-associated interstitial lung disease (RA-ILD), and an additional 20–30% may have subclinical RA-ILD (1–3). Furthermore, RA-ILD is associated with a median survival of less than three years after diagnosis (2), with two to ten times the odds of mortality compared to RA patients without ILD (4, 5). Identifying predictors of RA-ILD is crucial to better understand the underlying pathogenesis and to allow for earlier diagnosis and treatment with the goal of preventing irreversible lung damage. In the future, ascertaining predictors of RA-ILD may even build the foundation for interventions that can improve its poor survival by early detection or altering the natural history prior to clinically severe RA-ILD.
There are several gaps in the RA-ILD literature. First, no studies have investigated the association between lifestyle factors such as education or obesity on RA-ILD risk, despite evidence that they are risk factors for RA (6–9). Second, while many studies have shown smoking to be a risk factor for RA-ILD (10–16), a few have not found an association (17–19), possibly due to a threshold effect for smoking on RA-ILD risk. One study showed that smoking over 25 pack-years was associated with a dramatic increase in physiologic and radiologic abnormalities suggestive of RA-ILD compared to less than 25 pack-years, but this study did not explore additional smoking exposure thresholds or stratification groups, and it used pulmonary function abnormalities as the outcome instead of RA-ILD (10). Third, existing studies of RA-ILD risk have typically lacked comprehensive adjustment for many potential confounders (1, 5, 10–29), resulting in discordant results for certain risk factors including male sex, RA duration, rheumatoid factor (RF) positivity, disease activity, functional status, prednisone use, and rheumatoid nodules. Furthermore, the degree to which known lifestyle and clinical risk factors together predict risk of RA-ILD remains unknown.
To address these gaps, our objectives were threefold: (1) to elucidate the association of lifestyle factors such as education and obesity with subsequent risk of RA-ILD, (2) to define the pack-years threshold at which smoking increases risk of RA-ILD, and (3) to determine the degree to which a comprehensive assessment of known risk factors for RA-ILD predicts disease development. We hypothesized that lower education, obesity, and smoking over a threshold of 20 pack-years would be associated with increased risk of RA-ILD, but that all risk factors together would have only a modest ability to predict RA-ILD, pointing to the likely existence of unmeasured risk factors.
MATERIALS AND METHODS
Study Design and Participants
This nested, case-control study was performed within the Brigham Rheumatoid Arthritis Sequential Study (BRASS), an ongoing large, single-center, prospective registry of patients with RA since 2003 (30). With its low patient turnover, this population may have reasonable generalizability to other RA populations. For example, the mean age (57), proportion female (82%), proportion with new-onset RA (20%), and with high functional status (82%) are nearly identical to RA patients in the Consortium of Rheumatology Researchers of North America (CORRONA) registry (59, 75%, 20%, and 88% respectively) (30, 31). All RA cases met 1987 American College of Rheumatology (ACR) or 2010 ACR/European League Against Rheumatology criteria as determined by a rheumatologist (32, 33), and those with verified RA were eligible to participate (30). Blood samples, RA characteristics, and physician/patient-reported measures were collected at enrollment and semi-annually (30). This BRASS sub-study received approval from the Partners HealthCare Institutional Review Board (2016P000226), followed Strengthening The Reporting of Observational studies in Epidemiology (STROBE) guidelines for observational studies, and complied with the Declaration of Helsinki.
RA-ILD Cases and RA Non-ILD Controls
High-resolution chest computed tomography (CT) scans of BRASS participants performed for clinical care up until April 14, 2016 were reviewed by two attending thoracic radiologists and one attending pulmonologist. RA-ILD was defined as presence of interstitial lung abnormalities on the research read of chest CT, as described previously (28). In a previous study examining the same RA-ILD cases used in this analysis, the clinical significance of CT-defined ILD was found to be high in several ways. First, of 78 RA-ILD cases for whom clinical radiographic reports were available, 68 (87%) had features consistent with RA-ILD noted on the report. Second, 64 (75%) of the 85 RA-ILD cases had additional clinical evaluation and/or follow-up for the condition. In addition, 32 (38%) had changes to RA medications after CT scan. Finally, 32 (38%) of the RA-ILD cases died during study follow-up compared to only 126 (9%) in the previously analyzed RA population (28).
Controls were BRASS participants with absence of patient-reported ILD and absence of both billing codes and physician-reported history of asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, bronchiectasis, bronchiolitis obliterans-organizing pneumonia, drug-induced pneumonitis, or tuberculosis. We did not require that all controls have chest CTs performed since doing so could introduce selection bias due to the clinical indication of the imaging. Each case was matched to up to three controls on age (within 5 years), sex, RA duration (within 5 years), RF positivity, and time from exposure assessment to index date (within 6 months). We matched by the above known RA-ILD risk factors to improve power to investigate possible novel associations, knowing that this would slightly decrease the model’s ability to predict RA-ILD. Index date was the date of RA-ILD by the initial CT chest for the case and matched date for corresponding controls.
Exposures and Covariates
To ensure that assessment of exposures preceded the date of ILD, we identified the earliest study visit date containing these data occurring before the index date. Exposure and covariate variables obtained from BRASS study visits included age, sex, RA duration, race/ethnicity (White, non-Hispanic vs other), education (less than college degree vs. more), joint erosions (present vs absent on hand plain radiographs), rheumatoid nodules (present vs absent), body mass index (BMI) categories, smoking status (never, past, or current), smoking pack-years, disease activity score with 28-joint counts (DAS28-CRP) (remission [<2.6], low [2.6-<3.2], moderate [3.2-<5.2], or high [≥5.1]) (34), functional status by multi-dimensional health assessment questionnaire (MDHAQ) (0-<0.2, 0.2<−1.0, or ≥ 1.0) (35), and biologic disease modifying anti-rheumatic drug (DMARD), methotrexate, or prednisone use (never, past, current). RF positivity, anti-cyclic citrullinated peptide (anti-CCP) positivity, and C-reactive protein (CRP) (normal [<3 mg/L], low-positive [3-<10 mg/L], or high [≥10 mg/L]) came from study visit blood work.
Statistical Analysis
We compared continuous variables using Wilcoxon rank sum tests, and categorical variables using chi-squared tests. For the association analyses, conditional logistic regression models of each exposure were fitted to obtain odds ratios (OR) with 95% confidence intervals (CI) for RA-ILD case status, adjusting for anti-CCP, race/ethnicity, education, BMI (continuous), and smoking pack-years (continuous). Since disease activity and treatment closely interact, the main models of these factors did not adjust for each other. Exploratory sensitivity analyses added prednisone and methotrexate use to the disease activity model, and disease activity to each drug model, in order to investigate possible independent associations. For ordinal variables, we calculated p for trend by first calculating the median value within each category and then using those values as a continuous variable in a separate model. To determine the degree to which all risk factors predicted RA-ILD risk, we fit receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC). The threshold for statistical significance was a two-sided p<0.05. No participants were missing any data. Analyses were pre-specified in a protocol and performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Characteristics of RA-ILD Cases and Controls
Of the 1,100 RA cases included in BRASS, we identified 84 cases of confirmed RA-ILD and 233 matched RA non-ILD controls. Among controls, 60 (26%) had at least one chest CT performed, and an additional 76 (33%) had at least one chest x-ray. Therefore, the majority (58%) of controls had chest imaging performed that did not clinically reveal RA-ILD. Characteristics of controls with CT compared to without CT were similar, with none of the factors associated with RA-ILD being associated with receipt of chest CT in controls, including education (Supplementary Table S1). The median time between BRASS study visit where exposures/covariates were assessed and the index date of RA-ILD (or matched date) was 1.5 (IQR 0.6–2.5) years for RA-ILD cases and 1.9 (IQR 1.6–5.6) years for controls. At the time of exposure assessment, RA-ILD cases had more rheumatoid nodules, greater history of smoking, higher CRP, higher disease activity (DAS28-CRP), worse functional status (MDHAQ), less biologic DMARD and methotrexate use, and more prednisone use when compared to controls based on univariate analyses (Table 1).
Table 1. Characteristics of the 84 incident RA-ILD cases and their 233 RA non-ILD controls.
These characteristics were collected at the time of the earliest BRASS study visit.
| Number (%) |
|||
|---|---|---|---|
| Characteristic | RA-ILD Cases (N=84) | RA Controls (N=233) | p-value |
| Years to Index/Matched Date, median (IQR) | 1.5 (0.6,2.5) | 1.9 (1.6,5.6) | 0.84a |
| Age in years, mean (SD) | 67 (10) | 66 (11) | 0.41a |
| Female Sex | 65 (77) | 186 (80) | 0.64a |
| RA Duration in years, mean (SD) b | 20 (12) | 20 (11) | 0.99a |
| RF Positive | 73 (87) | 195 (84) | 0.48a |
| CCP Positive | 70 (83) | 190 (82) | 0.71 |
| White, non-Hispanic | 76 (90) | 219 (94) | 0.28 |
| Education Less than College | 30 (36) | 98 (42) | 0.31 |
| Joint Erosions | 49 (58) | 144 (62) | 0.58 |
| Rheumatoid Nodules | 42 (50) | 80 (34) | 0.01 |
| Body Mass Index, kg/m2 | 0.18 | ||
| <20 | 4 (5) | 16 (7) | |
| 20–<25 | 20 (23) | 77 (33) | |
| 25–<30 | 32 (38) | 87 (37) | |
| ≥30 | 28 (33) | 53 (23) | |
| Smoking Status | 0.03 | ||
| Never | 33 (39) | 124 (53) | |
| Past | 43 (51) | 100 (43) | |
| Current | 8 (10) | 9 (4) | |
| Smoking Pack-Years | <0.001 | ||
| Never Smoker | 36 (43) | 126 (54) | |
| 1–9 | 7 (8) | 45 (19) | |
| 10–19 | 11 (13) | 28 (12) | |
| 20–29 | 7 (8) | 21 (9) | |
| ≥30 | 23 (27) | 13 (6) | |
| C-Reactive Protein, mg/L | 0.006 | ||
| Normal (<3) | 33 (39) | 136 (58) | |
| Low-Positive (3–<10) | 31 (37) | 67 (29) | |
| High-Positive (≥10) | 20 (24) | 30 (13) | |
| Disease Activity Score-28-CRP | <0.001 | ||
| Remission (<2.6) | 27 (32) | 104 (45) | |
| Low (2.6–<3.2) | 4 (5) | 39 (17) | |
| Moderate (3.2–<5.1) | 37 (44) | 70 (30) | |
| High (≥5.1) | 16 (19) | 20 (9) | |
| MDHAQ Score | <0.001 | ||
| 0–<0.2 | 15 (18) | 64 (27) | |
| 0.2<–1.0 | 37 (44) | 127 (55) | |
| ≥1.0 | 32 (38) | 42 (18) | |
| Biologic DMARD Use | 0.03 | ||
| Never | 29 (35) | 76 (33) | |
| Past | 19 (23) | 27 (12) | |
| Current | 36 (43) | 130 (56) | |
| Methotrexate Use | 0.03 | ||
| Never | 18 (21) | 24 (10) | |
| Past | 32 (38) | 93 (40) | |
| Current | 34 (41) | 116 (50) | |
| Prednisone Use | 0.007 | ||
| Never | 5 (6) | 31 (13) | |
| Past | 42 (50) | 140 (60) | |
| Current | 37 (44) | 62 (27) | |
CRP = C-reactive protein, DMARD = disease-modifying anti-rheumatic drug, ILD = interstitial lung disease, IQR = interquartile range, MDHAQ = multi-dimensional health assessment questionnaire, RA = rheumatoid arthritis, RF = rheumatoid factor, SD = standard deviation
matched factor
Range was 1–46 years for cases and 2–47 years for controls. Median (IQR) was 20 (10,31) for cases and 18 (11,29) for controls.
Education and Obesity
After further adjusting for anti-CCP, race, and smoking, we found that participants with lower education had a decreased risk of RA-ILD compared to participants with college education or higher (Table 2). Obesity (BMI ≥30 kg/m2) was associated with an over two-fold increased risk of RA-ILD, with evidence of a dose-response relationship (p for trend 0.02) (Table 2).
Table 2. Association between education, obesity, and incident RA-ILD.
These data came from the 84 BRASS RA-ILD cases and 233 RA non-ILD controls.
| Characteristic | OR Conditioned on Matching Factorsa (95% CI) | Multivariableb OR (95% CI) |
|---|---|---|
| Education Less than College | 0.76 (0.46–1.26) | 0.53 (0.30–0.95) |
| Body Mass Index, kg/m2 | ||
| <20 | 0.98 (0.30–3.21) | 1.00 (0.29,3.43) |
| 20–<25 | (ref) | (ref) |
| 25–<30 | 1.35 (0.72–2.52) | 1.56 (0.79,3.06) |
| ≥30 | 2.05 (1.03–4.07) | 2.42 (1.11,5.24) |
All models were conditioned on matching factors (age, sex, RA duration, RF status, and time from study visit to index date). Bold values indicate statistical significance to p<0.05.
Also adjusting for anti-CCP, race/ethnicity, education (in BMI model), BMI (in education model), and smoking pack-years
Smoking Threshold
After adjustment, cumulative past smoking was not associated with RA-ILD (Table 3). However, current smoking was associated with over a three-fold increase in odds of RA-ILD compared to never smoking (Table 3). Furthermore, smoking 30 pack-years or higher was associated with a six-fold increased risk compared to nonsmokers (p for trend <0.001) (Table 3). In contrast, smoking pack-year levels less than 30 pack-years were not associated with increased risk of RA-ILD (Table 3).
Table 3. Association between smoking and incident RA-ILD.
These data came from the 84 BRASS RA-ILD cases and 233 RA non-ILD controls.
| Characteristic | OR Conditioned on Matching Factorsa (95% CI) | Multivariableb OR (95% CI) |
|---|---|---|
| Smoking Status | ||
| Never | (ref) | (ref) |
| Past | 1.56 (0.92–2.63) | 1.68 (0.97–2.92) |
| Current | 2.91 (1.08–7.85) | 3.27 (1.15–9.29) |
| Smoking Pack-Years | ||
| Never Smoker | (ref) | (ref) |
| 1–9 | 0.53 (0.22–1.26) | 0.54 (0.22–1.31) |
| 10–19 | 1.30 (0.59–2.89) | 1.42 (0.62–3.24) |
| 20–29 | 1.16 (0.42–3.19) | 1.44 (0.51–4.06) |
| ≥30 | 5.34 (2.50–11.5) | 6.06 (2.72–13.5) |
All models were conditioned on matching factors (age, sex, RA duration, RF status, and time from study visit to index date). Bold values indicate statistical significance to p<0.05.
Also adjusting for anti-CCP, race/ethnicity, education, and BMI
Other Risk Factors for RA-ILD
Other factors that remained statistically significant predictors of RA-ILD after adjustment included high-positive CRP, moderate or high disease activity (DAS28-CRP 3.2 or higher), poor functional status (MDHAQ ≥1.0), and current prednisone use, while past or current methotrexate use was associated with decreased risk of RA-ILD (Table 4). A sensitivity analysis adjusting disease activity for prednisone and methotrexate use showed that moderate and high disease activity remained statistically significant predictors of RA-ILD (OR 2.22, 95% CI 1.04–4.72 and OR 5.09, 95% CI 1.58–16.4), respectively, compared to remission (p for trend 0.003). Similarly, the association between prednisone and RA-ILD remained significant even after additionally adjusting for disease activity (OR 4.08, 95% CI 1.23–13.6).
Table 4. Association between previously-studied risk factors and incident RA-ILD.
These data came from the 84 BRASS RA-ILD cases and 233 RA non-ILD controls.
| Characteristic | OR Conditioned on Matching Factorsa (95% CI) | Multivariableb OR (95% CI) |
|---|---|---|
| White, non-Hispanic | 0.61 (0.24–1.54) | 0.57 (0.20–1.60) |
| Joint Erosions | 0.79 (0.45–1.40) | 0.74 (0.41,1.34) |
| Rheumatoid Nodules | 2.02 (1.15–3.55) | 1.66 (0.89,3.07) |
| C-Reactive Protein, mg/L | ||
| Normal (<3) | (ref) | (ref) |
| Low-Positive (3–<10) | 1.86 (1.04–3.34) | 1.67 (0.87,3.20) |
| High-Positive (≥10) | 2.79 (1.36–5.69) | 2.61 (1.21,5.64) |
| p for trend | 0.008 | 0.021 |
| Disease Activity Score-28-CRP | ||
| Remission (<2.6) | (ref) | (ref) |
| Low (2.6–<3.2) | 0.46 (0.15–1.37) | 0.47 (0.15–1.45) |
| Moderate (3.2–<5.1) | 2.28 (1.21–4.30) | 2.13 (1.09–4.19) |
| High (≥5.1) | 4.16 (1.65–10.5) | 4.28 (1.50–12.2) |
| p for trend | <0.001 | 0.002 |
| MDHAQ Score | ||
| 0–<0.2 | (ref) | (ref) |
| 0.2<–1.0 | 1.16 (0.58–2.34) | 1.07 (0.50,2.30) |
| ≥1.0 | 3.11 (1.48–6.54) | 3.10 (1.32,7.26) |
| p for trend | <0.001 | 0.004 |
| Biologic DMARD Use | ||
| Never | (ref) | (ref) |
| Past | 1.71 (0.82–3.55) | 1.47 (0.67–3.24) |
| Current | 0.69 (0.38–1.26) | 0.52 (0.27–1.02) |
| Methotrexate Use | ||
| Never | (ref) | (ref) |
| Past | 0.51 (0.24,1.06) | 0.38 (0.16–0.86) |
| Current | 0.45 (0.22–0.91) | 0.36 (0.17–0.77) |
| Prednisone Use | ||
| Never | (ref) | (ref) |
| Past | 2.03 (0.74–5.59) | 1.44 (0.48–4.34) |
| Current | 3.94 (1.36–11.4) | 3.58 (1.13–11.3) |
All models were conditioned on matching factors (age, sex, RA duration, RF status, and time from study visit to index date). Bold values indicate statistical significance to p<0.05.
Also adjusting for anti-CCP, race/ethnicity, education, BMI, and smoking pack-years
Ability to Predict RA-ILD
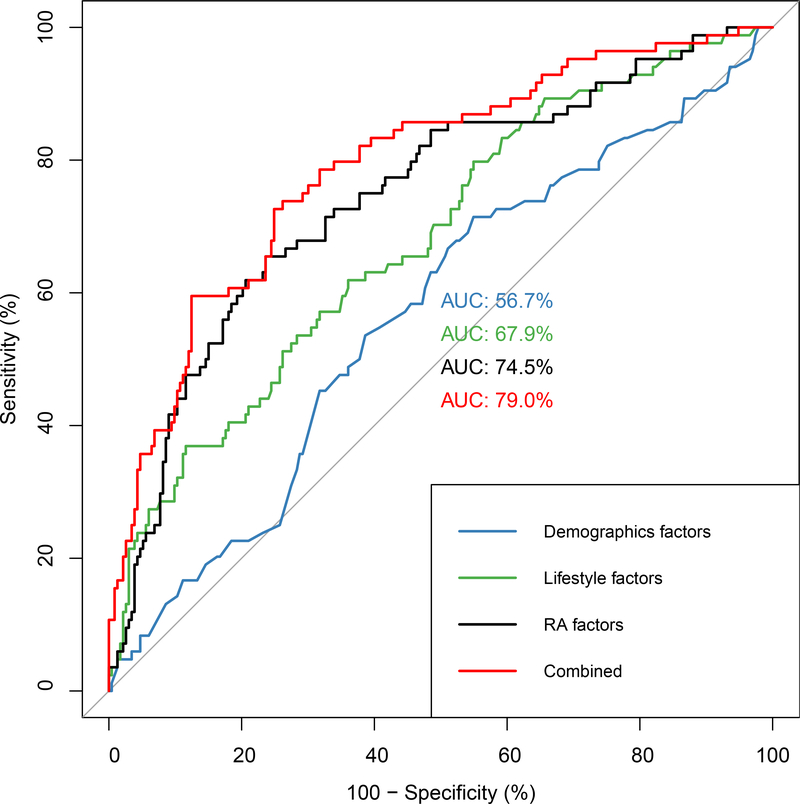
Together, these known demographic (age, race, education), lifestyle (BMI, smoking pack-years), and RA-related (RA duration, RF positivity, anti-CCP positivity, joint erosions, rheumatoid nodules, biologic DMARD use, methotrexate use, prednisone use, disease activity, functional status) risk factors explain a modest amount of an individual’s risk for RA-ILD (Figure 1). The AUC for all factors combined was 0.79 (95% CI 0.73–0.85).
Figure 1. Receiver operating characteristic curve for incident RA-ILD risk.
These curves were fit using the 84 RA-ILD cases and 233 RA non-ILD controls in BRASS using known demographic, lifestyle, and RA clinical risk factors.
DISCUSSION
This case-control study discovered several novel predictors for incident RA-ILD including obesity, higher education level, high-positive CRP, and poor functional status. We also made the new discovery that smoking beyond a threshold of 30 pack-years had a strong association with RA-ILD, while there was no evidence of an association for lower levels of smoking. Third, we confirmed existing RA-ILD risk factors such as disease activity, suggesting disease control could reduce RA-ILD risk, and prednisone, which should be used with caution in patients with RA who are at risk for RA-ILD. Finally, we demonstrated that currently-known clinical predictors of RA-ILD have only a modest ability to predict RA-ILD. These findings may shed light on the potential pathogenesis of RA-ILD, could improve patient-specific risk assessment and prevention efforts, and highlight the need for further studies on risk factors for RA-ILD.
We identified obesity and higher education as possible novel lifestyle risk factors for RA-ILD. Only one prior study reported data on the association between BMI and RA-ILD risk and found no association (29), but it had only 29 RA-ILD cases. Thus, the association between obesity and RA-ILD is a novel finding to this study. Obesity has a modest but positive association with risk of RA, supporting the biological plausibility of such an association with RA-ILD (8, 9). It is possible that the atelectasis or poorer-quality CT scans that occur in obese persons could result in misclassification of RA-ILD in either direction. Nevertheless, this discovery has important clinical implications, as obesity is now a second potentially modifiable risk factor for RA-ILD on which clinicians can counsel their patients.
The association between higher education and RA-ILD is also novel to this study. The one prior study of RA-ILD that reported data on education showed no association, though was limited by small sample size (17). This association between higher education and RA-ILD was surprising since higher socioeconomic status has been shown to have no association with ILD (36) or RA (37, 38). In fact, higher education has even been associated with a lower risk of RA (6, 7) and other autoimmune diseases like lupus (39). Thus, high medical literacy including willingness to undergo CT imaging may explain this association rather than a true biologic effect. Future studies should confirm the association between education and RA-ILD and investigate the mechanism for it, which may provide clues to the pathogenesis of RA-ILD. Conversely, this association may be explained by health literacy as a construct of social determinants of health.
We also identified high-positive CRP and poor functional status as novel clinical risk factors for RA-ILD. Two prior studies showed no association between CRP and RA-ILD in multivariable models, but only included CRP as a continuous variable instead of separating out high CRP from those with low or normal CRP (18, 19). The validity of this association is supported by the association with ESR with RA-ILD (10, 14, 16, 17). Thus, high-positive CRP may be a useful biomarker for predicting increased risk of developing RA-ILD. Similarly, we found reduced functional status (in particular, MDHAQ ≥1) was associated with increased risk of RA-ILD. This is consistent with a prior study that showed a univariate association between poor functional status and RA-ILD (10). Although two studies have shown no association between functional status and RA-ILD after adjusting for confounders, they were smaller than the current study (11, 17). Thus, this study showed high-positive CRP (≥10 mg/L) and poor functional status (MDHAQ ≥1) to be risk factors for RA-ILD that can aid clinicians in prognosticating which patients are at increased risk for RA-ILD.
Another key finding from this study is that smoking may exhibit a threshold effect in its relationship with RA-ILD. We found that smoking 30 pack-years or more was associated with a six fold increase in RA-ILD risk, whereas smoking under this threshold was not associated with increased risk of RA-ILD. Although most studies of RA-ILD risk show an association with smoking (10–13, 15, 16), some others do not (17–19). Based on our findings, one reason for this discrepancy could be that the smokers in the negative studies did not have sufficient (greater than 30 pack-year) smoking history. Alternatively, the lack of association could have resulted from lack of certain HLA-DR alleles, termed the “shared epitope,” as smoking may only increase RA-ILD risk in the presence of the shared epitope (14). Interestingly, this 30 pack-year threshold for RA-ILD differs from RA, where the relationship between smoking and RA risk increases at a threshold of 5–10 pack-years and plateaus at 20 pack-years (40). Such dissimilarity suggests that the mechanism for the association of smoking with RA differs from its association with RA-ILD. Additionally, current smokers had increased risk for RA-ILD, confirming the findings from two previous small, cross-sectional studies (11, 29). Together, these results underscore the importance of smoking cessation to prevent RA-ILD, especially in patients who have not yet reached the critical 30 pack-year threshold. Future studies of RA-ILD risk should consider stratifying by pack-years rather than ever/never smoking and investigate the pathophysiologic reasons for a threshold effect.
With its multivariable models, this study also clarified the association between several possible risk factors and RA-ILD. For example, some studies have shown a positive association for rheumatoid nodules (10, 14, 16) and disease activity (11, 14, 28), while other studies have shown no association between rheumatoid nodules (11, 17, 28) or disease activity (16–18) and risk of RA-ILD. We found that moderate to high disease activity was associated with increased risk of RA-ILD, suggesting that controlling disease activity can reduce risk of RA-ILD. We did not find an association with rheumatoid nodules, though matching on rheumatoid factor may have reduced the association. Similarly, some studies have shown an association between prednisone use and RA-ILD (14, 19, 21, 28), while others have not (11, 13, 18). In this study, even after accounting for disease activity, current prednisone use was associated with a fourfold increased risk for RA-ILD. Thus, providers should have caution in prescribing prednisone in RA or even RA-ILD patients.
The lack of association with methotrexate use, while initially surprising given the reported association between methotrexate and lung disease (41), has actually been shown repeatedly in prior studies of RA-ILD risk (11, 13, 14, 18, 19, 21, 28). In fact, three prior studies have even shown methotrexate may have a protective effect on RA-ILD risk, similar to this study (10, 16, 20). While it is possible that methotrexate may protect against RA-ILD given its therapeutic effects in RA, this decreased association might also simply represent channeling bias away from methotrexate use in patients with lung disease. Even so, given the lack of treatments for RA-ILD, further exploration of these two possibilities may be warranted.
Finally, despite discovery of these additional risk factors for RA, we found that these RA-ILD risk factors had only a modest AUC of 0.79. This finding is consistent with prior studies, whose AUCs ranged from 0.60 to 0.85 (13, 14, 29), and suggests a need to identify additional risk factors for RA-ILD. Fortunately, recent studies have shown promise for biomarkers like fine-specificity anti-citrullinated peptide antibodies (11, 42–45), matrix metalloproteinase 7 (13), surfactant protein D (13, 46), and pulmonary and activation-regulated chemokine (13), as well as genetic markers such as the HLA shared epitope (14) or MUC5B promoter variant (47). However, these biomarkers are not usually available in routine clinical care. Further studies incorporating these biologic and genetic markers are needed.
Strengths of this study include its inclusion of numerous previously studied risk factors for RA-ILD and their prospective collection. Nevertheless, there are several important limitations. First, ILD can begin insidiously before CT is obtained, so it is possible that the predictors may have resulted from RA-ILD. Even if this were true, data were collected a median of 1.5 years before clinical detection, so these factors could still identify patients in an earlier phase in the course of RA-ILD. Second, selection bias is possible since not all BRASS participants had chest CT scans available for review, since these were performed for clinical purposes. For example, some controls might have had unrecognized, subclinical ILD since chest CTs were performed on only 25% of controls, which would be expected to bias results toward the null. We mitigated this possibility by excluding controls with comorbidities that could be suggestive of ILD. Third, RA-ILD patients may be prone to differential follow-up compared to patients with RA and no ILD due to the serious nature of this condition. To mitigate any potential selection bias related to right/left censoring, our case-control study matched on time from exposure/covariate assessment to index/matched date. Fourth, matching controls on RA duration and RF positivity, while improving comparability of the groups in this study and power to detect novel associations, likely eliminated the association with anti-CCP and slightly lowered the calculated AUC since these factors were balanced between cases and controls by design. Finally, unmeasured factors exist and may be important for RA-ILD risk, such as biomarkers and genetic factors like the shared epitope and MUC5B variant.
In conclusion, we identified obesity, higher education, high-level CRP, and poor functional status as novel risk factors for RA-ILD. In addition, smoking 30 pack-years or greater was associated with a substantially increased risk of RA-ILD, whereas lower levels of smoking did not increase risk. These findings might improve our understanding of RA-ILD pathogenesis and prediction and also suggest weight loss and smoking cessation might prevent RA-ILD. However, all known clinical risk factors for RA-ILD have only a modest ability to predict RA-ILD development. Given the poor prognosis and limited available treatment options for RA-ILD, this finding emphasizes the critical need for future studies of clinical, biological, and genetic risk factors for RA-ILD.
Supplementary Material
ACKNOWLEDGEMENT
We thank Lily Martin for performing our technical review for this manuscript.
Funding: This work was supported by the Rheumatology Research Foundation K Supplement Award; National Institutes of Health [grant numbers K23 AR069688, K23 HL119558, R03 AR075886, R03 HL148484, L30 AR066953, P30 AR070253, and P30 AR072577]; and the R. Bruce and Joan M. Mickey Research Scholar Fund. The Brigham Rheumatoid Arthritis Sequential Study is funded by grants from Bristol-Myers Squibb, Crescendo Bioscience, and Sanofi. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare no conflicts of interest related to this work.
REFERENCES:
- 1.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156:528–35. [DOI] [PubMed] [Google Scholar]
- 2.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: A population-based study. Arthritis Rheum 2010;62:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Lokke A, Bendstrup E, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann Rheum Dis 2017;76:1700–6. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Cho SK, Choi CB, Choe JY, Chung WT, Hong SJ, et al. Impact of interstitial lung disease on mortality of patients with rheumatoid arthritis. Rheumatol Int 2017;37:1735–45. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L. Socioeconomic status and the risk of developing rheumatoid arthritis: Results from the swedish eira study. Ann Rheum Dis 2005;64:1588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghawi H, Crowson CS, Rand-Weaver J, Krusemark E, Gabriel SE, Juhn YJ. A novel measure of socioeconomic status using individual housing data to assess the association of ses with rheumatoid arthritis and its mortality: A population-based case-control study. BMJ Open 2015;5:e006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowson CS, Matteson EL, Davis JM 3rd, Gabriel SE. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: A systematic review and dose-response meta-analysis. Arthritis Res Ther 2015;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saag KG, Kolluri S, Koehnke RK, Georgou TA, Rachow JW, Hunninghake GW, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum 1996;39:1711–9. [DOI] [PubMed] [Google Scholar]
- 11.Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis 2014;73:1487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics--a large multicentre uk study. Rheumatology (Oxford) 2014;53:1676–82. [DOI] [PubMed] [Google Scholar]
- 13.Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med 2015;191:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Restrepo JF, del Rincon I, Battafarano DF, Haas RW, Doria M, Escalante A. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol 2015;34:1529–36. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Li H, Wu N, Dong X, Zheng Y. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 2017;36:817–23. [DOI] [PubMed] [Google Scholar]
- 16.Kiely P, Busby AD, Nikiphorou E, Sullivan K, Walsh DA, Creamer P, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the eras and eran inception cohorts. BMJ Open 2019;9:e028466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: Results from an inception cohort. Rheumatology (Oxford) 2010;49:1483–9. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Liang D, Zhao L, Li Y, Liu W, Ren Y, et al. Anti-cyclic citrullinated peptide antibody is associated with interstitial lung disease in patients with rheumatoid arthritis. PLoS One 2014;9:e92449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JX, Du CG. A retrospective study of clinical characteristics of interstitial lung disease associated with rheumatoid arthritis in chinese patients. Med Sci Monit 2015;21:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suissa S, Hudson M, Ernst P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum 2006;54:1435–9. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe F, Caplan L, Michaud K. Rheumatoid arthritis treatment and the risk of severe interstitial lung disease. Scand J Rheumatol 2007;36:172–8. [DOI] [PubMed] [Google Scholar]
- 22.Inui N, Enomoto N, Suda T, Kageyama Y, Watanabe H, Chida K. Anti-cyclic citrullinated peptide antibodies in lung diseases associated with rheumatoid arthritis. Clin Biochem 2008;41:1074–7. [DOI] [PubMed] [Google Scholar]
- 23.Sawada T, Inokuma S, Sato T, Otsuka T, Saeki Y, Takeuchi T, et al. Leflunomide-induced interstitial lung disease: Prevalence and risk factors in japanese patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1069–72. [DOI] [PubMed] [Google Scholar]
- 24.Shidara K, Hoshi D, Inoue E, Yamada T, Nakajima A, Taniguchi A, et al. Incidence of and risk factors for interstitial pneumonia in patients with rheumatoid arthritis in a large japanese observational cohort, iorra. Mod Rheumatol 2010;20:280–6. [DOI] [PubMed] [Google Scholar]
- 25.Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med 2012;106:1591–9. [DOI] [PubMed] [Google Scholar]
- 26.Rocha-Munoz AD, Ponce-Guarneros M, Gamez-Nava JI, Olivas-Flores EM, Mejia M, Juarez-Contreras P, et al. Anti-cyclic citrullinated peptide antibodies and severity of interstitial lung disease in women with rheumatoid arthritis. J Immunol Res 2015;2015:151626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurata I, Tsuboi H, Terasaki M, Shimizu M, Toko H, Honda F, et al. Effect of biological disease-modifying anti-rheumatic drugs on airway and interstitial lung disease in patients with rheumatoid arthritis. Intern Med 2019;58:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, et al. Rheumatoid arthritis disease activity predicting incident clinically-apparent ra-associated interstitial lung disease: A prospective cohort study. Arthritis Rheumatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salaffi F, Carotti M, Di Carlo M, Tardella M, Giovagnoni A. High-resolution computed tomography of the lung in patients with rheumatoid arthritis: Prevalence of interstitial lung disease involvement and determinants of abnormalities. Medicine (Baltimore) 2019;98:e17088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iannaccone CK, Lee YC, Cui J, Frits ML, Glass RJ, Plenge RM, et al. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: Data from the brigham and women’s hospital rheumatoid arthritis sequential study. Rheumatology (Oxford) 2011;50:40–6. [DOI] [PubMed] [Google Scholar]
- 31.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: Traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis 2010;69:1920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 33.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 34.Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, et al. Rheumatoid arthritis disease activity measures: American college of rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012;64:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pincus T, Keysor J, Sokka T, Krishnan E, Callahan LF. Patient questionnaires and formal education level as prospective predictors of mortality over 10 years in 97% of 1416 patients with rheumatoid arthritis from 15 united states private practices. J Rheumatol 2004;31:229–34. [PubMed] [Google Scholar]
- 36.Choi WI, Dauti S, Kim HJ, Park SH, Park JS, Lee CW. Risk factors for interstitial lung disease: A 9-year nationwide population-based study. BMC Pulm Med 2018;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bankhead C, Silman A, Barrett B, Scott D, Symmons D. Incidence of rheumatoid arthritis is not related to indicators of socioeconomic deprivation. J Rheumatol 1996;23:2039–42. [PubMed] [Google Scholar]
- 38.Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol 1999;26:47–54. [PubMed] [Google Scholar]
- 39.Karlson EW, Daltroy LH, Lew RA, Wright EA, Partridge AJ, Fossel AH, et al. The relationship of socioeconomic status, race, and modifiable risk factors to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum 1997;40:47–56. [DOI] [PubMed] [Google Scholar]
- 40.Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: A dose-response meta-analysis. Arthritis Res Ther 2014;16:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fragoulis GE, Conway R, Nikiphorou E. Methotrexate and interstitial lung disease: Controversies and questions. A narrative review of the literature. Rheumatology (Oxford) 2019;58:1900–6. [DOI] [PubMed] [Google Scholar]
- 42.Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, Oddis CV, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum 2013;65:869–79. [DOI] [PubMed] [Google Scholar]
- 43.Alunno A, Bistoni O, Pratesi F, La Paglia GMC, Puxeddu I, Migliorini P, et al. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology (Oxford) 2018;57:850–5. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Song S, Liu Y, Liu D, Lin Y, Ge S, et al. Autoreactive t cells to citrullinated hsp90 are associated with interstitial lung disease in rheumatoid arthritis. Int J Rheum Dis 2018;21:1398–405. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Liu C, Li L, Zhang F, Li Y, Zhang S. High levels of antibodies to citrullinated alpha-enolase peptide-1 (cep-1) identify erosions and interstitial lung disease (ild) in a chinese rheumatoid arthritis cohort. Clin Immunol 2019;200:10–5. [DOI] [PubMed] [Google Scholar]
- 46.White ES, Xia M, Murray S, Dyal R, Flaherty CM, Flaherty KR, et al. Plasma surfactant protein-d, matrix metalloproteinase-7, and osteopontin index distinguishes idiopathic pulmonary fibrosis from other idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2016;194:1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. Muc5b promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018;379:2209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.