Abstract
Antibody-mediated deposition of complement membrane attack complexes (MACs) on IFN-γ-primed human endothelial cells (ECs) triggers autocrine/paracrine IL-1β-mediated EC activation and IL-15 transpresentation to alloreactive effector memory T cells (TEM), changes that enable ECs to increase T cell proliferation and cytokine release. Here, we report use of single-cell microchip 32-plex proteomics to more deeply assess the functionality of the activated T cells and dependence upon EC-derived signals. Compared to control ECs, MAC-activated human ECs increase both the frequency and degree of polyfunctionality among both CD4+ and CD8+ proliferated TEM, assessed as secreted proteins. IFN-γ and TNF-α remain the predominant cytokines made by alloreactive TEM, but a few CD4+ TEM also made IL-4 while more CD8+ TEM made perforin and granzyme B. Increased polyfunctionality was attenuated by treatment of the MAC-activated ECs with anti-IL-15 blocking antibody more effectively than IL-1 receptor blockade. The increased polyfunctionality of T cells resulting from interactions with MAC-activated ECs may further link binding of donor specific antibody to T cell-mediated allograft pathologies.
Introduction
Allograft rejection is historically divided into cell-mediated and antibody-mediated categories, but these processes frequently occur in combination (mixed rejection), for example in allograft vasculopathy, and often with worse outcomes1,2. Human graft endothelial cells (ECs) are major participants in the effector phases of rejection because they alone among graft structural cells express high levels of class I and II MHC molecules that are the principal targets of donor specific antibody, and with bound peptides, the principal antigens directly recognized by alloreactive T cells3. ECs also express a range of costimulators that preferentially engage effector memory T cells (TEM), which is the circulating host T cell subset that correlates with T cell-mediated rejection. ECs can stimulate alloreactive CD8+ TEM to rapidly differentiate into cytotoxic T lymphocytes (CTL) that produce effector molecules, such as perforin, granzymes and cytokines,4–6 that kill grafts. Additionally, ECs rapidly stimulate different subpopulations of CD4+ to produce subsets of one or more cytokines that activate different effector cell types including CTLs, B cells, NK cells and macrophages, all of which may contribute to rejection7,8. T cell activation responses may be graded, depending on the strength of the antigen and costimulatory signals, so that stronger signals produce more potent effector cells that produce higher levels and more complex combinations of effector molecules.
We have reported that human alloantibodies, in the form of pooled pre-transplant broadly reactive panel reactive antibodies (PRA), bind to MHC molecules on IFN-γ-primed cultured ECs and, in a process that depends upon deposition and internalization of complement membrane attack complexes (MACs), results in transcription of pro-IL-1β, assembly of an NLRP3 inflammasome, processing and secretion of mature IL-1β. IFN-γ-priming of cultured ECs restores in situ features including expression of MHC molecules, inflammasome components, IL-15, and IL-15Rα. IL-1β then acts back on the ECs to increase their capacity to recruit and activate alloreactive CD4+ and CD8+ TEM, assessed as increased proliferation6,9. Among the IL-1β-induced effects is translocation of IL-15/IL-15Rα complexes from the nucleus, where they reside after IFN-γ-induction, to the cell surface where IL-15 can be transpresented to TEM, contributing to enhanced CD8+ but not CD4+ TEM proliferation6. These observations provide a putative mechanism through which donor-specific antibody (DSA) can augment T cells responses, contributing to mixed rejection. We have supported our in vitro observations using a human immune system mouse model of allograft vasculopathy. Although our prior analyses, which were performed on the total CD4+ and CD8+ TEM populations, showed an increase in both T cell proliferation and total cytokine production, they did not address whether individual T cells were more intensely activated. Functional heterogeneity of immune cells that may appear phenotypically similar can be uncovered by using single-cell analyses. Here, we report the use of single cell quantitative secreted proteome analysis to answer this question.
Results
PRA treatment of IFN-γ-primed human ECs under conditions permissive for complement activation significantly augmented the ability of ECs to stimulate isolated peripheral blood allogeneic CD4+ TEM and CD8+ TEM proliferation, assessed by CFSE dye dilution, and this effect of PRA was markedly reduced by pretreatment of ECs with inhibitors of IL-1 receptor (Figure 1A and 1B). In this experiment, we combined the CD4+ and CD8+ TEM, isolated at a 3:2 ratio in the co-cultures with ECs, extending our prior work in which these populations were tested separately6,9. Preventing recognition of surface IL-15/IL-15Rα with anti-IL-15 blocking antibody significantly reduced the augmentation of the CD8+ but not CD4+ TEM expansion. Furthermore, the alloantibody and complement-activated ECs had enhanced ability to induce augmented proliferation compared to normal sera and vehicle control treated ECs, measured by CFSE dye dilution (Supplemental Figure 1). These data indicate that the effect observed is due to alloantibody-mediated activation of complement on the ECs rather than antibody effects on the ECs alone or to Fc recognition.
Figure 1. Enhanced allogeneic TEM responding cells triggered by alloantibody and complement-activated ECs are quantitatively profiled for distinct cytokine secretion patterns and purified for single-cell highly multiplexed microchip-based proteomics.
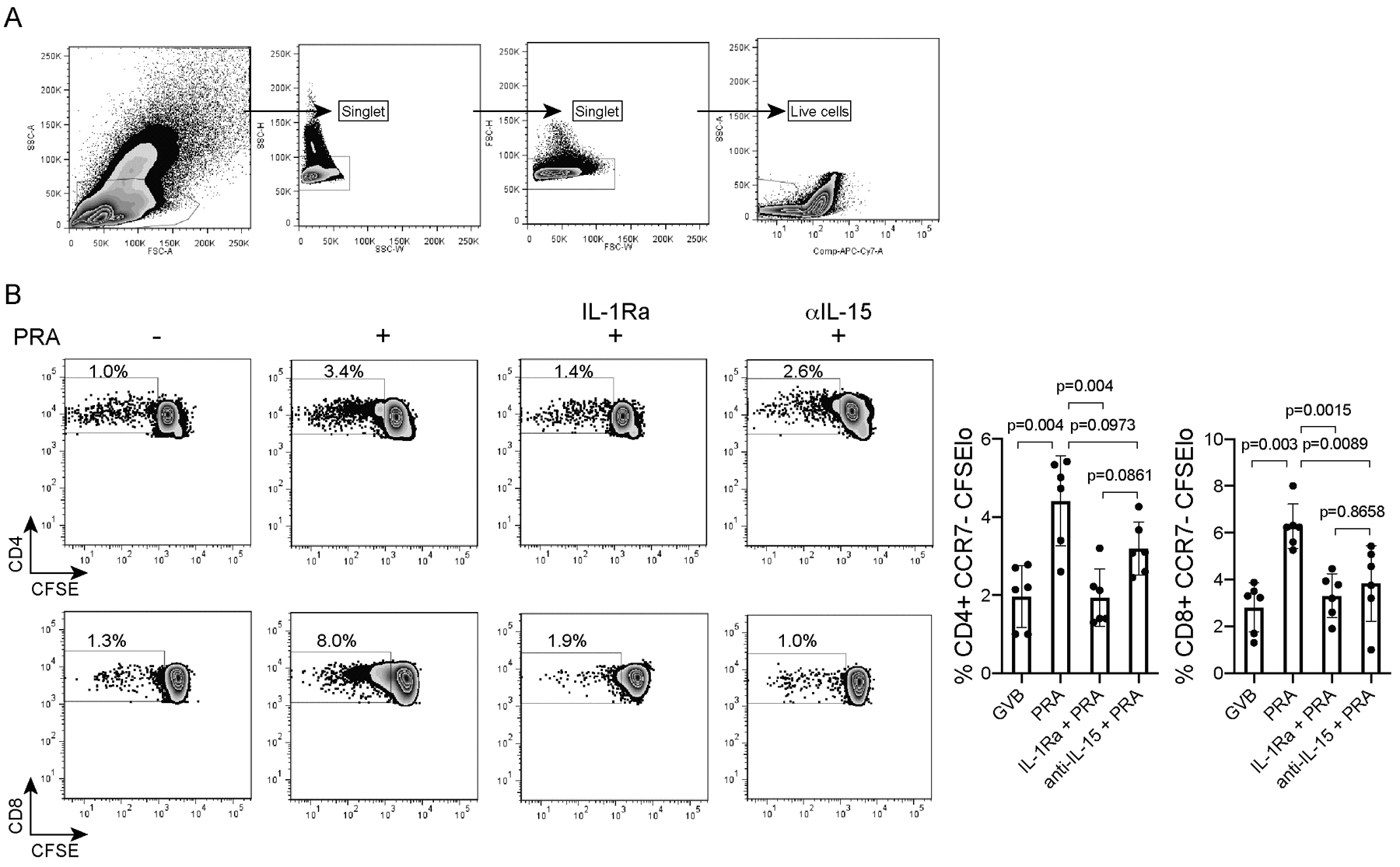
A) CFSE-labelled CD8+ and CD4+ effector memory T (TEM) cells from PBMCs of a single donor were analyzed by flow cytometry after 7-day co-culture with allogeneic HUVECs pre-treated with IL-1 Receptor antagonist (IL-1Ra), anti-IL-15 blocking antibody (αIL-15 mAb) or control prior to PRA sera or vehicle treatment for 6 hours (EC:CD4:CD8 ratio of 1:20:30). For all treatment groups, doublets were excluded and live cells were identified after viability dye staining. FACS plots show a representative individual experiment. B) Proliferation of live CD4+ and CD8+ TEM after EC:T cell co-culture was assessed by flow cytometry. CFSE dilution was assessed on day 7. The CD4+CCR7-CFSElo and CD8+CCR7-CFSElo TEM responders were sorted and CD4+ and CD8+ TEM were separated. Graphs show data from 6 independent experiments using 6 different PBMC and allogeneic HUVEC donors (mean ± SEM, 1-way ANOVA and Tukey’s multiple comparisons test). FACS plots show a representative individual experiment.
Proliferated live CD4+CCR7−CFSElo and CD8+CCR7−CFSElo TEM responders after 7 days of co-culture were purified by fluorescence-activated cell sorting (FACS) and cryopreserved prior to single cell cytokine analysis. Upon recovery from cryopreservation, TEM were stimulated briefly with anti-CD3 and anti-CD28 for 3 hours to resume the protein secretion pattern elicited by various stimulatory conditions and then subjected to single-cell 32-plex profiling using a fully validated panel of cytokines on the IsoPlexis single-cell platform10 (Figure 2A). Purified CD4+ TEM and CD8+ TEM that were not co-cultured with ECs were also profiled (“pre-coculture”) as controls. The latter populations did not produce any cytokines, likely due to the brevity of the antibody treatment used in the assay, which indicated that the proliferated populations had been altered by the culture with ECs.
Figure 2. Several distinct clusters of highly polyfunctional allogeneic CD4+ and CD8+ TEM subsets appear in response to PRA-treated ECs identified at single-cell resolution.
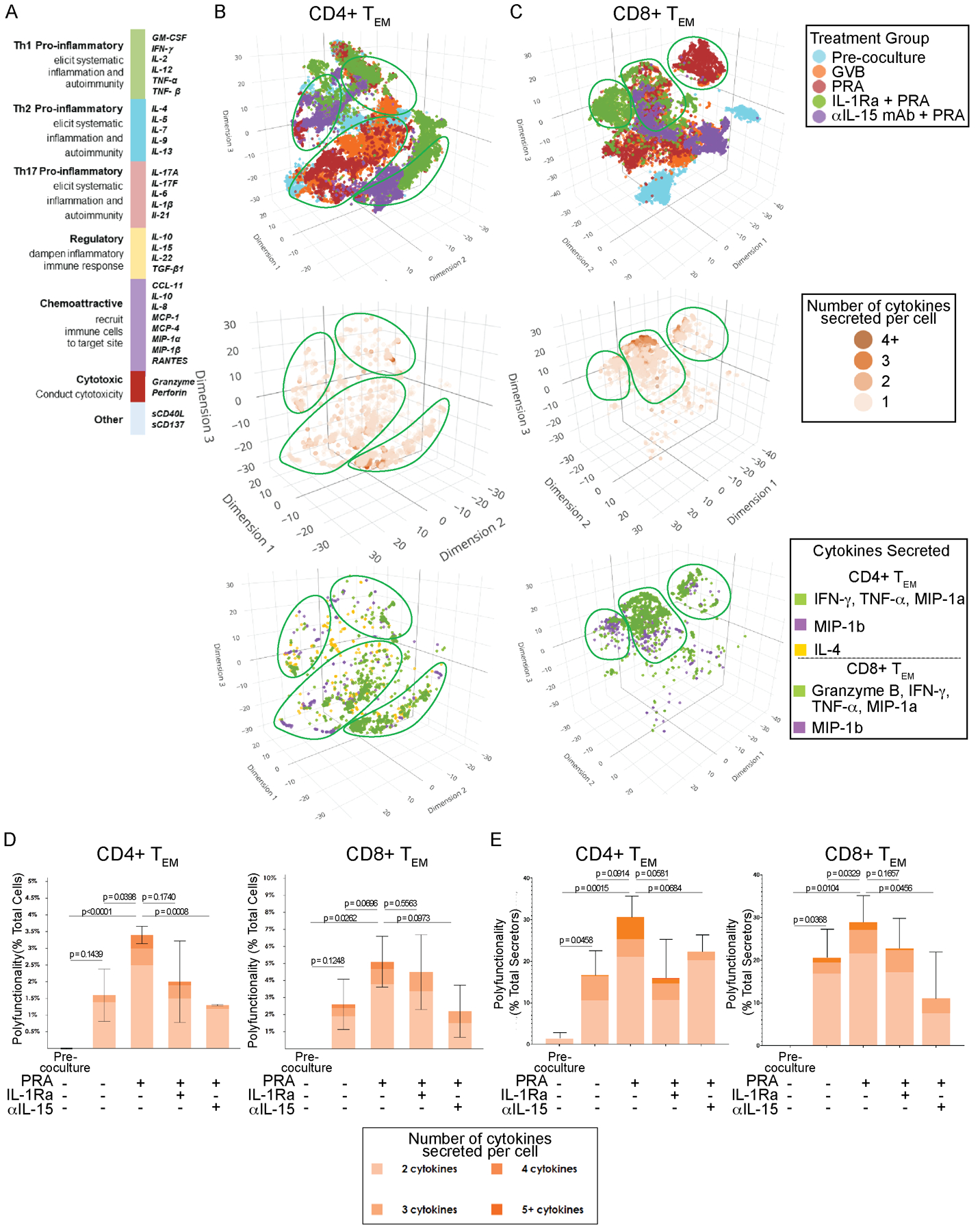
A) The cytokine secretion profiles of the proliferated allogeneic CD4+CCR7-CFSElo and CD8+CCR7-CFSElo TEM were assessed separately using IsoPlexis single-cell microchip proteomics platform. Each responding T cell was analyzed for true protein secretions across a broad validated 32-plex cytokine and chemokine panel includes immune functional groups Th1, Th2, Th17, regulatory, chemoattractive and cytotoxic. B) t-distributed stochastic neighbor embedding (t-SNE) visualization of CD4+ TEM responders activated by co-culture with allogeneic human ECs pre-treated with IL-1 Receptor antagonist (IL-1Ra), anti-IL-15 blocking antibody (αIL-15 mAb) or control prior to PRA sera or vehicle treatment. Purified CD4+ TEM that were not co-cultured with ECs were also profiled (“pre-coculture”). Polyfunctional T cell subsets, defined as secretors capable of coproducing at least two cytokines, and the cytokines secreted are identified at single cell resolution. Non-secretors were cells that did not secrete detectable levels of cytokine. Distinct clusters of highly polyfunctional T cell subsets are outlined. Data shown from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors. C) t-SNE visualization of CD8+ TEM responder polyfunctional and functional cytokine subsets. CD8+ TEM that were not co-cultured with allogeneic human ECs were also profiled (“pre-coculture”). Data shown from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors. D) The percent polyfunctionality of the total number of cells for each treatment group was calculated as the number of polyfunctional CD4+ or CD8+ TEM cells (≥2 cytokines secreted) out of the total number of cells, which also includes single-secretors and non-secretors. Graphs show data from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors (mean ± SEM, unpaired 2-tailed Student’s t-test). E) The percent polyfunctionality of the total secretors for each group was calculated as the number of polyfunctional CD4+ or CD8+ TEM cells out of the total number of secretors (≥1 cytokine secreted). t-SNE visualizations and graphs show data from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors (mean ± SEM, unpaired 2-tailed Student’s t-test).
Several distinct clusters of polyfunctional allogeneic CD4+ and CD8+ TEM subsets were generated in response to allogeneic EC activation as visualized by t-distributed stochastic neighbor embedding (t-SNE) of combined datasets from 4 PBMC and EC donors (Figure 2B and 2C). Individual T cells each represented by a dot are plotted on t-SNE based on number of cytokines secreted and the combinations of cytokines/chemokines the cell secretes. The major highly polyfunctional T cell subsets are outlined in green on each layer. The total percentage of polyfunctional single T cells of the total proliferated TEM sample regardless of the cytokine combination was computed (Figure 2D). To further understand the degree of polyfunctionality, the percentage of polyfunctional single T cells (≥2 cytokines) out of the total number of secretors (>1 cytokine) based on the number of cytokines co-secreted per cell was computed (Figure 2E). T cells stimulated by control IFN-γ-primed ECs showed low levels and degree of polyfunctionality (Figure 2D and 2E). There was significant upregulation in the number of allogeneic CD4+ and CD8+ TEM cells capable of co-secreting ≥2 cytokines (polyfunctional) and in response to PRA-activated ECs compared to control ECs (Figure 2D). While many proliferated T cells in all of the treatment groups did not appear to be secreting any detectable level of proteins (“non-secretors”) (Figure 2D), a greater percentage of T cells stimulated by PRA-activated ECs demonstrated higher degrees of polyfunctional secreting capabilities, with a significant number co-secreting 3 to 5 cytokines, compared to control, which were largely monosecretors or co-secreting only 2 to 3 cytokines (Figure 2E).
The signal intensity of cytokines secreted by every T cell above the detection threshold was plotted for each treatment group and the overall percentages of TEM cells within each group secreting protein at a strong signal intensity (>200 RFU) were computed (Figure 3A, percentages in bolded text). CD4+ and CD8+ TEM cells stimulated by PRA-activated ECs secreted proteins at a strong signal intensity (>200 RFU) at higher percentages compared to the other groups including GVB-control ECs. Furthermore, the percent of TEM strongly secreting a particular protein (>200 RFU) within each treatment group was also calculated (Figure 3A, percentages in unbolded text). By breaking down the overall signal intensity by each cytokine, we observed that individual T cells secreted more IFN-γ and TNF-α after co-culture with PRA-treated ECs compared to control ECs and that the CD4+ TEM were induced to secrete additional cytokines IL-4 and MIP-1β. These data suggest that PRA activation of ECs provide a stronger activation signal to alloreactive CD4+ and CD8+ TEM cells than do control ECs.
Figure 3. Proliferated TEM triggered by PRA-activated ECs have enhanced and distinct cytokines combinations leading to greater functional heterogeneity and polyfunctional cell subsets.
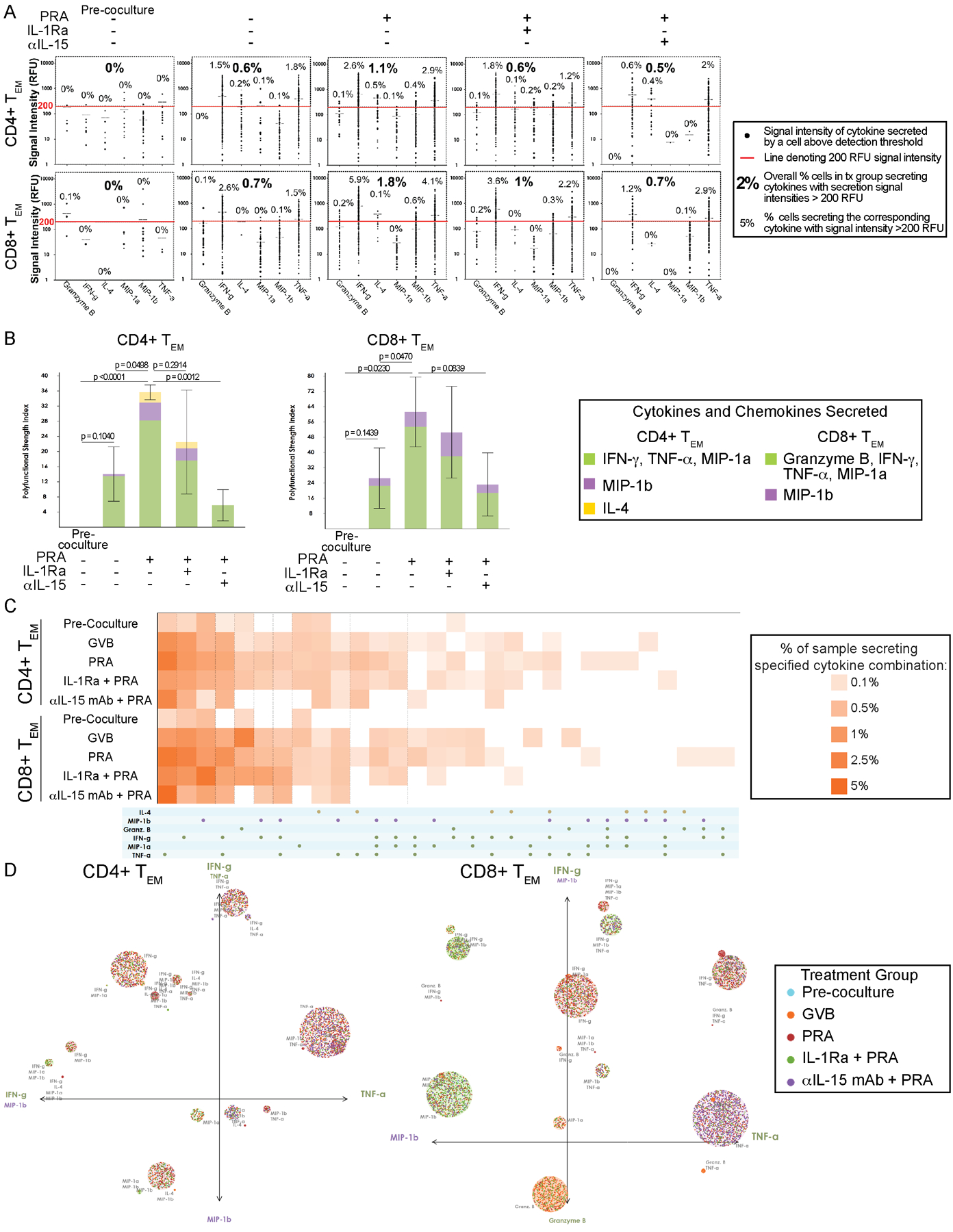
A) Single-cell cytokine signal distribution of CD4+ and CD8+ TEM activated by co-culture with allogeneic human ECs pre-treated with IL-1 Receptor antagonist (IL-1Ra), anti-IL-15 blocking antibody (αIL-15 mAb) or control prior to PRA sera or vehicle treatment. Each dot indicates the signal intensity of the cytokine that is secreted by the single T cells in the sample above the detection threshold. The overall percentage of cells with secretion signal intensities above 200 RFU is indicated for each treatment group in bolded text. For each cytokine detected, the percentage of cells secreting the corresponding cytokine at intensities greater than 200 RFU is indicated in unbolded text. Data shown from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors. B) The polyfunctional strength index (PSI), defined as the percentage of polyfunctional T cells (≥2 cytokines secreted) in the sample multiplied by the mean fluorescence signal intensity of the proteins secreted by those cells, and each cytokines’ contribution to the PSI are visualized. Graphs show data from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors (mean ± SEM, unpaired 2-tailed Student’s t-test, SEM). C) The various combinatorial cytokine secretions and corresponding percentage of CD4+ or CD8+ TEM responders in each the sample secreting the specified cytokine combinations are visualized by polyfunctional heatmap. Data shown from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors. D) The polyfunctional subsets of the responder CD4+ and CD8+ TEM driving are visualized by single-cell polyfunctional activation topology (PAT)- principal component analysis (PCA) plots. Each dot represents a single cell and are functionally clustered based on the cytokine secretion combination. The radius of each cluster is proportional to the secretion frequency of the corresponding group. The color of each cluster is weighted to the dominance of the T cells from a treatment group. The principal components are labeled according to their correlation with specific cytokines. Data shown from 4 independent experiments using 4 different PBMC and 4 allogeneic HUVEC donors.
We further analyzed the contributions of each secreted cytokine to the overall polyfunctionality profile by calculating the polyfunctional strength index (PSI) metric (Figure 3B). PSI is defined as the percentage of polyfunctional cells (co-secreting ≥2 proteins per cell) out of the total cells multiplied by the mean fluorescence intensity of the protein secreted by these polyfunctional cells. PSI is a single-cell proteomic metric that allows identification of unobserved deep functional heterogeneity of immune cells. It has recently been used to analyzed tumor-infiltrating CD8+ T cells to distinguish non-responders from responders of immune-checkpoint inhibitor therapy in addition to being used to analyzed pre-infusion anti-CD19 CAR-T cells to predicting clinical response and toxicities as well as cell product characterization for optimal manufacturing development11,12. Using PSI analysis, we observed that the dominant cytokines driving the polyfunctional strength for both CD4+ and CD8+ TEM were IFN-γ and TNF-α, characteristic of a type 1 response (Figure 3B). IL-4 and MIP-1β additionally contributed to the polyfunctionality of CD4+ TEM stimulated by PRA-activated ECs. Granzyme B, IFN-γ, TNF-α and MIP-1α were the dominant cytokines contributing to the increased polyfunctionality of CD8+ TEM activated by PRA-treated ECs. These data show that while the proliferated T cells are largely secreting combinations of type 1 cytokines (IFN-γ, TNF-α), additional cytokine IL-4 is observed to be secreted and contributing more to the polyfunctionality of the CD4+ T cells induced by the PRA-activated human ECs.
We next analyzed the roles of EC-derived IL-1 and IL-15 on the effects of T cell polyfunctionality. IL-1Ra pretreatment inhibited the ability of PRA-activated ECs to augment the CD4+ and CD8+ TEM proliferation response, as shown in Figure 1B, but had less effect than anti-IL-15 on the PRA-enhanced number of polyfunctional T responder cells and their degree of polyfunctionality (Figure 2D and 2E). This was initially surprising as IL-1Ra blocked translocation of IL-15/IL-15Rα complexes to the cell surface of isolated human ECs treated with PRA in culture6. Although activated T cells are not a major source of IL-1, they can produce TNF, which can replace IL-1 by acting on ECs, triggering NF-κB activation and subsequently IL-15/IL-15Rα translocation to the EC surface6. To determine if TNF was present in the EC:T cell co-cultures, we performed ELISA assays and readily detected TNF-α after 24 hours of co-culture, especially in the PRA-activated EC group compared to control and normal sera treated (Supplemental Figure 2). Blocking the recognition of surface IL-15 with anti-IL-15 blocking antibody inhibited the ability of PRA-activated ECs to enhance proliferation of CD8+ TEM, as shown in Figure 1B, and also inhibited the upregulation of the total number of polyfunctional cells (Figure 2D) and number of highly polyfunctional CD8+ TEM with a lesser effect on CD4+ TEM (Figure 2E). These data indicate that MAC-activated ECs leads to enhanced stimulation of highly polyfunctional TEM cells that is partly mediated by IL-15 transpresentation.
To better understand the alteration of specific polyfunctional subpopulations, we visualized cytokine production from all single cells across the four donors using a polyfunctional heatmap and polyfunctional activated topology principal component analysis (PAT PCA) analysis. In order to visualize the combinatorial differences rather than the intensity differences, the polyfunctional heatmap was used to display the cytokine combinations of polyfunctional cell subsets based on frequency (Figure 3C). CD4+ TEM and CD8+ TEM responders that were activated by PRA-treated ECs had the highest frequencies of most expressed cytokines (IFN-γ, TNF-α) and also polyfunctional subsets that co-produced combinations of cytokines. In particular, subsets secreting combinations including IL-4 for CD4+ TEM in the PRA group contributed to the enhanced polyfunctionality. To further visualize the distinct polyfunctional subsets within the activated TEM, we used PAT PCA visualizations that display each T cell, as a dot, within a group corresponding to the cytokines secreted (Figure 3D). The size of the group corresponds to the secretion frequency and the color is weighted to the dominance of proliferated T cells from a particular treatment group. As shown in Figure 3D, the majority of the groups are weighted to the T cell stimulated by PRA-activated ECs and the highly polyfunctional subsets, located farther along each axis, are dominated by T cells from the PRA-activated EC group. In addition, IL-4 was a major contributor to the enhanced number of polyfunctional subsets comprising the CD4+ TEM stimulated by PRA-activated ECs. IL-15 blocking antibody pretreatment was more effective than IL-1Ra pretreatment in markedly reducing the cytokine secretions, intensity and polyfunctional cytokine combinations in both CD4+ and CD8+ TEM induced by PRA-treated ECs. Overall, our data show that polyfunctional T cell subsets with distinct combinatorial cytokine secretions are enhanced in both CD4+ and CD8+ TEM co-cultured with PRA-activated ECs compared to control ECs, and that these responses are in part mediated by EC activation and transpresentation of IL-15.
Discussion
The findings reported here are part of our ongoing efforts to study how alloantibody and complement-mediated activation of graft ECs, seen in mixed rejection, enhances their immunogenicity and ability to induce an augmented T cell alloresponse. This co-incidence of antibody and T-cell mediated rejection is a major feature of allograft vasculopathy and late graft loss. Our principal new findings based on single-cell highly-multiplexed secreted proteomic profiling and the new alternative visualizations of the TEM cells activated by MAC-activated ECs revealed not only more a profound degree of activation but also enhanced heterogeneity of secretion combinations resulting in more highly polyfunctional subsets capable of co-secreting multiple cytokines. The increased breadth of response by the CD4+ TEM to include IL-4 in addition to Th1 cytokines (IFN-γ, TNF-α) supports the concept that CD4+ T cells exhibit a greater degree of plasticity compared to CD8+ T cells, even among circulating TEM subsets. While CD8+ TEM exhibited higher levels of activation, assessed by cytokine intensity, in addition to polyfunctionality, the identity of the cytokine profiles that contributed to the polyfunctional strength were largely unchanged. Highly polyfunctional CD4+ TEM may have increased ability to provide help to various effector populations such as CD8+ T cells, B cells, and innate effector cells, e.g. NK cells and macrophages.
Pre-treatment with anti-IL-15 blocking antibody diminished the degree of polyfunctionality and number of polyfunctional CD8+ TEM, in accordance with the effect of this pathway on CD8+ TEM proliferation. IL-1 receptor blockade inhibited the augmented TEM proliferation response, as expected, and appeared to have a lesser effect on polyfunctionality than proliferation. While our prior work showed that PRA-activation of ECs led to secreted IL-1, which activates NF-κB and induces translocation of IL-15/IL-15Rα to the EC surface, those experiments did not include TEM in the cultures. Activated T cells secreted TNF-α, which similarly to IL-1, can activate NF-κB and induces translocation of IL-15/ IL-15Rα complexes to the EC surface6. We detected low levels of TNF-α that appeared to be increased in the PRA-treated EC group compared to control group within 24 hours of EC:T cell co-culture (Supplemental Figure 2). This experiment provides one explanation for how T cells could bypass the effect of IL-1 signaling, but others, such as T cell CD154 engaging CD40 on ECs could also account for this. Regardless of the actual mechanism, the presence of T cells can replace the autocrine/paracrine IL-1 signaling we observed in isolated EC cultures allowing IL-15/IL-15Rα complexes to translocate to the EC plasma membrane so that transpresentation to T cells can occur. Furthermore, IL-15 signaling also surprisingly had an effect on the total number of CD4+ TEM polyfunctional cells despite the absence of an effect on the magnitude of the proliferative response. This could be a direct effect since CD4+ TEM express IL-2Rβ and common γ chain (γc) that form the receptor engaged by transpresented IL-15/IL-15Rα. Another possible explanation is an indirect effect due to reduced activation of CD8+ TEM on CD4+ TEM through an as of yet unknown mechanism. Thus, blocking IL-15 recognition on the surface of PRA/MAC-activated ECs appears to be the more effective strategy in inhibiting the increased proliferation and polyfunctional upregulation of alloreactive TEM response.
The single cell resolution of our studies revealed that a large number of the proliferated T cells did not actively secrete detectable levels of cytokines. These results bring into question the role and function of these proliferated T cells. Our findings are consistent with several possible explanations. These cells may have transiently expressed cytokine but have become silenced i.e. exhausted, or they may represent proliferated cytokine-driven bystanders. Regardless, these data suggest that increased proliferation does not simply correlate with increased cytokine expression.
In summary, our single-cell highly-multiplexed functional proteomics assessment of the allogeneic TEM allows identification of potential altered functions and heterogeneous polyfunctional responder subsets triggered by complement and alloantibody-activated ECs that may be key drivers of alloimmune rejection. Studies in cancer and other diseases have demonstrated that T cells capable of co-producing multiple cytokines/chemokines are the key effector cells in disease pathogenesis13–16. Other single cell expression approaches, such as single cell RNA-seq, provide insight into gene expression at the transcriptional level. However, transcription may not result in protein synthesis or secretion and single cell functional proteomics is required to determine the true protein secretory pattern from live cells, identify highly polyfunctional subsets of effector cells with unique cytokine signatures, and subsequently gain deeper insights into cellular functionality and kinetics that can be correlated to the in vivo setting. Our findings that augmented polyfunctionality in TEM cells resulting from interactions with MAC-activated ECs deepens our understanding of how alloantibody and complement-mediated enhancement of EC immunogenicity affects the alloimmune T cell response, mechanistically linking alloantibody to T-cell mediated allograft rejection.
Materials and Methods
Detailed experimental procedures are described in the Materials and Methods section in the Online Supporting Information. All protocols involving collection of and experimentation with human cells and tissues were approved by the Yale University Institutional Review Board.
Cell Culture, Reagents and Culture Treatments
Human EC responses to complement were elicited using discarded and pooled preparations of high titer PRA sera obtained from the Yale HLA tissue typing laboratory to treat serially passaged human umbilical vein ECs (HUVEC) cultures, as previously described6. HUVECs from a donor allogeneic to the PBMCs were pre-treated with human IFN-γ for 48 hours prior to treatment with complement permissive gelatin veronal buffer (GVB), 25% PRA in GVB, IL-1 receptor antagonist (IL-1Ra) + 25% PRA/GVB or anti-IL-15 blocking antibody + 25% PRA/GVB. Peripheral blood human CD8+CCR7−HLA-DR− and CD4+CCR7−HLA-DR− TEM lymphocytes were pre-labeled with CFSE dye and co-cultured with allogeneic HUVECs for 7 days (1:20:30 EC:CD4:CD8 ratio). T cell proliferation was assayed by CFSE dye dilution. CD4+CCR7−CFSElo and CD8+CCR7−CFSElo TEM were sorted by FACS after 7 days and cryopreserved prior to single cell 32-plex proteomics analysis.
Statistics
Data are expressed as mean ± SEM. Statistical analyses were performed using GraphPad Prism software. As indicated in figure legends, unpaired t tests were used to make statistical comparisons between two groups. A P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Gwedolyn Davis-Arrington for HUVEC isolation. This work was supported by NIH R01-HL051014 and U01-AI132895 to J.S.P.; NIH F30-AI138473–01A1 and NIH MSTP T32-GM136651 to C.B.X.
Abbreviations:
- CTL
cytotoxic T lymphocyte
- DSA
donor-specific antibody
- EC
endothelial cell
- FACS
fluorescence-activated cell sorting
- GVB
gelatin veronal buffer
- HUVEC
human umbilical vein endothelial cell
- IL-1Ra
IL-1 receptor antagonist
- MAC
membrane attack complex
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- PAT PCA
polyfunctional activated topology principal component analysis
- PRA
panel reactive antibodies
- PSI
polyfunctional strength index
- TEM
effector memory T cells
- Th cell
T helper cell
- t-SNE
t-distributed stochastic neighbor embedding
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. J.Z. is employed by and has equity ownership in IsoPlexis. S.M. is cofounder of, has equity ownership in, and holds patents with IsoPlexis. The other authors have no conflicts of interest to disclose.
Data availability statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Clerkin KJ, Restaino SW, Zorn E, Vasilescu ER, Marboe CC, Mancini DM. The effect of timing and graft dysfunction on survival and cardiac allograft vasculopathy in antibody-mediated rejection. J Heart Lung Transplant. 2016;35(9):1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kransdorf E, Kittleson M, Patel J, et al. Mixed Rejection: More Important Than Thought after Heart Transplantation. The Journal of Heart and Lung Transplantation. 2019;38(4, Supplement):S388. [Google Scholar]
- 3.Carman CV, Martinelli R. T Lymphocyte–Endothelial Interactions: Emerging Understanding of Trafficking and Antigen-Specific Immunity. Frontiers in Immunology. 2015;6(603). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dengler TJ, Pober JS. Human vascular endothelial cells stimulate memory but not naive CD8+ T cells to differentiate into CTL retaining an early activation phenotype. J Immunol. 2000;164(10):5146–5155. [DOI] [PubMed] [Google Scholar]
- 5.Biedermann BC, Pober JS. Human vascular endothelial cells favor clonal expansion of unusual alloreactive CTL. J Immunol. 1999;162(12):7022–7030. [PubMed] [Google Scholar]
- 6.Xie CB, Jiang B, Qin L, et al. Complement-activated interferon-γ-primed human endothelium transpresents interleukin-15 to CD8+ T cells. J Clin Invest. 2020;130(7):3437–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merola J, Jane-Wit DD, Pober JS. Recent advances in allograft vasculopathy. Curr Opin Organ Transplant. 2017;22(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175(8):4886–4896. [DOI] [PubMed] [Google Scholar]
- 9.Xie CB, Qin L, Li G, et al. Complement Membrane Attack Complexes Assemble NLRP3 Inflammasomes Triggering IL-1 Activation of IFN-gamma-Primed Human Endothelium. Circ Res. 2019;124(12):1747–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Paczkowski P, Mackay S, Ng C, Zhou J. Single-Cell Multiplexed Proteomics on the IsoLight Resolves Cellular Functional Heterogeneity to Reveal Clinical Responses of Cancer Patients to Immunotherapies. Methods Mol Biol. 2020;2055:413–431. [DOI] [PubMed] [Google Scholar]
- 11.Rossi J, Paczkowski P, Shen Y-W, et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 2018;132(8):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue Q, Bettini E, Paczkowski P, et al. Single-cell multiplexed cytokine profiling of CD19 CAR-T cells reveals a diverse landscape of polyfunctional antigen-specific response. Journal for ImmunoTherapy of Cancer. 2017;5(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8(4):247–258. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baitsch L, Baumgaertner P, Devêvre E, et al. Exhaustion of tumor-specific CD8⁺ T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. Journal of Experimental Medicine. 2007;204(10):2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


