Abstract
Background and aims:
The optimal time interval for diagnostic colonoscopy completion after an abnormal stool-based colorectal cancer (CRC) screening test is uncertain. We examined the association between time to colonoscopy and CRC outcomes among individuals who underwent diagnostic colonoscopy after abnormal stool-based screening.
Methods:
We performed a retrospective cohort study of Veterans age 50–75 years with an abnormal fecal occult blood test (FOBT) or fecal immunochemical test (FIT) between 1999 and 2010. We used multivariable Cox proportional hazards to generate CRC-specific incidence and mortality hazard ratios (HRs) and 95% confidence intervals (CI) for 3-month colonoscopy intervals, with 1–3 months as the reference group. Association of time to colonoscopy with late-stage CRC diagnosis was also examined.
Results:
Our cohort included 204,733 patients. Mean age was 61 years (SD: 6.9). Compared to patients who received a colonoscopy at 1–3 months, there was an increased CRC risk for patients who received a colonoscopy at: 13–15 months (HR=1.13, 95%CI:1.00–1.27), 16–18 months (HR=1.25, 95%CI:1.10–1.43), 19–21 months (HR=1.28, 95%CI:1.11–1.48), and 22–24 months (HR=1.26, 95%CI:1.07–1.47). Compared to patients who received a colonoscopy at 1–3 months, mortality risk was higher in groups who received a colonoscopy at: 19–21 months (HR=1.52, 95%CI:1.51–1.99) and 22–44 (HR=1.39, 95%CI:1.03–1.88). Odds for late stage CRC increased at 16 months.
Conclusions:
Increased time to colonoscopy is associated with higher risk of CRC incidence, death, and late stage CRC after abnormal FIT/FOBT. Interventions to improve CRC outcomes should emphasize diagnostic follow-up within 1 year of an abnormal FIT/FOBT result.
Keywords: prevention, Veterans Affairs, quality
LAY SUMMARY
Increased time to colonoscopy is associated with higher risk of colorectal cancer (CRC) diagnosis, CRC-related death, and advanced stage CRC after abnormal FIT/FOBT.
Graphical Abstract
INTRODUCTION
Colorectal cancer (CRC) is the second most common cause of cancer-related mortality among men and women in the United States (US), and there is strong evidence that screening reduces both incidence and CRC-related mortality.1, 2 The United States Preventive Services Task Force (USPSTF) recommends several screening modalities for CRC prevention and early detection, including the fecal immunochemical test (FIT) and fecal occult blood test (FOBT).2 Both tests are common non-invasive studies that, when abnormal (i.e. positive), require follow-up with diagnostic colonoscopy to evaluate for precancerous and cancerous colorectal lesions.3–7
Consequences of delays in colonoscopy completion after abnormal stool test results have not been widely studied. While poor outcomes such as late stage at presentation and increased mortality have been reported with failure to complete colonoscopy, influence of time to colonoscopy on these outcomes has not been well established.8–10 Existing data examining time to colonoscopy and clinical risk come from international settings or regional US cohorts and support a wide range of follow-up intervals (6 to 24 months).8, 9, 11–18 As a result, there are no national standards or federal quality mandates to guide patients, providers, or healthcare systems on the clinically acceptable period of time in which a diagnostic colonoscopy should be performed after an abnormal FIT/FOBT.7
As FIT use increases, and especially in the setting of the ongoing global coronavirus disease 2019 (COVID-19) pandemic that has necessitated greater reliance on non-invasive CRC screening modalities,19, 20 it is critical to further define best practice for optimal time to colonoscopic follow-up after abnormal results. Traditional barriers to follow-up including patient-factors (e.g., hesitancy about colonoscopy), provider roles (e.g., knowledge of results, referral for colonoscopy), and system-level factors (e.g., FIT tracking, provider reminders), are now compounded by competing priorities during the prolonged pandemic.21, 22 Thus, we conducted a national retrospective cohort study of US patients with abnormal FIT/FOBT results to examine the association between time to colonoscopy, CRC incidence, CRC stage at presentation, and CRC mortality. Findings will inform national standards for appropriate timing of colonoscopy after abnormal FIT/FOBT screening and interventions to improve CRC outcomes.
METHODS
Study Population and Data Collection
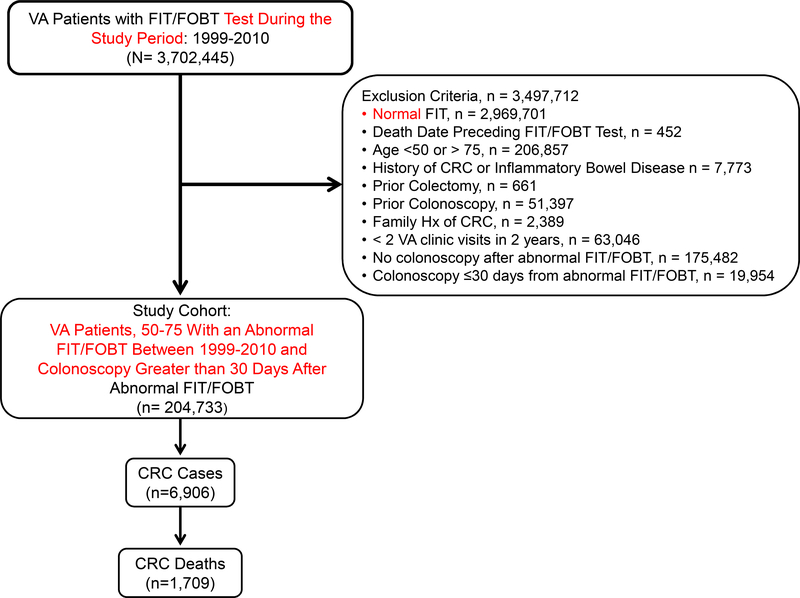
The Veterans Health Administration of the Department of Veterans Affairs (VA) is one of the largest integrated healthcare systems in the US and provides care for over 9 million Veterans annually through >1,200 healthcare facilities.23 The VA Corporate Data Warehouse (CDW) was created in 1999 and is the central data warehouse for VA electronic health records (EHR). The CDW houses patient demographic data, claims-based diagnoses, procedure codes, prescriptions, anthropometric measures, encounter and procedure notes, laboratory results, and pathology reports. We accessed CDW data to include patients that: (1) had an abnormal FIT or FOBT (FIT/FOBT) result between October 1, 1999 and December 31, 2010, (2) had no prior history of CRC or inflammatory bowel disease (Crohn’s or ulcerative colitis), and (3) were ages 50 to 75 at the time of abnormal FIT/FOBT. We excluded patients with: (1) a history of colonoscopy prior to the abnormal FIT/FOBT, (2) a history of colectomy prior to abnormal FIT/FOBT, (3) fewer than 2 VA primary care visits in the past 2 years, (4) no colonoscopy after abnormal FIT/FOBT, and/or (5) colonoscopy within 30 days of abnormal FIT/FOBT. We removed patients with an abnormal FIT/FOBT result who had a colonoscopy within 30 days of the abnormal FIT result to exclude the likely more severely symptomatic patients for whom FIT may have been ordered for diagnostic purposes, estimating that it takes approximately 30 days for patients to receive a diagnostic colonoscopy in the VA. Thus, the final analytic cohort consisted of individuals age 50 to 75 with an abnormal FIT/FOBT between 1999 and 2010 who completed a subsequent diagnostic colonoscopy greater than 30 days after the abnormal FIT/FOBT, and no prior history of CRC, inflammatory bowel disease, or colonoscopy prior to an abnormal stool blood test result. The study was approved by the VA Institutional Review board and Research and Development Committee at the San Diego Veterans Affairs Healthcare System.
Outcomes
We examined three primary outcomes: incident CRC, fatal CRC, and stage of CRC at presentation between October 1, 1999 and December 31, 2015. We identified incident CRC using the Oncology Domain database in the CDW, which contains cancer diagnoses from local VA cancer data abstractions24 and from patients who died from CRC-specific mortality. We ascertained fatal CRC from National Death Index cause-specific mortality data. Late stage CRC was defined as patients with CRC stages III and IV according to the American Joint Committee on Cancer staging system.
Exposure
Our primary exposure (predictor) variable was time to colonoscopy after abnormal FIT/FOBT. We identified colonoscopy uptake from Current Procedural Terminology (CPT) codes (Supplement Table 1). Our continuous exposure variable was defined as days to colonoscopy after abnormal FIT/FOBT. Subsequently, we categorized the variable into 3-month intervals following prior convention: 1–3 months, 4–6 months, 7–9 months, 10–12 months, 13–15 months, 16–18 months, 19–21 months, 22–24 months, and greater than 24 months, where 1 month equates to 30 days.8
Covariates
We ascertained demographic and clinical factors from the CDW. Demographic data included age at the time of the abnormal FIT/FOBT, sex, race, and ethnicity. We defined race and ethnicity as one variable with 6 mutually exclusive categories: non-Hispanic White (NHW), non-Hispanic Black (Black), Hispanic, Asian, American Indian or Alaska Native, and other (Native Hawaiian or other Pacific Islander, multiracial, and those that designated “other” race). Clinical variables were tobacco use, body mass index (BMI, kg/m2) and Charlson Comorbidity Index (CCI) at the time of abnormal FIT/FOBT (or within one year prior to the abnormal FIT for CCI). Tobacco use status was classified into never, former, current, and unknown categories as previously described.25 We used previously developed criteria to remove biologically implausible values for height and weight and to calculate BMI.26 We used the Deyo modification of the CCI for each patient, in which higher scores reflect greater disease burden and likelihood of death. 27 CCI was classified into 4 categories: 0, 1, 2, and ≥3.27
Statistical Analyses
We used descriptive statistics to describe patient demographic and clinical factors for the study cohort and characterize CRC outcome rates by time to colonoscopy. For the analysis examining the relationship between time to colonoscopy and incident and fatal CRC, we used multivariable Cox proportional hazards models to generate CRC-specific hazard ratios (HR) and 95% confidence intervals (CI) to measure the association. Our incident CRC analysis followed patients through CRC diagnosis, CRC death, other death, or the end of the study window (December 31, 2015). For mortality analyses, we followed patients to CRC death, other death, or the end of the study window. For stage at presentation, we performed a multivariable logistic regression to generate CRC-specific odds ratios (OR) and CI to measure the association between time to colonoscopy and late stage CRC diagnosis, compared to those without late stage CRC diagnosis. All multivariable models were adjusted for age at time of FIT/FOBT, sex, race/ethnicity, tobacco use, BMI (continuous), and CCI. We used colonoscopy completion within 1–3 months as our reference group, consistent with prior studies.28, 29
Statistical tests were 2-sided, and a P value less than 0.05 was considered statistically significant. We used Schoenfeld residuals to test the proportional hazard assumption.30 For these and all subsequently described analyses, HR or OR with CI not crossing unity were considered statistically significant. All analyses were performed with SAS software 9.4 (SAS Institute).
RESULTS
Descriptive characteristics of analytic cohort
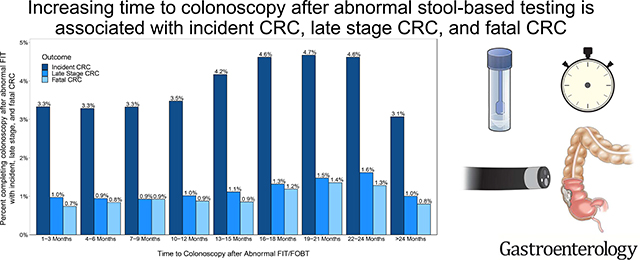
Our final analytic cohort included 204,733 Veterans with an abnormal FIT or FOBT result (Figure 1). In all, 6,906 were diagnosed with CRC and 1,709 died of CRC. The median study follow-up time was 9.2 years with an interquartile range (IQR) of 5.3 years. Mean age was 61.3 years (s.d. ± 6.9), 97% were male, 66% were NHW, and 19% were NHB. Tobacco use prevalence was 27%, and 44% of patients had a CCI score of 0. Mean BMI of the study cohort was 30.1 kg/m2 (s.d. ± 5.6) (Table 1). Rates of incident, late stage, and fatal CRC increased with time to colonoscopy after abnormal FIT/FOBT (Supplement Figure 1).
Figure 1:
Flowchart of inclusion and exclusion criteria for study cohort, 1999–2015
Table 1:
Demographic and clinical characteristics of study population
| Patient characteristic | Total n= 204,733 |
|---|---|
| Age, years (mean ± s.d.) | 61.3 ± 6.9 |
| Sex (n (%)) | |
| Female | 5453 (2.7) |
| Male | 199280 (97.3) |
| Race/ethnicity (n (%)) | |
| Non-Hispanic White | 135163 (66.0) |
| Non-Hispanic Black | 39111 (19.1) |
| Hispanic | 8112 (4.0) |
| Asian | 2055 (1.0) |
| American Indian | 1084 (0.5) |
| Multiracial/Other | 3256 (1.6) |
| Unknown | 15952 (7.8) |
| Smoking (n (%)) | |
| Never | 47326 (23.1) |
| Former | 41293 (20.2) |
| Current | 55382 (27.1) |
| Unknown | 60732 (29.7) |
| BMI, kg/m2 (mean ± s.d.) | 30.1 ± 5.6 |
| BMI Categorical (n (%)) | |
| Underweight | 1360 (0.7) |
| Normal | 31131 (15.2) |
| Overweight | 68381 (33.4) |
| Obese | 85093 (41.6) |
| Unknown | 18768 (9.2) |
| Charlson Comorbidity Index (n (%)) | |
| 0 | 89237 (43.6) |
| 1 | 59421 (29.0) |
| 2 | 25482 (12.5) |
| 3+ | 30358 (14.8) |
| Unknown | 235 (0.1) |
Association between time to colonoscopy and CRC incidence
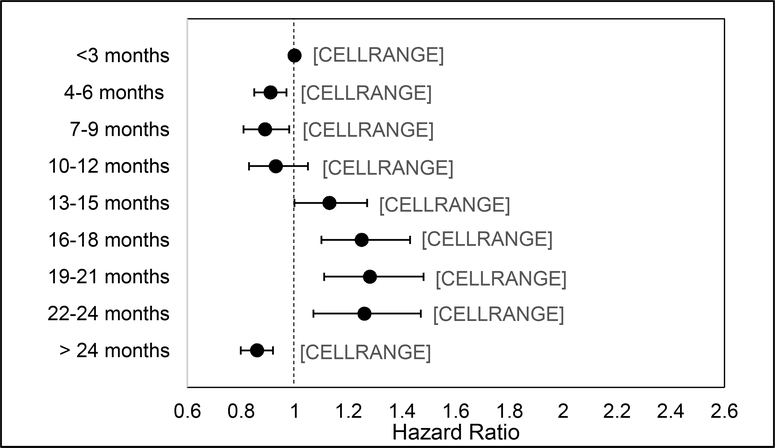
Table 2 provides results of the multivariable-adjusted model for the association between time to colonoscopy and CRC incidence. Overall, the risk for incident CRC increased with time to colonoscopy. Compared to patients who received a colonoscopy at 1–3 months, risk increased at 13–15 months (HR=1.13, 95%CI:1.00–1.27), 16–18 months (HR=1.25, 95%CI:1.10–1.43), 19–21 months (HR=1.28, 95%CI:1.11–1.48), and 22–24 months (HR=1.26, 95%CI:1.07–1.47). Risk of incident CRC was decreased for colonoscopy completion at 4–6 months (HR=0.91, 95%CI: 0.85–0.97), 7–9 months (HR=0.89, 95%CI: 0.81–0.98), and > 24 months (HR=0.86, 95%CI: 0.80–0.92). There was no difference in risk for patients with a colonoscopy at 10–12 months. (Figure 2).
Table 2:
Association between time to colonoscopy and risk of incident and fatal CRC among Veterans with an abnormal FIT/FOBT, represented as hazard ratios and 95% confidence intervals; 1999–2015 (n=204,733).
| Variable | No. of Patients | No. cases due to CRC | No. fatal CRC cases | Incident CRC Unadjusted HR (95% CI) | Incident CRC Adjusted HR (95% CI) | Fatal CRC Unadjusted HR (95% CI) | Fatal CRC Adjusted HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Time to Colonoscopy after abnormal FIT/FOBT | |||||||
| 1–3 months | 61,613 | 2050 | 454 | REF | REF | REF | REF |
| 4–6 months | 39,948 | 1312 | 335 | 0.93 (0.87–1.00) | 0.91 (0.85–0.97) | 1.00 (0.87–1.15) | 0.95 (0.83–1.10) |
| 7–9 months | 18,217 | 605 | 168 | 0.92 (0.84–1.01) | 0.89 (0.81–0.98) | 1.05 (0.88–1.26) | 1.00 (0.84–1.19) |
| 10–12 months | 10,187 | 354 | 89 | 0.96 (0.86–1.08) | 0.93 (0.83–1.05) | 0.99 (0.79–1.24) | 0.94 (0.74–1.17) |
| 13–15 months | 7,388 | 308 | 63 | 1.17 (1.03–1.31) | 1.13 (1.00–1.27) | 0.98 (0.75–1.27) | 0.93 (0.72–1.21) |
| 16–18 months | 5,478 | 253 | 65 | 1.29 (1.13–1.47) | 1.25 (1.10–1.43) | 1.36 (1.05–1.77) | 1.27 (0.98–1.65) |
| 19–21 months | 4,283 | 200 | 58 | 1.31 (1.13–1.51) | 1.28 (1.11–1.48) | 1.57 (1.19–2.06) | 1.52 (1.15–1.99) |
| 22–24 months | 3,595 | 166 | 46 | 1.28 (1.09–1.50) | 1.26 (1.07–1.47) | 1.44 (1.06–1.95) | 1.39 (1.03–1.88) |
| > 24 months | 54,024 | 1658 | 431 | 0.81 (0.76–0.86) | 0.86 (0.80–0.92) | 0.82 (0.72–0.93) | 0.89 (0.78–1.02) |
HR: Hazard Ratio; CI: Confidence Interval; No.: Number
Adjusted for age, sex, race/ethnicity, smoking, Charlson Comorbidity Index, and BMI
Figure 2:
Risk for incident CRC based on time to colonoscopy among US Veterans with abnormal FIT/FOBT, 1999–2015 (n=204,733)
Association between time to colonoscopy and CRC Mortality
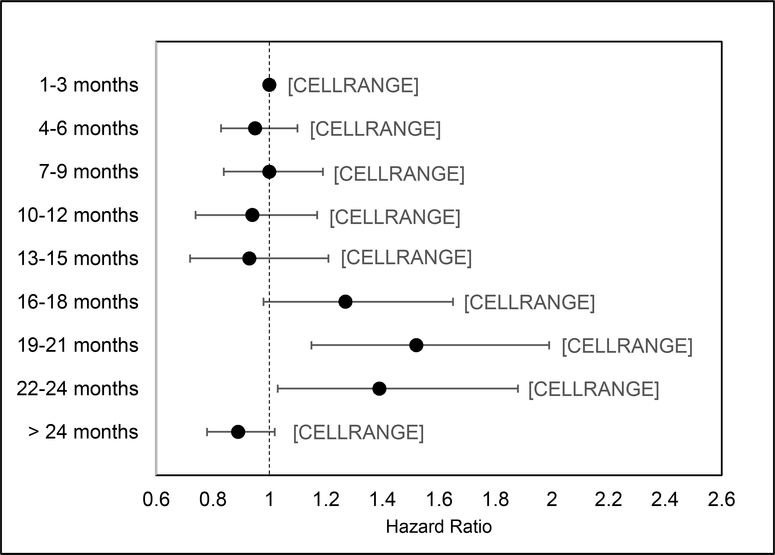
The results of the multivariable Cox proportional hazards model examining the relationship between time to colonoscopy and fatal CRC are shown in Table 2. Compared to patients who received a colonoscopy at 1–3 months, the risk of death increased with time to colonoscopy: 19–21 months (HR=1.52, 95%CI:1.51–1.99) and 22–24 (HR=1.39, 95%CI:1.03–1.88). There were no differences in the risk of CRC-related mortality for patients with a colonoscopy at the other time intervals. (Figure 3).
Figure 3:
Risk for fatal CRC based on time to colonoscopy among US Veterans with abnormal FIT/FOBT, 1999–2015; (n=204,733)
Association between time to colonoscopy and late stage colorectal diagnosis
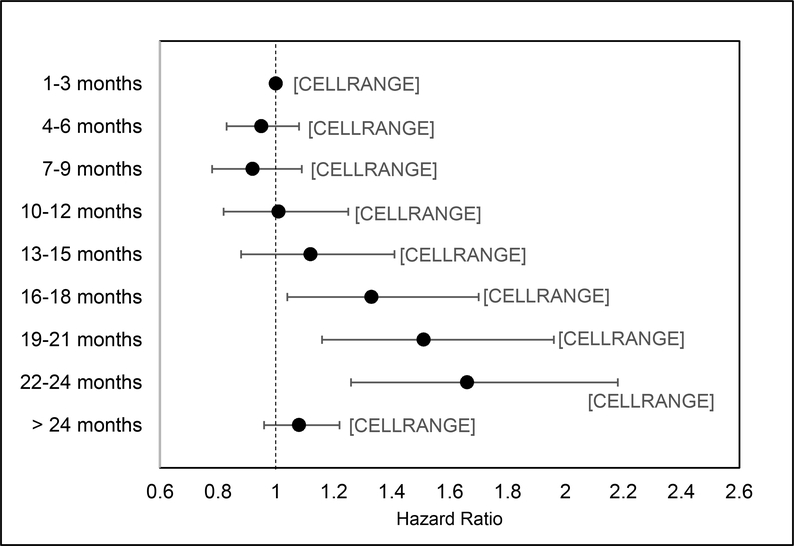
The results of the multivariable logistic regression model examining the relationship between time to colonoscopy and CRC stage at presentation are provided in Table 3. Compared to patients without late stage CRC at the time of diagnosis, the odds of advanced-stage diagnosis increased with time to colonoscopy when colonoscopy was delayed beyond 16–18 months: 16–18 months (OR=1.33, 95%CI: 1.04–1.70), 19–21 months (OR=1.51, 95%CI: 1.16–1.96) and 22–24 months (OR=1.66, 95%CI: 1.26–2.18). (Figure 4).
Table 3:
Association between time to colonoscopy and risk for late stage colorectal diagnosis among Veterans with an abnormal FIT/FOBT, 1999–2015 (n=204,271)
| Variable | No. of Patients | No. of Patients with Advanced CRC | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| Time to Colonoscopy after abnormal FIT/FOBT | ||||
| 1–3 months | 61,483 | 596 | REF | REF |
| 4–6 months | 39,851 | 376 | 0.97 (0.86–1.11) | 0.95 (0.83–1.08) |
| 7–9 months | 18,169 | 168 | 0.95 (0.80–1.13) | 0.92 (0.78–1.09) |
| 10–12 months | 10,165 | 103 | 1.05 (0.85–1.29) | 1.01 (0.82–1.25) |
| 13–15 months | 7,365 | 82 | 1.15 (0.91–1.45) | 1.12 (0.88–1.41) |
| 16–18 months | 5,464 | 72 | 1.36 (1.07–1.75) | 1.33 (1.04–1.70) |
| 19–21 months | 4,276 | 63 | 1.53 (1.18–1.98) | 1.51 (1.16–1.96) |
| 22–24 months | 3,589 | 58 | 1.68 (1.28–2.20) | 1.66 (1.26–2.18) |
| > 24 months | 53,909 | 539 | 1.03 (0.92–1.16) | 1.08 (0.96–1.22) |
Late stage colorectal cancer was defined as patients with CRC stages III and IV according to the American Joint Committee on Cancer staging system.
OR: Odds Ratio; CI: Confidence Interval; No.: Number
Adjusted for age, sex, race/ethnicity, smoking, Charlson Comorbidity Index, and BMI
Figure 4:
Association between time to colonoscopy and risk for late stage colorectal diagnosis among Veterans with an abnormal FIT/FOBT, 1999–2015 (n=204,271)
DISCUSSION
In a large cohort of over 204,000 US Veterans, increasing time to colonoscopy after abnormal FIT/FOBT was significantly associated with higher CRC incidence, CRC-related mortality, and late stage at CRC presentation. Patients who received diagnostic colonoscopy follow-up 13 to 24 months after abnormal FIT/FOBT were 1.1 to 1.3 times more likely to have a CRC diagnosis than individuals who received a colonoscopy 1–3 months after abnormal FIT/FOBT. The risk for CRC-related mortality increased 1.4 to 1.5 times when colonoscopy was delayed by 19 to 24 months. Odds of advanced stage at diagnosis was 1.3 to 1.7 times higher when colonoscopy was delayed beyond 16 months. For all outcomes, risk was increased when colonoscopy was delayed 13 to 24 months after abnormal FIT/FOBT. In secondary analyses, the likelihood of advanced CRC at the time of diagnosis was higher when colonoscopy was delayed beyond 16 months.
Most existing data examining the relationship between exposure to colonoscopy after abnormal stool-based screening and CRC outcomes come from international settings, do not investigate the role of time to colonoscopy, or support a range of follow-up intervals.6, 8, 9, 11, 13–18 Data from a 2017 Korean cohort demonstrated a 1.6-fold increased risk for CRC-specific death among patients who underwent colonoscopy (at an time) compared to those who did not.14 In the same patient population, CRC risk and advanced-stage disease increased when colonoscopy was delayed by more than 6 months; however the association between time to colonoscopy and death risk was not reported.13 A retrospective analysis in Israel suggested that the optimal time interval between an abnormal stool-based screening result and colonoscopy was 12 months to reduce the risk of CRC incidence and late stage diagnosis.11 In the same population-based screening program, the hazard of CRC-related mortality increased by 50% when colonoscopy was delayed greater than 12 months compared to 0–3 months.15
The few published reports from US studies have small cohort size or used either regional or state populations. One study that included 231 Veterans from a single VA facility reported an association between longer time to colonoscopy after FOBT and adenoma risk but was not powered to evaluate a malignancy endpoint.9 In a retrospective analysis of FIT-positive Kaiser Permanente patients, individuals who waited 10 to 12 months were more likely to have CRC and advanced-stage disease at the time of diagnostic colonoscopy when compared to FIT-positive individuals who underwent colonoscopy within 1 month.8 A microsimulation model to estimate the consequences of different times to colonoscopy after an abnormal FIT supported this time interval.10 Our study confirms an increased risk of CRC and advanced stage with time from colonoscopy but extends these findings by evaluating how time to colonoscopy impacts CRC-related mortality.8, 10–15, 31–33
The association between time to colonoscopy and CRC incidence reflects the two mechanisms by which FIT/FOBT can interfere with CRC tumorigenesis. CRC is the result of a series of sequential mutations that impact cell proliferation and apoptosis in the transition from normal colonic mucosa to polyp to CRC.34 Stool-based CRC screening modalities contribute to CRC prevention through the detection of colon and rectal polyps (usually adenomas) and the diagnosis of malignant lesions. Thus, the low rate of incident CRC among patients who underwent colonoscopy at 4–6 months and 7–9 months compared to 1–3 months most likely reflects a higher relative likelihood of prevalent CRC at the time of the FIT/FOBT in the 1–3 month group. Some of these individuals may have had additional symptoms concurrent with or after the abnormal FIT, prompting rapid colonoscopic follow-up.35 Although these differences appear small in absolute terms, the findings are clinically important due to the large number of individuals in the US that undergo stool-based CRC screening. Conversely, the increased incidence at 13–15 months and later most likely represents FIT/FOBT detection of advanced adenomas that transitioned to CRC in the interval between the abnormal FIT/FOBT result and colonoscopy, or cancers that were early and asymptomatic, and thus less likely to prompt more rapid follow up. That the risk of CRC incidence was not significantly increased at 10–12 months further supports this hypothesis and the tumorigenesis model. Patients with a nonadvanced adenomatous polyp at the time of FIT/FOBT may have had an abnormal result but required a longer dwell time than 10–12 months to transition to CRC.35 Given the relatively low estimated rate of adenoma to cancer progression, this group may also represent polyps that do not transition to CRC, as well as false positive FIT results and abnormal results that result from non-neoplastic lesions (i.e. hemorrhoids) in the lower gastrointestinal tract.7, 35
Our mortality findings coincide with the incidence results. Risk of mortality was elevated at 19 months after abnormal FIT/FOBT, which suggests less advanced neoplasia at the time of FIT/FOBT and stage migration in the interval between the abnormal FIT and colonoscopy. These findings are supported by our secondary analyses by stage and by prior studies that assessed stage at diagnosis but that were not able to evaluate a mortality outcome.8, 11 Beyond 16 months, there was increased odds for advanced stage, and this relationship was stronger as time to colonoscopy increased. As with incidence, patients with an abnormal FIT/FOBT caused by a slowly progressing polyp, non-progressing polyp, or non-neoplastic lesion likely represent the group that do not develop a CRC or death endpoint at 4–18 months or beyond 24 months. A lag between CRC incidence and CRC-related mortality is consistent with the CRC tumorigenesis model. The absence of significant findings for patients who underwent colonoscopy > 24 months after abnormal FIT/FOBT likely reflects a large and heterogenous group of patients who did not have an abnormal FIT/FOBT due to a polyp or malignancy, who had an abnormal FIT/FOBT due to a non-progressive adenoma, or who underwent colonoscopy for a reason other than the abnormal FIT/FOBT result years later. This interpretation is subject to potential survivor bias given that we were only able to study outcomes for individuals who survived to at least 24 months after abnormal FIT/FOBT. However, prevalent CRC or premalignant advanced adenomas at the time of an abnormal FIT/FOBT would have likely become apparent before 24 months. In addition, a lower percentage of advanced CRC (Table 3) is supportive of these explanations.
Currently, there is no national policy or standard for the clinically acceptable time interval between an abnormal FIT/FOBT result and diagnostic colonoscopy. Time to colonoscopic follow-up varies widely in practice and across healthcare settings.9, 21, 32, 36–42 Though data from our study and others suggest that the largest burden of CRC cases and deaths occur when colonoscopy is delayed several months after abnormal FIT/FOBT, we do not interpret these results to suggest that it is clinically appropriate to wait over a year for colonoscopic follow-up after abnormal FIT/FOBT. A recommended interval that is too long can contribute to polyp progression and stage migration of CRC, risking the need for more aggressive and morbid treatment, as well as less favorable outcomes. However, too short of a time interval could place undue burden on the patient and healthcare system. Thus, we feel that the strategy should be to intervene with colonoscopy well before 13 months and closer to 6 months after abnormal FIT. One systematic review and consortium has recommended setting a goal of 3 months and avoiding a delay of more than 6 months.32 Notably setting a goal of follow-up within 30 days likely contributed to the very high follow-up rates achieved by a large integrated healthcare system.43 Establishing national recommendations on follow-up intervals and requiring health systems to measure, monitor and report FIT/FOBT follow-up rates will help address national variation in FIT/FOBT follow-up rates. Although CRC screening is a Healthcare Effectiveness Data and Information Set (HEDIS) measure, there is currently no requirement to report follow-up of abnormal screening. As FIT/FOBT is a two-step process that is not truly complete for individuals with abnormal results until diagnostic colonoscopy is performed, there should also be HEDIS or similar quality metrics to make health systems accountable for follow-up.
There were many strengths to this study. The analyses included a racially and ethnically diverse sample of 204,733 patients with abnormal FIT/FOBT results resulting in 6,906 CRC cases and 1,709 CRC-related deaths over 16 years. The study size far exceeds prior studies that have examined the association between time to colonoscopy and CRC outcomes among patients with abnormal stool-based CRC screening results. In addition, we used validated approaches to capture both colonoscopy and CRC outcome data. Our study is also the first that we are aware of in the US to examine the relationship between time to colonoscopy and CRC-related mortality in individuals with abnormal CRC stool blood test results. The work will inform guidelines and standard of care for patients who undergo stool-based CRC screening. This guidance is especially relevant now in the setting of the COVID-19 pandemic and recommendations to increase use of non-colonoscopic screening modalities.44 Our findings emphasize the importance of colonoscopic follow-up and a need for attention to time to follow-up to avoid excess deaths from CRC during this time of limited access.
This study is not without limitations. First, our findings may be less generalizable to non-VA settings and to women given the largely male VA study population. However, the VA is one of few health systems in the US that provides access to a patient population of adequate size and data to perform these analyses. It is also reassuring that the absolute number of women included in the study was substantial (n=5453), especially compared to previous studies and the general lack of data on women. Second, despite including data on probable confounders, there is the potential for residual and unmeasured confounding in this retrospective cohort study. Third, we restricted our analyses to Veterans that had a follow-up colonoscopy in the VA.
In conclusion, among US veterans age 50 to 75 with an abnormal FIT/FOBT result, increased time to colonoscopy was significantly associated with CRC incidence and CRC-related mortality. These findings extend current knowledge about the clinical implications of time to diagnostic follow-up after abnormal FIT/FOBT. Further work should include interventions that address barriers to uptake of diagnostic colonoscopy after abnormal non-colonoscopic screening results and policies to encourage the routine measuring and monitoring of follow-up rates.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT:
The literature varies on the optimal time interval for diagnostic colonoscopy after an abnormal stool-based colorectal cancer test result (CRC) to maximize prevention of incident and fatal CRC.
NEW FINDINGS:
Time to colonoscopy was significantly associated with CRC incidence, CRC mortality, and advanced stage CRC in a retrospective cohort study of 204,733 adults with abnormal stool-based screening test results.
LIMITATIONS:
Study findings may be less generalizable to non-VA settings, and there is the potential for residual and unmeasured confounding in this retrospective cohort study.
IMPACT:
Time to colonoscopy after abnormal stool-based screening test results should be monitored as a quality metric. Patients should undergo colonoscopy well within 1 year to avoid poor outcomes.
Acknowledgements:
We would like to acknowledge Hanin Yassin, BS for her help with preparation of this submission.
Grant Support: This study was supported by National Institutes of Health U54 Partnership grants CA132384 and CA132379 (Martinez, Gupta, San Miguel), National Institutes of Health award number 5F32CA239360-02 (Demb), National Institutes of Health/National Cancer Institute award number R37 CA222866 (Gupta, Martinez), Department of Veterans Affairs Health Services Research and Development Award I01 HX001574 (Gupta), and National Institutes of Health/National Cancer Institute award number R03CA230947 (May).
Abbreviations:
- BMI
Body mass index
- CCI
Charlson Comorbidity Index
- CRC
colorectal cancer
- CI
confidence intervals
- COVID-19
coronavirus disease 2019
- CDW
Corporate Data Warehouse
- CPT
Current Procedural Terminology
- HER
electronic health records
- FOBT
fecal occult blood test
- FIT
fecal immunochemical test
- HRs
hazard ratios
- HEDIS
Healthcare Effectiveness Data and Information Set
- NHW
non-Hispanic White
- Black
non-Hispanic Black
- US
United States
- USPSTF
United States Preventive Services Task Force
- VA
Veterans Health Administration
Footnotes
Conflict of interest statement: There are no conflicts to report for any authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020. [DOI] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA : the journal of the American Medical Association. 2016;315(23):2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 3.Choi KS, Lee HY, Jun JK, et al. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. J Gastroenterol Hepatol 2012;27:1070–7. [DOI] [PubMed] [Google Scholar]
- 4.Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg 2008;95:1029–36. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JSB JH; Church TR; Snover DC; Bradley GM; Schuman LM; Ederer F Reducing Mortality from Colorectal Cancer by Screening for Fecal Occult Blood. The New England Journal of Medicine 1993;328:7. [DOI] [PubMed] [Google Scholar]
- 6.Libby G, Fraser CG, Carey FA, et al. Occult blood in faeces is associated with all-cause and non-colorectal cancer mortality. Gut 2018;67:2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;152:1217–1237.e3. [DOI] [PubMed] [Google Scholar]
- 8.Corley DA, Jensen CD, Quinn VP, et al. Association Between Time to Colonoscopy After a Positive Fecal Test Result and Risk of Colorectal Cancer and Cancer Stage at Diagnosis. JAMA 2017;317:1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gellad ZF, Almirall D, Provenzale D, et al. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci 2009;54:2497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meester RG, Zauber AG, Doubeni CA, et al. Consequences of Increasing Time to Colonoscopy Examination After Positive Result From Fecal Colorectal Cancer Screening Test. Clin Gastroenterol Hepatol 2016;14:1445–1451.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beshara A, Ahoroni M, Comanester D, et al. Association between time to colonoscopy after a positive guaiac fecal test result and risk of colorectal cancer and advanced stage disease at diagnosis. Int J Cancer 2019. [DOI] [PubMed] [Google Scholar]
- 12.Ferrat E, Le Breton J, Veerabudun K, et al. Colorectal cancer screening: factors associated with colonoscopy after a positive faecal occult blood test. Br J Cancer 2013;109:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YC, Fann JC, Chiang TH, et al. Time to Colonoscopy and Risk of Colorectal Cancer in Patients With Positive Results From Fecal Immunochemical Tests. Clin Gastroenterol Hepatol 2019;17:1332–1340 e3. [DOI] [PubMed] [Google Scholar]
- 14.Lee YC, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association Between Colorectal Cancer Mortality and Gradient Fecal Hemoglobin Concentration in Colonoscopy Noncompliers. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flugelman AA, Stein N, Segol O, et al. Delayed Colonoscopy Following a Positive Fecal Test Result and Cancer Mortality. JNCI Cancer Spectr 2019;3:pkz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaalby L, Rasmussen M, Zimmermann-Nielsen E, et al. Time to colonoscopy, cancer probability, and precursor lesions in the Danish colorectal cancer screening program. Clin Epidemiol 2019;11:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim NH, Lim JW, Kim S, et al. Association of time to colonoscopy after a positive fecal test result and fecal hemoglobin concentration with risk of advanced colorectal neoplasia. Dig Liver Dis 2019;51:589–594. [DOI] [PubMed] [Google Scholar]
- 18.Zorzi M, Hassan C, Capodaglio G, et al. Colonoscopy later than 270 days in a fecal immunochemical test-based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy 2020;52:871–876. [DOI] [PubMed] [Google Scholar]
- 19.Shaukat A, Church T. Colorectal cancer screening in the USA in the wake of COVID-19. Lancet Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EPIC helath research network. Preventive Cancer Screenings during COVID-19 Pandemic. Availble from: https://ehrn.org/wp-content/uploads/Preventive-Cancer-Screenings-during-COVID-19-Pandemic.pdf. Accessed: May 5, 2020.
- 21.May F, Yano EM, Provenzale D, et al. Barriers to Follow-Up Colonoscopies for Patients With Positive Results From Fecal Immunochemical Tests During Colorectal Cancer Screening. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 22.Selby K, Senore C, Wong M, et al. Interventions to ensure follow-up of positive fecal immunochemical tests: An international survey of screening programs. J Med Screen 2020:969141320904977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Providing care for Veterans. Accessed July 14, 2020. https://www.va.gov/health/aboutvha.asp.
- 24.Earles A, Liu L, Bustamante R, et al. Structured Approach for Evaluating Strategies for Cancer Ascertainment Using Large-Scale Electronic Health Record Data. JCO Clin Cancer Inform 2018;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res 2011;13:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noel PH, Copeland LA, Perrin RA, et al. VHA Corporate Data Warehouse height and weight data: opportunities and challenges for health services research. J Rehabil Res Dev 2010;47:739–50. [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 28.Partin MR, Gravely AA, Burgess JF Jr., et al. Contribution of patient, physician, and environmental factors to demographic and health variation in colonoscopy follow-up for abnormal colorectal cancer screening test results. Cancer 2017;123:3502–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes N, Hilsden RJ, Martel M, et al. Association Between Time to Colonoscopy After Positive Fecal Testing and Colorectal Cancer Outcomes: A Systematic Review. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SCHOENFELD D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. [Google Scholar]
- 31.Wattacheril J, Kramer JR, Richardson P, et al. Lagtimes in diagnosis and treatment of colorectal cancer: determinants and association with cancer stage and survival. Aliment Pharmacol Ther 2008;28:1166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: A systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin 2018;68:199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutter CM, Kim JJ, Meester RGS, et al. Effect of Time to Diagnostic Testing for Breast, Cervical, and Colorectal Cancer Screening Abnormalities on Screening Efficacy: A Modeling Study. Cancer Epidemiol Biomarkers Prev 2018;27:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology 2000;119:854–65. [DOI] [PubMed] [Google Scholar]
- 35.Brenner H, Hoffmeister M, Stegmaier C, et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut 2007;56:1585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson DJ, Lee JK, Boland CR, et al. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017;112:37–53. [DOI] [PubMed] [Google Scholar]
- 37.Miglioretti DL, Rutter CM, Bradford SC, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care 2008;46:S91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Issaka RB, Singh MH, Oshima SM, et al. Inadequate Utilization of Diagnostic Colonoscopy Following Abnormal FIT Results in an Integrated Safety-Net System. Am J Gastroenterol 2017;112:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correia A, Rabeneck L, Baxter NN, et al. Lack of follow-up colonoscopy after positive FOBT in an organized colorectal cancer screening program is associated with modifiable health care practices. Prev Med 2015;76:115–22. [DOI] [PubMed] [Google Scholar]
- 40.Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to Colonoscopy after Positive Fecal Blood Test in Four U.S. Health Care Systems. Cancer Epidemiol Biomarkers Prev 2016;25:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Partin MR, Burgess DJ, Burgess JF Jr., et al. Organizational predictors of colonoscopy follow-up for positive fecal occult blood test results: an observational study. Cancer Epidemiol Biomarkers Prev 2015;24:422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher DA, Jeffreys A, Coffman CJ, et al. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev 2006;15:1232–5. [DOI] [PubMed] [Google Scholar]
- 43.Selby K, Jensen CD, Zhao WK, et al. Strategies to Improve Follow-up After Positive Fecal Immunochemical Tests in a Community-Based Setting: A Mixed-Methods Study. Clin Transl Gastroenterol 2019;10:e00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks D, Issaka R, Itzkowitz S, et al. Reigniting Colorectal Cancer Screening As Communities Face And Respond To The COVID-19 Pandemic: A Playbook. Accessed July 12, 2020. https://nccrt.org/resource/a-playbook-for-reigniting-colorectal-cancer-screening-as-communities-respond-to-the-covid-19-pandemic/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.