Abstract
Purinergic signaling, a concept originally formulated by the late Geoffrey Burnstock (1929–2020), was found to modulate pathways in every physiological system. In metabolic disorders there is a role for both adenosine receptors and P2 (nucleotide) receptors, of which there are two classes, i.e. P2Y metabotropic and P2X ionotropic receptors. The individual roles of the 19 receptors encompassed by this family have been dissected - and in many cases the effects associated with specific cell types, including adipocytes, skeletal muscle, liver cells and immune cells. It is suggested that ligands selective for each of the four adenosine receptors (A1, A2A, A2B and A3), and several of the P2 subtypes (e.g. P2Y6 or P2X7 antagonists) might have therapeutic potential for treating diabetes and obesity. This is a developing story with some conflicting conclusions relevant to drug discovery, which we summarize here.
Keywords: adenosine receptors, P2Y receptors, P2X receptors, diabetes, obesity
1. Introduction
Type 2 diabetes (T2D) or diabetes mellitus is a cluster of metabolic disorders that are characterized by dysregulation of glucose metabolism due to decreased insulin sensitivity in peripheral metabolically active tissues and defects in insulin secretion from pancreatic β cells. An epidemic of T2D continues to rise due to a major shift towards physical inactivity and unhealthy eating habits increasing obesity in people worldwide. According to a national diabetes statistics report in 2020 (Centers for Disease Control, https://www.cdc.gov/diabetes/data/statistics-report/index.html, accessed Oct. 26, 2020), 34.2 million people, or 10.5% of the U.S. population, have diabetes. By 2035, approx. 600 million people worldwide are predicted to suffer from T2D. T2D also leads to severe pathophysiological complications such as coronary artery disease, stroke, atherosclerosis, diabetic nephropathy, diabetic neuropathy, diabetic retinopathy and certain types of cancer. Therefore, identifying promising pharmacological approaches to contain the onset and progression of T2D and associated complications is of utmost importance.
The purine nucleoside adenosine functions as a modulatory extracellular signaling molecule mediating a range of physiological and patho-physiological responses through its four target adenosine receptors (ARs). Caffeine and other alkylxanthines are the prototypical AR antagonists, and their effect in the human diet can be used to indicate the possible role of these receptors in diabetes. These four ARs constitute part of the larger purinergic signalome, which includes eight P2Y metabotropic and seven P2X ionotropic receptors that are activated by extracellular ATP and other nucleotides. Numerous studies [1–3] have indicated that purinergic tuning of metabolic processes has the potential to provide new treatments for T2D and obesity.
Adenosine, ATP and other nucleotides are released from cells as autocrine and paracrine mediators, in response to metabolic stress, inflammation and injury [4]. ARs, P2YRs and P2XRs are widely expressed in metabolically active tissues such as adipose tissue, skeletal muscle (SKM), liver, pancreas, brain and immune cells. Growing evidence highlights the critical role of purinergic signaling in the regulation of pathophysiological processes associated with T2D and associated co-morbidities [5]. Targeting purinergic receptors selectively for therapeutics of T2D and associated complications has been challenging due to the ubiquitous distribution and expression of the receptors and variable levels of the endogenous agonists. Furthermore, the intricate nature of purinergic signaling can contribute to unacceptable side effects and to pharmacological and genetic discrepancies between cellular models, animal models and human pathology. However, it is to be noted that each purinergic receptor might have different effects when activated tonically or clonically, and changes of purine receptor function often occur in pathological conditions [2]. In this review, we provide a background on the roles of purine nucleotide and nucleoside signaling in the pathophysiological mechanisms underlying T2D in different metabolic tissues regulating glucose and energy homeostasis (Tables 1 and 2).
Table 1.
Location and effects of adenosine receptors in diabetes and obesity.
| Receptor subtype | Location | Effect |
|---|---|---|
| Liver | ||
| A1 | Rat hepatocytes | Enhanced glycogenolysis [32] |
| A1 | Mouse liver | KO protected from ethanol induced fatty liver [33] |
| A1 | Rat hepatocytes | Enhanced glycogen incorporation and glucose clearance [34] |
| A2A | Rat hepatocytes | Enhanced gluconeogenesis [32] |
| A2A | Mouse whole body | KO increased NAFLD, hepatic inflammation [38] |
| A2A | Mouse whole body | KO mice on MCD diet increased NASH [39] |
| A2A | Mouse liver | Activation suppress inflammation cause by lipotoxicity [40, 41] |
| A2B | Mouse liver | KO protected from ethanol-induced fatty liver [33] |
| A2B | Mouse liver | Antagonism reduced glucose production during clamp studies [42] |
| A2B | Mouse liver | Activation inhibited hepatic lipogenesis [44] KO enhanced hepatic steatosis in HFD mice [44] |
| A2B | Mouse liver | KO impaired glucose tolerance and insulin sensitivity [45] Treatment with agonist improved metabolism [45] |
| A3 | Mouse STAM model | Activation by a prodrug protects against NASH [46] |
| A3 | Human studies | Agonist imparts anti-inflammatory effects in HCC and hepatitis [47] |
| A3 | Mouse NASH model | Agonist protects against NASH [48] |
| Skeletal muscle | ||
| A1 | Mouse skeletal muscle | Inhibited glucose uptake [56] |
| A1 | Rat soleus muscle | Enhanced insulin-mediated-glucose uptake [57] |
| A1 | Rat skeletal muscle | Antagonism reduced glucose uptake during muscle contractions [58] |
| A2B | Human myocytes | Enhanced glucose uptake [59] |
| A2B | Mouse skeletal muscle | Increased muscle mass and oxidative capacity [59] |
| A2B | Mouse skeletal muscle | Antagonism increased glucose uptake [42] |
| A2B | Mouse skeletal muscle | Antagonism decreased insulin sensitivity [60] |
| A2B | Mouse skeletal muscle | Increased insulin sensitivity in high sucrose fed insulin-resistant mice[60] |
| Adipose tissue | ||
| A1 | Rat adipocytes | Activation inhibits lipolysis [61–63] |
| A1 | Zucker rat whole body | Stimulation decreased plasma FFA and enhanced insulin stimulated glucose uptake [64, 65] |
| A1 | Mouse whole body | Excessive activation causes obesity [66] |
| A1 | Mouse whole body | Aged KO mice gained more weight on a HFD, higher food intake [68] |
| A1 | Mouse whole body | KO showed reduced age-associated fat accumulation [69] |
| A1 | Zucker rat whole body | Antagonist treatment improved glucose tolerance [70] |
| A2A | Mouse adipocytes | Enhanced browning of fat, protected from HFD induced obesity, improved whole body metabolism [71] |
| A2A | Mouse whole body | KO increased inflammation and insulin resistance [72]. |
| A2A | Mouse adipocytes | Activation enhanced food intake and inhibited thermogenesis [73–75] |
| A2B | Mouse whole body | Activation reduced inflammation and insulin resistance [76] |
| A2B | Mouse adipocytes | Activation protected against HFD-induced obesity [59] |
| A2B | Mouse adipocytes | KO increased weight gain and insulin resistance on a HFD [45] |
| A2B | Mouse adipocytes | Aged KO mice accumulated more visceral fat on a HFD [77, 78] |
| A2B | Mouse adipocytes | Activation reduced weight, improved insulin resistance [78, 79] |
| Pancreas | ||
| A1 | Mouse whole body | KO enhanced glucose-stimulated insulin release [67,86] |
| A1 | Rat islets | Antagonism increased insulin release [87] |
| A1 | Mouse islets | Enhanced secretion of insulin from islets of KO [69] |
| A2A | Mouse islets | Antagonism decreased insulin release [83] |
| A2A | INS-1 cells | Activation suppressed insulin release [88] |
| A2A | Zebrafish model of T1D | Activation increased P-cell proliferation [90] |
| A2A | Mouse-T1D model | Activation increased insulin release and ameliorated T1D [91] |
| A3 | P-TC6 cell line | Activation reduced cell proliferation and viability [83] |
| Hypothalamus | ||
| A1 | Mouse hypothalamus | Activation increased food intake, decreased energy expenditure [101] |
Table 2.
Location and effects of P2 receptors in diabetes and obesity.
| Receptor subtype | Location | Effect |
|---|---|---|
| Liver | ||
| P2Y1 | Rat hepatocytes | Stimulates glycogen phosphorylase [103] |
| P2Y13 | Mouse liver | Activation increased reverse cholesterol transport [104] |
| P2Y13 | Mouse liver | KO decreases hepatic HDL cholesterol uptake [105] |
| Skeletal muscle | ||
| P2Y6 | C2C12 cell line | Activation increased glucose uptake [108] |
| P2Y6 | Mouse skeletal muscle | KO decreased insulin-stimulated glucose uptake in obese mice, impaired glucose tolerance and insulin sensitivity [109] |
| Pancreas | ||
| P2X3 | Human beta cells | ATP increases calcium concentration [117] |
| P2Y1 | Human beta cells | ATP stimulate electrical activity, regulating calcium flux [115] |
| P2Y1 | STZ rats-perfused pancreas Activation caused insulin response same as control [124, 125] | |
| P2Y1 | Mouse islets | KO showed higher glucose-stimulated insulin response [126] |
| P2Y1 | Mouse islets | Activation decreased glucose-stimulated insulin response [127] |
| P2Y1 | MIN6 cell line | Activation increased insulin response [128] |
| P2Y6 | MIN6 cell line | Activation increased insulin response [129] |
| Adipose tissue | ||
| P2Y1 | Mesenchymal stem cells | Promotes adipogenic differentiation [130] |
| P2Y1 | Mouse whole body | KO decreased leptin production [147] |
| P2Y2 | Human adipocytes | Suppress basal lipolysis [134] |
| P2Y2 | Mouse whole body | KO protected from HFD-induced obesity, improved glucose tolerance, impaired adipocyte differentiation [135] |
| P2Y4 | Mesenchymal stem cells | Promotes adipogenic differentiation [130] |
| P2Y4 | Mouse whole body | KO increased adiponectin levels [147] |
| P2Y6 | Mouse adipocytes | Activation increased glucose uptake [108] |
| P2Y6 | Mouse adipose tissue | KO protected from diet induced obesity/inflammation [109] |
| P2Y13 | Mesenchymal stem cells | Promotes anti-adipogenic effect [136] |
| P2Y14 | Mouse whole body | KO protected from HFD induced insulin resistance, reduced macrophage infiltration in the liver [144] |
| P2Y14 | Mesenchymal stem cells | Promotes anti-adipogenic effect [131] |
| P2X7 | Mouse whole body | KO gained more weight, adipocyte hyperplasia, activation caused anti-adipogenic effect [137, 138, 131] |
| P2X7 | Mouse adipocytes | Antagonism suppresses NLRP3 inflammasome [141, 142] |
| P2X7 | Mouse whole body | KO protected from diet induced obesity, inflammation [143] |
| Hypothalamus | ||
| P2Y6 | Mouse AgRP neurons | Activation promoted feeding [153] |
| P2Y6 | Mouse AgRP neurons | Antagonist inhibited feeding in obesity [154] |
| P2Y6 | Mouse AgRP neurons | KO protected from diet-induced insulin resistance [154] |
1.1. Overview of purinergic signalome system
The term ‘purinergic’ was introduced by Geoffrey Burnstock in 1972 when he first proposed adenosine 5’-triphosphate (ATP) as a purinergic neurotransmitter in the gut and bladder [6, 7]. ATP can be released from diverse cell types by various mechanisms including release from the lysosome via exocytosis, co-release with other hormones, uncontrolled release from necrotic cells, controlled release through pannexin hemichannels and release through connexin hemichannels and P2X7 ion channels [8–13]. The mammalian genome codes for seven different ionotropic ligand-gated ion channel P2X receptors (P2X1–7) that are principally activated by ATP. The P2Y metabotropic G protein-coupled receptors (GPCRs) (P2Y1,2,4,6,11,12,13,14) are identified and characterized in mammals and are activated by a group of nucleotides and nucleotide sugars (ATP, ADP, UTP, UDP, UDP-glucose). P2Y receptors couple to different G-proteins, activating different intracellular signaling pathways, either the five members of the P2Y1-like subfamily (P2Y1,2,4,6 - Gq/11, Go, G12/13; P2Y11 - Gq/11 and Gs) or the three P2Y12-like receptors (P2Y12,13,14 - Gi/o).
ATP is dephosphorylated into adenosine in the extracellular space mainly by a two-step enzymatic sequence, beginning with CD39 (ectonucleoside triphosphate diphosphohydrolase 1: ENTPD1, NTPDase1) and three other NTPDases that convert ATP or ADP to AMP, followed by AMP hydrolysis by CD73 (ecto-5’-nucleotidase: NT5E) to adenosine. Other cell-surface associated enzymes such as pyrophosphatases, alkaline phosphatases and phosphodiesterases can also generate extracellular adenosine [14]. Adenosine can also be generated intracellularly by hydrolysis of AMP or S-adenosylhomocysteine and transported across cell membranes by equilibrative nucleoside transporters (ENTs) and concentrative nucleoside transporters (CNTs) that help to maintain extracellular adenosine levels. Reuptake of adenosine into cells is mediated by nucleoside transporters followed by rapid phosphorylation to AMP by adenosine kinase or deamination to inosine by adenosine deaminase (ADA).
Adenosine bind to and activates four purinergic (P1) receptors namely, A1, A2A, A2B and A3ARs. A1 and A2AARs have high affinity for adenosine, whereas A3AR and especially A2BAR have lower affinity [15]. A1 and A3ARs couple to pertussis toxin-sensitive Gi and Go proteins, and their activation decreases intracellular cAMP levels. Activation of Gs- and Golf-coupled A2A and A2BARs increases cAMP levels (Fig. 1). ARs can activate various signaling pathways such as mitogen activated protein kinase (MAPK) pathways comprising of extracellular signal-regulated kinase 1and 2 (ERK1 and ERK2), c-Jun-N-terminal kinase (JNK) and p38 MAPK (Fig. 1).
Figure 1.
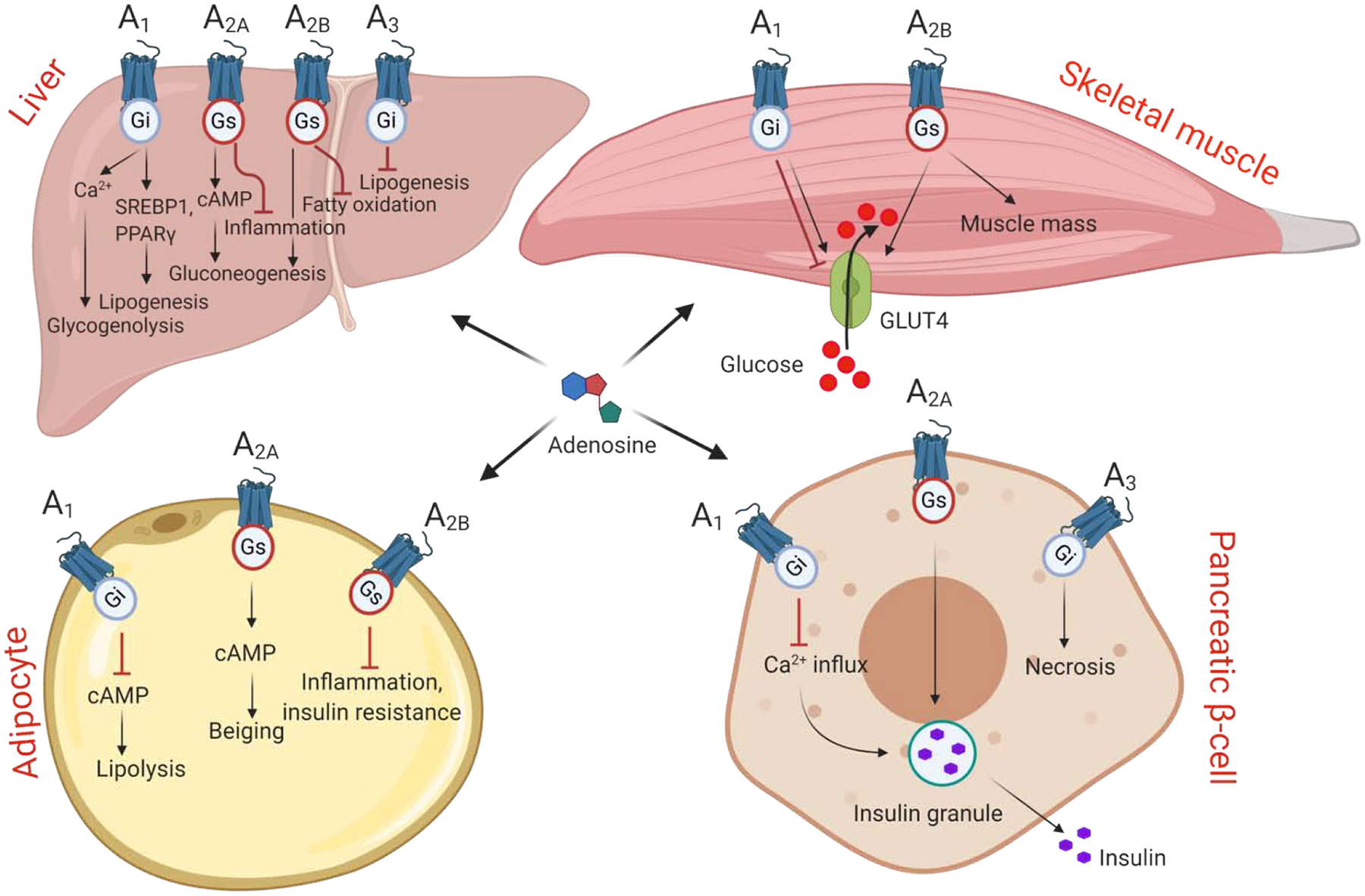
Modulation of metabolic processes by adenosine signaling.
Four adenosine receptors couple to Gi or Gs proteins and modulate the level of secondary messengers such as cAMP/Ca2+ to activate different signaling proteins. Metabolic processes regulated by adenosine receptors in liver, skeletal muscle, adipose tissue and pancreas. See Section 2 for details.
2. Adenosine receptors and diabetes
A fine balance between dietary intake, endogenous glucose release from liver, glucose uptake and utilization by SKM and adipose tissues is required to maintain glucose homeostasis in an organism. These processes are regulated by two main hormones released by pancreatic cells, i.e. insulin and glucagon. Other hormones of the pancreas such as somatostatin and amylin also affect blood glucose levels. Exogenous dietary intake stimulates insulin release from pancreatic β-cells enhancing glucose uptake by muscle and adipose tissues and inhibiting glucose production by the liver cells. Under conditions of obesity and T2D, insulin lacks its ability to clear plasma glucose due to the development of insulin resistance in peripheral tissues. Inhibition of lipolysis by insulin action on adipose tissue is also abolished, increasing plasma free fatty acid (FFA) levels that can accumulate in liver to cause non-alcoholic fatty liver disease (NAFLD). Infiltration of immune cells in different metabolic tissues and production of pro-inflammatory cytokines result in chronic low-grade inflammation associated with obesity and T2D. Genetic and pharmacological approaches undertaken to understand the contribution of ARs in the regulation of these processes in metabolically active organs are described below.
Coffee and caffeine in diabetes
Caffeine is a prototypical nonselective AR antagonist, and coffee is one of the most widely consumed beverages worldwide. Accumulating evidence suggest that persistent consumption of coffee is inversely correlated to the incidence of T2D [16–19], but little is known about the mechanisms responsible for this beneficial effect. Caffeine is one of the main components in coffee, but contrary to coffee’s beneficial effects, caffeine has been shown to have a deleterious effect on glucose metabolism in humans. A recent study demonstrated that acute caffeine ingestion impaired insulin sensitivity in healthy subjects [20]. In diabetic patients, acute administration of caffeine impaired postprandial glucose metabolism and insulin sensitivity [21]. In a crossover randomized clinical trial (RCT) in healthy individuals, caffeinated coffee consumption resulted in a higher area under curve (AUC) during 2 h for glucose and insulin compared to decaffeinated coffee [22]. In addition, insulin sensitivity was reduced by 40% in the caffeinated coffee group compared to decaffeinated coffee [22]. In overweight individuals, ingestion of caffeinated coffee lowered insulin levels and elevated glucose concentrations [23]. In a crossover RCT in subjects with T2D, consumption of caffeinated coffee resulted in a higher AUC for glucose compared to groups consuming water and decaffeinated coffee [24]. The subjects showed no difference in insulin sensitivity in this study [24]. Some studies did not find significant differences in glucose or insulin concentration following caffeinated coffee consumption [25]. On the contrary, long term trials to assess the effects of caffeine on glucose metabolism showed that caffeinated coffee ingestion may improve glycaemic metabolism by decreasing glucose levels and increasing insulin response [26–28]. Long term trials corroborate the epidemiologic studies showing a reduction of T2DM risk due to coffee consumption. These long-term beneficial effects on glucose metabolism may be due to anti-inflammatory/anti-oxidant effects of other constituents of coffee [29] or due to development of tolerance to the acute effects of caffeine consumption [27].
2.1. Liver
The liver plays a key role in the systemic regulation of glucose and lipid metabolism in both fasting and post-prandial states [30]. Circulating insulin’s action on liver controls blood glucose levels by activating glycogen synthesis and inhibiting gluconeogenesis. In pathological conditions of obesity and T2D, aberrant insulin action fails to decrease glucose production and lipid synthesis leading to hepatic insulin resistance [30]. Excessive triglyceride storage leads to the development of NAFLD, which may progress to nonalcoholic steatohepatitis (NASH). In studies of liver fibrosis and particularly the protective effects of A2A and A2B adenosine antagonists, the mRNA of all four ARs has been detected in various liver cells such as hepatocytes, hepatic stellate cells and Kupffer cells (resident macrophages) [31]. Current studies employing different pharmacological approaches and genetic animal models show contradictory effects of AR activation on liver function.
In isolated rat hepatocytes, A1AR activation enhanced glucose output mainly via Ca2+-mediated glycogenolysis [32]. A1AR was also shown to regulate hepatic lipogenesis and steatosis. A study by Peng et al., showed that A1AR activation enhanced hepatic lipogenesis and hence contributed to liver steatosis in an alcohol-induced fatty liver mouse model [33]. Mice lacking the A1AR were protected from developing fatty liver in this model [33]. However, a study reported that hyperglycemia increased A1AR gene expression in the liver of insulin-deficient diabetic rats [34]. This increased A1AR enhanced glycogen incorporation in hepatocytes from diabetic rats, leading to better blood glucose clearance [34].
Enhanced cAMP-mediated gluconeogenesis in rat hepatocytes was shown to be mediated by activation of the A2AAR [32]. Activation of A2AAR with a selective agonist (CGS21680) promoted glucose release from rat hepatocytes [32]. A2AAR activation resulted in anti-inflammatory effects [35, 36], whereas A2AAR deficiency enhanced pro-inflammatory responses [37]. Global A2AAR deficient mice displayed an increase in HFD-induced NAFLD and hepatic inflammation [38]. Lack of A2AAR in both hepatocytes and macrophages contributed to the enhanced HFD-induced inflammation [38].
Furthermore, A2AAR deficiency increased the abundance and the activity of sterol regulatory element-binding protein 1c (SREBP1c), elevating lipogenic events in mouse hepatocytes [38]. A2AAR deficient mice fed a methionine- and choline-deficient (MCD) diet displayed greater liver weight, increased severity of hepatic steatosis and liver inflammation compared to the controls [39]. Furthermore, enhanced MCD-induced NASH in A2AAR deficient mice was largely due to the increased inflammatory responses in macrophages [39]. The protective role of A2AAR for the development of NASH was largely attributed to the receptor’s effect on suppressing inflammation caused by lipotoxicity [40, 41].
However, the A2BAR has been gaining attention with respect to its role in regulating liver function. The A2BAR is associated with regulation of hepatic lipid and glucose metabolism. Lack of A2BAR protected mice from hepatic triglyceride accumulation and development of alcohol-induced fatty liver [33]. Antagonism of A2BAR increased expression of genes required for fatty acid metabolism [33]. Furthermore, A2BAR activation enhanced lipid accumulation in a cultured mouse hepatocyte cell line (AML-12) [33]. A2BAR inhibition by selective antagonist ATL-801 in diabetic KKA(Y) mice resulted in reduced glucose production during hyperinsulinemic-euglycemic clamp [42]. In macrophages from KKA(Y) mice, another A2BAR-selective antagonist, ATL-692, blocked the increase of IL-6 induced by non-selective agonist NECA, which did not occur with A1, A2A or A3 agonists. Thus, some of the beneficial effects of caffeine ingestion in KKA(Y) mice, and potentially in human T2D, may occur due to its blockade of the A2BAR [42]. Accordingly, a single nucleotide polymorphism (SNP) in A2BAR (1976T > C) has been identified to modulate the physiological effects of caffeine. The individuals with SNP-1976T > C displayed a higher postprandial glucose response after consuming caffeine and carbohydrate compared to carbohydrate alone [43].
A contrasting study showed that A2BAR activation inhibited hepatic lipogenesis via inhibiting sterol regulatory element binding protein-1 (SREBP-1). Lack of A2BAR led to the development of hepatic steatosis with elevated plasma triglyceride and cholesterol levels in high fat diet (HFD) fed mice [44]. However, adenovirus-mediated expression of hepatic A2BAR and its activation improved metabolism by reducing hepatic lipid synthesis [44]. A2BAR knockout (KO) mice on a HFD displayed impaired glucose tolerance and insulin sensitivity [45]. Treatment of wild-type (WT) mice on a HFD with an A2BAR agonist/partial agonist (BAY60–6553) improved glucose and insulin tolerance and decreased fasting blood glucose levels [45]. The impact of the A2BAR on lipid metabolism renders it a good drug target for the treatment of liver diseases, although both A2BAR agonists and antagonists have been under consideration for T2D treatment.
Although in mouse liver the level of A3AR mRNA expressed in stellate cells greatly exceeds that in hepatocytes [46], very few studies have been conducted to study the effect of A3AR on liver metabolism. A recent study showed that administration of an A3AR agonist in the form of a solubilizing prodrug (MRS7476, 5 mg/kg, p.o., b.i.d.) protected mice against NASH in the STAM mouse model [46]. The prodrug contained two succinyl ester groups that were cleaved in vivo to release the potent and highly selective A3AR agonist MRS5698. mRNA levels of human A3AR were 1.9-fold decreased in liver tissue from NAFLD patients compared to the control samples, indicating a possible role of A3AR in NAFLD pathophysiology [46]. Likewise, lack of A3AR in HFD fed mice increased gene expression of hepatic inflammation and steatosis markers [46]. This study was further supported by clinical studies that show A3AR agonists have anti-inflammatory effects in diseases such as hepatocellular carcinoma (HCC) and hepatitis [47]. An A3AR agonist, Cl-IB-MECA (namodenoson), has shown efficacy in a NASH model in the mouse [48] and is currently in a Phase 2 clinical trial for treating NASH.
2.2. Skeletal muscle
SKM is the largest insulin-sensitive organ as it comprises ~40% of total body mass in a healthy individual [49]. Due to its large mass, SKM accounts for ~30% of resting energy consumption and close to 100 % of enhanced energy consumption during exercise [49, 50]. High requirements for nutrients and fuel consumption render SKM a key contributor to systemic energy homeostasis. Following a meal, SKM is responsible for ~80% of the insulin-stimulated glucose uptake and disposal [51]. Glucose transported into myocytes via a GLUT4 transporter is immediately phosphorylated to a form that either gets stored as glycogen or enters the glycolytic pathway for oxidation. In insulin-resistant conditions, such as obesity and T2D, insulin-stimulated glucose uptake in SKM is greatly impaired [52, 53].
Contradictory studies have been reported with respect to the regulation of SKM glucose metabolism by adenosine signaling. Treatment of muscle preparations with ADA to degrade endogenous adenosine enhanced the insulin-induced glucose uptake and utilization [54, 55]. This inhibitory action of adenosine was observed to be mediated by A1AR in SKM [56]. By contrast, a study showed that activation of A1AR increased insulin-stimulated glucose uptake in rat soleus muscle [57]. AR antagonism by caffeine or a moderately A1AR-selective antagonist DPCPX (8-cyclopentyl-1,3-dipropylxanthine) resulted in a reduced rate of glucose uptake during muscle contractions [58]. Among the other ARs, few studies were conducted on A2BAR’s role in SKM metabolism. A recent study showed that A2BAR activation elevated glucose uptake in primary human myocytes and had an additive effect on insulin-mediated glucose uptake [59]. The authors also reported that SKM lacking A2BAR showed decreased expression of genes involved in mitochondrial biogenesis, function, and oxidative metabolism. However, pharmacological activation of A2BAR increased oxidative capacity [59]. A2BAR also plays a key role in muscle hypertrophy that may regulate whole body metabolism. Muscle mass was reduced in mice lacking A2BAR [59]. Pharmacological treatment of these mice with A2BAR agonist BAY60–6553 significantly increased muscle mass [59]. Interestingly, treatment of aged mice for 4 weeks with the same A2BAR agonist restored muscle mass to the elevated levels observed in young mice, protecting aging mice from muscle loss [59]. However, antagonism of the A2BAR during hyperinsulinemic-euglycemic clamp of diabetic KKA(y) mice increased glucose uptake in SKM [42]. Treatment of control animals with an A2BAR antagonist decreased insulin sensitivity, whereas an A2BAR antagonist rescued the insulin resistance phenotype in high sucrose-fed, insulin-resistant Wistar rats [60]. No detailed studies have been conducted to ascertain the role of A2AAR and A3AR in the regulation of SKM metabolism. Detailed in vivo studies with tissue-specific KOs of ARs are required to better understand the role of adenosine signaling in modulation of SKM metabolism.
2.3. Adipose tissue
Adipose tissue plays a very significant role in the pathophysiology of obesity and T2D, as it is a major organ that stores energy in the form of lipids and contributes to non-shivering thermogenesis [30]. During obesity and T2D conditions, dysfunction in adipose tissue is marked by increased lipolysis which leads to increased plasma FFAs. This causes increased uptake and storage of FFA by liver and SKM causing secondary insulin resistance [30]. Multiple studies have been conducted to understand the role of ARs in adipose tissue-metabolic processes. Activation of the A1AR has long been known to inhibit lipolysis in adipose tissue [61–63]. In Zucker rats, a genetic model of obesity, pharmacological stimulation of A1AR decreased circulating FFAs and improved insulin-stimulated glucose uptake by metabolically active peripheral tissues [64, 65]. Mechanistically, it has been demonstrated that activation of A1AR reduced cAMP levels in adipocytes that caused reduction in hormone-sensitive lipase and adipose triglyceride lipase activities inhibiting breakdown of triglycerides to FFAs [62]. These studies point toward beneficial effects of A1AR on whole body metabolism, and various A1AR agonists and partial agonists have been studied clinically for treatment of diabetes [155]. However, a study by Baraket et al., proposed that excessive A1AR activation may lead to development of obesity due to its inhibition of lipolysis [66]. A1AR KO mouse models have been used to decipher the receptor’s role in the development of obesity and regulation of whole-body metabolism. A1AR KO and WT mice kept on regular chow did not show any difference in body weight gain [67]. Similar results were found in another study where the authors demonstrated no difference in body weight in young (8 – 9 weeks of age) A1AR KO and WT mice on regular diet or HFD [68]. However, the authors reported higher body weight of aged A1AR KO (20 – 29 weeks of age) compared to WT mice fed either a regular or HFD due to higher food intake [68]. Similar changes in body weight were reported by a study, where old A1AR KO mice gained more weight than the controls [63]. However, Yang et al. demonstrated that lack of A1AR mitigated age-associated visceral adipose tissue accumulation due to reduced oxidative stress and inflammatory responses [69]. Consistent with the study, chronic treatment of Zucker obese rats with an A1AR antagonist (BW-1433) led to improved glucose tolerance [70]. These contrasting data highlight the need for the generation of adipose tissue-specific A1AR KO mouse models that can help to better understand the receptor’s role in regulation of lipolysis and its secondary effects on tissues such as liver and SKM.
A recent study showed A2AAR to be most abundant AR in mouse and human brown adipose tissue (BAT), the site of non-shivering thermogenesis upon cold exposure, suggesting the receptor’s role in regulation of thermogenesis [71]. The authors used pharmacological stimulation of the receptor and genetic approaches to show that A2AAR contributes to the browning of white fat. The activation of A2AAR also protected mice from development of diet-induced obesity by improving whole body metabolism [71]. A2AAR-deficient mice displayed enhanced adipose tissue inflammation and insulin resistance on HFD [72]. Disruption of A2AAR also resulted in enhanced palmitate-induced macrophage pro-inflammatory activation, suggesting a protective role of A2AAR in obesity-associated inflammation [72]. However, there are contrasting studies suggesting that A2AAR activation enhanced food intake and inhibited thermogenesis [73–75]. As with the A1AR, adipose-specific KO mouse models of A2AAR are warranted to understand the receptor’s role in the above-mentioned processes.
Similar to A2AAR, the A2BAR is also highly expressed in BAT. A2BAR activation was found to reduce adipocyte inflammation, as well as insulin resistance and islet destruction [76]. However, some beneficial results were also observed with A2BAR antagonists in a diabetic mouse strain [76].
Adipose-specific ablation of A2BAR reduced BAT-dependent energy expenditure, while A2BAR activation protected mice from diet-induced obesity [59]. The authors also reported high expression of A2BAR in BAT samples from lean compared to obese patients, and the receptor expression positively correlated with uncoupling protein 1 (UCP1) expression [59]. Consistent with this study, A2BAR KO mice gained more weight than the WT mice and developed insulin resistance on a HFD [45]. Increased accumulation of visceral adipose tissue was demonstrated in aged A2BAR KO mice [77, 78]. Pharmacological activation of A2BAR reduced weight, due to regulation of immune cells preventing cell death, and decreased adipogenesis, leading to improved insulin resistance [78, 79]. These studies conclude that an A2BAR agonist could potentially be used for therapeutic treatment of obesity and T2D (Fig. 1).
The role of A3AR in the regulation of adipose tissue metabolism and development of obesity and T2D is unknown.
2.4. Pancreas
T2D is characterized by a progressive decrease in β-cell function including impaired insulin secretion mainly due to rising lipotoxicity and hyperglycemia [80]. Several studies have demonstrated the importance of adenosine signaling in the regulation of β-cell function.
All four ARs are known to be expressed in mouse pancreatic tissue [81]. Cell lines derived from rat (INS-1) and mouse (β-TC6) insulin producing β-cells also express all four ARs [82, 83]. Earlier studies demonstrated the role of adenosine in stimulating insulin release from pancreatic islets. Treatment of rat pancreatic islets with adenosine decreased glucose-stimulated insulin release in a dose-dependent manner [84, 85]. Treatment with a high concentration of adenosine (100 μM) also decreased insulin secretion by mouse pancreatic islets in the presence of normal or a high glucose concentration [83]. Genetic ablation of A1AR in mice led to enhanced glucose-stimulated insulin release [67, 86]. Pharmacological antagonism of A1AR in rats showed similar effects by increasing insulin release by β-cells [87]. Mechanistically, increased cAMP levels due to inhibition of A1AR resulted in insulin release from β-cells [67, 86]. These studies were further confirmed by Yang et al., who showed enhanced secretion of insulin from islets of A1AR KO than the control mice [69]. Improved β-cell function in A1AR KO mice was attributed to reduced oxidative stress [69]. These studies strongly suggest that antagonism of A1AR could improve β-cell function in T2D conditions.
Treatment of mouse islets with adenosine also increased insulin secretion through A2AAR, and its antagonism by A2AAR-selective SCH58261 decreased insulin release [83]. By contrast, activation of A2AAR in INS-1 cells suppressed insulin release [88]. Thus, in vivo studies are required to understand the role of A2AAR in the regulation of β-cell function. Adenosine signaling can also affect β cell proliferation and survival, associated with the development of type 1 diabetes (T1D) [83, 89, 90]. Using a zebrafish model of T1D, Andersson et al. identified that NECA increased β-cell proliferation and restored normoglycemia via A2AAR activation [90]. Another study showed that A2AAR activation in mice ameliorated T1D by increasing insulin levels in pancreas as well as in circulation [91]. A2B receptor antagonists improve insulin secretion [2,21]. Potential association of A3AR with T1D was also suggested; treatment of the β-TC6 cell line with A3AR agonist Cl-IB-MECA, at very high concentrations (≥10 μM), reduced cell proliferation and viability [83]. A3AR agonists at low concentrations are associated with attenuating apoptosis. However, Cl-IB-MECA at high concentrations triggered β-TC6 cell death that could be partially prevented by antagonizing the receptor [83].
Increasing adenosine accumulation by inhibiting adenosine kinase can enhance β-cell proliferation [89]. Increased adenosine intracellular levels resulted in activation of mammalian target of rapamycin (mTOR) to promote β cell proliferation [89]. ADA inhibition can also increase adenosine levels, and elevated ADA activity in patients with T2D has been reported, suggesting its inhibition may provide therapeutic benefits [92–94].
In conclusion, available scientific data suggest that A1AR inhibition and A2AAR activation may prove useful as therapeutic approaches for increasing insulin secretion and treatment of diabetes (Fig. 1). However, the effects of A2BAR and A3AR modulators on the pancreas with respect to T2D require further validation.
2.5. Hypothalamus
Adenosine is a prototypic neuromodulator that can regulate neuronal activity including sleep, memory, anxiety, aggression, pain, locomotion, cardiac and immune functions, and neurodegenerative diseases [95–100]. However, few studies have been conducted to understand the role of neuronal ARs in regulation of energy homeostasis. A study by Wu et al. showed that HFD feeding increased adenosine levels in plasma, cerebrospinal fluid and hypothalamus of obese mice [101]. Among all ARs, expression levels of only A1AR was upregulated in the hypothalami of obese mice, suggesting A1AR’s role in the development of obesity [101]. A1AR activation or its over-expression in the paraventricular nucleus (PVN) region of the hypothalamus increased food intake and decreased energy expenditure in mice. Moreover, intracerebroventricular (i.c.v.) administration of caffeine reduced appetite in mice. The i.c.v. administration of caffeine for 7 days decreased obesity and plasma triglyceride levels to improve glucose tolerance in these mice [101].
3. P2 purinergic receptors and diabetes
Extracellular ATP activates two structurally and pharmacologically distinct families of P2 purinergic receptors - P2X and P2Y receptors. Various P2X and P2Y receptors are expressed in metabolically active tissues in human and mouse, suggesting their role in the regulation of glucose and energy homeostasis (Fig. 2).
Figure 2.
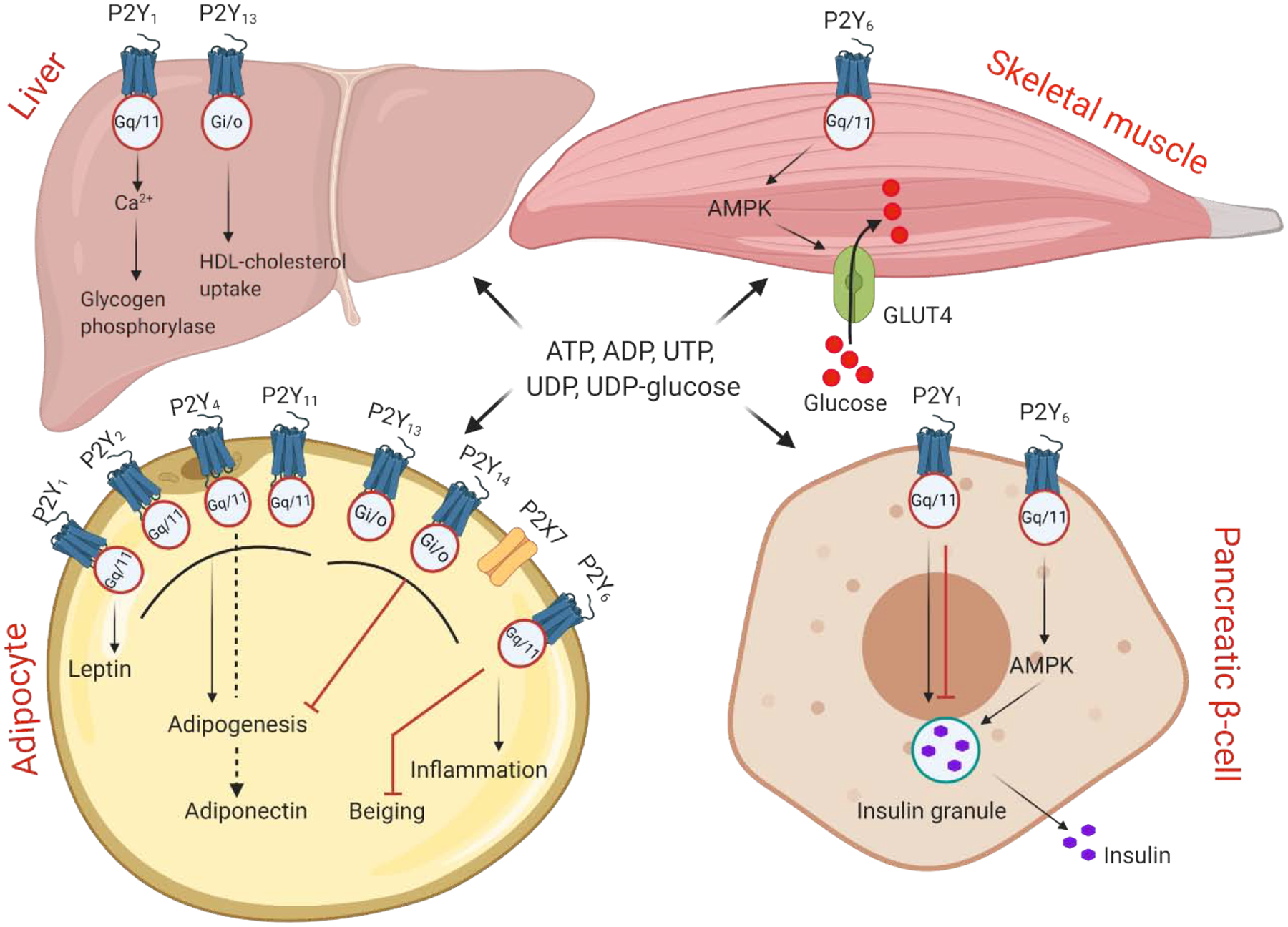
Regulation of metabolically active tissues functions by P2Y and P2X receptors.
Eight P2Y receptors are activated by receptor-specific nucleotide or nucleotide sugars. The receptors couple to different G-protein as indicated. Seven P2X receptors are membrane ion channels that are solely activated by ATP. Metabolic processes regulated by P2 receptors in liver, skeletal muscle, adipose tissue and pancreas. See Section 3 for details.
3.1. Liver
P2 receptors have been shown to regulate glucose and lipid metabolism in liver [102]. In isolated rat hepatocytes, P2Y1R activation stimulates glycogen phosphorylase by increasing cytoplasmic Ca2+ levels [103] and may contribute to hepatic glucose output. Purinergic signaling also modulates beneficial reverse cholesterol transport in liver. High density lipoprotein (HDL)-cholesterol levels in plasma were significantly decreased and their liver uptake increased after chronic P2Y13R activation by a partial agonist [104]. This conclusion was supported by Fabre et al. using P2Y13R KO mice [105]. Cangrelor (AR-C69931MX), a P2Y12R antagonist and approved antithrombotic agent that also acts as a partial P2Y13 agonist, stimulated hepatic HDL uptake in control mice and mice lacking the HDL-selective cholesteryl ester uptake system, but not in P2Y13R KO mice [73]. Knowledge of P2R function in the liver tissue associated with the development of diabetes and obesity is lacking. Liver-specific KO of these receptors should be generated to decipher their role in the liver pathophysiology and development of T2D.
3.2. Skeletal muscle
Purinergic receptors are expressed in human SKM fibers [106]. Enhanced glucose uptake in the C2C12 mouse SKM cell line was demonstrated after ATP stimulation, while nonselective P2R antagonist (suramin) ablated the effect, suggesting involvement of P2 receptors [107]. Balasubramanian et al. showed that stimulation of C2C12 cells with a P2Y6R dinucleotide agonist (MRS2957) significantly increased glucose uptake, and this stimulation was decreased by a selective P2Y6R antagonist (MRS2578) [108]. The enhancement of glucose uptake was mediated by AMP-activated protein kinase (AMPK) activation as treatment with an AMPK inhibitor abolished the effect [108]. These results were supported by a recent study where P2Y6R was KO specifically in SKM, and its effect on glucose metabolism was studied. Ablation of P2Y6R impaired glucose tolerance and insulin sensitivity and reduced insulin stimulated glucose uptake by mouse SKM (gastrocnemius) [109]. Thus, P2Y6R activation emerged as a potential means for improving SKM glucose uptake in diabetic subjects.
3.3. Pancreas
Insulin secretory vesicles contain adenine nucleotides, which are co-released with insulin during exocytosis [110–114]. Secreted ATP and ADP act in an autocrine manner in pancreatic β-cells by stimulating P2 receptors [115, 116]. ATP was proposed to act via the P2X3R by increasing intracellular calcium concentration [117]. Another study showed that ATP activates P2Y1R on human β-cells, stimulating electrical activity and coupling Ca2+ influx to Ca2+ release from endoplasmic reticulum stores [115, 118]. The P2Y1R acts on β-cells by activating protein kinase C [119]. Short ATP pulses stimulate, whereas longer exposure of β-cells to high concentrations of ATP can have negative feedback on the insulin secretion [117, 120–122]. This dual effect of ATP coordinates with Ca2+ oscillations [123]. Some earlier studies reported similar insulin responses to P2R agonist treatment in the perfused pancreas from T1D and T2D compared to control mouse groups. ADPβS, a P2Y1R agonist, induced a similar insulin response in the perfused pancreas of streptozotocin-treated (STZ) rats as in controls [124, 125]. These studies suggest that P2YR agonists might be useful for enhancing insulin release from glucose-unresponsive islets from diabetic animals. However, another study by Leon et al. indicated negative regulation of insulin secretion by P2Y1R activation. The authors observed a higher insulin response to a stimulatory glucose concentration in islets isolated from P2Y1R KO compared to control mice [126]. Similarly, Ohtani et al. demonstrated significant inhibition of insulin release at high glucose concentration by P2Y1R activation [127]. Inhibition of insulin release was also observed after P2Y6R activation in mouse islets [127]. By contrast, activation of mouse MIN6 cells by a P2Y1R agonist (2-MeSADP) and a P2Y6R agonist (Up3U) stimulated calcium-mediated insulin release [128]. The insulin secretion was blocked by P2Y1R and P2Y6R antagonists, respectively [128]. Mechanistically, P2Y6 agonists induced AMPK activation, which enhanced insulin release by MIN6 cells [129].
3.4. Adipocytes
P2X and P2Y receptors are expressed in various adipose depots and are involved in regulation of adipose tissue processes such as differentiation, browning, glucose uptake and thermogenesis. P2Y1, P2Y2, P2Y4 and P2Y11 receptor activation promoted adipogenic differentiation of bone marrow or adipose tissue derived stem cells [130–132]. P2Y1 and P2Y4 receptor activation by ADP and UTP, respectively, stimulated adipogenesis in human bone marrow-derived mesenchymal stem cells though activation of PPARγ, while the action of ectonucleotidases leading to increased AR activation resulted in osteogenic differentiation [133].
Human adipocytes display constitutive P2Y2R activity that suppresses basal lipolysis [134]. Pharmacological inhibition or knockdown of the P2Y2R increases cAMP levels and hence basal lipolysis [134]. P2Y2KO mice on a HFD were protected from diet-induced obesity, as the preadipocytes from KO mice differentiated less robustly than the WT cells [135]. Protection from obesity also resulted in reduced inflammation and improved glucose tolerance in P2Y2KO mice on a HFD [135]. Anti-adipogenic effects were exhibited upon P2Y13, P2Y14 and P2X7 receptor activation [131, 136]. Supporting an anti-adipogenic effect of the P2X7R, P2X7R KO mice exhibited increased body weight, adipocyte hyperplasia, ectopic lipid accumulation in pancreas [137]. Some studies indicate that P2X7R activation resulted in differentiation of stem cells into an osteoclast lineage rather than adipocytes [138]. P2X7R expression is enhanced in human adipose tissues from patients suffering from metabolic syndrome [139]. The P2X7R and NLRP3 inflammasome expression and IL-1β secretion were higher in obese subjects [140]. In metabolically unhealthy obese humans, a P2X7R antagonist blocked the adaptive Th17 polarization of Teff cells in visceral adipose tissue [141]. P2X7R activates and its antagonists suppress the NLRP3 inflammasome, which is a step leading to the pathology of diabetes [142]. Thus, P2X7R antagonists might be useful in treating diabetes [142]. In contrast, another study showed that P2X7 KO mice are not protected from diet-induced obesity, adipose tissue inflammation and associated metabolic dysfunction [143].
Interestingly, lack of P2Y14R in the whole body did not protect mice from diet-induced obesity [144]. However, P2Y14 KO mice were protected from HFD-induced insulin resistance in metabolic tissues such as liver, SKM and adipose tissue. Furthermore, the authors showed that lack of P2Y14R protected mice from macrophage infiltration in liver, thereby reducing obesity-associated inflammation [144]. Lack of P2Y14R in other metabolically active tissues and immune cells may contribute to the observed phenotype. Hence, an adipose tissue-specific P2Y14 KO mouse model may provide better understanding of the receptor’s role in the regulation of adipose tissue metabolism.
P2 receptors have been studied to decipher their role in glucose uptake in adipocytes. Stimulation of rat adipocytes with low concentrations of ATP inhibited insulin-stimulated glucose transport [145, 146]. A study showed that activation of the P2Y6R-AMPK pathway caused GLUT-4 translocation thereby enhancing glucose uptake in mouse white adipocytes [108]. A recent study showed that KO of P2Y6R in the whole body or in an adipocyte-specific manner protected mice from diet-induced obesity to improve glucose tolerance, insulin sensitivity and to reduce systemic inflammation. This improvement was associated with reduced JNK signaling with consequent increase of peroxisome proliferator-activated receptor (PPAR)-α and beiging of white fat [109]. This suggests that P2Y6R antagonists could be examined as a potential treatment of diabetes and obesity.
Purinergic signaling can also modulate production and secretion of adipokines critical for regulating metabolism in an organism. P2Y1R inhibition by highly selective antagonist MRS2500 decreased leptin production in isolated mouse white adipocytes [147]. Circulating leptin levels were reduced in P2Y1R KO mice, indicating that P2Y1R controls leptin production and secretion in white adipocytes [147]. Adiponectin expression and secretion in cardiac adipocytes was inhibited by a P2Y4R agonist (MRS4062), while adiponectin levels were increased in P2Y4R KO mice [148].
Mouse brown adipocytes express P2X and P2Y receptors [149]. P2X5R mRNA expression level is significantly higher in BAT compared to WAT and other mouse tissues [150]. Therefore, P2X5R is proposed as a novel cell surface marker for brown and beige adipocytes [150]. Furthermore, chronic cold exposure increased P2X5R expression in BAT and subcutaneous WAT, suggesting its role in thermogenesis [150, 151]. However, the mechanism of P2X5R action in different adipose tissues is largely unknown.
3.5. Hypothalamus
Over the last decade, interest in understanding the role of central nervous system (CNS) in the regulation of energy balance in mammals has been growing rapidly. Arcuate nucleus of the hypothalamus (ARH) in brain can respond to different nutrient signals from gut and adipose tissues to influence energy homeostasis. Activation of agouti-related peptide (AgRP) neurons in ARH regulates appetite by enhancing food intake [152]. A comprehensive study showed that P2Y6R is expressed in AgRP neurons and that P2Y6R activation by UDP increased AgRP firing rate and feeding in mice [153]. Pharmacological inhibition of P2Y6R in AgRP neurons by MRS2578 decreased food intake in mice [153]. Moreover, AgRP-P2Y6R KO mice are protected from obesity and insulin resistance on an obesogenic diet [154]. These studies show that P2Y6R in the CNS can be targeted to inhibit excessive feeding and to overcome development of obesity and T2D.
4. Conclusion
Present preclinical studies have highlighted the significance of adenosine signaling in the regulation of pathophysiological processes underlying diabetes. Among the Gs-coupled ARs, A2BAR stands out as a potential therapeutic target, as the receptor activation improves function of SKM and brown fat in aged as well as obese mice (Fig. 1). However, future studies are required to address A2BAR’s role in other metabolically active tissues such as liver and brain. A3AR has also emerged as a potential target providing protection against the development of NASH. However, its role in other metabolic tissues is largely unknown. Among P2Y and P2X receptors, inhibiting P2Y6R and P2X7R may provide therapeutic benefits against diet-induced obesity and diabetes (Fig. 2). A majority of the present studies utilized pharmacological manipulation or whole-body KO mouse models, which makes understanding of a receptor’s role in a tissue-specific manner highly complicated. Future studies are warranted to address this uncertainty. Numerous agonists and antagonists for purinergic receptors and inhibitors of enzymes regulating adenosine levels have been synthesized with the potential for use in preclinical and clinical studies targeting diabetes and associated complications [155–159].
Combining tissue-specific genetic approaches with pharmacological interventions may foster the development of novel drugs targeting purinergic signaling that may mitigate the development of diabetes and associated metabolic disorders.
Acknowledgment:
Funding from the NIDDK Intramural Research Program (ZIADK031116; ZIADK031117) is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Burnstock G and Gentile D, The involvement of purinergic signalling in obesity. Purinergic Signal, 2018. 14(2): p. 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G and Novak I, Purinergic signalling and diabetes. Purinergic Signal, 2013. 9(3): p. 307–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenstein A and Ravid K, G protein-coupled receptors and adipogenesis: a focus on adenosine receptors. J Cell Physiol, 2014. 229(4): p. 414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonioli L, et al. , Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer, 2013. 13(12): p. 842–57. [DOI] [PubMed] [Google Scholar]
- 5.Peleli M and Carlstrom M, Adenosine signaling in diabetes mellitus and associated cardiovascular and renal complications. Mol Aspects Med, 2017. 55: p. 62–74. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G, et al. , Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol, 1970. 40(4): p. 668–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G, Purinergic nerves. Pharmacol Rev, 1972. 24(3): p. 509–81. [PubMed] [Google Scholar]
- 8.MacDonald PE, et al. , Release of small transmitters through kiss-and-run fusion pores in rat pancreatic beta cells. Cell Metab, 2006. 4(4): p. 283–90. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, et al. , Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol, 2007. 9(8): p. 945–53. [DOI] [PubMed] [Google Scholar]
- 10.Chekeni FB, et al. , Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature, 2010. 467(7317): p. 863–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott MR, et al. , Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature, 2009. 461(7261): p. 282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anselmi F, et al. , ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A, 2008. 105(48): p. 18770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faigle M, et al. , ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One, 2008. 3(7): p. e2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonioli L, et al. , CD39 and CD73 in immunity and inflammation. Trends Mol Med, 2013. 19(6): p. 355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredholm BB, Adenosine--a physiological or pathophysiological agent? J Mol Med (Berl), 2014. 92(3): p. 201–6. [DOI] [PubMed] [Google Scholar]
- 16.van Dam RM and Hu FB, Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA, 2005. 294(1): p. 97–104. [DOI] [PubMed] [Google Scholar]
- 17.Reunanen A, Heliovaara M, and Aho K, Coffee consumption and risk of type 2 diabetes mellitus. Lancet, 2003. 361(9358): p. 702–3; author reply 703. [DOI] [PubMed] [Google Scholar]
- 18.Tuomilehto J, et al. , Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. JAMA, 2004. 291(10): p. 1213–9. [DOI] [PubMed] [Google Scholar]
- 19.Salazar-Martinez E, et al. , Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med, 2004. 140(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 20.Shi X, et al. , Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: a systematic review and meta-analysis. Nutr J, 2016. 15(1): p. 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane JD, et al. , Caffeine impairs glucose metabolism in type 2 diabetes. Diabetes Care, 2004. 27(8): p. 2047–8. [DOI] [PubMed] [Google Scholar]
- 22.Moisey LL, et al. , Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am J Clin Nutr, 2008. 87(5): p. 1254–61. [DOI] [PubMed] [Google Scholar]
- 23.Gavrieli A, et al. , Gender and body mass index modify the effect of increasing amounts of caffeinated coffee on postprandial glucose and insulin concentrations; a randomized, controlled, clinical trial. Metabolism, 2013. 62(8): p. 1099–106. [DOI] [PubMed] [Google Scholar]
- 24.Krebs JD, et al. , A cross-over study of the acute effects of espresso coffee on glucose tolerance and insulin sensitivity in people with type 2 diabetes mellitus. Metabolism, 2012. 61(9): p. 1231–7. [DOI] [PubMed] [Google Scholar]
- 25.van Dijk AE, et al. , Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care, 2009. 32(6): p. 1023–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wedick NM, et al. , Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr J, 2011. 10: p. 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dam RM, Pasman WJ, and Verhoef P, Effects of coffee consumption on fasting blood glucose and insulin concentrations: randomized controlled trials in healthy volunteers. Diabetes Care, 2004. 27(12): p. 2990–2. [DOI] [PubMed] [Google Scholar]
- 28.Ohnaka K, et al. , Effects of 16-week consumption of caffeinated and decaffeinated instant coffee on glucose metabolism in a randomized controlled trial. J Nutr Metab, 2012. 2012: p. 207426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding M, et al. , Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care, 2014. 37(2): p. 569–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden M and Shulman GI, The integrative biology of type 2 diabetes. Nature, 2019. 576(7785): p. 51–60. [DOI] [PubMed] [Google Scholar]
- 31.Fausther M, Extracellular adenosine: a critical signal in liver fibrosis. Am J Physiol Gastrointest Liver Physiol, 2018. 315(1): p. G12–G19. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Benitez E, et al. , Regulation of glycogen metabolism in hepatocytes through adenosine receptors. Role of Ca2+ and cAMP. Eur J Pharmacol, 2002. 437(3): p. 105–11. [DOI] [PubMed] [Google Scholar]
- 33.Peng Z, et al. , Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest, 2009. 119(3): p. 582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu IM, et al. , Increase in adenosine A1 receptor gene expression in the liver of streptozotocin-induced diabetic rats. Diabetes Metab Res Rev, 2003. 19(3): p. 209–15. [DOI] [PubMed] [Google Scholar]
- 35.Odashima M, et al. , Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology, 2005. 129(1): p. 26–33. [DOI] [PubMed] [Google Scholar]
- 36.Awad AS, et al. , Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol, 2006. 290(4): p. F828–37. [DOI] [PubMed] [Google Scholar]
- 37.Lukashev D, et al. , Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol, 2004. 173(1): p. 21–4. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y, et al. , Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity. Hepatology, 2018. 68(1): p. 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, et al. , Mice lacking adenosine 2A receptor reveal increased severity of MCD-induced NASH. J Endocrinol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imarisio C, et al. , Adenosine A(2a) receptor stimulation prevents hepatocyte lipotoxicity and non-alcoholic steatohepatitis (NASH) in rats. Clin Sci (Lond), 2012. 123(5): p. 323–32. [DOI] [PubMed] [Google Scholar]
- 41.Alchera E, et al. , Adenosine A2a receptor stimulation blocks development of nonalcoholic steatohepatitis in mice by multilevel inhibition of signals that cause immunolipotoxicity. Transl Res, 2017. 182: p. 75–87. [DOI] [PubMed] [Google Scholar]
- 42.Figler RA, et al. , Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes, 2011. 60(2): p. 669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banks NF, et al. , Genetic Polymorphisms in ADORA2A and CYP1A2 Influence Caffeine’s Effect on Postprandial Glycaemia. Sci Rep, 2019. 9(1): p. 10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koupenova M, et al. , A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation, 2012. 125(2): p. 354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnston-Cox H, et al. , The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One, 2012. 7(7): p. e40584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suresh RR, et al. , Design and in vivo activity of A3 adenosine receptor agonist prodrugs. Purinergic Signal, 2020, 16 p. 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fishman P, et al. , Pharmacological and therapeutic effects of A3 adenosine receptor agonists. Drug Discov Today, 2012. 17(7–8): p. 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fishman P, et al. , The A3 adenosine receptor agonist, namodenoson, ameliorates nonalcoholic steatohepatitis in mice. Int J Mol Med, 2019. 44(6): p. 2256–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zurlo F, et al. , Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest, 1990. 86(5): p. 1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallagher D, et al. , Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol, 1998. 275(2): p. E249–58. [DOI] [PubMed] [Google Scholar]
- 51.DeFronzo RA, et al. , The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes, 1981. 30(12): p. 1000–7. [DOI] [PubMed] [Google Scholar]
- 52.DeFronzo RA, et al. , Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest, 1985. 76(1): p. 149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitrakou A, et al. , Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes, 1990. 39(11): p. 1381–90. [DOI] [PubMed] [Google Scholar]
- 54.Leighton B, et al. , Effects of adenosine deaminase on the sensitivity of glucose transport, glycolysis and glycogen synthesis to insulin in muscles of the rat. Int J Biochem, 1988. 20(1): p. 23–7. [DOI] [PubMed] [Google Scholar]
- 55.Stace PB, et al. , Long term culture of rat soleus muscle in vitro. Its effects on glucose utilization and insulin sensitivity. FEBS Lett, 1990. 273(1–2): p. 91–4. [DOI] [PubMed] [Google Scholar]
- 56.Challiss RA, Richards SJ, and Budohoski L, Characterization of the adenosine receptor modulating insulin action in rat skeletal muscle. Eur J Pharmacol, 1992. 226(2): p. 121–8. [DOI] [PubMed] [Google Scholar]
- 57.Thong FS, et al. , Activation of the A1 adenosine receptor increases insulin-stimulated glucose transport in isolated rat soleus muscle. Appl Physiol Nutr Metab, 2007. 32(4): p. 701–10. [DOI] [PubMed] [Google Scholar]
- 58.Vergauwen L, Hespel P, and Richter EA, Adenosine receptors mediate synergistic stimulation of glucose uptake and transport by insulin and by contractions in rat skeletal muscle. J Clin Invest, 1994. 93(3): p. 974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gnad T, et al. , Adenosine/A2B Receptor Signaling Ameliorates the Effects of Aging and Counteracts Obesity. Cell Metab, 2020. 32(1): p. 56–70 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sacramento JF, et al. , A 2 Adenosine Receptors Mediate Whole-Body Insulin Sensitivity in a Prediabetes Animal Model: Primary Effects on Skeletal Muscle. Front Endocrinol (Lausanne), 2020. 11: p. 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhalla AK, et al. , Antilipolytic activity of a novel partial A1 adenosine receptor agonist devoid of cardiovascular effects: comparison with nicotinic acid. J Pharmacol Exp Ther, 2007. 321(1): p. 327–33. [DOI] [PubMed] [Google Scholar]
- 62.Dhalla AK, et al. , A1 adenosine receptor: role in diabetes and obesity. Handb Exp Pharmacol, 2009(193): p. 271–95. [DOI] [PubMed] [Google Scholar]
- 63.Johansson SM, et al. , Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol, 2008. 597(1–3): p. 92–101. [DOI] [PubMed] [Google Scholar]
- 64.Dhalla AK, et al. , A1 adenosine receptor partial agonist lowers plasma FFA and improves insulin resistance induced by high-fat diet in rodents. Am J Physiol Endocrinol Metab, 2007. 292(5): p. E1358–63. [DOI] [PubMed] [Google Scholar]
- 65.Schoelch C, et al. , Characterization of adenosine-A1 receptor-mediated antilipolysis in rats by tissue microdialysis, 1H-spectroscopy, and glucose clamp studies. Diabetes, 2004. 53(7): p. 1920–6. [DOI] [PubMed] [Google Scholar]
- 66.Barakat H, et al. , Differences in the expression of the adenosine A1 receptor in adipose tissue of obese black and white women. J Clin Endocrinol Metab, 2006. 91(5): p. 1882–6. [DOI] [PubMed] [Google Scholar]
- 67.Johansson SM, et al. , A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochem Pharmacol, 2007. 74(11): p. 1628–35. [DOI] [PubMed] [Google Scholar]
- 68.Faulhaber-Walter R, et al. , Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes, 2011. 60(10): p. 2578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang T, et al. , Abrogation of adenosine A1 receptor signalling improves metabolic regulation in mice by modulating oxidative stress and inflammatory responses. Diabetologia, 2015. 58(7): p. 1610–20. [DOI] [PubMed] [Google Scholar]
- 70.Xu B, et al. , A1 adenosine receptor antagonism improves glucose tolerance in Zucker rats. Am J Physiol, 1998. 274(2): p. E271–9. [DOI] [PubMed] [Google Scholar]
- 71.Gnad T, et al. , Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature, 2014. 516(7531): p. 395–9. [DOI] [PubMed] [Google Scholar]
- 72.Pei Y, et al. , Regulation of adipose tissue inflammation by adenosine 2A receptor in obese mice. J Endocrinol, 2018. 239(3): p. 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coupar IM and Tran BL, Effects of adenosine agonists on consumptive behaviour and body temperature. J Pharm Pharmacol, 2002. 54(2): p. 289–94. [DOI] [PubMed] [Google Scholar]
- 74.Fain JN, Pointer RH, and Ward WF, Effects of adenosine nucleosides on adenylate cyclase, phosphodiesterase, cyclic adenosine monophosphate accumulation, and lipolysis in fat cells. J Biol Chem, 1972. 247(21): p. 6866–72. [PubMed] [Google Scholar]
- 75.Schimmel RJ and McCarthy L, Role of adenosine as an endogenous regulator of respiration in hamster brown adipocytes. Am J Physiol, 1984. 246(3 Pt 1): p. C301–7. [DOI] [PubMed] [Google Scholar]
- 76.Merighi S, Borea PA, and Gessi S, Adenosine receptors and diabetes: Focus on the A(2B) adenosine receptor subtype. Pharmacol Res, 2015. 99: p. 229–36. [DOI] [PubMed] [Google Scholar]
- 77.Csoka B, et al. , A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes, 2014. 63(3): p. 850–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peleli M, et al. , In adenosine A2B knockouts acute treatment with inorganic nitrate improves glucose disposal, oxidative stress, and AMPK signaling in the liver. Front Physiol, 2015. 6: p. 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Csoka B, et al. , Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J, 2012. 26(1): p. 376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eizirik DL, Pasquali L, and Cnop M, Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol, 2020. 16(7): p. 349–362. [DOI] [PubMed] [Google Scholar]
- 81.Novak I, Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal, 2008. 4(3): p. 237–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Topfer M, et al. , Modulation of insulin release by adenosine A1 receptor agonists and antagonists in INS-1 cells: the possible contribution of 86Rb+ efflux and 45Ca2+ uptake. Cell Biochem Funct, 2008. 26(8): p. 833–43. [DOI] [PubMed] [Google Scholar]
- 83.Ohtani M, Oka T, and Ohura K, Possible involvement of A(2)A and A(3) receptors in modulation of insulin secretion and beta-cell survival in mouse pancreatic islets. Gen Comp Endocrinol, 2013. 187: p. 86–94. [DOI] [PubMed] [Google Scholar]
- 84.Bertrand G, Nenquin M, and Henquin JC, Comparison of the inhibition of insulin release by activation of adenosine and alpha 2-adrenergic receptors in rat beta-cells. Biochem J, 1989. 259(1): p. 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ismail NA, El Denshary EE, and Montague W, Adenosine and the regulation of insulin secretion by isolated rat islets of Langerhans. Biochem J, 1977. 164(2): p. 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salehi A, et al. , Absence of adenosine A1 receptors unmasks pulses of insulin release and prolongs those of glucagon and somatostatin. Life Sci, 2009. 85(11–12): p. 470–6. [DOI] [PubMed] [Google Scholar]
- 87.Zywert A, Szkudelska K, and Szkudelski T, Effects of adenosine A(1) receptor antagonism on insulin secretion from rat pancreatic islets. Physiol Res, 2011. 60(6): p. 905–11. [DOI] [PubMed] [Google Scholar]
- 88.Rusing D, Muller CE, and Verspohl EJ, The impact of adenosine and A(2B) receptors on glucose homoeostasis. J Pharm Pharmacol, 2006. 58(12): p. 1639–45. [DOI] [PubMed] [Google Scholar]
- 89.Annes JP, et al. , Adenosine kinase inhibition selectively promotes rodent and porcine islet beta-cell replication. Proc Natl Acad Sci U S A, 2012. 109(10): p. 3915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andersson O, et al. , Adenosine signaling promotes regeneration of pancreatic beta cells in vivo. Cell Metab, 2012. 15(6): p. 885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nemeth ZH, et al. , Adenosine receptor activation ameliorates type 1 diabetes. FASEB J, 2007. 21(10): p. 2379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khemka VK, et al. , Raised serum adenosine deaminase level in nonobese type 2 diabetes mellitus. ScientificWorldJournal, 2013. 2013: p. 404320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar R, et al. , Antihyperglycemic, antihyperlipidemic, anti-inflammatory and adenosine deaminase-lowering effects of garlic in patients with type 2 diabetes mellitus with obesity. Diabetes Metab Syndr Obes, 2013. 6: p. 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JG, et al. , Changes in Adenosine Deaminase Activity in Patients with Type 2 Diabetes Mellitus and Effect of DPP-4 Inhibitor Treatment on ADA Activity. Diabetes Metab J, 2011. 35(2): p. 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen JF, Eltzschig HK, and Fredholm BB, Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov, 2013. 12(4): p. 265–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schulte G and Fredholm BB, Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal, 2003. 15(9): p. 813–27. [DOI] [PubMed] [Google Scholar]
- 97.Cunha RA, How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem, 2016. 139(6): p. 1019–1055. [DOI] [PubMed] [Google Scholar]
- 98.Fredholm BB, et al. , Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol, 2000. 362(4–5): p. 364–74. [DOI] [PubMed] [Google Scholar]
- 99.Williams M, Adenosine: The prototypic neuromodulator. Neurochem Int, 1989. 14(3): p. 249–64. [DOI] [PubMed] [Google Scholar]
- 100.Fredholm BB, et al. , Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol, 2005. 45: p. 385–412. [DOI] [PubMed] [Google Scholar]
- 101.Wu L, et al. , Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice. Nat Commun, 2017. 8: p. 15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burnstock G, Vaughn B, and Robson SC, Purinergic signalling in the liver in health and disease. Purinergic Signal, 2014. 10(1): p. 51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dixon CJ, et al. , Regulation of rat hepatocyte function by P2Y receptors: focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5’-diphosphate. J Pharmacol Exp Ther, 2004. 311(1): p. 334–41. [DOI] [PubMed] [Google Scholar]
- 104.Serhan N, et al. , Chronic pharmacological activation of P2Y13 receptor in mice decreases HDL-cholesterol level by increasing hepatic HDL uptake and bile acid secretion. Biochim Biophys Acta, 2013. 1831(4): p. 719–25. [DOI] [PubMed] [Google Scholar]
- 105.Fabre AC, et al. , P2Y13 receptor is critical for reverse cholesterol transport. Hepatology, 2010. 52(4): p. 1477–83. [DOI] [PubMed] [Google Scholar]
- 106.Borno A, et al. , Purinergic receptors expressed in human skeletal muscle fibres. Purinergic Signal, 2012. 8(2): p. 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim MS, et al. , ATP stimulates glucose transport through activation of P2 purinergic receptors in C(2)C(12) skeletal muscle cells. Arch Biochem Biophys, 2002. 401(2): p. 205–14. [DOI] [PubMed] [Google Scholar]
- 108.Balasubramanian R, et al. , Enhancement of glucose uptake in mouse skeletal muscle cells and adipocytes by P2Y6 receptor agonists. PLoS One, 2014. 9(12): p. e116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jain S, Pydi SP, Toti KS, Robaye B, Idzko M, Gavrilova O, Wess J, Jacobson KA, Lack of Adipocyte Purinergic P2Y6 Receptor Greatly Improves Whole Body Glucose Homeostasis. Proc. Natl. Acad. Sci USA, 2020, 117 (48), p. 30763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hutton JC, Penn EJ, and Peshavaria M, Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J, 1983. 210(2): p. 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galvanovskis J, Braun M, and Rorsman P, Exocytosis from pancreatic beta-cells: mathematical modelling of the exit of low-molecular-weight granule content. Interface Focus, 2011. 1(1): p. 143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Braun M, et al. , Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells. J Gen Physiol, 2007. 129(3): p. 221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hazama A, Hayashi S, and Okada Y, Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch, 1998. 437(1): p. 31–5. [DOI] [PubMed] [Google Scholar]
- 114.Obermuller S, et al. , Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci, 2005. 118(Pt 18): p. 4271–82. [DOI] [PubMed] [Google Scholar]
- 115.Khan S, et al. , Autocrine activation of P2Y1 receptors couples Ca (2+) influx to Ca (2+) release in human pancreatic beta cells. Diabetologia, 2014. 57(12): p. 2535–45. [DOI] [PubMed] [Google Scholar]
- 116.Silva AM, et al. , Electrophysiological and immunocytochemical evidence for P2X purinergic receptors in pancreatic beta cells. Pancreas, 2008. 36(3): p. 279–83. [DOI] [PubMed] [Google Scholar]
- 117.Jacques-Silva MC, et al. , ATP-gated P2X3 receptors constitute a positive autocrine signal for insulin release in the human pancreatic beta cell. Proc Natl Acad Sci U S A, 2010. 107(14): p. 6465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tengholm A, Purinergic P2Y1 receptors take centre stage in autocrine stimulation of human beta cells. Diabetologia, 2014. 57(12): p. 2436–9. [DOI] [PubMed] [Google Scholar]
- 119.Wuttke A, Idevall-Hagren O, and Tengholm A, P2Y(1) receptor-dependent diacylglycerol signaling microdomains in beta cells promote insulin secretion. FASEB J, 2013. 27(4): p. 1610–20. [DOI] [PubMed] [Google Scholar]
- 120.Grapengiesser E, Dansk H, and Hellman B, Pulses of external ATP aid to the synchronization of pancreatic beta-cells by generating premature Ca(2+) oscillations. Biochem Pharmacol, 2004. 68(4): p. 667–74. [DOI] [PubMed] [Google Scholar]
- 121.Hellman B, Dansk H, and Grapengiesser E, Pancreatic beta-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab, 2004. 286(5): p. E759–65. [DOI] [PubMed] [Google Scholar]
- 122.Gylfe E, et al. , The neurotransmitter ATP triggers Ca2+ responses promoting coordination of pancreatic islet oscillations. Pancreas, 2012. 41(2): p. 258–63. [DOI] [PubMed] [Google Scholar]
- 123.Bauer C, et al. , ATP mediates a negative autocrine signal on stimulus-secretion coupling in mouse pancreatic beta-cells. Endocrine, 2019. 63(2): p. 270–283. [DOI] [PubMed] [Google Scholar]
- 124.Hillaire-Buys D, et al. , P2y purinoceptor responses of beta cells and vascular bed are preserved in diabetic rat pancreas. Br J Pharmacol, 1992. 106(3): p. 610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tang J, et al. , Preservation of insulin secretory responses to P2 purinoceptor agonists in Zucker diabetic fatty rats. Am J Physiol, 1996. 270(3 Pt 1): p. E504–12. [DOI] [PubMed] [Google Scholar]
- 126.Leon C, et al. , The P2Y(1) receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signal, 2005. 1(2): p. 145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohtani M, et al. , Evidence for the possible involvement of the P2Y(6) receptor in Ca (2+) mobilization and insulin secretion in mouse pancreatic islets. Purinergic Signal, 2008. 4(4): p. 365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Balasubramanian R, et al. , Activation of distinct P2Y receptor subtypes stimulates insulin secretion in MIN6 mouse pancreatic beta cells. Biochem Pharmacol, 2010. 79(9): p. 1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Balasubramanian R, et al. , AMP-activated protein kinase as regulator of P2Y(6) receptor-induced insulin secretion in mouse pancreatic beta-cells. Biochem Pharmacol, 2013. 85(7): p. 991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawano S, et al. , ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium, 2006. 39(4): p. 313–24. [DOI] [PubMed] [Google Scholar]
- 131.Zippel N, et al. , Purinergic receptors influence the differentiation of human mesenchymal stem cells. Stem Cells Dev, 2012. 21(6): p. 884–900. [DOI] [PubMed] [Google Scholar]
- 132.Li W, et al. , Regulation of the osteogenic and adipogenic differentiation of bone marrow-derived stromal cells by extracellular uridine triphosphate: The role of P2Y2 receptor and ERK1/2 signaling. Int J Mol Med, 2016. 37(1): p. 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ciciarello M, et al. , Extracellular purines promote the differentiation of human bone marrow-derived mesenchymal stem cells to the osteogenic and adipogenic lineages. Stem Cells Dev, 2013. 22(7): p. 1097–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ali SB, Turner JJO, and Fountain SJ, Constitutive P2Y2 receptor activity regulates basal lipolysis in human adipocytes. J Cell Sci, 2018. 131(22). [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y, et al. , P2Y2 Receptor Promotes High-Fat Diet-Induced Obesity. Front Endocrinol (Lausanne), 2020. 11: p. 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Biver G, et al. , Role of the P2Y13 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Stem Cells, 2013. 31(12): p. 2747–58. [DOI] [PubMed] [Google Scholar]
- 137.Beaucage KL, et al. , Loss of P2X7 nucleotide receptor function leads to abnormal fat distribution in mice. Purinergic Signal, 2014. 10(2): p. 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li W, et al. , Role of P2 × 7 receptor in the differentiation of bone marrow stromal cells into osteoblasts and adipocytes. Exp Cell Res, 2015. 339(2): p. 367–79. [DOI] [PubMed] [Google Scholar]
- 139.Madec S, et al. , Adipocyte P2X7 receptors expression: a role in modulating inflammatory response in subjects with metabolic syndrome? Atherosclerosis, 2011. 219(2): p. 552–8. [DOI] [PubMed] [Google Scholar]
- 140.Pandolfi J, et al. , Purinergic signaling modulates human visceral adipose inflammatory responses: implications in metabolically unhealthy obesity. J Leukoc Biol, 2015. 97(5): p. 941–949. [DOI] [PubMed] [Google Scholar]
- 141.Pandolfi JB, et al. , ATP-Induced Inflammation Drives Tissue-Resident Th17 Cells in Metabolically Unhealthy Obesity. J Immunol, 2016. 196(8): p. 3287–96. [DOI] [PubMed] [Google Scholar]
- 142.Wang D, et al. , P2X7 receptor mediates NLRP3 inflammasome activation in depression and diabetes. Cell Biosci, 2020. 10: p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun S, et al. , The ATP-P2X7 signaling axis is dispensable for obesity-associated inflammasome activation in adipose tissue. Diabetes, 2012. 61(6): p. 1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu J, et al. , GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet-induced obesity. J Immunol, 2012. 189(4): p. 1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chang KJ and Cuatrecasas P, Adenosine triphosphate-dependent inhibition of insulin-stimulated glucose transport in fat cells. Possible role of membrane phosphorylation. J Biol Chem, 1974. 249(10): p. 3170–80. [PubMed] [Google Scholar]
- 146.Halperin ML, Mak ML, and Taylor WM, Control of glucose transport in adipose tissue of the rat: role of insulin, ATP, and intracellular metabolites. Can J Biochem, 1978. 56(7): p. 708–12. [DOI] [PubMed] [Google Scholar]
- 147.Laplante MA, et al. , The purinergic P2Y1 receptor supports leptin secretion in adipose tissue. Endocrinology, 2010. 151(5): p. 2060–70. [DOI] [PubMed] [Google Scholar]
- 148.Lemaire A, et al. , Mouse P2Y4 Nucleotide Receptor Is a Negative Regulator of Cardiac Adipose-Derived Stem Cell Differentiation and Cardiac Fat Formation. Stem Cells Dev, 2017. 26(5): p. 363–373. [DOI] [PubMed] [Google Scholar]
- 149.Tozzi M and Novak I, Purinergic Receptors in Adipose Tissue As Potential Targets in Metabolic Disorders. Front Pharmacol, 2017. 8: p. 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ussar S, et al. , ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci Transl Med, 2014. 6(247): p. 247ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Razzoli M, et al. , Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Mol Metab, 2016. 5(1): p. 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krashes MJ, et al. , Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest, 2011. 121(4): p. 1424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Steculorum SM, et al. , Hypothalamic UDP Increases in Obesity and Promotes Feeding via P2Y6-Dependent Activation of AgRP Neurons. Cell, 2015. 162(6): p. 1404–17. [DOI] [PubMed] [Google Scholar]
- 154.Steculorum SM, et al. , Inhibition of P2Y6 Signaling in AgRP Neurons Reduces Food Intake and Improves Systemic Insulin Sensitivity in Obesity. Cell Rep, 2017. 18(7): p. 1587–1597. [DOI] [PubMed] [Google Scholar]
- 155.Jacobson KA, et al. , Historical and Current Adenosine Receptor Agonists in Preclinical and Clinical Development. Front Cell Neurosci, 2019. 13: p. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Toti KS, et al. , Pyrimidine Nucleotides Containing a (S)-Methanocarba Ring as P2Y6 Receptor Agonists. Medchemcomm, 2017. 8(10): p. 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gao ZG., T.D.K., Jain S, Yu J, Suresh RR, Jacobson KA, A1 Adenosine Receptor Agonists, Antagonists, and Allosteric Modulators, in The Adenosine receptors. 2018, Humana Press, Cham. p. 59–89. [Google Scholar]
- 158.Junker A, et al. , Structure-Activity Relationship of Purine and Pyrimidine Nucleotides as Ecto-5’-Nucleotidase (CD73) Inhibitors. J Med Chem, 2019. 62(7): p. 3677–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jung YH, et al. , Exploration of Alternative Scaffolds for P2Y14 Receptor Antagonists Containing a Biaryl Core. J Med Chem, 2020. 63(17): p. 9563–9589. [DOI] [PMC free article] [PubMed] [Google Scholar]


