Abstract
Introduction
Cholangiocarcinomas (CCAs) are biliary epithelial tumors with rising incidence over the past 3 decades. Early diagnosis of CCAs remains a significant challenge and the majority of patients present at an advanced stage. CCAs are heterogeneous tumors and currently available standard systemic therapy options are of limited effectiveness. Immune checkpoint inhibition (ICI) has transformed cancer therapy across a spectrum of malignancies. However, the response rate to ICI has been relatively disappointing in CCAs owing to its desmoplastic tumor microenvironment (TME).
Areas covered
Tumor microenvironment of CCAs comprises of innate and adaptive cells, stromal cells, and extracellular components (cytokines, chemokines, exosomes, etc.). This intricate microenvironment has multiple immunosuppressive elements that promoting tumor cell survival and therapeutic resistance. Accordingly, there is a need for the development of effective therapeutic strategies that target the TME. Herein, we review the components of the CCA TME, and potential therapies targeting the CCA TME.
Expert opinion
CCAs are desmoplastic tumors with a dense tumor microenvironment. An enhanced understanding of the various components of the CCA TME is essential in the effort to develop novel biomarkers for patient stratification as well as combination therapeutic strategies that target the tumor plus the TME.
Keywords: Cancer-associated fibroblasts, myeloid-derived suppressor cells, tumor-associated macrophages, immune checkpoint inhibition
1. INTRODUCTION
Cholangiocarcinomas (CCAs) are the second most common liver malignancy, originating from the epithelial cells of the biliary tract. CCAs are classified as intrahepatic (iCCA), perihilar (pCCA), or distal (dCCA) based on their anatomic location within the biliary tree [1, 2]. CCA is a dismal, difficult-to-diagnose disease with patients often presenting at a late stage. Patients with CCA are generally asymptomatic, and diagnosing CCA at an early stage remains a significant challenge. Consequently, presentation at an advanced stage precludes potentially curative surgical options. For patients who are not eligible for surgical resection or liver transplantation following neoadjuvant chemoradiation, systemic chemotherapy with gemcitabine and cisplatin is the standard of care. However, this combination has limited effectiveness with a median overall survival of approximately 12 months [3]. Tumor progression is largely dependent on the interactions between cancer cells and non-cancerous components in the tumor microenvironment (TME). The TME not only influences tumor development, but also impacts the sensitivity or resistance to therapeutic interventions [4]. CCAs are characterized by a prominent desmoplastic TME composed of various cell types (e.g. infiltrating immune cells and cancer-associated fibroblasts [CAFs]). This desmoplastic environment fosters tumor growth and therapeutic resistance [5]. Hence, targeting specific components of the TME is a promising therapeutic strategy. In this review, we provide an overview of the stromal and immune components of the CCA TME, and discuss potential therapeutic targets.
2. THE TUMOR IMMUNE MICROENVIRONMENT OF CHOLANGIOCARCINOMA
2.1. Tumor-associated macrophages (TAMs)
Macrophages are phagocytic innate immune cells that are extremely heterogeneous, and have different origins. Hepatic macrophages include Kupffer cells (KCs) which are tissue-resident macrophages, and recruited macrophages supplemented by blood-derived monocytes [6]. In tumor biology, tumor-associated macrophages (TAMs) are an integral component of the tumor immune contexture, and play an essential role in cancer progression and remodeling of the microenvironment [7]. In iCCA, KCs release tumor necrosis factor (TNFα) and promote cholangiocyte proliferation as well as carcinogenesis via activation of JNK signaling [8]. Increased infiltration of TAMs in human CCA tumor specimens has been associated with poor outcomes [9, 10]. In the TME, TAMs are recruited from circulating monocytes by chemokine C-C motif ligand 2 (CCL-2) and colony stimulating factor 1 (CSF-1) [11]. TAMs modulate the TME by releasing TNFα and interleukin (IL)-6 to support CCA cell growth [12]. TAMs also attract immunosuppressive cells such as regulatory T cells to the CCA TME [13].
In light of their pro-tumor role across a variety of malignancies, therapeutic targeting of TAMs has become an attractive anti-cancer approach. TAM depletion results in inhibition of WNT signaling with a resultant reduction in tumor burden in preclinical models of CCA [14]. There are several different approaches to inhibit or deplete TAMs in cancer, and various TAM targeting strategies are currently under investigation [15]. However, a recent study demonstrated that TAM blockade alone does not lead to tumor suppression in CCA due to a compensatory emergence of granulocytic-myeloid-derived suppressor cells (G-MDSCs) [16]. Cluster of differentiation 47 (CD47) is overexpressed in a number of different tumor types, and plays a role in tumor progression and metastasis via interaction with signal regulatory protein alpha (SIRPα), mainly expressed on macrophages. The CD47/ SIRPα axis functions as a protective signal employed by cancer cells to avoid phagocytic elimination [17]. CD47 has emerged as a myeloid checkpoint, and targeting the CD47/SIRPα axis has garnered attention of late [18–20]. Administration of anti-CD47 (B6H12.2) antibody decreased CCA colonization and infiltration of TAM in a mouse model of CCA [21]. By demonstrating that disruption of the CD47-SIRPα interaction promotes phagocytosis of tumor cells in CCA, this study indicated that CD47 may be a potential therapeutic target in CCA.
Macrophage c-mer tyrosine kinase (MerTK), upon activation in KC, activates hepatic stellate cells (HSCs) via a tumor growth factor-β (TGF-β) pathway with consequent liver fibrogenesis [22]. In a syngeneic murine colon adenocarcinoma tumor model, blockade of MerTK-mediated efferocytosis resulted in the accumulation of apoptotic cells within tumors and triggered a type I interferon response with activation of cGAMP synthase (cGAS)-stimulator of interferon genes (STING) signaling. Inhibiting MerTK-dependent uptake of dying cells by TAMs may yield a higher number of tumor antigens, thereby potentially supporting durable immune activation. Accordingly, blockade of MerTK in mice bearing tumor stimulated T cell cytotoxicity and achieved a synergistic effect when combined with anti-programmed cell death protein 1(PD-1) or anti-programmed cell death ligand-1(PD-L1) therapy [23]. These encouraging results indicate that CD47 and MerTK may be potential targets in CCA; nonetheless, additional studies are needed to evaluate the effectiveness of these emerging anti-cancer strategies in CCA.
2.2. Myeloid-derived suppressor cells (MDSCs)
MDSCs are immature myeloid cells with potent immunosuppressive properties, and expansion of MDSCs populations occurs in cancer [24]. MDSCs originate from the bone marrow, and accumulate in peripheral blood, lymphoid tissues, as well as the TME [25]. MDSCs inhibit cytotoxic T lymphocyte (CTL) and natural killer (NK) cell activation by expression of arginase (Arg1) and nitric oxide synthase 2 [26]. MDSCs also engage in crosstalk with regulatory T cells (Tregs) [27] and macrophages via immunosuppressive cytokines (e.g. IL-6 and IL-10 ), thereby promoting tumor immune evasion and immunotherapy resistance [28]. MDSCs comprise two large subsets: granulocytic or polymorphonuclear MDSCs and monocytic MDSCs (M-MDSCs). M-MDSC (defined as CD11b+CD14+/HLA-DR−) are significantly increased in peripheral blood of patients with CCA compared to healthy controls [29]. Data providing mechanistic insight vis-à-vis an immunosuppressive role of MDSCs in CCA progression or therapeutic targeting of MDSCs in CCA are limited. The majority of data elucidating MDSCs function in hepatopancreaticobiliary malignancies come from hepatocellular carcinoma (HCC) or pancreatic ductal adenocarcinoma (PDAC). In preclinical HCC models, MDSCs aggregate in the liver, and transform KCs to an immunosuppressive phenotype [30, 31]. Depletion of G-MDSCs using Ly6G monoclonal antibody in PDAC had a tumor suppressive effect via enhanced intratumoral accumulation of activated CD8+ T cells [32].
The liver-X receptor (LXR)/apolipoprotein E (ApoE) axis has recently been implicated in MDSC survival [33]. LXR activation reduces tumor growth and restrains tumor metastasis [34, 35]. Administration of the LXR agonist (RGX-104/GW3965) to tumor bearing mice significantly attenuated growth of several cancers including melanoma, glioblastoma, and lung cancer via enhanced MDSCs apoptosis [33]. Therapeutic targeting of TAMs and G-MDSCs either by Ly6G monoclonal antibody or the LXR agonist GW3965 augments ICI with anti-PD-1 [16]. The LXR agonist RGX-104 is currently under investigation in a phase I clinical trial in patients with advanced solid tumors (NCT02922764). Further studies are required to elucidate the mechanistic basis of MDSCs mediated immunosuppression and to investigate the therapeutic potential of agents targeting MDSCs in CCA.
2.3. Dendritic cells (DCs)
DCs function as antigen-presenting cells (APCs) which play an integral role in activation of the adaptive immune response. DCs are broadly categorized into two subsets: classical or conventional DCs (cDCs) and plasmacytoid DCs (pDC). cDCs originate from bone marrow precursors and have potent phagocytic properties [36]. In the TME, DCs activate the T cell response by capturing, processing, and cross-presenting neoantigens. However, tumor cells can transform DCs to an immature, immunosuppressive phenotype [37]. In CCA, infiltration of mature CD83+ DCs correlated with aggregation of CD4+/CD8+ T cells in the peritumoral region [38]. The presence of CD83+ DCs was also associated with improved patient outcomes. In contrast, the presence of CD1a (immature) DCs in the central tumor region is associated with a paucity of CD4+/CD8+ T cells [38]. FcεRI, a high affinity immunoglobulin E receptor, is employed by DCs for cross presentation and priming of CTLs [39]. There is a significant decrease in FcεRI+ monocytes and DCs in the peripheral blood of patients with CCA [40]. These findings indicate that DCs are dysfunctional in CCA and unable to restrain tumor progression.
There is a paucity of cDCs in human and murine PDAC, and this is associated with poor response to checkpoint inhibition [41]. Fms-related tyrosine kinase 3 ligand (Flt3L) augments DCs infiltration in tumors by enhancing DC proliferation and differentiation [42]. However, Flt3L monotherapy has had limited benefit in early phase clinical trials, likely due to lack of appropriate DC activation and licensing [42, 43]. Activation of CD40, a member of the TNF receptor superfamily, facilitates DC-mediated CTL activation and re-education of macrophages to an anti-tumor phenotype [44]. The combination of Flt3L and CD40 agonism stimulated a robust anti-tumor immune response and tumor regression in preclinical models of sarcoma, a poorly immunogenic tumor. The anti-tumor immune response was characterized by a dramatic increase in DCs as well as NK cells, NKT cells, and CD8+ T cells [45]. CDX-1140, a CD40 agonist, is currently under evaluation in a phase I clinical trial of advanced solid organ malignancies including CCA (NCT03329950). As the baseline CCA TME has a low density of DCs, the combination of CD40 and Flt3L agonism has the potential to boost the DC response, augment anti-tumor immunity, and sensitization to ICI in CCA.
2.4. Natural Killer cells (NK)
NK cells are innate lymphocytes that can track and destroy virally-infected and neoplastic cells without pre-stimulation. Upon recognizing neoplastic cells, NK cells release cytotoxic molecules (perforin, granzymes, and IFN-γ), and can induce target cell death by priming Fas ligand (FasL)/TNF-related apoptosis-inducing ligand [46, 47]. Preclinical studies have demonstrated that NK cell deficiency or impaired NK cell function is associated with tumor progression [48, 49]. In HCC, tumor infiltrating NK cells have fragmented mitochondria which impair their cytotoxicity with consequent tumor evasion of NK cell mediated tumor surveillance. Moreover, mitochondrial fragmentation in NK cells correlated with poor patient survival [50]. Natural killer group 2D (NKG2D), an activating NK cell receptor, and kills tumor cells by binding its ligand NKG2DL. Impairment of the NKG2D/NKG2DL axis assists tumor escape from immune surveillance. NKG2D receptor variants found in patients with primary sclerosing cholangitis have been reported to increase their susceptibility for CCA development [51, 52]. Moreover, the high expression of NKG2D ligands in human CCA is associated with improved disease-free and overall patient survival [53].
NK cell responses are regulated by inhibitory killer cell immunoglobulin-like receptors (KIRs) that engage HLA class I ligands. In tumor biology, KIRs are considered inhibitory checkpoints. In a multidimensional characterization of genes that encode KIRs, multiple alterations of KIR and HLA gene loci were identified in patients with CCA compared to controls [54]. For instance, co-carriage of KIR2DS1-HLA-C2 and KIR3DL1-HLA-Bw4Thr80 (low affinity) was identified as an independent predictor of poor outcomes [54]. These observations indicate that targeting KIR on NK cells is a potential immunotherapeutic option in CCA. The combination of lirilumab, an anti-KIR monoclonal antibody, and the anti-PD-1 monoclonal antibody nivolumab +/− the anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) monoclonal antibody ipilimumab is currently under investigation in a phase I clinical trial of patients with advanced solid organ malignancies including CCA (NCT03203876).
2.5. Tumor-infiltrating lymphocytes (TILs)
Tumor-infiltrating lymphocytes (TILs) include B lymphocytes, cytotoxic T cells (CD8+), and T helper cells (CD4+ T). The cell composition and molecular pattern of TILs remodel the CCA microenvironment, and shape cancer immune surveillance or immune escape. TIL infusion is an emerging option for CCA treatment. Adoptive transfer of CD4+ T helper 1 (Th-1) cell recognizing mutated neoantigen expressed by CCA cells achieved tumor regression [55]. An increase in CD8+ TILs is correlated with improved overall survival (OS) in CCA patients [56, 57]. In comparison, a high infiltration of CD4+ T was associated with favorable patient outcomes in CCA, whereas infiltration of Tregs is associated with poor OS in CCA [10, 56]. Down-regulation of FoxP3, a protein essential in the development and function of Tregs, in CCA cells resulted in a decrease in TGF-β1 and consequent improvement of effector T cell survival [58]. Similarly, overexpression of FoxP3 in PDAC cells upregulates PD-L1 transcription and recruits Tregs, thereby enhancing tumor immune invasion [59]. Expression of the immune checkpoint receptor PD-1 and its ligand PD-L1 is upregulated in surgically resected human CCA specimens. Tumor PD-L1 expression is correlated with poor tumor differentiation and advanced tumor stage [60–62]. However, the prevailing data indicate that the benefit of anti-PD-1 or anti-PD-L1 monotherapy may be limited to a small subset of CCA patients [63, 64].
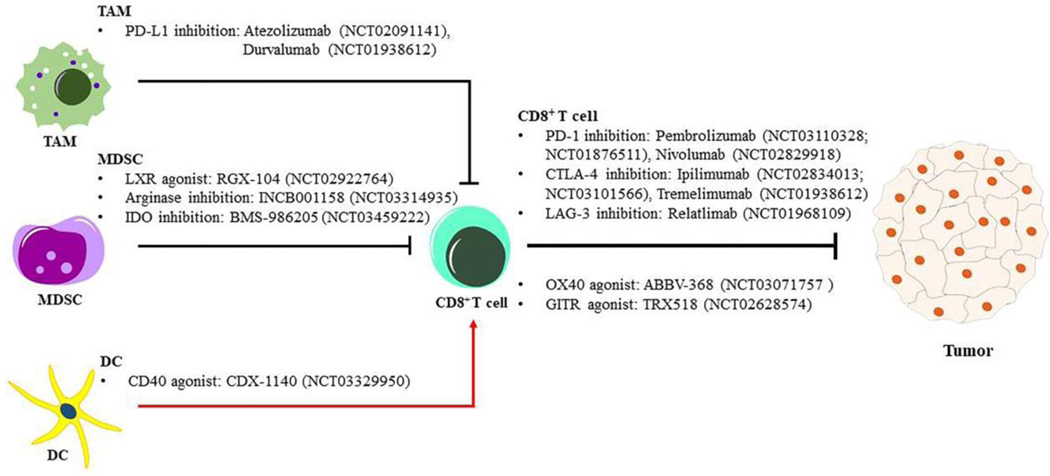
There are several potential immune checkpoint targets that are currently under investigation in preclinical and clinical studies (Figure 1). CTLA-4 is an inhibitory receptor that binds to CD80 which is expressed by APCs, and inhibits CTL activation. Expression of CTLA-4 and CD80 is increased in CCA and correlates with tumor recurrence and poor overall survival [65]. Multiple clinical trials assessing anti-PD-1 and anti-CTLA-4 in CCA are currently ongoing (NCT03473574, NCT03046862, and NCT03704480). Glucocorticoid-induced tumor necrosis factor receptor (GITR) is a co-stimulatory molecule that can enhance CTL effector function and attenuate Treg mediated immunosuppression [66]. GITR is over-expressed in TILs in CCA tumor tissues, and agonistic targeting of this checkpoint has the potential to enhance CTL activation [67]. TRX518, a GITR agonist, is currently under investigation in combination with pembrolizumab or nivolumab in a phase I clinical trial in patients with advanced solid tumors (NCT02628574) [66].
Figure 1: Schematic representation of immune cell targeting strategies in CCA.
Strategies overcoming the immunosuppressive tumor microenvironment are represented schematically. Inhibition or depletion tumor associated macrophage (TAM) and/or myeloid-derived suppressor cell (MDSC) abrogates the cytotoxic T (CD8+ T) exhaustion. Activates antigen-presenting cell (APC) like dendritic cell (DC) or stimulate the T cell activity accelerate anti-tumor response in CCA. CD40: cluster of differentiation 40; CTLA-4: cytotoxic T-lymphocyte associated protein 4; DCs: dendritic cells; GITR: glucocorticoid-induced tumor necrosis factor receptor; IDO: indoleamine 2,3-dioxygenase; LAG3: lymphocyte activation gene 3 protein; LXR: liver-X receptor; MDSCs: myeloid-derived suppressor cells; OX40: tumor necrosis factor receptor superfamily member 4; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; TAM: tumor associated macrophage.
3. NON-IMMUNE CELLULAR COMPONENTS OF THE CCA TME
3.1. Cancer‐associated fibroblasts (CAFs)
CCAs are desmoplastic tumors with a dense stroma. Cancer-associated fibroblasts (CAFs) comprise a major cellular component of the desmoplastic stroma of CCAs. CAFs are activated myofibroblasts that express α-smooth muscle actin (α-SMA) [68]. CAFs play a key role in mediating CCA growth and progression. Accordingly, α-SMA expression in the tumor stroma correlates with poor survival in patients with CCA [69, 70]. The expression of periostin, an extracellular matrix protein produced by α-SMA-positive CAFs in CCA, is higher in iCCA compared with control tissues [71]. Furthermore, enhanced periostin expression is a predictor of malignant progression in murine and human CCA, and correlates with poor patient outcomes [72, 73]. As CAFs play an essential role in CCA progression, targeting of CAFs has been proposed as a potential therapeutic strategy in CCA. Selective targeting of CAFs by navitoclax, a BH3 mimetic, induced CAF apoptosis with consequent reduction in tumor growth and metastasis and improved murine survival in a syngeneic rat model of CCA [74]. In a subsequent study, navitoclax inhibited tumor metastasis in vivo by blocking the secretion VEGF-A/C from activated CAFs in CCA [75]. Resveratrol [3,4’,5-trihydroxy-trans-stilbene (RV)], a polyphenol present in a variety of food products such as grapes and red wine, has also been employed to target CAFs [76]. Conditioned medium from CAFs pretreated with resveratrol had a reduction in IL-6 secretion as well as decreased proliferation and migration of CCA cell lines compared with control. Nintedanib, a small molecule inhibitor of multiple tyrosine kinases, is FDA-approved for treatment of idiopathic pulmonary fibrosis [77]. Nintedanib attenuated carbon tetrachloride induced liver fibrosis via suppression of HSC activation [78, 79]. Moreover, nintedanib has been shown to play an essential role in suppressing the activation of α-SMA+ CAFs in lung adenocarcinoma [80]. Preclinical evidence also supports a CAF suppressing effect of nintedanib in CCA. Nintedanib inhibited CAFs activation and reduced the secretion of cancer-promoting cytokines by CAFs (mainly IL-6, IL-8) in vitro and reduced tumor growth in a xenograft murine iCCA model [81]. These observations indicate that therapeutic targeting of CAFs is a promising approach for the treatment of CCA.
4. MEDIATORS: THE MESSENGERS IN THE CROSSTALK BETWEEN TUMOR CELLS AND TME
4.1. Small players with large roles: cytokines and chemokines
IL-6 plays a central role in the crosstalk between the tumor cells and TME cellular components. IL-6 is released by several cell populations including macrophages and CAFs [82, 83]. Intracellular IL-6 activation triggers canonical JAK/STAT3 signaling. As an upstream activator of STAT3, IL-6 has been reported to promote malignant transformation and metastasis of CCA [84]. Moreover, IL-6 alters the promoter methylation of epidermal growth factor receptor (EGFR), resulting in continuous EGFR activation, thereby driving CCA cell growth [85]. IL-6 also drives CCA proliferation via activation of ERK1/2-MAPK signaling [86]. Furthermore, systemic administration of IL-33 combined with biliary transduction of constitutively-activated AKT and yes-associated protein induced tumorigenesis in mice via an IL-6 dependent mechanism, indicating an essential role of IL-6 in CCA carcinogenesis [87]. Consistent with these preclinical observations, serum IL-6 levels correlate with poor patient outcomes [88]. A single cell-based study identified a subset of fibroblasts (CD146+ CAFs) that express high levels of IL-6 in iCCA. Moreover, the IL-6/IL-6R axis was enriched in CAFs and tumor cells [89]. As it plays an integral role in CCA proliferation and progression, targeting IL-6 signaling is an attractive putative therapeutic option in CCA. However, although preclinical data indicates an anti-tumor effect of IL-6 inhibition in pancreatic ductal adenocarcinoma, the limited data in CCA has been disappointing [90]. A single study demonstrated that pharmacologic blockade of IL-6R actually promoted, rather than hindered, CCA cell growth in vitro [91].
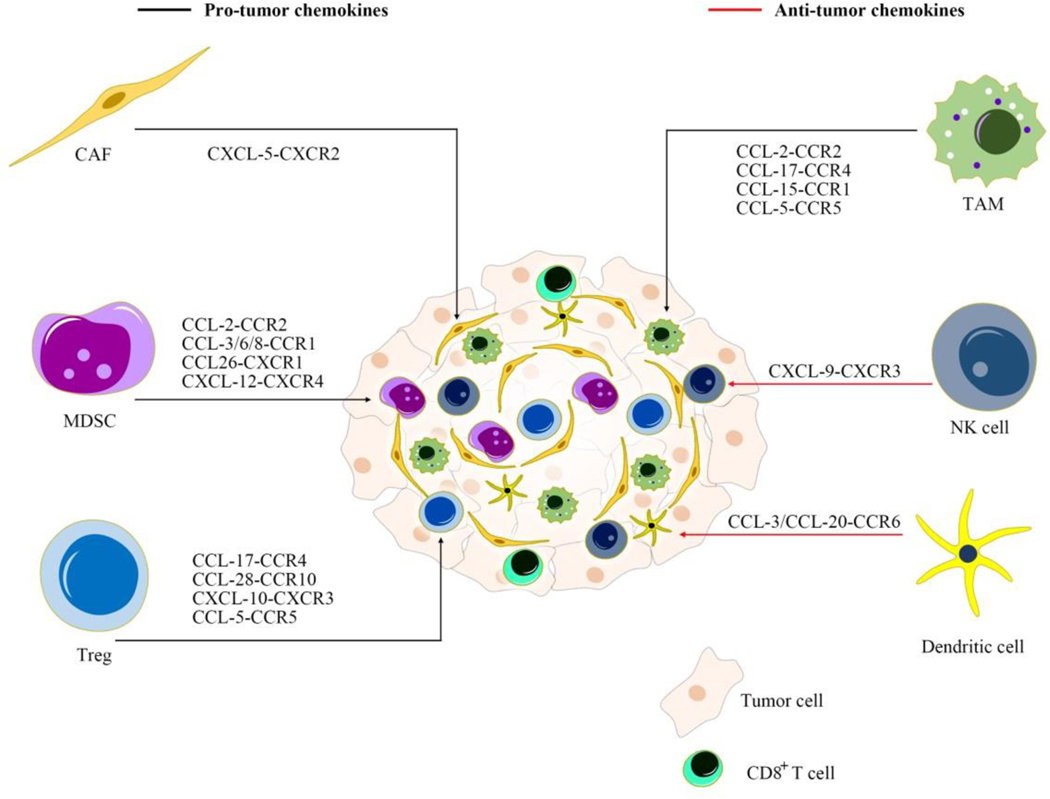
Several chemokines have been implicated in the tumor and immune microenvironment crosstalk. CCL-2, mainly secreted by CAFs, attracts MDSCs to the TME and fosters CCA growth [92]. Chemokine (C-C motif) ligand 28 (CCL-28), which is released by human cholangiocytes in response to inflammatory factors, recruits CCR10+ Tregs to limit hepatic inflammation [93]. Chemokine (C-X-C motif) ligand 9 (CXCL-9) regulates the recruitment of tumor-infiltrating NK cells in CCA. Patients with high CXCL-9 expression have a favorable overall survival following surgical resection compared to those with low CXCL-9 expression [94]. These observations indicate that inhibition of chemokines implicated in recruitment of immunosuppressive elements to the CCA TME is a potential therapeutic strategy. Likewise, augmenting chemokine signaling that attracts anti-tumor immune cells to the CCA TME has the potential to restrain CCA growth (Figure 2).
Figure 2: Targeting chemokine signaling as therapy for CCA.
Inhibition or activation of chemokine pathways influences recruitment of various immune cells. CAF: cancer-associated fibroblast; CCL: chemokine C-C motif ligand; CCR/CXCR: chemokine C-C motif/C-X-C motif receptor; MDSC: myeloid-derived suppressor cell; TAM: tumor-associated macrophage; Treg: regulatory T cell.
4.2. Small players with large roles: growth factors
Several growth factors have emerged as reciprocal mediators that contribute to cross-talk between CAFs and tumor cells in CCA. Platelet‐derived growth factors (PDGFs) exert a pro-tumor role in a paracrine signaling manner. PDGF‐BB released from myofibroblasts prevents TNF-α related apoptosis-inducing ligand (TRAIL)‐induced apoptosis of CCA cells by activating PDGF receptor β (PDGFRβ) on CCA cells [95]. PDGF-D, another member of the PDGF family produced by CCA cells, plays a crucial role in promoting CAF recruitment and activation by binding PDGFRβ expressed on CAFs [75, 96]. Accordingly, PDGFRβ blockade via imatinib, significantly impairs fibroblast recruitment in CCA. Heparin-binding epidermal growth factor (HB-EGF) secreted by CAFs, a ligand of epidermal growth factor receptor (EGFR), activates EGFR in CCA cells and promotes CCA cells migration and invasion in vitro. HB-EGF inhibition with a neutralizing antibody inhibits CCA progression [97].
As specific growth factors of vascular epithelial cells, the fundamental function of vascular endothelial growth factors (VEGFs) is to induce angiogenesis. VEGFs are highly expressed in CCA and correlate with poor patient outcomes [98, 99]. VEGF-D regulates the activities of stromal cells and aids tumor cell metastasis via lymphatic spread [100]. The expression of VEGF-C correlates with lymphatic invasion in intrahepatic CCA. Similarly, VEGF-C secreted by CAFs enhances the permeability of lymphatic endothelial cells (LECs), thereby inducing CCA lymphatic invasion and metastasis [75] [101]. Single cell transcriptomic analysis of tumors from liver cancer patients including 9 with iCCA demonstrated that VEGF plays an integral role in intratumoral diversity [102]. In this study, VEGF induced by hypoxia-inducible factor 1α (HIF1α) manipulated tumor endothelial cells, CAFs, and TAMs to drive tumor progression. The study findings implicate the VEGF axis in reprogramming of the TME to aid tumor progression. These observations indicate that VEGF inhibition may play a role in prevention of CCA progression and metastasis. Regorafenib, an oral multikinase inhibitor that targets VEGFR2, significantly suppressed CCA growth in vitro and in vivo [103]. However, regorafenib only had modest efficacy in patients. In a phase II clinical trial of patients with refractory biliary tract cancer (progressed on at least one line of systemic therapy; n=33), the stable disease and the objective response rates were 63.6% and 9.1%, respectively [104]. Pazopanib, a multikinase inhibitor that also inhibits VEGF, in combination with the MEK inhibitor Trametinib failed to achieve an improvement in progression free survival (PFS) in patients with advanced CCA [105]. A phase I/II clinical trial of regorafenib in combination with ICI (anti-PD-L1) is currently ongoing (NCT03475953). Future studies are needed to identify biomarkers that can potentially distinguish treatment responders form non-responders.
4.3. Small players with large roles: extracellular vesicles (EVs)
EVs are small membrane bound vesicles released by a variety of cell types into the extracellular milieu and facilitate intercellular communication by transporting intracellular components [106]. EVs contain complex cargo which consists of proteins, lipids, and nucleic acids (messenger RNA, microRNA, DNA) [107]. Once released from cells, EVs can regulate the function of recipient cells contributing to primary tumor formation and modulation of the TME [108]. EVs are also present in bile and have been implicated in regulation of cholangiocyte proliferation and in pathogenesis of cholangiopathies [109]. EVs also hold potential as a biomarker for CCA diagnosis. The median concentration of EVs is significantly higher in bile samples from patients with malignant biliary stenoses compared to patients with nonmalignant stenoses. Accordingly, the EV concentration of bile can distinguish between patients with malignant versus nonmalignant strictures with high diagnostic accuracy [110]. A biliary EV microRNA (miR)-based panel had a sensitivity of 67% and specificity of 96% for detection of CCA [111]. Proteomic analysis of serum EVs from human CCA cells compared to normal human cholangiocytes has also demonstrated a higher proportion of oncogenic proteins such as aminopeptidase N, pantetheinase, and polymeric immunoglobulin receptor; these oncogenic proteins had higher diagnostic capacity for detection of CCA [112]. Transcriptome analysis of EVs collected from serum and urine patients with CCA or healthy individuals has also demonstrated potential diagnostic capability as EVs derived from CCA patient specimens had differential RNA profiles compared to the disease control group [113].
An enhanced understanding of EVs and their cargo in CCA can facilitate design of novel therapeutics. Microrna (miR)-195 is downregulated in CCA cells and CAFs, and EVs transfer miR-195 from CAFs to CCA cells [114]. Furthermore, administration of CAF-derived EVs loaded with miR-195 resulted in tumor regression and improved murine survival [114]. miR-30e is also down-regulated in human CCA cells [115]. Treatment of CCA cells with miR-30e-enriched EVs resulted in attenuation of cell invasion and migration. Moreover, EV-mediated miR-30e transfer suppressed epithelial-mesenchymal transition by targeting Snail [115]. These studies indicate that EVs carrying designed cargos are a promising therapeutic for treatment of CCA.
5. CONCLUSION
CCA is a devastating malignancy with limited treatment options. Despite an increase in incidence, the overall 5-year survival remains abysmal at less than 10%. Tumor heterogeneity and the complex CCA TME with its cellular and molecular components pose significant barriers to the success of currently available treatment options. Accordingly, an enhanced understanding of the CCA TME and its components including immune cells, stromal elements, and extracellular factors, is essential in the effort to develop effective therapeutic strategies. Emerging evidence indicates that therapeutic targeting of the immune and nonimmune TME components holds significant promise.
6. EXPERT OPINION
CCA is a rare, highly aggressive malignancy with limited treatment options. The preponderance of patients present at an advanced stage and are not eligible for potentially curative surgical treatment options. The current practice standard for advanced CCA is systemic chemotherapy with gemcitabine and cisplatin. However, this regimen has a modest survival benefit with a median OS of 11.7 months in patients receiving the combination compared to 8.1 months for gemcitabine alone [3].
An enhanced understanding of the immunobiology of the tumor microenvironment has inaugurated the era of immune-oncology for treatment of cancer. However, the early results of immune checkpoint blockade monotherapy in CCA have been disappointing, likely owing to its dense, desmoplastic microenvironment that contains an abundance of immunosuppressive elements such as TAMs and MDSCs [16]. We have recently demonstrated that multiple layers of resistance involving elements of the innate and adaptive immune system contribute to tumor immune evasion in CCA. Accordingly, elucidating the cross-talk between immunosuppressive elements of the CCA TME, tumor cells, and the anti-tumor immune response is essential in the effort to develop effective therapies. Our current understanding of the CCA TME particularly the immune microenvironment is based largely on immunohistochemical analyses of resected human CCA specimens. Although these studies have been useful in imparting a global view of the CCA TME, our knowledge vis-à-vis the intricacies of microenvironment crosstalk is limited.
Advances in single cell biology including single cell transcriptomics and proteomics have illuminated variations at the cellular level that account for TME heterogeneity in a variety of malignancies. These variations, in turn, may underlie resistance to therapies. Therefore, technologies such as single cell RNA sequencing and mass cytometry can decode the heterogeneity of the CCA tumor ecosystem, and outline the functional characteristics of the innate and adaptive immune response in CCA. Although single cell based studies have begun to unravel the CCA TME, further work is necessary to examine the immune microenvironment in a comprehensive manner [89, 102]. Augmenting our understanding of the cellular and molecular components as well as the cell-cell communication in the CCA TME will aid design of novel therapeutic agents. Moreover, this will foster biomarker development which will guide selection of the appropriate therapy for a subset of patients.
The response rate to ICI monotherapy in CCA has been disappointing. Interim data analysis from KEYNOTE-158, an ongoing phase II clinical trial, demonstrated that patients treated with the anti-PD-1 therapy pembrolizumab had an objective response rate (ORR) of 5.8% (6/104 patients) [116]. PD-L1 expression did not correlate with response to therapy. Emerging results suggest that the response to ICI may vary according to the CCA subtype. In patients with biliary tract cancer (BTC) who had progressed on at least one line of systemic therapy, nivolumab had an ORR of 22%. The ORR in iCCA patients was 21% (6/28 patients). There were only 5 patients in the study with pCCA/dCCA, and two of these patients had a response to nivolumab [117]. The combination of nivolumab and ipilimumab was assessed in advanced BTC patients; the ORR in iCCA patients was 31% (5/16 patients). Notably, none of the pCCA/dCCA patients (n=10) in this study had a response to combinatorial ICI [118]. Accordingly, CCAs are poorly immunogenic or immune ‘cold’ tumors. Such a TME phenotype is typically characterized by immunosuppressive cells such as TAMs that prevent CTL infiltration to the tumor core [119]. Therefore, combinatorial immunotherapeutic strategies that target elements of the innate and adaptive immune system are likely to be more efficacious than single agent immunotherapies. A greater familiarity with the diverse immune and nonimmune components of the CCA TME will facilitate development of such strategies. The availability of immunocompetent preclinical mouse models that recapitulate the human disease is imperative to study potential therapeutics that target the CCA TME. We have generated a unique, syngeneic orthotopic mouse model of CCA to study the immunobiology of this desmoplastic malignancy and potential therapeutic targets in the CCA TME [120]. Humanized tumor models, such as patient-derived xenografts, can model the complexity of tumor development and progression as well as take into consideration TME factors. However, in these models primary human tumors are engrafted in immunodeficient mice, and investigation of immunotherapy agents requires an intact immune system. Patient-derived organoids can recapitulate tumor heterogeneity and molecular signatures, and therefore, represent an attractive alternative for preclinical assessment of novel therapeutics. Multicellular organoids comprise of tumor epithelium and endogenous immune stroma, and generation of such organoids would facilitate investigation of the CCA TME, specifically immuno-oncology studies [121]. In vitro, multicellular organoids contain variable immune elements. Characterization of these elements will be essential in the effort to employ multicellular organoids to assess immunotherapeutic strategies.
Article highlights.
CCA is a highly lethal, difficult-to-diagnose malignancy with limited treatment options.
Tumor-associated macrophages (TAMs) are an integral component of the CCA tumor immune contexture, and play an essential role in cancer progression and remodeling of the microenvironment.
Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells with potent immunosuppressive properties, and MDSC populations expand in a variety of malignancies including CCA.
The early results of immune checkpoint inhibition (ICI) monotherapy in CCA have been disappointing, likely owing to its dense, desmoplastic microenvironment that contains an abundance of immunosuppressive elements such as TAMs and MDSCs.
Cancer-associated fibroblasts (CAFs) play a crucial role in mediating CCA growth and progression, and therapeutic targeting of CAFs is a promising approach for the treatment of CCA.
Acknowledgments
Funding: This manuscript was funded by the National Cancer Institute (K08CA236874–01), American Gastroenterology Association Research Scholar Award, Center for Biomedical Discovery Team Science Award, Hepatobiliary Cancer SPORE (P50 CA210964) Career Enhancement Program, Mayo Center for Cell Signaling in Gastroenterology (P30DK084567), and the Mayo Foundation.
Declaration of Interest:
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Abbreviations:
- APCs
antigen-presenting cells
- ApoE
apolipoprotein E
- Arg1
arginase
- CAFs
cancer-associated fibroblasts
- CCA
cholangiocarcinoma
- CCL-2
chemokine (C-C motif) ligand 2
- CCL-28
chemokine (C-C motif) ligand 28
- CD47
cluster of differentiation 47
- cDCs
classical or conventional DCs
- cGAS
cGAMP synthase
- CSF-1
colony stimulating factor 1
- CTLs
cytotoxic T lymphocytes
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- CXCL-9
chemokine (C-X-C motif) ligand 9
- DCs
dendritic cells
- EVs
extracellular vesicles
- FasL
fas ligand
- Flt3L
fms-related tyrosine kinase 3 ligands
- GITR
glucocorticoid-induced tumor necrosis factor receptor
- G-MDSCs
granulocytic MDSCs
- HCC
hepatocellular carcinoma
- HIF1α
hypoxia-inducible factor 1α
- HSCs
hepatic stellate cells
- ICI
immune checkpoint inhibition
- IL
interleukin
- KCs
Kupffer cells
- KIRs
killer cell immunoglobulin-like receptors
- LXR
liver-X receptor
- MDSCs
myeloid-derived suppressor cells
- MerTK
macrophage c-mer tyrosine kinase
- M-MDSCs
monocytic MDSCs
- NK
natural killer
- NKG2D
natural killer group 2D
- OS
overall survival
- PD-1
programmed cell death protein 1
- PDAC
pancreatic ductal adenocarcinoma
- pDC
plasmacytoid DCs
- PD-L1
programmed cell death ligand-1
- PFS
progression free survival
- SIRPα
signal regulatory protein alpha
- STING
stimulator of interferon genes
- TAMs
tumor-associated macrophages
- TGF-β
tumor growth factor-β
- TILs
tumor-infiltrating lymphocytes
- TME
tumor microenvironment
- TNFα
tumor necrosis factor alpha
- Tregs
regulatory T cells
- VEGFs
vascular endothelial growth factors
- α-SMA
α-smooth muscle actin
Footnotes
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018. February;15(2):95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020. September;17(9):557–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. The New England journal of medicine 2010. April 8;362(14):1273–81. [DOI] [PubMed] [Google Scholar]
- 4.Jin M-Z, Jin W- L. The updated landscape of tumor microenvironment and drug repurposing. Signal transduction and targeted therapy 2020. 2020/08/25;5(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogdall D, Lewinska M, Andersen JB. Desmoplastic Tumor Microenvironment and Immunotherapy in Cholangiocarcinoma. Trends in cancer 2018. March;4(3):239–55. [DOI] [PubMed] [Google Scholar]
- 6.Dou L, Shi X, He X, et al. Macrophage Phenotype and Function in Liver Disorder. Front Immunol 2019;10:3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassetta L, Fragkogianni S, Sims AH, et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019. April 15;35(4):588–602.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan D, Huang S, Berger E, et al. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell 2017. June 12;31(6):771–89.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun D, Luo T, Dong P, et al. CD86(+)/CD206(+) tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ 2020;8:e8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitano Y, Okabe H, Yamashita YI, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. British journal of cancer 2018. January;118(2):171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013. February 1;73(3):1128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Techasen A, Loilome W, Namwat N, et al. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac J Cancer Prev 2012;13 Suppl:115–8. [PubMed] [Google Scholar]
- 13.Hasita H, Komohara Y, Okabe H, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci 2010. August;101(8):1913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulter L, Guest RV, Kendall TJ, et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. The Journal of clinical investigation 2015;125(3):1269–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 2018. December;17(12):887–904. [DOI] [PubMed] [Google Scholar]
- 16.Loeuillard E, Yang J, Buckarma E, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. The Journal of Clinical Investigation 2020. 10/01/;130(10):5380–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matlung HL, Szilagyi K, Barclay NA, et al. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunological reviews 2017. March;276(1):145–64. [DOI] [PubMed] [Google Scholar]
- 18.Weiskopf K, Jahchan NS, Schnorr PJ, et al. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest 2016. July 1;126(7):2610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012. April 24;109(17):6662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Xavy S, Mihardja S, et al. Targeting macrophage checkpoint inhibitor SIRPa for anticancer therapy. JCI insight 2020 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaeteewoottacharn K, Kariya R, Pothipan P, et al. Attenuation of CD47-SIRPalpha Signal in Cholangiocarcinoma Potentiates Tumor-Associated Macrophage-Mediated Phagocytosis and Suppresses Intrahepatic Metastasis. Transl Oncol 2019. February;12(2):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai B, Dongiovanni P, Corey KE, et al. Macrophage MerTK Promotes Liver Fibrosis in Nonalcoholic Steatohepatitis. Cell metabolism 2020. February 4;31(2):406–21.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Fei M, Zhang G, et al. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity 2020. February 18;52(2):357–73.e9. [DOI] [PubMed] [Google Scholar]
- 24.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer immunology research 2017. January;5(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nature immunology 2018. 2018/02/01;19(2):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol 2002. January 15;168(2):689–95. [DOI] [PubMed] [Google Scholar]
- 27.Siret C, Collignon A, Silvy F, et al. Deciphering the Crosstalk Between Myeloid-Derived Suppressor Cells and Regulatory T Cells in Pancreatic Ductal Adenocarcinoma. Frontiers in immunology 2020;10:3070–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beury DW, Parker KH, Nyandjo M, et al. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc Biol 2014;96(6):1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu XD, Hu J, Wang M, et al. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary & pancreatic diseases international : HBPD INT 2016. February;15(1):99–105. [DOI] [PubMed] [Google Scholar]
- 30.Ilkovitch D, Lopez DM. The liver is a site for tumor-induced myeloid-derived suppressor cell accumulation and immunosuppression. Cancer Res 2009. July 1;69(13):5514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacotte S, Slits F, Orci LA, et al. Impact of myeloid-derived suppressor cell on Kupffer cells from mouse livers with hepatocellular carcinoma. Oncoimmunology 2016;5(11):e1234565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stromnes IM, Brockenbrough JS, Izeradjene K, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 2014. November;63(11):1769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavazoie MF, Pollack I, Tanqueco R, et al. LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer. Cell 2018;172(4):825–40.e18.** This study demonstrates that LXR/ApoE agonism has a tumor suppressive effect via MDSC inhibition.
- 34.Guo D, Reinitz F, Youssef M, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov 2011. October;1(5):442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LXR agonism inhibits metastatic melanoma through activation of ApoE. Cancer Discov 2014. May;4(5):Of16. [DOI] [PubMed] [Google Scholar]
- 36.Wculek SK, Cueto FJ, Mujal AM, et al. Dendritic cells in cancer immunology and immunotherapy. Nature reviews Immunology 2020. January;20(1):7–24. [DOI] [PubMed] [Google Scholar]
- 37.Tran Janco JM, Lamichhane P, Karyampudi L, et al. Tumor-infiltrating dendritic cells in cancer pathogenesis. Journal of immunology (Baltimore, Md : 1950) 2015;194(7):2985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takagi S, Miyagawa S, Ichikawa E, et al. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Human pathology 2004. July;35(7):881–6. [DOI] [PubMed] [Google Scholar]
- 39.Platzer B, Elpek Kutlu G, Cremasco V, et al. IgE/FcεRI-Mediated Antigen Cross-Presentation by Dendritic Cells Enhances Anti-Tumor Immune Responses. Cell Reports 2015;10(9):1487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín-Sierra C, Martins R, Laranjeira P, et al. Functional Impairment of Circulating FcεRI(+) Monocytes and Myeloid Dendritic Cells in Hepatocellular Carcinoma and Cholangiocarcinoma Patients. Cytometry Part B, Clinical cytometry 2019. November;96(6):490–95. [DOI] [PubMed] [Google Scholar]
- 41.Hegde S, Krisnawan VE, Herzog BH, et al. Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer. Cancer Cell 2020. March 16;37(3):289–307.e9.** This study demonstrates that enhanced DC infliltration and activation can boost T cell immunity.
- 42.Freedman RS, Vadhan-Raj S, Butts C, et al. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clinical cancer research : an official journal of the American Association for Cancer Research 2003. November 1;9(14):5228–37. [PubMed] [Google Scholar]
- 43.Morse MA, Nair S, Fernandez-Casal M, et al. Preoperative mobilization of circulating dendritic cells by Flt3 ligand administration to patients with metastatic colon cancer. J Clin Oncol 2000. December 1;18(23):3883–93. [DOI] [PubMed] [Google Scholar]
- 44.Vonderheide RH. CD40 Agonist Antibodies in Cancer Immunotherapy. Annual review of medicine 2020. January 27;71:47–58. [DOI] [PubMed] [Google Scholar]
- 45.Borges L, Miller RE, Jones J, et al. Synergistic action of fms-like tyrosine kinase 3 ligand and CD40 ligand in the induction of dendritic cells and generation of antitumor immunity in vivo. J Immunol 1999. August 1;163(3):1289–97. [PubMed] [Google Scholar]
- 46.Mandal A, Viswanathan C. Natural killer cells: In health and disease. Hematology/oncology and stem cell therapy 2015. June;8(2):47–55. [DOI] [PubMed] [Google Scholar]
- 47.Chiossone L, Dumas PY, Vienne M, et al. Natural killer cells and other innate lymphoid cells in cancer. Nature reviews Immunology 2018. November;18(11):671–88. [DOI] [PubMed] [Google Scholar]
- 48.Peng L-s Zhang J-y, Teng Y-s, et al. Tumor-Associated Monocytes/Macrophages Impair NK-Cell Function via TGFβ1 in Human Gastric Cancer. Cancer immunology research 2017;5(3):248–56. [DOI] [PubMed] [Google Scholar]
- 49.Jun E, Song AY, Choi J-W, et al. Progressive Impairment of NK Cell Cytotoxic Degranulation Is Associated With TGF-β1 Deregulation and Disease Progression in Pancreatic Cancer. Frontiers in immunology 2019;10:1354–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng X, Qian Y, Fu B, et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nature immunology 2019. December;20(12):1656–67. [DOI] [PubMed] [Google Scholar]
- 51.Melum E, Karlsen TH, Schrumpf E, et al. Cholangiocarcinoma in primary sclerosing cholangitis is associated with NKG2D polymorphisms. Hepatology 2008. January;47(1):90–6. [DOI] [PubMed] [Google Scholar]
- 52.Wadsworth CA, Dixon PH, Taylor-Robinson S, et al. Polymorphisms in Natural Killer Cell Receptor Protein 2D (NKG2D) as a Risk Factor for Cholangiocarcinoma. Journal of clinical and experimental hepatology 2019. Mar-Apr;9(2):171–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsukagoshi M, Wada S, Yokobori T, et al. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci 2016. February;107(2):116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornillet M, Jansson H, Schaffer M, et al. Imbalance of Genes Encoding Natural Killer Immunoglobulin-Like Receptors and Human Leukocyte Antigen in Patients With Biliary Cancer. Gastroenterology 2019. October;157(4):1067–80.e9. [DOI] [PubMed] [Google Scholar]
- 55.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014. May 9;344(6184):641–5.* This study demonstrates that T cell infusion mediates tumor regression in CCA.
- 56.Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. British journal of cancer 2013. November 12;109(10):2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oshikiri T, Miyamoto M, Shichinohe T, et al. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol 2003. December;84(4):224–8. [DOI] [PubMed] [Google Scholar]
- 58.Ma C, Peng C, Lu X, et al. Downregulation of FOXP3 inhibits invasion and immune escape in cholangiocarcinoma. Biochem Biophys Res Commun 2015. March 6;458(2):234–9. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Li X, Wei X, et al. PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Signal transduction and targeted therapy 2020. April 17;5(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walter D, Herrmann E, Schnitzbauer AA, et al. PD-L1 expression in extrahepatic cholangiocarcinoma. Histopathology 2017. September;71(3):383–92. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Dong H, Ni S, et al. Programmed death-ligand 1 is upregulated in intrahepatic lymphoepithelioma-like cholangiocarcinoma. Oncotarget 2016. October 25;7(43):69749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye Y, Zhou L, Xie X, et al. Interaction of B7-H1 on intrahepatic cholangiocarcinoma cells with PD-1 on tumor-infiltrating T cells as a mechanism of immune evasion. J Surg Oncol 2009. November 1;100(6):500–4. [DOI] [PubMed] [Google Scholar]
- 63.Mody K, Starr J, Saul M, et al. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J Gastrointest Oncol 2019. December;10(6):1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang J, Yoo C, Jeong JH, et al. Efficacy and safety of pembrolizumab in patients with PD-L1 positive advanced biliary tract cancer (BTC): A prospective cohort study. Journal of Clinical Oncology 2019;37(15_suppl):4082–82. [Google Scholar]
- 65.Lim YJ, Koh J, Kim K, et al. Clinical Implications of Cytotoxic T Lymphocyte Antigen-4 Expression on Tumor Cells and Tumor-Infiltrating Lymphocytes in Extrahepatic Bile Duct Cancer Patients Undergoing Surgery Plus Adjuvant Chemoradiotherapy. Target Oncol 2017. April;12(2):211–18. [DOI] [PubMed] [Google Scholar]
- 66.Zappasodi R, Sirard C, Li Y, et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med 2019. May;25(5):759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou G, Sprengers D, Mancham S, et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. Journal of hepatology 2019. October;71(4):753–62. [DOI] [PubMed] [Google Scholar]
- 68.Vaquero J, Aoudjehane L, Fouassier L. Cancer-associated fibroblasts in cholangiocarcinoma. Curr Opin Gastroenterol 2020 Mar;36(2):63–69. [DOI] [PubMed] [Google Scholar]
- 69.Itou RA, Uyama N, Hirota S, et al. Immunohistochemical characterization of cancer-associated fibroblasts at the primary sites and in the metastatic lymph nodes of human intrahepatic cholangiocarcinoma. Human pathology 2019. January;83:77–89. [DOI] [PubMed] [Google Scholar]
- 70.Zhang XF, Dong M, Pan YH, et al. Expression pattern of cancer-associated fibroblast and its clinical relevance in intrahepatic cholangiocarcinoma. Human pathology 2017. July;65:92–100. [DOI] [PubMed] [Google Scholar]
- 71.Sirica AE, Almenara JA, Li C. Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications. Experimental and molecular pathology 2014. December;97(3):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manzanares M, Campbell DJW, Maldonado GT, et al. Overexpression of periostin and distinct mesothelin forms predict malignant progression in a rat cholangiocarcinoma model. Hepatol Commun 2018. February;2(2):155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Utispan K, Thuwajit P, Abiko Y, et al. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer 2010. January 24;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res 2013. January 15;73(2):897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cadamuro M, Brivio S, Mertens J, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. Journal of hepatology 2019;70(4):700–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thongchot S, Ferraresi A, Vidoni C, et al. Resveratrol interrupts the pro-invasive communication between cancer associated fibroblasts and cholangiocarcinoma cells. Cancer Lett 2018. August 28;430:160–71. [DOI] [PubMed] [Google Scholar]
- 77.Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. The New England journal of medicine 2015. August 20;373(8):782. [DOI] [PubMed] [Google Scholar]
- 78.Öztürk Akcora B, Storm G, Prakash J, et al. Tyrosine kinase inhibitor BIBF1120 ameliorates inflammation, angiogenesis and fibrosis in CCl(4)-induced liver fibrogenesis mouse model. Sci Rep-Uk 2017;7:44545–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wollin L, Togbe D, Ryffel B. Effects of Nintedanib in an Animal Model of Liver Fibrosis. Biomed Res Int 2020;2020:3867198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gabasa M, Ikemori R, Hilberg F, et al. Nintedanib selectively inhibits the activation and tumour-promoting effects of fibroblasts from lung adenocarcinoma patients. British journal of cancer 2017. October 10;117(8):1128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamanaka T, Harimoto N, Yokobori T, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. British journal of cancer 2020. February 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herbeuval JP, Lelievre E, Lambert C, et al. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol 2004. April 1;172(7):4630–6. [DOI] [PubMed] [Google Scholar]
- 83.Cho H, Seo Y, Loke KM, et al. Cancer-Stimulated CAFs Enhance Monocyte Differentiation and Protumoral TAM Activation via IL6 and GM-CSF Secretion. Clinical cancer research : an official journal of the American Association for Cancer Research 2018. November 1;24(21):5407–21. [DOI] [PubMed] [Google Scholar]
- 84.Isomoto H, Mott JL, Kobayashi S, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology 2007. January;132(1):384–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wehbe H, Henson R, Meng F, et al. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res 2006. November 1;66(21):10517–24. [DOI] [PubMed] [Google Scholar]
- 86.Frampton G, Invernizzi P, Bernuzzi F, et al. Interleukin-6-driven progranulin expression increases cholangiocarcinoma growth by an Akt-dependent mechanism. Gut 2012. February;61(2):268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada D, Rizvi S, Razumilava N, et al. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology 2015. May;61(5):1627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sripa B, Thinkhamrop B, Mairiang E, et al. Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS Negl Trop Dis 2012;6(5):e1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang M, Yang H, Wan L, et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. Journal of hepatology 2020;73(5):1118–30. [DOI] [PubMed] [Google Scholar]
- 90.Ware MB, McQuinn C, Zaidi MY, et al. Dual blockade of IL-6 and CTLA-4 regresses pancreatic tumors in a CD4+ T cell-dependent manner. bioRxiv 2020:2020.02.07.939199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kleinegger F, Hofer E, Wodlej C, et al. Pharmacologic IL-6Rα inhibition in cholangiocarcinoma promotes cancer cell growth and survival. Biochim Biophys Acta Mol Basis Dis 2019. February 1;1865(2):308–21. [DOI] [PubMed] [Google Scholar]
- 92.Lin Y, Li B, Yang X, et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia 2019. December;21(12):1133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eksteen B, Miles A, Curbishley SM, et al. Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10. J Immunol 2006. July 1;177(1):593–603. [DOI] [PubMed] [Google Scholar]
- 94.Fukuda Y, Asaoka T, Eguchi H, et al. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci 2020. February;111(2):323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes hedgehog survival signaling in cholangiocarcinoma cells. Hepatology 2011;54(6):2076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cadamuro M, Nardo G, Indraccolo S, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013;58(3):1042–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clapéron A, Mergey M, Aoudjehane L, et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 2013. December;58(6):2001–11. [DOI] [PubMed] [Google Scholar]
- 98.Vaeteewoottacharn K, Kariya R, Dana P, et al. Inhibition of carbonic anhydrase potentiates bevacizumab treatment in cholangiocarcinoma. Tumour Biol 2016. July;37(7):9023–35. [DOI] [PubMed] [Google Scholar]
- 99.Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. British journal of cancer 2008. January 29;98(2):418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stacker SA, Caesar C, Baldwin ME, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001. February;7(2):186–91. [DOI] [PubMed] [Google Scholar]
- 101.Aishima S, Nishihara Y, Iguchi T, et al. Lymphatic spread is related to VEGF-C expression and D2–40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2008. March;21(3):256–64. [DOI] [PubMed] [Google Scholar]
- 102.Ma L, Hernandez MO, Zhao Y, et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell 2019. October 14;36(4):418–30.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yeh CN, Chang YC, Su Y, et al. Identification of MALT1 as both a prognostic factor and a potential therapeutic target of regorafenib in cholangiocarcinoma patients. Oncotarget 2017. December 26;8(69):113444–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim RD, Sanoff HK, Poklepovic AS, et al. A multi-institutional phase 2 trial of regorafenib in refractory advanced biliary tract cancer. Cancer 2020. May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shroff RT, Yarchoan M, O’Connor A, et al. The oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma. British journal of cancer 2017. May 23;116(11):1402–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kosaka N, Yoshioka Y, Fujita Y, et al. Versatile roles of extracellular vesicles in cancer. J Clin Invest 2016. April 1;126(4):1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maas SLN, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends in cell biology 2017. March;27(3):172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becker A, Thakur BK, Weiss JM, et al. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016. December 12;30(6):836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Masyuk AI, Huang BQ, Ward CJ, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol 2010. October;299(4):G990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Severino V, Dumonceau JM, Delhaye M, et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology 2017. August;153(2):495–504 e8. [DOI] [PubMed] [Google Scholar]
- 111.Li L, Masica D, Ishida M, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 2014. September;60(3):896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arbelaiz A, Azkargorta M, Krawczyk M, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017. October;66(4):1125–43. [DOI] [PubMed] [Google Scholar]
- 113.Lapitz A, Arbelaiz A, O’Rourke CJ, et al. Patients with Cholangiocarcinoma Present Specific RNA Profiles in Serum and Urine Extracellular Vesicles Mirroring the Tumor Expression: Novel Liquid Biopsy Biomarkers for Disease Diagnosis. Cells 2020. March 14;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li L, Piontek K, Ishida M, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology 2017. February;65(2):501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ota Y, Takahashi K, Otake S, et al. Extracellular vesicle-encapsulated miR-30e suppresses cholangiocarcinoma cell invasion and migration via inhibiting epithelial-mesenchymal transition. Oncotarget 2018. March 27;9(23):16400–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Piha-Paul SA, Oh D- Y, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. International Journal of Cancer 2020;147(8):2190–98. [DOI] [PubMed] [Google Scholar]
- 117.Kim RD, Chung V, Alese OB, et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncology 2020;6(6):888–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klein O, Kee D, Nagrial A, et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients With Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncology 2020;6(9):1405–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018. May;24(5):541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rizvi S, Fischbach SR, Bronk SF, et al. YAP-associated chromosomal instability and cholangiocarcinoma in mice. Oncotarget 2018. January 19;9(5):5892–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neal JT, Li X, Zhu J, et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018. December 13;175(7):1972–88.e16.** This study demonstrates that the tumor immune microenviroment can be recapitulated in patient-derived organoids.