Abstract
The efficacy of the combination of a PARP inhibitor (PARPi) and an EZH2 inhibitor has been investigated in breast cancer cells with either BRCA1 mutation or BRCA2 mutation. However, earlier studies focused on the efficacy of this combination against BRCA-mutated but not BRCA-proficient breast cancer. Yang et al. observed that PARP1 depletion combined with EZH2 depletion via PRC2 depletion did not affect the growth of BRCA1/2 wild-type breast cancer cells in vitro. Moreover, Yang et al. reported that this combination stimulated synthetic viability of BRCA1/2-proficient breast cancer cells in vivo by regulating the tumor microenvironment to induce angiogenesis and differentiation of M2-type macrophages. The findings of Yang et al. provided evidence that both in vitro and animal models should be employed in the studies of PARPi combination therapies in order to involve the alteration of the tumor microenvironment in these investigations. These studies of PARP inhibition combined with EZH2 inhibition in breast cancer showed that this combination may benefit breast cancer patients carrying BRCA1-mutated tumor, but the combination may also enhance recurrence of BRCA2-mutated tumor and may even promote BRCA-proficient cancer cell survival. Therefore, BRCA1 mutation status should be used to select breast cancer patients for PARPi and EZH2 inhibitor combination treatment in clinical trials in the future.
Keywords: PRC2, macrophages, PARP inhibitor
Introduction
DNA repair deficiency and replication stress are both cellular stresses that contribute to genomic instability in breast cancer [1]. Poly(ADP-ribose) polymerase 1 (PARP1) plays a key role in regulating mechanisms to resolve DNA damage and replication fork stalling by consuming nicotinamide adenine dinucleotide (NAD+) to ADP(ribosyl)ate multiple proteins [2, 3]. Small-molecule PARP inhibitors (PARPis) are designed to compete with NAD+ for PARP1 and thus inhibit PARP1 enzymatic activity and trap PARP1 on DNA, inducing DNA damage and replication fork stalling as a result [3]. In addition to PARP1-mediated pathways, BRCA1/2 proteins also facilitate DNA repair and restoration of replication forks [4, 5]. Therefore, targeting PARP1 using PARPis can induce synthetic lethality in cancer cells with BRCA deficiencies [2]. Indeed, PARPis have been shown to prolong progression-free survival of breast cancer patients carrying germline deleterious BRCA1/2 mutations [6]. As the first strategy to successfully demonstrate synthetic lethality against BRCA-mutated breast cancer in the clinic [2], PARPis in cancer treatment have been extensively investigated to determine their effect on DNA damage repair and replication fork stalling [6]. However, the biological functions of PARP1 and BRCA1/2 proteins are not limited to DNA repair and gene transcription [7, 8]. For example, PARP1 also regulates NF-κB–mediated inflammation, mitosis, and cellular energetics, as it interacts with a variety of target proteins [8, 9]. In an investigation of the PARPi and EZH2 inhibitor combination, a recent study published in The FEBS Journal provides new insights showing that the interaction between tumor cells and the tumor microenvironment should be taken into consideration in the development of combinational therapies for targeting DNA damage repair pathways in cancer.
Therapeutic benefit of combination of PARPi and EZH2 inhibitor may be limited to BRCA1-mutated patients
Intrinsic and acquired PARPi resistance have been reported in germline BRCA-mutated cancer patients [10]; therefore, several combination therapies have been proposed to overcome PARPi resistance in breast cancer. Among the strategies to overcome PARPi resistance, the combination of a PARPi and an enhancer of zeste homolog 2 (EZH2) inhibitor showed synergism in BRCA-mutated breast cancer cell lines [11, 12]. EZH2 is a histone methyltransferase in polycomb repressive complex 2 (PRC2) [13]. PARP1-mediated poly-ADP(ribosyl)ation of EZH2 leads to dissociation of EZH2 from PRC2 in response to DNA damage in BRCA-mutated breast cancer cells [11, 12]. EZH2 and PARP1 also are promising targets because they were found to be co-overexpressed in about half of breast cancer patients [14], and high expression of PRC2 and PARP1 is associated with poor prognosis [15–17]. Yamaguchi et al. demonstrated that a DNA-damaging alkylating agent, methyl methanesulfonate, induced proteasome-mediated degradation of EZH2, whereas this EZH2 degradation could be reversed by combining methyl methanesulfonate with a PARPi [11]. Yamaguchi et al. further showed that the combination of a PARPi (olaparib) and an EZH2 inhibitor (GSK343) inhibited colony formation and tumor growth more than olaparib single-agent treatment did in BRCA1-mutated SUM149 breast cancer cells and a SUM149 orthotopic xenograft animal model [11]. However, in a BRCA2-depleted mouse breast cancer model, Rondinelli et al. demonstrated that the mice treated with the combination of a PARPi (olaparib) and an EZH2 inhibitor (GSK126) experienced early recurrence, and the tumor inhibition effect of the combination was similar to that of PARPi single-agent treatment [18]. Rondinelli et al. also showed that EZH2 played a critical role at the site of replication fork stalling in BRCA2-deficient tumor. These results suggest that the efficacy of the combination of an EZH2 inhibitor and a PARPi in breast cancer treatment is affected by the BRCA mutation status of the tumor.
While the studies published by Yamaguchi et al. and Rondinelli et al. focused on the effect of a PARPi combined with EZH2 inhibition on BRCA-mutated cancer cells [11, 18], Yang et al. reported that the tumor-promoting effect of PARP1 depletion combined with PRC2 depletion in BRCA-proficient breast cancer resulted from NF-κB signaling–induced angiogenesis and macrophage differentiation [19]. The PARP1 and PRC2 double-depleted cell was generated by knock-out of PARP1 and knock-down of PRC2 protein expression by small hairpin RNA targeting of either EZH2 or SUZ12 [19]. Interestingly, the function of PARP1 in DNA damage repair was not the key determining factor in the BRCA-proficient tumor growth promoted by PARP1 and PRC2 double depletion. Yang et al. further demonstrated that the PARP1 and PRC2 double-depleted tumor cells not only proliferated faster than single- depleted counterparts in intra-tumoral regions, but also activated NF-κB signaling that secreted cytokines and chemokines to induce angiogenesis and increase differentiation into tumor-promoting M2 macrophages [19]. On the basis of these publications, the PARPi and EZH2 inhibitor combination may only benefit patients bearing BRCA1-mutated tumor (Figure 1). Furthermore, as demonstrated by Yang et al. together with the previous work, the involvement of the tumor microenvironment revealed an inconsistency in overall cancer cell growth inhibition between in vitro experiments and animal models. Further in vivo investigation should be pursued to determine the effect of BRCA1/2 mutation status on the tumor response to PARPi and EZH2 inhibitor combination therapy.
Figure 1. Efficacy of PARP inhibitor combined with EZH2 inhibitor may depend on BRCA1/2 mutation status in breast cancer patients.
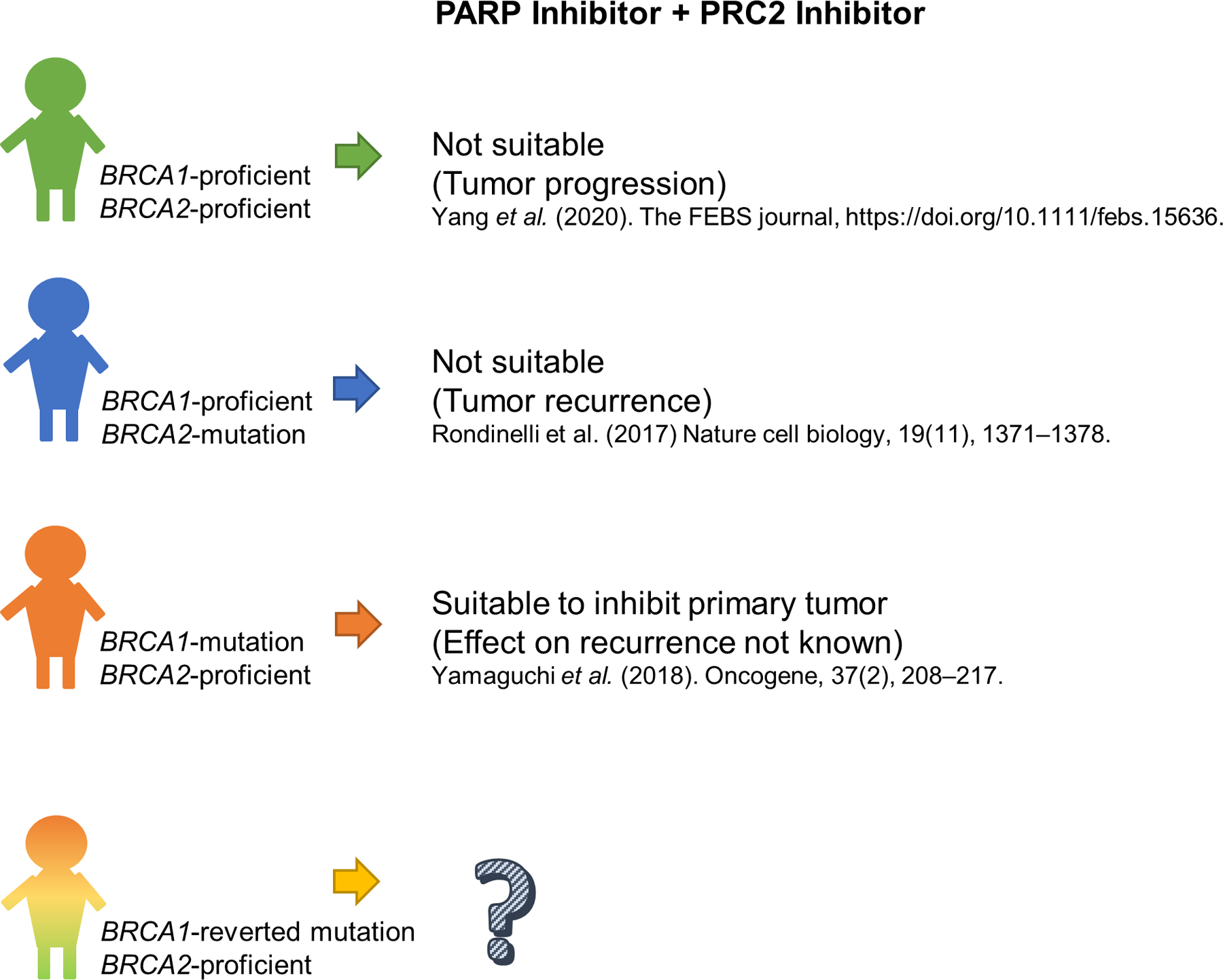
Using breast cancer cell lines in xenograft mouse models, studies showed that the tumor response to the PARP inhibitor and EZH2 inhibitor combination varies between models with differing BRCA1 and BRCA2 mutation status.
Conclusion
The combination of a PARPi and an EZH2 inhibitor has been evaluated in vitro and in vivo in breast cancer and ovarian cancer cells with varying BRCA mutation status [11, 14, 19, 20] and has been proposed for clinical trial investigation (ClinicalTrials.gov identifier NCT04355858). However, the effect of this combination on the tumor microenvironment has not been thoroughly investigated, and it is not clear whether the synthetic viability of PARPi and EZH2 inhibitor is limited to BRCA-proficient breast cancer patients. Moreover, secondary BRCA mutation has been observed in patients with acquired PARPi resistance [21], and the response in patients bearing BRCA-reverted cancer may not be similar to that in patients carrying wild-type BRCA tumor. It is also noteworthy that EZH2 has a non-catalytic function in cancer cells [22], and whether EZH2 inhibitors can fully suppress its biological functions to the same degree as EZH2 depletion is not well studied yet. In conclusion, the effects of a PARPi and an EZH2 inhibitor on the tumor microenvironment and the recurrence of tumors with varying BRCA mutation status should be further investigated to identify which patient population will benefit from the combination.
Acknowledgments
The manuscript was edited by Sarah Bronson, ELS, of the Research Medical Library at The University of Texas MD Anderson Cancer Center. M.-K. Chen is supported by the U.S. National Cancer Institute grant 5R01CA211615 (to Liuqing Yang).
Abbreviations:
- PARP1
poly(ADP-ribose) polymerase 1
- PARPi
PARP inhibitor
- EZH2
enhancer of zeste homolog 2
- PRC2
polycomb repressive complex 2
Footnotes
Conflicts of interest: The author has no conflicts of interest.
References
- 1.Duijf PHG, Nanayakkara D, Nones K, Srihari S, Kalimutho M & Khanna KK (2019) Mechanisms of Genomic Instability in Breast Cancer, Trends Mol Med. 25, 595–611. [DOI] [PubMed] [Google Scholar]
- 2.Helleday T (2011) The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings, Mol Oncol. 5, 387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pommier Y, O’Connor MJ & de Bono J (2016) Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action, Sci Transl Med. 8, 362ps17. [DOI] [PubMed] [Google Scholar]
- 4.Tarsounas M & Sung P (2020) The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication, Nat Rev Mol Cell Biol. 21, 284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fradet-Turcotte A, Sitz J, Grapton D & Orthwein A (2016) BRCA2 functions: from DNA repair to replication fork stabilization, Endocr Relat Cancer. 23, T1–T17. [DOI] [PubMed] [Google Scholar]
- 6.Yap TA, Plummer R, Azad NS & Helleday T (2019) The DNA Damaging Revolution: PARP Inhibitors and Beyond, Am Soc Clin Oncol Educ Book. 39, 185–195. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K & Miki Y (2004) Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage, Cancer science. 95, 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver AN & Yang ES (2013) Beyond DNA Repair: Additional Functions of PARP-1 in Cancer, Front Oncol. 3, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marti JM, Fernandez-Cortes M, Serrano-Saenz S, Zamudio-Martinez E, Delgado-Bellido D, Garcia-Diaz A & Oliver FJ (2020) The Multifactorial Role of PARP-1 in Tumor Microenvironment, Cancers (Basel). 12, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noordermeer SM & van Attikum H (2019) PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells, Trends Cell Biol. 29, 820–834. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi H, Du Y, Nakai K, Ding M, Chang SS, Hsu JL, Yao J, Wei Y, Nie L, Jiao S, Chang WC, Chen CH, Yu Y, Hortobagyi GN & Hung MC (2018) EZH2 contributes to the response to PARP inhibitors through its PARP-mediated poly-ADP ribosylation in breast cancer, Oncogene. 37, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruso LB, Martin KA, Lauretti E, Hulse M, Siciliano M, Lupey-Green LN, Abraham A, Skorski T & Tempera I (2018) Poly(ADP-ribose) Polymerase 1, PARP1, modifies EZH2 and inhibits EZH2 histone methyltransferase activity after DNA damage, Oncotarget. 9, 10585–10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jani KS, Jain SU, Ge EJ, Diehl KL, Lundgren SM, Muller MM, Lewis PW & Muir TW (2019) Histone H3 tail binds a unique sensing pocket in EZH2 to activate the PRC2 methyltransferase, Proc Natl Acad Sci U S A. 116, 8295–8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostafa NA, Fathi A, Haggag R, Ashour H & Salem RA (2018) Significance of combined analysis of PARP1 and EZH2 expression as potentially targetable biomarkers in breast cancer, Egyptian Journal of Pathology. 38, 266–274. [Google Scholar]
- 15.Martin CJ & Moorehead RA (2020) Polycomb repressor complex 2 function in breast cancer (Review), Int J Oncol. 57, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wassef M, Rodilla V, Teissandier A, Zeitouni B, Gruel N, Sadacca B, Irondelle M, Charruel M, Ducos B, Michaud A, Caron M, Marangoni E, Chavrier P, Le Tourneau C, Kamal M, Pasmant E, Vidaud M, Servant N, Reyal F, Meseure D, Vincent-Salomon A, Fre S & Margueron R (2015) Impaired PRC2 activity promotes transcriptional instability and favors breast tumorigenesis, Genes Dev. 29, 2547–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jene-Sanz A, Varaljai R, Vilkova AV, Khramtsova GF, Khramtsov AI, Olopade OI, Lopez-Bigas N & Benevolenskaya EV (2013) Expression of polycomb targets predicts breast cancer prognosis, Mol Cell Biol. 33, 3951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rondinelli B, Gogola E, Yucel H, Duarte AA, van de Ven M, van der Sluijs R, Konstantinopoulos PA, Jonkers J, Ceccaldi R, Rottenberg S & D’Andrea AD (2017) EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation, Nat Cell Biol. 19, 1371–1378. [DOI] [PubMed] [Google Scholar]
- 19.Yang AY, Choi EB, So Park M, Kim SK, Park MS & Kim MY (2020) PARP1 and PRC2 double deficiency promotes BRCA-proficient breast cancer growth by modification of the tumor microenvironment, FEBS J, doi: 10.1111/febs.15636. [DOI] [PubMed] [Google Scholar]
- 20.Karakashev S, Fukumoto T, Zhao B, Lin J, Wu S, Fatkhutdinov N, Park PH, Semenova G, Jean S, Cadungog MG, Borowsky ME, Kossenkov AV, Liu Q & Zhang R (2020) EZH2 Inhibition Sensitizes CARM1-High, Homologous Recombination Proficient Ovarian Cancers to PARP Inhibition, Cancer Cell. 37, 157–167 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobalina L, Armenia J, Irving E, O’Connor MJ & Forment JV (2020) A meta-analysis of reversion mutations in BRCA genes identifies signatures of DNA end-joining repair mechanisms driving therapy resistance, Ann Oncol, doi: 10.1016/j.annonc.2020.10.470. [DOI] [PubMed] [Google Scholar]
- 22.Koyen AE, Madden MZ, Park D, Minten EV, Kapoor-Vazirani P, Werner E, Pfister NT, Haji-Seyed-Javadi R, Zhang H, Xu J, Deng N, Duong DM, Pecen TJ, Frazier Z, Nagel ZD, Lazaro JB, Mouw KW, Seyfried NT, Moreno CS, Owonikoko TK, Deng X & Yu DS (2020) EZH2 has a non-catalytic and PRC2-independent role in stabilizing DDB2 to promote nucleotide excision repair, Oncogene. 39, 4798–4813. [DOI] [PMC free article] [PubMed] [Google Scholar]


