Abstract
APOBEC3 enzymes are key enzymes in our innate immune system regulating antiviral response in HIV and unfortunately adding diversity in cancer as they deaminate cytosine. Seven unique single and double domain APOBEC3s provide them with unique activity and specificity profiles for this deamination. Recent crystal and NMR structures of APOBEC3 complexes are unraveling the variety of epitopes involved in binding nucleic acids, including at the catalytic site, elsewhere on the catalytic domain and in the inactive N-terminal domain. The interplay between these diverse interactions is critical to uncovering the mechanisms by which APOBEC3s recognize and process their substrates.
Keywords: APOBEC3 proteins, ssDNA binding, deamination target sequence, RNA binding
Introduction:
APOBEC3s (apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3) is a family of human cytidine deaminases that catalyze the deamination of cytosine to uracil in the single stranded DNA(ssDNA) or ssRNA[1–5]. The APOBEC3 (A3) family consists of seven members; three of these enzymes (A3A, A3C, A3H) have a single zinc binding domain while the other four (A3B, A3D, A3F, A3G) have two domains. The two-domain A3s have a catalytically active C terminal domain (CTD) and a pseudo-catalytic N terminal domain (NTD) that binds to nucleic acids but does not have deaminase activity. Although NTDs have no deamination activity, they appear to be key for regulating the catalytic activity through increasing ssDNA binding affinity and promoting oligomerization[6]. These seven unique A3’s provide unique activity and specificity profiles for deamination.
A3s were first discovered due to their antiviral restriction of HIV[7–10]. HIV-1 counteracts A3 proteins by the accessory proteins Vif, which targets A3s for proteasomal degradation [8,11–16]. In addition to activity against retroviruses (including HIV-1), A3s are involved in the restriction of endogenous retrotransposons, especially LINE-1 elements. A3s also restrict DNA viruses including nuclear replicating ssDNA viruses such as adeno-associated virus[17] and dsDNA viruses such as hepatitis B virus, herpes viruses and HPV[18–21]. However, A3 activity can be a double-edged sword. In addition to inducing mutations on single-stranded viral genomes, A3s can cause mutations in host genomes when localization and/or activity of A3s is mis-regulated. Recent studies found that A3A, A3B and A3H could cause heterogeneities of human genome in various types of human cancer, including breast, bladder, head and neck, cervical, and lung cancer[22–30]. Moreover, when coupled with CRIPSR/Cas9, A3s are being leveraged as novel base editors to treat genetic diseases[31,32]. Therefore, insights of structures of A3 proteins and their complexes with substrates may contribute toward developing more effective antivirals and cancer therapeutics as well as base editing systems for gene therapy.
The structure of the catalytic domain of A3G (A3G-CTD) was the first domain to be solved by NMR and X-ray crystallography in 2008[33,34]. Since then more than fifty structures of A3 domains have been deposited in Protein Data Bank (PDB) (Supplementary Table 1) as of July 2020. However, these structures have been challenging to acquire due to poor solubility and tendency of these enzymes to aggregate. In the last several years however, complexes of A3 domains with DNA/RNA and full length A3 structures have finally been successfully determined.
In this review we will summarize the structural features and differences of individual A3 domains. These structural features provide the determinants of specificity for the deamination target sequences. We will further highlight the unique interactions RNA makes with A3H and finally discuss the most recently determined full-length double domain A3G structures. Thus this review will gives an overview of the structural basis for how these unique A3’s interact with DNA and RNA.
Structural features of individual A3 domains:
The consensus A3 domain structure contains six α-helices and five β-strands forming one β-sheet (Fig. 1a–c), and one Zn2+ is chelated by the histidine and cysteines in the deaminase motif which is essential for catalysis[1]. Each domain possesses consensus (H/C)-(A/V)-E-X24–30-P-C-X2-C cytidine deaminase motif although the N-terminal domain of the double-domain A3s are catalytically inactive[35]. The catalytic site centered by Zn2+ forms a positively charged pocket (Fig. 1a). Although the overall fold is conserved, subtle sequence differences among A3s have resulted in variations in loops length, structure, and flexibility as well as variations in surface charge, active site interactions, and oligomeric tendency (Figs. 1d and e). These variations underlie the functional characteristics of each A3 protein.
Figure 1: Recognition of the deamination target sequences.
a) Apo-form structure of the A3G-CTD (PDB ID: 2KEM). Electrostatic surface distribution showing positive (+5 kT/e, blue) and negative (−5 kT/e, red) regions. Helices and strands are labeled, and sidechains of His257, Glu259, Cys288 and Cys291 are shown as stick model. Zn2+ ion is shown as a purple sphere. b) Co-crystal structure of A3A with ssDNA (PDB ID: 5KEG). Three nucleotides of the deamination target ssDNA bound as U-shaped conformations. c) Co-crystal structure of a A3G catalytic domain variant (CTD2*) with ssDNA (PDB ID: 6BUX). ssDNA have more open and extended shape providing extra interaction with protein. In both A3A and A3G-CTD2*, the target cytosine (C0) is flipped out, and tightly packed under the Zn2+ ion by stacking aromatic rings with the Zn2+-chelating residue His70 and His257 in A3A and A3G, respectively. Proteins are represented as yellow cartoons; C, N, and O atoms are colored yellow, blue, and red, respectively, for amino acid residues of the protein. DNAs are represented as cartoons; nucleotides are colored blue and phosphate backbone are colored orange. d) and e) Summary of interactions between protein and ssDNA for A3A and A3G-CTD2*, respectively. f) Sequence alignment and structural representation of active site loops in APOBEC3 family. The sequence alignments of active site loops in A3 family are mapped on the ssDNA bound A3A crystal structure (PDB: 5KEG). The sequence similarity is colored according to figure legend.
Particularly, sequence differences in active site loops (loop 1, loop 3, loop 5 and loop 7) that surround the active site pocket (Fig. 1f) of catalytically active domains mainly contribute to the differential substrate specificity, binding affinity and deamination activity for ssDNA, as well as the distinct physiological functions in A3s[36]. For instance, comparing the structures of Z1 A3 domains (A3A, A3B-CTD and A3G-CTD), A3A has the shortest loop-1 appeared to keep the catalytic site open for substrate access, which may relate to A3A’s strong deaminase activity[37]. A3A and A3G-CTD share a unique feature in β-strand 2 as it is divided to two sections connected with a short loop [33,37,38]. Early structures of A3G-CTD suggested importance of loop-1 and loop-7 residues, including W211, R213, R215 and H216[33,39] and D316 and D317[34], respectively, for ssDNA-binding and deamination. A3B has been related to mutations of human genome in some cancer cells[27,28], yet A3B’s catalytic activity is low in vitro, which may be due to the closed conformation of its catalytic site seen in both solution NMR and crystal structures[40–42]. Therefore, systematic analysis of these active site loops will help reveal the structural mechanism of these functionally overlapping but distinct A3 proteins.
Structural determinants selecting deamination target sequences in ssDNA: A3A, B and G.
Although A3 domains share similar structure and overall fold, they have different catalytic activities, substrate preferences (Supplementary Table 2), and sequences specificities. A3 proteins deaminates 5’-TC and 5’-CC hot spots in single-stranded DNA (ssDNA) as well as HIV-1 proviral DNA[43–46]. Time-resolved NMR deamination assay indicated that A3G-CTD clearly preferred the 3’ cytidine as deamination target compared with the middle cytidine within a 5’-CCC motif[47]. Recent years, co-crystal structures of A3 proteins and substrate ssDNAs have emerged. The A3A-ssDNA complexes were published by both the Aihara and the Matsuo/Schiffer labs in 2017[48,49]. The Aihara lab co-crystallized inactive (E72A mutation) and C-terminal 4-residues truncation version of human A3A (Residues 1–195) with 15-nt ssDNA containing a 5’-TC deamination target (5’-AAAAAAATCGGGAAA)[48]. This crystal structure has 4 monomeric complexes in the asymmetric unit, and each shows clear electron density for either 5 nucleotides(5’-A−2T−1C0G+1G+2) or 6 nucleotides (5’-A−2T−1C0G+1G+2G+3) centered on the target cytosine (C0). The Schiffer and Matsuo labs also used an inactive A3A variant (E72A/C171A) for co-crystallization with 15-nt ssDNA (5’-TTTTTTTCTTTTTTT). There was a single A3A–ssDNA complex in the asymmetric unit of the 2.2 Å resolution structure where the target sequence 5’-T−1C0 was well ordered. In both structures, A3A-bound ssDNA adopts a U-shaped conformation anchored by the target cytosine C0 and T−1, with up and down-stream ssDNA bent away from the active site (Fig. 1b, d). The C0 and T−1 are accommodated in a deep groove formed by Loops-1, −3, −5 and −7 of A3A. The bound DNA adopts an irregular conformation, flipping out the nucleobase of the target cytidine (C0), to encircle the side chain of H29, which serves as a gate keeper to the active site (Fig. 1b, d).
The Aihara group also determined a co-crystal structure of a 7-nt ssDNA (5′-TTTTCAT) and a non-catalytic mutant (E255A) of A3B-CTD in which loop-1 was swapped with A3A loop-1 (A3Bctd-QMΔloop3-A3Aloop1) (PDB ID: 5TD5)[48]. The A3Bctd-QMΔloop3-A3Aloop1-ssDNA complex has a single nucleoprotein complex in the asymmetric unit and clear electron density for 4 nucleotides (5′-T−2T−1C0A+1). The overall DNA conformation and active site interactions look identical to that observed in aforementioned DNA-bound A3A structure[48], which was likely because this A3Bctd-QMΔloop3-A3Aloop1 protein contained a histidine from A3A (H29 in A3A) that provided ssDNA critical interactions to form the U-shape (Fig 2a, b). The Schiffer lab uncovered the underlying specificity of A3B by combining molecular modeling, dynamics simulations and deamination assays to discover that R211 in A3B-CTD plays the critical gate keeper role in DNA binding and deamination target specificity[50] (Fig 2c, d). The loop-1 amino acid sequence of A3B-CTD (203-NNDPLVLRRRQT-214) is significantly different from that of A3A (23-NNGIGRHKT-31) (Fig 2e). This study filled the gap for how ssDNA binds to the catalytic site of wild-type A3B-CTD, but more can provide a strategy to uncover substrate binding in other wild-type A3 domains.
Figure 2: Structural comparison of A3A-ssDNA and A3B-ssDNA.
The structural comparison of A3A-ssDNA (PDB: 5KEG) (a) and A3B-ssDNA model (c). Proteins are shown as cartoon representation (iceblue for A3A while green for A3B). ssDNAs are shown as orange sticks. Gatekeeper residues are shown as salmon sticks with critical intermolecular interactions shown as gray dashes. The intermolecular interactions of gatekeeper residue for coordinating DNA binding (b) H29 in A3A and(d) R211 in A3B. The different loop-1 residues in A3Bctd-QMΔloop3-A3Aloop1 structure (PDB: 5TD5; yellow sticks, cyan cartoon) and wild type A3B-ssDNA model are shown in (e).
Since A3G-CTD has relatively low affinity to ssDNA, to solve a co-crystal structure of A3G-CTD and ssDNA complex[51] the Matsuo lab generated a soluble, highly active variant of A3G-CTD with higher DNA binding affinity, namely CTD2. Previously in vitro experiments had found that A3G-CTD deaminated 5-nt 5’-TCCCA as fast as longer ssDNA substrates and deamination speed depended on pH with the fastest at pH 5.5, and identified H216 as the determinant of this pH dependency[52]. The CTD2-ssDNA complex structure supported these previous finding as all 5 nucleotides in the 5’-T−3C−2C−1C0A+1 target sequence had interaction with protein, and H216 had a π-π stacking interaction with A+1 which can be enforced by deprotonation of the histidine imidazole ring at lower pH (Fig. 1c, e). The role of H216 in the target sequence binding is similar to the corresponding H29 in A3A, and indeed A3A also deaminate faster at lower pH[53]. As Holden and co-workers suggested in their crystal structure of A3G-CTD[34], D316 and D317 recognized C−2C−1 through specific hydrogen bonds with nucleobases. W211 is unique to A3G-CTD, and it allows additional interaction with T−3 through π-π stacking interaction (Fig 1c, e). The Xiong lab took a different approach to generate co-crystals of A3G-CTD and ssDNA as they used a ssDNA binding protein, namely Pot1, to fuse with a soluble variant of A3G-CTD, and captured ssDNA through the Pot1-binding DNA sequence[54]. However, the Pot1-A3G-CTD fusion protein could not bind the deamination target sequence, instead an adenine of ssDNA from a neighboring asymmetric unit was interacting with W211, which authors suggested as an interaction during the search of target sequences. Recently, Cao lab has published NMR structures of A3G-CTD in complex with deamination product ssDNAs[55], presenting various nucleotide-protein interactions which may be used for non-catalytic binding. Although the A3-ssDNA complex structures have revealed specific interactions between protein and deamination target sequences during catalytic reaction, DNA-interactions during the search of the target sequence on a ssDNA are still elusive. Single molecule experiment such as optical tweezer[56] and atomic force microscopy[57] may be combined with structural information to show how A3 proteins “hops and slides” on ssDNA[58].
Interactions with RNA:
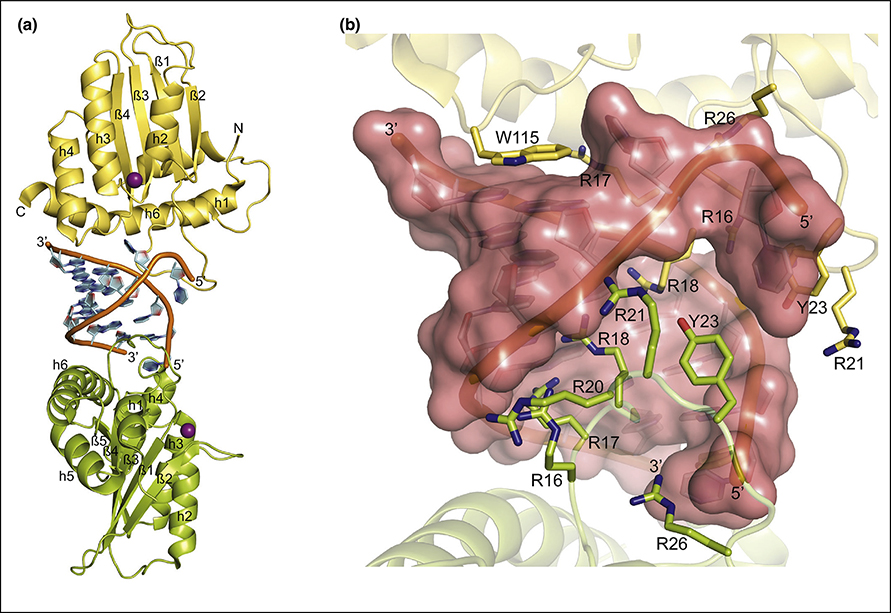
A3H, a single domain A3 and capable of restricting HIV-1 infection, has a unique structural feature as it forms dimer through binding a double-stranded RNA (dsRNA) without protein-protein interaction[30,59,60] (Fig. 3a). In the crystal structure of chimpanzee A3H (cpzA3H), bound 9-nt dsRNA was well ordered, and critical interactions between RNA and cmpA3H were determined, which included Y23 and W115 through π–π stacking interactions with nucleobases, and seven R/K residues in loop-1 fitting in the major groove of the dsRNA (Fig. 3b) forming non-specific interactions with the phosphate backbone[60]. Subsequently, Chen lab reported a crystal structure of a monomeric form of human A3H haplotype II variant that contained nine amino acid substitutions, among them W115A was the key substitution to be monomeric in solution[61]. The interactions of A3H with RNA involving residues remote from the active site indicates the more extensive interplay of the enzymes surface with recognizing nucleic acid sequences.
Figure 3: Crystal structure of the cpzA3H dimer with an RNA duplex.
A3H molecules are represented as yellow and green cartoons; C, N, and O atoms are colored yellow/green, blue, and red, respectively, for amino acid residues of the protein.; RNAs are represented as cartoon as well with color orange for phosphate back bone and light blue for ribose and bases. Zn2+ is shown as a purple sphere. a) Two molecules of cpzA3H (yellow and green) bound to dimeric dsRNA (PDB ID: 5Z98). b) Enlarge view of cpzA3H-dsRNA complex showing important residues involved in protein-RNA interactions. Two aromatic residues, Y23 and W115, provide critical driving forces through π–π stacking interactions with the aromatic rings of nucleobases. The arginine cluster fits in the dsRNA major groove. R16, R20, R21 and R26 in both chains lock the phosphate backbone. Surface of the dsRNA is shown in red.
Full-length double domain A3 structures:
The role of the inactive N-terminal domain of A3B, A3D, A3F and A3G in the full-length enzyme has been largely elusive. Since the N-terminal domain of human A3G (A3G-NTD) is profoundly insoluble, structure determination had been difficult. The Matsuo lab generated a soluble (and HIV-1 Vif binding) variant of A3G-NTD by substituting residues with corresponding ones in relatively soluble A3 domains, and solved solution NMR structure, mapped Vif-binding surfaces formed by loop-1, 3 and 7[62]. The Chen lab solved the crystal structure of a rhesus macaque A3G-NTD (rmA3G-NTD) variant with loop-8 replaced with four residues from human A3G-CTD to improve solubility[63]. The rmA3G-NTD variant formed a stable dimer in both solution and crystal, seems to be stabilized by RNA-binding at the dimerization interface[63].
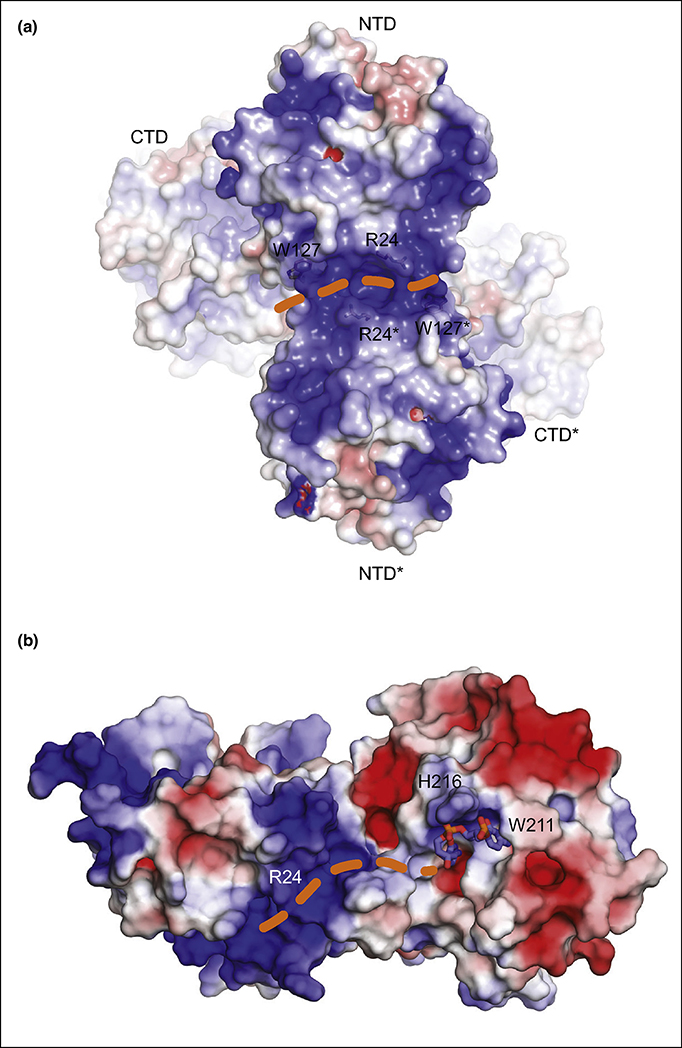
In 2020, the Chen lab determined structures of two soluble variants of full-length rhesus macaque A3G (rmA3G) for the first time, and these structures suggested mechanisms for both dimerization and possible RNA-binding surfaces of rmA3G[64] (Fig. 4a). Both NTD and CTD were folded in canonical A3 domain structures and connected through a short linker (R194-D198), although relative orientation between the NTD and the CTD were different by ~29° in these two rmA3G variant structures suggesting flexibility in relative orientation of two domains. These rmA3G variant formed different dimers in crystals through NTD-NTD (PDB ID: 6P3X) or NTD-CTD (PDB ID: 6P40) interactions[64]. The dimerization interface of 6P3X involves loop-1 (R24), loop-7 (Y124, Y125, F126, W127), helix-6 (N176, N177, K180, H181) from both subunits (Fig. 4a). Interestingly R24, K180 and H181 form a continuous positively charged patch at the dimerization interface (Fig. 4a). As previously suggested[65–67], W127 appeared on surface and accessible for direct interactions with RNA. The positively charged patch at the dimerization interface was important for RNA binging and stable dimerization of rmA3G. Amino acid sequence similarity and in vitro RNA-binding assays suggested the similar RNA binding/dimerization mechanism for human A3G although the importance of the positively charge patch and the dimerization for the restriction of HIV-1 infection was still elusive[64].
Figure 4: Crystal structures of a full-length rhesus macaque A3G variant and full-length human A3G variant.
a) Electrostatic surface distribution of the dimeric structure of rmA3G E/Q variant (PDB ID: 6P3X). Loop-1, loop-3 and helix-6 of the N-terminal domain (NTD) of both subunits form a positively charged dimerization interface where RNA likely binds. Orange dotted line shows a possible RNA-binding pathway. Sidechains of R24 and W127 of both subunits are presented as stick. b) Electrostatic surface distribution of the monomeric structural model of wild-type A3G generated based on the structure of sA3G* (PDB ID: 6WMA). 5’-dCdC binds near the active site interacting with residues including W211, R215, H216, T218 and D316. The dinucleotide is represented as sticks; atoms in nucleotides are colored blue, navy blue, red, and orange for C, N, O, and P, respectively. A positively charged channel is formed by NTD loop-1 and CTD loop-3, and R24 is located at the center of this channel. Orange dotted line indicates a possible ssDNA-binding pathway.
Most recently, the Matsuo/Schiffer labs determined a co-crystal structure of a soluble human A3G variant and deoxy-cytidine dinucleotide (PDB ID# 6WMA) (Fig. 4b)[68]. Two deoxy-cytidines, namely 5ʹ-C1C2 hereafter, were bound near the catalytic site, but distant from Zn2+, not positioned for deamination. The Watson-Crick face of C1 interacts with sA3G by three hydrogen bonds with V212 (loop-1) and D316 (loop-7), and the C1 ribose forms a hydrogen bond with W211. Additionally, the C1 has a π–π stacking interaction with W211. The Watson-Crick face of C2 also interacts with sA3G through three hydrogen bonds with R215, H216 and T218 through an ordered water molecule. Potentially the position and interactions of 5ʹ-C1C2 present a snapshot of the search of the deamination target sequence as A3G is moving along a ssDNA. The N-terminal domain of A3G has been suggested to provide additional affinity for ssDNA, and the sA3G:5ʹ-C1C2 complex structure indicated a positively charged channel between two domains which was directly connecting to the bound dinucleotide (Fig. 4b). R24 located in the middle of this channel appeared to be important for ssDNA binding as the substitution of R24 to alanine decreased both affinity for ssDNA and deamination efficiency in vitro. To reveal detail interactions between the NTD and DNA as well as the relative orientation of the NTD and the CTD upon the binding to a ssDNA, additional structures of full-length A3G bound to longer ssDNA must be determined.
Conclusions:
The A3 family of enzymes interactions with DNA and RNA are a complex combination of catalytic specificity and potentially nonspecific recognition whose role in specificity is still being characterized. Active site loops (loop 1, 3, 5 and 7), which have direct contacts with ssDNA, have shown the most conformational changes in complex compared to apo structures. The dynamics of these loops might be the key for defining the substrate specificities and functional variation among A3s. In addition, these structures revealed differences in the conformation of bound ssDNA (U-shape in A3A and chimeric A3B; linear in A3G). Hence, the differences in the secondary structure of substrate DNA may provide fundamental insights into the mechanisms by which A3s recognize their specific substrates. Interactions with nucleic acids remote from the active site, both within the catalytic domain as observed in A3H or within the non-catalytic domain NTD domain of A3G and likely the other double domain A3B, A3D and A3F, likely fine tune the specific interactions of the enzymes with their substrates within the cellular compartment within which they exist.
There are still many unanswered questions into the specificity and mechanisms of actions by which A3’s function and the role of the non-catalytic domain. Most likely single molecule experiments such as atomic force microscopy, fluorescence spectroscopy and optical tweezers combined with mutational analyses will provide invaluable insights, which are missing from ensemble studies or static structures of A3 enzymes. In addition, the elusive structures of the A3-HIV-Vif-E3 ligase complexes are beginning to be accessible through both single molecule Cryo electron microscopy and tomography. These technologies potentially even permitting the visualization of snapshots of A3s deaminating HIV-1 ssDNA in HIV-1 capsid or within a cellular compartment in near future. Through such characterization A3’s will likely be leveraged as therapeutic targets, either for inhibition as in cancer or in exquisitely specific base editors when coupled with Cas9.
Supplementary Material
Highlights.
Specificity of APOBEC3 proteins is determined by 4 active site loops
ssDNA adopts different conformations dependent on the APOBEC3 enzyme
Regions remote from the active site, including the N-terminal domain, contribute to substrate DNA and RNA binding sites.
Acknowledgments
Funding:
This work was supported by in part with grant from the U.S. National Institutes of Health R01GM118474/R01AI150478 for CAS and HM. For A.M. and H.M., this project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Research Ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases)
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Declaration of Interest:
The authors declare no conflict of interest.
No conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Betts L, Xiang S, Short SA, Wolfenden R, Carter CW Jr.: Cytidine deaminase. The 2.3 A crystal structure of an enzyme: transition-state analog complex. J Mol Biol 1994, 235:635–656. [DOI] [PubMed] [Google Scholar]
- 2.Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N: An anthropoid-specific locus of orphan C to U RNA-editing enzymes on chromosome 22. Genomics 2002, 79:285–296. [DOI] [PubMed] [Google Scholar]
- 3.Wedekind JE, Dance GS, Sowden MP, Smith HC: Messenger RNA editing in mammals: new members of the APOBEC family seeking roles in the family business. Trends Genet 2003, 19:207–216. [DOI] [PubMed] [Google Scholar]
- 4.Conticello SG, Thomas CJ, Petersen-Mahrt SK, Neuberger MS: Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol Biol Evol 2005, 22:367–377. [DOI] [PubMed] [Google Scholar]
- 5.LaRue RS, Andresdottir V, Blanchard Y, Conticello SG, Derse D, Emerman M, Greene WC, Jonsson SR, Landau NR, Lochelt M, et al. : Guidelines for naming nonprimate APOBEC3 genes and proteins. J Virol 2009, 83:494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green AM, Weitzman MD: The spectrum of APOBEC3 activity: From anti-viral agents to anti-cancer opportunities. DNA Repair (Amst) 2019, 83:102700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D: Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 2003, 424:99–103. [DOI] [PubMed] [Google Scholar]
- 8.Sheehy AM, Gaddis NC, Malim MH: The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat Med 2003, 9:1404–1407. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L: The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 2003, 424:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecossier D, Bouchonnet F, Clavel F, Hance AJ: Hypermutation of HIV-1 DNA in the absence of the Vif protein. Science 2003, 300:1112. [DOI] [PubMed] [Google Scholar]
- 11.Conticello SG, Harris RS, Neuberger MS: The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol 2003, 13:2009–2013. [DOI] [PubMed] [Google Scholar]
- 12.Marin M, Rose KM, Kozak SL, Kabat D: HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat Med 2003, 9:1398–1403. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF: Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 2003, 302:1056–1060. [DOI] [PubMed] [Google Scholar]
- 14.Wiegand HL, Doehle BP, Bogerd HP, Cullen BR: A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J 2004, 23:2451–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Sarkis PT, Luo K, Yu Y, Yu XF: Regulation of Apobec3F and human immunodeficiency virus type 1 Vif by Vif-Cul5-ElonB/C E3 ubiquitin ligase. J Virol 2005, 79:9579–9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirakawa K, Takaori-Kondo A, Kobayashi M, Tomonaga M, Izumi T, Fukunaga K, Sasada A, Abudu A, Miyauchi Y, Akari H, et al. : Ubiquitination of APOBEC3 proteins by the Vif-Cullin5-ElonginB-ElonginC complex. Virology 2006, 344:263–266. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Lilley CE, Yu Q, Lee DV, Chou J, Narvaiza I, Landau NR, Weitzman MD: APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr Biol 2006, 16:480–485. [DOI] [PubMed] [Google Scholar]
- 18.Janahi EM, McGarvey MJ: The inhibition of hepatitis B virus by APOBEC cytidine deaminases. J Viral Hepat 2013, 20:821–828. [DOI] [PubMed] [Google Scholar]
- 19.Vieira VC, Leonard B, White EA, Starrett GJ, Temiz NA, Lorenz LD, Lee D, Soares MA, Lambert PF, Howley PM, et al. : Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B. MBio 2014, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Wakae K, Kitamura K, Aoyama S, Liu G, Koura M, Monjurul AM, Kukimoto I, Muramatsu M: APOBEC3 deaminases induce hypermutation in human papillomavirus 16 DNA upon beta interferon stimulation. J Virol 2014, 88:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suspene R, Aynaud MM, Koch S, Pasdeloup D, Labetoulle M, Gaertner B, Vartanian JP, Meyerhans A, Wain-Hobson S: Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. J Virol 2011, 85:7594–7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buisson R, Langenbucher A, Bowen D, Kwan EE, Benes CH, Zou L, Lawrence MS: Passenger hotspot mutations in cancer driven by APOBEC3A and mesoscale genomic features. Science 2019, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, Kiezun A, Kryukov GV, Carter SL, Saksena G, et al. : An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet 2013, 45:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannataro VL, Gaffney SG, Sasaki T, Issaeva N, Grewal NKS, Grandis JR, Yarbrough WG, Burtness B, Anderson KS, Townsend JP: APOBEC-induced mutations and their cancer effect size in head and neck squamous cell carcinoma. Oncogene 2019, 38:3475–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petljak M, Alexandrov LB, Brammeld JS, Price S, Wedge DC, Grossmann S, Dawson KJ, Ju YS, Iorio F, Tubio JMC, et al. : Characterizing Mutational Signatures in Human Cancer Cell Lines Reveals Episodic APOBEC Mutagenesis. Cell 2019, 176:1282–1294 e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanton C, McGranahan N, Starrett GJ, Harris RS: APOBEC Enzymes: Mutagenic Fuel for Cancer Evolution and Heterogeneity. Cancer Discov 2015, 5:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burns MB, Lackey L, Carpenter MA, Rathore A, Land AM, Leonard B, Refsland EW, Kotandeniya D, Tretyakova N, Nikas JB, et al. : APOBEC3B is an enzymatic source of mutation in breast cancer. Nature 2013, 494:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns MB, Temiz NA, Harris RS: Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet 2013, 45:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buisson R, Lawrence MS, Benes CH, Zou L: APOBEC3A and APOBEC3B Activities Render Cancer Cells Susceptible to ATR Inhibition. Cancer Res 2017, 77:4567–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaban NM, Shi K, Lauer KV, Carpenter MA, Richards CM, Salamango D, Wang J, Lopresti MW, Banerjee S, Levin-Klein R, et al. : The Antiviral and Cancer Genomic DNA Deaminase APOBEC3H Is Regulated by an RNA-Mediated Dimerization Mechanism. Mol Cell 2018, 69:75–86 e79.* Crystal structure of human APOBEC3H haplotype II. It formed a dimeric structure that was similar to the pig-tailed macaque A3H and chimpanzee A3H structures. Biochemical studies suggested that double-stranded RNA (ssRNA) binding was required for packaging into viral particles and antiviral activity, whereas RNA-binding minimized DNA deamination activity.
- 31.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR: Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY, et al. : Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353. [DOI] [PubMed] [Google Scholar]
- 33.Chen KM, Harjes E, Gross PJ, Fahmy A, Lu Y, Shindo K, Harris RS, Matsuo H: Structure of the DNA deaminase domain of the HIV-1 restriction factor APOBEC3G. Nature 2008, 452:116–119. [DOI] [PubMed] [Google Scholar]
- 34.Holden LG, Prochnow C, Chang YP, Bransteitter R, Chelico L, Sen U, Stevens RC, Goodman MF, Chen XS: Crystal structure of the anti-viral APOBEC3G catalytic domain and functional implications. Nature 2008, 456:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haché G, Liddament MT, Harris RS: The retroviral hypermutation specificity of APOBEC3F and APOBEC3G is governed by the C-terminal DNA cytosine deaminase domain. J Biol Chem 2005, 280:10920–10924. [DOI] [PubMed] [Google Scholar]
- 36.Silvas TV, Schiffer CA: APOBEC3s: DNA-editing human cytidine deaminases. Protein Sci 2019, 28:1552–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byeon IJ, Ahn J, Mitra M, Byeon CH, Hercik K, Hritz J, Charlton LM, Levin JG, Gronenborn AM: NMR structure of human restriction factor APOBEC3A reveals substrate binding and enzyme specificity. Nat Commun 2013, 4:1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang Y, Xiao X, Li SX, Wolfe A, Chen XS: Molecular Interactions of a DNA Modifying Enzyme APOBEC3F Catalytic Domain with a Single-Stranded DNA. J Mol Biol 2018, 430:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen KM, Martemyanova N, Lu Y, Shindo K, Matsuo H, Harris RS: Extensive mutagenesis experiments corroborate a structural model for the DNA deaminase domain of APOBEC3G. FEBS Lett 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi K, Carpenter MA, Kurahashi K, Harris RS, Aihara H: Crystal Structure of the DNA Deaminase APOBEC3B Catalytic Domain. J Biol Chem 2015, 290:28120–28130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byeon IJ, Byeon CH, Wu T, Mitra M, Singer D, Levin JG, Gronenborn AM: Nuclear Magnetic Resonance Structure of the APOBEC3B Catalytic Domain: Structural Basis for Substrate Binding and DNA Deaminase Activity. Biochemistry 2016, 55:2944–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi K, Demir O, Carpenter MA, Wagner J, Kurahashi K, Harris RS, Amaro RE, Aihara H: Conformational Switch Regulates the DNA Cytosine Deaminase Activity of Human APOBEC3B. Sci Rep 2017, 7:17415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beale RC, Petersen-Mahrt SK, Watt IN, Harris RS, Rada C, Neuberger MS: Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: correlation with mutation spectra in vivo. J Mol Biol 2004, 337:585–596. [DOI] [PubMed] [Google Scholar]
- 44.Langlois MA, Beale RC, Conticello SG, Neuberger MS: Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities. Nucleic Acids Res 2005, 33:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang Y, Siew LM, Wang X, Han Y, Lampen R, Zheng YH: Human cytidine deaminase APOBEC3H restricts HIV-1 replication. J Biol Chem 2008, 283:11606–11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH: Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol 2004, 14:1392–1396. [DOI] [PubMed] [Google Scholar]
- 47.Furukawa A, Nagata T, Matsugami A, Habu Y, Sugiyama R, Hayashi F, Kobayashi N, Yokoyama S, Takaku H, Katahira M: Structure, interaction and real-time monitoring of the enzymatic reaction of wild-type APOBEC3G. EMBO J 2009, 28:440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi K, Carpenter MA, Banerjee S, Shaban NM, Kurahashi K, Salamango DJ, McCann JL, Starrett GJ, Duffy JV, Demir O, et al. : Structural basis for targeted DNA cytosine deamination and mutagenesis by APOBEC3A and APOBEC3B. Nat Struct Mol Biol 2017, 24:131–139.* Co-crystal structure of an inactive (E72A mutation) human A3A and 15-nt ssDNA. The 3.1 Å resolution A3A-ssDNA structure showed clear electron density for either 5 (5’-A−2T−1C0G+1G+2) or 6 (5’-A−2T−1C0G+1G+2G+3) nucleotides centered on the target cytosine (C0). A3A-bound ssDNA adopts a U-shaped conformation by encircling the side chain of H29. The co-crystal structure of a non-catalytic mutant (E255A) of A3B-CTD in which loop-1 was swapped with A3A loop-1 (A3Bctd-QMΔloop3-A3Aloop1) and 7-nt ssDNA (5′-TTTTCAT) was also determined. The overall DNA conformation and active site interactions looked identical to that observed in the ssDNA-bound A3A structure.
- 49.Kouno T, Silvas TV, Hilbert BJ, Shandilya SMD, Bohn MF, Kelch BA, Royer WE, Somasundaran M, Kurt Yilmaz N, Matsuo H, et al. : Crystal structure of APOBEC3A bound to single-stranded DNA reveals structural basis for cytidine deamination and specificity. Nat Commun 2017, 8:15024.* Co-crystal structure of human A3A (E72A/C171A) variant and-15 nt ssDNA (5’-TTTTTTTCTTTTTTT). There was a single A3A–ssDNA complex in the asymmetric unit of the 2.2 Å resolution structure where the target sequence 5’-T−1C0T+1 was well ordered in the electron density. The ssDNA adopted a U-shaped conformation.
- 50.Hou S, Silvas T, Leidner F, Nalivaika EA, Matsuo H, Kurt Yilmaz N, Schiffer CA: Structural analysis of the active site and DNA binding of human cytidine deaminase APOBEC3B. J Chem Theory Comput 2018, 10.1021/acs.jctc.8b00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maiti A, Myint W, Kanai T, Delviks-Frankenberry K, Sierra Rodriguez C, Pathak VK, Schiffer CA, Matsuo H: Crystal structure of the catalytic domain of HIV-1 restriction factor APOBEC3G in complex with ssDNA. Nat Commun 2018, 9:2460.* Co-crystal structure of a soluble variant of A3G-CTD and 9-nt ssDNA (5’-AATCCCAAA). This co-crystal structure revealed that five nucleotides 5’-T−3C−2C−1C0A+1 containing the deamination target sequence had interaction with protein. A+1 had π-π stacking interaction with H216 which can be enforced by deprotonation of the histidine imidazole ring at lower pH enabling higher enzymatic activity. C−2C−1 were recognized through hydrogen bonds with D316 and D317. W211 is unique to A3G-CTD, and it allows additional interaction with T−3 through π-π stacking interaction.
- 52.Harjes S, Solomon WC, Li M, Chen KM, Harjes E, Harris RS, Matsuo H: Impact of H216 on the DNA binding and catalytic activities of the HIV restriction factor APOBEC3G. J Virol 2013, 87:7008–7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito F, Fu Y, Kao SA, Yang H, Chen XS: Family-Wide Comparative Analysis of Cytidine and Methylcytidine Deamination by Eleven Human APOBEC Proteins. J Mol Biol 2017, 429:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziegler SJ, Liu C, Landau M, Buzovetsky O, Desimmie BA, Zhao Q, Sasaki T, Burdick RC, Pathak VK, Anderson KS, et al. : Insights into DNA substrate selection by APOBEC3G from structural, biochemical, and functional studies. PLoS One 2018, 13:e0195048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan X, Lan W, Wang C, Cao C: Structural Investigations on the Interactions between Cytidine Deaminase Human APOBEC3G and DNA. Chem Asian J 2019, 14:2235–2241. [DOI] [PubMed] [Google Scholar]
- 56.Morse M, Huo R, Feng Y, Rouzina I, Chelico L, Williams MC: Dimerization regulates both deaminase-dependent and deaminase-independent HIV-1 restriction by APOBEC3G. Nat Commun 2017, 8:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shlyakhtenko LS, Lushnikov AY, Miyagi A, Li M, Harris RS, Lyubchenko YL: Nanoscale structure and dynamics of ABOBEC3G complexes with single-stranded DNA. Biochemistry 2012, 51:6432–6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chelico L, Pham P, Calabrese P, Goodman MF: APOBEC3G DNA deaminase acts processively 3’ --> 5’ on single-stranded DNA. Nat Struct Mol Biol 2006, 13:392–399. [DOI] [PubMed] [Google Scholar]
- 59.Bohn JA, Thummar K, York A, Raymond A, Brown WC, Bieniasz PD, Hatziioannou T, Smith JL: APOBEC3H structure reveals an unusual mechanism of interaction with duplex RNA. Nat Commun 2017, 8:1021.* This is the pig-tailed macaque APOBEC3H crystal structure. This structure showed, for the first time, a unique feature of A3H as it formed dimer through binding a double-stranded RNA without protein-protein interaction.
- 60.Matsuoka T, Nagae T, Ode H, Awazu H, Kurosawa T, Hamano A, Matsuoka K, Hachiya A, Imahashi M, Yokomaku Y, et al. : Structural basis of chimpanzee APOBEC3H dimerization stabilized by double-stranded RNA. Nucleic Acids Res 2018, 46:10368–10379.* Crystal structure of chimpanzee A3H (cpzA3H). The crystal structure of cpzA3H showed well ordered 9-nt dsRNA, and critical interactions between RNA and protein were determined. Y23 and W115 form π–π stacking interactions with nucleobases, while seven R/K residues in loop-1 fit in the major groove of the dsRNA forming non-specific interactions with the phosphate backbone.
- 61.Ito F, Yang H, Xiao X, Li SX, Wolfe A, Zirkle B, Arutiunian V, Chen XS: Understanding the Structure, Multimerization, Subcellular Localization and mC Selectivity of a Genomic Mutator and Anti-HIV Factor APOBEC3H. Sci Rep 2018, 8:3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kouno T, Luengas EM, Shigematsu M, Shandilya SM, Zhang J, Chen L, Hara M, Schiffer CA, Harris RS, Matsuo H: Structure of the Vif-binding domain of the antiviral enzyme APOBEC3G. Nat Struct Mol Biol 2015, 22:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao X, Li SX, Yang H, Chen XS: Crystal structures of APOBEC3G N-domain alone and its complex with DNA. Nat Commun 2016, 7:12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang H, Ito F, Wolfe AD, Li S, Mohammadzadeh N, Love RP, Yan M, Zirkle B, Gaba A, Chelico L, et al. : Understanding the structural basis of HIV-1 restriction by the full length double-domain APOBEC3G. Nat Commun 2020, 11:632.* Crystal structures of two soluble variants of full-length rhesus macaque APOBEC3G (rmA3G). These rmA3G variants formed different dimers in crystals through NTD-NTD (PDB ID: 6P3X) or NTD-CTD (PDB ID: 6P40) interactions, and suggested a possible RNA-binding surface at the dimerization interface (PDB ID: 6P3X). Relative orientation between the NTD and the CTD were different by ~29° in these two rmA3G dimer structures suggesting flexibility in relative orientation of two domains.
- 65.Huthoff H, Malim MH: Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and Virion encapsidation. J Virol 2007, 81:3807–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chelico L, Prochnow C, Erie DA, Chen XS, Goodman MF: Structural model for deoxycytidine deamination mechanisms of the HIV-1 inactivation enzyme APOBEC3G. J Biol Chem 2010, 285:16195–16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friew YN, Boyko V, Hu WS, Pathak VK: Intracellular interactions between APOBEC3G, RNA, and HIV-1 Gag: APOBEC3G multimerization is dependent on its association with RNA. Retrovirology 2009, 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maiti A, Myint W, Delviks-Frankenberry KA, Hou S, Kanai T, Balachandran V, Sierra Rodriguez C, Tripathi R, Kurt Yilmaz N, Pathak VK, et al. : Crystal structure of a soluble APOBEC3G variant suggests ssDNA to bind in a channel that extends between the two domains. J Mol Biol 2020, 10.1016/j.jmb.2020.10.020.* Co-crystal structure of a soluble human APOBEC3G variant (sA3G) and di-deoxy-cytidine (PDB ID:6WMA). The NTD was rotated 90° relative to the CTD along the major axis of the molecule, an orientation that forms a positively charged channel connected to the CTD catalytic site. sA3G was shown to bind a deoxy-cytidine dinucleotide near the catalytic Zn2+, yet not in the catalytic position. A structure-based mechanism was suggested to explain why A3G exhibits a 3’ to 5’ directional preference in processive deamination.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.