Abstract
Background:
Atrial fibrillation (AF) and heart failure (HF) often accompany one another and each is independently associated with poor outcomes. However, the association between AF burden and outcomes is poorly understood.
Objectives:
We aimed to describe the association between device-based AF burden and HF clinical outcomes.
Methods:
We used a nationwide, remote monitoring database of cardiac implantable electronic devices (CIEDs), linked to Medicare claims. We included patients with non-permanent AF, undergoing new CIED implant, stratified by baseline HF. The outcomes were new-onset HF, HF hospitalization, and all-cause mortality at 1 and 3 years.
Results:
We identified 39,710 patients who met inclusion criteria (n=25,054 with HF; 14,656 without HF). Patients with HF were younger (mean age 76.3 vs. 78.5, p<.001), more often male (65% vs. 54%, p<.001), and had higher mean CHA2DS2-VASc scores (5.4 vs. 4.1, p<.001). Among those without HF, increasing device-based AF burden was significantly associated with increased risk of new-onset HF (adjusted HR 1.09 per 10% AF burden, 95% CI 1.06–1.12, p<.001) and all-cause mortality (adjusted HR 1.05 per 10% AF burden, 95% CI 1.01–1.10, 0.012). Among patients with HF, increasing AF burden was significantly associated with increased risk of HF hospitalization (adjusted HR 1.05 per 10% AF burden, 95% CI 1.04–1.06, p<.001) and all-cause mortality (adjusted HR 1.06 per 10% AF burden, 95% CI 1.05–1.08, p<.001).
Conclusions:
Among older patients with AF receiving CIEDs, increasing AF burden is significantly associated with increasing risk of adverse HF outcomes and all-cause mortality.
Keywords: atrial fibrillation, heart failure, arrhythmia burden, outcomes, Medicare, remote monitoring
Graphical Abstract
Introduction
Atrial fibrillation (AF) is closely-linked to an increased risk of stroke and death.1 However, these relationships have historically been based on an AF diagnosis made in clinic, with 12-lead electrocardiography – typically requiring a relatively high burden of the arrhythmia (i.e., persistent) for diagnosis. However, AF is often a progressive disease with great variability in AF burden.2, 3 Yet measuring the relationship between AF burden and clinical outcomes has been limited in large populations due to reliance on clinical encounter diagnostic tools, variability in AF measurement approaches, and insufficient statistical power to detect differences.4
Patients with AF are at increased risk of heart failure (HF), and the conditions together are associated with particularly poor outcomes.5 However, the evidence for worse outcomes is primarily derived from observational and administrative cohorts, where AF is treated as a binary diagnosis irrespective of the chronicity, severity, or burden – characteristics that can impact outcomes.6 Therefore, we aimed to measure the relationship between AF burden and HF outcomes in a large, broad cohort of patients without persistent or permanent AF undergoing de novo cardiac implantable electronic device (CIED) implantation. We focused on patients with non-permanent AF in order to isolate the association of outcomes with different amounts of non-continuous AF and avoid the additional biases associated with typically sicker patients with longstanding-persistent and permanent AF.7 The objectives of this study were to measure the association between AF burden and (1) new-onset HF and all-cause mortality among patients without baseline HF, and (2) HF hospitalization and all-cause mortality among patients with HF at baseline.
Methods
Data Sources
We linked CIED data from the Merlin.net™ database (Abbott, Chicago, IL, USA) for de novo implantations between 04/01/2010 and 12/31/2016 and follow-up through 12/31/2017 to Medicare claims from 2009–2017 accessed through the Centers for Medicare and Medicaid Services (CMS) Virtual Research Data Center (VRDC). Medicare claims included inpatient, outpatient, and carrier claims, Part D prescription drug records, and the corresponding Master Beneficiary Summary Files (MBSF). The inpatient files contain claims for inpatient services. The outpatient files contain claims for outpatient services. The carrier files contain non-institutional provider claims for services rendered in any setting. Each of these files includes dates of service, diagnosis and procedure codes. The MBSF contains demographics, birth and death dates, and program eligibility and enrollment. The Merlin.net™ database contains patient-level implantation dates, and records containing daily or weekly averaged atrial arrhythmia measures derived from remote device monitoring.
Analytic Timeline
The analytic timeline is shown in the Supplemental Material, Figure S1. Implantation was defined as Day 1. Day 1–30 was defined as a blanking period to exclude perioperative influences on AF burden; this was followed by a period to assess baseline AF burden, as exposure, from days 31–60. Day 61 is defined as the index date. The follow up time for outcomes and AF burden reassessment was 1 and 3 years after the index date. Comorbid conditions and prior history were assessed during the 12 months prior to the index date.
Study Population
We included patients who were implanted with a dual-chamber implantable cardioverter defibrillator (ICD) or pacemaker (PM), or cardiac resynchronization therapy (CRT) device, were 65 years or older at the time of CIED implant, utilized Merlin.net™ remote monitoring from 4/1/2010–12/31/2016, and who were successfully linked to Medicare claims. Briefly, we used inpatient and outpatient Medicare claims data between 2010–2016 to identify patients who received a CIED using ICD procedure codes. We then linked those Medicare implant records to the Merlin.net™ database using date of birth, sex and implantation date, and selected matches based on best agreement between data sources (see Supplemental Material, Table S1).
From Medicare records, we only included patients who had a clinical diagnosis of AF based on ICD-9-CM 427.31 or ICD-10-CM I48.0*–I48.2*, I48.91 found in any position on an inpatient, outpatient or carrier claim in the 12 months prior to index date, had continuous fee-for-service Medicare enrollment during the 12 months prior to index date, and had at least 1 qualifying 30-day period of AF burden records for cohort inclusion. Patients were excluded if they had persistent AF measured from day 31–60 post-implant defined as daily duration in AF ≥98% (see below).
Study Variables
AF burden was defined as the total daily atrial tachycardia/atrial fibrillation duration (seconds per week). Where only weekly burden was available, this was divided by 7 for average daily AF burden. For the primary analysis, we assessed baseline exposure AF burden on days 31–60 post-implant. Only patients without persistent AF during this exposure period were included in the study cohort. We defined AF burden in two ways: 1) daily percentage in AF and 2) maximum duration of any single AF episode.8 For each, we measured as continuous and categorical variables. Continuous AF burden in this dataset was observed to have a skewed distribution towards extreme low and high values (Supplemental Table, S2). However, additional transformation would limit analytic approaches and clinical interpretation. Therefore, we identified cut-offs for AF burden based on this distribution and clinical appropriateness. For the baseline assessment, we defined percent in AF categories as follows: 0, (0, 5], and (5 to 98) and maximum duration categories as follows: 0 to 59 seconds, 1 to 59 minutes, 1 to 23.5 hours, and ≥24 hours. As a sensitivity analysis, the percent in AF and maximum duration of an AF episode were also measured as time-dependent variables every 30 days. The 4 categories for time-dependent percent in AF were defined as follows: 0, (0, 5], (5, 98), and [98, 100].
Demographics were derived from the MBSF, and CIED type from the Merlin.net™ database. We searched carrier claims in the year prior to the index date for comorbid conditions and to derive the CHA2DS2-VASc scores based on validated coding algorithms (see Supplemental Material, Table S3).9, 10 Comorbidities included dementia, diabetes mellitus, ischemic heart disease, peripheral vascular disease, congestive HF, cerebrovascular disease, hypertension, chronic obstructive pulmonary disease, renal disease, stroke/TIA, myocardial infarction, cancer (metastatic or non-metastatic), and valvular heart disease. CHA2DS2-VASc score was calculated as follows: one point each for age 65 – 74, female, history of HF, hypertension, vascular disease [peripheral vascular disease or ischemic heart disease], and diabetes and 2 points each for age ≥75 and history of stroke/TIA.
Outcomes
The primary outcomes of interest for our analysis were new-onset HF and all-cause mortality among patients with no prior diagnosis of HF at baseline, and HF hospitalization and all-cause mortality among patients with a prior diagnosis of HF at baseline (prevalent HF). New-onset HF was defined as the occurrence of a HF diagnosis code in any position (ICD-9-CM 428.*, 402.x1, 404.x1 or 404.x3 or ICD-10-CM I50.*, I11.0, I13.0, I13.2) on a single inpatient claim or at least three outpatient claims within 20 months in patients with no prior HF recorded at baseline or the prior 12 months. The date of the incident diagnosis was defined as the earlier of (1) the discharge date of the earliest inpatient diagnosis or (2) the through date of the third outpatient or carrier diagnosis.11 HF hospitalization was defined as primary diagnosis of ICD-9-CM 402.x1, 404.x1, 404.x3 or 428.* or ICD-10-CM I50.*, I11.0, I13.0, or I13.2 on an inpatient Medicare claim. All-cause mortality was determined based on the death date recorded in the Medicare MBSF files.
All outcomes for primary analysis were assessed for 1 year following the index date. We censored data for patients at (a) 1 year after the index date, (b) the end of claims data availability (12/31/2017), (c) the date when enrollment in fee-for-service Medicare ended and (d) the death date for non-death outcomes. For time-varying sensitivity analyses, we conducted both 1- and 3-year analyses.
Statistical analysis
For baseline characteristics, categorical variables are presented as frequencies with percentages and continuous variables as means with standard deviations. We described baseline characteristics of the overall study cohort, and stratified by those with prior heart failure and those without prior heart failure. The differences in baseline characteristics by HF subgroups were tested for using χ2 tests for categorical variables and Wilcoxon rank sum tests for continuous variables.
Kaplan-Meier methods were used to estimate mortality. For all other outcomes, we calculated incidence based on estimates from the cumulative incidence function, which accounts for the competing risk of mortality, which is high in this population. The new onset HF outcome was analyzed in the subset of beneficiaries with no prior HF at baseline. We calculated cumulative incidence overall and stratified by sex, and we used log rank test for mortality and Gray’s test for other outcomes to assess differences between groups.
Cox proportional hazards models were used to examine univariate and multivariable associations of AF burden measured as percentage of time in AF and the maximum duration of AF (continuous and categorical variables) during the baseline period with mortality and cardiovascular outcomes. For multivariable modeling, we fully adjusted for demographics and comorbid conditions listed in the baseline characteristics table. We tested for differences in the effect of AF burden by sex by including interactions between the sex variable and AF burden variables for each outcome in separate outcome models. For all outcomes, we tested the linearity of the AF percentage and maximum duration continuous variables’ functional form using the supremum test. For all analyses, we used a 2-tailed α = .05 to establish statistical significance and reported 95% confidence intervals. All analyses utilized SAS enterprise guide 7.1 (SAS Institute Inc.). This study was approved by the Duke University Institutional Review Board. The research reported in this paper adhered to human research guidelines according to Helsinki Declaration as revised in 2013.
Sensitivity analysis
We conducted several sensitivity analyses. First, we tested for differences in the effect of AF burden by device type by including interactions between the low vs high-voltage device variable and AF burden variables in all outcome models. Second, we used time-dependent Cox models with percentage in AF and maximum AF duration as time-varying variables calculated for each 30-day period out to follow-up of 3 years. Third, we described baseline characteristics and reran the baseline AF burden Cox models in the broader eligible study population not requiring clinical claims-based AF at baseline. Finally, we incorporated restricted cubic splines into models and generated hazard ratio plots by continuous AF variables if non-linearity is indicated.
Funding
The analysis was designed and performed by the authors independent from the sponsor. The authors are solely responsible for the study design, analysis, interpretation of data, writing of the report, and in the decision to submit the paper for publication. The sponsor provided technical input on use of Merlin.net™ database. The corresponding and senior authors had full access to all the data in the study and final responsibility for the decision to submit for publication.
Results
Overall, 39,710 patients with a clinical diagnosis of non-persistent AF and a de novo CIED fulfilled the inclusion criteria and were successfully linked across data sets. Mean age was 77.1 (SD 8.7) years, 61% (n=24,119) were male, and 63% (n=25,054) had HF at baseline (Table 1). Patients with HF at baseline were younger (mean age 76.3 vs. 78.5, p<.001), more often male (65% vs. 54%, p<.001), and had higher CHA2DS2-VASc scores (5.4 vs. 4.1, p<.001). Among the overall cohort of 39,710 patients, the mean (standard deviation) for number of daily AF burden measurements per patient was 872.08 (511.04). Additional data on baseline AF burden, by percentage and maximal duration, are show in Supplemental Material Tables, S2 and S4. Baseline characteristics, stratified by AF burden, are shown in Supplemental Material Table S5.
Table 1.
Baseline Characteristics of the study population, stratified by baseline HF status.
| Overall | HF | No HF | P-value | |
|---|---|---|---|---|
| N=39,710 | N=25,054 | N=14,656 | ||
| Age, years [mean(SD)] | 77.1(8.7) | 76.3(8.9) | 78.5(8.0) | <.001 |
| Male | 24,119(60.7) | 16,245(64.8) | 7,874(53.7) | <.001 |
| Race/ethnicity | <.001 | |||
| White | 36,710(92.4) | 22,848(91.2) | 13,862(94.6) | |
| Black | 1,997(5.0) | 1,568(6.3) | 429(2.9) | |
| Other | 319(0.8) | 198(0.8) | 121(0.8) | |
| Device Voltage | <.001 | |||
| Low Voltage(pacemaker) | 20,156(50.8) | 8,248(32.9) | 11,908(81.3) | |
| High Voltage(defibrillator) | 19,554(49.2) | 16,806(67.1) | 2,748(18.8) | |
| Device Type | <.001 | |||
| Dual Chamber Pacemaker | 17,797(44.8) | 6,417(25.6) | 11,380(77.6) | |
| CRT-P | 2,359(5.9) | 1,831(7.3) | 528(3.6) | |
| Dual Chamber ICD | 7,750(19.5) | 5,715(22.8) | 2,035(13.9) | |
| CRT-D | 11,804(29.7) | 11,091(44.3) | 713(4.9) | |
| Comorbid conditions | ||||
| Dementia | 1,328(3.3) | 822(3.3) | 506(3.5) | .36 |
| Diabetes Mellitus | 15,366(38.7) | 11,258(44.9) | 4,108(28.0) | <.001 |
| Ischemic heart disease | 28,654(72.2) | 20,606(82.2) | 8,048(54.9) | <.001 |
| Peripheral vascular disease | 9,132(23.0) | 6,727(26.9) | 2,405(16.4) | <.001 |
| Congestive Heart failure | 25,054(63.1) | 25,054(100) | 0 | - |
| Cerebrovascular disease | 7,028(17.7) | 4,643(18.5) | 2,385(16.3) | <.001 |
| Hypertension | 35,940(90.5) | 23,133(92.3) | 12,807(87.4) | <.001 |
| Chronic obstructive pulmonary disease | 16,094(40.5) | 12,377(49.4) | 3,717(25.4) | <.001 |
| Renal disease | 13,169(33.2) | 10,464(41.8) | 2,705(18.5) | <.001 |
| Stroke/TIA | 3,820(9.6) | 2,539(10.1) | 1,281(8.7) | <.001 |
| Myocardial infarction | 12,405(31.2) | 10,008(39.9) | 2,397(16.4) | <.001 |
| Cancer | 4,167(10.5) | 2,730(10.9) | 1,437(9.8) | <.001 |
| Valvular heart disease | 16,437(41.4) | 12,123(48.4) | 4,314(29.4) | <.001 |
| CHA2DS2-VASc score, Mean(SD) | 4.9(1.3) | 5.4(1.2) | 4.1(1.2) | <.001 |
| Score<=2 | 1,356(3.4) | 101(0.4) | 1,255(8.6) | <.001 |
| Score=3 | 4,122(10.4) | 961(3.8) | 3,161(21.6) | <.001 |
| Score>=4 | 34,232(86.2) | 23,992(95.8) | 10,240(69.9) | <.001 |
| AF Burden at Baseline (days 31–60) | ||||
| Percent in AF, mean (SD) | 6.43(18.69) | 6.60(19.32) | 6.13(17.56) | 0.013 |
| Maximum duration, mean minutes (SD) | 402.53 (2569.83) | 467.72(3027.64) | 291.08(1484.58) | <.001 |
Baseline characteristics, co-morbidities, stratified by HF at baseline. Values are presented as n(%) unless otherwise specified.
HF: heart failure; SD: standard deviation; ICD: implantable cardioverter-defibrillator; CRT: cardiac resynchronization therapy; TIA: transient ischemic attack.
New Onset Heart Failure and Mortality
Among patients without HF at baseline (n=14,656) 10.1% of patients developed new-onset HF and 4.3% died at 1 year. As AF burden increased, there was a significantly-increased 1-year risk of both new-onset HF (adjusted HR 1.089 per 10 percentage points AF burden, 95% CI 1.063–1.115, p<.001) and all-cause mortality (adjusted HR 1.053 per 10% AF burden, 95% CI 1.011–1.096, 0.012; Table 2). When measured by maximum AF duration, and when stratified categorically, by daily percent in AF (i.e. 0, 0–5, and 5–98), the relationships remained significant (Figure 1, Figure 2A, Supplemental Material Table S6).
Table 2.
Association between AF burden and outcomes among patients with and without heart failure at baseline.
| Unadjusted Model | Adjusted Model | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Patients with no heart failure at baseline | ||||
| New Onset HF at 1 Year | ||||
| Percent in AF, per 10 | 1.076(1.050, 1.101) | <.001 | 1.089 (1.063, 1.115) | <.001 |
| Maximum Duration, per 1 hr | 1.002(1.000, 1.003) | 0.012 | 1.002(1.000, 1.002) | 0.005 |
| Mortality at 1 Year | ||||
| Percent in AF, per 10 | 1.035 (0.994, 1.077) | 0.100 | 1.053(1.011, 1.096) | 0.012 |
| Maximum Duration, per 1 hr | 0.998(0.993, 1.003) | 0.461 | 0.999(0.995, 1.003) | 0.326 |
| Patients with heart failure at baseline | ||||
| HF Hospitalization at 1 Year | ||||
| Percent in AF, per 10 | 1.044(1.034, 1.054) | <.001 | 1.048(1.038, 1.059) | <.001 |
| Maximum Duration, per 1 hr | 1.001(1.000, 1.001) | <.001 | 1.001(1.000, 1.001) | 0.004 |
| Mortality at 1 Year | ||||
| Percent in AF, per 10 | 1.064(1.047, 1.081) | <.001 | 1.063(1.046, 1.080) | <.001 |
| Maximum Duration, per 1 hr | 1.001(1.000, 1.001) | 0.024 | 1.001(1.000, 1.001) | 0.005 |
AF: atrial fibrillation; HF: heart failure; CI: confidence interval.
Figure 1.
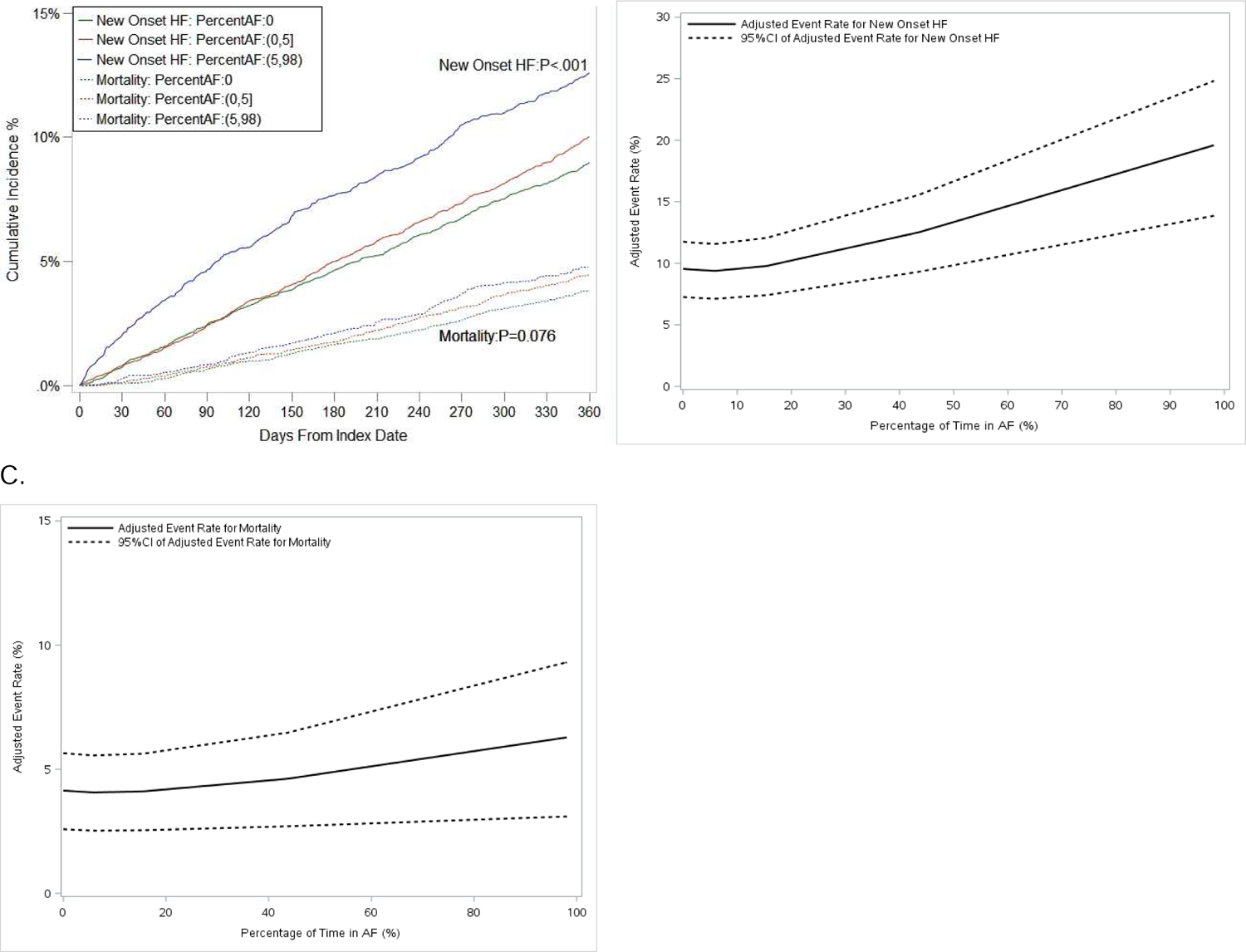
Cumulative incidence curves of new-onset heart failure (HF) and mortality, stratified by atrial fibrillation (AF) burden (categorical, percent in AF) (A), and adjusted event rates of new-onset HF (B) and mortality (C) among patients without HF at baseline. Parentheses indicate exclusive borders; brackets indicate inclusive borders. CI 5 confidence interval.
Figure 2.
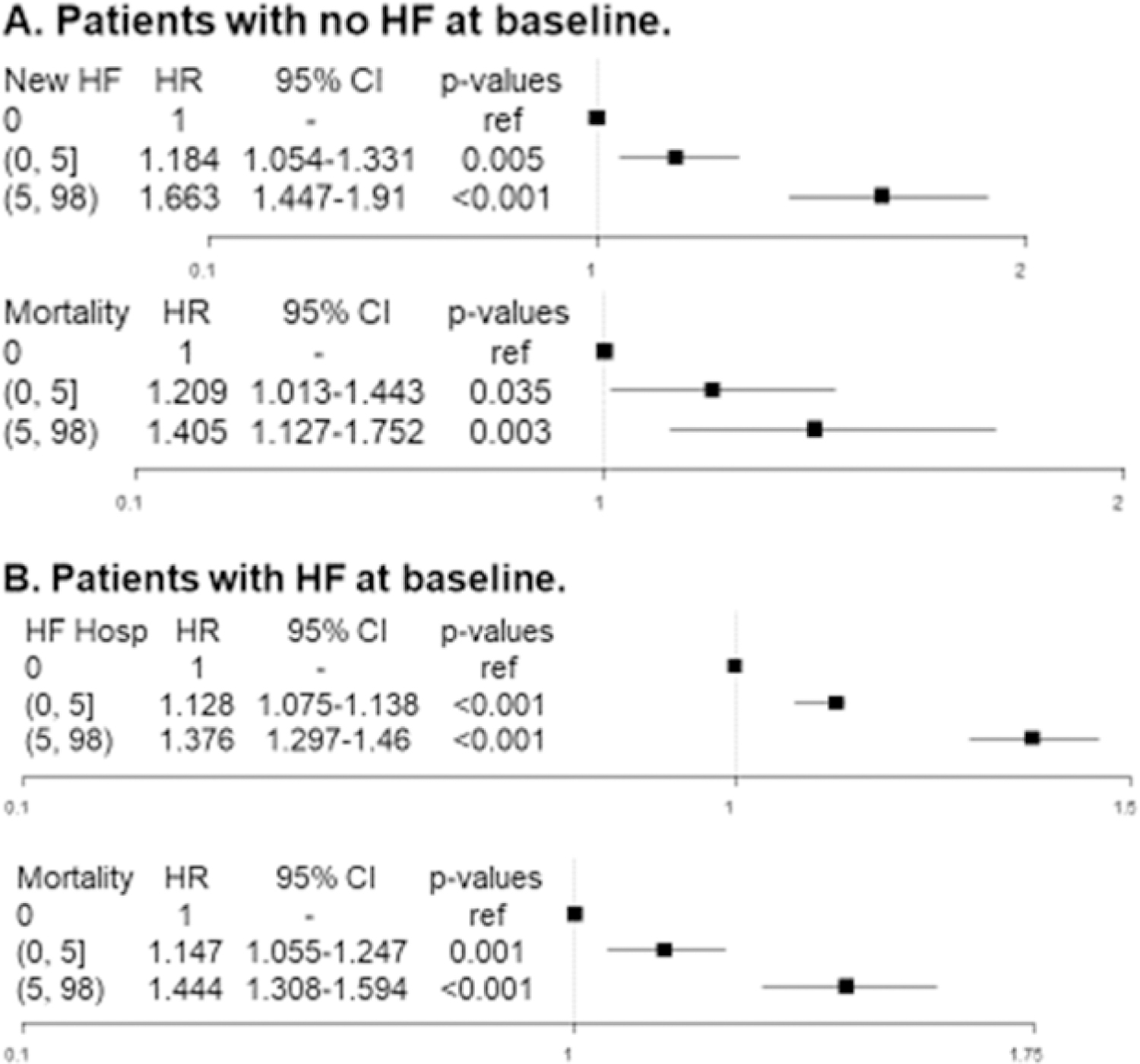
(Forest plots of new heart failure (HF) and all-cause mortality among patients with no HF at baseline (A) and HF hospitalization and mortality among Q7 patients with pre-existing HF (B) by percentage of atrial fibrillation burden. Parentheses indicate exclusive borders; brackets indicate inclusive borders. CI 5 confidence interval; HR 5 hazard ratio..
Heart Failure Hospitalization and Mortality
Among patients with HF at baseline (n=25,054), HF hospitalization occurred in 35.1% and 11.8% died at 1 year. In unadjusted and adjusted analyses, AF burden was significantly associated with both HF hospitalization (adjusted HR 1.048 per 10 percentage points AF burden, 95% CI 1.038–1.059, p<.001) and all-cause mortality (adjusted HR 1.063 per 10 percentage points AF burden, 95% CI 1.046–1.080, p<.001, Table 2). When measured by maximum episode duration, and stratified categorically by percent in AF (0, 0–5, 5–98), the relationships remained significant (Figure 2B, Figure 3, Supplemental Material Table S7).
Figure 3.
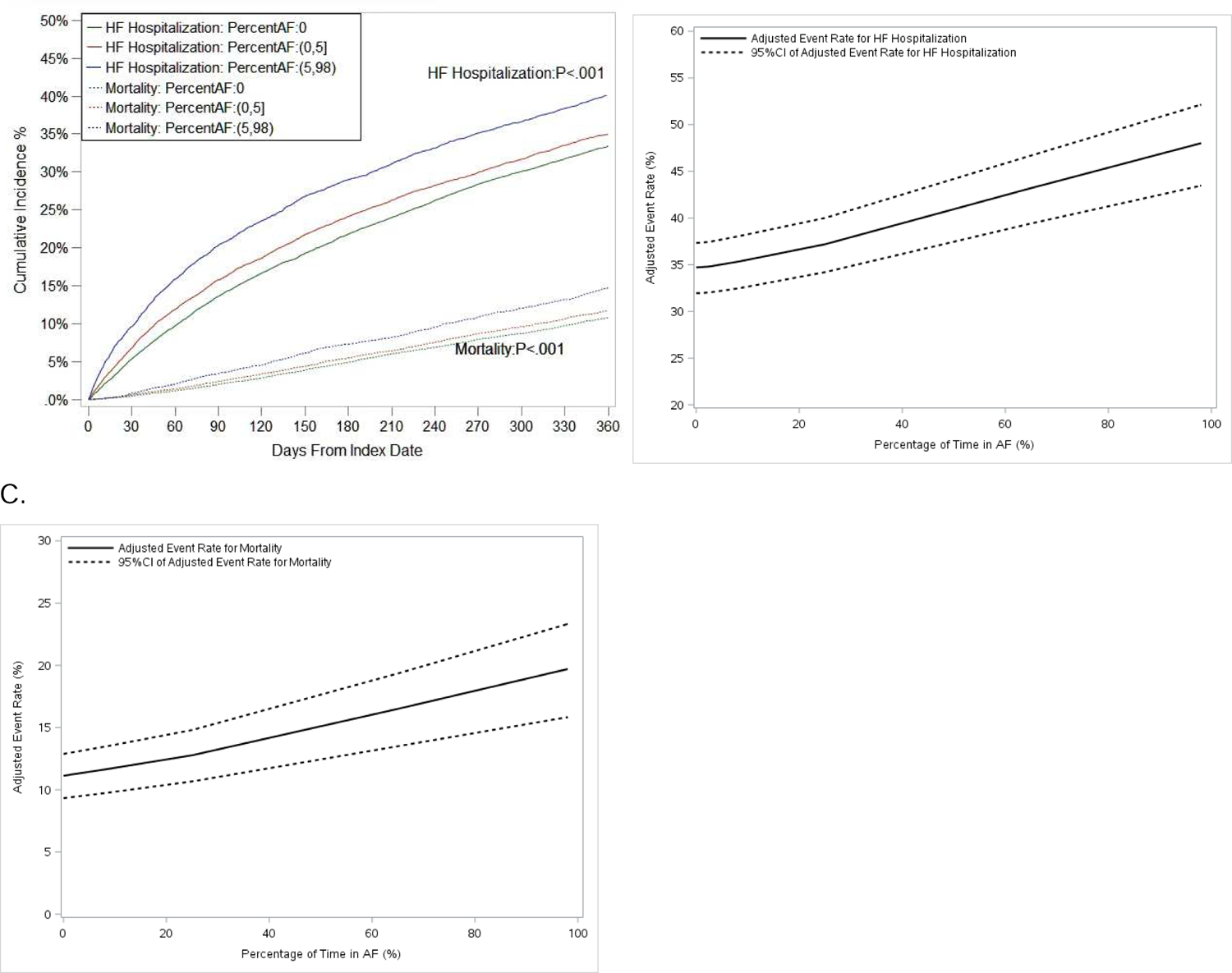
Cumulative incidence curves of heart failure (HF) hospitalization and mortality, stratified by atrial fibrillation (AF) burden (categorical, percent in AF) (A), and adjusted event rates of HF hospitalization (B) and mortality (C) among patients with HF at baseline. Parentheses indicate exclusive borders; brackets indicate inclusive borders. CI 5 confidence interval.
All models indicated linear functional form for AF percentage in all outcomes and for maximum duration in the model of all-cause mortality among patients with no prior diagnosis of HF at baseline based on supremum test and restricted cubic spline testing.
Sensitivity Analyses
Interaction testing among patients with HF at baseline, including between sex and AF burden, and device type and AF burden, for outcomes of new-onset HF and all-cause mortality, were not significant. In Cox models with percentage in AF and maximum AF duration as time-varying variables calculated at 30-day periods, we assessed 3-year outcomes in both cohorts, among patients without and with HF at baseline. All observed adjusted hazard ratios were consistent with the primary analysis for the outcomes of new-onset HF (among no HF at baseline), HF hospitalization (among HF at baseline), and all-cause mortality (in each cohort; Supplemental Material, Table S8).
Exploratory testing of linearity, based on maximum AF episode duration, demonstrated possible non-linearity for the endpoints of new-onset HF (among patients without HF at baseline), and for HF hospitalization and all-cause mortality (among patients with HF at baseline). Restricted cubic splines analyses for these endpoints demonstrated a possible plateau at certain maximum durations (approximately 18 hours; Supplemental Material, Figure S2).
Discussion
This is the first, nation-wide, large-scale analysis of continuous electrocardiographic data, to quantify the association between device-based AF burden and HF-related clinical outcomes. There are several major findings from these analyses of more than 39,000 patients with pre-existing clinical AF and an implanted cardiac device. First, increasing AF burden, by continuous and categorical measures, is associated with increasing risk of new-onset HF and HF hospitalization. Second, increasing AF burden is associated with increasing risk of all-cause mortality, among patients with and without HF at baseline. Finally, rates of adverse outcomes in patients with CIEDs and AF are high in general. Within 1 year, 10% develop HF and 10% with prior HF experience mortality. Our findings have important implications for the care of these high-risk patients.
Patients with AF are at risk for a variety of adverse cardiovascular outcomes, including increased risk of stroke, systemic embolism, cognitive impairment, HF, and death. While stroke is a devastating complication of AF and often receives the greatest attention, the occurrences of HF events and death are 2–3 fold more common in comparison.12 Early clinical trials failed to identify superior outcomes amongst these clinical events in HF and non-HF patients with AF randomized to pharmacologic rhythm control (i.e., antiarrhythmic drugs).13 However, more recent randomized clinical trials of catheter ablation in patients with HF and reduced ejection fraction have shown that maintenance of sinus rhythm can improve clinical outcomes in patients with AF and HF, including mortality.14 Moreover, the improvement in outcomes in these trials of catheter ablation does not appear to be limited to patients with complete elimination of AF. Among trials that reported AF burden within this meta-analysis, AF burdens at study conclusion were still as high as 25% in those patients with HF that underwent catheter ablation. Additionally, trials show that not all patients with AF and HF respond to catheter ablation, as treatment effect can be heterogeneous.15 One reason may be disease chronicity – data from the GENETIC-AF trial demonstrated that the longer patients have AF prior to HF diagnosis (and vice-versa), the worse their observed clinical outcomes.6 Further studies are needed to better identify those patients with AF and HF that are most likely to benefit from invasive rhythm control.
The present data from a nationwide cohort of patients with CIEDs demonstrate that escalating AF burden matters – more AF is associated with worse HF outcomes and worse all-cause mortality. Moreover, for clinicians seeking a threshold at which intervention should be implemented, we found increasing risk with increasing burden without a threshold or inflection point – even a small increase in AF above 0% heightens risk of adverse outcomes.
Combined, these observations support what many have observed anecdotally in clinical practice – patients with lower AF burden do better. While clinical dogma often dictates that the sickest patients tend to benefit the most from aggressive therapy, patients with less severe AF tend to respond better to catheter ablation. This may reflect more favorable AF substrate, via less atrial myopathy and thus better response to therapy, however, the present analysis is not designed to elucidate mechanistic insights. In considering the natural disease progression towards worsening AF, these data support earlier, more aggressive interventions for patients with lower AF burden and existing HF,16 or those at risk of developing new HF, although identifying the latter remains a challenge.
We did not observe an interaction between AF burden and CIED device type – that is, increasing AF burden was associated with increasing risk of adverse events irrespective of PM, ICD, or CRT implant, which represents a surrogate for severity of cardiac structural disease. It is likely that older patients with AF undergoing CIED implant have such significant risk of adverse events at baseline, the additional impact of device type is modest. While patients with preserved left ventricular function may not qualify for a high-voltage device (i.e., defibrillator), they may still have HF with preserved or mildly-reduced ejection fraction or other cardiovascular comorbidities associated with increased risk of all-cause mortality. Nevertheless, there may be additional absolute risk of adverse events among patients with high-voltage devices, owing the morbidity that led to the implantation of such a device; however, our data does not suggest a significantly modified effect of AF burden on outcome among those patients.
Older patients with a diagnosis of AF undergoing CIED implant are likely at high risk of adverse events for a number of reasons. First, they are at increased risk of stroke-related death and are treated with oral anticoagulants, which increase bleeding-associated risks.17 Second, they have additional cardiomyopathy and/or underlying conduction disease, which is leading to CIED implant, increasing overall morbidity and mortality. Lastly, their hospitalization for CIED implant may trigger subsequent adverse events, which have been shown to represent an inflection point in the course of older patients’ disease.18
Limitations
The cohort only includes Medicare patients with CIEDs from a single manufacturer and may not reflect outcomes in younger patients, those with CIEDs from different manufacturer, or broader patients with AF (without CIEDs, and/or without HF). As this was a population cohort study, case-by-base electrocardiographic verification was not performed for arrhythmia episodes, though medical treatments support clinical diagnoses of AF were present. Additionally, these findings are adjusted observations of the relationship between AF burden and cannot prove cause and effect. Lastly, the distribution of AF burden is skewed to very high and very low; while this may limit interpretation of AF burden as a continuous measure, we observed consistent results when it was measured categorically.
Conclusions
Among older patients with AF receiving CIEDs, increasing AF burden is significantly associated with increasing risk of adverse HF outcomes and all-cause mortality. These data suggest that interventions which reduce AF burden earlier in the disease progression, may reduce the risks of HF-associated events and death.
Supplementary Material
Acknowledgements
This work was supported by a grant from Abbott. Dr. Steinberg was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL143156. The content is solely the responsibility of the authors and does not necessarily represent the views of the sponsor or the National Institutes of Health.
Conflict of Interest
The following relationships exist related to this presentation: BS reports research support from Abbott, Boston Scientific, and Janssen; consulting to Janssen, AltaThera, Merit Medical, Bayer, and speaking for NACCME. YN is an employee of Abbott and holds Abbott stock. JPP is supported by R01HL128595 from the National Heart, Lung and Blood Institute and receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Myokardia, Sanofi, Philips, and Up-to-Date. The remaining co-authors did not report any relevant disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolf PA, Dawber TR, Thomas HE Jr., Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 2.Deng H, Bai Y, Shantsila A, et al. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clinical Research in Cardiology 2017;106:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieuwlaat R, Prins MH, Le Heuzey JY, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J 2008;29:1181–1189. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg BA, Piccini JP. When Low-Risk Atrial Fibrillation Is Not So Low Risk: Beast of Burden. JAMA cardiology 2018;3:558–560. [DOI] [PubMed] [Google Scholar]
- 5.Khazanie P, Liang L, Qualls LG, et al. Outcomes of medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart failure 2014;2:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccini JP, Abraham WT, Dufton C, et al. Bucindolol for the Maintenance of Sinus Rhythm in a Genotype-Defined HF Population: The GENETIC-AF Trial. JACC Heart failure 2019;7:586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J 2015;36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gelder IC, Healey JS, Crijns H, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 9.Birman-Deych E, Waterman AD, Yan Y, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care 2005;43:480–485. [DOI] [PubMed] [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 11.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med 2008;168:418–424. [DOI] [PubMed] [Google Scholar]
- 12.Piccini JP, Hammill BG, Sinner MF, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 14.Turagam MK, Garg J, Whang W, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med 2019;170:41–50. [DOI] [PubMed] [Google Scholar]
- 15.Jones DG, Haldar SK, Hussain W, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med 2020. [DOI] [PubMed]
- 17.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turakhia MP, Solomon MD, Jhaveri M, et al. Burden, timing, and relationship of cardiovascular hospitalization to mortality among Medicare beneficiaries with newly diagnosed atrial fibrillation. Am Heart J 2013;166:573–580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


