Abstract
Study Objective.
Provider misconceptions regarding intrauterine device safety for adolescents and young women can unnecessarily limit contraceptive options offered; we sought to evaluate rates of N gonorrhaeae or C trachomatis diagnoses among young women adopting intrauterine devices.
Design.
Secondary analysis of a cluster-randomized provider educational trial.
Setting.
40 U.S.-based reproductive health centers.
Participants.
1,350 participants aged 18–25 seeking contraceptive care were followed for 12-months.
Interventions.
The parent study assessed the impact of a provider training on evidence-based contraceptive counseling.
Main Outcome Measures.
We assessed incidence of N gonorrhaeae or C trachomatis (GC/CT) diagnoses by IUD use and sexually transmitted infection risk factors using Cox regression modeling and generalized estimating equations.
Results.
204 participants had GC/CT history at baseline; 103 received a new GC/CT diagnosis over 12-month follow-up. IUDs were initiated by 194 participants. Incidence of GC/CT diagnosis was 10.0 per 100 person-years during IUD use vs. 8.0 otherwise. In adjusted models, IUD use (aHR 1.31, 95% CI 0.71–2.40), adolescent age (aHR 1.28, 95% CI 0.72–2.27), history of GC/CT (aHR 1.23, 95% CI 0.75–2.00) and intervention status (aHR 1.12, 95% CI 0.74–1.71) were not associated with GC/CT diagnosis; however, new GC/CT diagnosis rates were significantly higher among individuals reporting multiple partners at baseline (aHR 2.0, 95%CI 1.34–2.98).
Conclusion.
In this young study population with GC/CT history, this use of intrauterine devices was safe and did not lead to increased GC/CT diagnoses. However, results highlighted the importance of dual sexually transmitted infection and pregnancy protection for participants with multiple partners.
Keywords: adolescent, contraception, intrauterine device, Neisseria gonorrhoeae, Chlamydia trachomatis, sexually transmitted diseases
Introduction
Intrauterine devices (IUDs) are an important option within the range of contraceptive choices available to adolescents and young adults.1–5 In recent years, medical professional organizations, including the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and the Centers for Disease Control and Prevention (CDC) have made recommendations supporting IUD use by adolescents and nulliparous women.6–8 Nevertheless, national studies show some contraceptive providers are reluctant to offer IUDs to adolescents and young women, at least in part due to the misconception that the method would be unsafe for them because they are at elevated sexually transmitted infection (STI) risk.9–14 Such provider practices may unnecessarily limit contraceptive options among adolescents and young women.14,15
Despite the lack of robust supporting evidence, some providers believe IUDs increase women’s risk of pelvic inflammatory disease (PID) and tubal infertility through biologic and behavioral factors: facilitating bacteria from the lower to upper genital tract and greater likelihood of STI acquisition through reduced condom usage.16,17 Evidence on the biologic risk potential of the IUD includes a meta-analysis of randomized trials including over 20,000 initiators which found no excess risk of PID among IUD users at low risk of STI, and identified only a small increased PID risk in the 20 days immediately post-placement.18 More recent literature reports risk of PID at the time of IUD insertion as very small and unrelated to IUD placement with same-day STI testing in a clinical trial population,19 and that PID remains rare even with IUD placement during asymptomatic STI.20–22 The CDC Selected Practice Recommendations for Contraceptive Use and Medical Eligibility Criteria for Contraceptive Use support same-day STI screening without delaying IUD insertion among women with risk factors for STIs in the absence of purulent cervicitis or known chlamydial or gonorrheal infection.23–25 Fewer studies have assessed concurrent use of condoms with the IUD. However, a few rigorous studies have shown that use of IUDs and implants did not compromise concurrent condom use, although dual use, concurrent use of hormonal and barrier methods for pregnancy and STI prevention, is low among most contraceptive users.26–28 Nevertheless, a large well-conducted observational study identified a small but statistically significant difference in STI incidence between long-acting reversible contraceptive (LARC) initiators and women initiating other non-LARC methods (3.9% vs. 2.0%).26
Given the conflicting evidence and continuing concerns that increasing IUD access among young people will result in increased STI incidence,29 this objective of this study was to assess the incidence of new diagnoses of N gonorrhaeae or C trachomatis (GC/CT) by IUD use over a 12-month period. This secondary analysis used data from a cluster randomized trial of a provider training intervention to increase contraceptive access, including to the IUD, on adolescent and young women’s STI outcomes.1 Prior analyses showed the intervention increased access to IUDs and the implant among both adolescents and young adults, aged 18–25 years,1,30 without undermining dual use.27 This analysis adds to the evidence by measuring the role of STI risk factors in IUD counseling and selection among this sample as well as GC/CT diagnoses.
Materials and Methods
We conducted a secondary analysis of data from a cluster-randomized trial of a clinic staff educational intervention to increase access to the full range of contraceptive methods, including copper and levonorgestrel-releasing IUDs and progestin subdermal implants, among women aged 18–25. Briefly, the study recruited participants from 40 Planned Parenthood health centers across the United States that had at least 400 contraceptive patients annually, had no established contraceptive educational intervention programs, and did not share staff with another study site. Twenty-three sites recruited family planning or general reproductive health patients and 17 recruited post-abortion patients. The intervention consisted of a half-day Continuing Medical Education-accredited training course from the University of California, San Francisco to improve providers’ contraceptive knowledge and skills, including IUD eligibility for women at elevated STI risk and those with comorbidities. The training covered patient-centered counseling focused on patient preference, non-judgmental care, clinic capacity for service provision, CDC U.S. medical eligibility criteria for IUD use,24 and ethical issues such as IUD removal at patient request. Clinicians received hands-on training on IUD placement and removal and a review of complex cases. All staff at intervention clinics (n=20) underwent training while control clinics (n=20) did not. Participant recruitment began in May 2011, was staggered across health centers, and follow-up data collection was completed in May 2013. The study design and primary results of the intervention are published elsewhere in detail.1
Women aged 18–25 visiting study clinics were eligible to participate if they were at risk of pregnancy (sexually active within the prior three months), were receiving contraceptive counseling, and did not desire pregnancy within the next 12 months. At baseline, participants completed a self-administered questionnaire including items on STI history, sexual partners, and contraceptive method counseling and selection. Participants completed quarterly follow-up surveys via phone or online, including questions about IUD use and new STI diagnoses. Data on IUD placements and removals and new STI diagnoses were also collected from medical record review from Planned Parenthood records covering the one-year study period. Sample size for the trial was calculated to estimate the primary study outcome contraceptive method choice by study arm.1
This study was approved by the Committee on Human Research of the University of California, San Francisco and the Allendale Investigational Review Board and was registered with ClinicalTrials.gov (NCT01360216). All participants provided written informed consent.
Measures
The primary outcome of this analysis was new diagnosis of GC/CT during the 12-month study follow-up. New diagnoses of GC/CT were captured from two sources: via self-report on quarterly surveys and from medical record data. We focused on GC/CT as the primary etiologic agents of PID.31 Participating health centers used United States Food and Drug Administration (FDA)-approved nucleic acid-based tests for STI diagnoses, and tests were administered per clinic protocol. STIs identified at study enrollment were treated following clinical guidelines and were not included as new diagnoses.32 We also examined new PID diagnoses captured by self-report on quarterly surveys in exploratory analyses. Timing of survey-reported incident GC/CT and PID diagnoses was assigned to the midpoint between surveys; timing of new diagnoses captured via medical records were assigned to the testing date.
Independent variables measured at baseline were age (adolescent, aged 18–19 versus young adult, aged 20–25), history of GC/CT, history of STIs, and multiple sexual partners, or new sexual partner. History of STIs was assessed as ever diagnosed with N gonorrhaeae, C trachomatis, genital herpes simplex virus, human papillomavirus or genital warts, T vaginalis, syphilis, hepatitis B or C, human immunodeficiency virus, or other STI, and categorized as ‘yes’, ‘no’, or ‘unknown.’ History of PID was captured from medical records review data and survey (categorized as ‘yes’, ‘no’ or ‘unknown’). Participants reported the number of sexual partners they had (multiple vs. one/none) and whether they had a new sexual partner (yes vs. no) within three months prior to study enrollment. Covariates included race/ethnicity (self-identified White, Latina, Black or other), insurance type (private, Medicaid/state, none, or don’t know), parity (parous vs. nulliparous), practice setting (family planning vs. abortion) and study arm (intervention vs. control health center) at study enrollment. IUD use was measured as a time-varying covariate over study follow-up via survey and medical record review, so that we could sequentially model IUD use prior to GC/CT diagnoses.
Analyses
The analytic population for examining new GC/CT diagnoses included participants who had completed at least one follow-up survey (n=1,356; 90.4%), excluding six participants missing data for primary covariates (Figure 1), resulting in an analytic sample of 1,350. Sociodemographic characteristics of participants in our analytic sample did not differ from those excluded (not shown). We estimated the 12-month cumulative incidence of GC/CT with life table analysis and GC/CT incidence by IUD use with Kaplan-Meier survival curves. IUD use and intervention effect on time to first GC/CT diagnosis were modeled using Cox proportional hazard regression models with robust cluster variance estimation to account for clustering by site. Schoenfeld residuals were calculated to confirm the proportional hazards assumption. Participants contributed data to the survival analyses until new diagnosis of GC/CT, loss to follow-up or study exit at 12-months.
Figure 1.
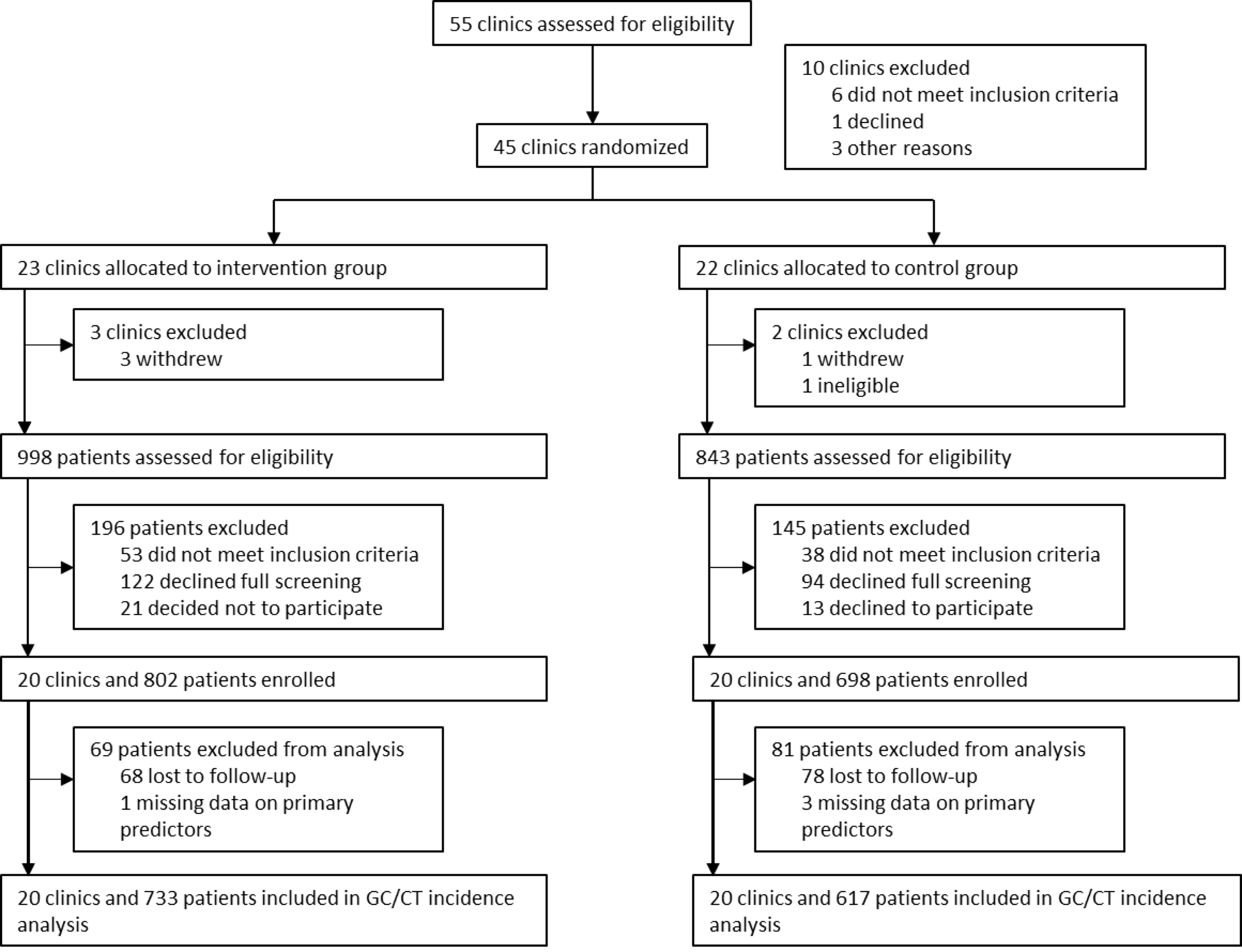
Schematic Diagram of Study Participant Selection and Analytic Samples
We explored new PID diagnoses during study follow-up among women with at least one follow-up survey, excluding 15 who did not provide data on PID diagnosis during study follow-up (n=1,341). Very few participants reported new PID diagnoses; we calculated simple cumulative incidence by arm using life table analysis. Women were censored when they reported PID diagnoses, were lost to follow up, or exited the study.
Finally, we explored a few steps prior to IUD use, that is, contraceptive counseling and method selection, to assess any differences by STI risk factors, including age, history of GC/CT, history of STI, and multiple or new sexual partners, using multivariable logistic regression analysis with generalized estimating equations (GEE) to account for the clustered study design. We tested for statistical interactions between the provider educational intervention and these characteristics, to assess any differential intervention effect on the relationship between these factors and outcomes, estimating separate models with multiple sexual partners and new partners, and history of GC/CT and history of any STI. Finally, to contextualize our findings on multiple partners, GC/CT diagnosis, and IUD selection, we describe the relationship between multiple partners at baseline and contraceptive method selected.
All analyses were conducted in Stata v16 (StataCorp, College Station, TX), using robust standard errors, and differences were considered statistically significant at p<0.05.
Results
Participant Characteristics
Of the 1,350 women comprising our analytic sample, 733 enrolled in intervention clinics and 617 in control clinics (Figure 1). Twenty-three percent were teens aged 18–19 (Table 1); most were unmarried (94%; not shown). Approximately one-fifth of participants reported multiple sexual partners (21%) or a new partner (22%) in the past three months. About one-quarter of participants reported any STI history (24%), with 15% reporting a prior diagnosis of N gonorrhoeae or C trachomatis. Few participants reported PID history (3%). Self-perceived STI risk was low, with 94% reporting being ‘unlikely’ or ‘very unlikely’ to get an STI over the following year. Condom use at last sex was 31.4%.27 Diagnosis of GC/CT at the baseline visit was 3.3%, and these cases were excluded from outcome assessment, as were the 4.4% diagnosed for any STI. 194 participants (14.4%) initiated an IUD during the study.
Table 1.
Participant Characteristics at Study Enrollment by Intervention Group
| Intervention | Control | Total | ||||
|---|---|---|---|---|---|---|
| n=733 | n=617 | n=1,350 | ||||
| n | % | n | % | n | % | |
| Age Group | ||||||
| Teen (age 18–19) | 555 | 75.7 | 487 | 78.9 | 1042 | 77.2 |
| Young adult (age 20–25) | 178 | 24.3 | 130 | 21.1 | 308 | 22.8 |
| Race/ethnicity | ||||||
| White | 364 | 49.7 | 304 | 49.3 | 668 | 49.5 |
| Latina | 181 | 24.7 | 184 | 29.8 | 365 | 24.7 |
| Black | 107 | 14.6 | 96 | 15.6 | 203 | 15.0 |
| Other | 81 | 11.1 | 33 | 5.4 | 114 | 8.4 |
| Educational attainment (n=1341) | ||||||
| High school or less | 520 | 71.4 | 450 | 73.4 | 970 | 72.3 |
| Some college | 104 | 14.3 | 85 | 13.9 | 189 | 14.1 |
| College degree | 104 | 14.3 | 78 | 12.7 | 182 | 13.6 |
| Health insurance type | ||||||
| Private | 226 | 30.8 | 185 | 30.0 | 411 | 30.4 |
| Medicaid/State | 198 | 27.0 | 163 | 26.4 | 361 | 26.7 |
| None | 276 | 37.7 | 238 | 38.6 | 514 | 38.1 |
| Don’t know | 33 | 4.5 | 31 | 5.0 | 65 | 4.7 |
| Nulliparous (n=1348) | 547 | 74.7 | 423 | 68.7 | 970 | 72.0 |
| Condom use at last sex (n=1345) | 219 | 30.0 | 203 | 33.1 | 422 | 31.4 |
| Any unprotected sex (past 3 months; n=1344) | 463 | 63.7 | 406 | 65.8 | 869 | 64.7 |
| New partner (past 3 months; n=1346) | 171 | 23.4 | 129 | 21.0 | 300 | 22.3 |
| Multiple partners | 160 | 21.8 | 123 | 19.9 | 283 | 21.0 |
| Perception of STI Risk (n=1345) | ||||||
| Very Likely | 7 | 1.0 | 9 | 1.5 | 16 | 1.2 |
| Likely | 34 | 4.7 | 27 | 4.4 | 61 | 4.5 |
| Unlikely | 256 | 35.1 | 197 | 32.0 | 453 | 33.7 |
| Very unlikely | 433 | 59.3 | 382 | 62.1 | 815 | 60.6 |
| History of CT/GC | ||||||
| Yes | 106 | 14.5 | 98 | 15.9 | 204 | 15.1 |
| No | 604 | 82.4 | 494 | 80.1 | 1098 | 81.3 |
| Unknown | 23 | 3.1 | 25 | 4.1 | 48 | 3.6 |
| History of any STI | ||||||
| Yes | 174 | 23.7 | 144 | 23.3 | 318 | 23.6 |
| No | 522 | 71.2 | 432 | 70.0 | 954 | 70.7 |
| Unknown | 37 | 5.1 | 41 | 6.7 | 78 | 5.8 |
| History of PID | ||||||
| Yes | 24 | 3.3 | 16 | 2.6 | 40 | 3.0 |
| No | 696 | 95.0 | 581 | 94.2 | 1277 | 94.6 |
| Don’t know | 13 | 1.8 | 20 | 3.2 | 33 | 2.4 |
| Practice setting | ||||||
| Family Planning | 452 | 61.7 | 342 | 55.4 | 794 | 58.8 |
| Abortion | 281 | 38.3 | 275 | 44.6 | 556 | 41.2 |
| IUD use initiated within first 6 mo | 104 | 14.2 | 90 | 14.6 | 194 | 14.4 |
GC/CT: N gonorrhoeae or C trachomatis, PID: pelvic inflammatory disease. No significant differences were identified by study arm.
New N gonorrhoeae or C trachomatis Diagnoses
Overall, 103 participants experienced a new diagnosis of either N gonorrhoeae (n=14) or C trachomatis (n=96) over the 12-month follow-up, with 7 co-infections. 90 new diagnoses were reported on surveys, and 29 were captured from medical records, with 16 captured on both sources. Incidence of new GC/CT diagnosis was 10.0 per 100 person-years during IUD use compared to 8.0 otherwise, and IUD use was not associated with time to new GC/CT diagnosis (aHR 1.31, 95% CI 0.71–2.40) (Table 2). New GC/CT diagnosis rates did not differ significantly by age group (aHR 1.28, 95% CI 0.72–2.27), prior history of GC/CT (aHR 1.23, 95% CI 0.75–2.00), or study arm (aHR 1.12, 95% CI 0.74–1.71); however, report of multiple partners at baseline was associated with two-fold the hazard of new GC/CT diagnosis (aHR 2.00, 95% CI 1.34–2.98; Figure 2). Results were similar for separate models with new partner rather than multiple partners (aHR 1.97, 95% CI 1.37–2.84; full model not shown).
Table 2.
Predictors of Time to New N gonorrhoeae or C trachomatis Diagnoses (Hazard Ratios from Cox Proportional Hazards models)
| Incidence per 100PY | Unadjusted Models (n=1350) | Adjusted Modela (n=1350) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | ||
| IUD Use | |||||
| Yes | 10.0 | 1.27 (0.75–2.17) | 0.38 | 1.31 (0.74–1.71) | 0.59 |
| No | 8.0 | REF | - | REF | - |
| Age Group | |||||
| Teen (age 18–19) | 10.0 | 1.34 (0.83–2.18) | 0.23 | 1.28 (0.72–2.27) | 0.40 |
| Young adult (age 20–25) | 7.7 | REF | - | REF | - |
| History of GC/CT | |||||
| Yes | 11.5 | 1.51 (0.96–2.39) | 0.08 | 1.23 (0.75–2.00) | 0.40 |
| No | 7.8 | REF | - | REF | - |
| Multiple partners, past 3m | |||||
| Yes | 13.5 | 1.92 (1.32–2.79) | <0.01 | 2.00 (1.34–2.98) | <0.01 |
| No | 6.9 | REF | - | REF | - |
| Study arm | |||||
| Intervention | 8.6 | 1.09 (0.71–1.68) | 0.69 | 1.12 (0.74–1.71) | 0.59 |
| Control | 7.8 | REF | - | REF | - |
Covariates included: race/ethnicity, insurance status, parity, practice setting; HR=Hazard Ratio; CI=Confidence Intervals; GC/CT: N gonorrhoeae or C trachomatis.
Figure 2.
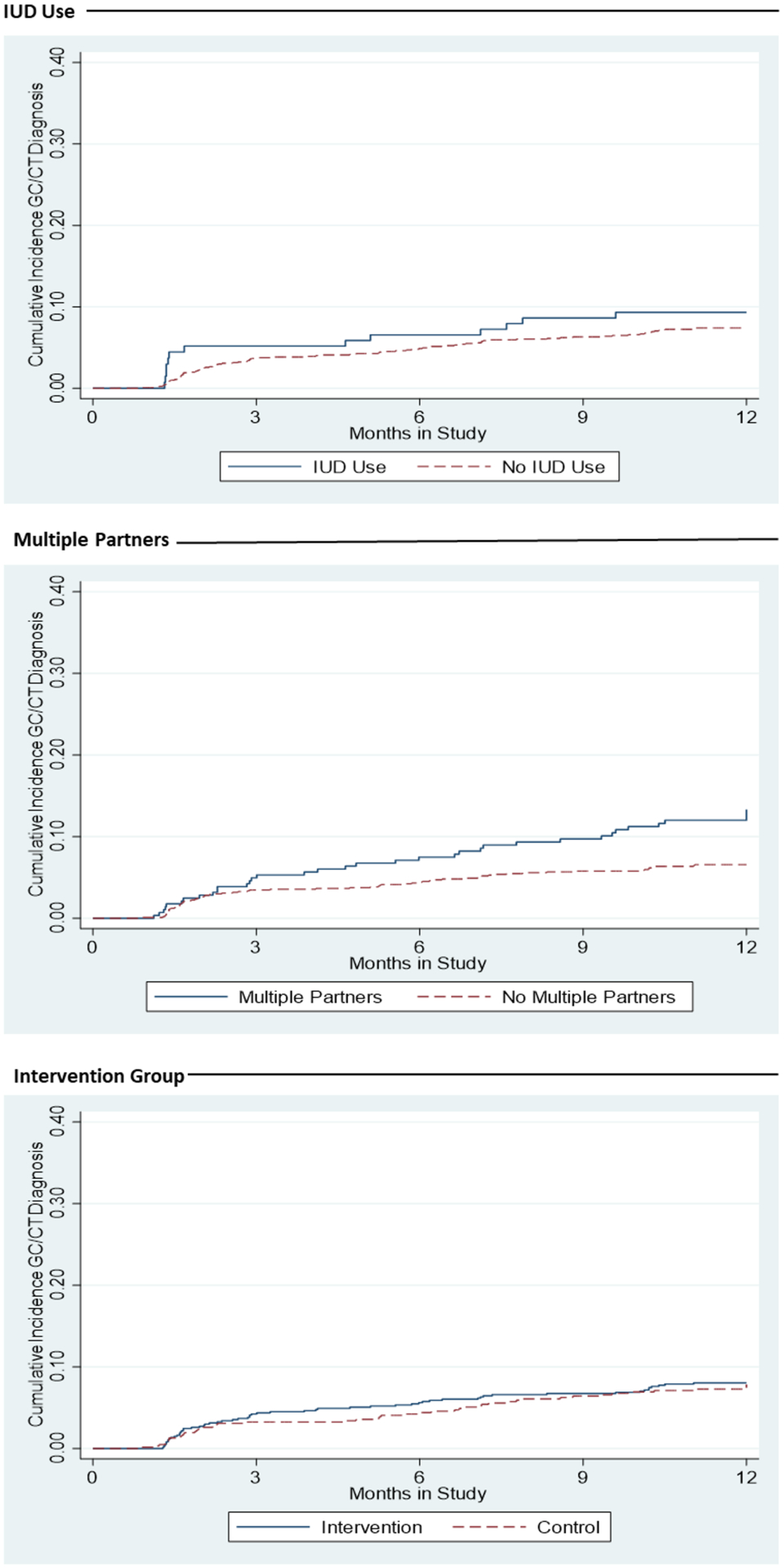
Cumulative Incidence of New GC/CT Diagnoses by IUD Use, Multiple Partners and Intervention Status during 12-Month Study Follow Up
New Pelvic Inflammatory Disease Diagnoses
The cumulative incidence of PID at 12-months was 0.09% overall. No study diagnoses of PID were reported during IUD use, and incidence of new PID diagnosis was 0.98 per 100 person years among those not using the IUD.
IUD Counseling and Method Choice by STI Risk Factors
In our analyses of IUD counseling and method choice, the provider educational intervention was associated with an over 3-fold increased odds of IUD counseling in multivariable analyses (aOR 3.29, 95% CI 2.37–4.56; Table S1), with no significant difference by history of GC/CT (aOR 1.10, 95% CI 0.83–1.47) or separately by history of any STI (aOR 1.19, 95% CI 0.93–1.53; not shown). Women with multiple partners were similarly likely to be counseled on the IUD (aOR 0.80, 95% CI 0.61–1.05), and additionally had significantly higher odds of counseling on condom use (aOR 1.30, 95% CI: 1.00–1.69, p=0.047; not shown). Similar results were identified in models with new sexual partner in place of multiple partners (aOR 0.84, 95% CI 0.68–1.05; full model not shown). Models including an interaction term for intervention and history of GC/CT showed no differential effect (p=0.78; not shown). Likewise in main effects analyses, IUD method choice did not differ significantly by history of GC/CT (aOR 1.15, 95% CI: 0.81–1.63) or separately by history of any STI (aOR 1.32, 95% CI 0.93–1.87; not shown), and the interaction term with history of GC/CT was not significant (P=0.44; not shown). Significantly fewer women who reported multiple partners at baseline chose an IUD (aOR 0.66, 95% CI 0.46–0.96), with similar results from models including new sexual partner (aOR 0.76, 95% CI 0.51–1.09; not shown). Across all contraceptive methods, women reporting multiple partners were significantly more likely to choose condoms (24.4% vs. 19.8%) or the patch (7.1% vs. 3.9%), whereas there was no difference in selection of the oral contraceptive pill, ring, shot, or implant (not shown).
Discussion
IUD use among adolescents and young women did not result in increased GC/CT diagnoses within the context of a provider educational intervention trial to increase access to IUDs and implants among this population.
Our finding of no significant difference in new diagnoses by IUD use and history of GC/CT among adolescents and young women support current guidelines on the safety of intrauterine contraception for young women and women with prior STI history.6,8,24 Our findings are consistent with a recent smaller retrospective review of electronic medical records of among 422 urban adolescents which identified no elevated risk of C trachomatis among LARC users versus non-LARC method users.33 However, they contrast with results from the CHOICE Project, a large observational cohort study.26 The CHOICE analysis similarly explored the role of age, new partner, and STI history on STI incidence, and found a significantly increased odds of STI for women under age 25. The difference observed between our study and the CHOICE project was likely influenced by the differing study populations and designs, including potential selection effects by contraceptive method that may be occurring in observational designs.
In our study, women who reported multiple sexual partners had significantly lower odds of IUD selection and nearly twice the rate of GC/CT. Interventions among youth to increase condom use and reduce the number of sexual partners have significantly reduced STI incidence.34 Our prior research showed increasing LARC access among teens and young adults does not compromise dual method use, and provider counseling on condoms at baseline is associated with higher dual method use at 12 months and at last sex.27 However, only 14% of study participants reported dual method use, suggesting that additional efforts are necessary to improve concurrent condom use among this population to improve STI prevention including provision of non-judgmental counseling.26
Consistent with evidence-based guidelines,7,35 providers in our study counseled young women on IUDs irrespective of age or prior history of GC/CT.11,23,36 However, other research suggests that there are ongoing provider misperceptions about IUD use and risk of subsequent infection.9,11,35,37 Therefore, ensuring access to a broad range of contraceptive methods regardless of age or STI history is an important target for continued intervention. Patient-centered contraceptive counseling approaches incorporating shared decision-making based on current evidence combined with individual patient preferences are needed to safeguard reproductive autonomy.38
Our results support the safety of IUD initiation among adolescents and young adults following clinical practice guidelines, in conjunction with efforts to improve concurrent condom use for STI prevention.26 Robust designs are necessary to study IUD use by young women in U.S. who are at elevated risk of STIs and to inform concerns that increasing IUD access among this population will result in increased STI incidence.29 Research that universally tests study participants at baseline and follow-up could provide informative evidence.
Strengths of the current study include a randomized design and the national study population of young low-income women at risk of pregnancy and STI, with high-quality data and study retention.39 However, several limitations to our analysis exist. Although we captured STI diagnosis and treatment using both medical record review and via self-report, we did not systematically test all participants for STIs. Women who initiated IUDs during the study may have been more likely to undergo STI testing compared to other participants. Steiner et al. found new LARC users were more likely to have received STI testing compared to non-users of contraception.40 However, our findings of no difference in GC/CT by study arm and IUD use during the study would thus be conservative, as greater scrutiny would have resulted in higher STI detection among IUD users. Furthermore, study participants may not have returned to the clinic during follow-up, resulting in lack of medical record data.
Important limitations are relevant to our exploratory analysis of PID by intervention arm and IUD use. PID is diagnosed by clinical criteria (e.g., pelvic pain, tenderness, lack of other cause), without use of specific objective technique, although the diagnosis may be supported by fever, discharge, lab findings (CG/GC in <50%, leukocytes on microscopy), or findings on endometrial biopsy, sonography or laparoscopy.25 Clinical diagnosis is sufficient for treatment initiation and public health reporting, but has low sensitivity and specificity.41 This exploratory outcome is further limited by the lack of medical record capture on PID diagnosis during study follow-up; all follow-up diagnosis data for PID was from participant self-report which may be subject to social desirability or ascertainment biases. Few patients reported PID diagnoses when surveyed. Although accuracy of self-reported history of PID has not been studied, self-reported history of CT infection commonly yields false negative and false positive results.42 Given the complexity of its clinical case definition, this is also plausible for PID. Given the low PID rate observed and our sample size, interpretation of study results must acknowledge the precision of our effect estimates; a larger sample would provide a more precise analysis.43 Finally, our results may not be generalizable to different patient and provider contexts.
Conclusions
Our study results showing no significant increase in GC/CT diagnoses among adolescent and young adult IUD users contributes to the growing body of evidence supporting efforts to improve counseling and access to IUDs among all women, including those at high risk of pregnancy and STI acquisition. Improving provider knowledge and skills for evidence-based IUD selection, in conjunction with counseling on STI risk reduction, is an important strategy to improve young women’s access to the full range of contraceptive methods.
Supplementary Material
Acknowledgements:
This work was supported by the William and Flora Hewlett Foundation [grant number: 2010-5442, 2012-7650, 2013-8607], and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant numbers: K99HD086232, K12HD052163 and K12HD001259]. Teva Pharmaceuticals Industries and Bayer HealthCare provided educational samples of intrauterine devices for the training. The study sponsors had no role in study design, collection, analysis and interpretation of data, writing of the report, and in the decision to submit the article for publication. We thank Johanna Morfesis and Planned Parenthood investigators and research coordinators at these affiliates: Northern, Central and Southern New Jersey; Columbia Willamette; Great Northwest and Hawaiian Islands; Greater Ohio; Greater Washington and North Idaho; Mar Monte; Michigan; Minnesota, North Dakota, South Dakota; Mt. Baker; Northern California; Pacific Southwest; Pasadena and San Gabriel Valley; Rocky Mountains; South Atlantic; Southeastern Pennsylvania; Southern New England; and Southwest and Central Florida. All these contributors received compensation. We thank Dr. Charles McCulloch for design expertise. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of Planned Parenthood Federation of America, Inc.
Footnotes
Disclosure statement: The authors report no conflict of interest.
References:
- 1.Harper CC, Rocca CH, Thompson KM, et al. Reductions in pregnancy rates in the USA with long-acting reversible contraception: a cluster randomised trial. Lancet (London, England) 2015;386:562–8. [DOI] [PubMed] [Google Scholar]
- 2.Peipert JF, Madden T, Allsworth JE, Secura GM. Preventing unintended pregnancies by providing no-cost contraception. Obstetrics and gynecology 2012;120:1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. [DOI] [PubMed] [Google Scholar]
- 4.Jatlaoui TC, Riley HE, Curtis KM. The safety of intrauterine devices among young women: a systematic review. Contraception 2017;95:17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricketts S, Klingler G, Schwalberg R. Game change in Colorado: widespread use of long-acting reversible contraceptives and rapid decline in births among young, low-income women. Perspect Sex Reprod Health 2014;46:125–32. [DOI] [PubMed] [Google Scholar]
- 6.Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2012;120:983–8. [DOI] [PubMed] [Google Scholar]
- 7.Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (CDC). U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013;62:1–60. [PubMed] [Google Scholar]
- 8.Ott MA, Sucato GS. Contraception for adolescents. Pediatrics 2014;134:e1257–81. [DOI] [PubMed] [Google Scholar]
- 9.Harper CC, Stratton L, Raine TR, et al. Counseling and provision of long-acting reversible contraception in the US: national survey of nurse practitioners. Prev Med 2013;57:883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper CC, Henderson JT, Raine TR, et al. Evidence-based IUD practice: family physicians and obstetrician-gynecologists. Fam Med 2012;44:637–45. [PMC free article] [PubMed] [Google Scholar]
- 11.Biggs MA, Harper CC, Malvin J, Brindis CD. Factors influencing the provision of long-acting reversible contraception in California. Obstetrics and gynecology 2014;123:593–602. [DOI] [PubMed] [Google Scholar]
- 12.Stanwood NL, Garrett JM, Konrad TR. Obstetrician-gynecologists and the intrauterine device: a survey of attitudes and practice. Obstetrics and gynecology 2002;99:275–80. [DOI] [PubMed] [Google Scholar]
- 13.Luchowski AT, Anderson BL, Power ML, Raglan GB, Espey E, Schulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception 2014;89:572–7. [DOI] [PubMed] [Google Scholar]
- 14.STDs in Adolescents and Young Adults. Centers for Disease Control and Prevention, 2017. (Accessed April 26, 2018, at https://www.cdc.gov/std/stats16/adolescents.htm.)
- 15.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med 2016;374:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra-Infante F, Guzman-Rodriguez R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med 2001;345:561–7. [DOI] [PubMed] [Google Scholar]
- 17.Sufrin CB, Postlethwaite D, Armstrong MA, Merchant M, Wendt JM, Steinauer JE. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstetrics and gynecology 2012;120:1314–21. [DOI] [PubMed] [Google Scholar]
- 18.Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet (London, England) 1992;339:785–8. [DOI] [PubMed] [Google Scholar]
- 19.Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement. American journal of obstetrics and gynecology 2016;215:599.e1–.e6. [DOI] [PubMed] [Google Scholar]
- 20.Sufrin CB, Averbach SH. Testing for sexually transmitted infections at intrauterine device insertion: an evidence-based approach. Clinical obstetrics and gynecology 2014;57:682–93. [DOI] [PubMed] [Google Scholar]
- 21.Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Positive Testing for Neisseria gonorrhoeae and Chlamydia trachomatis and the Risk of Pelvic Inflammatory Disease in IUD Users. J Womens Health (Larchmt) 2015;24:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections. Contraception 2016;94:701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Division of Reproductive Health. U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013;62:1–60. [PubMed] [Google Scholar]
- 24.Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep 2016;65:1–103. [DOI] [PubMed] [Google Scholar]
- 25.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 26.McNicholas CP, Klugman JB, Zhao Q, Peipert JF. Condom use and incident sexually transmitted infection after initiation of long-acting reversible contraception. American journal of obstetrics and gynecology 2017;217:672.e1–.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Ayadi AM, Rocca CH, Kohn JE, et al. The impact of an IUD and implant intervention on dual method use among young women: Results from a cluster randomized trial. Prev Med 2017;94:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rattray C, Wiener J, Legardy-Williams J, et al. Effects of initiating a contraceptive implant on subsequent condom use: A randomized controlled trial. Contraception 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner RJ, Liddon N, Swartzendruber AL, Rasberry CN, Sales JM. Long-Acting Reversible Contraception and Condom Use Among Female US High School Students: Implications for Sexually Transmitted Infection Prevention. JAMA Pediatr 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbs SE, Rocca CH, Bednarek P, Thompson KMJ, Darney PD, Harper CC. Long-Acting Reversible Contraception Counseling and Use for Older Adolescents and Nulliparous Women. J Adolesc Health 2016;59:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soper DE. Pelvic inflammatory disease. Obstetrics and gynecology 2010;116:419–28. [DOI] [PubMed] [Google Scholar]
- 32.Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010;59:1–110. [PubMed] [Google Scholar]
- 33.Mendoza RM, Garbers S, Lin S, Stockwell MS, Warren M, Gold MA. Chlamydia Infection Among Adolescent Long-Acting Reversible Contraceptive and Shorter-Acting Hormonal Contraceptive Users Receiving Services at New York City School-Based Health Centers. LID - S1083–3188(19)30291–8 [pii] LID - 10.1016/j.jpag.2019.09.006 [doi]. [DOI] [PubMed] [Google Scholar]
- 34.Petrova D, Garcia-Retamero R. Effective Evidence-Based Programs For Preventing Sexually-Transmitted Infections: A Meta-Analysis. Curr HIV Res 2015;13:432–8. [DOI] [PubMed] [Google Scholar]
- 35.Callegari LS, Darney BG, Godfrey EM, Sementi O, Dunsmoor-Su R, Prager SW. Evidence-based selection of candidates for the levonorgestrel intrauterine device (IUD). J Am Board Fam Med 2014;27:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tepper NK, Steenland MW, Marchbanks PA, Curtis KM. Laboratory screening prior to initiating contraception: a systematic review. Contraception 2013;87:645–9. [DOI] [PubMed] [Google Scholar]
- 37.Morgan IA, Zapata LB, Curtis KM, Whiteman MK. Health Care Provider Attitudes about the Safety of “Quick Start” Initiation of Long-Acting Reversible Contraception for Adolescents. J Pediatr Adolesc Gynecol 2019;32:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dehlendorf C, Krajewski C, Borrero S. Contraceptive counseling: best practices to ensure quality communication and enable effective contraceptive use. Clinical obstetrics and gynecology 2014;57:659–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez LM, Grey TW, Tolley EE, Chen M. Brief educational strategies for improving contraception use in young people. Cochrane Database Syst Rev 2016;3:Cd012025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner RJ, Pazol K, Swartzendruber A, et al. Use of Long-Acting Reversible Contraception Among Adolescent and Young Adult Women and Receipt of Sexually Transmitted Infection/Human Immunodeficiency Virus-Related Services. J Adolesc Health 2018;62:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubacher D, Grimes DA, Gemzell-Danielsson K. Pitfalls of research linking the intrauterine device to pelvic inflammatory disease. Obstetrics and gynecology 2013;121:1091–8. [DOI] [PubMed] [Google Scholar]
- 42.Frisse AC, Marrazzo JM, Tutlam NT, et al. Validity of self-reported history of Chlamydia trachomatis infection. American journal of obstetrics and gynecology 2017;216:393.e1–.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumi J, Wittes JT. Through the looking glass: understanding non-inferiority. Trials 2011;12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


