Abstract
Background
Recent experimental work has shown that phthalates may increase inflammation. Prior research has not examined the role of exposure to phthalates in relation to inflammatory status among postmenopausal women who are at higher risk of developing inflammation-related chronic disorders.
Objectives
We aimed to examine the associations of urinary phthalate biomarker concentrations with circulating levels of c-reactive protein [CRP] and interleukin-6 [IL-6] among 443 postmenopausal women selected into a breast cancer case-control study nested within the Women’s Health Initiative (WHI).
Methods
A total of 13 phthalate metabolites were measured in urine samples provided at WHI enrollment from 1993–1998. We also measured baseline levels of CRP and IL-6 in these women’s serum or plasma samples. Multivariable linear models were used to investigate the role of each phthalate biomarker in relation to CRP and IL-6, adjusting for potential confounding factors and specifically evaluating the role of BMI.
Results
In adjusted models we observed positive associations of monocarboxynonyl phthalate (MCNP) with CRP (β= 0.092; 95% CI 0.026, 0.158) and IL-6 (β = 0.108; 95% CI 0.013, 0.204). These positive associations were attenuated and non-significant, however, after further adjustment for body mass index (BMI). In contrast, we observed inverse associations of monoethyl phthalate (MEP) (β = −0.019; 95% CI −0.036, −0.001) and monobenzyl phthalate (MBzP) (b = −0.034; 95% CI −0.058, −0.010) with CRP levels only after adjustment for BMI. Other phthalate biomarkers examined were not significantly associated with either CRP or IL-6 levels.
Conclusions
Overall, these results do not suggest an important role for phthalates in promoting an inflammatory response. Future prospective studies are warranted to improve understanding of these associations, particularly in clarifying the role of BMI.
Keywords: Phthalates, Biomarkers, Epidemiology, Postmenopausal, Inflammation, CRP, IL-6
Introduction
Mounting scientific evidence suggests potentially harmful relationships between phthalate exposure and inflammation. Phthalates are man-made chemicals that are added to plastics and can be found in many everyday household, personal care, medical, and child products.(1) Detectable concentrations of urinary phthalate biomarkers are present among the vast majority of the U.S. population and indicate wide variation in exposure.(2) Acute inflammation is a normal response to a novel foreign substance in the body.(3) However, chronic, systemic inflammation also can occur and is associated with rheumatoid arthritis,(4) Alzheimer’s disease,(5) diabetes,(5,6) cancer,(5–7) cardiovascular disease,(6,8) and osteoporosis.(9) These conditions are prevalent among postmenopausal women, thus highlighting the importance of understanding inflammation and their sequelae in this population
Animal and cell-based studies report increases in the production of pro-inflammatory cytokines in response to phthalate exposures.(10–17) Epidemiological studies also support positive associations between urinary phthalate metabolite concentrations and allergic conditions associated with heightened immune response, including asthma, rhinitis, and eczema (17). The inflammatory effects of phthalates may be mediated through actions of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). Phthalate exposure may activate NF-kB, which signals the production of pro-inflammatory cytokines, such as interleukin-6 (IL-6).(13) NF-kB also is shown to stimulate production of c-reactive protein (CRP), a marker of systemic inflammation, in a process mediated via IL-6 and interleukin 1 beta (IL-1β).(18) Additionally, phthalates may trigger inflammatory responses through their interactions with the estrogen receptor (19) or PPARs, (20–21) as well as their induction of oxidative stress. (22–23)
Epidemiological studies in young adult populations, largely within pregnancy cohorts, indicate positive associations between some, but not all, phthalate biomarkers and CRP and/or IL-6.(24–26) Furthermore, the direction of these associations is inconsistent across studies. To our knowledge, prior studies have not examined relationships between urinary phthalate biomarker concentrations and circulating levels of inflammatory biomarkers in postmenopausal women. Postmenopausal women are of particular interest because they are both highly exposed to phthalates, with creatinine-adjusted urinary biomarker concentrations exceeding those among similarly aged males,(1) and also at potentially high risk for negative health consequences if phthalate exposure increases inflammation. Our study is the first to examine the relationship between phthalate exposure and inflammation in postmenopausal women. We investigated the associations of thirteen urinary phthalate biomarkers with two circulating inflammatory biomarkers (CRP, IL-6) using baseline data from a subset of Women’s Health Initiative (WHI) participants.
Materials and Methods
Study Population
WHI is a large-scale national study of postmenopausal women.(27) WHI conducted three clinical trials (CT) as well as a separate observational study (OS), enrolling a total of 161,808 postmenopausal women 50–79 years old at WHI baseline.(28,29) After the clinical trials ended in 2005, consenting CT and OS participants have continued to provide follow-up data. Study investigators also conducted a bone density study at three clinical sites, which included 11,020 participants enrolled in the CT or OS and from whom a first morning void spot urine sample was collected. Our nested case-control study selected invasive breast cancer cases (N=419) and 2:1 matched controls (N=838) from among WHI bone density sub-study participants.(28,30)
We included participants in the nested case-control study with baseline measures of 1) urinary phthalate biomarker data, and 2) circulating CRP or IL-6 . Participants were excluded if they were missing baseline covariate information. Inflammatory biomarker data for our analyses were obtained from multiple, separate, WHI ancillary studies of selected participants; data generated from these studies were returned to the WHI and incorporated into its data resource. Thus, our analysis includes 443 women (414 with CRP and 163 with IL-6) selected from our nested case-control study (Figure 1).
Figure 1.
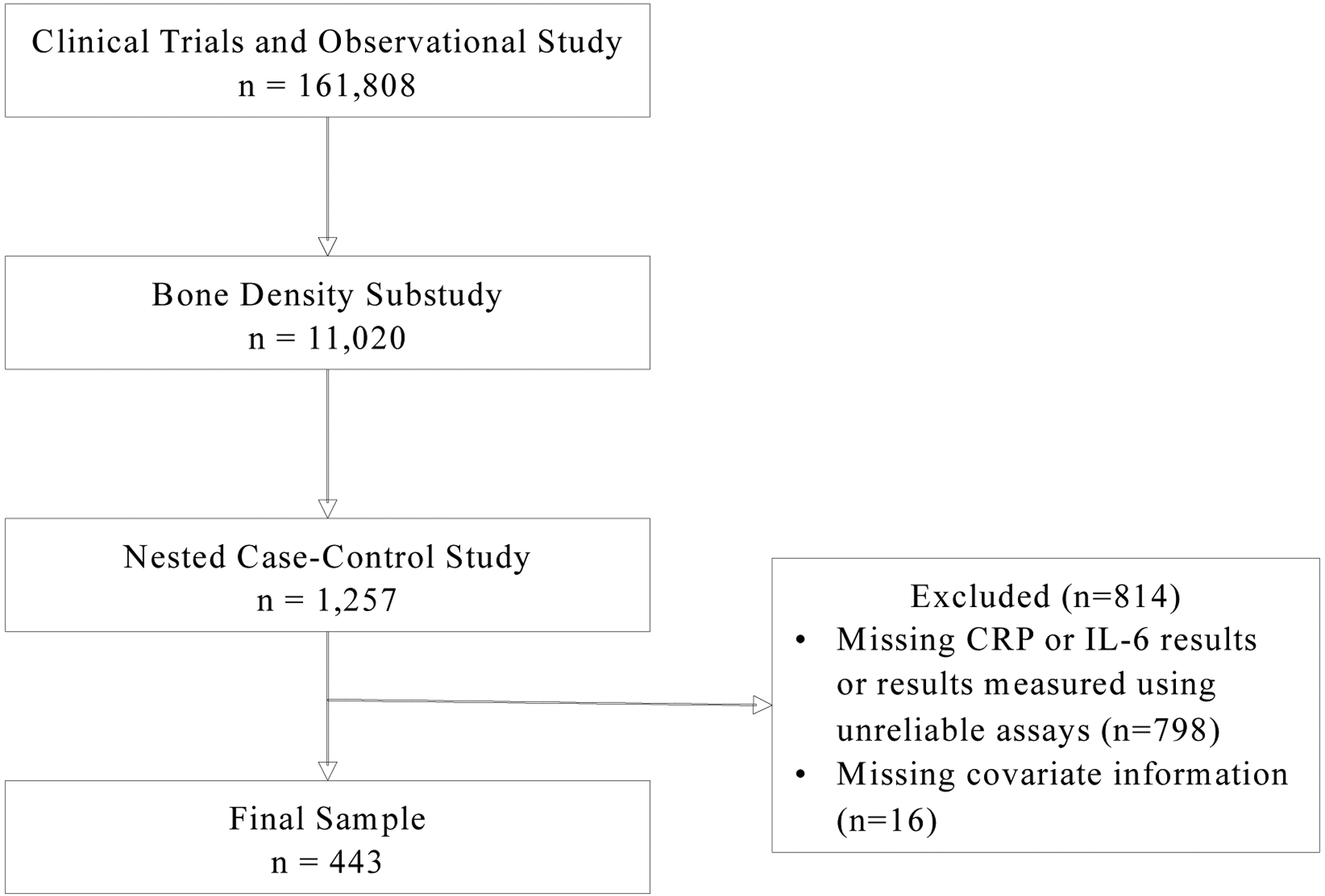
Study population ascertainment for the analysis of phthalate exposure and inflammation biomarker levels in postmenopausal women
Written informed consent was provided by participants upon WHI enrollment.(31) Institutional review boards (IRB) at each clinical site approved the WHI, and our secondary analysis was approved by the University of Massachusetts Amherst IRB. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory in the analysis of samples did not constitute engagement in human subject research.
Measurement of urinary phthalate metabolites
Urinary phthalate metabolites were measured as a biomarker of phthalate exposure. Participants collected first morning void urinary samples at home and refrigerated them until their baseline visit scheduled for later that day. During the clinic visit, samples were acquired, processed, and frozen in polypropylene cryovials by trained personnel until they were ready for shipment to McKesson Bioservices (now Fisher Bioservices, Rockville, MD), where they were stored at −80°C until transferred to research laboratories for biomarker measurements. Participant samples used for the WHI nested-case control study were sent from Fisher BioServices to the CDC for processing and analysis. Urine samples were analyzed for thirteen phthalate metabolites (monoethyl phthalate [MEP], monobutyl phthalate [MBP], mono-hydroxybutyl phthalate [MHBP], monoisobutyl phthalate [MiBP], mono-hydroxyisobutyl phthalate [MHiBP], monobenzyl phthalate [MBzP], monocarboxypropyl phthalate [MCPP], mono (2-ethylhexyl) phthalate [MEHP], mono (2-ethyl-5-hydroxyhexyl) phthalate [MEHHP], mono (2-ethyl-5-oxohexyl) phthalate [MEOHP], mono (2-ethyl-5-carboxypentyl) phthalate [MECPP], monocarboxyoctyl phthalate [MCOP], and monocarboxynonyl phthalate [MCNP]) using enzymatic deconjugation of the glucuronidated analytes, followed by assessment of exposure levels using on-line solid phase extraction and high performance liquid chromatography-electrospray ionization-tandem mass spectrometry. The laboratory was masked to disease status and participant identity. Average of within-batch CVs were 5.4% for MBP, 6.1% for MBzP, 4.7% for MCNP, 6.3% for MCOP, 5.8% for MCPP, 4.3% for MECPP, 5.4% for MEHHP, 19.5% for MEHP, 6.0% for MEOHP, 3.1% for MEP, 9.0% for MHBP, 21.9% for MHiBP, and 10.3% for MiBP.(28) Phthalate metabolite concentrations below the limit of detection (LOD) were given a value equal to the limit of detection (LOD) / √2.(28) Only five phthalate metabolites had samples whose concentrations were below the LOD (MBP = 0.07%, MEHP = 0.63%, MHBP = 0.43%, MHiBP = 1.56%, MiBP = 0.46%).(28) For metabolites of the same parent compound, we created a summary biomarker by dividing each metabolite of a single parent by its molecular weight and then summing across metabolites.(28,32) Sum of di-n-butyl phthalate (ΣDBP) was calculated as the molar sum of MBP and MHBP, sum of di-isobutyl phthalate (ΣDiBP) was calculated as the molar sum of MiBP and MHiBP, and sum of di(2-ethylhexyl) phthalate (ΣDEHP) was calculated as the molar sum of MEHP, MEHHP, MEOHP, and MECPP.(28)
Measurement of inflammatory biomarkers
Fasting blood samples were collected at baseline. Participants were asked to refrain from smoking, taking aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), or partaking in strenuous physical activity for 48 hours prior to their visit. To separate plasma (ethylenediaminetetraacetic acid [EDTA] or citrate) and serum from the blood, serum samples were left to clot at 4°C for 1 hr. and all samples were centrifuged for 10 min. Separated serum and plasma were aliquoted and frozen at −70°C for 2 hours prior to shipping to McKesson Bioservices for storage at −80°C until transfer to research laboratories for biomarker measurements.
Source (“raw”) CRP and IL-6 outcome data were from nine and six different research laboratories, respectively, although assay methods were often similar across laboratories. Details on the laboratories, original studies, assay methods, specimen type, and CV as well as the number of observations included in the present analysis along with the distribution of the measured CRP or IL-6 values are provided in Supplemental Tables 1 and 2. Average intra-assay CVs ranged from 1.9% to 9.2% for assays measuring CRP,(33) and 4% to 23% for assays measuring IL-6.(33)
Inflammatory biomarker outcome harmonization
Initially, we explored whether the measured values of CRP or IL-6 varied by specimen type or the laboratory in which the assays were performed. We fit linear regression models with the biomarker as the dependent variable and the specimen type (i.e. serum, EDTA plasma, or citrate plasma), laboratory, and days between sample collection and biomarker measurement as independent variables (Supplemental Table 3). These analyses suggested variation in CRP associated with days between sample collection and biomarker measurement and variation in IL-6 associated with the laboratory.
We harmonized the measured biomarker values by fitting separate multiple predictor linear regression models of the natural log transformed CRP and IL-6 values. These models included age, smoking status, BMI, use of anti-inflammatory medications within 48 hours prior to blood draw, laboratory, and storage time (i.e. the time between blood draw and assay) as independent variables. The predicted values of ln-CRP and ln-IL-6 generated by these models then were used as the dependent variables in our regression models to evaluate associations between phthalate biomarkers and inflammatory biomarkers, as described below. When a participant had more than one measurement of CRP or IL-6 available from separate ancillary studies, we preferentially selected CRP measures made in serum and IL-6 measures made in EDTA plasma, given that these were most frequent specimen types used. If a participant had multiple values available for a single biomarker and specimen type, we randomly selected one for inclusion in these analyses.
Assessment of covariates
Sociodemographic and behavioral characteristics, as well as medical history were obtained from self-report using a questionnaire. Physical measurements (e.g. height, weight) were conducted by trained staff and participants’ current medications were recorded by a research nurse. Information on all potential covariates were ascertained at baseline. We included age,(24,34,35) urine creatinine,(24) race/ethnicity,(24,26) neighborhood socioeconomic status (SES) index,(35) smoking status,(35) alcohol intake,(35) physical activity, (28,36) and BMI(24–26,35) as potential covariates based on prior epidemiological studies. Neighborhood SES index is a composite measure calculated using the following variables: 1) percentage of adults older than 25 with less than a high school education, 2) percentage of male unemployment, 3) percentage of households with income below the poverty line, 4) percentage of households receiving public assistance, 5) percentage of households with children headed only by a female, and 6) median household income.(37)
Statistical analysis
In two sets of main analyses, we performed multiple variable linear regression to estimate the associations of the dependent variable defined as circulating CRP (n=414) and IL-6 (n=163), respectively, in relationship to phthalate exposures, crudely and after adjustment for selected covariates. Phthalate biomarkers (i.e. individual metabolites or their molar sums) and inflammation biomarkers were natural log transformed to improve normality; geometric means were calculated on phthalate biomarkers standardized by creatinine to account for differences in urinary dilution. In preliminary analyses, we compared our CRP outcome (n=414) and IL-6 outcome (n=163) samples with each other and with participants in the bone mineral density sub-study (n=11,020). Descriptive statistics and independent group’s hypothesis tests (chi square or T tests) were calculated as appropriate. In our main analyses, for each dependent variable and each primary phthalate predictor, we estimated two multiple variable predictor models: “Model 1” and “Model 1 + BMI”. If BMI lies on the causal pathway, then adjustment for BMI in our analysis of phthalate biomarkers and inflammatory biomarkers would be inappropriate. Therefore, we decided a priori to adjust for a set of potential confounders and then to separately examine the impact of further adjustment for BMI. “Model 1” investigated the association of the dependent variable with the phthalate predictor of interest adjusted for selected confounding. “Model 1 + BMI” was fit to investigate the hypothesized role BMI as confounder of a priori interest. In selecting covariates for inclusion in “Model 1”, we included variables that produced ≥10% change in the estimated beta for the phthalate predictors. This yielded as control variables: age, urinary creatinine, neighborhood SES index, alcohol intake, and smoking status. As this was a hypothesis generating analysis, we did not adjust for multiple comparisons. As a sensitivity analysis, we repeated our multivariable linear regression analyses using participants who were not habitual non-steroidal anti-inflammatory drug (NSAID) users (i.e. salicylates [and salicylate combinations], cyclooxygenase 2 [COX-2] inhibitors, phenylbutazones, NSAIA combinations, HMG-COA Reductase Inhibitors [and HMG-COA Reductase Inhibitor combinations]). Because CVs for some IL-6 assays were high and thus may have generated unreliable results, we repeated analyses for IL-6 including data from assays with CVs ≤15% (N=91). All analyses were performed in SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Bone density sub-study participants with available CRP and/or IL-6 values were generally similar to the overall bone density sub-study population (Table 1). Bone density sub-study participants with CRP values were more likely to be non-white and have a lower educational level than those without CRP values. Similarly, bone density sub-study participants with IL-6 values were more likely to be have a lower education level than those without IL-6 values. The distributions of phthalate biomarkers, CRP, and IL-6 are presented in Table 2 and indicate variability in these measurements within our population.
Table 1.
Distribution of baseline sociodemographic and behavioral characteristics in our sub-sample of Women’s Health Initiative (WHI) participants compared to the bone mineral density (BMD) sub-study from which they were selected
| CRP sample (n=414) | p-valuea | IL-6 sample (n=163) | p-valuea | BMD sample (n=11,020) | |
|---|---|---|---|---|---|
| Age, years; Mean (SD) | 63.1 (7.0) | 0.98 | 63.5 (6.5) | 0.51 | 63.2 (7.4) |
| White; N (%) | 247 (59.7) | <.0001 | 119 (73.0) | 0.25 | 8,454 (76.8) |
| Education level; N (%) | <.0001 | <.0001 | |||
| Less than high school degree | 121 (29.5) | 44 (27.7) | 993 (9.1) | ||
| Post high school/some college | 155 (37.8) | 57 (35.9) | 6,613 (60.4) | ||
| College degree or higher | 134 (32.7) | 58 (36.5) | 3,342 (30.5) | ||
| Annual Income <$35,000; N (%) | 214 (54.6) | 0.63 | 80 (52.3) | 0.38 | 5,704 (55.8) |
| Alcohol intake; N(%) | 0.78 | 0.54 | |||
| 0 drinks per week | 164 (39.6) | 57 (35.0) | 4,276 (39.2) | ||
| <1 drink per week | 141 (34.1) | 61 (37.4) | 3,538 (32.4) | ||
| 1–6 drinks per week | 76 (18.4) | 31 (19.0) | 2,215 (20.3) | ||
| 7+ drinks per week | 33 (8.0) | 14 (8.6) | 889 (8.1) | ||
| Smoking status; N (%) | 0.32 | 0.27 | |||
| Never smoked | 241 (58.2) | 83 (50.9) | 5,932 (54.6) | ||
| Past smoker | 144 (34.8) | 70 (42.9) | 4,047 (37.2) | ||
| Current smoker | 29 (7.0) | 10 (6.1) | 888 (8.2) | ||
| Body mass index, kg/m2; Mean (SD) | 28.7 (6.0) | 0.14 | 28.4 (5.5) | 0.76 | 28.3 (6.0) |
| Physical activity level, MET hrs/week; Mean (SD) | 11.5 (13.5) | 0.97 | 12.1 (13.6) | 0.53 | 11.4 (13.8) |
| Current NSAID use; N(%) | 173 (41.2) | 0.94 | 75 (46.0) | 0.30 | 4,626 (42.0) |
Abbreviations used: NSAID, nonsteroidal anti-inflammatory drug; SES, socioeconomic; MET, metabolic equivalent; BMD, bone mineral density
p-values are for the comparison of CRP and IL-6 samples to the BMD sample
Table 2.
Distribution of baseline creatinine-standardized urinary phthalate biomarker concentrations and circulating inflammatory biomarker levels in the Women’s Health Initiative (WHI) Study (n=443)a,b
| Biomarkersc | Mean | SD | Min | 25th percentile | 75th percentile | Max |
|---|---|---|---|---|---|---|
| DEHP, umol/g creatinine | 0.0033 | 0.0050 | 0.0002 | 0.0016 | 0.0038 | 0.083 |
| DBP, umol/g creatinine | 0.0025 | 0.0029 | 0.0001 | 0.0010 | 0.0031 | 0.036 |
| DiBP, umol/g creatinine | 0.0003 | 0.0004 | 0.00003 | 0.0001 | 0.0003 | 0.0046 |
| MEP, ug/g creatinine | 3.76 | 12.57 | 0.088 | 0.54 | 2.59 | 130.05 |
| MBzP, ug/g creatinine | 0.23 | 0.24 | 0.013 | 0.099 | 0.27 | 2.15 |
| MCPP, ug/g creatinine | 0.061 | 0.088 | 0.0072 | 0.028 | 0.062 | 1.15 |
| MCOP, ug/g creatinine | 0.084 | 0.19 | 0.0090 | 0.032 | 0.077 | 2.56 |
| MCNP, ug/g creatinine | 0.059 | 0.14 | 0.0067 | 0.023 | 0.056 | 2.37 |
| CRP, mg/Ld | 2.88 | 1.81 | 0.46 | 2.02 | 4.04 | 27.03 |
| IL-6, pg/mLe | 2.08 | 1.63 | 0.92 | 1.51 | 2.43 | 13.02 |
Statistics for phthalate biomarkers were calculated on natural log-transformed values and were back-transformed to original scale for presentation
Statistics presented for inflammatory biomarkers are based on the harmonized data
ΣDBP was calculated as the molar sum of MBP and MHBP; ΣDiBP was calculated as the molar sum of MiBP and MHiBP; ΣDEHP was calculated as the molar sum of MEHP, MEHHP, MEOHP, and MECPP
n=414
n=163
Abbreviations used: MEP, mono-ethyl phthalate; MBzP, monobenzyl phthalate; MCOP, monocarboxyoctyl phthalate; MCNP, monocarboxynonyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; DBP, di-n-butyl phthalate; DiBP, di-isobutyl phthalate; DEHP, di(2-ethylhexyl) phthalate; CRP, C-reactive protein; IL-6, interleukin-6
Most phthalate biomarkers were not statistically significantly associated with circulating CRP or IL-6 levels after adjustment for covariates (Table 3). However, we did observe statistically significant, positive associations between MCNP and CRP (β = 0.092; 95% CI 0.026, 0.158) and IL-6 (β = 0.0092; 95% CI −.0003, 0.186) in Model 1; these associations were attenuated and non-significant with additional adjustment for BMI. A borderline significant association between MCOP and CRP was observed in Model 1 (β = 0.063; 95% CI −0.003, 0.129); this also was attenuated and non-significant with additional adjustment for BMI. We observed a statistically significant, negative association between MEP and CRP (b = −0.019; 95% CI −0.036, −0.001) as well as between MBzP and CRP (b = −0.034; 95% CI −0.058, −0.010) only in models adjusted for BMI. We observed similar results when repeating analyses among participants who did not habitually use NSAIDs (Supplemental Table 6), except for a borderline significant positive association between DiBP and IL-6, and when restricting analyses to IL-6 results from assays with CVs less than 15% (Supplemental Table 7).
Table 3.
Multivariable-adjusted estimated associations between urinary phthalate biomarker concentrations and circulating inflammatory biomarkers
| CRP (n=414) | IL-6 (n=163) | |||||||
|---|---|---|---|---|---|---|---|---|
| Multivariable adjusted (Model 1)a | Model 1 + BMI | Multivariable adjusted (Model 1)a | Model 1 + BMI | |||||
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| DEHPb | 0.036 (−0.030, 0.102) | 0.29 | −0.010 (−0.038, 0.017) | 0.45 | 0.022 (−0.070, 0.113) | 0.64 | −0.020 (−0.100, 0.059) | 0.61 |
| DBPb | −0.001 (−0.057, 0.056) | 0.98 | −0.020 (−0.043, 0.003) | 0.09 | 0.007 (−0.081, 0.095) | 0.87 | 0.003 (−0.072, 0.079) | 0.93 |
| DiBPb | 0.012 (−0.047, 0.070) | 0.70 | −0.020 (−0.045, 0.004) | 0.10 | 0.064 (−0.024, 0.152) | 0.15 | 0.043 (−0.033, 0.119) | 0.26 |
| MEP | −0.009 (−0.052, 0.034) | 0.68 | −0.019 (−0.036, −0.001) | 0.04 | −0.027 (−0.084, 0.030) | 0.35 | −0.023 (−0.072, 0.025) | 0.34 |
| MBzP | 0.026 (−0.033, 0.084) | 0.39 | −0.034 (−0.058, −0.010) | 0.01 | −0.022 (−0.110, 0.065) | 0.62 | −0.044 (−0.119, 0.031) | 0.25 |
| MCPP | 0.020 (−0.047, 0.088) | 0.55 | −0.005 (−0.033, 0.022) | 0.70 | −0.036 (−0.139, 0.067) | 0.49 | −0.045 (−0.133, 0.044) | 0.32 |
| MCOP | 0.063 (−0.003, 0.129) | 0.06 | 0.017 (−0.010, 0.045) | 0.21 | 0.015 (−0.073, 0.103) | 0.73 | −0.042 (−0.119, 0.034) | 0.27 |
| MCNP | 0.092 (0.026, 0.158) | 0.01 | 0.021 (−0.006, 0.049) | 0.13 | 0.092 (−0.003, 0.186) | 0.06 | 0.032 (−0.051, 0.116) | 0.45 |
Adjusted for age, creatinine, alcohol intake, neighborhood socioeconomic status index, and smoking status;
ΣDBP was calculated as the molar sum of MBP and MHBP; ΣDiBP was calculated as the molar sum of MiBP and MHiBP; ΣDEHP was calculated as the molar sum of MEHP, MEHHP, MEOHP, and MECPP
Abbreviations used: MEP, mono-ethyl phthalate; MBzP, monobenzyl phthalate; MCOP, monocarboxyoctyl phthalate; MCNP, monocarboxynonyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; DBP, di-n-butyl phthalate; DiBP, di-isobutyl phthalate; DEHP, di(2-ethylhexyl) phthalate; CRP, C-reactive protein; IL-6, interleukin-6
Discussion
Overall, we did not observe strong associations between urinary phthalate biomarkers and CRP or IL-6 in our sample of postmenopausal women from WHI. MCNP was significantly, positively associated with CRP and IL-6, with each 10% increase in urinary MCNP associated with a 0.9% higher CRP or IL-6; however, these associations were attenuated after adjustment for BMI. These findings differ from those of Ferguson et al (2014) in a population of pregnant women, who reported higher IL-6 levels (%Δ = 16.8, 95% CI: 2.69, 32.9, p=0.02) and CRP levels (%Δ = 10.1, 95% CI: −0.86, 22.2, p=0.08) associated with an increase of 2.4 ng/mL in MCNP, while adjusting for BMI.(25) We found statistically significant, negative associations of MEP and MBzP with CRP only after adjustment for BMI. In a study of participants from the National Health and Nutrition Examination Survey (NHANES),(24) Ferguson et al (2011) also reported negative associations between MEP and CRP (β per 1 mg/L increase in MEP = −0.020; 95% CI −0.040, 0.0003, p=0.05), yet a positive association between MBzP and CRP. The substantial differences in study population across these studies and ours (i.e. pregnant women, randomly selected healthy adults, and postmenopausal woman) likely affect the underlying presence of inflammation, and possibly biasing results toward null associations in populations with elevated inflammation due to pregnancy or aging. Additional research will be helpful in determining whether true direction of association as well as the biological mechanisms underlying such associations.
The parent phthalate of MCNP is used as a component of food packaging materials.(38,39) Similarly, MEP is a metabolite of diethyl phthalate and MBzP is a metabolite of benzylbutyl phthalate (BBP), which is often used to add flexibility to food packaging.(40–42) As such, confounding by BMI is possible, given that packaged and processed foods are associated with obesity, which is itself positively associated with inflammation.(6) Alternatively, there is growing evidence that phthalates directly affect weight gain,(43,44) and thus obesity may be a step on the causal pathway linking phthalates to inflammation. We observed that associations between MCNP and inflammatory markers were attenuated when adjusting for BMI, indicating that at least some of the association between phthalate exposure and inflammation may be through a pathway that includes elevated BMI. Future prospective studies will be useful in clarifying these mechanisms.
We observed null findings across all other phthalate biomarkers, which differs from prior epidemiological studies reporting statistically significant associations between MiBP, MEHHP and CRP levels,(24) as well as between MCPP and IL-6 levels.(26) Our analysis included only a single measurement of each urinary phthalate biomarker, while other studies incorporated up to 4 repeated measurements. Because phthalates are quickly metabolized, with a half-life of 3 to 18 hours,(45) a single urine sample may not accurately reflect participants’ long-term exposure. This high within-person variability leads to non-differential misclassification, and therefore an attenuation of associations.
Our results must be interpreted in the context of additional limitations, notably our reliance on inflammatory biomarker levels that were derived from multiple WHI ancillary studies. It is possible that by design our sample population is not representative of the WHI study population or the general population. Although we used baseline measurements in which participants were free of disease, incidence of future disease is likely to be higher in our subsample than in the WHI study population due to the sampling criteria of the ancillary studies. Compared to the WHI bone density sub-study from which our participants were selected, at enrollment our sample differed significantly by race/ethnicity and education level but no other characteristics. The small number of participants in racial/ethnic subgroups prohibits within-subgroup analyses, although our results should be broadly generalizable to the population of U.S. women. Further, although the biological mechanism between phthalate exposure and inflammation is not established, we do not expect that mechanisms linking phthalate exposure to inflammation would vary by age, race/ethnicity, or SES. Thus, our results are likely to be externally valid. While not statistically significant, we also observed variation in inflammatory biomarker levels by specimen type, lab, assay method, and storage time. Importantly, some assays for IL-6 reported high CVs, which may have introduced measurement error; however, our results were similar in analyses restricted to IL-6 measurements derived from assays with CV<15%. We used regression modeling to harmonize assay measurements across studies, although, it is possible that some measurement error remains among our predicted CRP and IL-6 values. Lastly, our sample size was moderate, especially for the IL-6 analyses.
There are several strengths to our analysis. First, this is the only study found in published literature to assess associations between phthalate biomarkers and inflammatory markers among postmenopausal women. As postmenopausal women are at higher risk for developing inflammation-related chronic conditions (e.g. diabetes, rheumatoid arthritis, etc.), women in this age group could be especially at risk for inflammatory effects triggered by phthalate exposure, if any. Second, we were able to adjust for a large number of confounders in our analyses, although residual confounding is a potential concern in any study. Also, we measured phthalate biomarker data using a validated assay at a highly experienced laboratory.
5. Conclusion
Overall, our study does not suggest an important role for phthalates in promoting an inflammatory response. We did observe some statistically significant results that merit further study along with careful consideration of the role of obesity. Importantly, these results should be interpreted in light of the limitations noted, including lack of temporality and potential effects from combining measurements of CRP and IL-6 made from different laboratories. Further work in large, prospective studies with multiple assessments of phthalate biomarkers, adiposity and inflammatory phenotypes will be helpful for understanding whether phthalate exposure could affect inflammation in older women.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the contributions of the following institutions and individuals:
WHI Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis
CDC: Xiaoyun Ye, Manori Silva, Ella Samandar, Jim Preau, Tao Jia
Funding:
This work was supported by the National Institute of Environmental Health Sciences (R01ES024731). The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Competing Financial Interests
The authors declare they have no actual or potential competing financial interests.
Research Approval
Written informed consent was provided by participants upon WHI enrollment. Institutional review boards at each clinical site approved the WHI, and this particular analysis was approved by the University of Massachusetts Amherst IRB. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory in the analysis of samples did not constitute engagement in human subjects research.
References
- 1.Department of Health and Human Services, Center for Disease Control and Prevention. Fourth National Report National Report on Human Exposure to Environmental Chemicals. 2009.
- 2.Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental Health Perspectives. 2004;112:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. British Journal of Nutrition. 2015;114:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Rheumatoid Arthritis (RA) [Internet]. [cited 2019 Oct 1]. Available from: https://www.cdc.gov/arthritis/basics/rheumatoid-arthritis.html
- 5.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Research Reviews. 2011;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity & inflammation: The linking mechanism & the complications. Archives of Medical Science. 2017;13:851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crusz SM, Balkwill FR. Inflammation and cancer: Advances and new agents. Nature Reviews Clinical Oncology. 2015;12:584–96. [DOI] [PubMed] [Google Scholar]
- 8.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II-2–II10. [DOI] [PubMed] [Google Scholar]
- 9.Ginaldi L, Mengoli LP, De Martinis M. Osteoporosis, inflammation and ageing. Handbook on Immunosenescence: Basic Understanding and Clinical Applications. 2009;2:1–5. [Google Scholar]
- 10.Zhou L, Chen H, Xu Q, Han X, Zhao Y, Song X, et al. The effect of di-2-ethylhexyl phthalate on inflammation and lipid metabolic disorder in rats. Ecotoxicology and Environmental Safety. 2019;170:391–8. [DOI] [PubMed] [Google Scholar]
- 11.Zheng SJ, Tian HJ, Cao J, Gao YQ. Exposure to di(n-butyl)phthalate and benzo(a)pyrene alters IL-1β secretion and subset expression of testicular macrophages, resulting in decreased testosterone production in rats. Toxicology and Applied Pharmacology. 2010;248:28–37. [DOI] [PubMed] [Google Scholar]
- 12.Jepsen KF, Abildtrup A, Larsen ST. Monophthalates promote IL-6 and IL-8 production in the human epithelial cell line A549. Toxicology in Vitro. 2004;18:265–9. [DOI] [PubMed] [Google Scholar]
- 13.Nishioka J, Iwahara C, Kawasaki M, Yoshizaki F, Nakayama H, Takamori K, et al. Di-(2-ethylhexyl) phthalate induces production of inflammatory molecules in human macrophages. Inflammation Research. 2012;61:69–78. [DOI] [PubMed] [Google Scholar]
- 14.Rael LT, Bar-Or R, Ambruso DR, Mains CW, Slone DS, Craun ML, et al. Phthalate esters used as plasticizers in packed red blood cell storage bags may lead to progressive toxin exposure and the release of pro-inflammatory cytokines. Oxidative Medicine and Cellular Longevity. 2009;2:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman-Jensen A, Gregory C, Singh A. Household Food Security in the United States in 2017. Department of Agriculture, Economic Research Service. 2018. [Google Scholar]
- 16.Kocbach Bølling A, Ovrevik J, Samuelsen JT, Holme JA, Rakkestad KE, Mathisen GH, et al. Mono-2-ethylhexylphthalate (MEHP) induces TNF-α release and macrophage differentiation through different signalling pathways in RAW264.7 cells. Toxicology Letters. 2012;209:43–50. [DOI] [PubMed] [Google Scholar]
- 17.Bølling AK, Sripada K, Becher R, Bekö G. Phthalate exposure and allergic diseases: Review of epidemiological and experimental evidence. Environment International. 2020;139:105706. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPβ and signal transducer and activator of transcription-3. Immunology. 2003;108:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazioti M The potential role of endocrine disrupting chemicals in cellulite. Medical Hypotheses. 2018;116:132–5. [DOI] [PubMed] [Google Scholar]
- 20.Huang Q, Zhang H, Chen Y-J, Chi Y-L, Dong S. The Inflammation Response to DEHP through PPARγ in Endometrial Cells. Int J Environ Res Public Health [Internet]. 2016. [cited 2021 Mar 1];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4808981/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee C-H, Aoyama T, et al. Di(2-ethylhexyl)phthalate Induces Hepatic Tumorigenesis through a Peroxisome Proliferator-activated Receptor α-independent Pathway. Journal of Occupational Health. 2007;49:172–82. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Z, Ji K, Shen X, Zhang W, Wang R, Xu W, et al. Di(2-ethylhexyl) phthalate promotes hepatic fibrosis by regulation of oxidative stress and inflammation responses in rats. Environmental Toxicology and Pharmacology. 2019;68:109–19. [DOI] [PubMed] [Google Scholar]
- 23.Duan Y, Wang L, Han L, Wang B, Sun H, Chen L, et al. Exposure to phthalates in patients with diabetes and its association with oxidative stress, adiponectin, and inflammatory cytokines. Environment International. 2017;109:53–63. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environmental Research. 2011;111:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson KK, Cantonwine DE, Rivera-González LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV., et al. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environmental Science and Technology. 2014;48:7018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson KK, McElrath TF, Mukherjee B, Loch-Caruso R, Meeker JD. Associations between maternal biomarkers of phthalate exposure and inflammation using repeated measurements across pregnancy. PLoS ONE. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Women’s Health Initiative. About WHI [Internet]. [cited 2019 Oct 10]. Available from: https://www.whi.org/about/SitePages/AboutWHI.aspx
- 28.Reeves KW, Santana MD, Manson JAE, Hankinson SE, Zoeller RT, Bigelow C, et al. Predictors of urinary phthalate biomarker concentrations in postmenopausal women. Environmental Research. 2019;169:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women’s Health Initiative recruitment methods and results. Annals of Epidemiology. 2003;13:S18–77. [DOI] [PubMed] [Google Scholar]
- 30.Reeves K, Dias S, Manson JE, Hankinson SE, Zoeller RT, Bigelow C, et al. Urinary Phthalate Biomarker Concentrations and Postmenopausal Breast Cancer Risk. Journal of the National Cancer Institute. 2019;111:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA - Journal of the American Medical Association. 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 32.Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, et al. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environmental Science and Technology. 2014;48:8881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Women’s Health Initiative. Blood and Urine Collection, Processing and Shipment. Women’s Health Initiative. 1997. [Google Scholar]
- 34.Bai PY, Wittert G, Taylor AW, Martin SA, Milne RW, Jenkins AJ, et al. The association between total phthalate concentration and non-communicable diseases and chronic inflammation in South Australian urban dwelling men. Environmental Research. 2017;158:366–72. [DOI] [PubMed] [Google Scholar]
- 35.Choi YJ, Ha KH, Kim DJ. Exposure to bisphenol A is directly associated with inflammation in healthy Korean adults. Environmental Science and Pollution Research. 2017; [DOI] [PubMed] [Google Scholar]
- 36.Hamer M, Sabia S, Batty GD, Shipley MJ, Tabák AG, Singh-Manoux A, et al. Physical activity and inflammatory markers over 10 years: Follow-up in men and women from the whitehall II cohort study. Circulation. 2012;126:928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin BA, Eibner C, Bird CE, Jewell A, Margolis K, Shih R, et al. The relationship between urban sprawl and coronary heart disease in women. Health and Place. 2013;20:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Di-isononyl Phthalate. National Biomontoring Program. 2017.
- 39.U.S. Food & Drug Administration. Code of Federal Regulations. Silver Spring; 2019. page 175. [Google Scholar]
- 40.Zhang L, Jiang DG, Sui HX, Wu PG, Liu AD, Yang DJ, et al. Dietary exposure to benzyl butyl phthalate in China. Biomedical and Environmental Sciences. 2016;29:365–73. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Biomonitoring Summary. National Biomontoring Program. 2017. [Google Scholar]
- 42.Agency for Toxic Substances and Disease Registry. Diethyl Phthalate. Toxic Substances Portal. 2011.
- 43.Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: A prospective investigation in US women. International Journal of Obesity. 2014;38:1532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Díaz Santana MV, Hankinson SE, Bigelow C, Sturgeon SR, Zoeller RT, Tinker L, et al. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: A prospective cohort study. Environmental Health: A Global Access Science Source. 2019;18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environment International. 2015;85:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


