Summary
Objective:
To evaluate factors influencing reproductive decision-making in families containing multiple individuals with epilepsy.
Methods:
One hundred forty-nine adults with epilepsy and 149 adult biological relatives without epilepsy from families containing multiple affected individuals completed a self-administered questionnaire. Participants answered questions regarding their belief in a genetic cause of epilepsy (genetic attribution) and estimated risk of epilepsy in an offspring of an affected person. Participants rated factors for their influence on their reproductive plans, with responses ranging from “much more likely” to “much less likely” to want to have a child. Those with epilepsy were asked, “Do you think you would have wanted more (or any) children if you had not had epilepsy?”
Results:
Participants with epilepsy had fewer offspring than their unaffected relatives (mean 1.2 vs. 1.9, p=0.002), and this difference persisted among persons who had been married. Estimates of risk of epilepsy in an offspring of an affected parent were higher among participants with epilepsy than among relatives without epilepsy (mean 27.2 vs. 19.6, p=0.002). Nineteen percent of participants with epilepsy responded that they would have wanted more children if they had not had epilepsy. Twenty-five percent of participants with epilepsy responded that “the chance of having a child with epilepsy” or “having epilepsy in your family” made them less likely to want to have a child. Having these genetic concerns was significantly associated with greater genetic attribution and estimated risk of epilepsy in offspring of an affected parent.
Significance:
People with epilepsy have fewer children than their biological relatives without epilepsy. Beliefs about genetic causes of epilepsy contribute to concerns and decisions to limit child-bearing. These beliefs should be addressed in genetic counseling to ensure that true risks to offspring and reproductive options are well understood.
Keywords: genetic attribution, genetic counseling, epidemiology, psychosocial
Introduction
Multiple population-based studies have found reduced birth rates among persons with epilepsy.1–4 Previous research has also found a lower likelihood of pregnancy among persons with epilepsy than among their unaffected same-sex siblings.5 This phenomenon may be attributed to biological obstacles,6–12 psychosocial factors, or a combination. Psychosocial influences include reduced marriage rates among both men and women with epilepsy5,13 and concerns that lead people with epilepsy to choose to have fewer or no children. Several studies have found that women14–17 and men17 with epilepsy report having decided to limit reproduction because they have epilepsy. Top concerns of women with epilepsy include the effects of anti-seizure medications (ASMs) and seizures on a developing fetus, reduced seizure control during pregnancy, and risk of a child developing epilepsy.14–17 In previous studies, the decision to have fewer children due to epilepsy was associated with higher levels of concern about having a child with epilepsy,16,17 and in one study, with the effect of having epilepsy on the ability to care for a child.17 Studies have also investigated perceived risk of epilepsy in the offspring of an affected parent and its relationship to reproductive decisions.16,17 One study found that compared with women who did not change their reproductive plans, those who decided to have fewer children because they had epilepsy were more likely to estimate a risk of epilepsy greater than 20% among offspring of an affected parent.16 However, another study found no significant difference in risk estimates between those who chose to have fewer children and those who did not.17
This paper expands on previous research by investigating how genetic attribution, the belief that the cause of one’s disorder is genetic (regardless of actual cause), may contribute to this complex process involved in child-bearing plans. We used data from a survey administered to members of families containing multiple individuals with epilepsy. This study sample provides unique insight because of the participants’ prior participation in genetic research and strong belief in a genetic origin of their epilepsy. Previous research within these families has shown an association of genetic attribution with the psychosocial impacts of epilepsy18,19 and highlighted the importance of reproductive concerns in considering the potential impacts of genetic testing.20
In this paper, we address the following questions:
How do participants with epilepsy differ in number of offspring compared to their unaffected biological relatives?
How do participants with epilepsy and their unaffected relatives estimate risk of epilepsy in an offspring of an affected parent?
What are the concerns of people from families with multiple affected individuals when making reproductive decisions?
How does “genetic attribution” (the participant’s perception that his or her epilepsy had a genetic cause) influence reproductive decision-making?
Methods
Study sample
The study sample comprised individuals who participated in the Epilepsy Family Study of Columbia University (EFSCU), a long-term investigation that began in the 1980s as a familial aggregation study and evolved into a genetic linkage study.21 Eligibility for the linkage study required that each family contain either a sibling pair or three or more individuals with nonacquired epilepsy. After an initial interview with a proband or family informant, we screened each family member (or parent, in the case of young children) individually for seizure occurrence and performed a complete diagnostic assessment in those who screened positive, including a semistructured diagnostic interview22 and review of medical records whenever possible. To determine which family members would be included, we extended the families into larger pedigrees using sequential ascertainment.23 This involved including all first-degree relatives of each person determined to have had epilepsy; for example, if the mother of a participant had epilepsy, we attempted to enroll her parents and siblings, and if any of her siblings was affected, we included all offspring of that sibling. We continued this process until we identified a sibship in which no one was affected. Participants were recruited from 1997–2006 through physician referrals, advertisements through the Epilepsy Foundation, and a study website.21
For the present study, participants were deemed eligible if they met the following criteria: prior participation in the EFSCU study, current age between 18–79 years, ability to complete a self-administered survey in English, and consent to be contacted for future research. Participants completed the survey between 2013 and 2015, either online (Survey Monkey, Inc., Palo Alto, California, U.S.A., www.surveymonkey.com) or on paper (Appendix S1).
The present analysis was restricted to participants with epilepsy and their biological relatives without epilepsy and excluded participants who married into these families. The Columbia University Irving Medical Center Institutional Review Board approved the study.
History of epilepsy
Participants were classified as having epilepsy based on their responses to either of two survey questions. The first question asked, “Which of your biological relatives have had epilepsy or a seizure disorder?” with “yourself” as the first in the list. The second question was, “Have you ever been told that you had epilepsy or a seizure disorder?” Self-reported data were used as opposed to diagnoses from the original genetic study because up to 20 years had elapsed since the data from the original study were collected, and some participants developed epilepsy in the interim. In addition, we reasoned that participants’ perceptions of whether they had epilepsy may be most relevant for consideration of reproductive concerns. As previously reported, self-reports of epilepsy agreed very well (kappa = 0.86) with diagnoses of epilepsy in the genetic study, which were based on comprehensive collection of clinical data.19 Among 186 participants who self-reported epilepsy in the current survey, 18 were not previously classified as having epilepsy. Seven of these had new onset of epilepsy since our previous contact with them and the remaining 11 had had febrile seizures or other events that led them to perceive they had epilepsy and thus to self-report epilepsy in the current study. Conversely, eight participants previously classified as having epilepsy did not self-report epilepsy and therefore were excluded from our current analyses. Upon review of our previous information, their original diagnoses appeared doubtful.
Genetics and genetic attribution
All participants were asked to indicate if they had ever had clinical genetic testing for any condition, and if so, for what reason, where it was done, and what results they received. As previously described,19,24 three questions were used to evaluate genetic causal attribution of epilepsy, i.e., the extent to which participants believed epilepsy to be caused by genetics. All participants answered two questions asking their opinion on the role of genetics in causing epilepsy in their family (no/small, medium, big), and their opinion on their chance of having an epilepsy-related mutation (no/small, moderate, high). Participants with epilepsy answered an additional question asking how much they believed genetics had influenced their risk of developing epilepsy (none, some, strong). We created a scale variable by averaging the responses to the three questions (Cronbach’s alpha=0.77 among persons with epilepsy).19 The scale was not normally distributed; hence, scale values were categorized as high (3), moderate (2–2.9) or low (<2) for analysis.
Number of offspring
All participants were asked, “How many biological children do you have,” and numerical responses were recorded.
Estimates of epilepsy risk in offspring
All participants were asked to assign a numerical value (zero to 100) to the question, “Out of 100 children with a mother or father with epilepsy, how many will eventually develop epilepsy?”
Reproductive concerns
Participants were asked to rate factors according to their effect on their decisions about having biological children or future plans to have biological children, with five possible responses: “made me much less likely to want to have a child,” “made me less likely to want to have a child,” “no effect at all,” “made me more likely to want to have a child,” and “made me much more likely to want to have a child.” Participants both with and without epilepsy rated the concerns “the chance of having a child with epilepsy,” “having epilepsy in your family” and “advice from your doctor.” Only persons with epilepsy rated the concern, “the effect of having epilepsy on your ability to care for a child.” Five additional concerns related to pregnancy were asked only of women with epilepsy.
Reproductive plans
Participants with epilepsy were asked, “Do you think you would have wanted more (or any) children if you had not had epilepsy?” with responses “yes,” “no,” or “don’t know.”
Statistical analysis
We estimated prevalence ratios (PRs) through Poisson regression models with robust standard errors, using generalized estimating equation (GEE) models to adjust for the non-independence of individuals within the same family. We first compared mean number of offspring among participants with epilepsy versus their biological relatives without epilepsy. To assess potential confounding, we evaluated associations with the predictor (history of epilepsy) and the outcome (number of children) and adjusted for variables that were associated with both predictor and outcome, using a p-value of 0.2 (Tables S1 and S2). Potential confounders included sex, age (18–34 years, 35–49 years, ≥ 50 years), education (college graduate or higher, less than college graduate), religion (Catholic, Protestant, Other [Jewish/Buddhist/Hindu/other], None/atheist/prefer not to say), and marital status (ever, never).
We compared participants with epilepsy and their unaffected biological relatives in their estimates of epilepsy risk in an offspring of an affected parent. We also compared participants with epilepsy and unaffected relatives in their responses to two genetics-related concerns, “having a child with epilepsy” and “having epilepsy in your family.” For these analyses, the response categories “made me much less likely to want to have a child,” and “made me less likely to want to have a child” were combined.
Further analyses were restricted to participants with epilepsy. We evaluated responses to the question, “Would you have wanted more (or any) children if you had not had epilepsy?” according to predictors including demographic variables, time since last seizure (<5, ≥5 years), lifetime number of seizures (≤20, 21–100, >100), total number of affected family members (≥4, <4), the genetic attribution scale variable, and risk estimates in an offspring of an affected parent.
We examined the prevalence of all concerns among those with epilepsy, aggregating response categories “made me much less likely to want to have a child,” and “made me less likely to want to have a child.” We examined responses to the genetics-related concerns in relation to estimated risk of epilepsy in offspring of an affected parent and the genetic attribution scale variable.
We used IBM SPSS Statistics for Windows, Version 22.0 for analysis.
Results
The sample for the study included 774 people (after excluding those who married into the families). Of these, 589 (76%) were reached to invite them to participate and 379 completed the survey, for an overall participation rate of 49%. Those classified as having epilepsy in the original study were more likely to participate (54%) than were their biological relatives without epilepsy (45%) (p=0.01). The participation rate was also higher in women than in men (53% vs. 44%, p=0.01), in college graduates than in others (58% vs. 47%, p=0.02), and in those who identified as white, non-Hispanic than in others (50% vs. 37%, p=0.07). Participants were also older than non-participants (average 51 vs. 45 years, p<0.001).
We excluded 76 individuals (37 with epilepsy, 39 unaffected relatives) because they were known to have affected offspring at the time of the initial linkage study. Exclusion of these persons was necessary to eliminate selection bias. Due to the methods used for enrollment (described above), having an affected offspring might have been the reason an individual was included in the linkage study. Persons with an affected offspring must, by definition, have one or more offspring, leading to inflation in the apparent reproductive rates.
The final pool of participants included 298 participants from 94 families (149 with epilepsy and 149 biological relatives without epilepsy) with an average of three participants (range 1–19) per family. Among the 149 biological relatives, 59 were full siblings of the participants with epilepsy, and the remaining 90 were other relative types (e.g., half-siblings, cousins, nieces or nephews, aunts or uncles). Among all participants, 56% were female, 54% college graduates, and 93% white, non-Hispanic. Mean age was 48.7 years (SD=14.3).
Of the 94 families included in this study, 19 had clinical symptoms consistent with autosomal dominant epilepsy with auditory features (ADEAF)25, 10 of which had been found in our previous research to have mutations in the leucine-rich, glioma inactivated 1 (LGI1) gene. We offered clinical genetic testing for LGI1 with pre- and post-test genetic counseling to participants in these families. However, this offer was extended after participants completed the survey; none were offered this testing before survey completion. Of the 84 families without mutations in LGI1, 48 were included in other research studies involving genome sequencing and two were identified to have epilepsy-related pathogenic variants in other genes. These variants were identified after the participants completed the surveys for this study.
In the survey, 26 (9%) of the 298 participants responded that they had had clinical genetic testing for epilepsy. However, all but one participant responded that this testing was through the genetic linkage study (a misinterpretation, since the study involved linkage analysis and not clinical genetic testing). The remaining participant indicated that he or she had received genetic testing for “known epilepsy genes” through his or her doctor (possibly a gene panel), and the result was “negative.”
Participants with vs. without epilepsy: number of offspring
Participants with epilepsy had significantly fewer offspring than their biological relatives without epilepsy (mean 1.2 vs. 1.9, PR=0.7, 95% confidence interval [CI] 0.51–0.86, p=0.002, Table 1). The results were very similar when restricted to participants with epilepsy and unaffected full siblings (mean 1.2 vs. 1.8, PR= 0.7, 95% CI 0.55–0.84, p<0.001). Although the proportion ever married was slightly lower among people with epilepsy (75%) than their unaffected full siblings (85%) or other relatives (78%), the difference was not significant (p=0.30, Table S2). The difference in number of offspring between persons with and without epilepsy persisted when restricted to participants who had been married (mean 1.6 vs. 2.3, PR=0.7, 95% CI 0.56–0.88, p=0.002, Table 1). The difference also persisted when restricted to persons with epilepsy and unaffected full siblings who had been married (mean 1.6 vs. 2.2, PR=0.8, 95% CI 0.63–0.89, p=0.001).
Table 1.
Mean number of offspring among participants with epilepsy and biological relatives without epilepsy, stratified by marriage
| na | Mean number of offspring | SEM | PR (95% CI) | |
|---|---|---|---|---|
| Total | ||||
| Participants with epilepsy | 144 | 1.24 | 0.11 | 0.7 (0.51–0.86) |
| Biological relatives without epilepsy | 149 | 1.89 | 0.29 | 1.0 (ref.) |
| Full siblings | 59 | 1.83 | 0.19 | |
| Other relatives | 90 | 1.92 | 0.40 | |
| Ever married | ||||
| Participants with epilepsy | 106 | 1.62 | 0.12 | 0.7 (0.56–0.88) |
| Biological relatives without epilepsy | 119 | 2.31 | 0.30 | 1.0 (ref.) |
| Full siblings | 49 | 2.16 | 0.17 | |
| Other relatives | 70 | 2.41 | 0.45 |
PR, prevalence ratio, CI, confidence interval, ref., referent
Total n’s vary among different variables because of missing data. Out of 149 participants with epilepsy, 5 did not report number of offspring.
Among all demographic variables, religion was the only potential confounder of the association between number of offspring and history of epilepsy. Participants who were Catholic or Protestant had more children than others (Table S1) and people with epilepsy were less likely to be Catholic or Protestant than relatives without epilepsy (Table S2). However, adjustment for religion did not alter the findings. Although age and education were also associated with number of offspring (Table S1), these variables did not differ between people with epilepsy and unaffected relatives (Table S2).
Participants with vs. without epilepsy: risk estimates and genetics-related concerns
Average estimated risk of epilepsy in offspring of an affected parent was higher among participants with epilepsy (27%) than their unaffected full siblings (20%) or other relatives (19%) (p=0.01). Participants with epilepsy were more likely to estimate risk ≥50% than were their unaffected relatives (Table 2).
Table 2.
Estimated risk of epilepsy in offspring of affected parent and genetics-related concerns among participants with epilepsy, full siblings without epilepsy, and other biological relatives without epilepsy
| Participants with epilepsy | Unaffected biological relatives | ||||||
|---|---|---|---|---|---|---|---|
| Full siblings | Othersb | ||||||
| na | % | na | % | na | % | p-value | |
| Participant’s estimated risk of epilepsy in offspring of affected parent | |||||||
| ≥50% | 38 | 30.4 | 9 | 18.4 | 16 | 21.6 | 0.01 |
| 25–49% | 22 | 17.6 | 5 | 10.2 | 11 | 14.9 | |
| 10–24% | 35 | 28.0 | 19 | 38.8 | 15 | 20.3 | |
| <10% | 30 | 24.0 | 16 | 32.7 | 32 | 43.2 | |
| Response that concern would “make me less likely to want to have a child” | |||||||
| Chance of having a child with epilepsy | 139 | 25.9 | 54 | 11.1 | 86 | 18.6 | 0.05 |
| Having epilepsy in your family | 139 | 25.2 | 53 | 11.3 | 86 | 19.8 | 0.09 |
Total n’s vary among different variables because of missing data.
Other relatives listed as half-siblings, cousins, nieces, nephews, aunts or uncles
Since risk of epilepsy in offspring could reasonably be expected to differ depending on the number of affected persons in the family, we examined risk estimates within strata defined by number of affected family members (<4, ≥4) based on data from our previous genetic study. Risk estimates were higher in families containing ≥4 affected persons than in those with fewer, both among people with epilepsy (34% vs. 23%, p=0.01) and unaffected relatives (full siblings 27% vs. 15%, p=0.01; other relatives 20%, vs. 17%, p=0.59). Estimated risks were higher among people with epilepsy than unaffected family members within each stratum of number of affected relatives.
For genetics-related concerns, the proportions who responded the concern made them “less likely” or “much less likely” to want to have a child were greater among participants with epilepsy than among unaffected relatives (Table 2).
Participants with epilepsy: reproductive plans
Overall, 19% of all participants with epilepsy responded they “would have wanted more (or any) children if they had not had epilepsy.” Women were nearly three times as likely as men to say “yes” to this question (PR=2.6, p=0.02, Table 3). Participants who reported having had more than 100 lifetime seizures were significantly more likely to say “yes” than those who reported fewer than 20 seizures (PR=2.9, p=0.01, Table 3). Those who had ≥4 affected family members were also more likely than those with <4 affected family members to say “yes” (PR=2.2, p=0.04, Table 3). Compared with people with the lowest genetic attribution score, those with the highest score were 3.5 times as likely, and those with an intermediate score 4.6 times as likely to answer “yes” (Table 3).
Table 3.
Proportion of participants with epilepsy who responded “yes” to “Do you think you would have wanted more (or any) children if you had not had epilepsy?” by demographic variables, epilepsy-related variables, number of affected family members, genetic attribution scale and risk estimates
| na | % yes | p-value | PR (95% CI) | |
|---|---|---|---|---|
| All participants with epilepsy | 148 | 18.9% | n/a | n/a |
| Women | 86 | 25.6% | 0.02 | 2.6 (1.15–6.06) |
| Men | 62 | 9.7% | (ref.) | 1.0 (ref.) |
| Age 18–34 years | 28 | 25.0% | 0.36 | 1.5 (0.63–3.60) |
| Age 35–49 years | 42 | 19.0% | 0.74 | 1.1 (0.53–2.49) |
| Age ≥50 years | 78 | 16.7% | (ref.) | 1.0 (ref.) |
| College graduate or higher | 80 | 21.3% | 0.43 | 1.3 (0.68–2.46) |
| Less than college graduate | 67 | 16.4% | (ref.) | 1.0 (ref.) |
| Catholic | 30 | 13.3% | 0.36 | 0.6 (0.15–1.96) |
| Protestant | 51 | 19.6% | 0.63 | 0.8 (0.35–1.89) |
| Otherb | 28 | 21.4% | 0.79 | 0.9 (0.35–2.21) |
| None/atheist/prefer not to say | 33 | 24.2% | (ref.) | 1.0 (ref.) |
| Ever married | 110 | 19.1% | 0.92 | 1.0 (0.50–2.15) |
| Never married | 38 | 18.4% | (ref.) | 1.0 (ref.) |
| Last seizure <5 years ago | 68 | 25.0% | 0.29 | 1.5 (0.71–3.19) |
| Last seizure ≥5 years ago | 54 | 16.7% | (ref.) | 1.0 (ref.) |
| Lifetime number of seizures | ||||
| >100 | 27 | 40.7% | 0.01 | 2.9 (1.32–6.37) |
| 21–100 | 28 | 14.3% | 0.98 | 1.0 (0.36–2.91) |
| ≤20 | 64 | 14.1% | (ref.) | 1.0 (ref.) |
| Total number of affected family members | ||||
| ≥4 | 61 | 27.9% | 0.04 | 2.2 (1.04–4.77) |
| <4 | 80 | 12.5% | (ref.) | 1.0 (ref.) |
| Genetic attribution scalec | ||||
| High (3) | 46 | 19.6% | 0.10 | 3.5 (0.78–15.88) |
| Moderate (2–2.9) | 66 | 25.8% | 0.03 | 4.6 (1.19–18.01) |
| Low (<2) | 36 | 5.6% | (ref.) | 1.0 (ref.) |
| Participant’s estimate of risk of epilepsy in offspring of affected parent | ||||
| ≥50% | 38 | 21.1% | 0.40 | 0.7 (0.31–1.61) |
| 25–49% | 21 | 19.0% | 0.37 | 0.6 (0.23–1.72) |
| 10–24% | 35 | 11.4% | 0.08 | 0.4 (0.13–1.14) |
| <10% | 30 | 30.0% | (ref.) | 1.0 (ref.) |
PR, prevalence ratio, CI, confidence interval, ref., referent
Total n’s vary among different variables because of missing data.
Other religions listed as Jewish, Hindu, Buddhist, or “Other.”
Genetic attribution questions, each coded from 1 to 3: “In your opinion, how big a role has genetics had in causing the epilepsy in your family?” “In your opinion, what do you think the chances are that you have a change or mutation in a gene that affects risk for epilepsy?” and “How much do you think genetics or inheritance influenced your risk of developing epilepsy?” Scale values were obtained by averaging the responses to the three questions and then categorized.
Participants with epilepsy: reproductive concerns
For each reproductive factor, we examined the proportion of participants who responded the concern made them “less likely” or “much less likely” to want to have a child (Figure 1). Among women with epilepsy, 37–56% were concerned with pregnancy-related complications.
Figure 1:
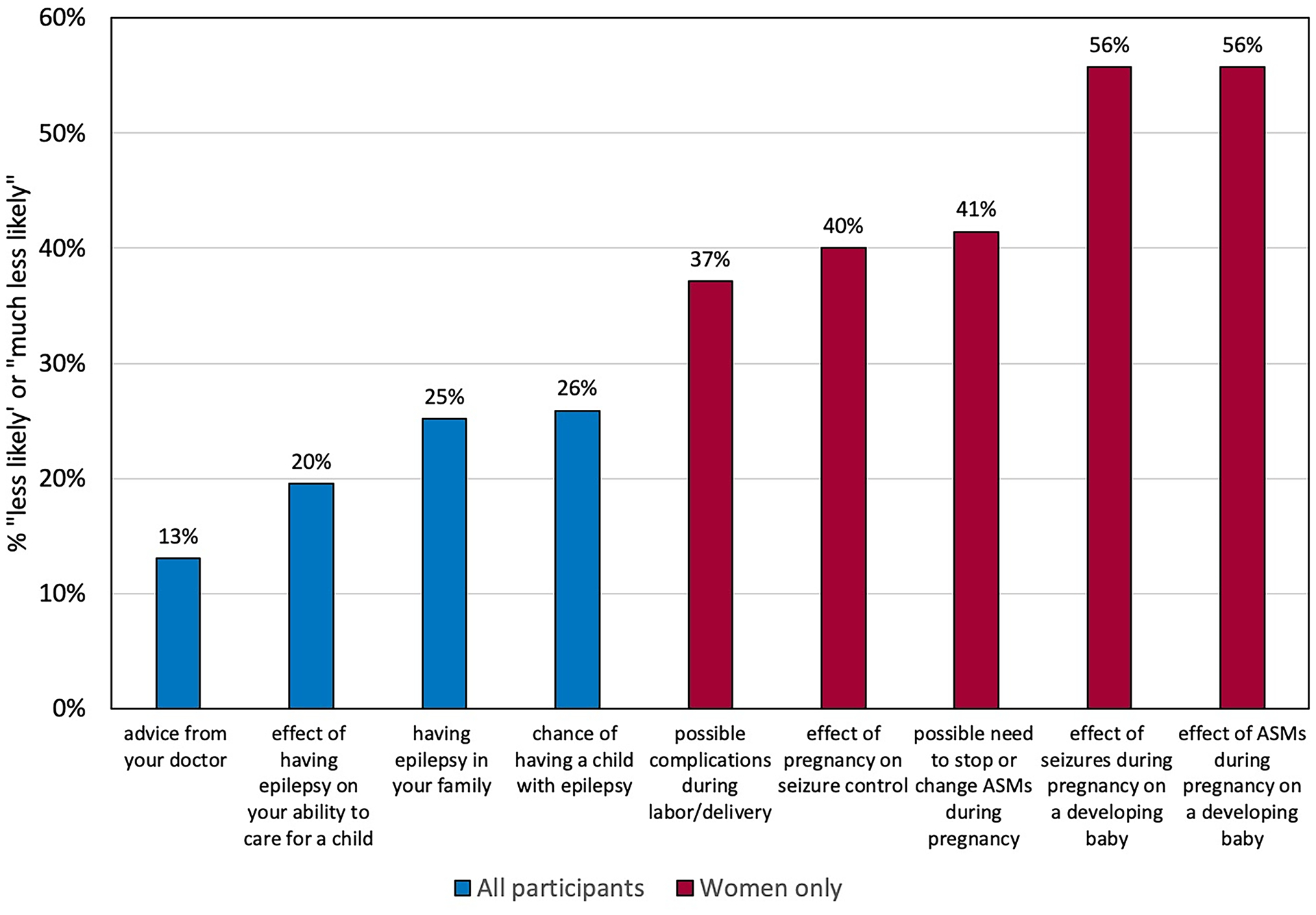
Percent of participants who responded that the factor made them “less likely” or “much less likely” to want to have a child. Abbreviations: ASMs, anti-seizure medications.
Among both men and women with epilepsy, approximately 25% of participants were concerned about the genetics-related factors. The prevalence of genetics-related concerns increased with increasing estimates of offspring epilepsy risk (Figure 2) and with increasing genetic attribution score (Figure 3).
Figure 2:
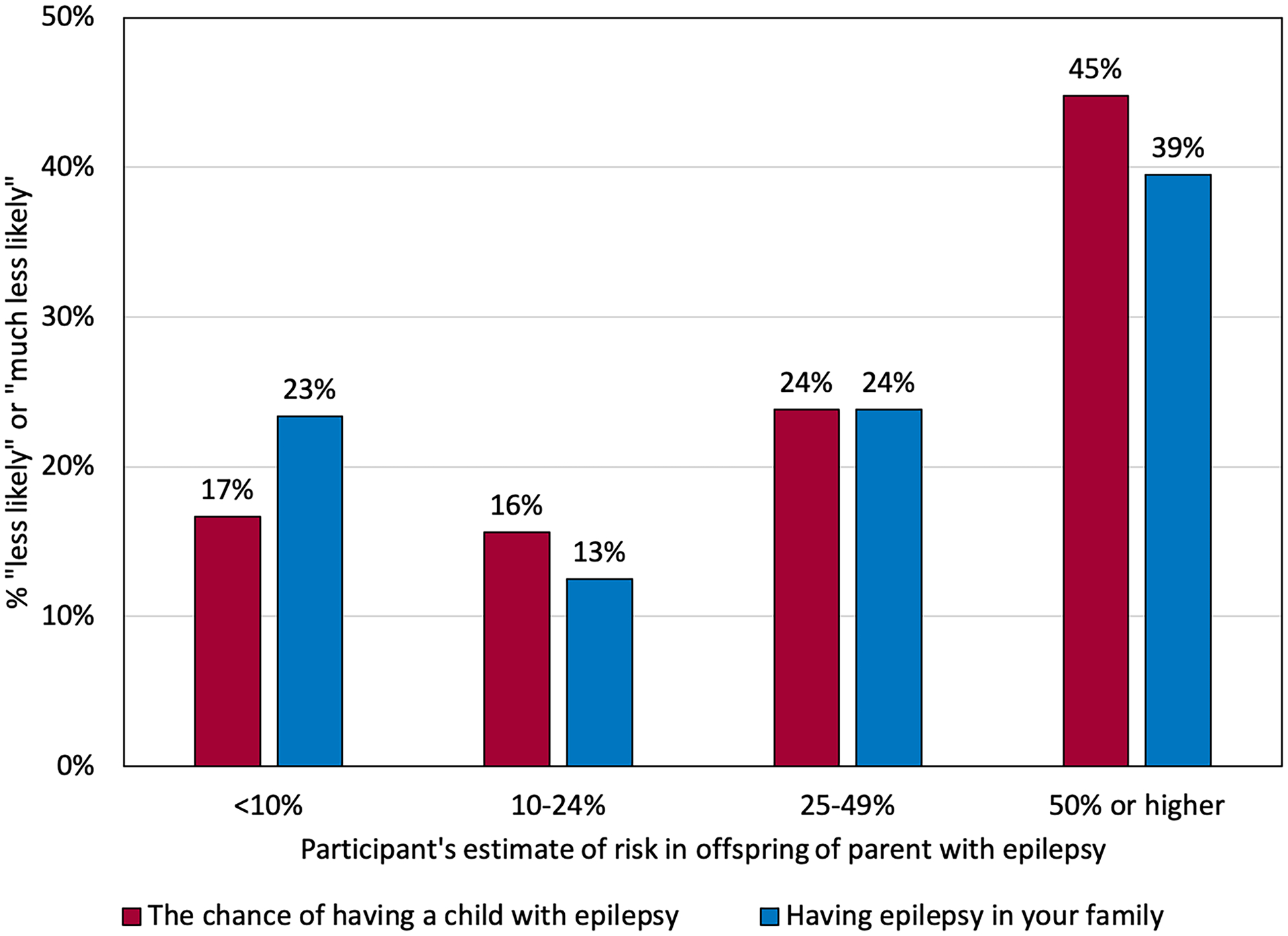
Percent of participants who responded that the genetics-related factors made them “less likely” or “much less likely” to want to have a child, according to estimated risk of epilepsy in offspring of a parent with epilepsy. P-values from GEE models: p=0.006 (the chance of having a child with epilepsy), p=0.04 (having epilepsy in the family).
Figure 3:
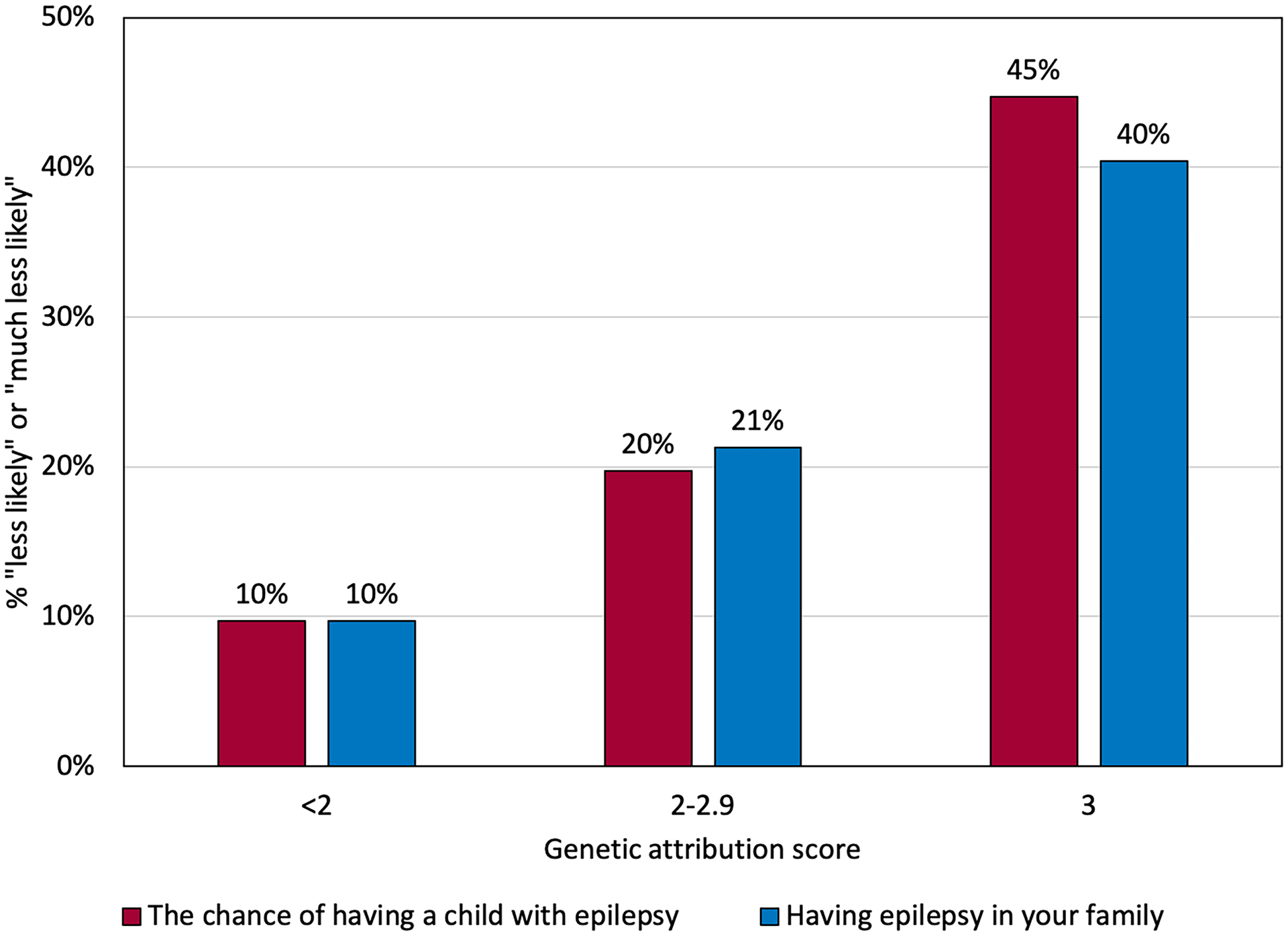
Percent of participants who responded that the genetics-related factors made them “less likely” or “much less likely” to want to have a child, according to genetic attribution scale. 47/139 (34%) participants scored 3 on the scale, 61/139 (44%) scored 2–2.9, and 31/139 (22%) scored <2. P-values from GEE models: p=0.008 (the chance of having a child with epilepsy), p=0.019 (having epilepsy in the family).
Discussion
People with epilepsy in these families had fewer children than their siblings or other biological relatives without epilepsy. Our findings align with previous reports of reduced birth rates among persons with epilepsy in the general population1–4 and reduced likelihood of pregnancy among persons with epilepsy compared to same-sex siblings.5 Reduced marriage rates among those with epilepsy did not explain our findings.5,13
Risk estimates of epilepsy in offspring of an affected parent were higher among participants with epilepsy than their unaffected relatives. The mean estimate of risk among those with epilepsy (27%) was very similar to that in another study of patients from an outpatient clinic in the United States (26%).17 In population-based data, risk in offspring of parents with epilepsy was not greater than 10% even in the highest risk subgroup.26 Risks are likely higher in families with multiple affected persons such as those we studied; however, we are unable to estimate these risks because of the way the families were selected – having an affected offspring was one of the criteria used to select families for the linkage study. To assess the impact of having affected relatives on participants’ risk estimates, we stratified by number of people with epilepsy in each family. Risk estimates were higher among participants (both with and without epilepsy) in families containing ≥4 affected persons than in those with fewer, reflecting participants’ impression (likely correct) of higher offspring risk in families with more affected persons. In both groups of families, participants with epilepsy estimated higher risks in offspring of an affected parent than did their unaffected relatives, reflecting greater concerns about having a child with epilepsy among people with epilepsy than among their unaffected relatives.
The proportion of participants who responded they would have wanted more (or any) children if they had not had epilepsy (19%) is somewhat lower than in previous studies, which reported that 25–38% of participants decided to have fewer children due to epilepsy.14–17 Our study differs from previous studies because of the older age of many of our participants, who were looking back on their reproductive histories rather than reporting plans for the future. Also, our survey question might have had a negative or stigmatizing connotation. Nevertheless, participants with higher genetic attribution scores were more likely to say “yes” (Table 3), suggesting that those who believed their epilepsy had a genetic cause were more likely than others to limit desired childbearing due to epilepsy.
More than half of the women in our study were concerned about the possible impact of seizures and ASMs during pregnancy on the developing fetus. Having these concerns may indirectly reflect a deficit of knowledge or confusion regarding these topics.27 Our findings emphasize the importance of preconception counseling for women with epilepsy as they face complex issues in epilepsy management during pregnancy.28,29
Genetics-related concerns (“the chance of having a child with epilepsy” and “having epilepsy in your family”) were more prevalent among participants with epilepsy than their relatives without epilepsy (Table 2). Additionally, among participants with epilepsy, the prevalence of genetics-related concerns increased with increases in estimated risk of epilepsy in the offspring of an affected person (Figure 2) and genetic attribution scores (Figure 3). These findings are similar to those in a previous study, in which estimated risk in the offspring of an affected person was significantly increased among participants who indicated that the risk of having a child with epilepsy factored into their decision to limit childbearing.17 Our findings suggest that persons who more strongly believe their epilepsy is caused by genetics are more likely than others to have concerns that reduce their desire to have children.
Almost none of our participants had had clinical genetic testing when they completed the survey between 2013 and 2015. Today, genetic testing is increasingly accessible to people with epilepsy and could have an important impact on reproductive decision-making. Discovery of a pathogenic variant through clinical genetic testing in a person with epilepsy informs the likelihood that an offspring will inherit it. Unaffected family members may also be tested to learn whether they carry the variant, to inform the chance their offspring will also carry it. If a confirmed pathogenic variant is identified in a person with epilepsy or one of his or her relatives, prenatal testing or preimplantation genetic diagnosis can be offered to minimize the risk in his or her offspring.
However, the implications of genetic test results for reproductive decision-making in epilepsy depend heavily on understanding of the relationship of a pathogenic variant to epilepsy phenotypes. For many of the identified genes associated with epilepsy, penetrance is reduced and expressivity is variable, i.e., different epilepsy phenotypes are observed among different persons with pathogenic variants in the same gene. An important example of this problem is in genetic epilepsy with febrile seizures plus (GEFS+), in which penetrance of variants in SCN1A is incomplete (so that some offspring with the variant remain unaffected) and phenotypes in affected offspring can range from simple age-limited febrile seizures to severe epileptic encephalopathies.30 Clearly, choices about reproduction in the context of genetic testing are complex and should be informed by genetic counseling by professionals with specific knowledge about epilepsy.31
In addition, we emphasize that only a fraction of the potential genetic causes of epilepsy have been identified so far. Causative variants have been identified in very few of the families studied here, despite investigation with whole genome or whole exome sequencing in more than half of them. Although these families were recruited for our studies because they contained multiple affected individuals (and thus appeared likely to be segregating high penetrance causative variants), the underlying genetic contributions may be more complex than we originally assumed. Education and counseling regarding offspring risk in such cases must clarify that failure to identify a pathogenic variant does not imply that genes do not contribute to the cause of epilepsy in an individual or family. In this context, the risk to offspring is likely to be higher than in the general population, but specific risk estimates are not available.
This study has several limitations. We did not ask whether participants actively limited their reproduction due to epilepsy, nor what specific actions they might have taken to do so. The participants of this study come from rare families with multiple individuals with epilepsy and are therefore unrepresentative of all persons with epilepsy. Furthermore, our participants were highly educated, ethnically homogeneous, and recruited into the study based on their interest and participation in genetic research. It would be important to assess the impact of genetic beliefs on reproductive decision-making in a larger, more diverse, and more representative sample of people with epilepsy. Our sample size was relatively small, limiting statistical power for some associations we wished to test.
Despite limitations, our findings reveal an important relationship between beliefs about genetic influences on epilepsy and reproductive decision-making among men and women with epilepsy. With increasing interest and application of genetics in clinical contexts, informing patients about the true risks for epilepsy in offspring, and providing genetic testing and reproductive options when available are extremely important. Obtaining accurate information about risks to offspring and developing ways to make it accessible to diverse groups of people with epilepsy should be a priority.
Supplementary Material
Table S1. Mean number of offspring among participants with epilepsy and their biological relatives without epilepsy, by demographic variables.
Table S2. Distribution of participants with epilepsy and unaffected biological relatives without epilepsy, by demographic variables.
Appendix S1. Selected extracts from questionnaire.
Key Points.
In families with multiple affected individuals, people with epilepsy have fewer children than their biological relatives without epilepsy.
Within these families, people with epilepsy estimate higher risk of epilepsy in offspring of affected persons than do their relatives without epilepsy.
Those with epilepsy who believe it had a genetic cause are more likely than others to limit desired childbearing due to epilepsy.
The impact of concern about having a child with epilepsy on reproductive decisions increases with greater belief in a genetic cause of epilepsy.
Acknowledgments
This research was supported by National Institute of Health (NIH) grants T35 AG044303, R01 NS078419, R01 NS104076 and RM1 HG007257. We thank the participants who generously donated their time to participate in this study.
Footnotes
Conflict of Interest
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Wallace H, Shorvon S, Tallis R. Age-specific incidence and prevalence rates of treated epilepsy in an unselected population of 2,052,922 and age-specific fertility rates of women with epilepsy. Lancet 1998;352:1970–1973. [DOI] [PubMed] [Google Scholar]
- 2.Farmen AH, Grundt JH, Tomson T, et al. Age-specific birth rates in women with epilepsy: a population-based study. Brain Behav 2016;6:e00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artama M, Isojarvi JI, Raitanen J, et al. Birth rate among patients with epilepsy: a nationwide population-based cohort study in Finland. Am J Epidemiol 2004;159:1057–1063. [DOI] [PubMed] [Google Scholar]
- 4.Webber MP, Hauser WA, Ottman R, et al. Fertility in persons with epilepsy: 1935–1974. Epilepsia 1986;27:746–752. [DOI] [PubMed] [Google Scholar]
- 5.Schupf N, Ottman R. Likelihood of pregnancy in individuals with idiopathic/cryptogenic epilepsy: social and biologic influences. Epilepsia 1994;35:750–756. [DOI] [PubMed] [Google Scholar]
- 6.Harden CL, Pennell PB. Neuroendocrine considerations in the treatment of men and women with epilepsy. Lancet Neurol 2013;12:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artama M, Isojarvi JI, Auvinen A. Antiepileptic drug use and birth rate in patients with epilepsy--a population-based cohort study in Finland. Hum Reprod 2006;21:2290–2295. [DOI] [PubMed] [Google Scholar]
- 8.Pennell PB, French JA, Harden CL, et al. Fertility and Birth Outcomes in Women With Epilepsy Seeking Pregnancy. JAMA Neurol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol 2008;64:200–211. [DOI] [PubMed] [Google Scholar]
- 10.Isojarvi JI, Laatikainen TJ, Knip M, et al. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol 1996;39:579–584. [DOI] [PubMed] [Google Scholar]
- 11.Isojarvi JI, Rattya J, Myllyla VV, et al. Valproate, lamotrigine, and insulin-mediated risks in women with epilepsy. Ann Neurol 1998;43:446–451. [DOI] [PubMed] [Google Scholar]
- 12.Tomson T, Battino D, Bonizzoni E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol 2018;17:530–538. [DOI] [PubMed] [Google Scholar]
- 13.Dansky LV, Andermann E, Andermann F. Marriage and fertility in epileptic patients. Epilepsia 1980;21:261–271. [DOI] [PubMed] [Google Scholar]
- 14.Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different lifestages: results of the ‘Ideal World’ survey. Seizure 2003;12:502–507. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez B, Gibson P, Kustra R. Epilepsy and women’s health issues: unmet needs--survey results from women with epilepsy. Epilepsy Behav 2007;10:163–169. [DOI] [PubMed] [Google Scholar]
- 16.Lee SM, Nam HW, Kim EN, et al. Pregnancy-related knowledge, risk perception, and reproductive decision making of women with epilepsy in Korea. Seizure 2013;22:834–839. [DOI] [PubMed] [Google Scholar]
- 17.Helbig KL, Bernhardt BA, Conway LJ, et al. Genetic risk perception and reproductive decision making among people with epilepsy. Epilepsia 2010;51:1874–1877. [DOI] [PubMed] [Google Scholar]
- 18.Sabatello M, Phelan JC, Hesdorffer DC, et al. Genetic causal attribution of epilepsy and its implications for felt stigma. Epilepsia 2015;56:1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garofalo DC, Sorge ST, Hesdorffer DC, et al. Genetic attribution and perceived impact of epilepsy in multiplex epilepsy families. Epilepsia 2019;60:2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shostak S, Zarhin D, Ottman R. What’s at stake? Genetic information from the perspective of people with epilepsy and their family members. Soc Sci Med 2011;73:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottman R, Berenson K, Barker-Cummings C. Recruitment of families for genetic studies of epilepsy. Epilepsia 2005;46:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians’ diagnoses. Epilepsia 1990;31:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannings C, Thompson EA. Ascertainment in the sequential sampling of pedigrees. Clin Genet 1977;12:208–212. [DOI] [PubMed] [Google Scholar]
- 24.Sorge ST, Hesdorffer DC, Phelan JC, et al. Depression and genetic causal attribution of epilepsy in multiplex epilepsy families. Epilepsia 2016;57:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottman R, Winawer MR, Kalachikov S, et al. LGI1 mutations in autosomal dominant partial epilepsy with auditory features. Neurology 2004;62:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peljto AL, Barker-Cummings C, Vasoli VM, et al. Familial risk of epilepsy: a population-based study. Brain 2014;137:795–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrath A, Sharpe L, Lah S, et al. Pregnancy-related knowledge and information needs of women with epilepsy: a systematic review. Epilepsy Behav 2014;31:246–255. [DOI] [PubMed] [Google Scholar]
- 28.Stephen LJ, Harden C, Tomson T, et al. Management of epilepsy in women. Lancet Neurol 2019;18:481–491. [DOI] [PubMed] [Google Scholar]
- 29.Tomson T, Battino D, Bromley R, et al. Management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epileptic Disord 2019;21:497–517. [DOI] [PubMed] [Google Scholar]
- 30.Poduri A, Sheidley BR, Shostak S, et al. Genetic testing in the epilepsies-developments and dilemmas. Nat Rev Neurol 2014;10:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poduri A When Should Genetic Testing Be Performed in Epilepsy Patients? Epilepsy Curr 2017;17:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean number of offspring among participants with epilepsy and their biological relatives without epilepsy, by demographic variables.
Table S2. Distribution of participants with epilepsy and unaffected biological relatives without epilepsy, by demographic variables.
Appendix S1. Selected extracts from questionnaire.


