Abstract
Multidrug resistant (MDR) Gram-negative bacteria are an urgent global health threat. We report on the design and evaluation of a xenosiderophore-conjugated cationic random copolymer (pGQ-DG) which exhibits selective antibacterial activity against Pseudomonas aeruginosa (P. aeruginosa) by targeting select outer membrane (OM) receptors for scavenging xenosiderophores such as deferoxamine (DFO), while possessing favorable cytocompatibility and exhibiting low hemolysis, to enhance and safely damage the bacterial OM. pGQ-DG demonstrated synergistic properties in combination with vancomycin (VAN) when evaluated in vitro against P. aeruginosa. In addition, pGQ-DG plus VAN cleared the P. aeruginosa infection and efficiently accelerated healing in a murine wound healing model as effectively as colistin, suggesting that this strategy could serve as an alternative to colistin against MDR bacteria.
Keywords: Pseudomonas aeruginosa, deferoxamine:gallium, vancomycin, amphiphilic, polymeric antimicrobials, wound healing
Graphical Abstract

1. Introduction
ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) account for the majority of hospital acquired infections [1]. These infections often develop into MDR isolates, making their treatment extremely challenging in the face of limited number of new antibiotics [2], [3], [4]. Gram-negative P. aeruginosa is one of the most common pathogen-causing infection in burn wounds [5] and is difficult to eradicate thoroughly due to its propensity to frequently develop MDR during treatment [6]. Though polymyxins are currently used as a last resort defense against difficult MDR pathogens [7], plasmid-mediated resistance to colistin has been reported [8, 9] and research into alternative antibacterial treatments that do not rely on new drug discovery screens is urgently needed.
Many antibiotics available in the market are potent against Gram-positive bacteria but exhibit poor activity against Gram-negative bacteria because transport of the drug across the outer lipopolysaccharide (LPS) membrane of Gram-negative bacteria is tightly regulated, whereby small hydrophilic molecules are able to diffuse through open porins but larger amphiphilic molecules typically require specific transporters [10, 11]. In fact, the majority of open porins in the outer membrane of P. aeruginosa exclude most molecules >600 Da and makes it difficult for potent but poorly permeable antibiotics to exert significant activity [12], [13], [14]. One promising strategy to overcome this cell permeability barrier is to disrupt the integrity of the OM via ionic interactions with polycationic materials, though these are often non-specific and damaging to both mammalian and bacterial cells [15, 16].
Most effective antibacterial polymers are typically amphiphilic and characterized by an abundance of cationic and hydrophobic residues [17]. Highly promising polymers possessing these properties are those formed from quaternary ammonium salts (PQAS). Quaternary ammonium salts are cationic moieties containing alkyl groups, which when incorporated into a polymer, allow for adjustable hydrophobic properties. The cationic charge is needed for the polymer to electrostatically interact preferentially with anionic bacterial membranes over zwitterionic human cell membranes [18]. Next, hydrophobic alkyl chains in the polymer insert into the cell membrane to disrupt its integrity and ultimately results in cell death. During the last two decades, PQAS have become a research hotspot, as they have been reported to be nonvolatile, chemically stable, and safer due to their inability to permeate through the skin [19]. In addition to PQAS, guanidine-containing cationic polymers have also been reported to possess antimicrobial properties and favorable cytocompatibility compared to other polycations [20], [21], [22]. Other strategies to minimize host toxicity to antibacterial cationic polymers have also included balancing the charge density to be more toxic to bacteria over mammalian cells or incorporating siderophores to selectively target membrane-disrupting polymers to Gram-negative bacteria [23, 24].
Given the essential role of iron in bacterial physiology and pathogenicity, targeting bacteria through iron uptake transport systems mediated by siderophores have become especially attractive strategies for the development of selective antibacterial polymers. For example, bacterial uptake systems often cannot distinguish between Ga(III) and Fe(III), therefore the complexation of DFO to Ga(III), i.e. DFO:Ga, has been shown to inhibit the growth of many bacterial and fungal species by interfering with iron-dependent metabolic pathways [25]. Since Ga(III) cannot be reduced, its uptake essentially inactivates all iron-dependent sequential reduction and oxidation processes in the cell. Interestingly, previous work by others have found that Ga(III) is preferentially taken up over Fe(III) in P. aeruginosa and that a solution of gallium nitrate alone could inhibit P. aeruginosa growth and biofilm formation in vitro and in murine lung infection models [26, 27]. Whether complexed to nitrate, maltolate, protoporphyrin IX or DFO, Ga(III) displayed antibacterial activity under various culture conditions [28], [29], [30], [31].
In this paper, a triple combination anti-pseudomonal approach based on a random PQAS copolymer design was investigated by 1) incorporating cationic guanidine moieties and short alkyl quaternary ammonium salts to disrupt the bacterial membrane, 2) conjugating DG to the random copolymer to further promote interactions with P. aeruginosa and disrupt iron metabolism, and 3) enhancing bacteria sensitivity to vancomycin (VAN), a Gram-positive glycopeptide (MW 1449 g/mol) known to be inactive against P. aeruginosa due to its poor permeability across the OM. An especially appealing feature of this polymeric antimicrobial combination strategy is that synthesis of the random copolymer backbone is conducted as a simple one-pot reaction via Reversible Addition—Fragmentation chain Transfer (RAFT) polymerization of three acrylate monomers: Boc-protected 2-aminoethyl acrylate (Boc-AEA), N-[3-(Dimethylamino) propyl] methacrylamide (DMAPMA) and pentafluorophenyl-protected acrylate (PFPA), followed by post-modifications of respective monomers to introduce cationic guanidine groups, amphiphilic alkyl quaternary ammonium salts (QAS), and DFO as a xenosiderophore for iron chelation [32], [33], [34] (Fig. 1A and Scheme S1). To demonstrate superior polymer performance, we also fabricated equivalent appropriate cationic and amphiphilic homopolymer controls (pGEA and pQDH respectively) as described in the Materials and Methods section. The final targeted random copolymer, pGQ-DFO, is amphiphilic and water soluble. Synthesis and characterization of pGQ-DFO complexed to Ga(III) to form pGQ-DG, mechanism of action, antimicrobial activity, and biocompatibility were investigated in vitro against two gram-negative bacteria, P. aeruginosa PAO1 and Escherichia coli ATCC 25922 (E. coli). To investigate the superior antibacterial properties of pGQ-DG plus VAN in vivo, this three-prong strategy was furthermore evaluated against a murine wound healing infection model of P. aeruginosa.
Fig. 1.

Schematic illustration and chemical structures of all polymers synthesized, including homopolymers pGEA and pQDH and random copolymers pGQ and pGQ-DG.
2. Materials and methods
2.1. Polymer synthesis and physical characterization
The detailed synthesis of cationic poly(2-guanidinoethylacrylate) (pGEA) and amphiphilic poly[(2-methacrylamido) propyltetrahexyldimethylammonium bromide] (pQDH) homopolymers as well as random copolymers pGQ-DG and pGQ are summarized in Scheme S1. All the polymers synthesized were subjected to characterization by nuclear magnetic resonance (NMR) on a Bruker Ascend 400 MHz NMR spectrometer to confirm their molecular structures and degree of polymerizations (Figure S1–S7). Polymers were also subjected to gel permeation chromatography (GPC) to establish their apparent molecular weight and polydispersity index (PDI) with respect to PEG standards. Zeta potentials (ζ) of polymers were measured with a Malvern Zetasizer Nano ZS instrument (Malvern, UK).
2.2. Bacterial strains and culture conditions
Gram-negative reference strains of P. aeruginosa ATCC 15692 (PAO1) and Escherichia coli (E. coli, ATCC 25922) were obtained from American Type Culture Collection (ATCC). Bacteria was first inoculated into cationic-adjusted Mueller-Hinton agar (MHA) plates from stock solution stored at −80°C and cultured under aerobic conditions at 37 °C for 24 h. A colony was picked and sub-cultured in cation-adjusted Mueller-Hinton broth medium (MHB) and grown to stationary phase at 37 °C in an incubator shaker prior to all experiments. The concentration of bacteria was monitored by measuring the optical density at 600 nm (OD600). All growth media and Milli-Q water used for bacterial cultures were sterilized by autoclave prior to use. Sterile polypropylene culture tubes and sterile polystyrene 96-well plates used for culturing were manufactured by VWR and Coming Incorporated respectively.
2.3. Minimum inhibitory concentration (MIC) assays
The MIC, defined as the lowest polymer concentration tested to inhibit bacterial growth, was determined for all polymers synthesized using a standard microbroth dilution assay. Briefly, a 135 μL aliquot of antimicrobials serially diluted in MHB media was mixed with 15 μL aliquot of bacterial solution into a 96-well plate. The bacterial suspension was diluted to an OD600 value of 0.01 in MHB medium and the final OD600 value in the 96-well plate was 0.001. The plate was subsequently incubated at 37 °C for 18 h. The bacterial sample (As) was assayed by measuring OD600 using a microplate reader. Wells with only medium served as negative controls (AN) and bacteria grown in medium without the addition of polymers served as positive controls (AP). The resulting mean OD600 is reported, where the error bars are the standard deviation (SD) of the mean obtained from three independent replicates. Relative growth of bacteria was calculated using the following formula:
2.4. Mammalian cytocompatibility assays
The cytotoxicity of all polymers synthesized was assessed by the metabolism-based resazurin assay. Mouse macrophage J774A.1 cells were seeded in a 96-well plate at a density of 5000 cells per well. Cells were incubated in DMEM supplemented with 10% Fetal bovine serum (FBS) and 1% penicillin/ streptomycin at 37 °C in 5% CO2 for 24 h. Next, cells were incubated with growth medium containing polymers at various concentrations. After 24 h, the substrate resazurin was dissolved in cell culture medium at a concentration of 44 mM and 100 μL was added to each well and cultured for 4 h. Cell viability was determined by measuring fluorescence emission at 590 nm (excitation at 560 nm). Fluorescence intensities from the wells without cells were used as blanks (Flblank) and the fluorescence intensity from control cells without treatment were used to represent 100% cell viability (Flcontrol). All the measurements were conducted in triplicate and cell viability was determined as follows:
Hemolytic activity of the polymers was conducted on sheep red blood cells (RBCs) and assessed by a hemoglobin release assay. Sheep RBCs were first washed three times in PBS (10 mM pH 7.4) and diluted to a final concentration of 2 × 107 cells per mL. Aliquots (100 μL) of the RBC solution were seeded into a 96-well plate containing 100 μL of polymers at various concentrations (0.01–10 mg/mL) and incubated in a humidified atmosphere containing 5% CO2 at 37°C for 2 h. Samples were then centrifuged to pellet intact RBCs and hemoglobin released into the supernatant was quantified by UV-Vis absorbance at 405 nm (As). Data was normalized with respect to a PBS negative control representing 0% hemolysis (AN) and 1% Triton X-100 positive control representing 100% hemolysis (Ap). Each hemolysis assay was independently repeated three times using different stock solutions. Hemolytic activity is reported as the concentration observed to lyse 20% of RBCs (HC20). The percentage of hemolysis was calculated using the formula:
2.5. Outer membrane permeabilization studies with nitrocefin
Outer membrane (OM) permeability of P. aeruginosa PAO1 was assessed through the established nitrocefin assay [35] by treating bacteria with 0.5 mg/mL polymer (this corresponds to a concentration > MIC for either pGQ-DG or pGQ). Briefly, bacteria cells in the stationary phase were collected, suspended in 10 mM PBS to an OD = 0.1 in a 96-well microplate and incubated with 0.2 mM nitrocefin and 0.5 mg/mL polymer. Control experiment consisted of only treating the bacterial suspension with nitrocefin. Hydrolysis of nitrocefin was monitored by measuring the increasement of OD490 (Δ(OD490) at 37 °C for 3 h. Studies were carried out in triplicates.
2.6. Membrane potential analysis with flow cytometry
Membrane potential was determined by flow cytometry using the BacLight Bacterial Membrane Potential Kit from Invitrogen following manufacturer’s instructions. At low concentrations, the dye DiOC2(3) exhibits green fluorescence in bacterial cells but become more concentrated in healthy cells that are maintaining a membrane potential. As concentration increases in these intact cells, the dye self-associates and the fluorescence emission shifts to red. The red and green fluorescence bacterial populations corresponding to intact and disrupted membranes can be easily distinguished using a flow cytometer. P. aeruginosa cells were first inoculated to mid-log phase. Viable cells were then diluted to 1 × 107 CFU/mL in PBS and either 0.5 mg/mL polymer (pGQ-DG or pGQ) or 5 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP) as a control representing fully depolarized membranes was added. After 1 h incubation at 37 °C, 30 μM DiOC2(3) dye was added to all samples. Bacteria were then assayed by flow cytometry using a 488 nm laser and emission filters for fluorescein and the Texas Red dye. Membrane potential analysis was analyzed by taking the ratio of cells exhibiting red fluorescence to green fluorescence. Gates were drawn based on the untreated (polarized) and CCCP-treated (fully depolarized) controls. Data are representative of two independent assays completed in duplicates.
2.7. Bacterial morphological studies with TEM and SEM
Bacterial morphology was assessed through images obtained by scanning electron microscopy (SEM, FE-SEM FEI Teneo) and transmission electron microscopy (TEM, JEOL JEM-2100). Bacteria (OD600 = 0.1) and polymer solution at 2×MIC were co-incubated for 3 h. After incubation, bacteria solutions were washed three times with PBS and fixed with 4% paraformaldehyde for 4 h. After centrifugation, bacteria cells were fixed with 2% paraformaldehyde for another 1h, centrifuged again and washed with PBS two more times. Collected bacterial cells were dehydrated by sequential treatments through 20% incremental ethanol washes for 10 minutes up to 100% ethanol. Several drops of the suspension were placed on a Formvar/carbon-coated 200 mesh copper grid and left to dry at room temperature prior to observation by TEM. To obtain SEM images, a drop of a bacterial solution was dripped on a silicon wafer and coated with gold and viewed by SEM.
2.8. Antibacterial time-kill assays
Antibacterial activity for pGQ-DG was investigated through a time-kill assay. Bacterial strains of P. aeruginosa (PAO1) and E. coli (ATCC 25922) were inoculated and cultured until the OD600 reached a value of 0.4–0.6 in MHB medium. Tubes containing an initial concentration of 5×105 CFU/mL bacteria were mixed with pGQ-DG at 2×MIC prepared in PBS solution. Samples were incubated at 37°C with shaking (250 rpm) and 100 μL aliquots from each bacterial tube was sampled at various times (0, 2, 4, 6 h) and immediately serially diluted with saline and plated onto MH agar plates using a spreader. The MH agar plates were incubated between 16–18 h at 37 °C, after which single colonies were counted and CFU per milliliter (CFU/mL) was calculated.
2.9. Live/dead bacterial imaging assays
Bacteria cells were inoculated in 3 mL MHB medium, grown to stationary phase at 37 °C, harvested by centrifugation (8000 rpm, 10 min), and washed with saline 3x prior to resuspending cells in fresh medium to a predetermined concentration. Next 900 μL of bacterial solution (OD600 = 0.1) was mixed with 100 μL of pGQ-DG to a final concentration 2× MIC or with PBS as a control and incubated at 37 °C with shaking (250 rpm) for 2 h. The cell suspension was then centrifuged (8000 rpm, 10 min) and washed with saline. Live/dead bacteria were visualized by staining the membrane with SYTO 13 (30 min) and propidium iodide (PI, 20 min) to identify dead bacteria. Bacteria cells were then fixed on a poly-D-lysine coated chambered cover glass and imaged with a Carl Zeiss LSM 710.
2.10. Potentiation properties of pGQ-DG with VAN
The MICs of VAN with pGQ-DG was evaluated against P. aeruginosa PAO1, wherein the Fractional Inhibitory Concentration Index (FICI) for each combination evaluated was calculated. Briefly, a 135 μL aliquot of MHB medium containing serially diluted antibiotic and polymer was added to each well containing 15 μL aliquot of bacterial solutions in a 96-well plate. The plate was subsequently incubated at 37 °C for 18 h prior to assessing bacterial growth by measuring OD600. Using the microdilution checkerboard method, the FICI was calculated using the following equation:
where MICA and MICB are the MICs of drugs A and B alone and MICCA and MICCB are the concentrations of the combination drugs in wells corresponding to an MIC. A FICI ≤0.5 indicates synergy, FICI of 0.5–4 indicates indeterminate effects and FICI > 4 indicates antagonism [36].
2.11. In vivo murine wound healing infection model and histological analysis
To evaluate the antibacterial efficacy of pGQ-DG with VAN in vivo, a murine wound healing infection model was used (n=6 mice/group, 6-8 weeks old BALB/c female mice with an average weight of 15–20 g). All the procedures involving animal use were performed with the approval of Institutional Animal Care and Use Committee (IACUC) at the University of Georgia. Five formulations (saline control, pGQ-DG at 2 mg/mL (at concentrations corresponding to the IC50), colistin at 0.8 mg/mL, VAN at 0.8 mg/mL, and VAN plus pGQ-DG at 0.8 and 2 mg/mL respectively). Circular skin wounds with a diameter of ca. 0.8 cm were made to the dorsal skin of each mouse by incision and the skin was removed using stainless-steel scissors as previously conducted [37]. Next, P. aeruginosa PAO1 suspension (50 μL) containing 1 × 108 CFU/mL in sterilized saline was dripped onto the wound site and covered with a sterilized cotton gauze moistened with PBS to keep the bacteria growing. The infection was confirmed on day 0 post-surgery by sampling the wound area with a sterile swab, plating on MH agar, and incubating for 16 h at 37 °C. Afterwards, solutions containing the antimicrobial formulations noted above (50 μL) were dripped onto the wound and allowed to soak in prior to covering with a band-aid. Wound closure for each mouse was assessed with a digital camera on days 0, 2, 4, 6, 8, and 10. The healing progress of the wound was assessed by the following equation:
where A0 is the original wound area and At is the wound area at specified time point. For histology images, mice were euthanized at the final time point and the skin including wound tissue with adjacent normal skin was collected and fixed with 10% paraformaldehyde solution. The tissue samples were analyzed by H&E staining and images were taken with an optical microscope.
2.12. Statistical analysis.
All statistical analyses and calculations were performed using GraphPad Prism software. Data are presented as the Mean ± SD. One-way analysis of variance (ANOVA) was used as a statistical mean to analyze the differences among group means. In all evaluations p<0.05 was used as the criterion for statistical significance.
3. Results and Discussion
3.1. Polymer synthesis and characterization
Complete synthetic details and physical characterization of polymers at each step are fully described in the Supplementary Information (Scheme S1, Figure S1–S7). In general, the one-pot copolymerization of three monomers Boc-AEA, DMAPMA, and PFPA generates the random copolymer p(BocAEA-r-DMAPMA-r-PFPA) and ultimately forms the backbone of p(GEAx-r-QDHy-r-DGz), i.e. pGQ-DG. Similarly, the copolymerization of two monomers Boc-AEA and DMAPMA generates the random copolymer p(BocAEA-r-DMAPMA) to form the backbone of p(GEAx-r-QDHy), i.e. pGQ. Two additional homopolymers of equivalent length containing cationic guanidine groups (pGEA) and amphiphilic alkyl quaternary ammonium salts (pQDH) were also fabricated. Graphical and chemical representations of each polymer synthesized is summarized in Fig. 1. The zeta potential (ζ) of pGQ-DG was +40.7 mV and +38.9 mV for pGQ, homopolymer pGEA possessed a charge of +32.2 mV and pQDH possessed a charge of +23.5 mV, a value slightly lower due to presence of the short alkyl side chains. The final DG concentration on pGQ-DG was determined to be 300 μM per 1 mg/mL polymer. This was calculated by measuring the final concentration of gallium present in the polymer using atomic absorption spectroscopy (AAS), calculating the final concentration of DFO in the polymer by complexing it to Fe(III) and measuring UV-Vis absorption property at 430 nm, and taking the ratio to calculate the average number of DG per pGQ-DG. All physical characterization data for the polymers is summarized in Table 1.
Table 1.
Physical characterization, antibacterial activity and cytocompatibility of polymeric antimicrobials synthesized.
| Samples | Mn* | ζ | Numbers of Units † | MIC (μg/mL) | IC50 | HC20 | |||
|---|---|---|---|---|---|---|---|---|---|
| GEA | QDH | DG | P. aeruginosa | E. coli | J774A.1 | RBC | |||
| (g/mol) | mV | PAO1 | ATCC 25922 | (μg/mL) | (μg/mL) | ||||
| pGEA | 5400 | 29.2±1.5 | 25 | – | – | 512 | 512 | 100 | 100 |
| pQDH | 6300 | 23.5±2.3 | – | 20 | – | >2000 | 512 | 5000 | >5000 |
| pGQ | 13500 | 46.7±6.5 | 22 | 25 | – | 512 | 512 | 2000 | 5000 |
| pGQ-DG | 10200 | 38.9±3.3 | 20 | 22 | 4 | 128 | 512 | 2000 | 5000 |
based on GPC using PEG as standards.
based on 1H NMR; DG was calculated based on AAS of gallium and UV-Vis of DFO:Fe(III) at 430 nm in the final polymer
HC20: concentration causing 20% lysis (hemolysis) of RBC relative to controls
3.2. Minimum inhibitory concentration assays
Broth microdilution testing was used to determine the MIC of polymers against two Gram-negative bacteria: P. aeruginosa PAO1 and E. coli ATCC 25922 (Figure S8). These two strains were specifically chosen because PAO1 has been reported to express proper OM receptors capable of binding DFO complexes as it scavenges for iron [29]. In contrast, E. coli ATCC 25922 does not appear to possess OM receptors for DFO complexes [24, 25] and serves as a useful control for assessing the effect of incorporating DG in the targeted polymer.
Homopolymers pGEA and pQDH both exhibited similar antibacterial activity against E. coli at MICs of 512 μg/mL. Against P. aeruginosa, pGEA also had an MIC of 512 μg/mL but pQDH failed to exhibit any activity (MIC>2000 μg/mL) against the bacteria. The untargeted pGQ exhibited the same MIC of 512 μg/mL against both P. aeruginosa and E. coli. The incorporation of DG into pGQ-DG resulted in higher antimicrobial activity against P. aeruginosa (MIC of 128 μg/mL) but stayed at 512 μg/mL for E. coli. Overall, Gram-negative E. coli sensitivity to all the polymers synthesized was similar whereas P. aeruginosa sensitivity to pGQ-DG was four-fold enhanced compared to pGQ. Polymers containing QAS are reported to display antibacterial activity when the alkyl length > C4 [38], however the sensitivity can differ drastically between P. aeruginosa and E. coli due to intrinsic differences in their respective LPS structures and variable permeability properties [39], as was observed for pQDH wherein E. coli displayed >4 fold sensitivity compared to P. aeruginosa.
Previous work reported that free Ga(III) and DG exhibit similar MICs of 32 μM against P. aeruginosa PAO1 under iron-deficient conditions [29]. In contrast, we conducted our MIC assays for Ga(III) and DG in normal iron containing MHB medium and found that the MIC for Ga(III) was 640 μM and no antibacterial activity for DG was found at concentrations as high as 4 mM (Figure S9). This is likely because under iron-starved conditions, P. aeruginosa responds by upregulating either the expression of appropriate OM receptors for xenosiderophores or native siderophores (e.g. pyochelin and pyoverdine) for scavenging iron [40]. Under normal iron containing conditions, Ga(III) added actually competes with environmental iron for complexation with native siderophores, which explains the higher MIC observed (640 vs. 32 μM). DG exhibited little activity since the presence of excess environmental iron most likely resulted in the upregulation of OM receptors for native siderophores rather than xenosiderophores.
The antibacterial effect of conjugated pGQ-DG was compared to pGQ plus equivalent free DG against P. aeruginosa. The conjugated pGQ-DG resulted in an MIC of 128 μg/mL, corresponding to an equivalent DG concentration of approximately 38 μM. There was no enhanced antibacterial activity when equivalent pGQ plus Ga(III) or equivalent pGQ plus DG was added simultaneously to the media (Figure S10). These results confirm that DG conjugation to pGQ is necessary for the enhanced antibacterial effect observed in pGQ-DG. All MIC data for the polymers and controls synthesized are summarized in Table 1.
3.3. Mammalian cytocompatibility assays
Cell toxicity and hemolysis assays were used to assess cytocompatibility of polymers. Amphiphilic pQDH (IC50 of 5 mg/mL) was less toxic to J774A.1 mouse macrophage cells than cationic pGEA (IC50 of 100 μg/mL). Furthermore, since mammalian cells do not express receptors for DG, the IC50 of pGQ and pGQ-DG was confirmed to be similar for both random copolymers (2 mg/mL), thus making them less toxic to mammalian cells than cationic pGEA homopolymer (Figure S11A). Free Ga(III) or DG exhibited no toxicity to mammalian cells, up to the highest concentration tested (10 mM) (Figure S11C). Interestingly, even though the zeta potential of pGQ (i.e. pGEA-r-QDH) is +46.7 mV and that of pGQ-DG (i.e. pGEA-r-QDH-r-DG) is +38.9 mV compared to pGEA at +29.2 mV, both random polymers were still less cytotoxic to mammalian cells. This implies that the charge distribution of a polymer as well as its zeta potential contribute to the overall toxicity observed.
The hemolysis activity was conducted by incubating polymers with sheep RBC over the concentration range of 0.01–10 mg/mL for 2 h to determine HC20, defined as the concentration of the polymer capable of causing 20% lysis of RBC (Figure S11B). Similarly, the HC20 for cationic pGEA was 0.1 mg/mL, representing a concentration at least 50-fold more damaging to red cells than pQDH (HC20 > 5 mg/mL), pGQ (HC20 of 5 mg/mL), or pGQ-DG (HC20 of 5 mg/mL). Free Ga(III) or DG exhibited no RBC lysis over the same incubation period, up to the highest concentration tested (10 mM) (Figure S11D).
Ideally, an antimicrobial compound that is intended for medical applications should exhibit minimal activity against mammalian cells and high antimicrobial activity (low MIC) against bacteria. By taking into account the cytotoxicity and hemolysis profiles of all the polymers prepared and weighing that data against the MIC data, pGQ-DG exhibited the best balance of low mammalian toxicity and high antibacterial activity, making it the best candidate of all the polymers to move forward. All mammalian cytocompatibility data for the polymers are summarized in Table 1.
3.4. Mechanism of action
To investigate the mechanism of action of pGQ and pGQ-DG on the integrity of the bacterial membrane, a series of assays were conducted against P. aeruginosa. The nitrocefin assay can be used to detect for the presence of β-lactamase, an enzyme capable of hydrolyzing the dye and causing its color to change from yellow to red. In Gram-negative bacteria, mature β-lactamases are located primarily in the periplasmic space and cannot effectively cross the OM, making nitrocefin a useful reporter for successful permeabilization of gram-negative bacterial cells [41, 42]. In the presence of a permeabilizer, nitrocefin is able to cross into the periplasm wherein β-lactamase enzymes present can readily cleave it, inducing a change in nitrocefin’s absorbance properties that can be monitored at 490 nm. As shown in Fig. 2A Δ(OD490) significantly increased only upon co-incubation of nitrocefin with pGQ-DG and pGQ. Overall, bacteria treated with pGQ-DG showed highest OM permeability over the time tested, which agrees with results previously reported for membrane disrupting Pluronic F127-DG compared to F127 [24]. This data confirms that pGQ-DG can physically damage the outer membrane of P. aeruginosa.
Fig. 2.

In vitro evaluation of bacterial membrane damage by the polymeric antimicrobials. (A) Kinetics of nitrocefin hydrolysis with polymeric antimicrobials treatment. (B) Membrane potential of treated P. aeruginosa were measured in the presence of DiOC2(3) by flow cytometry, Red/green fluorescence ratio was calculated using population mean fluorescence intensities for bacteria in the presence of polymeric antimicrobials. SEM and TEM images of P. aeruginosa before (C, E) and after treatments (D, F) with pGQ-DG for 3h. The insert image in E shows a bacterium with intact OM and the insert image in F shows a bacterium with damaged membrane.
Maintaining proper cell membrane potential is vital for bacteria survival. To determine whether pGQ-DG can depolarize the bacterial cell membrane, the dynamic change of the potential across the P. aeruginosa membrane was monitored by flow cytometry using a bacterial membrane potential kit. DiOC2(3) is a dye molecule that emits green fluorescence in bacteria cells but as it becomes more concentrated in healthy cells capable of maintaining a membrane potential, the dye self-associates and the fluorescence emission shifts to red. This ratio of red to green fluorescence was employed to evaluate the membrane potential of cells in the presence of pGQ-DG and pGQ. The proton ionophore (CCCP) served as a positive control to completely depolarize the membrane. Overall, both pGQ-DG and pGQ can similarly depolarize the bacterial membrane (Fig. 2B). Although pGQ-DG displayed enhanced OM permeability compared to pGQ based on the nitrocefin assay, the membrane potential assay demonstrates that the enhanced permeability is not due to membrane depolarization by DG but depends rather on the positively charged GQ units in the polymers. This data makes sense since the role of DG is mainly to serve as a targeting ligand for select OM receptors therefore the presence of DG units increased the sensitivity of P. aeruginosa to the polymers over E. coli, likely due to expression differences in OM receptors for DG between the two bacteria.
To visualize the morphology of P. aeruginosa cells before and after treatment with pGQ-DG (2×MIC for 3 h), SEM and TEM images were obtained. For the control untreated group, no obvious morphological changes were observed, wherein cells displayed a smooth rod-shaped surface and intact membranes (Fig. 2C). In contrast, after treatment with pGQ-DG, cell integrity was visibly compromised and poorly defined (Fig. 2D). Similar results were demonstrated by TEM analysis, wherein the faint outline of the intact OM can be observed in Fig. 2E compared to the poorly defined OM observed in Fig. 2F. Overall, the combined data suggests that the antibacterial mechanism of pGQ-DG involves binding to OM receptors of DG leading to enhanced disruption of the bacterial cell wall by pGQ.
3.5. Antibacterial Time-kill Assays
Since pGQ-DG and pGQ exhibit different MICs towards P. aeruginosa, we compared the antibacterial activity of these two polymers at the same concentration of 0.5 mg/mL against PAO1. After 2 h incubation, pGQ-DG eradicated 80% of the bacteria (MIC of 128 μg/mL against P. aeruginosa) whereas pGQ (MIC of 512 μg/mL) was not as effective at decreasing the bacteria number under the same incubation period (Figure S12). To further investigate the antibacterial properties of our lead polymer pGQ-DG, time-kill assays were conducted in both P. aeruginosa and E. coli at 2×MIC for each strain and colony forming units (CFU) were assessed at 0, 2, 4, and 6 h time points. After 4 h incubation, the number of viable bacteria decreased by 99.9% for P. aeruginosa and E. coli compared to their respective 0 h timepoint (Fig. 3A); representative MH agar plates for each strain showing the comparative visual decrease in CFU after 4 h of treatment with pGQ-DG compared to t=0 controls are shown in Fig. 3B. To further confirm the antibacterial properties of pGQ-DG, live/dead bacterial planktonic cells were imaged by CLSM. All bacteria were stained with SYTO 13 (a green fluorescence nucleic acid stain) and dead bacteria were identified with red propidium iodide (PI). Images of individual channels captured for control cells (untreated) and cells treated with pGQ-DG at 2 h can be found in Figure S13–S14. As can be seen from the merged images summarized in Fig. 3C, viable bacterial cells remained visibly green throughout untreated samples whereas samples treated with pGQ-DG were stained red or yellow. These results confirm that pGQ-DG can effectively kill planktonic bacterial cells.
Fig. 3.
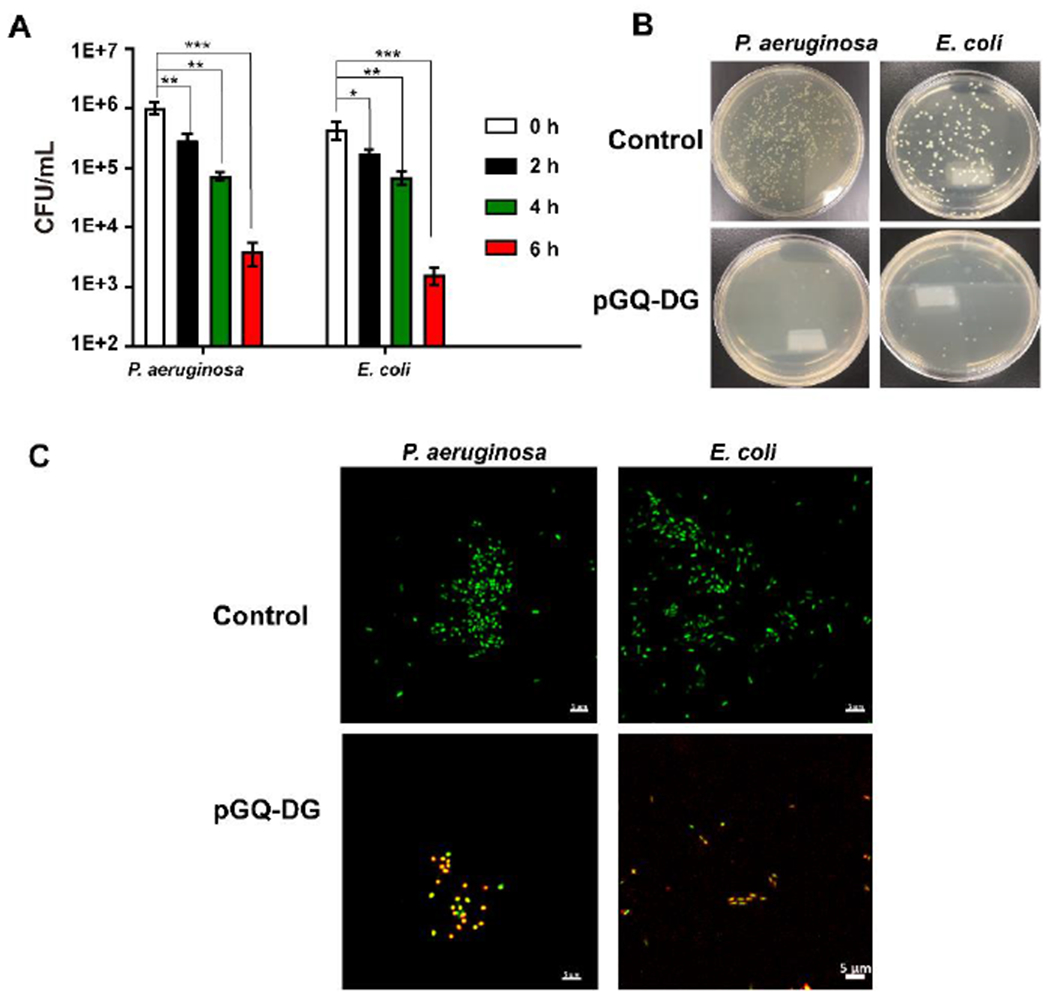
In vitro antibacterial evaluation of amphiphilic pGQ-DG. (A) Bactericidal time-kill curves of pGQ-DG at 2×MIC against P. aeruginosa PAO1 and E. coli ATCC 25922; error bars represent mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. (B) Bacterial viability evaluated by agar plate dilution method before (top) after treatments (bottom) after 2 h. (C) Merged CLSM images of Live/Dead bacteria before and after pGQ-DG treatment for 2 h. SYTO 13 (green) was used to label both live and dead bacteria and propidium iodide (red) was the stain used to identify dead bacteria (C); scale bar: 5 μm.
3.6. Potentiation properties of pGQ-DG plus VAN
To explore the combined effect of physically damaging the cell membrane with poorly permeable antibiotics, the potentiation properties of pGQ-DG at sub-MIC concentration was investigated against P. aeruginosa and E. coli using VAN as a model drug (Fig. 4). VAN is a potent glycopeptide antibiotic (MW 1449 Da) capable of inhibiting cell wall biosynthesis in Gram-positive pathogens by targeting the d-Ala-d-Ala terminus of peptidoglycan (PG) [43]. In contrast, VAN is inactive against Gram-negative pathogens due to poor diffusion through open porins (which typically have a size limit of 600 Da) before reaching its target site in the periplasm. The MIC for VAN and VAN plus pGQ-DG was determined, and the FICI was calculated to determine whether there was a synergistic interaction. As shown in Fig. 4, the FICI value of sub-MIC concentrations of pGQ-DG plus VAN was 0.31 in P. aeruginosa, which confirms synergy. In contrast, when E. coli was treated with sub-MIC concentrations of pGQ-DG plus VAN, the MIC of VAN decreased but the FICI was above 0.5, suggesting indeterminate rather than synergistic effects with the polymer. At sub-MIC, pGQ-DG enhanced the antibacterial activity of VAN against P. aeruginosa but was inconclusive for E. coli. This result also demonstrated that E. coli exhibits approximately 12-fold higher OM permeability than P. aeruginosa to the same antibiotics [39]. In addition, two carbapenem-resistant strains of P. aeruginosa were also tested and results similarly revealed that pGQ-DG enhanced the antibacterial activity of VAN in these MDR strains (Figure S15). A disruption of the P. aeruginosa OM would therefore be expected to result in more significant change in its OM permeability compared to E. coli.
Fig. 4.

Dose response curves and FICI for VAN alone and combined with pGQ-DG against two Gram-negative bacterial strains, P. aeruginosa and E. coli.
3.7. In vivo murine wound healing infection model of P. aeruginosa
The antibacterial properties of pGQ-DG plus VAN were investigated in vivo through a murine wound healing infection model of P. aeruginosa PAO1. The formulations evaluated were saline, VAN, colistin, pGQ-DG, and pGQ-DG plus VAN. A graphical representation of the experimental setup is summarized in Fig. 5A, wherein Day 0 corresponds to the first day mice were treated with antibacterial agents. Based on the IC50, 2 mg/mL pGQ-DG was used in the infection wound. Two commercially available antibiotics VAN and colistin were also examined in parallel and used at same concentration 0.8 mg/mL. To quantitatively compare the performance of various treatments, the bacteria around the wounds were cultured and counted at different time points (Fig. 5B–5C). VAN is a significant antibiotic for the treatment of infections caused by Gram-positive bacteria but is ineffective against P. aeruginosa bacterial infection. In contrast, colistin is a potent polymyxin currently used as a last resort defense against difficult MDR Gram-negative pathogens and show higher antimicrobial activity against P. aeruginosa [7]. Animals treated with pGQ-DG and VAN cleared the infection and healed much more rapidly based on wound size compared to infected mice treated with pGQ-DG alone after 10 days (Fig. 5D). In vivo therapeutic bacterial inhibition and injury recovery by pGQ-DG plus VAN was similar to colistin-treated mice but possess an important advantage in that polymeric antimicrobials have a lower probability of causing bacterial resistance due to costly restructuring of the OM by the organism [44]. The results confirm that pGQ-DG plus VAN can effectively clear P. aeruginosa infections and accelerate wound healing similar to colistin.
Fig. 5.

In vivo analysis for the cationic polymer pGQ-DG in P. aeruginosa-infected mouse knife injury model. (A) Schematic illustration for the construction of mouse knife injury model and the therapeutic profile. (B) Typical photographs of the wound at day 0, 4 and 10 after different treatment; P. aeruginosa was isolated from the wound sites and then cultured on agar at day 0 and 10. (C) Normalized number of viable P. aeruginosa isolated from wound sites at different days after different treatments, and their corresponding wound sizes (relative area versus initial area). Error bars represent the standard deviation of six repeated measurements (D). *p < 0.05, **p < 0.01.
Rate of healing for infected tissues treated with different formulations were compared on days 5 and 10 by histology (Fig. 6). At the early infection stage (day 5), wounds treated with saline and VAN displayed more neutrophils and the skin had damaged epidermis or even lacked epidermis compared to day 10. On day 5, wounds treated with pGQ-DG, pGQ-DG plus VAN, and colistin had neutrophils present still but this was also accompanied by obvious re-epithelialization of the infection sites compared to controls. This is based on the presence of many more fibroblasts (which is related to the formation of the epidermis), and hair follicles and sweat glands present in those groups compared to saline and VAN controls. By day 10, following a total of 7 treatments, pGQ-DG plus VAN and colistin effectively cleared the P. aeruginosa infection (Fig. 5) and efficiently accelerated wound healing in mice (Fig. 6).
Fig. 6.

Histologic analysis of the P. aeruginosa-infected knife injury tissue by hematoxylin and eosin (H&E) staining for different treating groups. The black arrows point to the epidermis, the yellow arrows point to fibroblasts, the white arrows point to neutrophils, and the red arrows point to hair follicles. By day 10, re-epithelialization of the tissue was accelerated in mice treated with pGQ-DG plus VAN and colistin compared to other groups. Scale bar 100 μm.
Conclusions
In conclusion, a triple combination anti-pseudomonal approach based on a random copolymer design termed pGQ-DG was investigated by 1) incorporating cationic guanidine moieties and short alkyl quaternary ammonium salts to disrupt the bacterial membrane, 2) conjugating DG to the random copolymer to further promote interactions with P. aeruginosa and disrupt iron metabolism, and 3) enhancing bacteria sensitivity to VAN. An especially appealing feature of this polymeric antimicrobial combination strategy is that synthesis of the random copolymer backbone is conducted as a simple one-pot reaction via RAFT polymerization of three acrylate monomers, followed by facile post-modifications to introduce cationic guanidine groups, amphiphilic QAS, and DFO as a xenosiderophore for iron chelation. The inhibition mechanism of pGQ-DG is likely due to disturbance of the OM via a combination of electrostatic and hydrophobic interactions coupled with selective binding via DG and resulting enhanced permeability of VAN as a result of the membrane damage. The advantage of this synergistic effect was observed in vitro and in vivo in a wound healing infection model of P. aeruginosa. Overall pGQ-DG plus VAN cleared the P. aeruginosa infection and efficiently accelerated wound healing in mice as effectively as colistin, suggesting that this strategy could serve as an alternative to colistin against MDR bacteria.
Supplementary Material
Statement of Significance.
P. aeruginosa exhibits intrinsic antibiotic resistance due to limited permeability of its outer membrane (OM). A triple combination antipseudomonal approach was investigated by 1) selectively targeting P. aeruginosa through the complex DFO:gallium, 2) disrupting the OM through a cationic random copolymer, and 3) enhancing bacteria sensitivity to VAN as a result of the OM disruption. Synthesis and characterization of the lead polymer pGQ-DG, mechanism of action, antimicrobial activity, and biocompatibility were investigated in vitro and in vivo. Overall pGQ-DG plus VAN cleared the P. aeruginosa infection and accelerated wound healing in mice as effectively as colistin, suggesting that this strategy could serve as an alternative to colistin against multidrug resistant P. aeruginosa.
Acknowledgements
This work was supported in part by NIH grant R01DK099596 awarded to M.P. Xiong.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Pendleton JN, Gorman SP, Gilmore BF, Clinical relevance of the ESKAPE pathogens, Expert Rev. Anti-infect. Ther. 11 (2013) 297–308. [DOI] [PubMed] [Google Scholar]
- [2].Rice LB, Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE, J. Infect. Dis. 197 (2008) 1079–1081. [DOI] [PubMed] [Google Scholar]
- [3].Lee JH, Jeong SH, Cha SS, Lee SH, A lack of drugs for antibiotic-resistant Gram-negative bacteria, Nat. Rev. Drug Discov. 6 (2007) 938–938. [Google Scholar]
- [4].Lautenbach E, Polk RE, Resistant gram-negative bacilli: A neglected healthcare crisis? Am. J. Health-Syst. Pharm. 64 (2007) S3–S21. [DOI] [PubMed] [Google Scholar]
- [5].Azzopardi EA, Azzopardi E, Camilleri L, Villapalos J, Boyce DE, Dziewulski P, Dickson WA, Whitaker IS, Gram Negative Wound Infection in Hospitalised Adult Burn Patients-Systematic Review and Metanalysis, PLOS ONE 9 (2014)e95042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Livermore DM, Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: Our worst nightmare? Clin. Infect. Dis. 34 (2002) 634–640. [DOI] [PubMed] [Google Scholar]
- [7].Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J, Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America, Clin. Infect. Dis. 48 (2009) 1–12. [DOI] [PubMed] [Google Scholar]
- [8].Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J, Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study, Lancet Infect. Dis. 16 (2016) 161–168. [DOI] [PubMed] [Google Scholar]
- [9].Srinivas P, Rivard K, Polymyxin Resistance in Gram-negative Pathogens, Curr. Infect. Dis. Rep.19 (2017) 38. [DOI] [PubMed] [Google Scholar]
- [10].Hancock REW, Speert DP, Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment, Drug Resist. Updat. 3 (2000) 247–255. [DOI] [PubMed] [Google Scholar]
- [11].Hancock REW, Bell A, Antibiotic uptake into gram-negative bacteria, Eur. J. Clin. Microbiol. Infect. Dis. 7 (1988) 713–720. [DOI] [PubMed] [Google Scholar]
- [12].Benz R, Hancock REW, Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes, Biochim. Biophys. Acta Biomembr. 646 (1981) 298–308. [DOI] [PubMed] [Google Scholar]
- [13].Hancock REW, Decad GM, Nikaido H, Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01, Biochim. Biophys. Acta Biomembr. 554 (1979) 323–331. [DOI] [PubMed] [Google Scholar]
- [14].Novikova OD, Solovyeva TF, Nonspecific porins of the outer membrane of Gram-negative bacteria: Structure and functions, Biochem. (Mosc) Suppl. Ser. A Membr. Cell Biol. 3 (2009) 3–15. [Google Scholar]
- [15].Li C, Lewis MR, Gilbert AB, Noel MD, Scoville DH, Allman GW, Savage PB, Antimicrobial activities of amine-and guanidine-functionalized cholic acid derivatives, Antimicrob. Agents Chemother. 43 (1999) 1347–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vaara M, Agents that increase the permeability of the outer membrane, Microbiol. Rev. 56 (1992) 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takahashi H, Caputo GA, Vemparala S, Kuroda K, Synthetic Random Copolymers as a Molecular Platform To Mimic Host-Defense Antimicrobial Peptides, Bioconjug. Chem. 28 (2017) 1340–1350. [DOI] [PubMed] [Google Scholar]
- [18].Al-Ahmad A, Laird D, Zou P, Tomakidi P, Steinberg T, Lienkamp K, Nature-Inspired Antimicrobial Polymers - Assessment of Their Potential for Biomedical Applications, Plos One 8 (2013) e73812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dizman B, Elasri MO, Mathias LJ, Synthesis and antimicrobial activities of new water-soluble bis-quaternary ammonium methacrylate polymers, J. Appl. Polym. Sci. 94 (2004) 635–642. [Google Scholar]
- [20].Andreev K, Bianchi C, Laursen JS, Citterio L, Hein-Kristensen L, Gram L, Kuzmenko I, Olsen CA, Gidalevitz D, Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes, Biochim. Biophys. Acta Biomembr.. 1838 (2014) 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Locock KES, Michl TD, Valentin JDP, Vasilev K, Hayball JD, Qu Y, Traven A, Griesser HJ, Meagher L, Haeussler M, Guanylated Polymethacrylates: A Class of Potent Antimicrobial Polymers with Low Hemolytic Activity, Biomacromolecules 14 (2013) 4021–4031. [DOI] [PubMed] [Google Scholar]
- [22].Banerjee SL, Das S, Bhattacharya K, Kundu M, Mandal M, Singha NK, Ag NPs incorporated self-healable thermoresponsive hydrogel using precise structural “Interlocking” complex of polyelectrolyte BCPs: A potential new wound healing material, Chemical Engineering Journal 405 (2021) 126436. [Google Scholar]
- [23].Qiao J, Liu Z, Purro M, Xiong MP, Antibacterial and Potentiation Properties of Charge-Optimized Polyrotaxanes for Combating Opportunistic Bacteria. J Mater Chem B 6 (2018) 5353–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Purro M, Qiao J, Liu Z, Ashcraft M, Xiong MP, Desferrioxamine:gallium-pluronic micelles increase outer membrane permeability and potentiate antibiotic activity against Pseudomonas aeruginosa, Chem. Commun. 54 (2018) 13929–13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Minandri F, Bonchi C, Frangipani E, Imperi F, Visca P, Promises and failures of gallium as an antibacterial agent, Future Microbiol. 9 (2014) 379–397. [DOI] [PubMed] [Google Scholar]
- [26].Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK, The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity, J. Clin. Invest. 117 (2007) 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Goss CH, Kaneko Y, Khuu L, Anderson GD, Ravishankar S, Aitken ML, Lechtzin N, Zhou G, Czyz DM, McLean K, Olakanmi O, Shuman HA, Teresi M, Wilhelm E, Caldwell E, Salipante SJ, Hornick DB, Siehnel RJ, Becker L, Britigan BE, Singh PK, Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections, Sci. Transl. Med. 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].DeLeon K, Balldin F, Watters C, Hamood A, Griswold J, Sreedharan S, Rumbaugh KP, Gallium Maltolate Treatment Eradicates Pseudomonas aeruginosa Infection in Thermally Injured Mice, Antimicrob. Agents. Chemother. 53 (2009) 1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, Greenberg EP, Banin E, The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent, Proc. Natl. Acad. Scic U.S.A 105 (2008) 16761–16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Antunes LCS, Imperi F, Minandri F, Visca P, In Vitro and In Vivo Antimicrobial Activities of Gallium Nitrate against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents. Chemother. 56 (2012) 5961–5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hijazi S, Visaggio D, Pirolo M, Frangipani E, Bernstein L, Visca P, Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens, Front. Cell. Infect. Microbiol. 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu Z, Qiao J, Nagy T, Xiong MP, ROS-triggered degradable iron-chelating nanogels: Safely improving iron elimination in vivo. J. Control. Release 283 (2018) 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu Z, Purro M, Qiao J, Xiong MP, Multifunctional Polymeric Micelles for Combining Chelation and Detection of Iron in Living Cells. Adv. Healthc. Mater. 6 (2017)1700162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu Z, Simchick GA, Qiao J, Ashcraft MM, Cui S, Nagy T, Zhao Q, Xiong MP, Reactive Oxygen Species-Triggered Dissociation of a Polyrotaxane-Based Nanochelator for Enhanced Clearance of Systemic and Hepatic Iron. ACS Nano (2020) 10.1021/acsnano.0c01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Eriksson M, Nielsen PE, Good L, Cell permeabilization and uptake of antisense peptide-peptide nucleic acid (PNA) into Escherichia coli. J. Biol. Chem. 277 (2002) 7144–7. [DOI] [PubMed] [Google Scholar]
- [36].Odds FC, Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52 (2003) 1–1. [DOI] [PubMed] [Google Scholar]
- [37].Chen WY, Chang HY, Lu JK, Huang YC, Harroun SG, Tseng YT, Li YJ, Huang CC, Chang HT, Self-Assembly of Antimicrobial Peptides on Gold Nanodots: Against Multidrug-Resistant Bacteria and Wound-Healing Application, Adv. Funct. Mater. 25 (2015) 7189–7199. [Google Scholar]
- [38].Zhang AQ, Liu QQ, Lei YF, Hong SH, Lin YL, Synthesis and antimicrobial activities of acrylamide polymers containing quaternary ammonium salts on bacteria and phytopathogenic fungi. React. Funct. Polym. 88 (2015) 39–46. [Google Scholar]
- [39].Zgurskaya HI, Lopez CA, Gnanakaran S, Permeability Barrier of Gram-Negative Cell Envelopes and Approaches To Bypass It, ACS Infect. Dis. 1 (2015)512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cornelis P, Dingemans J, Pseudomonas aeruginosa adapts its iron uptake strategies in function of the type of infections. Front. Cell. Infect. Mibrol. 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Epand RF, Pollard JE, Wright JO, Savage PB, Epand RM, Depolarization, Bacterial Membrane Composition, and the Antimicrobial Action of Ceragenins, Antimicrob. Agents Chemother. 54 (2010) 3708–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Eriksson M, Nielsen PE, Good L, Cell permeabilization and uptake of antisense peptide-peptide nucleic acid (PNA) into Escherichia coli, J. Biol. Chem. 277 (2002) 7144–7147. [DOI] [PubMed] [Google Scholar]
- [43].Walsh CT, Fisher SL, Park IS, Prahalad M, Wu Z, Bacterial resistance to vancomycin: Five genes and one missing hydrogen bond tell the story, Chem. Biol. 3 (1996) 21–28. [DOI] [PubMed] [Google Scholar]
- [44].Kamaruzzaman NF, Tan LP, Hamdan RH, Choong SS, Wong WK, Gibson AJ, Chivu A, Pina M. d. F, Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J .Mol. Sci. 20 (2019) 2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


