Abstract
Background and aims:
Atherosclerosis progression and regression studies are related to its prevention and treatment. Although we have gained extensive knowledge on germline phospholipid transfer protein (PLTP) deficiency, the effect of inducible PLTP deficiency in atherosclerosis remains unexplored.
Methods:
We generated inducible PLTP (iPLTP)-knockout (KO) mice and measured their plasma lipid levels after feeding a normal chow or a Western-type diet. Adenovirus associated virus-proprotein convertase subtilisin/kexin type 9 (AAV-PCSK9) was used to induce hypercholesterolemia in the mice. Collars were placed around the common carotid arteries, and atherosclerosis progression and regression in the carotid arteries and aortic roots were evaluated.
Results:
On a normal chow diet, iPLTP-KO mice exhibited decreased cholesterol, phospholipid, apoA-I, and apoB levels compared with control mice. Furthermore, the overall amount of high-density lipoprotein (HDL) particles was reduced in these mice, but this effect was more profound for larger HDL particles. On a Western-type diet, iPLTP-KO mice again exhibited reduced levels of all tested lipids, even though the basal lipid levels were increased. Additionally, these mice displayed significantly reduced atherosclerotic plaque sizes with increased plaque stability. Importantly, inducible PLTP deficiency significantly ameliorated atherosclerosis by reducing the size of established plaques and the number of macrophages in the plaques without causing lipid accumulation in the liver.
Conclusions:
Induced PLTP deficiency in adult mice reduces plasma total cholesterol and triglycerides, prevents atherosclerosis progression, and promotes atherosclerosis regression. Thus, PLTP inhibition is a promising therapeutic approach for atherosclerosis.
Keywords: Inducible PLTP gene knockout mice, Lipoprotein metabolism, Atherosclerosis progression and regression, Stability of atherosclerotic plaque
1. Introduction
Plasma phospholipid transfer protein (PLTP) mediates the transfer of phospholipids from very low-density lipoproteins (VLDLs) and chylomicrons into high-density lipoproteins (HDLs) [1]. PLTP can also act as a putative fusion factor to enlarge HDLs [2]. Thus, PLTP activity influences both non-HDL and HDL metabolism. Importantly, human genome-wide association studies have shown that human PLTP levels are positively associated with plasma cholesterol levels [3].
Increased PLTP expression has been established in different pathologies associated with increased risk of human coronary artery disease (CAD), including obesity, insulin resistance, and type II diabetes. Similarly, we have reported that CAD patients increased their serum PLTP activity [4]. In the last decade, the majority of human studies have demonstrated a positive association between plasma PLTP activity and atherosclerosis. Vergeer et al. reported that lower hepatic PLTP transcription and plasma PLTP activity led to a decreased risk of cardiovascular events among five cohorts comprising 4658 patients and 11, 459 controls [5]. In a Framingham Heart Study, Robins et al. [6] found that higher plasma PLTP activity predicted first cardiovascular events, defined as fatal or non-fatal coronary heart disease or stroke, in men. Moreover, PLTP activity has been positively correlated with left ventricular systolic dysfunction [7]. After controlling for a variety of baseline variables, we identified plasma PLTP activity as a strong and independent predictor of all-cause mortality within 5 years in a cohort of 170 high-risk diabetic men [8].
In mouse models, germline PLTP deficiency reduced atherosclerotic lesion size [9], whereas its overexpression showed the opposite effect [10]. The germline PLTP-knockout (KO) mice have provided extensive information on the role of PLTP in plasma lipoprotein metabolism and atherogenesis. However, germline deficiency does not necessarily represent the best technical approach to study the function of a protein or enzyme in vivo, particularly in adult mice. Indeed, we found the difference between germling deficiency and inducible deficiency on serine palmitoyltransferase [11,12]. Another example for this was liver kinase B1 deficiency [13,14]. Germline deficiency might not overcome some compensating effects, which could be initialed at early stage of life and could influence the phenotypes, observed in adult. Importantly, germline deficiency could not allow us to evaluate atherosclerosis regression.
Atherosclerosis progression is related to disease prevention, whereas atherosclerosis regression is related to disease treatment. Atherosclerosis regression studies are currently in their initial stages and much progress is needed. In this study, we have generated a mouse model in which PLTP deficiency is inducible during adulthood, thus providing a tool to evaluate both atherosclerosis progression and regression.
2. Materials and methods
2.1. Mice and diets
In this study, the previously generated PLTP-Flox-△Neo mice [15] were crossed with ubiquitin C-Cre-estrogen receptor T2 (UBC-Cre-ERT2) transgenic mice. Tamoxifen was used to induce the estrogen receptor, which subsequently induced the UBC promoter-mediated Cre recombinase expression (Supplementary Fig. 1A). Tamoxifen (80 μg/g) was intraperitoneally injected into male and female PLTP-Flox-△Neo/UBC-Cre-ERT2 mice (12–16 weeks old), a total of three times every other day to produce the global inducible PLTP-knockout (iPLTP-KO) mice. Tamoxifen injected PLTP-Flox-△Neo mice were used as a control. The genetic background of the mice used in this study was C57BL/6. The mice were fed either a normal chow (Research Diets, Inc.) or a Western-type diet (0.15% cholesterol, 20% saturated fat; Research Diets, Inc.). All animal experiments were conducted under the approval of the SUNY Downstate Medical Center IACUC.
2.2. AAV8-D377Y-mPCSK9 treatment
Adenovirus-associated virus proprotein convertase subtilisin/kexin type 9 (AAV8-D377Y-mPCSK9 (Penn Vector, University of Pennsylvania) was injected to create an LDL receptor deficiency-like phenotype in WT and iPLTP-KO mice, as previously reported [16]. AAV8-D377Y-mPCSK9 was diluted to a concentration of 2 × 1011 vector genomes in 100 μL sterile saline. To study atherosclerosis progression, the 100 μL solution was intravenously injected into mice on the Western-type diet to reach plasma cholesterol levels of approximately 500 mg/dL. To study atherosclerosis regression, 180 μL of the solution was intravenously injected into mice on the Western-type diet to maintain plasma cholesterol levels greater than 1000 mg/dL.
2.3. Lipid and lipoprotein measurements
Fasting plasma samples were collected for fast protein liquid chromatography (FPLC) separation and lipid measurements. Plasma total cholesterol, phospholipids, and triglycerides were assayed by enzymatic methods (Wako Pure Chemical Industries Ltd., Osaka, Japan). Plasma apoE, apoB, and apoA-I levels were determined as follows. Briefly, 0.2 μL plasma was separated by 4–15% SDS gel electrophoresis and immunoblotted with polyclonal antibodies against apoE (Abcam), apoB (US Biological), and apoA-I (Biodesign).
2.4. Plasma lipoproteins analysis with nuclear magnetic resonance (NMR) spectroscopy
Plasma lipoprotein analysis was conducted on a 600 MHz NMR spectrometer (Bruker Biospin), as previously reported [17] with some minor modifications. Briefly, 110 μL of each plasma sample was mixed with 110 μL of 0.085 M phosphate buffer containing 10% D2O [18], and 200 μL of the mixture was transferred into a 3-mm NMR tube for NMR analysis. A total of 151 plasma parameters were quantified using a server-based software package (Bruker Biospin), including 112 lipoprotein parameters (main fractions, subclasses, and their compositional components) and 39 small metabolites (e.g., amino acids, ketone bodies, glucose, carboxylic acids, ethanol). An additional 186 ratio-parameters (e.g., cholesterol-to-triglyceride ratio, percentage of triglycerides, and cholesterol in total lipids) were calculated from the quantitative lipoprotein data. A total of 337 quantitative parameters were obtained and collectively employed to define the metabolomic phenotypes of each plasma sample.
2.5. Native polyacrylamide gel electrophoresis (PAGE) for plasma lipoproteins
Native PAGE was performed with a previously reported system [19] using three sets of plasma samples (WT, iPLTP-KO, and iPLTP-KO + recombinant PLTP [rPLTP, 10 μg/mL]). All samples were incubated at 37 °C for 4 h, and 50 μL of each sample was loaded into the gel for PAGE.
2.6. PLTP activity assay
Plasma from tamoxifen treated PLTP-Flox-△Neo mice (control) and PLTP-Flox-△Neo/UBC-Cre-ERT2 mice (experimental), male and female, was used to measure PLTP activity as previously reported [20]. Briefly, combine 2 μl donor and 50 μl acceptor with 3 μl mouse plasma and 45 μl 10 mM tris, 150 mM NaCl, 1 mM EDTA, pH 7.4. The mixture was incubated at 37 °C for 20 min. Read fluorescence intensity on spectrometer at excitation wavelength of 465 nm and emission wavelength of 535 nm.
2.7. Carotid collar positioning surgery
Collars were prepared from Silastic tubing (Dow Corning) and were autoclaved until further use. Mice were anesthetized by intraperitoneal injection of ketamine/xylazine. The anterior cervical triangles were accessed through a sagittal anterior neck incision. The right carotid sheath was opened, and the common carotid artery was dissected from the surrounding connective tissue, avoiding damage to the vagus nerves and carotid bodies. Collars were placed bilaterally around the common carotid arteries, and their axial edges were approximated by placement of two circumferential silk ties. Subsequently, the skin incision was closed, and the animals were returned to their cage to recover from the anesthesia.
2.8. Atherosclerosis progression study
Supplementary Fig. 1A depicts the strategy for the atherosclerosis progression study. PLTP-Flox-△Neo (control) and PLTP-Flox-△Neo/UBC-Cre-ERT2 (experimental) mice were first treated with AAV8-D377Y-mPCSK9. After 1 week, plasma cholesterol levels were measured, and all mice exhibited LDL receptor deficiency-like cholesterol levels (~150 mg/dL). The mice were then fed the Western-type diet for 2 weeks, and plasma lipids were measured to confirm hypercholesterolemia. Then, the mice were injected with tamoxifen (80 μg/g) a total of three times every other day to deplete PLTP. The mice remained on the Western-type for 5 more weeks, and then carotid collar surgery was performed. Atherosclerotic lesions were measured 2 and 4-weeks after surgery, and the lesion contents and plaque stability were measured 10-weeks after surgery.
2.9. Atherosclerotic plaque size measurement
Carotid samples were fixed in 4% formaldehyde for 24 h and embedded in optimal cutting temperature (OCT) compound. Samples were cut into a 6-μm thickness at 50-μm intervals. Sections were stained with hematoxylin and eosin (H&E) for analysis of overall morphology.
For the en face assays, the entire aorta was removed, cleaned, opened longitudinally, and then stained with oil-red O for 30 min at room temperature. The percentage of the stained area was determined using image analysis software (Image-Pro-Plus).
2.10. Atherosclerotic plaque content measurements and stability evaluation
Frozen sections were stained with oil-red O (Sigma) to determine the lipid content, and Masson’s trichrome staining was used to visualize the collagen content (Thermo Scientific). Sections were incubated with rabbit anti-mouse α-smooth muscle cell (α-SMC) antibody (1:200, Sigma) or rat anti-mouse monocyte/macrophage antibody (MOMA)-2 (1:100, Abcam) to detect SMCs and macrophages, respectively. The vulnerability index was calculated as macrophages (%) + lipids (%)/SMCs (%) + collagen (%), as previously described [21]. Staining areas were quantified with Image-Pro-Plus software.
2.11. Atherosclerosis regression study
Supplementary Figure 1B depicts the strategy for the atherosclerosis regression study. PLTP-Flox-△Neo/UBC-Cre-ERT2 and PLTP-Flox-△Neo mice were first treated with AAV8-D377Y-mPCSK9, and all mice were fed the Western-type diet for 20 weeks. At week 16, the carotid collar positioning surgery was performed, as described above. At week 20, a set of mice were sacrificed for atherosclerotic baseline establishment. The rest of the mice were injected with tamoxifen (80 μg/g) a total of three times every other day to deplete PLTP. Then, all the mice were fed a normal chow diet for another 8 weeks, and the atherosclerotic lesions were measured at week 28.
2.12. Statistical analysis
Each experiment was conducted at least three times. Data were expressed as the mean ± standard deviation (SD). Differences between two groups were analyzed by the unpaired, two-tailed Student’s t-test, and differences among multiple groups were assessed by analysis of variance (ANOVA), followed by the Student-Newman-Keuls test. p-values < 0.05 were considered significant.
3. Results
3.1. Inducible PLTP deficiency decreases plasma PLTP activity
We previously generated homozygous PLTP-Flox-△Neo mice and found that these animals have normal plasma PLTP activity and cholesterol and phospholipid levels [15]. Next, we crossed these PLTP-Flox-△Neo mice with ubiquitin C-Cre-estrogen receptor T2 (UBC-Cre-ERT2) transgenic mice (Supplementary Fig. 2A and B) to obtain PLTP-Flox-△Neo/UBC-Cre-ERT2 transgenic mice. After tamoxifen treatment, we obtained global iPLTP KO mice with a 90% reduction in plasma PLTP activity (Supplementary Fig. 2C), compared with tamoxifen treated PLTP-Flox-△Neo mice in both male and female mice. There was no gender difference.
3.2. Inducible PLTP deficiency decreases mouse plasma cholesterol and phospholipid levels
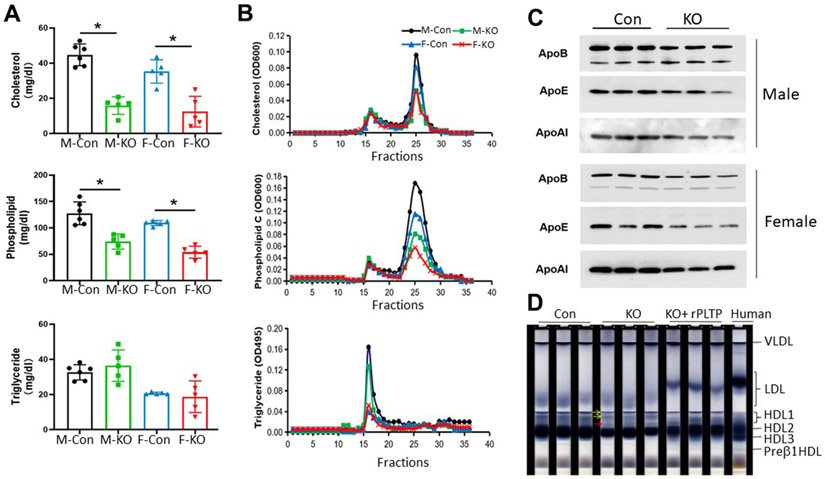
We found that iPLTP deficiency in both male and female mice significantly decreased plasma cholesterol (65% and 64%, p < 0.001; Fig. 1A) and phospholipid (42% and 51%, p < 0.01; Fig. 1A) levels, but it had no significant effect on triglyceride levels (Fig. 1A).
Fig. 1. Plasma lipoprotein analysis of iPLTP-KO and control mice on chow diet.
(A) Plasma cholesterol, phospholipid, and triglyceride measurements in male and female iPLTP-KO and control mice. (B) Fast protein liquid chromatography analysis of plasma lipid distribution using pooled plasma samples from male and female iPLTP-KO or control mice. (C) Western blot of apoA-I, apoB (top band, apoB100; bottom band, apoB48), and apoE levels. (D) Native polyacrylamide gel electrophoresis for lipoprotein separation. Mouse and human plasma (50 μL) samples were pre-stained with Sudan Black and then run on our system (Materials and Methods). The two large HDLs (yellow arrows) not existing in human plasma were detected in mouse plasma samples. Inducible PLTP deficiency resulted in a new HDL particle formation (red arrow). Addition of rPLTP into iPLTP-KO mouse plasma resulted in the formation of human-like LDL and a preβ1-HDL. M-Con, male controls; M-KO, male iPLTP-KO mice; F-Con, female controls; F–KO, female iPLTP-KO mice. Values the are mean ± standard deviation (n = 5, *p < 0.01).
Plasma lipid distributions were also examined by FPLC using pooled plasma samples. We observed decreased plasma cholesterol levels in the HDL fraction, but not in the non-HDL fraction, from iPLTP-KO mice compared with those of the controls (Fig. 1B). This was also observed for total phospholipid distribution (Fig. 1B). Although female mice had lower triglyceride levels than male mice, there was no difference between WT and iPLTP-KO mice, regardless of sex (Fig. 1B).
Next, we assessed plasma apolipoprotein levels by SDS-PAGE and immunoblot and found that both male and female iPLTP-KO mice exhibited significantly reduced apoB (25–30%, p < 0.01); apoE (40–60%, p < 0.01), and apoA-I (30–40%, p < 0.01) levels compared with sex-matched controls (Fig. 1C and Supplementary Fig. 3A). Collectively, induced PLTP deficiency had a significant effect on all lipoproteins in adult mice.
Because plasma PLTP mediates the transfer of lipids from VLDL into HDL, PLTP activity can influence both VLDL and HDL levels. We used NMR to isolate five VLDL subclasses and four HDL subclasses to precisely observe VLDL and HDL alterations. We then measured triglyceride levels of VLDLs and cholesterol and phospholipid levels of HDLs. Inducible PLTP deficiency significantly reduced triglyceride levels of VLDL5, the smallest VLDL particle (Supplementary Table 1). This was also observed for VLDL4, the second smallest VLDL particle; however, the difference was not statistically significant. The deficiency elicited a more profound effect on the reduction of all HDL particles; however, it was much stronger for larger HDLs. Collectively, inducible PLTP deficiency had significant effects on small VLDLs and large HDLs.
To precisely examine the alterations of lipoproteins, particularly HDLs, we used our previously developed native PAGE system to separate lipoproteins. As previously reported [19], our native PAGE system separated total human HDL into four fractions (preβ1-HDL [5%], HDL1 [larger ones, 5%], HDL2 [50%], and HDL3 [40%]), as well as an LDL fraction (Fig. 1D). Mice have a different lipoprotein pattern compared to humans. They have a major HDL (90%) with a similar migration rate as that of human HDL2. Mice also have large and very large HDLs, which do not exist in human plasma. Importantly, mice have no human-like preβ1-HDL and human-like HDL3 (Fig. 1D). We found that iPLTP-KO mice exhibited reduced levels of two large HDLs and the major HDL; however, a new fraction of HDL was present. Additionally, these mice exhibited reduced LDL which has a faster migration rate than that of humans (Fig. 1D). Interestingly, we found that the addition of rPLTP into the iPLTP-KO plasma resulted in human-like LDL and preβ1-HDL (Fig. 1D), suggesting that rPLTP can mediate lipoprotein conversion in vitro.
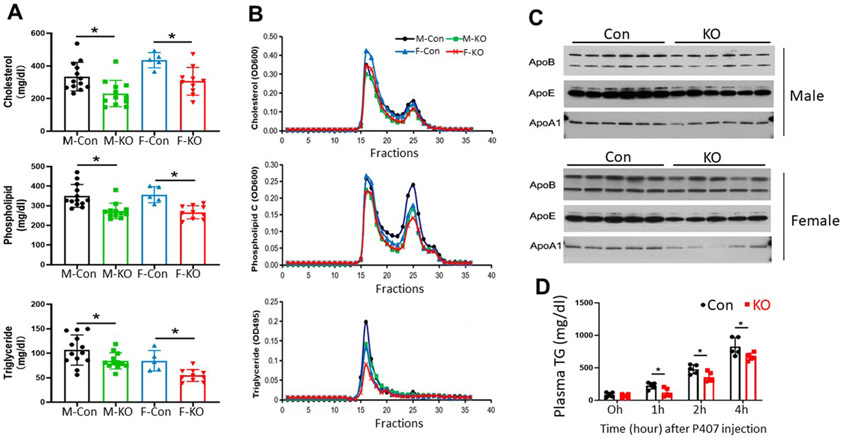
We next used AAV8-D377Y-mPCSK9 intravenous injection to create an LDL receptor deficiency-like phenotype in our control (PLTP-Flox-△Neo) and experimental (PLTP-Flox-△Neo/UBC-Cre-ERT2) mice to examine the effect of inducible PLTP deficiency on plasma lipoprotein levels during hyperlipidemia. The mice were fed the Western-type diet (Materials and methods), and plasma lipids were measured after 3 weeks. All control mice had high plasma cholesterol levels (340–480 mg/dL) and females had higher cholesterol levels than males (Fig. 2A), indicating hypercholesterolemia. However, these effects were not as severe as those observed in LDL-receptor-KO mice, with plasma cholesterol levels reaching extremely high levels (1500–2500 mg/dL) while on the Western-type diet [22].
Fig. 2. Plasma lipoprotein analysis of iPLTP-KO and control mice on the Western-type diet.
(A) Plasma cholesterol, phospholipid, and triglyceride measurements in male and female iPLTP-KO and control mice. (B) Fast protein liquid chromatography analysis of plasma lipid distribution using pooled plasma samples from male and female iPLTP-KO or control mice. (C) Western blot of apoA-I, apoB (top band, apoB100; bottom band, apoB48), and apoE levels. (D) VLDL production measurements of mice. Male iPLTP-KO and control mice fed the Western-type diet for 3 weeks. Mice were fasted for 16 h and then intraperitoneally injected with Poloxamer 407 (1 mg/g) to block VLDL clearance. Plasma triglyceride tri was measured at 0, 1, 2, and 4 hour. Con, controls; KO, PLTP KO mice. h, hour. Values are the mean ± standard deviation (n = 5–6), *p < 0.01).
We found that iPLTP-KO male and female mice showed significant decreases in plasma cholesterol (33–38%, p < 0.01), phospholipid (21–36%, p < 0.01), and triglyceride (25–35%, p < 0.01) levels compared with the controls (Fig. 2A). These changes were confirmed by FPLC (Fig. 2B), and apoB, apoE, and apoA-I levels were significantly reduced (Fig. 2C and Supplementary Fig. 3B). Collectively, PLTP deficiency induced in adult mice with PCSK9 overexpression and the Western-type diet had a significant effect on all circulating lipoproteins.
To determine whether iPLTP had an effect on VLDL production, we evaluated liver triglyceride production rate using Poloxamer 407 to block VLDL clearance from the circulation. We found that total PLTP depletion significantly reduced triglyceride production (Fig. 2D).
3.3. Inducible PLTP deficiency attenuates carotid artery atherogenesis
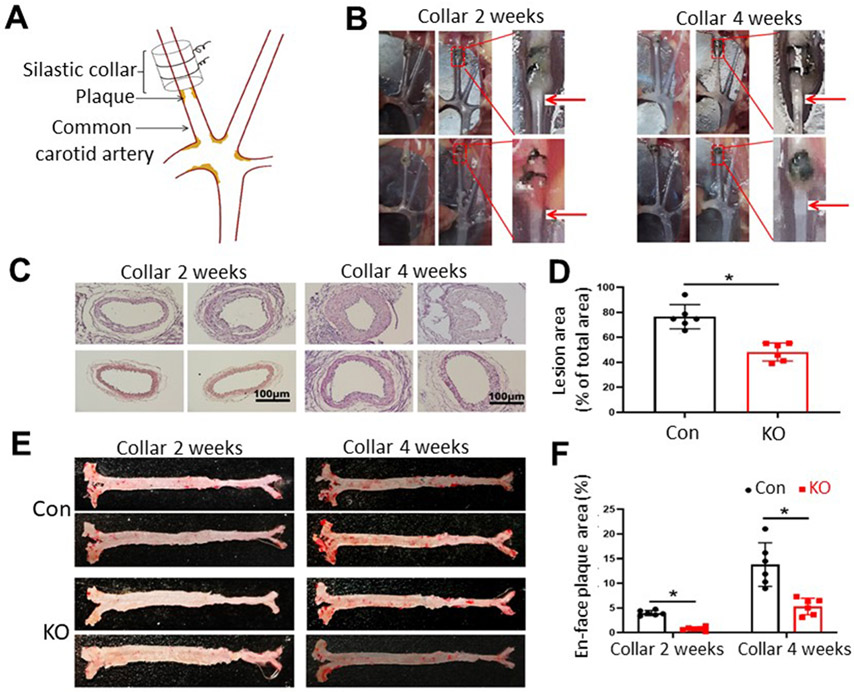
In order to investigate the effect of inducible PLTP deficiency in the early stage of atherogenesis, we performed carotid color surgery on AAV8-D377Y-mPCSK-treated mice fed a Western-type diet (Fig. 3A). These mice exhibited minor hypercholesterolemia (Fig. 2A), and the carotid collar placement accelerated the development of atherosclerosis. All control mice had detectable atherosclerotic lesions 2 weeks after collar placement, whereas iPLTP-KO mice did not (Fig. 3B and C). After 4-weeks, both control and iPLTP-KO mice had lesions; however, iPLTP-KO mice had significantly smaller lesions than controls (Fig. 3B-D). Next, we stained whole aortas with Oil Red O, 2 and 4 weeks after collar placement, and found that iPLTP-KO mice displayed barely detectable aortic lesions at 2 weeks in contrast to control mice (Fig. 3E and F). At 4 weeks, iPLTP-KO mice exhibited accumulated aortic lesions; however, control mice still had significantly more lesions (Fig. 3E and F). Importantly, the Western-type diet did not cause more triglyceride accumulation in the PLTP-deficient livers (Supplementary Fig. 4A).
Fig. 3. Atherosclerosis progression in male iPLTP-KO and control mice.
We used the strategy described in Supplementary Figure IA. (A) Illustration of carotid collar surgery. (B) Collared carotid artery with atherosclerotic plaques (red arrows). (C) Hematoxylin and eosin staining of collared carotid artery, 2 and 4 weeks after carotid collar surgery. At 2 weeks, all control mice exhibited lesions (8/8), whereas iPLTP-KO mice did not (0/8). At 4 weeks, both mice exhibited lesions, but iPLTP-KO mice had smaller-sized lesions than controls. (D) Quantification of lesion size 4 weeks after surgery. (E) En face aortic plaque analysis after Oil Red O staining. (F) Quantification of whole-aortic lesion area (en face). Con, controls; KO, iPLTP-KO mice. Values are the mean ± standard deviation (n = 6, *p < 0.01).
3.4. Inducible PLTP deficiency stabilizes atherosclerotic plaques
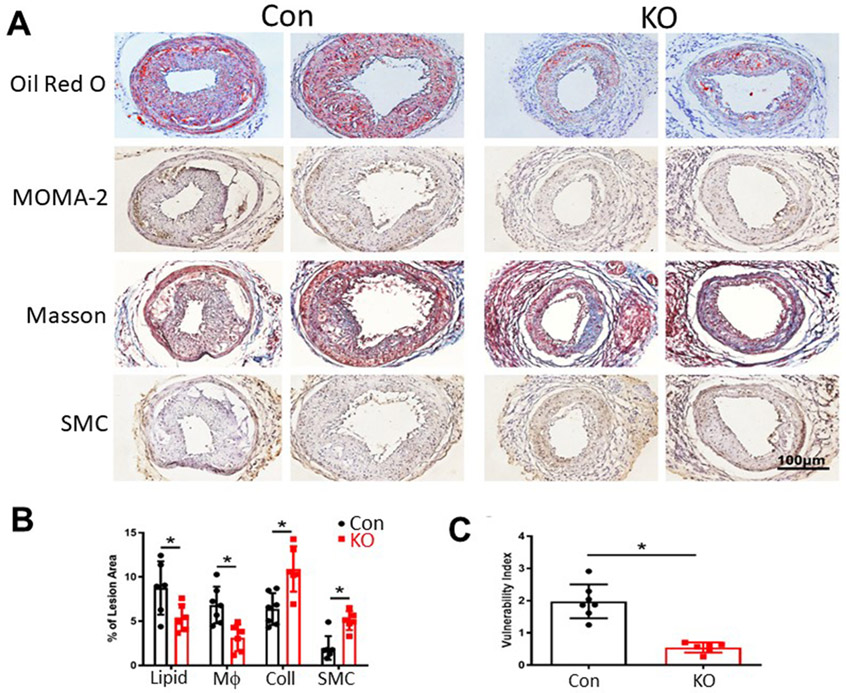
Next, we evaluated whether inducible PLTP deficiency played a role in lipid content as well as the contents of macrphages, smooth muscle cells (SMC) and collagen in atherosclerotic plaques after 10 weeks of the Western-type diet. We found that iPLTP-KO mice had significantly lower lipid and macrophage levels and significantly higher collagen and SMC levels compared with the controls (Fig. 4A and B). Based on these measurements, we calculated the plaque vulnerability index, which was significantly lower for iPLTP-KO mice (p < 0.01; Fig. 4C), indicating that inducible PLTP deficiency increased atherosclerotic plaque stability.
Fig. 4. Atherosclerotic plaque lipid, macrophage, collagen, and smooth muscle cell (SMC) staining and plaque stability analysis.
(A) Frozen sections were used. Lipids were stained with Oil Red O; macrophages were immunostained with rat anti-mouse-monocyte/macrophage antibody (MOMA2); collagen was stained with Masson’s trichrome; and SMCs were immunostained with rabbit anti-mouse oc-SMC antibody. (B) Staining quantification. (C) Vulnerability index calculation. Con, controls; KO, iPLTP-KO mice. Coll, collage; Mϕ, macrophage. Values are the mean ± standard deviation (n = 6–7, *p < 0.01).
3.5. Inducible PLTP deficiency promotes atherosclerosis regression
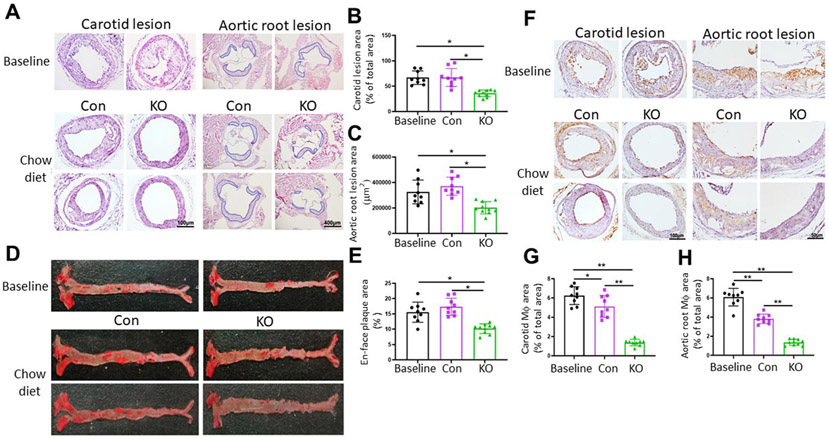
We used male PLTP-Flox-△Neo/UBC-Cre-ERT2 and PLTP-Flox-△Neo mice to study atherosclerosis regression. To achieve severe hyperlipidemia and to fully induce atherosclerotic lesions, we increased the AAV8-D377Y-mPCSK9 dose by 80%, resulting in plasma cholesterol levels greater than 1000 mg/dL (Supplementary Fig. 5), which were-close to those observed in the LDL receptor deficiency-like condition with the Western-type diet. After 20 weeks, a set of the mice were sacrificed for atherosclerotic baseline establishment. We found well-developed plaques in both the carotid arteries and aortic roots (Fig. 5A). The rest of the mice were treated with tamoxifen for PLTP depletion in PLTP-Flox-△Neo/UBC-Cre-ERT2 mice, and PLTP-Flox-△Neo mice were used as controls. The mice were fed chow diet for another 8 weeks, and the lesions were examined at week 28 (Supplementary Fig. 1B). Similar to a previous report [23], normal chow diet replacement had no effect on lesion size (Fig. 5A-E). However, PLTP deficiency significantly reduced lesion size in the carotid artery, aortic root, and whole aorta (Fig. 5A-E), indicating that it effectively ameliorated atherosclerosis. Moreover, we monitored plasma cholesterol levels during the entire 28 weeks and found that they were significantly reduced at week 22 (Supplementary Fig. 5), which was possibly due to the tamoxifen treatment and normal chow diet replacement.
Fig. 5. Atherosclerosis regression in male iPLTP-KO and control mice.
We used the strategy described in Supplementary Fig. 1B. (A) Hematoxylin and eosin staining of collared carotid artery and aortic root with atherosclerotic plaques (lesion areas highlighted by blue lines). (B and C) Quantification of lesion area of collared carotid artery and aortic root. D. Whole aorta (en face) with atherosclerotic plaques stained with Oil Red O. (E) Quantification of whole-aortic lesion area (en face). (F) Frozen aortic root sections were immunostained with rat anti-mouse monocyte/macrophage antibody (MOMA-2). (G) Quantification of macrophages in carotid plaques. (H) Quantification of macrophages in aortic root plaques. Con, controls; KO, iPLTP-KO mice. Values are the mean ± standard deviation (n = 9–10, *p < 0.01).
We also found that the chow diet replacement significantly reduced the number of macrophages (Fig. 5F-H) in carotid artery and aortic root plaques compared with baseline levels, although the plaque sizes were not significantly altered (Fig. 5A-C). Importantly, PLTP deficiency significantly reduced the number of macrophages in these plaques (Fig. 5F-H), suggesting that PLTP depletion might promote macrophage efferocytosis within the plaques. We also measured liver triglyceride levels at the end of the study and again found no accumulation in the PLTP-deficient livers (Supplementary Fig. 4B).
4. Discussion
In this study, we found that global inducible PLTP deficiency 1) caused a significant reduction in cholesterol, phospholipid, apoA-I, apoB, and apoE levels as well as the VLDL production rate; 2) significantly attenuated atherosclerosis development in a PCSK9-overexpression mouse model; 3) promoted atherosclerotic plaque stability; and 4) significantly ameliorated atherosclerosis, as demonstrated by diminished lesion sizes and plaque macrophages. These results indicate that it is possible to manipulate plasma lipoprotein levels toward an anti-atherogenic profile by inhibiting PLTP activity before and after the formation of atherosclerosis.
Using our previous germline PLTP-KO mice, we discovered that PLTP deficiency reduced atherosclerotic lesion size [9]. However, this concept was not tested in mice with PLTP deficiency during adulthood. Although germline-deficient animals were used to study disease prevention (i.e., inhibition of atherosclerosis progression), they could not be used to study disease treatment, (i.e., regression of fully developed atherosclerosis). There are certain approaches to deplete genes in adulthood. Besides inducible Cre expression, adenovirus associated virus-mediated shRNA, specific monoclonal antibody treatment, and antisense oligonucleotide were used to deplete genes in adult animals. In this study, we used iPLTP-KO mice to examine the effects of PLTP depletion during adulthood and its impact on lipoprotein metabolism and atherosclerosis during both disease progression and regression.
We used carotid collar placement surgery to accelerate the development of atherosclerosis in our mouse model, and like most of the researchers, we used root assay to evaluate the atherosclerotic lesions. The whole process required 3-4-months. In our previous studies, atherosclerosis analyses could be conducted in LDL receptor-KO mice until after 4 months of the Western-type diet [22] or in apoE-KO mice until after 6 months of the chow diet [24]. However, carotid collar placement allowed us to examine atherosclerotic lesions at a very early stage during atherosclerosis development, and we found that inducible PLTP deficiency prevented lesion formation at 2-weeks (Fig. 3B and C). Additionally, the surgery was simple, and no mortality was observed. We also evaluated the content, size, and stability of well-developed plaques at 10-weeks (Fig. 4).
PCSK9 overexpression and the Western type diet feeding in mice can initial minor hypercholesterolemia (plasma cholesterol levels are 340–480 mg/dl) (Fig. 2A). This approach provides a way to enable hypercholesterolemia becoming more controllable. We can very precisely see the initiation of the atherosclerosis under minor hypercholesterolemia. Indeed, 2-week on the Western type diet, PLTP deficient mice have no observable atherosclerotic lesions, while the control mice have obvious lesions (Fig. 3B and C).
Inducible PLTP deficiency has similar effect as germline PLTP deficiency on reducing atherosclerosis progression (Fig. 3) [9]. The reduction of VLDL production (Fig. 2D) [9], which is a source of HDL pool in the circulation [25], could be the reason for the attenuation. We also found that inducible PLTP deficiency has similar effect as germline PLTP deficiency on increasing atherosclerotic plaque stability [26]. As reported in our previous study [26], PLTP expression promotes receptor-interacting protein 3 recruitment of macrophages in cytoplasm and induces the formation of reactive oxygen species, thus inducing the instability of the plaques. In line with this, previously, we also reported that germline PLTP depletion induces changes in vitamin E distribution between lipoprotein classes, inducing more protection of VLDL and LDL particles against oxidation [27], this could also be a reason for reducing atherosclerosis progression and inducing plaque stability observed in inducible PLTP KO mice.
There are at least four models for atherosclerosis regression studies: 1) a transplant model, in which plaque-bearing aortic segments are transferred into normolipidemic mice [28], 2) the Reversa model, in which LDL production can be conditionally reduced in LDL receptor-KO mice [29], 3) an acute treatment model, in which apoE-KO mice are injected with apoA1 [30], and 4) an LDL receptor antisense oligonucleotide treatment model.
In this study, we used the Reversa model with AAV8-D377Y-mPCSK9-mediated LDL deficiency [16]. This approach not only allowed us to evaluate atherosclerosis progression but also regression. We depleted PLTP when mouse atherosclerotic plaques were well developed, which occurred in AAV8-D377Y-mPCSK9-treated control mice after 5 months on the Western-type diet. To our surprise, switching from the Western-type diet to the normal chow diet did not result in atherosclerotic plaque size changes (Fig. 5A-E). This was consistent with a previous report [23] but different from another [16]. However, normal chow diet replacement significantly reduced the plaque macrophage content (Fig. 5F-H). Importantly, PLTP depletion effectively reduced lesion size and the number of macrophages, suggesting that PLTP deficiency promoted atherosclerosis regression (Fig. 5). Lacking PLTP would promote macrophage efferocytosis within plaques and would reduce monocyte entry to subendothelial space. This was the first time to show that inducible PLTP deficiency-mediated atherosclerosis regression, implicating a translational value for atherosclerosis treatment.
PLTP deficiency may cause atherosclerosis regression via HDL regulation. Although both iPLTP-KO and germline PLTP-KO mice exhibit reduced plasma HDL levels, this effect is smaller in iPLTP-KO mice. Germline PLTP-KO mice also show a marked reduction in apo-AI (85%) [31], whereas iPLTP-KO mice only show a 30–40% reduction (Fig. 1C and Supplementary Fig. 3A). This may be because germline PLTP-KO mice have complete loss of PLTP activity earlier in life, which may affect other pathways involving apoA-I and HDL. Recent research has indicated that HDL particles are very heterogeneous, and that increasing total HDL cholesterol does not reduce cardiovascular disease risk [32]. Thus, it is important to characterize each HDL subclass in terms of its pro- or anti-atherogenicity. Both germline PLTP-KO and transgenic mice have reduced HDL levels [31,33]; however, the former has smaller-sized HDLs [20]. In this study, we used NMR spectroscopy to isolate four HDL subclasses and measured their cholesterol and phospholipid levels. Inducible PLTP deficiency had different effects on HDL reduction (Supplementary Table 1), thus, we used our native PAGE system for HDL characterization. We found that inducible PLTP deficiency reduced all HDLs but also generated a new HDL particle (Fig. 1D). Interestingly, exogenous rPLTP shifted the iPLTP-KO mouse lipoprotein profile toward a human-like change due to the appearance of pre-β1-HDL, HDL3, and bigger LDL (Fig. 1D). The significance of these phenomena and their relevance to atherogenesis warrant further investigation.
PLTP may play a role in reverse cholesterol transport (RCT) which impacts atherosclerosis regression [34]. However, a relatively recent report has indicated that PLTP overexpression and depletion reduce HDL levels and cholesterol efflux capacity without impacting macrophage-mediated RCT [35].
We knew that PLTP has an intracellular effect on VLDL production [9] which can be regulated [36]. In concert with the increase in triglyceride synthesis, the increased PLTP activity permits triglyceride incorporation into large VLDLs [37]. Again, in this study, we found that iPLTP-KO mice exhibited reduced VLDL production (Fig. 2D). However, the precise mechanism remains unclear. NMR analysis showed that PLTP deficiency significantly reduced small VLDL triglyceride levels (Supplementary Table 1), suggesting an intracellular effect on small VLDL assembly and secretion. This finding warrants further investigation.
Another difference between germline PLTP-KO and iPLTP-KO mice is that VLDL production is not affected in germline PLTP-KO mice when crossed with germline LDL receptor-KO mice [9], whereas VLDL production is inhibited in adult iPLTP-KO mice in response to LDL receptor dysfunction via PCSK9 overexpression (Fig. 2D). Furthermore, germline PLTP deficiency prevents apoB secretion [9]. LDL receptor can interact with nascent VLDL within the secretory pathway, regulating apoB secretion [38]. Germline LDL receptor deficiency seems to act as a suppressor mutation preventing PLTP deficiency-mediated reduction of apoB secretion in the secretory pathway [9]. Regarding AAV8-mediated PCSK9 overexpression in hepatocytes, PCSK9 may only impact LDL receptor on the surface of cells [39] and not in the secretory pathway. Moreover, germline LDL receptor deficiency may also have undefined effects during mouse development and may not necessarily reflect the situation during adulthood.
In summary, inducible PLTP deficiency in adult mice results in reduced plasma cholesterol and atherosclerotic plaque size as well as plaque stability. Importantly, it also reverses established atherosclerosis and provides a practicable approach for the treatment of atherosclerosis.
Supplementary Material
Acknowledgments
The authors are grateful to Mr. Ziwen Wang, for his assistance with the illustration of the carotid collar surgery.
Financial support
This work was supported by a Veterans Affairs (USA) Merit Award (BX000900 to Xian-Cheng Jiang) and grants from the National Institutes of Health (USA) (RO1HL139582 and RO1HL149730 to Xian-Cheng Jiang).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2021.03.011.
References
- [1].Tall AR, Krumholz S, Olivecrona T, Deckelbaum RJ, Plasma phospholipid transfer protein enhances transfer and exchange of phospholipids between very low density lipoproteins and high density lipoproteins during lipolysis, J. Lipid Res 26 (1985) 842–851. [PubMed] [Google Scholar]
- [2].Jauhiainen M, Metso J, Pahlman R, Blomqvist S, van Tol A, Ehnholm C, Human plasma phospholipid transfer protein causes high density lipoprotein conversion, J. Biol. Chem 268 (1993) 4032–4036. [PubMed] [Google Scholar]
- [3].Teslovich TM, et al. , Nature 466 (2010) 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schlitt A, Bickel C, Thumma P, Blankenberg S, Rupprecht HJ, Meyer J, Jiang XC, High plasma phospholipid transfer protein levels as a risk factor for coronary artery disease, Arterioscler. Thromb. Vasc. Biol 23 (2003) 1857–1862. [DOI] [PubMed] [Google Scholar]
- [5].Vergeer M, Boekholdt SM, Sandhu MS, Ricketts SL, Wareham NJ, Brown MJ, de Faire U, Leander K, Gigante B, Kavousi M, Hofman A, Uitterlinden AG, van Duijn CM, Witteman JC, Jukema JW, Schadt EE, van der Schoot E, Kastelein JJ, Khaw KT, Dullaart RP, van Tol A, Trip MD, Dallinga-Thie GM, Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility, Circulation 122 (2010) 470–477. [DOI] [PubMed] [Google Scholar]
- [6].Robins SJ, Lyass A, Brocia RW, Massaro JM, Vasan RS, Plasma lipid transfer proteins and cardiovascular disease. The Framingham Heart Study, Atherosclerosis 228 (2013) 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cavusoglu E, Marmur JD, Chhabra S, Chopra V, Eng C, Jiang XC, Relation of baseline plasma phospholipid transfer protein (PLTP) activity to left ventricular systolic dysfunction in patients referred for coronary angiography, Atherosclerosis 207 (2009) 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cavusoglu E, Marmur JD, Chhabra S, Hojjati MR, Yanamadala S, Chopra V, Eng C, Jiang XC, Elevated baseline plasma phospholipid protein (PLTP) levels are an independent predictor of long-term all-cause mortality in patients with diabetes mellitus and known or suspected coronary artery disease, Atherosclerosis 239 (2015) 503–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jiang XC, Qin S, Qiao C, Kawano K, Lin M, Skold A, Xiao X, Tall AR, Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency, Nat. Med 7 (2001) 847–852. [DOI] [PubMed] [Google Scholar]
- [10].van Haperen R, van Tol A, van Gent T, Scheek L, Visser P, van der Kamp A, Grosveld F, de Crom R, Increased risk of atherosclerosis by elevated plasma levels of phospholipid transfer protein, J. Biol. Chem 277 (2002) 48938–48943. [DOI] [PubMed] [Google Scholar]
- [11].Li Z, Li Y, Chakraborty M, Fan Y, Bui HH, Peake DA, Kuo MS, Xiao X, Cao G, Jiang XC, Liver-specific deficiency of serine palmitoyltransferase subunit 2 decreases plasma sphingomyelin and increases apolipoprotein E levels, J. Biol. Chem 284 (2009) 27010–27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li Z, Kabir I, Jiang H, Zhou H, Libien J, Zeng J, Stanek A, Ou P, Li KR, Zhang S, Bui HH, Kuo MS, Park TS, Kim B, Worgall TS, Huan C, Jiang XC, Liver serine palmitoyltransferase activity deficiency in early life impairs adherens junctions and promotes tumorigenesis, Hepatology 64 (2016) 2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC, The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin, Science 310 (2005) 1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Woods A, Heslegrave AJ, Muckett PJ, Levene AP, Clements M, Mobberley M, Ryder TA, Abu-Hayyeh S, Williamson C, Goldin RD, Ashworth A, Withers DJ, Carling D, LKB1 is required for hepatic bile acid transport and canalicular membrane integrity in mice, Biochem. J 434 (2011) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yazdanyar A, Quan W, Jiang XC, Liver-specific phospholipid transfer protein deficiency reduces high-density lipoprotein and non-high-density lipoprotein production, Arterioscler. Thromb. Vasc. Biol 9 (2013) 2058–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peled M, Nishi H, Weinstock A, Barrett TJ, Zhou F, Quezada A, Fisher EA, A wild-type mouse-based model for the regression of inflammation in atherosclerosis, PloS One 12 (2017), e0173975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jimenez B, Holmes E, Heude C, Tolson RF, Harvey N, Lodge SL, Chetwynd AJ, Cannet C, Fang F, Pearce JTM, Lewis MR, Viant MR, Lindon JC, Spraul M, Schafer H, Nicholson JK, Quantitative lipoprotein subclass and low molecular weight metabolite analysis in human serum and plasma by (1)H NMR spectroscopy in a multilaboratory trial, Anal. Chem 90 (2018) 11962–11971. [DOI] [PubMed] [Google Scholar]
- [18].Jiang L, Huang J, Wang Y, Tang H, Eliminating the dication-induced intersample chemical-shift variations for NMR-based biofluid metabonomic analysis, Analyst 137 (2012) 4209–4219. [DOI] [PubMed] [Google Scholar]
- [19].Chen Y, Dong J, Zhang X, Chen X, Wang L, Chen H, Ge J, Jiang XC, Evacetrapib reduces prebeta-1 HDL in patients with atherosclerotic cardiovascular disease or diabetes, Atherosclerosis 285 (2019) 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiang H, Yazdanyar A, Lou B, Chen Y, Zhao X, Li R, Hoang Bui H, Kuo MS, Navab M, Qin S, Li Z, Jin W, Jiang XC, Adipocyte phospholipid transfer protein and lipoprotein metabolism, Arterioscler. Thromb. Vasc. Biol 35 (2015) 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shiomi M, Ito T, Hirouchi Y, Enomoto M, Fibromuscular cap composition is important for the stability of established atherosclerotic plaques in mature WHHL rabbits treated with statins, Atherosclerosis 157 (2001) 75–84. [DOI] [PubMed] [Google Scholar]
- [22].Jiang H, Li Z, Huan C, Jiang XC, Macrophage lysophosphatidylcholine acyltransferase 3 deficiency-mediated inflammation is not sufficient to induce atherosclerosis in a mouse model, Front Cardiovasc Med 5 (2018) 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Basu D, Hu Y, Huggins LA, Mullick AE, Graham MJ, Wietecha T, Barnhart S, Mogul A, Pfeiffer K, Zirlik A, Fisher EA, Bornfeldt KE, Willecke F, Goldberg IJ, Novel reversible model of atherosclerosis and regression using oligonucleotide regulation of the LDL receptor, Circ. Res 122 (2018) 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fan Y, Shi F, Liu J, Dong J, Bui HH, Peake DA, Kuo MS, Cao G, Jiang XC, Selective reduction in the sphingomyelin content of atherogenic lipoproteins inhibits their retention in murine aortas and the subsequent development of atherosclerosis, Arterioscler. Thromb. Vasc. Biol 30 (2010) 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rader DJ, Molecular regulation of HDL metabolism and function: implications for novel therapies, J. Clin. Invest 116 (2006) 3090–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang K, Liu X, Yu Y, Luo T, Wang L, Ge C, Liu X, Song J, Jiang X, Zhang Y, Qin S, Zhang M, Phospholipid transfer protein destabilizes mouse atherosclerotic plaque, Arterioscler. Thromb. Vasc. Biol 34 (2014) 2537–2544. [DOI] [PubMed] [Google Scholar]
- [27].Jiang XC, Tall AR, Qin S, Lin M, Schneider M, Lalanne F, Deckert V, Desrumaux C, Athias A, Witztum JL, Lagrost L, Phospholipid transfer protein deficiency protects circulating lipoproteins from oxidation due to the enhanced accumulation of vitamin E, J. Biol. Chem 277 (2002) 31850–31856. [DOI] [PubMed] [Google Scholar]
- [28].Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo JG, Fallon JT, Fisher EA, Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model, J. Vasc. Surg 34 (2001) 541–547. [DOI] [PubMed] [Google Scholar]
- [29].Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA, Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques, Circulation 123 (2011) 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hewing B, Parathath S, Barrett T, Chung WK, Astudillo YM, Hamada T, Ramkhelawon B, Tallant TC, Yusufishaq MS, Didonato JA, Huang Y, Buffa J, Berisha SZ, Smith JD, Hazen SL, Fisher EA, Effects of native and myeloperoxidase-modified apolipoprotein a-I on reverse cholesterol transport and atherosclerosis in mice, Arterioscler. Thromb. Vasc. Biol 34 (2014) 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiang XC, Bruce C, Mar J, Lin M, Ji Y, Francone OL, Tall AR, Targeted mutation of plasma phospholipid transfer protein gene markedly reduces high-density lipoprotein levels, J. Clin. Invest 103 (1999) 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B, Investigators I, Effects of torcetrapib in patients at high risk for coronary events, N. Engl. J. Med 357 (2007) 2109–2122. [DOI] [PubMed] [Google Scholar]
- [33].Foger B, Santamarina-Fojo S, Shamburek RD, Parrot CL, Talley GD, Brewer HB Jr., Plasma phospholipid transfer protein. Adenovirus-mediated overexpression in mice leads to decreased plasma high density lipoprotein (HDL) and enhanced hepatic uptake of phospholipids and cholesteryl esters from HDL, J. Biol. Chem 272 (1997) 27393–27400. [DOI] [PubMed] [Google Scholar]
- [34].Fisher EA, Regression of atherosclerosis: the journey from the liver to the plaque and back, Arterioscler. Thromb. Vasc. Biol 36 (2016) 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kuwano T, Bi X, Cipollari E, Yasuda T, Lagor WR, Szapary HJ, Tohyama J, Millar JS, Billheimer JT, Lyssenko NN, Rader DJ, Overexpression and deletion of phospholipid transfer protein reduce HDL mass and cholesterol efflux capacity but not macrophage reverse cholesterol transport, J. Lipid Res 58 (2017) 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yu Y, Lei X, Jiang H, Li Z, Creemers JWM, Zhang M, Qin S, Jin W, Jiang XC, Prodomain of furin promotes phospholipid transfer protein proteasomal degradation in hepatocytes, J Am Heart Assoc 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Okazaki H, Goldstein JL, Brown MS, Liang G, LXR-SREBP-1c-phospholipid transfer protein axis controls very low density lipoprotein (VLDL) particle size, J. Biol. Chem 285 (2010) 6801–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Twisk J, Gillian-Daniel DL, Tebon A, Wang L, Barrett PH, Attie AD, The role of the LDL receptor in apolipoprotein B secretion, J. Clin. Invest 105 (2000) 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang DW, Garuti R, Tang WJ, Cohen JC, Hobbs HH, Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 13045–13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.