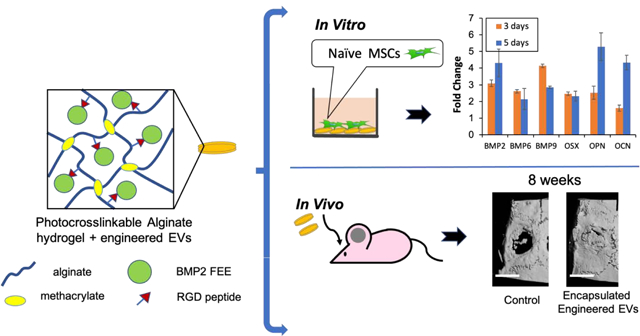
Abstract
Mesenchymal stem cell (MSC) derived extracellular vesicles (EVs) in their naïve and engineered forms have emerged as potential alternatives to stem cell therapy. While they have a defined therapeutic potential, the spatial and temporal control of their activity in vivo remains a challenge. The objective of this study was to devise a methodology to encapsulate EVs in 3D hydrogels for prolonged delivery. To achieve this, we have leveraged the MSC EV interactions with ECM proteins and their derivative peptides. Using osteoinductive functionally engineered EVs (FEEs) derived from MSCs, we show that FEEs bind to mimetic peptides from collagen (DGEA, GFPGER) and fibronectin (RGD). In in vitro experiments, photocrosslinkable alginate hydrogels containing RGD were able to encapsulate, tether and retain the FEEs over a period of 7 days while maintaining the structural integrity and osteoinductive functionality of the EVs. When employed in a calvarial defect model in vivo, alginate-RGD hydrogels containing the FEEs enhanced bone regeneration by a factor of 4 compared to controls lacking FEEs and by a factor of 2 compared to controls lacking the tethering peptide. These results show that EVs can be tethered to biomaterials to promote bone repair and the importance of prolonged delivery in vivo. Results also provide a prelude to the possible use of this technology for controlled delivery of EVs for other regenerative medicine applications.
Keywords: Exosomes, Extracellular vesicles, Integrins, Alginate, Controlled release, Bone regeneration
Graphical Abstract
Introduction:
Extracellular vesicles (EVs) are nano scale vesicles (40–150nm) secreted by cells that contribute to intracellular communication [1]. Although originally believed to be mediators of cellular homeostasis by secreting cellular waste [2], recent literature demonstrates their specific roles in modulating cellular function in immunology and regenerative medicine [2, 3]. EVs, when endocytosed by effector cells, trigger a cellular response designated by the parental cell to the target cell [4, 5].
Mesenchymal stem cells (MSCs) play a central role in immunomodulation and regeneration. They are, therefore, widely recognized as candidates for cell-based regenerative medicine. Many effects of human MSC (HMSC) therapy are attributable to paracrine effects of the HMSC secretome [6–8]. More specifically, HMSC-derived EVs have been implicated as the drivers behind the effects of the HMSC secretome [9–11]. Therefore, MSC EVs have high therapeutic potential for immunomodulation and for tissue regeneration [12].
EVs derived from lineage-specified MSCs induce lineage-specific changes in target naïve MSCs [13–15]. These observations suggest that EVs cargo is selective and can direct tissue-specific gene regulatory programs. This important discovery has been confirmed by several laboratories independently [16–18]. A recent study by Narayanan et.al indicates that MSC EV function supersedes the extracellular matrix (ECM) derived signals, suggesting the importance of EV signaling and its potential in regenerative medicine applications [19].
Despite many demonstrations of their regenerative potential, MSC derived EVs are not a global solution to all therapeutic needs. For example, naïve MSC EVs are minimally osteoinductive [20]. However, when the MSCs are subjected to osteogenic differentiation, their derivative EVs acquire enhanced osteoinductive properties [14, 16]. To achieve tissue-specific results, MSC EVs may require engineering to contain cargo with specific functional roles. Evidence for this has been presented using BMP2 overexpression in HMSCs to precondition HMSCs to produce functionally engineered EVs (FEEs) that promote bone repair by potentiating the BMP2 signaling cascade in targeted cells and tissues[21].
The intended biological effects of engineered or naïve EVs can be achieved when they are made available to targeted cells or tissues during a defined period of time [22]. Previous studies of EV delivery have explored topical application [23], systemic [24], intrathecal [25], intravitreal injections [26] and intranasal delivery mechanisms [27]. There are several perceived limitations to these approaches. Primarily, they require large quantities of EVs. The delivery routes involve contact or exposure with ‘bystander’ tissues that contribute to poor efficiency and potentiating ectopic effects especially in the case of FEEs. MSC EVs are endocytosed by several cell types via a common mechanism that involves the cell surface heparin sulfate proteoglycan receptors (HSPGs)[15]. Therefore, localization of FEEs to the target site is important to minimize off target effects.
To achieve localization or site-specificity, EVs from different MSC sources have been entrapped within alginate [28], chitosan [23], pluronic [29] and silk blended[30] hydrogels to promote vascularization and/or facilitate regeneration of tissues such as skin, bone and cardiac muscle. These physical entrapment methods result in EVs being released within hours of placement at the desired site [22]. While this approach may be suitable for immunomodulatory effects immediately post injury, a prolonged release mechanism is required to more optimally direct tissue regeneration. To facilitate cellularization, scaffolds designed for regenerative medicine contain pore sizes of many μm. The average EV particle size is 150nm. This size disparity alone invalidates the potential physical restriction of EV release from hydrogels and is an inherent limitation to achieving delayed (or controlled) release of the EVs.
Based on these observations, the present investigation explores a mechanistic approach to sustained delivery of EVs. EV interactions with extracellular matrix (ECM) protein domains present in ECM proteins such as type I collagen and fibronectin have been leveraged to tether FEEs to hydrogels. For this study, we have chosen alginate as a carrier for EV delivery as alginate has been shown in published studies to be a biologically neutral material with tunable mechanical and biodegradable properties capable of supporting cellularization and tissue repair (reviewed in [31]) . The biochemical interaction of integral EVs membrane receptors with ECM protein domain-containing hydrogels is presently investigated to enable sustained delivery of osteoinductive FEEs[21] in a rat calvarial defect model of bone repair.
Materials and Methods:
Cell culture:
Primary HMSCs were obtained from Lonza and cultured in αMEM basal media (Gibco) supplemented with 20% fetal bovine serum (FBS, Gibco) 1% L-glutamate (Gibco) and 1% antibiotic, antimycotic solution (Gibco)[21].
BMP2 overexpressing HMSCs:
HMSCs constitutively expressing BMP2 used here have been previously characterized [21]. Briefly, HMSCs were transduced with lentiviral particles containing a BMP2 expression plasmid under the control of EF1α promoter and BMP2 expressing cells were selected for stable expression using puromycin. In parallel, a control vector without the BMP2 gene was also transduced in HMSCs. The increase in expression of the BMP2 was verified by qRT PCR and by the presence of BMP2 protein in the secretome (Supplementary Figure 1).
Isolation and characterization of EVs:
Functionally engineered EVs (FEEs) were isolated from the BMP2 expressing HMSCs as per standardized protocols[13, 14, 21]. Before isolation, the HMSCs were washed in serum free basal medium and cultured for 24 hours in serum free basal medium. The conditioned medium was harvested and centrifuged at 1500×g to remove cellular debris. The medium was then concentrated fivefold (20% of original volume) using a 100KDa cutoff spin filter (Millipore) and EVs were harvested from this concentrated medium using the ExoQuick TC isolation reagent (System Biosciences). The isolated EVs were characterized for the presence of markers CD63 (1/250, Abcam), HSP70 (1/250, Abcam) and TSG101 (1/250, Abcam) by immunoblotting. Cell lysates were used as comparative controls. The presence of integrins α5 (1/250, Abcam) and αV (1/250, Santacruz Biotechnology) in the EV lysates was also evaluated by immunoblotting. Nano particle tracking analysis (NTA) was performed to obtain the size distribution of the isolated EVs and immunoelectron microscopy was performed with CD63 primary antibody (1/100, Abcam) and 20nm gold conjugated secondary antibody (1/1000, Abcam) for morphological verification and to confirm the presence of the EV marker in the isolated vesicles.
Binding of EVs to ECM, Type I collagen, Fibronectin and their derivative peptides:
For all qualitative and quantitative experiments, FEEs were fluorescently labeled using the cell tracker red/green labeling kit (Thermo Scientific). For qualitative evaluation of EV binding to cell-secreted ECM, HMSCs were grown to confluence in cover slips placed in 6-well culture dishes. At 100% confluence, the cover slips were decellularized to leave behind the ECM as per our previously published protocols [32]. The decellularized coverslips were incubated with 1×106 Fluorescently labeled EVs for 2 hours at room temperature, washed 3× in sterile PBS and fixed in 4% neutral buffered formalin. The adherent cells were then immunostained with anti-type I collagen (1/100, Abcam) or anti-fibronectin (1/1000, Sigma) antibodies and imaged using a Zeiss LSM 710 Meta confocal microscope.
Quantitative binding experiments were performed in 96 well assay plates coated with 5μg/well Type I collagen (BD Biosciences), Fibronectin (Sigma), RGD peptide, DGEA peptide or GFPGER peptide. The peptides were synthesized by the UIC research resource center core facility. Wells were coated with type I collagen, fibronectin or the relevant peptides overnight as per published and standardized protocols [33]. Fluorescently labeled FEEs were then added in increasing doses to the wells (n=6 per group) and incubated for 2 hours at room temperature. The wells were washed 3× with PBS, fixed in 4% neutral buffered formalin and the fluorescence from the bound EVs was quantitatively measured using a fluorescent plate reader (BioTek). For competition binding experiments, the FEEs were pretreated with 2mM RGD, DGEA or GFPGER peptides for 1 hour prior to their addition and incubation with the coated plates.
For measurement of release kinetics of bound FEEs from the coated plates, the coated plates were incubated with saturable number of EVs derived from the binding curve as per the procedure described above (1×108 EVs). The plates were then incubated with 100μl PBS for the indicated time points and the fluorescence from the released EVs was quantitatively measured (n=6) using a BioTek fluorescent plate reader. After each reading, the PBS solution was replaced with fresh 100μl PBS and iterative measures were recorded. The results were plotted cumulatively as percentage of fluorescence from the bound EVs at time zero.
Generation of photocrosslinkable alginate hydrogels:
Sodium alginate was methacrylated and conjugated to the RGD peptide. Alginate was dissolved in MES buffer (0.1M MES, 0.3M NaCl, pH:6.5) at 1%w/v. EDC and sulfo-NHS were dissolved at 1.5mg/ml and 0.84mg/ml respectively. The GGGGRGDY peptide was mixed with the solution at a concentration of 10μmoles/g of sodium alginate. Four residues of glycine were added to the amino terminal side of the RGD for flexibility during NHS-ester crosslinking to the alginate backbone. To generate photocrosslinkable alginate, the peptide conjugated alginate was dissolved in deionized water at 2.5%w/v and equal volume of methacrylic anhydride was added to the solution. The solution was stirred continuously for 72 hours and pH maintained at 7.5 by addition of 10N NaOH. Once dissolved, the solution was mixed with twice the volume of 100% ethanol to precipitate the methacrylated alginate. The precipitated alginate was separated by repeated centrifugation at 4000rpm and airdried at room temperature. Once conjugated, the alginate mixture was dialyzed against deionized water and lyophilized. The presence of methacrylate and RGD was verified by NMR spectroscopy and the presence of the peptide was further verified by a Bradford Protein Assay (BioRad) as per the manufacturer’s protocol. For the generation of hydrogels, alginate +/− RGD peptide was dissolved in PBS at the desired w/v in 96 well assay plates and polymerized using long range UV (312nm) exposure for 2 minutes in the presence of the initiator (VA-086). For EV encapsulation the required number of EVs were constituted in the PBS solution prior to adding it to the alginate monomers to keep the same final volume. The alginate EV mixture was then treated similar to the other groups for the process of gelation.
EV release kinetics from alginate hydrogels:
Fluorescently labeled FEEs (5×108 FEE particles (counted using NTA)) were mixed with alginate control (A, no peptide motifs) and alginate-RGD (A-RGD) monomers (2 or 4% w/v) and then polymerized using a light source emitting long range ultraviolet (UV) light (Thermo Scientific). The experiments were performed in 96 well plates using 50μl total hydrogel per well. The percentage of FEEs released from the hydrogel was calculated by measuring the fluorescence of the released FEEs at stipulated time points with respect to the fluorescence from 4×108 FEE particles served as the 100% fluorescence reference. After each time point the PBS covering the hydrogel was harvested for fluorescence measurement and replaced with fresh PBS solution. A cumulative release plot was plotted to observe the rate of release from the various groups (n= 6/group and time point).
Functional studies of encapsulated FEEs:
For these experiments, the FEEs were encapsulated in A-RGD hydrogels as described above. The same dimensions of the hydrogels and the same number of FEEs were used. To observe the function of the released FEEs, the experiment was performed in Transwell® dishes where 100,000 HMSCs were seeded in the bottom well and the control or EV containing hydrogels were placed in the top well. To alternatively evaluate the functionality of the FEEs bound to the hydrogel matrix on cells that colonize the hydrogel, the cells were seeded on to the hydrogels directly. The cells were cultured for up to 24 hours to observe the FEE endocytosis and for 3 and 5 days to observe osteoinductive gene expression by qRT PCR and for alkaline phosphatase (ALP) activity.
For quantitation of osteogenic differentiation and calcium deposition, 50,000 HMSCs were seeded on to 4% A-RGD hydrogels +/− FEEs and cultured in the presence of osteogenic differentiation medium comprising of ascorbic acid (50μM), β-glycerophosphate (10mM) and dexamethasone (1μM) as per our previously published protocols [14]. 1 and 2 weeks post culture, the samples were fixed in neutral buffered 4% formalin followed by alizarin red staining. Quantitation of the staining was performed as per published protocol [34]. Briefly, 250μl of 10% (v/v) acetic acid was added to each hydrogel and incubated at room temperature for 30 minutes. The samples were vortexed for 30 seconds and heated to 85oC for 10 min followed by incubation on ice for 5min. The samples were then centrifuged for 15 min at 20,000g and the supernatant was removed and transferred to a tube containing 10% ammonium hydroxide (to neutralize the pH). 100μl aliquots were then read in triplicate for each sample at 405nm in a 96-well plate reader (BioTek).
For the qualitative endocytosis experiments, the cells were fixed in neutral buffered formalin, permeablized and counter stained for actin (Phalloidin FITC, SIGMA, 1/2500) imaged using a Zeiss LSM 710 Meta confocal microscope. For gene expression analyses, RNA was isolated from the cells at 3 and 5 days followed by reverse transcription and qRT PCR for osteoinductive genes (table 1). Fold change in gene expression was calculated using the DDCt method with respect to cells similarly treated but without EVs (n=4). For the ALP assay, the total protein was isolated from the cells and the ALP activity was measured using an assay kit (Abcam) as per the manufacturer’s protocol.
Table 1:
List of Primers used:
| Gene Name | Forward primer sequence (5’–3’) | Reverse primer sequence (5’–3’) |
|---|---|---|
| GAPDH | CAGGGCTGCTTTTAACTCTGG | TGGGTGGAATCATATTGGAACA |
| BMP2 | ACTACCAGAAACGAGTGGGAA | GCATCTGTTCTCGGAAAACCT |
| BMP6 | TGTTGGACACCCGTGTAGTAT | AACCCACAGATTGCTAGTGGC |
| BMP9 | AGAACGTGAAGGTGGATTTCC | CGCACAATGTTGGACGCTG |
| OSX | CCTCTGCGGGACTCAACAAC | AGCCCATTAGTGCTTGTAAAGG |
| OPN | GAAGTTTCGCAGACCTGACAT | GTATGCACCATTCAACTCCTCG |
| OCN | CACTCCTCGCCCTATTGGC | CCCTCCTGCTTGGACACAAAG |
Rat calvarial bone defect model:
All animal experiments were performed in accordance with protocols approved by the UIC animal care committee (ACC, Assurance no: A3460.01). All groups and time points contained 6 defects per group. Briefly, the rats were anesthetized intraperitoneally using Ketamine (80–100mg/kg)/Xylazine (10mg/kg). Using aseptic technique, a vertical incision was made in the head at the midline to expose the calvarial bone. The connective tissue was removed and two 5mm calvarial defects were created bilaterally in the calvarium without dura perforation using a trephine burr. 4% Alginate (A) and A-RGD hydrogels with/without FEEs were generated as described earlier. Each defect received 5×108 FEEs encapsulated in a 5mm (diameter) hydrogel. Each hydrogel was 50μl in volume. Four- and 8-weeks post-surgery, the rats were euthanized by carbon dioxide asphyxiation and cervical dislocation. The calvaria were dissected, fixed in neutral buffered 4% formalin and scanned using a Scanco40 μCT scanner. The data from the scan was quantitatively analyzed using a custom MatLab software to obtain BV/TV data for all the groups and time points. The samples were then decalcified in 10% EDTA solution, embedded in paraffin, and 10μm sections were subjected to H&E staining and immunohistochemistry (IHC) for bone sialo protein (BSP, 1/250 Abcam), dentin matrix protein 1 (DMP1, 1/250, Santacruz Biotechnology) and osteocalcin (OCN, 1/250, Abcam).
Statistics:
The normal distribution of the data obtained from the experiments was evaluated using the Shapiro-Wilk test. For experiments involving two groups, student’s t-test with a confidence interval of 95% was utilized. For the experiments involving comparison of more than two groups, one-way ANOVA was performed with a confidence interval of 95%. Pairwise comparisons were performed using Tukey’s ad-hoc test with a confidence interval of 95%.
Results:
Characterization of FEEs:
The EVs isolated from BMP2-expressing HMSCs demonstrated the expression of exosomal markers CD63, TSG101 and HSP 70 by immunoblotting (Figure 1A). Tubulin was used as a negative control and was observed only in cell lysates and not in EV lysates. The size distribution of the isolated EVs was evaluated by NTA and had an average particle size of 140–160nm across different EV preparations throughout the course of this study (Figure 1B). The isolated FEEs were also characterized for morphology by transmission electron microscopy. Results presented in Figure 1C show the presence of CD63 immunolabeled EVs. The HMSC endocytosis of isolated FEEs was verified qualitatively using fluorescently labeled FEEs (Figure 1D). Overall, the results indicated that these isolated FEEs adhere to the international society for EV research (ISEV) standard for qualifying as exosomes [35, 36]. However, as exosomes also share similar properties with other types of vesicles, we have referred to them as EVs.
Figure 1. Characterization of EVs:
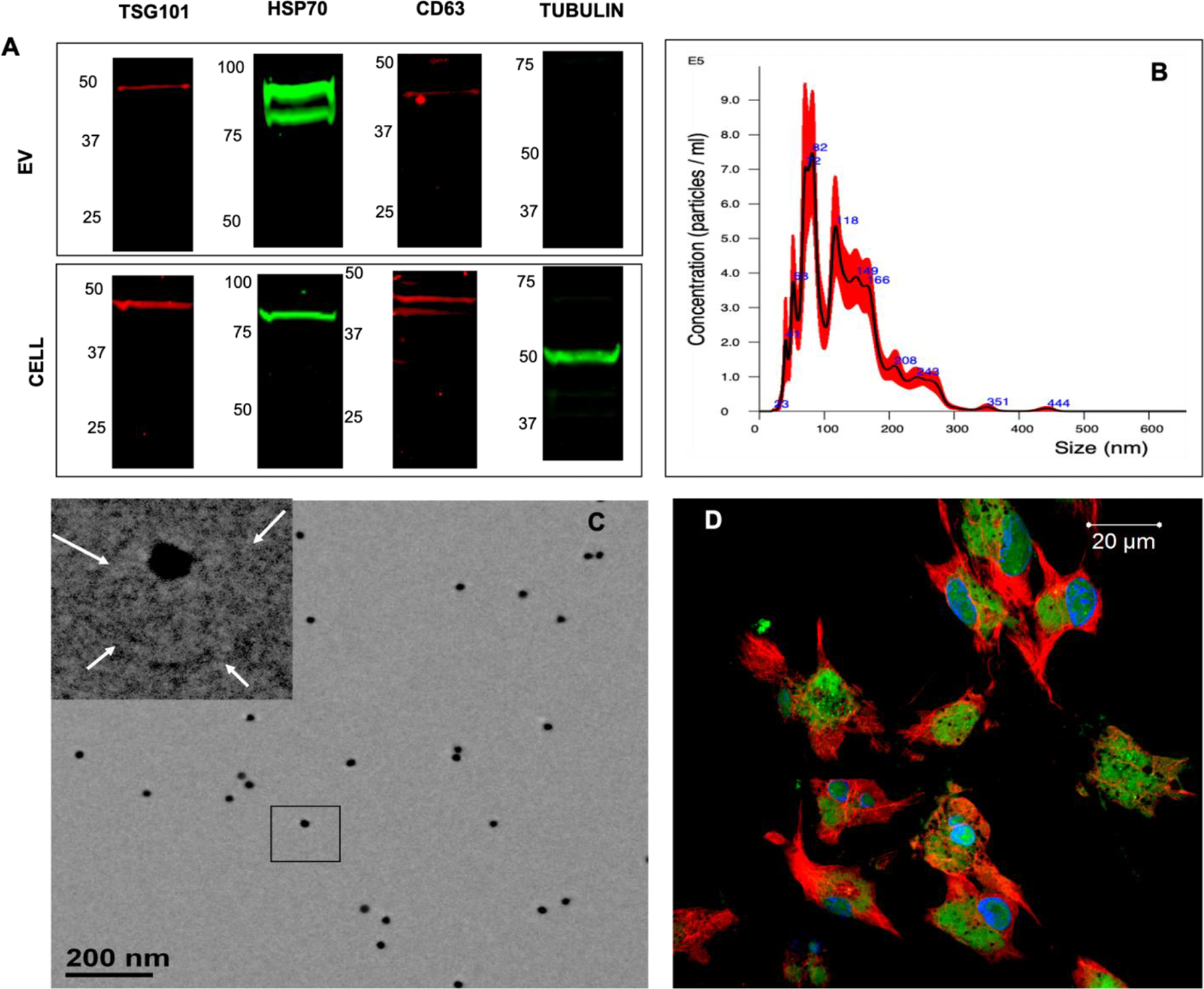
A) Immunoblots of FEE and cell lysates for the presence of TSG101, HSP70, CD63 exosomal marker proteins as well as for the intracellular protein control tubulin. Note the absence of tubulin in the EV lysates. B) Representative NTA plot of FEEs indicating the size distribution of the isolated EVs. C) Representative TEM image of FEEs immunolabeled for CD63 (20 nm gold dots). The insert represents the boxed area. The arrows in the insert point to the EV membrane. D) Confocal micrograph showing a representative image of the uptake of the fluorescently labeled FEEs (green) by HMSCs fluorescently stained with tubulin antibody (red) and DAPI nuclear stain (blue).
Binding of FEEs to ECM components:
EV membranes are subsets of the parental cell’s plasma membrane. Therefore, we hypothesized that EVs could bind to the ECM of HMSCs. Qualitative microscopic studies with decellularized HMSC ECM indicated that fluorescently labeled FEEs co-localized with Type I collagen and fibronectin (Figures 2A and 2C). FEEs bound to these proteins in a dose dependent and saturable manner (Figures 2B and 2D).
Figure 2. Binding of FEEs to ECM proteins:
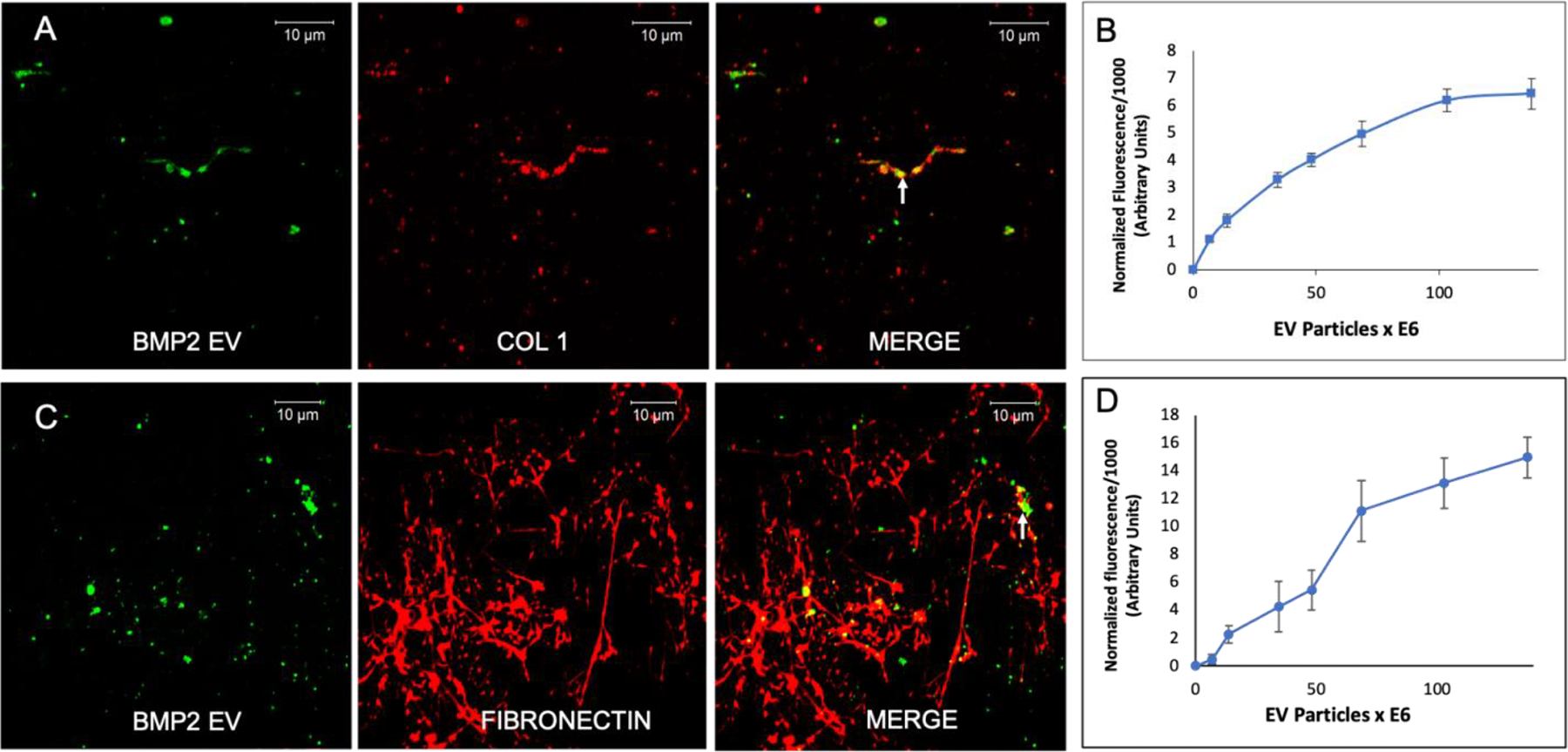
A) Confocal micrograph showing a representative image of the binding of fluorescently labeled FEEs (green) to the decellularized ECM of HMSCs immunostained for type I collagen (red). Arrow in merged image points to a representative area of colocalization. Scale bar represents 10μm. B) Graphical representation of dose-dependent and saturable binding of fluorescently labeled FEEs to type I collagen coated assay plates (data points represent mean +/− SD, n=6). C) Confocal micrograph showing a representative image of the binding of fluorescently labeled FEEs (green) to the decellularized ECM of HMSCs immunostained for fibronectin (red). Arrow in merged image points to a representative area of colocalization. Scale bar represents 10μm. D) Graphical representation of dose-dependent and saturable binding of fluorescently labeled FEEs to fibronectin coated assay plates (data points represent mean +/− SD, n=6).
The RGD domain of fibronectin and the collagen mimetic peptides DGEA and GFPGER support integrin-mediated cell binding. In an effort to identify if the FEEs could bind to these peptides, quantitative binding experiments were performed in assay plates coated with these peptides. Results presented in Figure 3A indicate that the FEEs bind dose dependently to these peptides and no statistical difference was observed in the binding affinity of the FEEs to each of the individual peptides. In a competition experiment, FEEs were pre-incubated with 2mM peptides. Their interactions with type I collagen and fibronectin demonstrated significant reduction in binding compared to controls (Figure 3B, C). Binding of the FEEs to type I collagen and fibronectin was significantly reduced upon pre-treatment with the derivative peptides but not abrogated indicating that the evaluated peptides may not represent the sole EV binding domains of these ECM proteins. However, these peptide domains contribute significantly to EV binding to ECM proteins.
Figure 3. Binding of FEEs to ECM derivative peptides (2D kinetics):
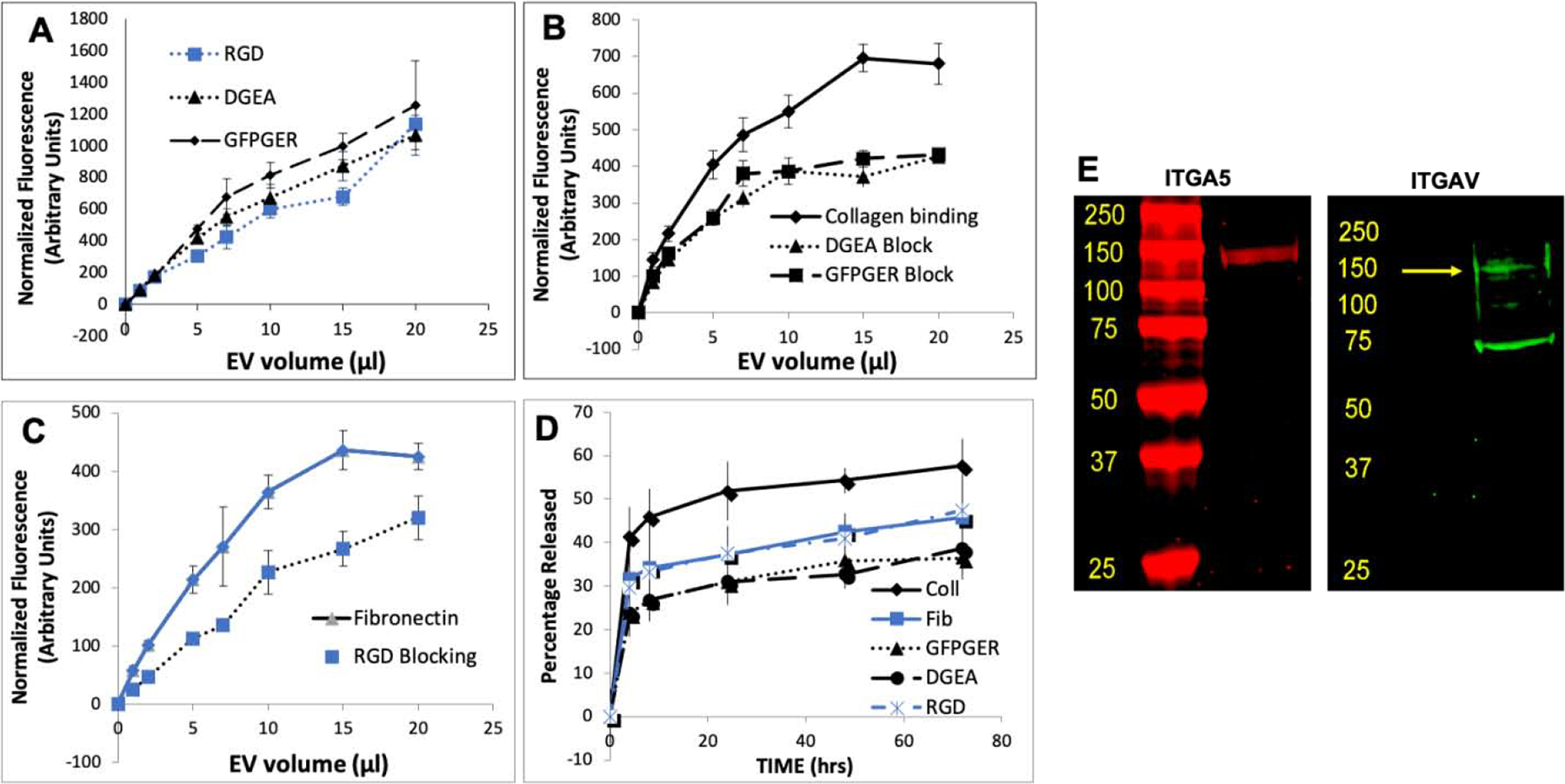
A) Graphical representation of dose-dependent binding of fluorescently labeled FEEs to RGD, DGEA and GFPGER peptide coated assay plates. B) Graphical representation of dose-dependent binding of FEEs (with or without pre-treatment with collagen mimetic peptides DGEA and GFPGER) to type I collagen. Note the reduction in binding to type I collagen when the FEEs are pre-treated with the peptides. C) Graphical representation of dose-dependent binding of FEEs (with or without pre-treatment with RGD peptide) to fibronectin. Note the reduction in binding to fibronectin when the FEEs are pre-treated with the RGD peptide. D) Graphical representation of the fluorescently labeled FEE release from type I collagen, fibronectin, RGD, DGEA, and GFPGER coated assay plates over time. In all the graphs, the blue lines represent fibronectin and/or derivative peptides and black lines represent type I collagen and/or derivative peptides. For all graphs, data points represent mean +/− SD, n=6, * represents statistical significance of the peptide treated group to untreated control group at all concentrations calculated using Tukey’s ad hoc test post ANOVA. E) Immunoblots of FEE lysates probed for the presence of ITGA5 and ITGAV proteins. Yellow arrow in the ITGAV blot points to the expected molecular weight of the protein.
Release kinetics studies indicated that the EV release from type I collagen was faster compared to that of fibronectin and the other peptides (Figure 3D). All the groups showed statistically significant differences with respect to the data from type I collagen bound FEEs. No significance was observed between the fibronectin group and the peptide groups. These results, coupled with the binding studies, indicated the potential of the peptides to serve as EV carriers possibly via ECM-domain binding receptors on EV membranes.
Cellular binding to these peptides is integrin mediated [37]. Therefore, we evaluated and identified that integrins α5 and αv, the alpha subunits that contribute to type I collagen and fibronectin binding [37], are present in EVs. Immunoblotting of FEE protein lysates indicated that the α5 and αv integrins were present in EVs (Figure 3E) implicating that ECM and ECM derived peptide binding of EVs may be mediated by EV resident integrins.
To incorporate these peptides as EV carriers in hydrogels, we generated photocrosslinkable alginate hydrogels with RGD peptides linked to the backbone in a proof-of-concept approach. Supplementary Figure 2 shows photo polymerization (Supplementary Figure 2A) and a pictorial representation of the alginate hydrogels (supplementary Figure 2B) used in the hydrogel experiments. SEM was used to observe the ultrastructure of the 4% alginate hydrogels with and without encapsulated FEEs and no morphological changes were observed among the groups (Supplementary Figure 2C). NMR spectroscopy was used to verify the presence of methacrylate and RGD in the alginate backbone (Supplementary Figures 2D and 2E). While the presence of methacrylate was clearly observed, NMR for the RGD peptide presence showed the characteristic peaks muted or slightly shifted (arrows and brackets in Supplementary Figure 2E). The presence of RGD was therefore, confirmed by Bradford protein assay (supplementary Figure 2E). This assay clearly indicated the presence of the RGD peptide in the samples. The dimensions of the hydrogels and the amount of FEEs loaded within the constructs were kept constant to allow for comparison across in vitro and in vivo experiments. FEEs were encapsulated in 2% and 4% photo crosslinked alginate hydrogels +/− RGD peptide and the release of fluorescently labeled FEEs from the hydrogels was measured over time (Figure 4A). Results indicated that the presence of the RGD peptide in the alginate backbone significantly improved EV retention by retarding the hydrogel release of EVs. Both the 2% and 4% A-RGD groups showed statistical significance at all time points with respect to their RGD lacking counterparts (P<0.05). The release kinetics of the 2% A-RGD hydrogels was equivalent to that of 4% A hydrogels and the 4%-A-RGD hydrogel showed the greatest EV retention. Approximately 80% of EVs were retained within the 4%-A-RGD hydrogel after 1 week. The 4% A-RGD group also showed significantly more FEEs retained and slower release profile compared to the 2% A-RGD group.
Figure 4. Release and integrity of alginate hydrogel encapsulated FEEs in a 3D system using RGD as a tether:
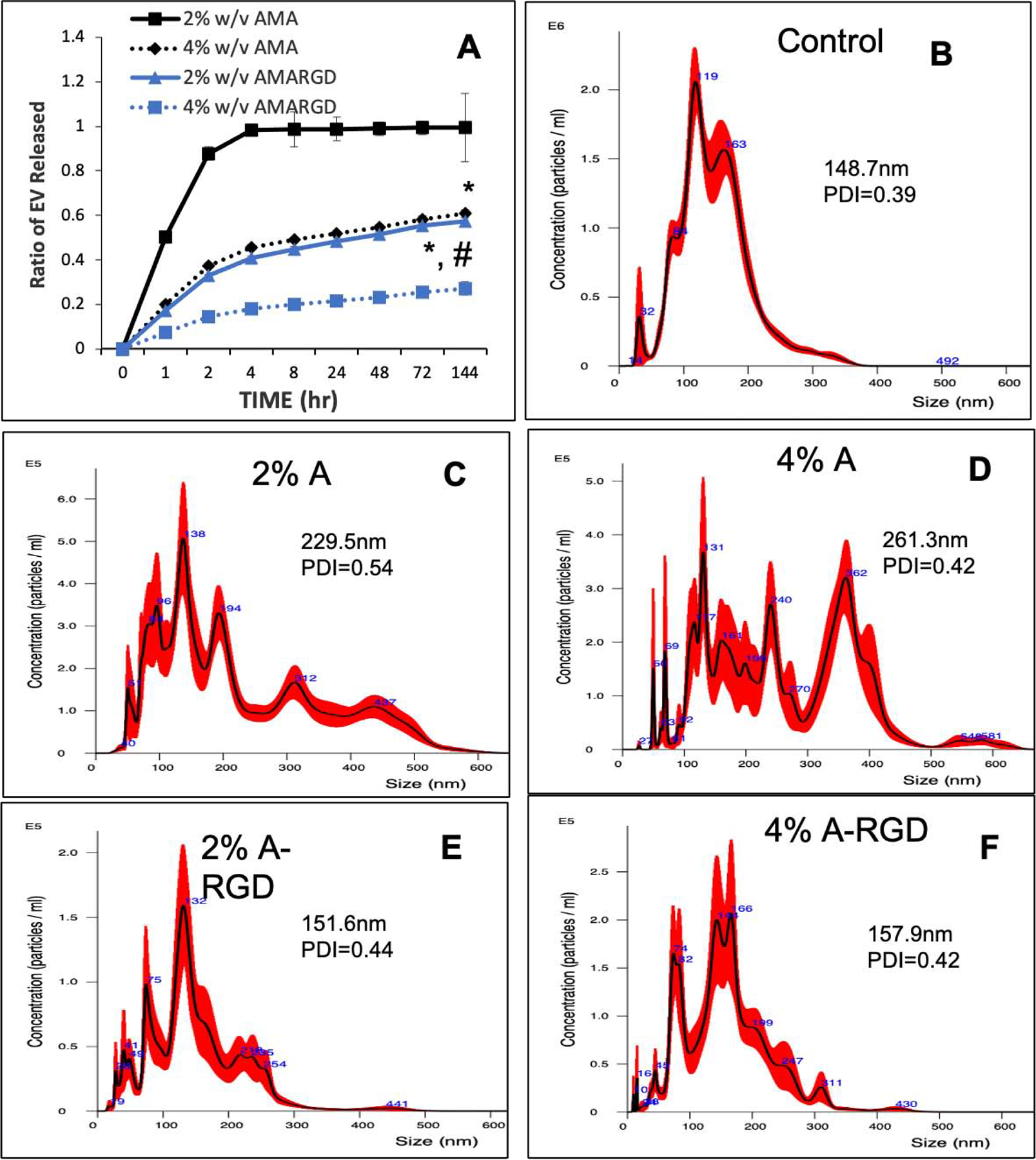
A) Graphical representation of 3D encapsulated fluorescently labeled FEE release from 2% (blue lines) and 4% (black lines) alginate hydrogels +/− RGD peptide over time. Data points represent mean +/− SD, n=6. * represents statistical significance with respect to RGD vs no RGD containing hydrogel pairs and # represents statistical significance of the 4% A-RGD group compared to the 2% A-RGD group calculated using Tukey’s ad hoc test post ANOVA. B, C, D, E and F) Representative NTA plots of FEEs released from each of the hydrogel groups indicating EV size distribution before (control (B)) and after release from the 2% (C) and 4% (D) alginate hydrogels as well as the 2% and 4% alginate hydrogels containing the RGD peptide (E and F respectively). The inserts in the figure show average particle size and the poly dispersity index (PDI).
The released EVs function is dependent on their physical integrity. Thus, the released FEEs from each time point were collected cumulatively and their size distribution was analyzed by NTA (Figures 4B, 4C, 4D, 4E and 4F). EVs released from RGD containing hydrogels showed a size distribution resembling EVs size distribution obtained before encapsulation with FEEs encapsulated with the 4% A-RGD hydrogel showing the best overall profile in terms of similarity to the control. However, the size distribution from the non-RGD containing hydrogels included what appeared to be EV aggregates with a larger average particle diameter. In terms of the polydispersity index (PDI), the 2% and 4% A-RGD hydrogels were similar to that of the pre-encapsulation control sample. Together, these results indicated that the EV integrity is retained following release from the RGD containing hydrogels.
Functionality of hydrogel encapsulated FEEs:
The functionality of the encapsulated FEEs was evaluated quantitatively and qualitatively. Based on the release profile and the EV integrity data obtained from NTA analysis, we chose 4% A-RGD hydrogels to perform these experiments. Scanning electron microscopy images presented in supplementary Figure 2C show that the morphology of these alginate hydrogels is not affected by the presence of the RGD peptide and the FEEs. We first evaluated hydrogel-released FEEs. This experiment involved no contact between the hydrogels containing FEEs and the recipient HMSCs and was performed in a Transwell® chamber with the hydrogel placed in the upper chamber and the HMSCs seeded below (Figure 5A). Empty hydrogels in the same setup served as controls.
Figure 5. In vitro functionality of hydrogel encapsulated FEEs:
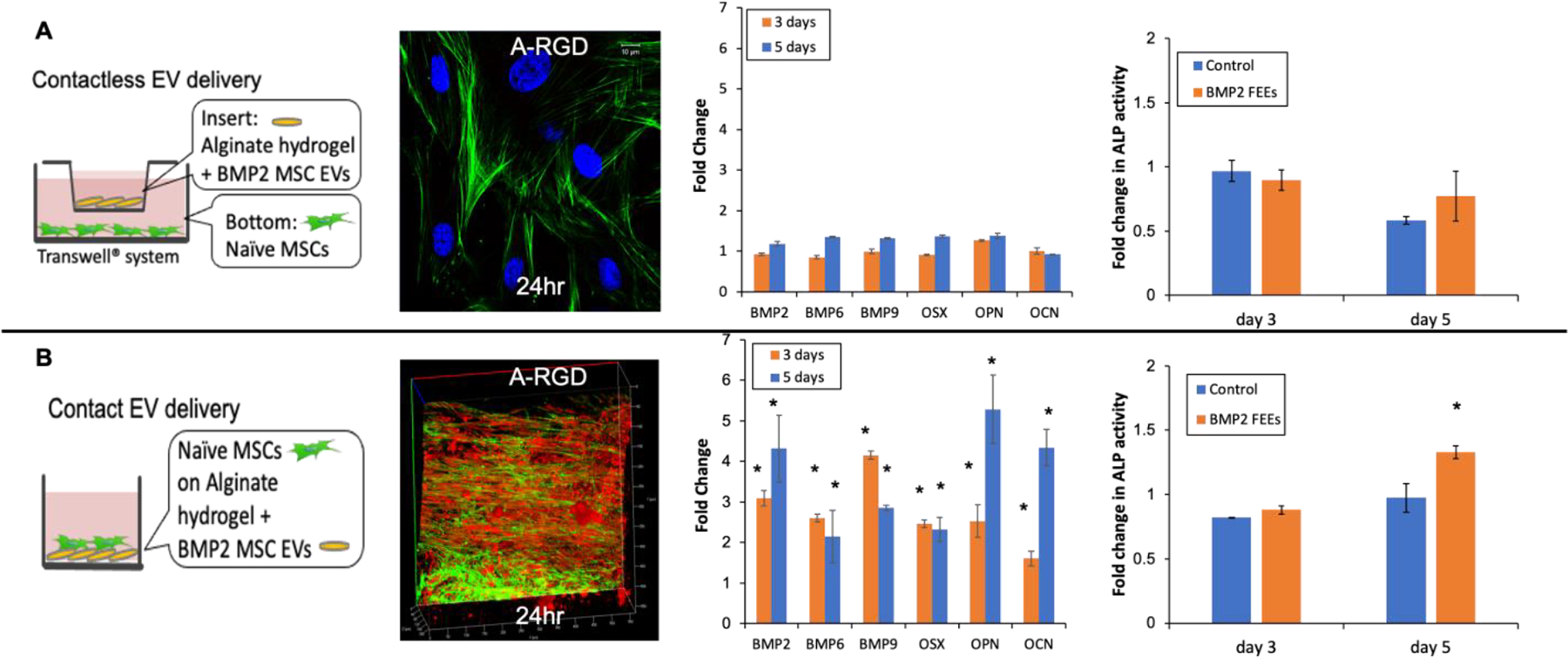
A) A schematic representation of the contactless experimental setup followed by confocal micrograph showing a representative image of the uptake of the fluorescently labeled FEEs (red) released from 4% A-RGD alginate hydrogels by HMSCs fluorescently stained with actin (green) and DAPI (blue) at 24 hours post the no contact experiment start time. Note the absence of EV presence in the cells indicating that minimal amounts of EVs were released from the hydrogels. The following graph depicts fold change in osteogenic gene expression at 3 and 5 days post the no contact experiment start time. No significant changes in expression levels were noted in comparison to control setup lacking EVs. The final graph shows fold change in alkaline phosphatase (ALP) activity in HMSCs 3 and 5 days the start of the contactless experiment. No significant change was observed with respect to the control group. B) A schematic representation of the contact experimental setup followed by 3D Confocal micrograph showing a representative image of the uptake of the fluorescently labeled BMP2 FEEs (red) by HMSCs fluorescently stained with actin (green) at 24 hours post the contact experiment start time. The following graph depicts fold change in osteogenic gene expression at 3 and 5 days post the contact experiment start time. The final graph shows fold change in ALP activity in HMSCs 3 and 5 days the start of the contact experiment. For all graphical figures, data points represent mean fold change +/− SD. * represents statistical significance with respect to the control group measured by student’s t-test.
In agreement with the results from the release profile experiments, no EVs were observed to be endocytosed by the HMSCs at 24 hours, indicating effective retention of the FEEs by the hydrogel (Figure 5A). In this experiment, the FEEs were fluorescently labeled in red and the cellular actin was stained with phalloidin FITC (green). The absence of red fluorescence-stained EVs in Figure 4A indicates the absence of endocytosed EVs within the cells. However, in experiments using hydrogels without RGD, released and endocytosed FEEs could be observed by confocal microscopy (Supplementary Figure 3). Three and five days after HMSC culture opposing the A-RDG hydrogels, negligible changes in osteoinductive gene expression and alkaline phosphatase (ALP) activity were observed. (Figure 5A). This result is aligned with the negligible number of EVs were released from this hydrogel over 3 – 5 days.
In contrast, when HMSCs were seeded in direct contact with 4% A-RGD hydrogels, the FEEs (Figure 5B), endocytosis of the encapsulated FEEs was observed by 3D confocal microscopy (Figure 5B). qRT PCR of representative osteoinductive gene expression in HMSCs indicated that direct seeding (contact with osteoinductive FEEs [21]) onto 4% A-RGD hydrogels triggered the osteogenic differentiation of the naïve HMSCs when compared to HMSCs seeded on A-RGD hydrogels lacking FEEs as observed by osteogenic gene expression and ALP activity assays (Figure 5B). Note that these experiments cannot be performed using the alginate hydrogels lacking the RGD peptide because the alginate hydrogels do not support the attachment and proliferation of HMSCs. Overall, these in vitro studies confirmed that the 4% A-RGD hydrogel was able to successfully encapsulate the osteoinductive FEEs, support HMSC attachment and promote osteoinductive differentiation of the naïve HMSCs.
Functionality of alginate encapsulated FEEs in vivo:
The ability of the 4% A-RGD hydrogels to deliver functionally active FEEs in vivo to direct bone regeneration was evaluated in a rat calvarial defect model. For these experiments, four groups were tested: alginate (A), alginate-RGD (A-RGD), alginate + FEEs (A+FEE) and alginate-RGD+FEEs (A-RGD+FEE). The osteoinductive ability of the FEEs was evaluated at 4 and 8 weeks. Quantitative μCT analysis indicated that alginate and A-RGD hydrogels by themselves were not osteoinductive over the study period as indicated by the absence of bone formation (Figures 6A, 6B).
Figure 6. In vivo functionality of the encapsulated FEEs:
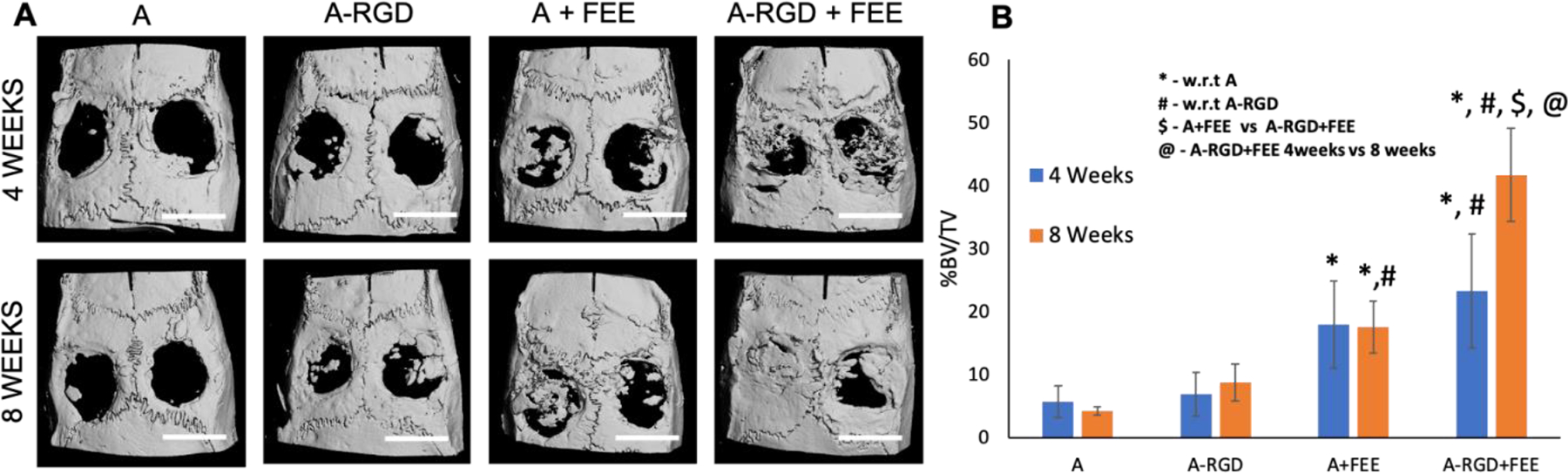
A) Representative μCT images of 5 mm calvarial defects that were treated with alginate hydrogels (+/−FEEs) and alginate-RGD hydrogels +/− FEEs at 4-weeks and 8-weeks post wounding. Scale bar in all μCT images represents 5mm. B) Volumetric quantitation of the μCT data expressed as percentage bone volume regenerated to total void volume (n=6 defects per group and per time point). * represents statistical significance with respect to the alginate alone control group (no RGD, no FEEs) and # represents statistical significance with respect to the A-RGD alone (no FEEs) group. $ represents statistical significance for A+FEE versus A-RGD+FEE. * represents statistical significance for A-RGD+FEE 4 weeks versus A-RGD+FEE 8 weeks calculated using Tukey’s post hoc test post ANOVA.
At 4 weeks, the regenerated bone volume for both the A+FEE group and A-RGD+FEE group showed significantly increased bone formation compared to alginate alone control. This indicated that at this time point, the FEE functionality was not affected by the presence/absence of the RGD tethering peptide. At 8 weeks, the A-RGD+FEE group showed significantly greater bone regeneration when compared to either the alginate or A-RGD groups. At 8 weeks, there was a clear, statistically significant difference in the volume of bone regenerated by the A-RGD+FEE group compared to A+FEE group. The regenerated bone volume in A+FEE group (lacking the RGD tethering peptide), did not increase between 4 and 8 weeks. In contrast, the volume of bone regenerated in the A-RGD+FEE group increased between 4 and 8 weeks (Figure 6B) and suggested the effective retention and distribution of FEEs by this scaffold.
Complimentary histological evaluation of the calvarial defects (Figure 7) reiterated the μCT observations that there was minimal bone presence in the control groups lacking FEEs (denoted by black arrows in Figure 7) and the presence of abundant residual alginate hydrogel (designated by yellow arrows in Figure 7). Immunofluorescence staining of bone-specific ECM proteins dentin matrix protein 1 (DMP1, Figure 8), bone sialoprotein (BSP, Figure 9) and osteocalcin (OCN, Figure 10) of these control groups also failed to reveal broad or remarkable DMP1 or BSP expression, indicating a generalized lack of bone formation. The extent of bone formation observed by μCT analysis at 4 weeks for the A+FEE and the A-RGD+FEE groups was evident histologically (Figure 7). The osteogenic effect of the FEEs in both of these groups was noted by the presence of bone (black arrows in Figure 7) approximating the alginate hydrogels. The osteogenic response was also indicated by the increased expression of DMP1, BSP and OCN (Figures 8, 9 and 10 respectively). Overall, these results confirmed the ability of the A-RGD-FEE hydrogels with prolonged delivery of the osteoinductive FEEs to increase bone regeneration in vivo.
Figure 7. Histological evaluation of calvarial defects:
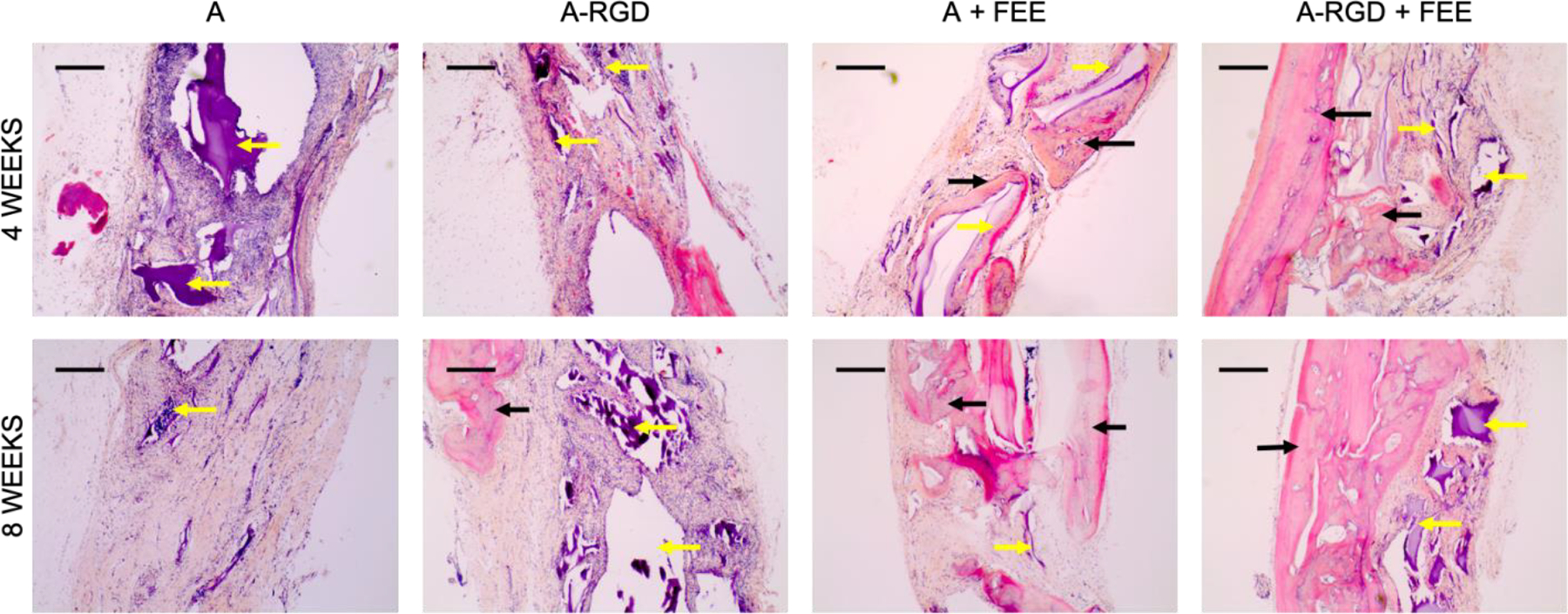
Images are representative light microscopy images of H&E stained demineralized calvarial samples of defects treated with alginate hydrogels (+/−FEEs) and alginate-RGD hydrogels +/− FEEs at 4- and 8-weeks post wounding. The black arrows in the images point to regenerated bone tissue. The yellow arrows in the images point to alginate hydrogel. Scale bar represents 100μm in all images.
Figure 8. IHC of calvarial defects:
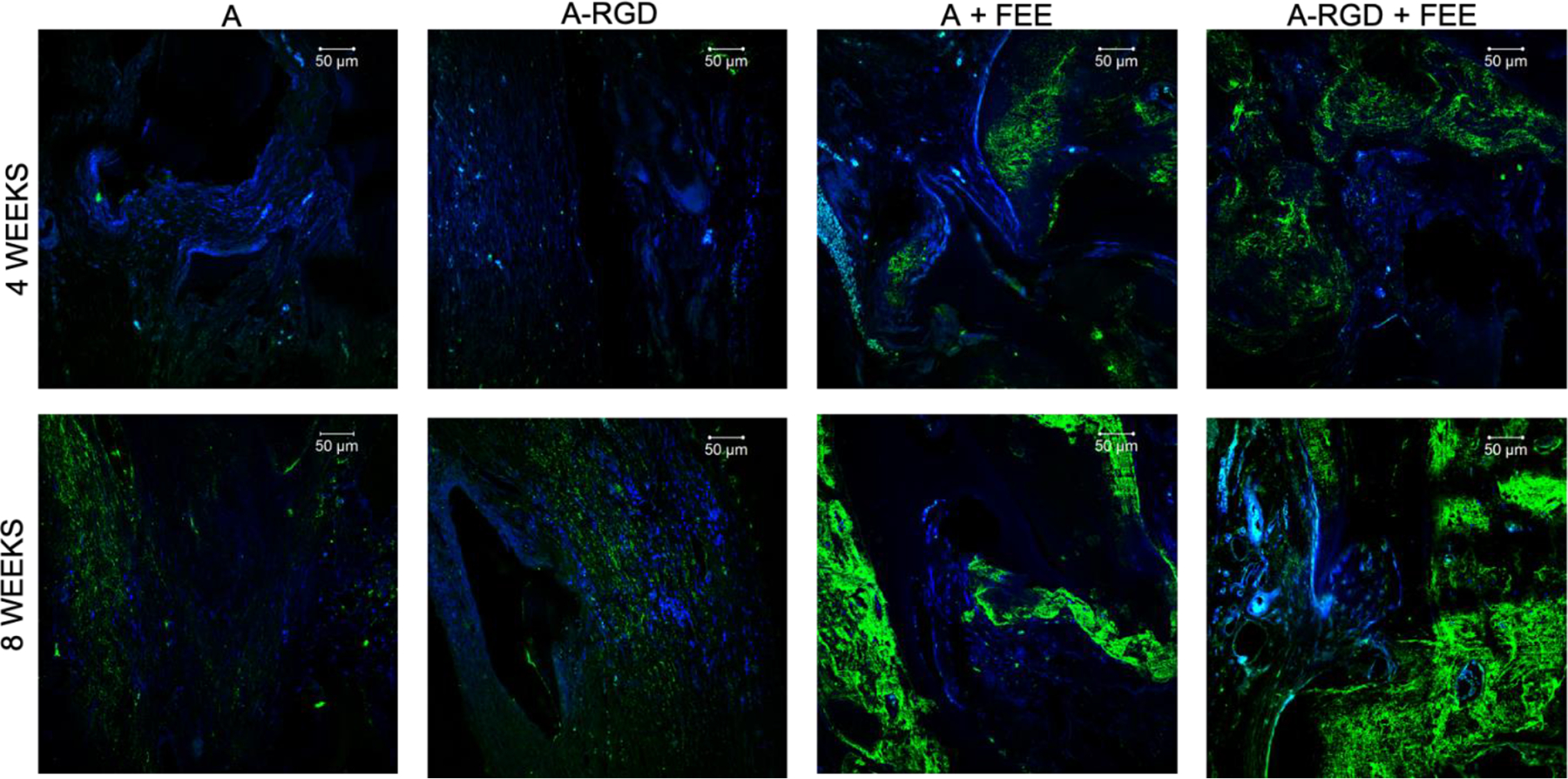
Images represent the expression levels of osteoinductive marker protein DMP1 in the calvarial sections from the demineralized calvarial samples of defects treated with alginate hydrogels (+/− FEEs) and alginate-RGD hydrogels +/− FEEs at 4- and 8-weeks post wounding. Scale bar represents 50μm in all images. In all images green represents DMP1 presence in the section and the nuclei are stained in blue.
Figure 9. IHC of calvarial defects:
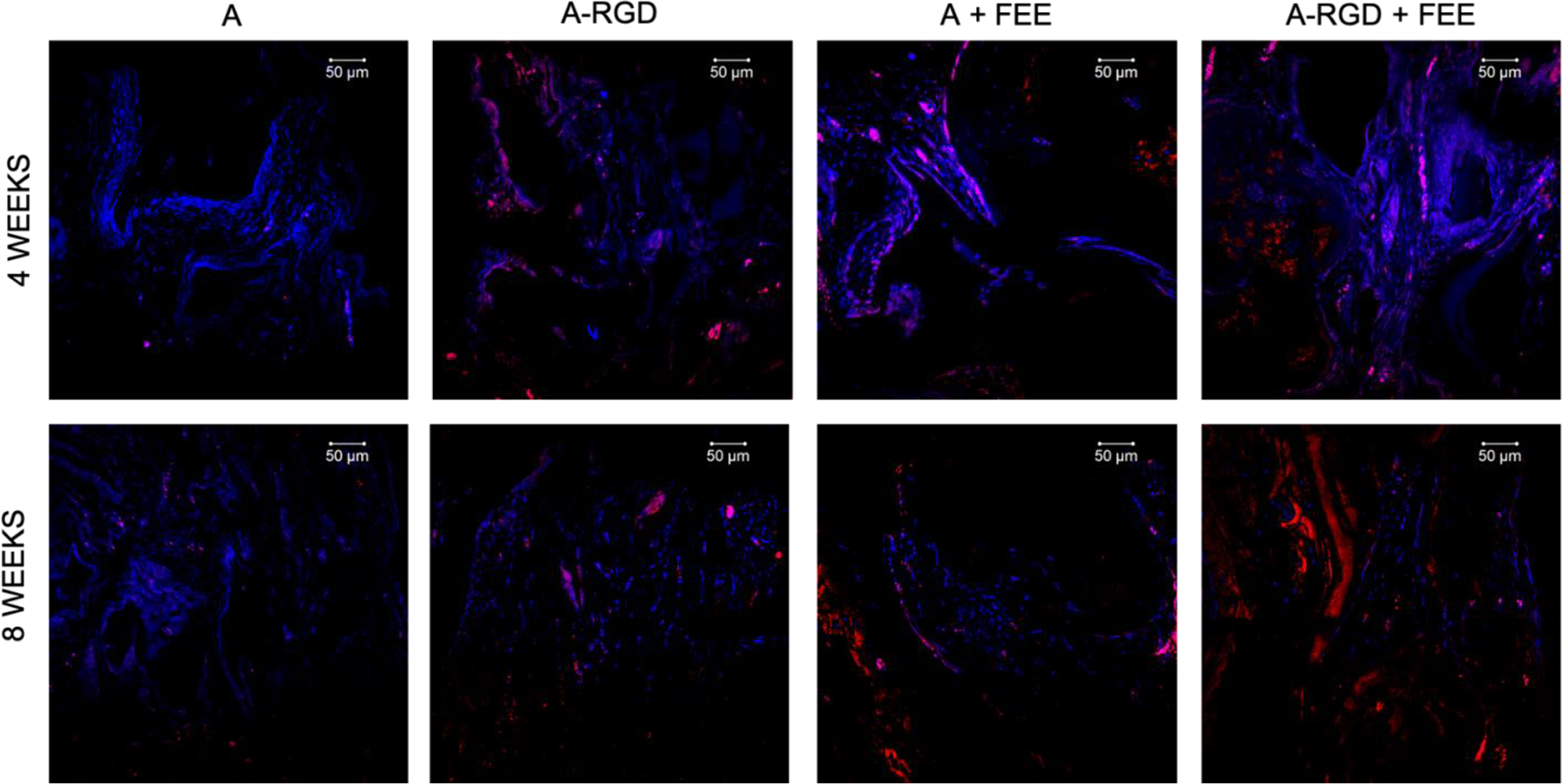
Images represent the expression levels of osteoinductive marker protein BSP in the calvarial sections from the demineralized calvarial samples of defects treated with alginate hydrogels (+/− FEEs) and alginate-RGD hydrogels +/− FEEs at 4- and 8-weeks post wounding. Scale bar represents 50μm in all images. In all images red represents BSP presence in the section and the nuclei are stained in blue.
Figure 10. IHC of calvarial defects:
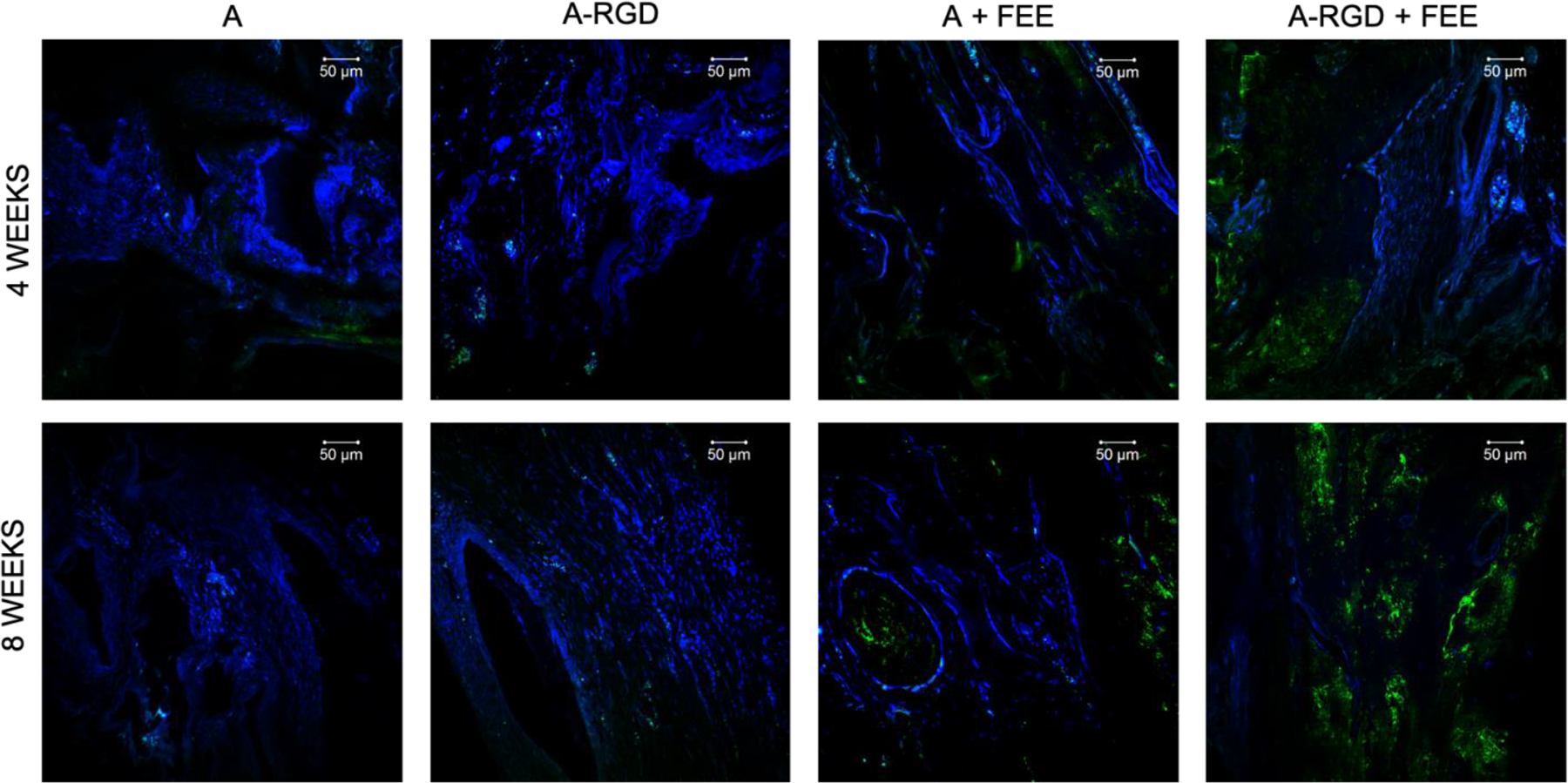
Images represent the expression levels of osteoinductive marker protein OCN in the calvarial sections from the demineralized calvarial samples of defects treated with alginate hydrogels (+/− FEEs) and alginate-RGD hydrogels +/− FEEs at 4- and 8-weeks post wounding. Scale bar represents 50μm in all images. In all images green represents OCN presence in the section and the nuclei are stained in blue.
Discussion:
The therapeutic success of MSC-derived EVs in their native state or engineered for a specific function is predicated upon the ability to deliver them in a safe and effective manner. Efficient delivery reduces the number of EVs required to achieve the desired function as well as prevents ectopic activity, especially when functionally engineered EVs (FEEs) are involved. In this study, we sought to understand the dynamics of EV interactions with the ECM and devise ways to leverage those interactions for regenerative medicine applications.
As a proof-of-concept approach, we have evaluated EV encapsulation in alginate hydrogels. Over the past several years, multiple studies have shown the versatility, biocompatibility and safety of alginate as an effective biomaterial[38–41]. The mechanical properties of this hydrogel can be tailored by varying the weight percentages of the monomer as well as by the degree of photo crosslinking. Furthermore, this hydrogel (and others) can be formulated as an injectable material, a bulk gel (or solid), or used as a bio ink to create 3D-printed scaffolds. These attributes render alginate as an attractive candidate for initial studies of EV encapsulation. Although the results presented in this study highlight the potential of alginate, we envision that the principles outlined herein may be broadly applicable for various hydrogel formulations that can incorporate the carrier peptides.
To demonstrate engineered and directed functionality of EVs, this study adopted the use of previously characterized osteoinductive engineered EVs derived from HMSCs constitutively expressing BMP2 [21]. The osteoinductive properties of this FEE is driven in part by its miRNA composition and it has demonstrated function to enhance bone repair.
We demonstrated that the integrin membrane proteins of this FEE contribute to its binding to cell-secreted type I collagen and fibronectin and to derived peptides in a dose dependent, saturable manner. A recent review of EV membrane interactions with cells and ECM indicated a possible link between extracellular fibronectin and EVs and their role in EV recycling[42]. Our results affirm the role of integrin membrane receptor – ECM interaction and suggests that MSC EV or FEE binding to endogenous ligands can be leveraged to enhance the efficacy of EVs in regenerative medicine.
Here, we have used one such peptide, RGD as an EV tether. Previous studies have chemically conjugated or engineered the RGD peptide to EVs [43, 44] to target them to cellular integrins. This interesting approach may have advantages. However, with MSC EVs endocytosed by target cells via the heparin sulfate proteoglycan (HSPG) receptors on the plasma membrane of the target cells[45, 46], it is possible that targeting the cellular integrins by adding an RGD peptide to the EV membrane may affect the natural endocytic process of MSC EVs. In this study, we have identified that integrins present on MSC EVs can bind to ECM derivative peptides and the pre-treatment of the EVs with the peptides reduced EV binding to their respective ECM proteins. The significance of EV membrane integrins has been documented in cancer cells and has been implicated in tumor progression [47]. EV resident integrins in MSCs require further characterization from the perspective of their binding to the remodeling ECM of healing tissues or the therapeutic tethering and retaining FEEs within an engineered scaffold as successfully demonstrated here using alginate hydrogels. Increasing adhesive peptide amounts by varying the weight percentage of alginate increased the retention of EVs such that approximately 80% of EVs were retained after one week in the 4% A-RGD-FEE group. This enhanced retention may prolong EV presence at healing sites leading to increased efficiency of delivery as well as functionality.
RGD containing scaffolds for bone tissue engineering typically are used to target cell adhesion, proliferation and retention[48]; here, the generated scaffolds may serve a dual purpose. They possess the ability to promote host cell attachment and proliferation by means of unused RGD domains within the alginate and also to retain EVs. In vivo, EVs exist tethered to the cell secreted ECM[49] and are made available be utilized by the colonizing cells. Similarly, the EVs tethered by means of RGD-integrin interaction to the alginate biomaterial was endocytosed by colonizing MSCs. When an engineered EV is introduced to the system tethered to the scaffold similar to the natural ECM as presented in this study, the colonizing cell function can be effectively modified. In this case, enhanced osteoinduction supported increased bone regeneration in vivo.
The BMP2 FEE utilized in this study has been previously characterized for its in vitro and in vivo osteoinductive properties and hence has served as an ideal platform for the evaluation of tethering and delivery mechanisms[21]. The functional studies conducted in vitro indicate that the encapsulated EVs trigger HMSC osteoblastic differentiation. This study also indicates that the adhesive peptide-tethered FEEs were active following endocytosis by ECM adherent HMSCS in vitro. Thus, the implantation of FEEs within a tethering scaffold continuously osteogenesis between 4 and 8 weeks when compared to FEEs in the alginate scaffold lacking the tethering RGD peptides. At 8 weeks, we interpret the RGD containing hydrogel as continuing to release FEE and promote further bone regeneration. The engineering of a measurable delay in the release of the FEEs from A-RGD hydrogels and the associated prolonged osteoinductive signaling for bone regeneration is an important advance in the potential use of EVs for tissue engineering.
Previous studies have shown that MSC EVs are endocytosed by recipient cells in a dose dependent and saturable manner [14, 45, 46]. This saturation effect implies that an overabundance of EVs may not necessarily provide increased efficiency. To this point, alginate hydrogels carrying FEEs (without RGD tether) may have released all EVs rapidly as indicated by bone regeneration at 4 weeks. Yet, the effect of the FEEs was similar to the slow releasing hydrogel containing the RGD tether indicating the saturable nature of EV activity as well as the benefits of prolonged EV release using this system. The μCT data for bone repair was validated histologically as well as by IHC for osteoinductive protein markers that confirmed the beneficial effects of prolonged release of the FEEs.
The immunohistochemical results showed increased presence of osteoinductive proteins DMP1, BSP and OCN in the FEE treated groups in line with their expected activity [21]. The role of DMP1[50], BSP[51, 52] and OCN[52] with respect to bone formation, repair and regeneration has been well established. Therefore, their upregulation in the presence of FEEs indicates continued osteoinductive stimulation of the host system resulting in better regeneration of tissue. While the groups containing FEEs showed enhanced presence of these proteins, only the group with tethered FEEs showed an increase in protein levels between 4 and 8 weeks lending further support to the quantitative μCT results. Taken together, the in vivo results indicated the benefits of tethering FEEs to biomaterials as well as the benefits of prolonged delivery for bone regenerative applications.
In addition to EV delivery, the in vivo results also indicated the ability of the RGD tethered alginate hydrogels to be degraded over time. This was evident from the histological images that showed new bone formation around and within the islands of hydrogel in the regenerating tissue. We predict that the degradation rate of the alginate with the infiltration of host cells may also contribute to the release kinetics in vivo. Nevertheless, tethering promoted enhanced retention and prolonged release. Recent studies have encapsulated EVs within alginate hydrogels for regenerative medicine applications to effect regeneration of skin [53] and for the treatment of myocardial infraction [54]. Both of these studies rely on the immediate effect of the EVs to impart anti-inflammatory and anti-apoptotic activity. While quick delivery of EVs may suit these applications, for engineering applications that require a prolonged presence and for applications that utilize FEEs tethering may prove advantageous as evidenced by our in vivo results.
With the mechanical property of alginate highly flexible, it’s possible to alter peptide concentrations as well as alginate monomer concentrations to precisely control release kinetics to suit different applications. Furthermore, with different peptides eliciting different kinetics, it may even be possible to facilitate a biphasic/multiphasic release profile of multiple FEEs from the same hydrogel that target different cellular processes. We believe that these areas can be active targets of future studies by us as well as other groups.
Conclusion:
In summary, this study presents evidence that engineered MSC EVs interact dose dependently and saturably with ECM proteins type I collagen and fibronectin and their derivative adhesive peptides and that this interaction can be leveraged to control the release of functional EVs from hydrogels to promote regeneration in vivo over a prolonged period of time. While bone has been utilized as a model system in this study, we envision the applicability and the extrapolation of this methodology to enhance repair/regeneration of multiple tissues.
Supplementary Material
Signifiacne statement.
The beneficial effects of human MSC (HMSC) therapy are attributable to paracrine effects of the HMSC derived EVs. While EV engineering has the potential to impact several fields of regenerative medicine, targeted delivery of the engineered EVs with spatial and temporal control is necessary to prevent off-target effects and enhance tissue specificity. Here, we have leveraged the interactions of MSC EVs with ECM proteins to develop a tethering system that can be utilized to prolong EV delivery in vivo while maintaining the structural and functional integrity of the EVs Our work has provided a tunable platform for EV delivery that we envision can be formulated as an injectable material or a bulk hydrogel suitable for regenerative medicine applications.
Acknowledgement:
This work was funded by NIH R01DE027404 to Ravindran and Gajendrareddy. We would like to acknowledge the support of the UIC research resources center (RRC) for their assistance with electron and confocal microscopy as well as for the NTA and NMR analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors do not have any conflicts of interest to report.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference:
- [1].Park IH, Micic ID, Jeon IH, A study of 23 unicameral bone cysts of the calcaneus: open chip allogeneic bone graft versus percutaneous injection of bone powder with autogenous bone marrow, Foot & ankle international 29(2) (2008) 164–70. [DOI] [PubMed] [Google Scholar]
- [2].Johnstone RM, Mathew A, Mason AB, Teng K, Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins, Journal of cellular physiology 147(1) (1991) 27–36. [DOI] [PubMed] [Google Scholar]
- [3].Azmi AS, Bao B, Sarkar FH, Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review, Cancer Metastasis Rev 32(3–4) (2013) 623–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M, Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1, The Journal of biological chemistry 288(24) (2013) 17713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nat Cell Biol 9(6) (2007) 654–9. [DOI] [PubMed] [Google Scholar]
- [6].Yao Y, Huang J, Geng Y, Qian H, Wang F, Liu X, Shang M, Nie S, Liu N, Du X, Dong J, Ma C, Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts, PloS one 10(6) (2015) e0129164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dai W, Hale SL, Kloner RA, Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats, Regenerative medicine 2(1) (2007) 63–8. [DOI] [PubMed] [Google Scholar]
- [8].Gnecchi M, Zhang Z, Ni A, Dzau VJ, Paracrine mechanisms in adult stem cell signaling and therapy, Circulation research 103(11) (2008) 1204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK, Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury, Stem cell research 4(3) (2010) 214–22. [DOI] [PubMed] [Google Scholar]
- [10].Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N, Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats, PloS one 7(9) (2012) e44092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K, Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling, Immunology letters 147(1–2) (2012) 47–54. [DOI] [PubMed] [Google Scholar]
- [12].Cooper LF, Ravindran S, Huang CC, Kang M, A Role for Exosomes in Craniofacial Tissue Engineering and Regeneration, Frontiers in physiology 10 (2019) 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang C-C, Narayanan R, Alapati S, Ravindran S, Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration, Biomaterials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Narayanan R, Huang CC, Ravindran S, Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells, Stem Cells Int 2016 (2016) 3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang C-C, Kang M, Narayanan R, DiPietro LA, Cooper LF, Gajendrareddy P, Ravindran S, Evaluating the Endocytosis and Lineage-Specification Properties of Mesenchymal Stem Cell Derived Extracellular Vesicles for Targeted Therapeutic Applications, Frontiers in pharmacology 11(163) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cui Y, Luan J, Li H, Zhou X, Han J, Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression, FEBS letters 590(1) (2016) 185–92. [DOI] [PubMed] [Google Scholar]
- [17].Martins M, Ribeiro D, Martins A, Reis RL, Neves NM, Extracellular Vesicles Derived from Osteogenically Induced Human Bone Marrow Mesenchymal Stem Cells Can Modulate Lineage Commitment, Stem cell reports 6(3) (2016) 284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang J, Liu X, Li H, Chen C, Hu B, Niu X, Li Q, Zhao B, Xie Z, Wang Y, Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway, Stem cell research & therapy 7(1) (2016) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Narayanan K, Kumar S, Padmanabhan P, Gulyas B, Wan ACA, Rajendran VM, Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation, Biomaterials 182 (2018) 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qin Y, Wang L, Gao Z, Chen G, Zhang C, Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo, Scientific reports 6 (2016) 21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang CC, Kang M, Lu Y, Shirazi S, Diaz JI, Cooper LF, Gajendrareddy P, Ravindran S, Functionally engineered extracellular vesicles improve bone regeneration, Acta Biomater 109 (2020) 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Riau AK, Ong HS, Yam GHF, Mehta JS, Sustained Delivery System for Stem Cell-Derived Exosomes, Frontiers in pharmacology 10 (2019) 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ, Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model, Stem Cells Transl Med 6(3) (2017) 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shimoda A, Ueda K, Nishiumi S, Murata-Kamiya N, Mukai SA, Sawada S, Azuma T, Hatakeyama M, Akiyoshi K, Exosomes as nanocarriers for systemic delivery of the Helicobacter pylori virulence factor CagA, Scientific reports 6 (2016) 18346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shiue SJ, Rau RH, Shiue HS, Hung YW, Li ZX, Yang KD, Cheng JK, Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury-induced pain in rats, Pain 160(1) (2019) 210–223. [DOI] [PubMed] [Google Scholar]
- [26].Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang C-C, Feng L, Zelka R, Lopez J, Sharma M, Roth S, Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion, Biomaterials (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK, Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus, Proceedings of the National Academy of Sciences of the United States of America 114(17) (2017) E3536–E3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ, Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model, Theranostics 7(1) (2017) 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, Mao C, Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration, Theranostics 9(1) (2019) 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu N, Wang L, Guan J, Tang C, He N, Zhang W, Fu S, Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model, Int J Biol Macromol 117 (2018) 102–107. [DOI] [PubMed] [Google Scholar]
- [31].Neves MI, Moroni L, Barrias CC, Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments, Front Bioeng Biotechnol 8 (2020) 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang CC, Narayanan R, Warshawsky N, Ravindran S, Dual ECM Biomimetic Scaffolds for Dental Pulp Regenerative Applications, Front Physiol 9 (2018) 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ravindran S, Gao Q, Ramachandran A, Blond S, Predescu SA, George A, Stress chaperone GRP-78 functions in mineralized matrix formation, J Biol Chem 286(11) (2011) 8729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tavakol S, Ragerdi Kashani I, Azami M, Khoshzaban A, Tavakol B, Kharrazi S, Ebrahimi S, Rezayat Sorkhabadi SM, In vitro and in vivo investigations on bone regeneration potential of laminated hydroxyapatite/gelatin nanocomposite scaffold along with DBM, Journal of Nanoparticle Research 14(12) (2012) 1265. [Google Scholar]
- [35].Shah R, Patel T, Freedman JE, Circulating Extracellular Vesicles in Human Disease, N Engl J Med 379(10) (2018) 958–966. [DOI] [PubMed] [Google Scholar]
- [36].Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK, Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, Journal of extracellular vesicles 7(1) (2018) 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Plow EF, Haas TA, Zhang L, Loftus J, Smith JW, Ligand binding to integrins, J Biol Chem 275(29) (2000) 21785–8. [DOI] [PubMed] [Google Scholar]
- [38].Garske DS, Schmidt-Bleek K, Ellinghaus A, Dienelt A, Gu L, Mooney DJ, Duda GN, Cipitria A, Alginate Hydrogels for In Vivo Bone Regeneration: The Immune Competence of the Animal Model Matters, Tissue engineering. Part A (2020). [DOI] [PubMed] [Google Scholar]
- [39].Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, Li S, Deng Y, He N, Injectable hydrogels for cartilage and bone tissue engineering, Bone Res 5 (2017) 17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sun J, Tan H, Alginate-Based Biomaterials for Regenerative Medicine Applications, Materials (Basel) 6(4) (2013) 1285–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Augst AD, Kong HJ, Mooney DJ, Alginate hydrogels as biomaterials, Macromol Biosci 6(8) (2006) 623–33. [DOI] [PubMed] [Google Scholar]
- [42].Buzás EI, Tóth EÁ, Sódar BW, Szabó-Taylor KÉ, Molecular interactions at the surface of extracellular vesicles, Seminars in Immunopathology 40(5) (2018) 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang H, Wu J, Wu J, Fan Q, Zhou J, Wu J, Liu S, Zang J, Ye J, Xiao M, Tian T, Gao J, Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice, J Nanobiotechnology 17(1) (2019) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang J, Li W, Lu Z, Zhang L, Hu Y, Li Q, Du W, Feng X, Jia H, Liu BF, The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis, Nanoscale 9(40) (2017) 15598–15605. [DOI] [PubMed] [Google Scholar]
- [45].Huang CC, Kang M, Narayanan R, DiPietro LA, Cooper LF, Gajendrareddy P, Ravindran S, Evaluating the Endocytosis and Lineage-Specification Properties of Mesenchymal Stem Cell Derived Extracellular Vesicles for Targeted Therapeutic Applications, Front Pharmacol 11 (2020) 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Huang CC, Narayanan R, Alapati S, Ravindran S, Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration, Biomaterials 111 (2016) 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Paolillo M, Schinelli S, Integrins and Exosomes, a Dangerous Liaison in Cancer Progression, Cancers (Basel) 9(8) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Z, Dong L, Han L, Wang K, Lu X, Fang L, Qu S, Chan CW, Self-assembled Biodegradable Nanoparticles and Polysaccharides as Biomimetic ECM Nanostructures for the Synergistic effect of RGD and BMP-2 on Bone Formation, Sci Rep 6 (2016) 25090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, Stolz DB, Badylak SF, Matrix-bound nanovesicles within ECM bioscaffolds, Sci Adv 2(6) (2016) e1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE, Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism, Nat Genet 38(11) (2006) 1310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gorski JP, Wang A, Lovitch D, Law D, Powell K, Midura RJ, Extracellular bone acidic glycoprotein-75 defines condensed mesenchyme regions to be mineralized and localizes with bone sialoprotein during intramembranous bone formation, J Biol Chem 279(24) (2004) 25455–63. [DOI] [PubMed] [Google Scholar]
- [52].Baht GS, Hunter GK, Goldberg HA, Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation, Matrix Biol 27(7) (2008) 600–8. [DOI] [PubMed] [Google Scholar]
- [53].Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S, Akbariqomi M, Sanikhani NS, Absalan M, Tavoosidana G, Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study, J Biomed Mater Res A 108(3) (2020) 545–556. [DOI] [PubMed] [Google Scholar]
- [54].Lv K, Li Q, Zhang L, Wang Y, Zhong Z, Zhao J, Lin X, Wang J, Zhu K, Xiao C, Ke C, Zhong S, Wu X, Chen J, Yu H, Zhu W, Li X, Wang B, Tang R, Wang J, Huang J, Hu X, Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction, Theranostics 9(24) (2019) 7403–7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


