Abstract
Spray drying is a technique that can be used to stabilize biopharmaceuticals, such as vaccines, within dry particles. Compared to liquid pharmaceutical products, dry powder has the potential to reduce costs associated with refrigerated storage and transportation. In this study, spray drying was investigated for processing an adjuvanted tuberculosis subunit vaccine, formulated as an oil-in-water nanoemulsion, into a dry powder composed of microparticles. Applying in-silico approaches to the development of formulation and processing conditions, successful encapsulation of the adjuvanted vaccine within amorphous microparticles was achieved in only one iteration, with high retention (>90%) of both the antigen and adjuvant system. Moisture-controlled stability studies on the powder were conducted over 26 months at temperatures up to 40°C. Results showed that the powder was physically stable after 26 months of storage for all tested temperatures. Adjuvant system integrity was maintained at temperatures up to 25 °C after 26 months and after one month of storage at 40 °C. The spray-dried product demonstrated improved antigen thermostability when stored above refrigerated temperatures as compared to the liquid product. These results demonstrate the feasibility of spray drying as a method of encapsulating and stabilizing an adjuvanted vaccine.
Keywords: nano-encapsulation, physical stability, gel microparticles, vaccine spray drying, adjuvant emulsion
Graphical Abstract
Introduction
An important aspect of disease control is intervention by inducing widespread protective immunity through vaccination. Liquid pharmaceutical formulations, such as vaccines, usually must be kept refrigerated during transportation and storage in order to maintain their potency. Widespread global immunization is limited because vaccines often require refrigeration to remain effective [1]. Exposure to higher temperatures can lead to loss of potency of the active pharmaceutical ingredient and the possible formation of unsafe byproducts. Eliminating the refrigeration requirement would facilitate greater global distribution through cost reduction of storage and transportation. This would be especially beneficial towards interventions for diseases that are prevalent in population-dense locations that lack the required infrastructure to maintain refrigeration. Thermostability can be improved by converting a liquid product into a solid dosage form that can be rehydrated for administration as needed. Desiccation processing methods, such as spray drying, have been widely used in the food processing, chemical, and pharmaceutical industries to preserve temperature-sensitive components [2]. Spray drying produces a dry powder made up of microparticles through the drying of atomized droplets via a drying gas. Once the solvent has evaporated, the dried particles are separated from the drying gas, e.g. via a cyclone.
Thermostability of vaccines can be improved by stabilization via spray drying. Experimental dry powder vaccines have been developed for the intervention of measles [3], influenza [4, 5, 6, 7], anthrax [8], herpes [9], whooping cough [10], and tuberculosis [11, 12]. The immunogenicity of spray-dried vaccines can be maintained provided an appropriate stabilizing excipient(s) and drying conditions are used. Successfully stabilized dry powder vaccines have been reported to elicit similar immunogenicity profiles as their liquid counterparts after reconstitution when administered in animal models [3, 4, 7, 10]. Ideally, a simple, low cost formulation with one stabilizing excipient is used to improve stability, however, an excipient system with optimized stabilizing properties consisting of multiple stabilizers may be required for biologics which are difficult to stabilize [11, 13].
A disease whose eradication would be greatly advanced with the existence of a thermostable vaccine is tuberculosis (TB). The World Health Organization has noted that TB is the leading cause of death from a single infectious agent, having caused an estimated 1.5 million deaths in 2018 [14]. Approximately one in four people globally is infected with latent TB, wherein the immune system has not eliminated the pathogen and instead has contained it within granulomas [15]. Ten percent of those with latent TB are expected to develop active TB within their lifetime [14, 16]. There is currently no vaccine that is fully effective in preventing either TB incidence or latent TB becoming active in adults [14]. Due to these significant drawbacks to the current intervention strategy, an alternative TB vaccine must be developed. The WHO has explicitly noted that new TB vaccines must improve upon the current BCG vaccine with demonstrated efficacy in adolescent and adult subjects with and without latent tuberculosis, and must be safe for all subjects, including those with suppressed immune systems [16]. Subunit vaccines typically have better safety profiles than live attenuated vaccines; however, they often do not elicit strong immune responses on their own. Thus, if administered via parenteral injection, an adjuvant may be required to stimulate a robust immune response [17].
A vaccine candidate under development by the Infectious Disease Research Institute (Seattle, WA, USA), ID93+GLA-SE, is a subunit TB vaccine based on a recombinant fusion protein, ID93, and a nanoemulsion adjuvant system, GLA-SE. ID93 is composed of four Mycobacterium tuberculosis antigens, Rv3619, Rv1813, Rv3620, and Rv2608, all associated with virulence or latency [18]. The adjuvant system is formulated as a nanoemulsion and is comprised of glucopyranosyl lipid A (GLA), a synthetic Toll-like receptor 4 agonist, and a squalene oil-in-water stable emulsion (SE). Development and characterization of the GLA-SE system is discussed elsewhere [19]. The ID93+GLA-SE vaccine candidate has shown promising preclinical [20] and clinical results [21, 22] when administered three times as a liquid via injection.
To improve thermal stability, a liquid product may be converted into a solid dosage form through processing methods such as lyophilization or spray drying. Lyophilization, also known as freeze-drying, removes water by freezing the product and then removing the water through sublimation, leaving behind a dry ‘cake’. A previous study investigated lyophilization of the ID93+GLA-SE vaccine in a single vial as a method of improving thermal stability [23]. Reconstitution of the lyophilized vaccine showed that physicochemical characteristics, such as ID93 and GLA integrity, were maintained. Further formulation development led to several lyophilized vaccine candidates [24], which were stable after three months storage at 37 °C and still elicited immune response in mice after storage at 50 °C for three months. The best-performing candidate utilized a trehalose-Tris excipient system to stabilize the vaccine [24] and is currently undergoing a Phase I clinical trial [25]. Success in lyophilization of the ID93+GLA-SE vaccine led to consideration of spray drying as an alternative technique to produce a dry vaccine product. Spray drying is more cost-effective than lyophilization as it is a faster, more scalable process with reduced operation and installation costs [26, 27]. Nevertheless, there are technical difficulties associated with spray drying the ID93+GLA-SE formulation. One challenge is due to the structure of the ID93+GLA-SE vaccine as a nanoemulsion with a phospholipid emulsifier. Its emulsion droplets must be encapsulated with high efficiency within particles during the spray drying. Another challenge is to stabilize both the ID93 and GLA-SE active components of the vaccine within the dry powder for long-term room temperature stability. Both challenges must be overcome to produce a successful spray-dried vaccine powder.
Stabilization requires an appropriate stabilizer and optimal spray drying conditions to ensure that desiccation does not lead to losses or changes in vaccine component structure. There has been successful stabilization via spray drying of vaccines formulated as dispersions. Enveloped viral vector vaccines [6], an outer membrane vesicle vaccine [10], and a subunit vaccine consisting of an antigen and liposomal adjuvant system [12] have all been stabilized through spray drying with the disaccharide trehalose as an excipient. Trehalose acted as a glass stabilizer to stabilize both the protein and lipid components of the vaccines in order to create a successful dry powder product. Trehalose is a commonly used glass stabilizer due to its high glass transition temperature, high solubility [28], low toxicity [29], and ability to act as both a protein [30] and liposome [31] stabilizer during spray drying. The glass transition temperature (Tg) can be defined as the temperature at which the properties of a brittle amorphous material undergo a reversible change to more rubber-like. Glass stabilizers are hypothesized to physically stabilize proteins during drying and storage through two main mechanisms: water replacement and vitrification [2, 30, 32, 33]. Water replacement theory states that biologic structure is maintained in water through hydrogen bonding. During drying, the hydrogen bonds between the water and the biologic are replaced by hydrogen bonds with the glass stabilizer. To inhibit protein denaturing, the glass stabilizer must be capable of forming many hydrogen bonds and maintain an amorphous state in order to match the biologic structure [2, 30]. Vitrification theory states that the biologic is rendered immobile within a glassy matrix [2, 30]. Protein stability is largely dependent on the molecular mobility of the stabilizer at temperatures near the glass transition temperature [2, 30]. It has been reported that glass stabilizers also stabilize the phospholipid layer of liposomes through both the water replacement and vitrification mechanisms [31]. Further refinement of these theories includes the consideration of the effect of α relaxation (global relaxation) versus β relaxation (local relaxation) processes on glass stability [34].
In this paper, we stabilize the tuberculosis subunit vaccine ID93+GLA-SE via spray drying using trehalose as a stabilizer. A successful spray-dried ID93+GLA-SE product must stabilize the ID93 protein and GLA long-term as well as encapsulate the GLA-SE nanoemulsion droplets with high efficiency. An in-silico engineering approach was used to calculate spray drying process parameters based on material properties to minimize vaccine component losses. The resulting powder was placed on a stability study at various temperatures for 26 months and analyzed to assess both the physical stability of the powder and the chemical stability of the vaccine components.
Theory
Particle Design
Use of a glass stabilizer is required to stabilize both the ID93 protein and the GLA-SE nanoemulsion droplets. Trehalose was chosen as the glass stabilizing excipient for this study due to its relatively high Tg and demonstrated ability to stabilize spray-dried proteins and lipid-based dispersions. A relatively high solute concentration (100 mg/mL) of the excipient was chosen. Increasing the solids content increases the generated particle size. Larger particles have a lower surface-to-volume ratio, which increases the probability for nanoemulsion droplets to be embedded within the particle. This is because a lower surface area will decrease availability for the droplets to accumulate on the surface. Increased encapsulating agent concentration supports an earlier shell formation [35], thus trapping the nanoemulsion droplets within the interior of the forming particle. Additionally, studies on the spray drying of phages [13] and an HSV-2 vaccine [9] have demonstrated that spray drying with an increased trehalose concentration in the feedstock provides better stabilization than a lower concentration.
An amorphous solid phase is desired in order to promote stabilization of the antigen, agonist, and phospholipid membrane of the adjuvant system. A study on the lyophilization of the GLA-SE vaccine have shown that crystalline lyophilisate increased GLA-SE emulsion droplet size after reconstitution [24]. Amorphous trehalose microparticles can be designed to be stable for storage at a specific temperature by ensuring low molecular mobility. With a low molecular mobility, the amorphous structure will maintain its shape as a brittle glass. The molecular mobility of these materials is a function of the storage temperature [36]. Designing a powder for storage at or below the Kauzmann temperature - that is, the temperature at which the molecular mobility is insignificant - is a method used to maximize physical stability. The Kauzmann temperature is approximately 50 K below the glass transition temperature, Tg, for pharmaceutical powders [37]. The success criteria for the lyophilized formulation was long-term stability at 37 °C. Hence, the Tg of the spray-dried powder must be ≥ 88 °C. As described by the Gordon-Taylor equation [38], Tg for sugar-water mixtures can be determined based on their mass fractions and an empirical parameter k. Chen et al. [39] modelled the trehalose-water glass transition curve as a function of powder moisture content by fitting literature data to the Gordon-Taylor equation. For low water content systems, a k of 5.9 was determined based on a trehalose Tg of 387.1 K [39, 40] and a water Tg of ~138 K [39]. For a spray-dried powder with a Tg greater than or equal to 88 °C, the moisture content of the spray-dried trehalose powder must not exceed 2-3%. The moisture sorption isotherm of trehalose indicates that subjecting trehalose powder to 10% relative humidity (RH) leads to an approximate 2-3% moisture content based on moisture sorption isotherms of amorphous trehalose at 25 to 30 °C [41]. Water sorption of amorphous sugars decrease with increasing temperature for a given RH, however, Hancock and Dalton [42] demonstrated that at low RH (<20 % RH), the water sorption isotherms for amorphous trehalose stored at 30 and 50 °C are very similar.
Therefore, in order to obtain a Tg greater than or equal to 88 °C, the relative humidity at the collection point of the spray dryer must be less than 10%. Additionally, some level of moisture content may be necessary for protein stability as over-drying may lead to increased degradation [43]. Another critical processing parameter is the spray dryer outlet temperature. This must be much lower than the Tg to prevent powder adhesion to the dryer surfaces and crystallization of the resulting powder. Experiments involving twin fluid atomizers to spray dry oil-in-water emulsions reported that a high air-liquid-ratio will decrease the emulsion size distribution [44], where the air-liquid ratio is defined as the ratio of the atomizing gas flow rate to the liquid feed flow rate.
Based on these calculations, the inlet temperature, drying gas flow rate, and air-liquid ratio of the atomizer were calculated iteratively until acceptable outlet conditions were reached. Additionally, a relatively low inlet drying gas temperature of 65 °C was prioritized. The droplet temperature was predicted to be near the wet bulb temperature of a pure water droplet in air at 65 °C, i.e. approximately 28 °C. A low droplet temperature was chosen in order to prevent possible evaporation of the nanoemulsion droplets during the spray drying process and the deactivation of GLA and ID93. The assumption that the droplet temperature is approximately the wet bulb temperature is valid provided that the water evaporation from the droplet surface is not significantly hindered by the forming particle surface. The particle will reach the drying gas temperature once dry. However, this exposure to higher temperatures is not expected to occur until further downstream in the dryer, where the drying gas temperature is cooler than at the inlet.
Particle Formation and Structure
The encapsulation of very small particles or droplets within a protective material via spray drying has been summarized elsewhere [45]. This process, known as microencapsulation or nanoencapsulation via spray drying, has been studied for the main uses of protecting the encapsulated product from the surrounding environment, encapsulating a toxic product, or controlling the rate of release of the encapsulated product. Spray drying has been shown to successfully encapsulate food ingredients [46, 47, 48] as well as lipid-based drug delivery systems [49] as dry emulsions. Briefly, oil-in-water emulsions can be spray-dried to produce a powder containing the encapsulated droplets. The properties of these particles are dependent on the processing parameters, as well as on the properties of both the encapsulated ingredient and the encapsulating agent [45].
The distribution of components within a particle and the particle’s morphology are dependent on the material properties of the formulation and processing conditions of the spray dryer. Appropriate processing conditions, such as drying temperature, is contingent on the properties of the given formulation. A sufficiently high temperature must be used to form a thin shell around the drying droplets early in the drying process in order to entrap the emulsion droplets and obtain a high encapsulation efficiency. However, the temperature must also be low enough to prevent possible evaporation of emulsion droplets or inactivation of temperature-sensitive components [50]. Encapsulation efficiency, defined here as the mass of the dispersed phase retained within the powder after spray drying, has been reported to increase with the concentration of the encapsulating agent [51, 52]. An encapsulated ingredient to encapsulating agent ratio of 1:4 is often used in the spray drying of emulsions in order to obtain a high encapsulation efficiency [53, 54, 55]. These findings can be applied to the encapsulation of vaccines formulated as dispersions via spray drying.
Based on Wang et al.’s [35] findings, a high encapsulation efficiency is achieved when initial shell formation occurs quickly. An early shell formation will prevent the emulsion droplets from reaching the surface of the drying droplet and thus be lost during the evaporative process. Droplet drying time, τD, can be approximated using Eq. 1, where d0 is the initial droplet diameter given in μm and κ is the evaporation rate given in μm2/s [56].
| 1 |
In order to predict the distribution of components, a particle formation model that describes the radial distribution of components within the atomized droplets during the drying process was used, in which a dimensionless Peclet number, Pe, was defined as Eq. 2, where Di refers to the diffusion coefficient of component i and κ stands for the evaporation rate of the solvent [57]. For simple particle formation models the evaporation rate can be approximated as that of a pure solvent droplet when subjected to the dryer inlet temperature and humidity. Therefore, Pe is a parameter affected by both material properties of the components and processing parameters and can be used to predict final particle morphology [57]. The use of more complex models [58, 59] was considered outside the scope of this study.
| 2 |
For the nanoemulsion system studied in this case, the nanoemulsion droplets can be treated as nanoparticles, assuming they remain stable in an aqueous dispersion. The diffusion coefficient of nanoparticles can be approximated through the Stokes-Einstein equation. Based on this equation, larger nanoparticles and nanodroplets will have a low diffusion coefficient, typically much lower than that of dissolved molecules, leading to a much larger Pe.
In general, for a component with low Pe, material migration of the given component inside of an atomized droplet is quick relative to the speed of the receding droplet surface [57]. Hence, solutes with a low Pe distribute evenly in the atomized droplet during evaporation, typically forming spherical, solid particles if they do not crystallize. For components with very large Pe, such as nanoemulsion droplets, the diffusion is slow compared to the evaporation rate, rendering the component effectively immobile, and thus the component will accumulate near the surface as the atomized droplet dries. Using the particle formation model, it is possible to manipulate the distribution of the main components trehalose and the nanoemulsion droplets within the spray-dried powder.
Materials and Methods
Materials
Trehalose dihydrate (CAS 6138-23-4; Fisher Scientific Ottawa, ON, Canada) with a purity of 98% was used as the excipient for spray drying. Tris(hydroxymethyl)aminomethane (Tris) (CAS 77-86-1; Sigma Aldrich, Oakville, ON, Canada) and hydrochloric acid (CAS 7647-01-0; Sigma Aldrich, Oakville, ON, Canada) were used as a buffer system. Formulations were made with HPLC grade water (CAS 7732-18-5; Fisher Scientific Ottawa, ON, Canada).
The ID93 antigen and GLA-SE adjuvant components of the vaccine were produced separately. The ID93 recombinant fusion protein has a molecular weight of 93 kDa [20] and an estimated 95% of the antigen is associated with the adjuvant system [60]. The construction, expression, and purification of ID93 has been described previously [20]. Briefly, ID93 was expressed in E. coli, purified under denaturing conditions by chromatography, and analyzed by SDS-PAGE. ID93 protein in buffered Tris solution at pH 8.0 was stored in aliquots of 1.2 mg/mL at −80 °C prior to use. GLA-SE was formulated with a squalene concentration of 10% v/v, 19 mg/ml dimyristoyl-sn-glycero-3-phosphocholine (DMPC) as an emulsifier, 50 μg/mL of GLA, 0.9 mg/ml Pluronic F68, and 0.5 mg/ml Vitamin E. This nanoemulsion was stored in a refrigerator prior to use. Manufacture of GLA-SE generally followed the same procedure as described in Orr et al. [23], except that the oil phase in the present work included the addition of α-tocopherol (0.05% w/v), and that glycerol and buffer were omitted from the aqueous phase. The initial emulsion droplet size was determined to be 90.6 nm in diameter, with a polydispersity index of 0.06.
Two formulations were prepared for spray drying; an adjuvant system-only formulation and a formulation containing both the antigen and adjuvant system. The former will hereafter be referred to as spray-dried trehalose+GLA-SE (SD-TG) and the latter as spray-dried trehalose+GLA-SE+ID93 (SD-TGI). The adjuvant system-only formulation was investigated for applications where different antigens can be added to the dose after reconstitution of the adjuvant system. The feedstock was prepared by first dissolving trehalose to 200 mg/mL and Tris to 40 mM in HPLC grade water. This solution was then pH adjusted to a pH of 7.5±0.1 using hydrochloric acid. A separate emulsion of GLA-SE or GLA-SE+ID93 was diluted with water to twice the working concentration (4% [v/v] squalene, 20 μg/mL GLA; 8 μg/mL ID93). The trehalose-Tris solution and the nanoemulsion were then mixed in a 1:1 ratio such that the final concentrations, summarized in Table 1, were achieved. A liquid covialed ID93+GLA-SE formulation (2% [v/v] squalene, 10 μg/mL GLA, and 4 μg/mL ID93, buffered in 20mM Tris, pH 8.0) was used in this study for comparison of ID93 retention. Additionally, a lyophilized vaccine candidate of ID93+GLA-SE was established in prior stabilization work and also used in this study for comparison [24]. It utilized an excipient combination of 10% trehalose (w/v) and 20 mM Tris buffered to a pH of 7.5.
Table 1.
Final concentration of the main components in the adjuvant-only SD-TG and antigen containing SD-TGI feedstock formulations
| Component | Feedstock Concentration | |
|---|---|---|
| SD-TG | SD-TGI | |
| Trehalose | 100 mg/mL | 100 mg/mL |
| Tris (buffer) | 2.42 mg/mL (20 mM) | 2.42 mg/mL (20 mM) |
| Squalene | 17.2 mg/mL | 17.2 mg/mL |
| GLA | 10 μg/mL | 10 μg/mL |
| ID93 | - | 4 μg/mL |
Methods
Spray Drying
Spray drying was conducted using a custom research spray dryer [61] with a twin fluid atomizer operated at an air-liquid ratio of 8, which corresponds to an atomized droplet diameter of approximately 9 μm [37]. The outlet humidity target was less than 10% RH to achieve a Tg greater than 88 °C. A spray dryer process mass and energy balance model [62] was used to determine processing conditions to achieve an outlet temperature of 36 °C and outlet RH of 7%. The feedstock was supplied to the atomizer at a rate of 0.6 mL/min using a peristaltic pump (Model 77200-60; Cole-Parmer, Montreal, QC, Canada). The atomized droplets dried in an air flow rate of 200 SLPM, where the drying gas temperature at the inlet was 65 °C. Dry particles were separated from the air by a cyclone, and powder was collected in glass jars. These jars were sealed and stored in an environmental chamber set to 25 °C and 7% RH until powder packaging.
The diffusion coefficient for trehalose in water at 100 mg/ml is 5×10−10 m2/s [57]. For an inlet temperature of 65 °C the evaporation rate is approximately 3.7×10−9 m2/s [56], which corresponds to Pe=0.9 for trehalose, based on Eq. (2). Solutes with a low Pe are expected to distribute evenly in the droplet, which is desired in order to increase trehalose coverage of the nanoemulsion droplets to facilitate stabilization during the drying process. The Pe for trehalose was approximately 1, suggesting that the chosen inlet temperature of 65 °C is appropriate. The GLA-SE droplet size has been measured as approximately 92 nm [24]. The diffusion coefficient of GLA-SE nanoemulsion droplets with a diameter of 92 nm has been measured via dynamic light scattering as 5.3×10−12 m2/s (data not shown). For a theoretical evaporation rate of 4×10−9 m2/s, the Pe of the nanoemulsion droplets is ~ 95. The nanoemulsion droplets are expected to accumulate near the surface of the atomized droplet. At a very high Pe, the nanoemulsion droplets are expected to accumulate and possibly coalesce near the surface due to the force from the receding particle surface and convective flux of water towards the surface. Based on similar studies, trehalose is expected to form solid, spherical amorphous particles when spray-dried [57, 63].
Stability Study
The design targets for the spray-dried formulation required that the vaccine concentration and integrity be retained post-spray drying and that the powder maintains stability for at least three months at 40 °C or 24 months at 25 °C. Stability of the spray-dried powder was assessed using the following preliminary acceptance criteria: distinct particles with no sign of fusing, maintenance of amorphous solid state, moisture content change less than 1%, emulsion droplet size change less than or equal to 50%, polydispersity index less than 0.2, squalene content loss less than or equal to 20%, GLA content loss less than or equal to 20%, and ID93 is present. Similar criteria were used previously for the assessment of the lyophilized candidates [24].
The powders were stored at −20 °C, 2-8 °C, 25 °C, and 40 °C. The stability arm was chosen for 40 °C rather than 37 °C as recommended by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for pharmaceutical stability studies [64]. Similarly, time points were chosen based on the ICH guidelines. Characterization tests for chemical stability of the reconstituted powder were conducted at the beginning of the study, and then at multiple time points after storage at two weeks, one month, two months, three months, six months, nine months, 13.5 months, 19 months, and 26 months. Similarly, characterization tests for physical stability of the dry powder were conducted at the beginning of the study, and after one month, three months, twelve months and 26 months of storage.
Dry Powder Packaging for Stability Study
Spray-dried powder will equilibrate to the new water activity when placed in an environment with a different RH than its initial value [65]. Desiccants, such as silica gel, are hygroscopic agents that can be used to modify or buffer the RH within a closed package. Chemical degradation due to a change in RH has been shown to be mitigated by the inclusion of desiccant within the package [65]. Desiccants can be equilibrated to the desired moisture content when placed in an environmental chamber at the desired RH value [2]. The time required to equilibrate is influenced by several factors, such as the difference in RH value and temperature and the permeability of the packaging seals.
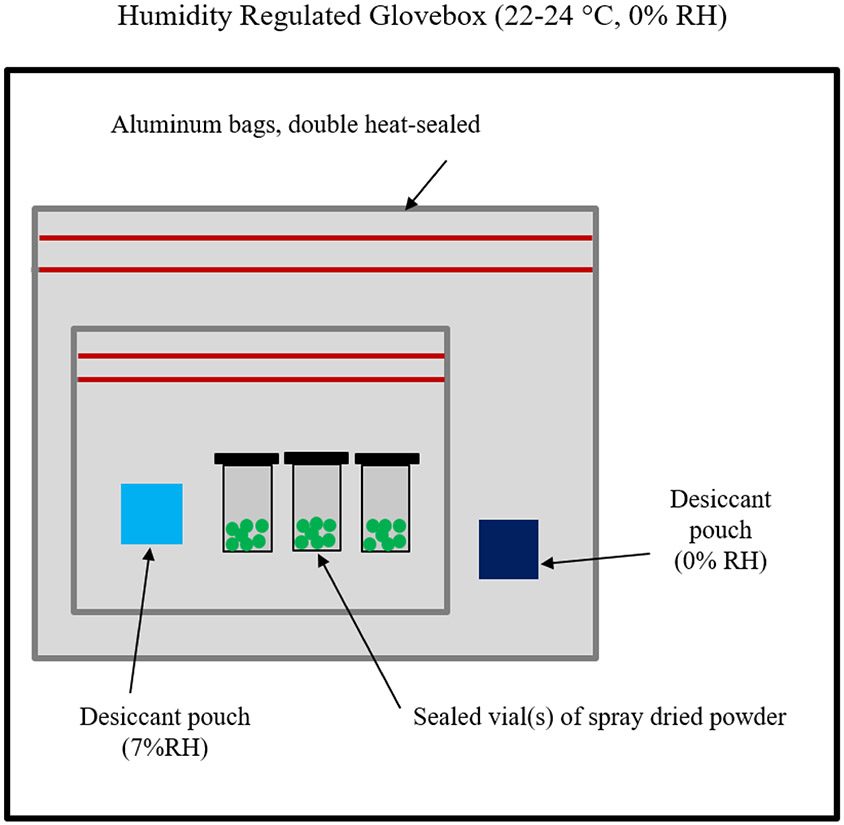
A packaging process involving the use of desiccants was utilized to prevent moisture uptake in the powder during temperature-controlled storage of the stability study. Packaging preparation involved placing silica gel pouches into an environmental chamber set to 25 °C and 7% RH for 3-4 days in order to equilibrate the desiccant to the outlet RH of the spray dryer. An equal number of silica gel pouches were equilibrated to 0% RH in a regulated glove box over the same time period. The packaging process took place within a custom glovebox set to 0% RH. The powder was aliquoted into low bind snap cap tubes (Product Z768820; Sigma Aldrich, Oakville, ON, Canada). The powder-containing tubes were then placed into an aluminum bag, along with a 7% RH desiccant pouch to prevent powder moisture changes. However, moisture transfer rates through the seals increase with a larger difference between the environments. To counteract this, the aluminum bag was then double heat-sealed and placed into another aluminum bag, along with a 0% RH desiccant pouch to minimize the moisture difference between the innermost package and the external environment. This external bag was also double heat-sealed and labelled. A simplified schematic summarizing the configuration is shown in Figure 1.
Figure 1.
Simplified schematic for packaging protocol to ensure powder moisture content control during long-term powder storage
Dry Powder Characterization
Field Emission Scanning Electron Microscopy (Zeiss Sigma FE-SEM; Carl Zeiss, Oberkochen, Germany) was used to determine whether powder morphology changed over time. Powder samples were dispersed directly onto aluminum SEM stubs (Product 16111; Ted Pella, Inc.; Redding, CA, USA) and pressed against the surface of the stub to intentionally produce cracked particles for imaging. To prevent damage to the electron microscope, these samples were placed in a desiccator connected to an in-house vacuum system for 2-4 days to remove the exposed nanoemulsion droplets. Subsequently, the samples were sputtered with a coating of 80% gold and 20% palladium (Leica ACE600 Carbon/Metal Coater; Concord, ON, Canada) to a thickness of 10-15 nm. Images ranging from magnifications of 500 to 20000× were taken at a working distance of 5.3-6.3 mm using an accelerating voltage of 3-4 kV.
The solid phase of the powder was monitored by Raman spectroscopy to assess whether or not the spray-dried powder remained amorphous during storage. A custom dispersive Raman spectroscopy system was utilized for this purpose. The system included a 671 nm diode-pumped solid-state laser (Ventus Solo MPC6000; Laser Quantum, Stockport, UK). A detailed description of a similar apparatus has been published elsewhere. [66]. Samples were placed in a closed sample chamber under nitrogen to prevent moisture exposure. All spectra were measured at a temperature of 22.0-23.0 °C and at less than 5% RH. In addition to measured SD-TG and SD-TGI powder samples, Raman spectra analysis was also conducted on neat amorphous and crystalline trehalose powder samples as references. Similarly, reference spectra were also obtained for liquid samples of squalene and crystalline Tris powder samples. A deconvolution process was used to assess the contributions and solid phase of each component. The deconvolution process is described elsewhere [67].
Karl Fisher Calorimetry (Karl Fisher Coulometric Titrator Model C30; Mettler Toledo; Mississauga, ON, Canada) was used to measure the water content of powder samples by mass. Results were displayed as a percentage based on the measured sample mass. Oven temperature for the method was 110 °C. Moisture content of the powder was determined by measuring and averaging results of two vials of the same powder. Analysis of experiments to determine moisture content of a HYDRANAL water standard (Honeywell; Mexico City, Mexico) showed that the machine variance was 0.3%.
Reconstituted Powder Characterization
At each stability time point, approximately 108 mg of spray-dried powder was reconstituted with the appropriate volume of freshly dispensed MilliQ water to yield sample concentrations expected in the liquid drug product. The exact reconstitution volume was based off the exact mass of powder in each vial and an empirically determined expansion factor. Each test was performed in triplicate on separate vials of reconstituted powder for the first three months. Remaining timepoints were performed on single vials. Replicates of measurements of the feedstock were made from the same vial. The stability study involved storage of the spray-dried powders at several storage temperatures: −20, 2-8, 25 and 40 °C. For each temperature, at a given time point the powders were reconstituted and assessed for nanoemulsion droplet diameter, polydispersity index, squalene concentration, GLA concentration, ID93 presence, and pH. Unlike other chemical characterization tests, osmolality was only measured initially, after three months, 13.5 months, 19 months, and 26 months for all storage temperatures.
A dynamic light scattering technique (Zetasizer APS; Malvern, UK) was used to measure the mean hydrodynamic diameter and polydispersity of the nanoemulsion droplets in the liquid formulations reconstituted from spray-dried powders. Details of the measurement process have been described elsewhere [68]. Liquid feedstock measurements were from one sample analyzed three times.
GLA content in the powder was quantified using a reversed-phase HPLC method. Separation of GLA from the sample was performed on an Agilent 1200 series HPLC (Agilent Technologies; Santa Clara, CA, USA) equipped with a silica-based, C18 reversed-phase column (Atlantis T3 Column; Waters; Elstree, UK) with a gradient from 50% mobile phase B to 90% mobile phase B over 18 minutes and a flowrate of 1.0 mL/min. Mobile phase A contained 75:15:10 (v/v/v) methanol:chloroform:water, 1% (v/v) acetic acid, and 20 mM ammonium acetate. Mobile phase B contained 50:50 (v/v) methanol:chloroform, 1% (v/v) acetic acid, and 20 mM ammonium acetate. Samples were diluted 10-fold in mobile phase B, and the injection volume was 100 μL. Column temperature was held constant at 30 °C. A charged aerosol detector (Corona CAD; ESA Biosciences; Chelmsford, MA, USA) was used for analyte detection. A standard curve was prepared from GLA in mobile phase B. The peak heights from the standards were fit with a second order polynomial per the charged aerosol detector manufacturer’s directions. Sample analyte concentrations were calculated by interpolation. Liquid feedstock measurements were done in two replicates, each analyzed once.
Squalene content in the powder was quantified using a reversed-phase HPLC method. Separation of squalene from the sample was done with the same equipment and the same mobile phases used for GLA as described above. Samples were diluted 100-fold in mobile phase B, and 10 μL of volume was injected with a gradient from 50% mobile phase B to 90% mobile phase B over 30 minutes and a flowrate of 1.0 mL/min. Column temperature was held constant at 30 °C. The peak area from the standards were fit with a second order polynomial per the charged aerosol detector manufacturer’s directions. Sample analyte concentrations were calculated by interpolation. Liquid feedstock measurements were completed in two replicates, each analyzed once.
SDS-PAGE was used to detect the presence of the ID93 protein in the samples. Samples were reduced in LDS buffer (NP0007; Thermo Fisher Scientific, Waltham, MA, USA) spiked with β-Mercaptoethanol to a final concentration of 1.25% (v/v). Samples were heated at 90 °C for 15 minutes and then cooled to room temperature. Once cooled and centrifuged at 2000 rpm for 30 seconds, samples and molecular weight marker (LC5925; Thermo Fisher Scientific, Waltham, MA, USA) were run in 4-20% Tris-Glycine gels (XP04205BOX; Thermo Fisher Scientific, Waltham, MA, USA). Gels were silver stained (PROTSIL1-1KT; Sigma Aldrich, St. Louis, MO, USA) and imaged (AlphaImager EC; Protein Simple, San Jose, CA, USA). At each time point, two SDS-PAGE samples were prepared and analyzed per vial to confirm the presence of the ID93 protein.
Reconstituted powder pH was measured using a pH meter (Orion ROSS Ultra Semi-micro pH Electrode, Thermo Fisher Scientific, Waltham, MA, USA). Liquid feedstock measurements consisted of one sample. Measurements of reconstituted samples were performed in triplicate for the first three months; measurements were performed once for the remaining timepoints.
Osmolality of the reconstituted powder was measured using an osmometer (Model 2020; Advanced instruments, Norwood, MA, USA) to determine if the reconstituted powder was isotonic and suitable for injection. Liquid feedstock measurements consisted of one sample. Measurements of reconstituted samples were performed in triplicate for the first three months; measurements were performed once for the remaining timepoints.
Statistical Analysis
Mean results are reported, and the indicated error is the standard deviation of replicate measurements. Number of replicates for each method are indicated above. A two-tailed student’s t-test was used for analysis, where statistically insignificant differences were reported for p>0.05.
Results
Powder Manufacturing
Process calculations predicted the nominal solids throughput in the spray dryer to be 75 mg/min. During powder manufacturing for the stability study, the actual rate to produce SD-TG and SD-TGI powders was 49 mg/min and 45 mg/min, corresponding to spray drying yields of 65% and 60%, respectively. Considering the relatively small spray drying batch size of 16.4 g and 17.3 g, produced over 5.5 hours and 6.5 hours, respectively, the yield was typical and well within the acceptable range for early development.
The measured properties of the formulations before and after spray drying (post-reconstitution) are shown in Table 2. The target values given are the same as for the lyophilized vaccine product. All measured vaccine properties were well within the target values. Average nanoemulsion droplet size was nearly the same as the liquid vaccine product. Hydrodynamic diameter increased only slightly by 2-3% after spray drying. The polydispersity index of the nanoemulsion droplets did not change significantly (p>0.05) over the course of spray drying for either formulation.
Table 2.
Chemical and colloidal properties before and after spray drying for adjuvant-only (SD-TG) and antigen containing (SD-TGI) formulations.
| ID | Nanoemulsion Droplet Diameter (nm) |
Polydispersity Index |
Squalene Content (mg/mL) |
GLA Content (μg/mL) |
ID93 | pH | Osmolality (mOsmol/kg) |
|
|---|---|---|---|---|---|---|---|---|
| Target | 120 ± 40 | <0.2 | 13.6 – 20.5 | 10 ± 2.5 | Present | 7.5 – 8.5 | 315 – 415 | |
| SD-TG | Feedstock Liquid | 95.3 ± 0.9 | 0.05 ± 0.04 | 19.1 ± 0.4 | 9.2 ± 0.5 | N/A | 7.62 | 383 |
| Reconstituted Powder | 96.9 ± 0.2 | 0.07 ± 0.02 | 18.8 ± 0.3 | 9.5 ± 0.4 | N/A | 7.57 ± 0.04 | 376 ± 8 | |
| SD-TGI | Feedstock Liquid | 94.9 ± 0.8 | 0.06 ± 0.01 | 18.8 ± 0.3 | 9.3 ± 0.4 | Present | 7.56 | 389 |
| Reconstituted Powder | 97.6 ± 1.1 | 0.09 ± 0.01 | 17.3 ± 0.6 | 9.0 ± 0.4 | Present | 7.55 ± 0.01 | 351 ± 8 | |
Squalene content levels before and after spray drying are very similar, indicating a high encapsulation efficiency. The results show high retention (>90%) of the squalene component for both formulations. The high retention indicates that the evaporation losses during low temperature spray drying for this system are less than 10%. The GLA content did not change significantly (p>0.05) over the course of spray drying for either formulation, indicating that the GLA component, a synthetic lipid, was successfully stabilized by the trehalose. The retention of GLA content indicates that this component does not degrade over the course of spray drying, which was a concern, as GLA is sensitive to thermal stress [24]. Similarly, ID93 protein was shown to be present in both the liquid and reconstituted formulations. Likewise, the pH did not change significantly (p>0.05) over the course of spray drying for either formulation.
Physicochemical Stability
Particle Morphology
Representative SEM images for the spray-dried SD-TG and SD-TGI formulations are shown in Figure 2. Analysis of the SEM images indicates that spray drying the formulations produced a polydisperse powder sample with microparticles ranging in geometric diameter from ~1-20 μm. These images show that the spray-dried microparticles were spherical with surfaces ranging from smooth to slightly dimpled. The spray-dried powders containing the emulsion droplets were relatively flowable and easy to handle. The similarity of the microparticles from the SD-TG and SD-TGI formulations indicates that the presence of the protein ID93 does not affect the particle morphology. Microparticles of neat trehalose spray-dried from a 100 mg/mL aqueous solution under the same processing conditions are also shown in Figure 2 for comparison. The trehalose particles have an outer particle morphology very similar to those of the SD-TG and SD-TGI formulation, that is, spherical particles with smooth or slightly dimpled surfaces and a similar geometric diameter. Spray-dried bacteriophage encapsulated in trehalose [13, 69] and outer membrane vesicle pertussis vaccine spray-dried with trehalose [10] produced particles with a similar exterior morphology. These particles, composed mainly of trehalose, were also spherical, had a similar geometric diameter range and exhibited a smooth or dimpled surface.
Figure 2.
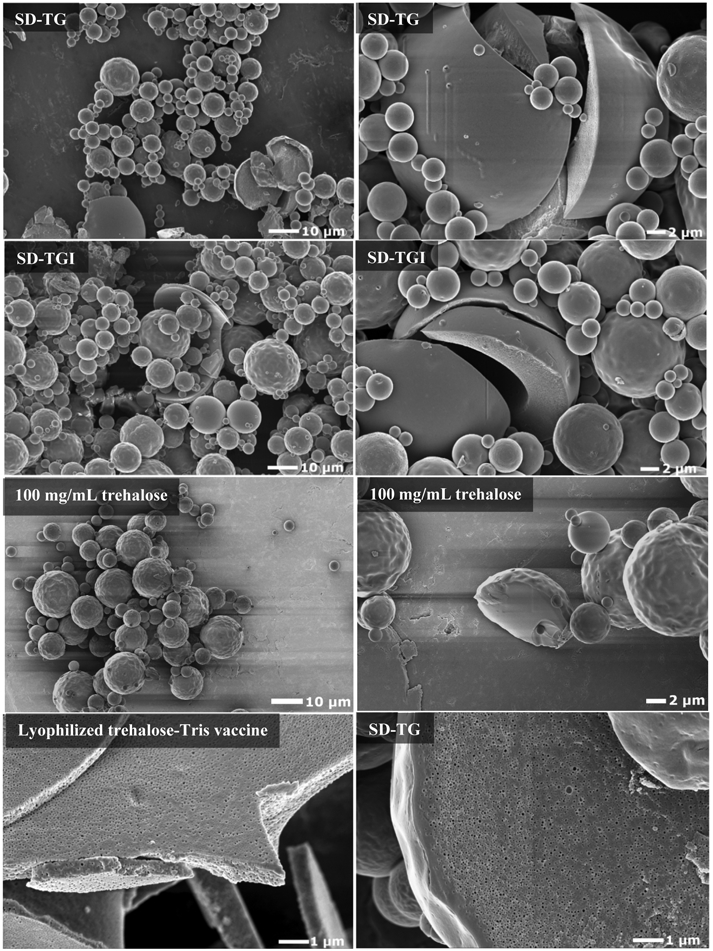
Comparison of morphology for the adjuvant-only SD-TG formulation, antigen-containing SD-TGI formulation, spray-dried trehalose and the lyophlized vaccine. External morphology of all spray-dried powders shows similar spherical, polydisperse particles that are not fused. Interior structure of the lyophilized vaccine and the spray-dried particles shows similar voids left by the encapsulated emulsion droplets. Scales are provided on the respective images.
SEM images of the cracked microparticles of SD-TG and SD-TGI all show that these particles can be hollow or solid, with a range of shell thicknesses within a given sample. The images show that the interior structure of this shell differs from shells formed with spray-dried trehalose. Trehalose particles show a solid interior whereas the interior of the SD-TG and SD-TGI particles appears to be foam-like with numerous voids within the trehalose matrix. These voids observed in the SEM images of SD-TG and SD-TGI also appear in images of the lyophilized trehalose-Tris formulation (Figure 2). The geometric diameter of these voids appears to be approximately the same as that of the nanoemulsion droplet hydrodynamic diameter. It can be concluded that these voids are left behind by nanoemulsion droplets that evaporated during the imaging preparation process. Higher magnification images of the surface (not shown) show few of these voids on the surface of the particle.
Similar interior morphologies have been shown in SEM images particles generated by emulsion encapsulation via spray drying of isoeugenol in a glucose syrup matrix [70], spray drying of D-Limonene emulsion with different combinations of the excipients gum arabic, maltodextrin, and trehalose [71], and spray drying of fish oil in a whey protein isolate matrix [72]. The particles in the latter paper are especially like the particles shown in this study. Both studies generated intact thin-shelled particles with the emulsion droplets embedded within the particle wall. A central void was also found in the particles generated in a study on surface formation of drying emulsions [35].
There appears to be a slight gradient in nanoemulsion droplet distribution within the particle, with a higher concentration of droplets closer to the surface of the particle than to the center, as demonstrated by the cracked SD-TG microparticle in Figure 2. As per the theoretical particle formation theory calculations previously discussed, the Pe number of the nanoemulsion droplets is >>1 and the Pe of trehalose is ≈1. Theory suggests that the nanoemulsion droplets accumulated near the surface of the drying droplet because the nanoemulsion droplets were unable to diffuse to the centre faster than the particle was forming. The lack of visual nanoemulsion droplet aggregation and relatively even distribution in the SD-TG and SD-TGI particles suggests that an appropriate drying temperature was chosen.
Similar SEM images of the powder samples were taken over the course of the stability study. Images of the SD-TGI formulation after three months’ and 26 months’ storage at 40 °C are shown in Figure 3. A lower magnification view, shown in Figure 3a, demonstrates that the sample stored at 40 °C has maintained overall exterior particle structure even after 26 months of storage. High magnification images of particle exteriors (not shown) indicated that smaller particles may experience some bridging. As shown in Figure 3b, the interior structure of the particle is also maintained after storage at 40 °C, as the voids left by the nanoemulsion droplets are clearly distinct. Cavities within the central void are too large to be nanoemulsion droplets; they may be caused by ruptured nanoemulsion droplets, which led to the pockmarked inner surface. The pockmarked inner surface was not consistently exhibited in cracked particles. The morphology for the samples stored at lower temperatures also displayed a maintenance of interior and exterior particle structure over 26 months of storage, however, no particle bridging was apparent in the samples stored at lower temperatures.
Figure 3.
SEM images of antigen containing SD-TGI powder after 3 months and 26 months of storage at 40 °C indicating A) sample maintains overall external morphology after accelerated storage, and B) interior particle structure is maintained. Scales are provided on the respective images.
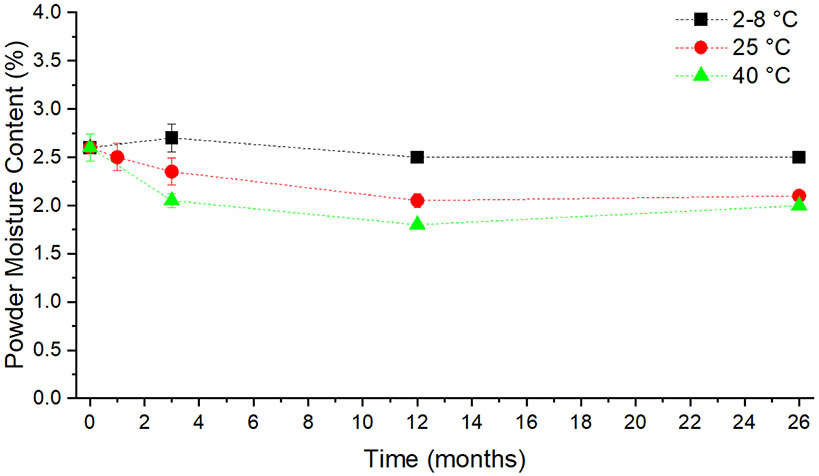
Powder Moisture Content
Maintenance of moisture content is critical to avoid lowering of the Tg, which would result in powder crystallization. Crystallization of amorphous spray-dried powder will inactivate the biological components [2]. The moisture content of the powder by wet basis after manufacture was measured to be 2.6 ± 0.1% in this study. Moisture content of the SD-TGI powder after 26 months of storage, as given in Figure 4, did not change significantly (p>0.05) for all storage temperatures.
Figure 4.
Graph indicating the powder moisture content of the antigen containing SD-TGI powder sample after 26 months of storage at 2-8, 25, and 40 °C. The minimal change at each temperature arm of the stability study indicates that the moisture content of the powder is preserved. Results shown represent mean values and error bars indicate the standard deviation of the measurements (n=2).
Solid State Analysis
Raman spectroscopy analysis was completed on SD-TG and SD-TGI powders at various time points during the stability study in order to confirm the presence of initially formulated components within the sample and to determine any changes in solid phase. Sample spectra for SD-TG powder obtained at timepoint 0, as well as reference spectra for amorphous trehalose, squalene, crystalline Tris, and crystalline trehalose, are shown in Figure 5. The ID93 protein and GLA contributions were not considered due to their low mass fraction.
Figure 5.
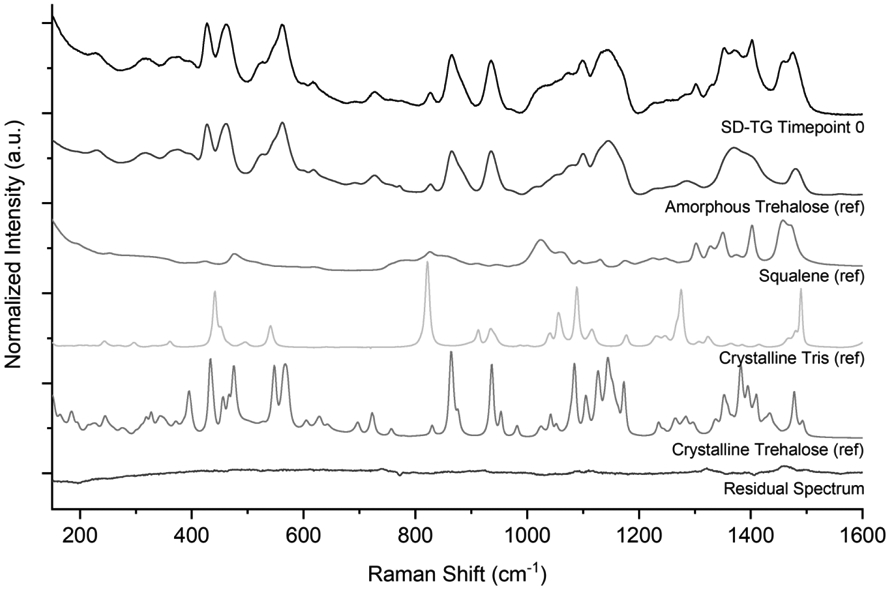
Normalized Raman spectrum of the adjuvant-only SD-TG powder sample, and reference spectra of amorphous trehalose, squalene, Tris, and crystalline trehalose. Also shown is the normalized residual spectrum, obtained by subtracting individual component contributions from the raw measured SD-TG spectrum.
Inspection of the sample spectra showed the trehalose component to be completely amorphous. This is evidenced by the presence of amorphous trehalose characteristic peaks at 425, 460, 560, 865, 935, and 1145 cm−1 in the sample and further supported by the lack of crystalline trehalose characteristic peaks at approximately 395, 455, 695, 980, and 1235 cm−1, to cite a few examples. The squalene in the sample was identified by its characteristic peak appearing at 1400 cm−1. The solid phase of trehalose is further confirmed by the low normalized intensity of the residual spectrum, shown in Figure 5. The residual spectrum was obtained by subtracting the reference spectra for amorphous trehalose (mass fraction 81%), squalene (mass fraction 14%) and Tris (mass fraction 2%) from the sample spectrum. Similar analysis was completed on the SD-TGI powder (not shown), also confirming the amorphous structure of the trehalose component and the presence of squalene and Tris buffer in the sample. It is expected that SD-TG and SD-TGI have similar results, as the only difference in formulation between the samples is the presence of ID93 in the latter.
Sample spectra and the respective residual spectrum after deconvolution for SD-TGI at time point 0 and after 26 months of storage at 2-8, 25, and 40 °C are shown in Figure 6. The spectra for all storage temperatures were very similar, indicating that the solid phase of the powder had not measurably changed over the course of the stability study. Indeed, deconvolution of the SD-TG and SD-TGI spectra collected at different points of the stability study confirmed that all samples showed presence of squalene and that trehalose remained amorphous, as indicated by the relatively low intensity residual spectra. This ties into the moisture content data, in that if moisture content did not rise, it is unlikely that the trehalose crystallized as the Tg was theoretically >88 °C.
Figure 6.
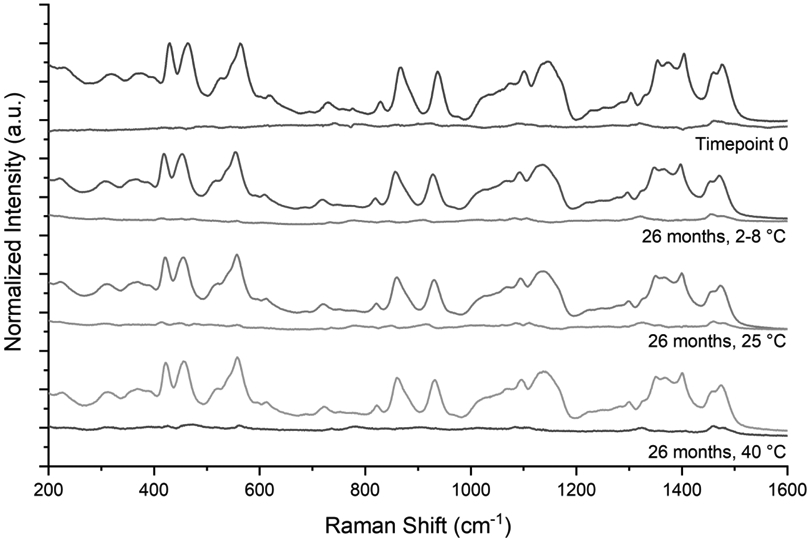
Normalized Raman spectra of the antigen containing SD-TGI powder formulation, shown after 26 months of storage at 2-8, 25, and 40 °C, and at time point 0. The residual spectrum after deconvolution is given below the respective sample spectrum. The relatively low residual spectrum for each sample indicates that the SD-TGI powder stored for 26 months does not undergo any solid phase changes for all storage temperatures.
Emulsion Droplet Size and Distribution
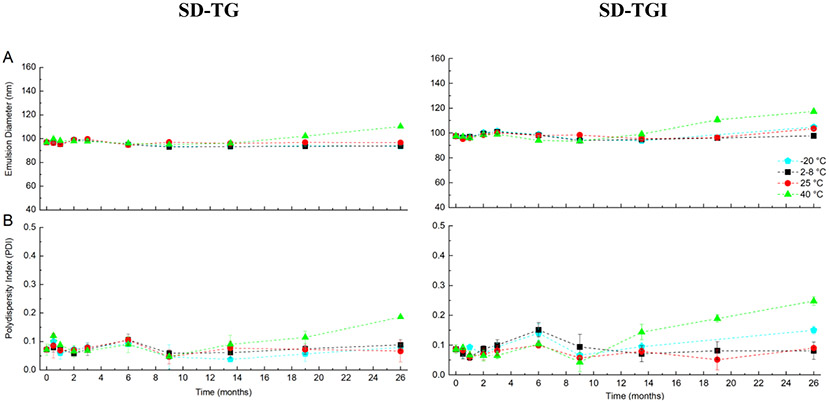
Properties of the emulsion droplet size of the GLA-SE vehicle over 26 months of the stability study are shown in Figure 7. The changes are all within the pre-determined target stability criteria mentioned above; that is, <50% change in droplet size; and polydispersity index <0.2 after at least three months storage’ at 40 °C and 24 months at 25 °C. Both the initial change in emulsion diameter and the subsequent size change over storage are important in evaluating a spray-dried emulsion. After three months’ storage, droplet size increased only 1-3% for the SD-TG formulation. Similarly, droplet size increased only 2-4% for the SD-TGI formulation. The polydispersity index did not change significantly over the course of three months at any given storage temperature (p>0.05). The nearly constant droplet diameter and polydispersity index indicates that the nanoemulsion droplets did not coalesce or shrink during the three-month storage or on reconstitution, even at the highest temperature of 40°C, proving that the nanoemulsion droplets were well protected by the trehalose stabilizer as designed.
Figure 7.
Plots showing A) nanoemulsion droplet diameter size and B) polydispersity index of droplets for the reconstituted adjuvant-only SD-TG powder (left) and reconstituted antigen containing SD-TGI powder (right) stored over 26 months at the indicated temperatures. Legend indicates the storage temperatures for the powder. Parameters are within stability target of ≤50% change in emulsion size and ≤0.2 polydispersity index for at least 3 months at 40 °C and 24 months at 25 °C. Results shown represent mean values and error bars indicate the standard deviation of the measurements (n=3).
Nanoemulsion droplet diameter for all samples stored at −20, 2-8, and 25 °C after 26 months was within −4 to 7% of the initial measurement. The droplet size of the SD-TG and SD-TGI samples stored at 40 °C for 26 months experienced a slightly greater increase of 14% and 20%, respectively. These results show that the powder is well within the <50% change in diameter stability criteria even after long term storage at high temperatures. Similarly, the polydispersity index remains below 0.2 after 26 months of storage for all samples except the SD-TGI sample stored at 40 °C. Low measured polydispersity index indicates that the nanoemulsion droplet diameter distribution remains relatively monodisperse over the course of the stability study.
Squalene Content
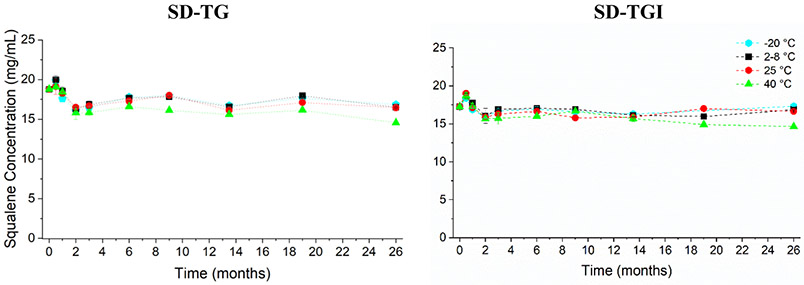
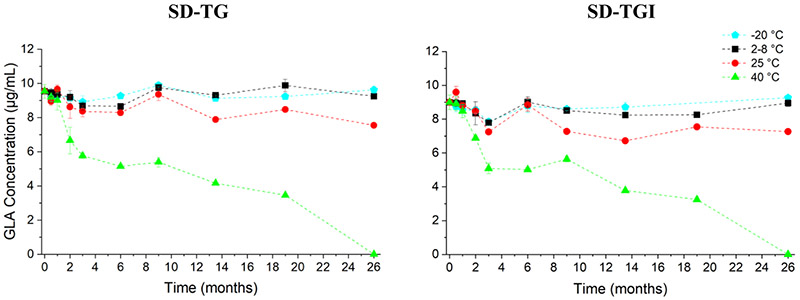
Squalene retention of the SD-TG and SD-TGI powders over 26 months of the stability study are shown in Figure 8. The changes are all within the pre-determined target <20% loss for squalene content for at least three months at 40 °C or 24 months at 25 °C, as defined previously. Squalene content was preserved after three months at 40 °C, with 84% retention for the SD-TG formulation and 91% retention for the SD-TGI formulation, when compared to the initial measurement. After 26 months of storage, the squalene content for the SD-TG formulation was 88% to 90% retained when stored at −20, 2-8, and 25 °C. Similarly, the squalene content for the SD-TGI formulation was 96 to 100% retained after 26 months of storage at −20, 2-8, and 25 °C. Squalene retention for the SD-TG and SD-TGI formulations after 26 months of storage at 40 °C was 78% and 85%, respectively. Reported increases in squalene content may be due to assay variability.
Figure 8.
Plots showing squalene content of the reconstituted adjuvant-only SD-TG powder (left) and reconstituted antigen containing SD-TGI powder (right) stored over 26 months at the indicated temperatures. Legend indicates the storage temperatures for the powder. Parameters are within stability target of ≤20% squalene loss for at least 3 months at 40 °C and 24 months at 25 °C., indicating that the squalene component of the vaccine is stabilized within the dry powder. Results shown represent mean values and error bars indicate the standard deviation of the measurements (n=3).
GLA Content
The pre-determined target stability criteria for the agonist GLA was <20% loss of GLA after three months storage at 40 °C. GLA retention of the SD-TG and SD-TGI powders over 26 months of the stability study are shown in Figure 9. GLA retention is high for storage at −20 and 2-8 °C after three months for both powders (87-93% retained). A decrease of GLA concentration of 40% for the SD-TG formulation and 43% for SD-TGI formulation stored at 40 °C for three months was observed.
Figure 9.
Plots showing GLA content of the reconstituted adjuvant-only SD-TG powder (left) and reconstituted antigen containing SD-TGI powder (right) stored over 26 months at the indicated temperatures. Legend indicates the storage temperatures for the powder. Results shown represent mean values and error bars indicate the standard deviation of the measurements (n=3).
GLA content did not decrease significantly (p>0.05) for the SD-TG and SD-TGI formulations after 26 months of storage at −20 and 2-8 °C. Retention of GLA for the SD-TG and SD-TGI formulations stored at 25 °C was 79% and 81%, respectively. No GLA was detected in either formulation after storage at 40 °C for 26 months.
ID93 Content
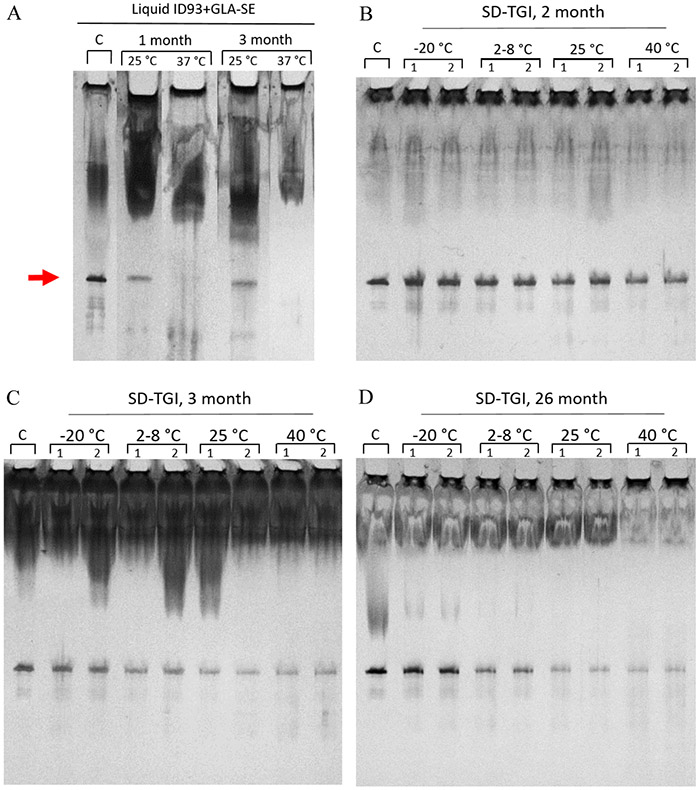
SDS-PAGE results of the samples over the course of the stability study were compared to a two-vial ID93+GLA-SE control. The SDS-PAGE results of a liquid ID93+GLA-SE formulation after one and three months of storage at 25 and 37 °C are shown for comparison in Figure 10a. Oil smear shown at the top of the gels was due to GLA-SE [73]. The ID93 band of the liquid formulation after one and three months of storage at 37 °C is no longer present and shown with reduced intensity at 25 °C storage as compared to the control. The presence of the ID93 band in the SD-TGI samples, as shown in Figure 10b, confirms that ID93 is present in the spray-dried product at all storage temperatures at two months of storage. Additionally, all samples measured at two months of storage show similar band intensity to the control. Similarly, all SD-TGI samples showed similar band intensity to the control at timepoint 0, after two weeks and one month of storage (data not shown). The reduced intensity of the band in the 25 and 40 °C samples in Figure 10c as compared to the control may suggest that there is some loss in ID93 protein after three months’ storage. However, the ID93 band is still visible in all samples stored for 26 months, as shown in Figure 10d.
Figure 10.
SDS-PAGE results of A) the ID93+GLA-SE liquid formulation after one and three months of storage at 25 and 37 °C, and the SD-TGI powder after storage at −20, 2-8, 25, and 40 °C for B) two months, C) three months, and D) 26 months. A lane of two-vial ID93+GLA-SE control sample (“C”) is shown for comparison at each timepoint. The red arrow points to the ID93 protein band.
pH and Osmolality
A previous study found that increased aggregation of ID93 occurs at and below neutral pH [24]. Formulating the vaccine at pH 7.5 or 8 minimizes aggregation and maximizes the temperature at which unfolding occurs [24]. Overall, pH of the reconstituted liquid trended slightly downward over time for all temperatures. After three months of storage, pH change was between 0.07 and 0.16 pH units for all samples. After storage for 26 months at −20, 2-8, and 25 °C, the greatest pH change from timepoint 0 was 0.09 pH units. A greater pH decrease (0.4 pH units) was evident in reconstituted SD-TG and SD-TGI powders after storage at 40 °C for 26 months.
Osmolality was monitored through the stability study to ensure that the reconstituted formulation is isotonic and suitable for injection. Change in osmolality was insignificant for all SD-TG samples after 26 months of storage. Change in osmolality was insignificant for SD-TGI samples stored at −20, 2-8, and 25 °C for 26 months and increased 6% for the sample stored at 40 °C. The osmolality of all samples for both formulations remained within the 300±100 mOsmol/kg target.
Discussion
This study reports the investigation of a thermostable presentation of ID93+GLA-SE, an adjuvanted subunit TB vaccine candidate, via spray drying. The vaccine candidate is formulated as a nanoemulsion, where the emulsion is stabilized by a phospholipid emulsifier that forms a monolayer at the oil-water interface. Spray drying a vaccine into a dry powder form can greatly improve long term stability over its liquid counterpart; however, loss of efficacy can occur due to suboptimal formulation and processing parameters, causing loss or structural change of the vaccine components during spray drying [74].
Our results showed that utilizing trehalose as a stabilizer during spray drying of the ID93+GLA-SE vaccine candidate successfully preserved integrity of the adjuvant, with evidence of antigen retention. Crucially, the nanoemulsion droplets maintained their size on reconstitution after spray drying. The lack of membrane fusing suggests that the chosen processing parameters effectively facilitated the stabilization at the phospholipid monolayer membrane of the GLA-SE nanoemulsion droplets within the trehalose matrix. The overall preservation of nanoemulsion droplet diameter is critical as it is has been shown that smaller nanoemulsion droplets are more stable over time than large ones [75]. Additionally, the ID93+GLA-SE vaccine candidate targets uptake by dendritic cells to induce immunity and particle size is known to influence dendritic cell uptake.
The maintenance of droplet size also shows that the shearing forces on the formulation due to the twin fluid atomizer were not sufficient to cause droplet size change. Conversely, spray drying of H56/CAF01, which was formulated as a liposomal dispersion, resulted in size change of the CAF01 adjuvant [12]. H56/CAF01 was also spray-dried with trehalose in the study, further indicating that stabilization must also consider the processing parameters rather than just the stabilizing excipient. Similarly, the method of manufacture plays a role in preserving phospholipid membrane integrity. Spray drying the vaccine showed little change in GLA-SE droplet diameter; however, after lyophilization, GLA-SE diameter increased 31% and 18% for trehalose-containing and sucrose-containing lead candidates, respectively [24]. The discrepancy in diameter changes for the formulations with similar excipients but differing manufacturing methods indicates that there are additional stresses in the lyophilization process that may cause the nanoemulsion droplets to coalesce.
In addition to nanoemulsion droplet size preservation, results show that a high encapsulation of squalene within the spray-dried particles was achieved, further suggesting that trehalose was an appropriate choice of wall material for this vaccine candidate. High retention of GLA further signifies that trehalose provided suitable protection during desiccation. Preservation of these components during spray drying was a concern as both the emulsion size and content needed to be stabilized during the desiccation process. The chosen drying temperature was clearly not too high as the integrity of these components was preserved. Protection of these components against desiccation stress is likely due to glass stabilization by trehalose. Similar stabilizing performance was shown in the lyophilization study, where GLA concentration did not significantly decrease below the target after lyophilization for any of the samples [24]. High encapsulation efficiencies via spray drying have been previously achieved for nanoemulsions at the 100 nm scale. Nanoemulsions containing the drug tioconazole were formulated with a mean size of 182 nm and spray-dried using lactose as an excipient [76]. The authors reported that nanoemulsion size was preserved and that there was no loss of drug over the spray drying process.
Further indication that appropriate formulation and processing conditions were utilized in spray drying the vaccine candidate was the lack of emulsion droplets accumulated on the outer surface of the particles. Particles with a high surface oil content have been reported to have small protrusions on the outer surface [77] or exhibit a pockmarked surface [35, 77]. The authors of these studies suggested that the protrusions are caused by the covered oil droplets and the pockmarks are from the surface oil droplets rupturing, leaving behind small craters in the outer particle wall. As neither of these surface morphologies is seen in the SEM images of either the SD-TG or the SD-TGI particles, it appears that few nanoemulsion droplets accumulated directly on the outer surface.
Trehalose has been previously successfully utilized as a stabilizer to confer thermostability to spray-dried lipid-based vaccines. Kanoija et al. [5] spray-dried several formulations of an influenza vaccine with trehalose as an excipient and held the powder at 60 °C for three months. For all tested formulations, greater than 86% of the antigen was retained after the stability study. Conversely, the liquid influenza vaccine when stored under the same conditions lost all antigenicity after only 5 days. However, an appropriate stabilizer is dependent on the structure of the vaccine. Toniolo et al. [6] spray-dried enveloped and non-enveloped viral vaccines using combinations of trehalose, mannitol, dextran, and lactose as stabilizers and stored the powders at 37 °C. The different types of vaccines had different levels of titer remaining for the same processing conditions and stabilizer used. Trehalose and trehalose mixtures stabilized the enveloped vaccines best, whereas the non-enveloped viral vaccine was best stabilized by the mannitol-dextran mixture. Given such differences, the optimal formulation depends on the vaccine type. However, success of long-term vaccine stabilization is also dependent on the spray drying parameters. Analysis of HSV-2 vaccine spray-dried with either trehalose or sucrose as the stabilizing excipient under various processing conditions after storage at 45 °C over 10 days showed differences in activity loss among samples [9]. The HSV-2 vaccine powders stabilized with sucrose had a large range of activity loss depending on the spray drying parameters used, indicating that the processing conditions will affect the long-term stabilization of the vaccine. To this end, our study assessed both processing losses during spray drying and the long-term stability of the spray-dried SD-TG and SD-TGI powders to determine if the chosen processing conditions and stabilizer were appropriate for conferring thermostability to this vaccine candidate.
This study showed that the powder remained physically stable throughout the stability study for all storage temperatures. For instance, moisture content of the spray-dried vaccine powder remained low throughout the stability study. The protective packaging method likely conferred protection of powders against moisture uptake. Proper packaging is required to preserve powder integrity, as shown for spray-dried anthrax vaccine powders held at 25 and 40 °C for three months [8], where the moisture content of the anthrax vaccine powders was reportedly within 0.5% of initial measurement after three months of storage due to the use of an effective packaging method. Similarly, Price et al. [78] stored spray-dried BCG vaccine powders at different temperatures up to one year. The vaccine powders were stored in either a scintillation vial or a protective packaging system that utilized desiccant, reactive oxygen species scavengers, and inert gas. Powders stored at 40 °C without humidity protection showed significant titer losses.
The long-term stability study results also showed that the SD-TGI powder maintained amorphous structure over 26 months even with added thermal stress. As shown in these results, trehalose, when stored dry, does not crystallize. This was also shown for spray-dried anthrax vaccine powders using a packaging system including desiccant [8]. These anthrax vaccine powders also used trehalose as the main stabilizing excipient. The moisture content of the powders remained close to the initial measurement, and the powders remained amorphous. Vandenheuval et al. [69] spray-dried bacteriophages in trehalose powders and stored them at 4 and 25 °C at 0 and 54% RH. They found that phage was inactivated due to the crystallization of amorphous trehalose. This result is due to the storage of unprotected trehalose-based powders at high humidity. The spray-dried SD-TG and SD-TGI powders did not crystallize in this study, indicating that the packing system effectively protected the powders from crystallization caused by exposure to high humidity.
Additionally, images of the particles obtained over the course of the stability study showed that interior and exterior morphology was overall preserved. Some bridging was shown between the small particles of the spray-dried vaccine powder stored at 40 °C. Bridging between particles is due to transition to a more energy favorable state through surface area reduction. However, bridging is expected after long term storage at high temperatures as bridging is facilitated by increased molecular mobility due to increased temperature. The overall lack of particle fusing, shape change, and maintenance of internal structure after 26 months of storage at 40 °C indicates physical stability was achieved.
One benefit of spray drying as compared to lyophilization is that spray drying allows for the design of solid dosage forms suitable for inhalation. Both interior and exterior particle structure was maintained for the samples stored at 25 °C for 26 months, with no clear indication of particle bridging. The preservation of the physical state of the spray-dried powder suggests that it could maintain aerosol performance even after long-term storage. However, this study focused on assessing the feasibility of a spray-dried ID93+GLA-SE formulation for reconstitution; as such the formulation was not optimized for pulmonary delivery. The relatively large size of the particles in this study are outside of the generally accepted respirable range for particles (1-5 μm) [27]. Additionally, trehalose-based powders are usually very cohesive and thus lack the dispersibility required for pulmonary administration. Optimization of a powder for respiratory delivery requires that the particles are within respirable range and the powder is dispersible. Improvement of powder dispersibility can be achieved through the addition of a compatible dispersibility enhancer to the formulation. The development of a spray-dried ID93+GLA-SE presentation suitable for pulmonary administration has been discussed elsewhere [79].
The results of the stability study on the spray-dried powders showed that the integrity of the adjuvant system was maintained after 26 months of storage at 25 °C and the antigen was still present after 26 months of storage at 40 °C. The stability criteria for stabilization of the adjuvant system was clearly met as the system exhibited long term room-temperature stability. The spray-dried powder also exhibited varying degrees of short-term high temperature stability. The emulsion droplet size was maintained for both samples (<50% change) for 26 months of storage and the polydispersity index was maintained (<0.2) for 19 months at 40 °C. For comparison, the droplet size of the 2-vial clinical presentation of ID93+GLA-SE increased to over 240 nm after only three months’ storage at 37 °C [24]. Similarly, squalene was retained in the SD-TG and SD-TGI powders after storage at 40 °C for 19 months and 26 months, respectively. GLA was retained after one month of storage at 40 °C, however, approximately 40% GLA losses were shown after three months of storage. Nevertheless, this result still demonstrates an improvement in thermostability as a stability study on a covialed liquid ID93+GLA-SE product showed greater than 50% GLA loss after three months of storage at 25 °C and complete degradation of GLA when stored at 37 °C for three months [24]. Images of stained gels indicate that spray-dried product’s ID93 band intensity is similar to that of a control after two months of storage at 40 °C, and the ID93 band is still present after holding the spray-dried product at 40 °C for 26 months, albeit at a reduced intensity. Conversely, the liquid product’s ID93 band cannot be detected after only one month of storage at 37 °C. This result clearly indicates that the spray-dried formulation has improved thermostability of the ID93 protein.
These stability results can also be compared to a stability study completed on the proof of concept lyophilized candidate and the lead lyophilized candidates [24]. Compared to three-month stability studies at 37 °C conducted on lead lyophilized candidates and liquid single-vial presentation, the spray-dried powders have a comparable or better performance regarding droplet size change [24]. Additionally, the droplet diameter of the spray-dried formulations remained under 120 nm over 26 months for all storage temperatures whereas the droplet diameter of the proof-of-concept lyophilized candidate increased to over 200 nm after three months’ storage at 37 °C [24]. The approximate 40% loss in GLA in the spray-dried samples after three months of storage is comparable to the reported 48% GLA loss in the lyophilized proof-of-concept formulation when stored at 37 °C for three months. However, the lead lyophilized candidates ranged from no losses up to 36% GLA loss under the same storage conditions, depending on the formulation. Change in pH for the samples stored at 40 °C for three months was 0.16 pH units or less; comparatively, the lead lyophilized vaccine candidates stored at 37 °C for three months showed similar pH decreases of less than 0.12 pH units [24]. The pH of the liquid, two-vial and lyophilized proof-of-concept samples exhibited pH decreases of ≥0.2 pH units. Thus, the spray-dried formulations appear to offer greater protection against pH change than the liquid vaccine presentations and similar protection as the lead lyophilized candidates.
The lead lyophilized candidates generally outperform the spray-dried formulations in terms of GLA and possibly ID93 retention at high temperature storage. However, these lead lyophilized candidates were developed and tested after an extensive design-of-experiments approach. The spray-dried candidates in this paper are a first iteration and clearly exhibit improved protection against degradation as compared to the liquid vaccine. Additionally, the WHO has developed a controlled temperature chain (CTC) program that allows vaccines to be temporarily kept outside of the typical cold chain requirement of 2-8 °C provided that the vaccine is stable when exposed to at least 40 °C for a minimum of three days [80]. Comparatively, the stability criteria given in this study are much stricter than the WHO CTC requirements. While the spray-dried powders were not stable after three months of storage at 40 °C, the exhibited powder stability for one month at 40 °C does exceed the minimum three-day stability requirement as set by the WHO. Overall the physicochemical results of the spray-dried vaccine powder after storage appear promising; however, in vivo studies are needed to verify vaccine potency after storage.
Conclusion
Two versions of an adjuvanted tuberculosis vaccine, one without the antigen and one without, were spray-dried and the stability of the dry powder product was evaluated over 26 months. To the authors’ best knowledge, these are the first published results regarding the stabilization of a subunit vaccine formulated as a nanoemulsion at the 100-nm diameter scale using trehalose via spray drying and suggesting stability potential at high temperatures.
This study showed that integrity of the adjuvant system was preserved over the spray drying process, with evidence of antigen retention. Given the small scale, the efficiency of encapsulation and preservation of the nanoemulsion droplets is especially notable. Additionally, characterization of the spray-dried formulation indicated that the adjuvant system was stable after one month of storage at 40 °C, with the suggested antigen retention after two months at 40 °C similar to a two-vial liquid clinical presentation of the vaccine candidate. These results are especially meaningful in light of global health applications as the refrigeration infrastructure required for vaccine distribution is not always available. A dry powder product that is stable short term at high temperatures alleviates the necessity for such refrigeration infrastructure at the final step of distribution in remote locations. Besides, formulating the vaccine as a dry powder and reconstituting as needed will significantly reduce the costs associated with transport and storage. Immunogenicity studies have not been carried out on the spray-dried vaccine; however, the spray-dried vaccine appears to be comparable to the lyophilized vaccine, which is currently undergoing a Phase I clinical trial [25], in terms of biophysical characterization.
The spray-dried adjuvant system was shown to be stable after 26 months of storage at temperatures up to 25 °C. The dry powder presentation of the oil-in-water adjuvant system can be combined with subunit vaccines for other diseases. In comparison to the lyophilization treatment of these biological formulations, which has been proven to be a beneficial method of storage [24], spray drying presents a promising alternative with its potentially lower processing cost and improved scalability [26]. The increased production capability of spray drying can be utilized to stockpile larger amounts of thermostable adjuvant or vaccine for pandemic situations. Additionally, spray drying, unlike lyophilization, allows for the engineering of inhalable properties that open doors to other delivery routes, such as pulmonary and intranasal administration. Our dry powder product was physically stable after 26 months of storage at a high temperature up to 40 °C. The demonstrated preservation of the spray-dried powder suggests retention of aerosol performance after long-term storage at high temperatures; however, further work is required to optimize the formulation for inhalable delivery.
Acknowledgements
This work was supported by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract HHSN272201400041C. The authors would like to thank Leanne Millburn for assistance with data collection and Tony Phan for emulsion preparation.
Footnotes
Disclosures
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].World Health Organization (WHO), "Temperature sensitivity of vaccines," August 2006. [Online]. Available: https://apps.who.int/iris/handle/10665/69387. [Accessed 17 April 2020].
- [2].Carrigy NB and Vehring R, "Engineering stable spray-dried biologic powder for inhalation," in Pharmaceutical Inhalation Aerosol Technology (3rd ed.), Hickey AJ and da Rocha S, Eds., Boca Raton, CRC Press, 2019, pp. 291–326. [Google Scholar]
- [3].Argarkhedkar S, Kulkarni PS, Winston S, Sievers R, Dhere RM, Gunale B, Powell K, Rota P. a., Papania M and MDVP author group, "Safety and immunogenicity of dry powder measles vaccine administered by inhalation: A randomized controlled Phase 1 clinical trial," Vaccine, vol. 32, pp. 6791–6797, 2014. [DOI] [PubMed] [Google Scholar]
- [4].Sou T, Morton DAV, Williamson M, Meeusen EN, Kaminskas LM and McIntosh MP, "Spray-dried influenza antigen with trehalose and leucine produces an aerosolizable powder vaccine formulation that induces strong systemic and mucosal immunity after pulmonary administration," Journal of Aerosol Medicine and Pulmonary Drug Delivery, vol. 28, no. 5, pp. 361–371, 2015. [DOI] [PubMed] [Google Scholar]
- [5].Kanojia G, Willems G-J, Frijlink HW, Kersten GFA, Soema PC and Amorij J-P, "A design of experiment approach to predict product and process parameters for a spray dried influenza vaccine," International Journal of Pharmaceutics, vol. 511, pp. 198–1111, 2016. [DOI] [PubMed] [Google Scholar]
- [6].Toniolo SP, Afkhami S, Mahmood A, Fradin C, Lichty BD, Miller MS, Xing Z, Cranston ED and Thompson MR, "Excipient selection for thermally stable enveloped and non-enveloped viral vaccine platforms in dry powders," International Journal of Pharmaceutics, vol. 561, pp. 66–73, 2019. [DOI] [PubMed] [Google Scholar]
- [7].Zhu C, Shoji Y, Burke M, Hartman CE, Chichester JA, Breit J, Yusibov V, Chen D and Lal M, "Stabilization of HAC1 Influenza Vaccine by Spray Drying," Pharmaceutical Research, vol. 31, pp. 3006–3018, 2014. [DOI] [PubMed] [Google Scholar]
- [8].Jones RM, Burke M, Dubose D, Chichester JA, Manceva S, Horsey A, Streatfield SJ, Breit J and Yusibov V, "Stability and pre-formulation development of a plant-produced anthrax vaccine candidate," Vaccine, vol. 35, pp. 5463–5470, 2017. [DOI] [PubMed] [Google Scholar]
- [9].LeClair DA, Li L, Rahman N, Cranston ED, Xing Z and Thompson MR, "Stabilization of HSV-2 viral candidate by spray drying," International Journal of Pharmaceutics, vol. 569, pp. 1–10, 2019. [DOI] [PubMed] [Google Scholar]
- [10].Kanojia G, Raeven RHM, van der Maas L, Bindels THE , van Riet E, Metz B, Soema PC, ten Have R, Frijlink HW, Amorij J-P and Kersten GFA, "Development of a thermostable spray dried outer membrane vesicle pertussis vaccine for pulmonary immunization," Journal of Controlled Release, vol. 286, pp. 167–178, 2018. [DOI] [PubMed] [Google Scholar]
- [11].Jin TH, Tsao E, Goudsmit J, Dheenadhayalan V and Sadoff J, "Stabilizing formulations for inhalable powders of adenovirus 35-vectored tuberculosis (TB) vaccine (AERAS-402)," Vaccine, vol. 28, pp. 4639–4375, 2010. [DOI] [PubMed] [Google Scholar]
- [12].Thakur A, Ingvarsson PT, Schmidt ST, Rose F, Andersen P, Christensen D and Foged C, "Immnological and physical evaluation of the multistage tuberculosis subunit vaccine candidate H56/CAF01 formulated as a spray dried powder," Vaccine, vol. 36, pp. 3331–3339, 2018. [DOI] [PubMed] [Google Scholar]
- [13].Carrigy NB, Liang L, Wang H, Kariuki S, Nagel TE, Connerton IF and Vehring R, "Trileucine and pullulan improve anti-Campylobacter bacteriophage stability in engineered spray-dried microparticles," Annals of Biomedical Engineering, vol. 48, pp. 1169–1180, 2019. [DOI] [PubMed] [Google Scholar]
- [14].World Health Organization, "Global Tuberculosis Report," World Health Organization, Geneva, 2019. [Google Scholar]
- [15].Flynn JL and Chan J, "Immunology of Tuberculosis," Annual Review of Immunology, vol. 19, no. 1, pp. 93–129, 2001. [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization, "WHO Preferred Product Characteristics for New Tuberculosis Vaccines," World Health Organization, Geneva, 2018. [Google Scholar]
- [17].Reed SG, Bertholet S, Coler RN and Friede M, "New horizons in adjuvants for vaccine development," Trends in Immunology, vol. 30, no. 1, pp. 23–32, 2009. [DOI] [PubMed] [Google Scholar]
- [18].Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvick TS, Coler RN and Reed SG, "Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis," Journal of Immunology, vol. 181, no. 11, pp. 7948–7957, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Plessner Windish H, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS and Reed SG, "Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant," Public Library of Science One, vol. 6, no. 1, p. e16333, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bertholet S, Ireton GC, Ordway DJ, Plessner Windish H, Pine SO, Kahn M, Phan T, Orme IM, Vedvick TS, Baldwin SL, Coler RN and Reed SG, "A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobaterium tuberculosis," Science Translational Medicine, vol. 2, no. 53, pp. 53–74, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, Rolf T, Lu L, Alter G, Hokey D, Jayashankar L, Walker R, Snowden MA, Evans T, Ginsberg A and Reed SG, "The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial," Nature Partner Journals, vol. 3, no. 34, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L, Vergara J, Mabwe S, Bilek N, Geldenhuys H, Kany-Kany Luabeya A, Ellis R, Ginsberg AM, Hanekom WA, Reed SG, Coler RN, Scriba TJ, Hatherill M and TBVPX-114 study team, "Safety and immunogenicty of the novel tuberculosis vaccine ID93+GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial," Lancet Respiratory Medicine, vol. 6, no. 4, pp. 287–298, 2018. [DOI] [PubMed] [Google Scholar]
- [23].Orr MT, Kramer RM, Barnes LV, Dowling QM, Desbien AL, Beebe EA, Laurance JD, Fox CB, Reed SG, Coler RN and Vedvick TS, "Elimination of the cold-chain dependance of a nanoemulsion adjuvant vaccine against tuberculois by lyophilization," Journal of Controlled Release, vol. 10, no. 177, pp. 20–26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kramer RM, Archer MC, Dubois N, Dubois Cauwelaert N, Beebe EA, Huang P.-w. D., Dowling QM, Schwartz AM, Fedor DM, Vedvick TS and Fox CB, "Development of a thermostable nanoemulsion adjuvanted vaccine against tuberculosis using a design-of-experiments approach," International Journal of Nanomedicine, vol. 13, pp. 3689–3711, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Infectious Disease Research Institute, "Identifier: NCT03722472, Phase 1 Clinical Trial of Single-Vial ID93 + GLA-SE in Healthy Adults," 3 September 2019. [Online]. Available: https://clinicaltrials.gov/ct2/show/NCT03722472?term=id93&draw=2&rank=1. [Accessed 23 April 2020].
- [26].Schwartzbach H, "Achieving aseptic drying with spray drying technologies," Pharmaceutical Technology Europe, vol. 23, no. 9, 2011. [Google Scholar]
- [27].Kanojia G, ten Have R, Soema PC, Frijlink H, Amorij P and Kersten G, "Developments in forumulation and delivery of spray dried vaccines," Human Vaccines & Immunotherapeutics, vol. 13, no. 10, pp. 2364–2378, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lammert AM, Schmidt SJ and Day GA, "Water activity and solubility of trehalose," Food Chemistry, vol. 61, no. 1, pp. 139–144, 1998. [Google Scholar]
- [29].Richards AB, Krakowka S, Dexter LB, Schmid H, Wolterbeek APM, Waalkens-Berendsen DH, Shigoyuki A and Kurimoto M, "Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies," Food and Chemical Toxicology, vol. 40, pp. 871–898, 2002. [DOI] [PubMed] [Google Scholar]
- [30].Grasmeijer N, Stankovic M, de Waard H, Frijlink HW and Hinrichs WLJ, "Unraveling protein stabilization mechanisms: vitrification and water replacement in a glass transition temperature controlled system," Biochimica et Biophysica Acta, vol. 1834, no. 4, pp. 763–769, 2013. [DOI] [PubMed] [Google Scholar]
- [31].Ingvarsson PT, Yang M, Nielsen HM, Rantanen J and Foged C, "Stabilization of liposomes during spray drying," Expert Opinion on Drug Delivery, vol. 8, no. 3, pp. 375–388, 2011. [DOI] [PubMed] [Google Scholar]
- [32].Ohtake S and Wang J, "Trehalose: current use and future applications," Journal of Pharmaceutical Sciences, vol. 100, no. 6, pp. 2020–2053, 2011. [DOI] [PubMed] [Google Scholar]
- [33].Tonnis WF, Mensink MA, de Jager A, van der Voort Maarschalk K, Frijlink HW and Hinrichs WL, "Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins," Molecular Pharmaceutics, vol. 12, pp. 684–694, 2015. [DOI] [PubMed] [Google Scholar]
- [34].Cicerone MT, Pikal MJ and Qian KK, "Stabilization of proteins in solid form," Advanced Drug Delivery Reviews, vol. 93, pp. 14–24, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang Y, Che L, Fu N, Chen XD and Selomulya C, "Surface formation phenomena of DHA-containing emulsion during convective droplet drying," Journal of Food Engineering, vol. 150, pp. 50–61, 2015. [Google Scholar]
- [36].Zhou D, Zhang GG, Law D, Grant DJ and Schmitt EA, "Physical stability of amorphous pharmaceuticals: Importance of configurational thermodynamic quantities and molecular mobility," Journal of Pharmaceutical Sciences, vol. 91, no. 8, pp. 1863–1872, August 2000. [DOI] [PubMed] [Google Scholar]
- [37].Hoe S, Ivey JW, Boraey MA, Shamsaddini-Shahrbabak A, Javaheri E, Sadaf M, Finlay WH and Vehring R, "Use of a fundamental approach to spray-drying formulation design to facilitate the development of multi-component dry powder aerosols for respiratory drug delivery," Pharmaceutical Research, vol. 32, no. 2, pp. 449–465, February 2014. [DOI] [PubMed] [Google Scholar]
- [38].Gordon M and Taylor JS, "Ideal copolymers and the second-order transitions of synthetic rubbers. i. non-crystalline copolymers," Journal of Applied Chemistry, vol. 2, no. 9, pp. 493–500, September 1952. [Google Scholar]
- [39].Chen T, Fowler A and Toner M, "Literature review: supplemented phase diagram of the trehalose-water binary mixture," Cryobiology, vol. 40, no. 3, pp. 277–282, 200. [DOI] [PubMed] [Google Scholar]
- [40].Crowe LM, Reid DS and Crowe JH, "Is trehalose special for preserving dry biomaterials?," Biophysical Journal, vol. 71, pp. 2087–2093, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roe KD and Labuza TP, "Glass transition and crystallization of amorphous trehalose-sucrose mixtures," International Journal of Food Properties, vol. 8, no. 3, pp. 559–574, 2005. [Google Scholar]
- [42].Hancock BC and Dalton CR, "The effect of temperature on water vapor sorption by some amorphous pharmaceutical sugars," Pharmaceutical Development and Technology, vol. 4, no. 1, pp. 125–131, 1999. [DOI] [PubMed] [Google Scholar]
- [43].Mensink MA, Frijlink HW, van der Voort Maarschalk K and Hinrichs WLJ, "How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions," European Journal of Pharmaceutics and Biopharmaceutics, vol. 114, pp. 288–295, 2017. [DOI] [PubMed] [Google Scholar]
- [44].Munoz-Ibanez M, Azagoh C, Dubey BN, Dumoulin E and Turchiuli C, "Changes in oil-in-water emulsion size distribution during the atomization step in spray-drying encapsulation," Journal of Food Engineering, vol. 167, pp. 122–132, 2015. [Google Scholar]
- [45].Arpagaus C, Collenburg A, Rutti D, Assadpour E and Jafari SM, "Nano spray drying for encapsulation of pharmaceuticals," International Journal of Pharmaceutics, vol. 546, pp. 194–214, 2018. [DOI] [PubMed] [Google Scholar]
- [46].Encina C, Vergara C, Gimenez B, Oyarzun-Ampuero F and Robert P, "Conventional spray-drying and future trends for the microencapsulation of fish oil," Trends in Food Science & Technology, vol. 56, pp. 46–60, 2016. [Google Scholar]
- [47].Sarkar A, Arfsten J, Golay P-A, Acquistapace S and Heinrich E, "Microstructure and long-term stability of spray dried emulsions with ultra-high oil content," Food Hydrocolloids, vol. 52, pp. 857–867, 2016. [Google Scholar]
- [48].Pang Y, Duan X, Ren G and Liu W, "Comparative study on different drying methods of fish oil microcapsules," Journal of Food Quality, pp. 1–7, 2017. [Google Scholar]
- [49].Re M-I, "Formulating Drug Delivery Systems by Spray Drying," Drying Technology, vol. 24, pp. 433–446, 2006. [Google Scholar]
- [50].Ziaee A, Albadarin AB, Padrela L, Femmer T, O'Reilly E and Walker G, "Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches," European Journal of Pharmaceutical Sciences, vol. 127, pp. 300–318, 2019. [DOI] [PubMed] [Google Scholar]
- [51].Gharsallaoui A, Roudaut G, Chambin O, Voilley A and Saurel R, "Applications of spray-drying in microencapsulation of food ingedients: An overview," Food Research International, vol. 2007, pp. 1107–1121, 2007. [Google Scholar]
- [52].Linke A, Balke T and Kohlus R, "Identification of key factors determining the surface oil concentration of encapsulated lipid particles produced by spray drying," in 21st International Drying Symposium, Valencia, Spain, 2018. [Google Scholar]
- [53].Jafari SM, Assadpoor E, He Y and Bhandari B, "Encapsulation efficiency of food flavours and oils during spray drying," Drying Technology, vol. 26, no. 7, pp. 816–835, 2008. [Google Scholar]
- [54].Encina C, Marquez-Ruiz G, Holgado F, Gimenez B, Vergara C and Robert P, "Effect of spray-drying with organic solvents on the encapsulation, release and stability of fish oil," Food Chemistry, vol. 263, pp. 283–291, 2018. [DOI] [PubMed] [Google Scholar]
- [55].Bringas-Lantigua M, Exposito-Molina I, Reineccius GA, Lopez-Hernandez O and Pino JA, "Influence of spray-dryer air temperatures on encapsulated mandarin oil," Drying Technology, vol. 29, pp. 520–526, 2011. [Google Scholar]
- [56].Vehring R, "Pharmaceutical Particle Engineering via Spray Drying," Pharmaceutical Research, vol. 25, no. 5, pp. 999–1022, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vehring R, Foss WR and Lechuga-Ballesteros D, "Particle formation in spray drying," Aerosol Science, vol. 38, pp. 728–746, 2007. [Google Scholar]
- [58].Poozesh S, Lu K and Marsac PJ, "On the particle formation in spray drying process for bio-pharmaceutical applications: Interrogating a new model via computational fluid dynamics," International Journal of Heat and Mass Transfer, vol. 122, pp. 863–873, 2018. [Google Scholar]
- [59].Larbi Z, Sadoun N, Si-Ahmed E.-k. and Legrand J, "An efficient numerical prediction of the crust onset of a drying colloidal drop," International Journal of Heat and Mass Transfer, Vols. 165, Part A, p. 1210613, 2021. [Google Scholar]
- [60].Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG and Coler RN, "Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis," Journal of Controlled Release, vol. 172, pp. 190–200, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ivey J, Bhambri P, Lewis D, Church T, Finlay W and Vehring R, "Dried corticosteroid particle formation from evaporating monodisperse propellant solution droplets," in AAPS Annual Meeting and Exposition, Denver, 2016. [Google Scholar]
- [62].Ivey JW and Vehring R, "The use of modeling in spray drying of emulsions and suspensions accelerates formulation and process development," Computers and Chemical Engineering, vol. 34, pp. 1036–1040, 2010. [Google Scholar]
- [63].Wang H, Nobes DS and Vehring R, "Particle Surface Roughness Improves Colloidal Stability of Pressured Pharmaceutical Suspensions," Pharmaceutical Research, vol. 36, no. 43, 2019. [DOI] [PubMed] [Google Scholar]
- [64].International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use, "ICH Harmonised Tripartite Guideline - Stability Testing of New Drug Substances and Products Q1A(R2)," ICH, 2003. [Google Scholar]
- [65].Waterman KC and MacDonald BC, "Package selection for moisture protection for solid, oral drug products," Journal of Pharmaceutical Sciences, vol. 99, no. 11, pp. 4437–4452, 2010. [DOI] [PubMed] [Google Scholar]
- [66].Wang H, Barona D, Oladepo S, Williams L, Hoe S, Lechuga-Ballesteros D and Vehring R, "Macro-Raman spectroscopy for bulk composition and homogeneity analysis of multi-component pharmaceutical powders," Journal of Pharmaceutical and Biomedical Analysis, vol. 141, pp. 180–191, 2017. [DOI] [PubMed] [Google Scholar]
- [67].Vehring R, "Red-excitation dispersive Raman spectroscopy is a suitable technique for solid-state analysis of respirable pharmaceutical powders," Applied Spectroscopy , vol. 59, no. 3, pp. 286–292, 2005. [DOI] [PubMed] [Google Scholar]
- [68].Chan MY, Dowling QM, Sivananthan SJ and Kramer RM, "Particle sizing of nanoparticle adjuvant formulations by dynamic light scattering (DLS) and nanoparticle tracking analysis (NTA)," Methods in Molecular Biology, vol. 1494, pp. 239–252, 2017. [DOI] [PubMed] [Google Scholar]
- [69].Vandenheuvel D, Meesus J, Lavigne R and Van den Mooter G, "Instability of bacteriophages in spray-dried trehalose powders is caused by crystallization of the matrix," International Journal of Pharmaceutics, vol. 472, pp. 202–205, 2014. [DOI] [PubMed] [Google Scholar]
- [70].Krogsgard Nielsen C, Kjems J, Mygind T, Snabe T, Schwarz K, Serfert Y and Meyer RL, "Enhancing the antibacterial efficacy of isoeugenol by emulsion encapsulation," International Journal of Food Microbiology, vol. 229, pp. 7–14, 2016. [DOI] [PubMed] [Google Scholar]
- [71].Paramita V, Iida K, Yoshii H and Furuta T, "Effect of additives on the morphoolgy of spray-dried powder," Drying Technology, vol. 28, no. 3, pp. 323–329, 2010. [Google Scholar]
- [72].Wang Y, Liu W, Chen XD and Selomulya C, "Micro-encapsulation and stabilization of DHA containing fishoil in protein-based emulsion through mono-disperse droplet spray dryer," Journal of Food Engineering, vol. 175, pp. 74–84, 2016. [Google Scholar]
- [73].Schwartz AM, Chan MY, Fedor DM and Sivananthan SJ, "Staining and transfer techniques for SDS-PAGE Gels to minimize oil-in-water emulsion adjuvant interference," in Vaccine Adjuvants. Methods in Molecular Biology , vol 1494, New York, Humana Press, 2017, pp. 273–283. [DOI] [PubMed] [Google Scholar]
- [74].Ingvarsson PT, Schmidt ST, Christensen D, Larsen NB, Hindrichs WLJ, Andersen P, Rantanen J, Nielson HM, Yang M and Foged C, "Designing CAF-adjuvanted dry powder vaccines: Spray drying preserves the adjuvant activity of CAF01," Journal of Controlled Release, vol. 167, pp. 256–264, 2013. [DOI] [PubMed] [Google Scholar]
- [75].Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG and Vedvick TS, "Monitorung the effects of component structure and source on formulation stability and adjuvant activity of oil-in-water emulsions," Colloids and Surfaces B; Biointerfaces, vol. 65, pp. 98–105, 2008. [DOI] [PubMed] [Google Scholar]
- [76].Ribeiro RF, Motta MH, Harter APG, Flores FC, Beck RCR, Schaffazick SR and de Bona da Silva C, "Spray-dried powders improve the controlled relase of antifungal tioconazole-loaded polymeric nanocapsules compared to with lyophilized products," Materials Science and Engineering C, vol. 59, pp. 875–884, 2016. [DOI] [PubMed] [Google Scholar]
- [77].Jones JR, Prime D, Leaper MC, Richardson DJ, Rielly CD and Stapley AGF, "Effect of processing variables and bulk composition on the surface composition of spray dried powders of a model food system," Journal of Food Engineering, vol. 118, pp. 19–30, 2013. [Google Scholar]
- [78].Price DN, Kunda NK, Ellis R and Muttil P, "Design and optimization of a temperature-stable dry powder BCG vaccine," Pharmaceutical Research, vol. 37, no. 11, pp. 1–14, 2020. [DOI] [PubMed] [Google Scholar]
- [79].Gomez M, McCollum J, Wang H, Ordoubadi M, Jar C, Carrigy NB, Barona D, Tetreau I, Archer M, Gerhardt A, Press C, Fox CB, Kramer RM and Vehring R, "Development of a formulation platform for a spray-dried, inhalable tuberculosis vaccine candidate," International Journal of Pharmaceutics, vol. 593, p. 120121, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].World Health Organization, "Annex 5 Guidelines on the stability evaluation of vaccines for use under extended controlled temperature conditions," WHO Technical Report Series No. 999, 2016. [Google Scholar]