Abstract
Introduction
During pregnancy, SARS-CoV-2 infection may cause an abnormal development of the placenta, thus influencing maternal and fetal outcomes. Few studies have reported data on placental morphology and histology in infected pregnant patients, although not compared with carefully matched controls. The aim of this study is to compare placental morphology and histology of pregnant women affected by SARS-CoV-2 to non-infected controls.
Methods
This is a prospective multicenter case-control study on 64 pregnant women affected by SARS-CoV-2 who delivered at term or late-preterm. Data were collected about pregnancy course, maternal and fetal outcomes, placental biometry and macro- and microscopical morphology. 64 not-infected women were identified as controls, matched by age, body mass index and ethnicity.
Results
Cases and controls had similar fetal and maternal outcomes. No significant differences were observed in placental macro- or microscopical morphology between the two groups. In the cases treated with antivirals, chloroquine, LMWH or antibiotics, placentas were heavier but not more efficient than the non-treated, since the fetal/placental weight ratio did not differ. Moreover, delayed villous maturation was more frequent in treated women, although not significantly. The newborns whose mothers received oxygen therapy as treatment had higher levels of umbilical cord pO₂ at birth.
Discussion
In this prospective case-control study, SARS-CoV-2 infection during the third trimester did not influence placental histological pattern. Pharmacological and oxygen therapy administered to women affected by this viral infection could impact maternal and fetal outcomes and be associated to placental histological alterations.
Keywords: SARS-CoV-2 infection, Placental histological lesions, COVID-19, Pregnancy
Abbreviations: COVID-19, SARS-CoV-2 disease 19; BMI, body mass index; ICP, intrahepatic cholestasis of pregnancy; GDM, gestational diabetes mellitus; IUGR, intrauterine growth restriction; PTL, pre-term labor; PPH, post-partum hemorrhage; FVM, fetal vascular malperfusion; MVM, maternal vascular malperfusion; NICU, neonatal intensive care unit; LMWH, low molecular weight heparin; SaO₂, oxygen saturation; PaO₂/FiO₂, arterial oxygen partial pressure / fraction of inspired oxygen
1. Introduction
The placenta represents a highly perfused compartment separating maternal and fetal circulations. The infection with SARS-CoV-2 has the potential to increase inflammatory and oxidative stress in the placenta, thus compromising both pregnancy evolution and fetal development [1]. Indeed, pregnancies complicated by SARS-CoV-2 infection have an increased risk of miscarriage, preterm birth, pre-eclampsia and stillbirth [[2], [3], [4]]. These pregnancy-related pathologies have long been known to be associated with an abnormal placental development.
To date, a viral infection of the placenta by SARS-CoV-2 has been suggested in small case series [[5], [6], [7], [8]]. Our group has recently described the SARS-CoV-2 genome in two term placentas out of 31 pregnancies with COVID [9]. In that study, three cases of vertical transmission were identified and documented [9], accompanied by a strong inflammatory response in maternal and umbilical plasma as well as in the placenta. Although various parameters are still under analysis to constitute evidence of vertical transmission [10], intrauterine viral exposure has been reported in different studies [[11], [12], [13], [14]]. Methodological issues however still need to be effectively addressed to make this evidence strong.
Placental morphological and histological examination may contribute significant clues about placental viral exposure and its consequences. However, as of today, limited data are available from infected pregnant patients, mainly in severe cases not compared with carefully matched controls. In particular, placentas from SARS-CoV-2 positive neonates showed chronic intervillositis together with macrophages CD68+ infiltration and SARS-CoV-2 spike protein's mRNA in placental syncytiotrophoblast cells [14].
Fetal vascular malperfusion (FVM) was also found in placentas of SARS-CoV-2 infected women [[15], [16], [17]]. Interestingly, in a study by Mulvey et al. the placentas were negative for viral RNA and spike proteins’ presence [17].
Moreover, evidence of villous edema and retroplacental hematoma [1], as well as histiocytic intervillositis have been reported in placental syncytiotrophoblast cells where SARS-CoV-2 was predominantly concentrated [18].
Although Patberg and colleagues reported that FVM and maternal vascular malperfusions (MVM) may be more frequent in placentas of SARS-CoV-2 infected patients [19], no specific pathological pattern results from placentas of SARS-CoV-2-positive women according to the few retrospective studies available [[14], [15], [16], [17], [18], [20]].
In order to evaluate SARS-CoV-2 related placental damage and its possible harmful effect on pregnancy and neonatal outcomes, we conducted a prospective case-control study to compare the placental morphology and histology in pregnant women affected by SARS-CoV-2 with non-infected controls at the time of delivery.
2. Methods
This is a prospective multicenter case-control study on 64 pregnant women with a confirmed SARS-CoV-2 infection during pregnancy. Pregnant women were enrolled from March until August 2020 at Sacco Hospital and Buzzi Hospital in Milan, and at San Gerardo Hospital in Monza. Only pregnant women who delivered at term or late preterm (≥34 gestational weeks) were included.
SARS-CoV-2 negative controls were retrospectively selected from a Buzzi Hospital database, obtained from consecutively collected placentas.
An equal to cases (n = 64) number of women, who consecutively delivered in 2019, prior to SARS-CoV-2 appearance in Italy, were matched for ethnicity, age group (18–29, 30–39 and ≥40 years old) and pre-pregnancy Body Mass Index (BMI- normal weight: 18–24,99 kg/m2, overweight: 25–29,99 kg/m2, obese: ≥ 30 kg/m2) to the cases. The enrollment was performed picking the first case from a chronologically ordered database, while respecting the inclusion criteria and delivering at term or late preterm.
2.1. Clinical data collection
Demographic, biometric (pre-gestational BMI) and ethnicity (Caucasian, Chinese-Asian, Middle Eastern, African, South American) characteristics were recorded for each enrolled woman.
Obstetrical data included parity, pregnancy onset, gestational age at the time of infection and at delivery, mode of delivery with or without any induction of labor, and the presence or absence of any pregnancy-related pathologies (e.g. of maternal origin: Intrahepatic Cholestasis of Pregnancy- ICP, hypertensive disorders, thyroid diseases or Gestational Diabetes Mellitus- GDM; of fetal/neonatal origin: IntraUterine Growth Restriction- IUGR, PreTerm Labor- PTL), and delivery complications (postpartum hemorrhage - PPH).
The severity degree of the infection was defined as follows:
-
•
asymptomatic: no symptoms (with negative imaging when performed);
-
•
mild: one or more symptoms among fever, cough, pharyngeal pain, headache, myalgia, nausea, emesis, diarrhea, anosmia, ageusia, but no dyspnea (abnormal imaging when performed);
-
•
moderate: evidence of lower respiratory disease by clinical assessment or imaging and Oxygen saturation (SaO₂) ≥ 94% on room air;
-
•
severe: one or more symptoms among SaO₂ ≤ 94% in ambient air, PaO₂/FiO₂ <300 mmHg (i.e. arterial oxygen partial pressure/fraction of inspired oxygen), respiratory rate >30/minute or pneumonia involving more than 50% of the lungs' volume at X-ray scan.
Neonatal data were also collected: umbilical arterial pH and pO₂ at delivery, neonatal weight, APGAR score at 5 min, NICU admission.
Cases underwent a clinical evaluation of vital signs and symptoms, and a radiological chest x-ray when required. Pharmacological management before and/or after delivery (antivirals, hydroxychloroquine, LMWH, antibiotics or their combinations, oxygen) was recorded to be included in our analysis.
2.2. Biological samples collection and analyses
All placentas, both from cases and controls, were stored and analyzed at the ‘Pathology Unit’ of the Luigi Sacco Hospital- ASST Fatebenefratelli-Sacco in Milan.
Placental weight was recorded, whilst placental efficiency was calculated as fetal/placental weight ratio [21].
Macroscopical and microscopical lesions were described according to the Amsterdam Placental Workshop Group 2014 classification [22].
Considering the main placental patterns observed, we divided the histological features into six classes:
-
•
Normal, when placentas did not show any specific alteration;
-
•
Delayed Villous Maturation, according to the Amsterdam consensus definition (a monotonous villous population with centrally placed capillaries and decreased vasculosyncytial membranes, in at least 30% of one full-thickness parenchymal slide);
-
•
Fetal Vascular Malperfusion, if thrombosis, segmental avascular villi, villous stromal vascular karyorrhexis, vascular intramural fibrin deposition, stem vessel obliteration/fibromuscular sclerosis and/or vascular ectasia were observed;
-
•
Chorioamnionitis, considering acute forms;
-
•
Maternal Vascular Malperfusion, including placental hypoplasia, infarction (considered when larger than 5% in non-peripheral placental zones), retroplacental hemorrhage, distal villous hypoplasia and accelerated villous maturation;
-
•
Chronic inflammation, when placentas showed features attributable to chronic deciduitis or high and low grade chronic villitis of unknown etiology.
The criteria used for grading and staging were applied for each category.
After removing both the maternal decidua and all the fetal membranes, the umbilical cord was trimmed from the placental disc. Then histological multiple samples were collected as follows: two samples were trimmed from the membranes, three from the umbilical cord, one from the umbilical cord's insertion and five from the placental cotyledons.
After the fixation with formalin, the presence of the SARS-CoV-2 infection was evaluated in 47 placentas by detecting the viral RNA with a PCR technique. Total RNA was extracted from 3 unstained slides (5 μm thick) using Quick-RNA FFPE Miniprep [Zymo Research, Irvine, CA, USA] in elution volume of 30 μL.
The WHO/Charité SARS-CoV-2 Real-Time RT-PCR E-gene assay [Berlin, Germany] was adapted using a qPCRBIO Probe 1-Step Go Master Mix [PCR Biosystems]. Human RNase P was used as an internal control to confirm RNA was adequately extracted and conserved. Positive sample were confirmed using the CE-IVD Logix Smart COVID-19 kit [Co-Diagnostic, Salt Lake City, Utah, USA]. According to the literature, cycle threshold values less than 40 were considered positive.
The positivity to SARS-CoV-2 viral infection was established by two consecutive and positive PCR experiments.
2.3. Ethics declaration
The protocol was approved by the local ‘Medical Ethical and Institutional Review Board’ [Comitato Etico Milano Area 1, protocol n° 15408, March 11, 2020]. We obtained a written informed consent to collect personal data and biological samples, and to perform the analyses, according to CARE guidelines and in compliance with the Declaration of Helsinki principles.
The authors have no competing interests to declare.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
2.4. Statistical analyses
Maternal demographic, biometrics, obstetrical and clinical characteristics, pregnancy outcomes and neonatal data displayed a non-normal distribution (Kolmogorov-Smirnov test). They were thus compared among three or more independent study groups (Kruskal-Wallis test) or among two independent study groups (Mann-Whitney U test).
Chi-square analyses were performed to compare among groups the ethnicity, parity, pregnancy onset, pregnancy related diseases, delivery mode, induction of labor, APGAR score at 5 min < 7, NICU admission data and the placental histological diagnoses, applying the Yates continuity correction.
A two-way between-group ANOVA (ANalysis Of VAriance) was conducted to explore the impact of the symptomatic course of SARS-CoV-2 infection, the pharmacologic therapy and the evidence of the virus' presence in placenta (independent variables), as individual or joint effect, on cord pO 2 concentration at birth (dependent variables); and applied with the Levene's assumptions. A Tukey's HSD test was run as post-hoc test.
Correlations between the analyzed variables were assessed using the Spearman's Rank Order Correlation-rho.
Participants’ baseline characteristics were presented as frequencies ± percentages - for categorical variables, or mean ± standard deviations - for quantitative continuous variables.
Comparisons between groups and correlations were considered statistically significant when p-value ≤ 0.05.
Statistical analyses were performed using SPSS (v.25.00, IBM Statistics, Armonk/NY, USA).
3. Results
3.1. Clinical data analysis
Table 1-A presents the main features of the study population, comparing cases and controls. The two groups were comparable for all the evaluated parameters.
Table 1- A.
Population features.
| Features | Cases (n=64) | Controls (n=64) | p-value |
|---|---|---|---|
| Age (yrs) | 31.9 ± 5.5 | 32.1 ± 4.8 | 0.862 |
| BMI (Kg/m2) | 25.3 ± 5.0 | 24.4 ± 4.7 | 0.345 |
| Ethnicity | 1 | ||
| Caucasian | 40 (62.5%) | 40 (62.5%) | |
| Chinese-Asian | 8 (12.5%) | 8 (12.5%) | |
| Middle Eastern | 7 (10.9%) | 7 (10.9%) | |
| African | 3 (4.7%) | 3 (4.7%) | |
| South American | 6 (9.4%) | 6 (9.4%) | |
| Nulliparas | 37 (57.8%) | 42 (65.6%) | 0.363 |
| Spontaneous pregnancy onset | 63 (98.4%) | 62 (69.9%) | 0.559 |
| Gestational age at delivery | 1 | ||
| ≥ 37 wks | 61 (95.3%) | 61 (95.3%) | |
| 34-37 wks | 3 (4.7%) | 3 (4.7%) | |
| Pregnancy related diseases | 0.640 | ||
| GDM2 | 7 (10.9%) | 10 (15.6%) | |
| IUGR³ | 2 (3.1%) | 3 (4.7%) | |
| Thyroid disease | 5 (7.8%) | 3 (4.7%) | |
| Hypertensive disorders | 1 (1.6%) | 3 (4.7%) | |
| PTL | 1 (1.6%) | 0 (0%) | |
| ICP | 1 (1.6%) | 0 (0%) | |
| Delivery mode | 0.445 | ||
| Vaginal delivery | 42 (65.6%) | 35 (54.7%) | |
| Cesarean section | 20 (31.2%) | 26 (40.6%) | |
| Vacuum-assisted vaginal delivery | 2 (3.1%) | 3 (4.7%) | |
| Induction of labour | 21 (32.8%) | 13 (20.3%) | 0.109 |
| Newborn weight (g) | 3160.6 ± 449.4 | 3237.4 ± 479.5 | 0.309 |
| APGAR score at 5 minutes <7 | 0 (0%) | 0 (0%) | 1 |
| NICU admission | 1 (1.6%) | 3 (4.7%) | 0.310 |
Continuous variables are expressed as mean ± standard deviation (SD). All the other features are expressed as categorical variables in frequencies and their percentages in the brackets.
Table 1-B presents the specific features of the cases’ population.
Table 1- B.
SARS CoV-2 positive cases features
| Features | |
|---|---|
| Gestational age at infection diagnosis (wks) | 37.6 ± 4.1 |
| Gestational age at delivery (wks) | 38.9 ± 1.2 |
| Cord pH | 7.32 ± 0.09 |
| Cord pO₂ (mmHg) | 33.24 ± 14.83 |
| SARS-CoV-2 infection course | |
| Asymptomatic | 35 (54.7%) |
| Mild | 14 (21.9%) |
| Moderate | 12 (18.7%) |
| Severe | 3 (4.7%) |
| Oxygen therapy | |
| None | 49 (76.6%) |
| Nasal cannulae | 2 (3.1%) |
| Venturi mask | 2 (3.1%) |
| Pharmacologic therapy | |
| None | 23 (35.9%) |
| Antivirals+Chloroquine+LMWH | 8 (12.5%) |
| Antibiotics added | 11 (17.2%) |
| Only LMWH | 6 (9.4%) |
| Only antibiotics | 9 (14.1%) |
| PCR positive placentas | 7 (14.9%) |
Continuous variables are expressed as mean ± standard deviation (SD). All the other features are expressed as categorical variables in frequencies and their percentages in the brackets.
Six patients acquired SARS-CoV-2 infection between 18 and 33 gestational weeks, and they were negative at the time of delivery. They did not differ from the rest of the study's population for the main analyzed features and results.
For all other cases, the infection was diagnosed in the immediate weeks prior to delivery.
SARS-CoV-2 neonatal infection was tested with a molecular nasopharyngeal swab performed within 2 h of birth in 51 children born from mothers whose swab was positive at the time of delivery. Only one of them resulted as “weak positive”, while the other fifty were negative. The same test was repeated 24–48 h after birth and no more positive results were observed. The newborn who resulted weak positive at birth always stayed asymptomatic and his molecular nasopharyngeal swabs collected at 48 h, 72 h and 10 days after birth were negative.
Treatments were based on the hospital protocols and involved the administration of oxygen (via a nasal cannula or a Venturi mask), or a drug treatment with antiretroviral drugs (lopinavir/ritonavir 400 mg twice for 7 days) in association with hydroxychloroquine (200 mg once for 7 days) and LMWH (4000–6000 UI daily up to 20 days postpartum) and/or antibiotics (ampicillin or cephalosporins).
Oxygen supplementation was required for the 3 severe cases and for one moderate case.
Moreover, the radiological investigation revealed interstitial pneumonia in 16 cases, being associated both to a mild or to a severe clinical presentation.
3.2. Histological placental examination
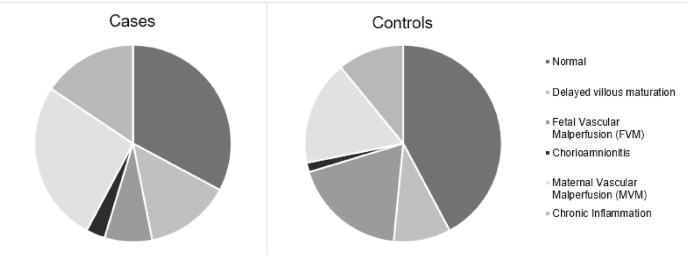
The results of the histological examination are presented as pie charts in Fig. 1 .
Fig. 1.
Placental histological diagnoses.
No significant differences were recorded regarding histological features. In particular, normal placental features were equally distributed in cases (32.8%) and controls (42.2%, p = 0.273.); delayed villous maturation was as frequent in cases (14.1%) as in controls (9.4%, p = 0.410); cases developed chorioamnionitis with frequencies comparable to controls (3.1 vs. 1.6% respectively, p = 0.559); chronic inflammation distribution was comparable between cases (15.6%) and controls (10.9%, p = 0.435); MVM had a similar frequency in cases (26.6%) and controls (17.2%, p = 0.200); FVM appeared less frequent in cases than controls, though not reaching statistical significance (7.8% vs. 18.8% respectively, p = 0.068).
In the FVM group, there were no cases of high grade FVM. In the Chronic Inflammation category, the number of cases with high grade villitis were similar to those of controls (data not shown) (see Fig. 2 ).
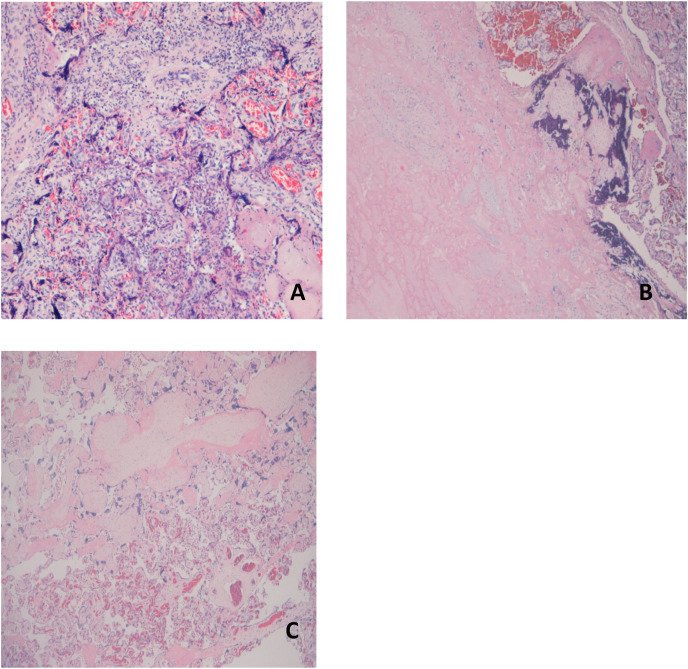
Fig. 2.
Presents the microscopic lesions found in SARS-CoV-2 infected patients.
A - A picture from a High Grade Villitis of Unknown Etiology (HG-VUE, EE, 10x): note the diffuse lymphohistiocytic component.
B - Maternal Vascular Malperfusion: an area with «ghost » villi typical of older infarcts (left).
C - Fetal Vascular Malperfusion: a field of avascular, fibrotic villi (top), typically seen in a segmental FVM
3.3. Symptomatic versus Asymptomatic cases
We then focused on the cases’ group comparing the macro- and microscopical placental features in women with an asymptomatic SARS-CoV-2 infection to those who developed any symptom. Moreover, we evaluated fetal oxygenation comparing umbilical arterial blood pO₂ levels between symptomatic or not patients (Table 2 ).
Table 2.
Asymptomatic vs. symptomatic patients’ placentas and pO₂ levels.
| Features | Asymptomatic (n=35) | Symptomatic (n=29) | p-value |
|---|---|---|---|
| Gestational Age at delivery (wks) | 39.0 ± 1.1 | 38.8 ± 1.4 | 0.470 |
| Placental Weight (g) | 487.3 ± 88.2 | 487.3 ± 81.1 | 0.418 |
| Fetal Placental/Weight ratio | 6.6 ± 1.0 | 6.5 ± 0.8 | 0.513 |
| Placental Diagnoses | |||
| Normal | 13 (37.1%) | 8 (27.6%) | 0.418 |
| Delayed Villous Maturation | 4 (11.4%) | 5 (17.2%) | 0.505 |
| Fetal Vascular Malperfusion (FVM) | 4 (11.4%) | 1 (3.4%) | 0.236 |
| Chorioamnionitis | 0 (0%) | 2 (6.9%) | 0.114 |
| Chronic Inflammation | 6 (17.1%) | 4 (13.8%) | 0.713 |
| Maternal Vascular Malperfusion (MVM) | 8 (22.9%) | 9 (31.0%) | 0.461 |
| Umbilical artery pO₂ (mmHg) | 29.23 ± 12.17 | 38.90 ± 16.60 | 0.030 |
The significantly higher umbilical cord pO2 levels recorded in symptomatic patients (38.90 ± 16.60 vs. 29.23 ± 12.17 mmHg, p = 0.030) is likely due to oxygen therapy provided to some symptomatic patients; more data are needed to support this hypothesis.
3.4. Pharmacologically treated versus Not treated cases
We also compared placental biometry and histology and cord pO₂ levels between cases treated with any pharmacologic therapy to those who did not receive drugs ( Table 3 ).
Table 3.
Pharmacologically treated vs. not treated patients’ placentas and pO₂ levels.
| Features | Any therapy (n=34) | No therapy (n=23) | p-value |
|---|---|---|---|
| Gestational age at delivery (wks) | 38.9 ± 1.3 | 38.7 ± 1.1 | 0.454 |
| Placental Weight (g) | 506.1 ± 85.5 | 453.6 ± 69.5 | 0.032 |
| Fetal Placental/Weight ratio | 6.4 ± 0.9 | 6.7 ± 0.9 | 0.258 |
| Placental Diagnoses | |||
| Normal | 12 (35.3%) | 8 (34.8%) | 0.968 |
| Delayed Villous Maturation | 8 (23.5%) | 1 (4.3%) | 0.051 |
| Fetal Vascular Malperfusion (FVM) | 0 (0%) | 1 (4.3%) | 0.220 |
| Chorioamnionitis | 0 (0%) | 2 (8.7%) | 0.080 |
| Chronic Inflammation | 5 (14.7%) | 5 (21.7%) | 0.493 |
| Maternal Vascular Malperfusion (MVM) | 9 (26.5%) | 6 (26.1%) | 0.974 |
| Cord pO₂ (mmHg) | 33.24 ± 14.83 | 34.82 ± 15.98 | 0.810 |
Continuous variables are expressed as mean ± standard deviation (SD). All the other features are expressed as categorical variables in frequencies and their percentages in the brackets.
Placentas of treated women were significantly heavier (506.1 ± 85.5 vs. 453.6 ± 69.5, p = 0.032) but not more efficient since the fetal/placental weight ratio did not differ significantly.
No relevant differences resulted from the histological diagnoses between these two groups. The delayed villous maturation was more frequent in treated women, approaching statistical significance (23.5% vs. 4.3%, p = 0.051).
3.5. Cases with PCR Positive versus Negative placentas
From the PCR analysis performed on 47 placentas, only seven were found positive.
When comparing SARS-CoV-2 PCR positive versus negative placentas, we found no differences in gestational age at delivery (38.6 ± 1.0 vs. 38.9 ± 1.2 weeks, p = 0.267), placental weight (428.3 ± 102.2 vs. 494.5 ± 80.1 g, p = 0.143), fetal/placental weight ratio (7.1 ± 0.8 vs. 6.5 ± 0.9, p = 0.069), umbilical artery pO₂ (41.03 ± 20.98 vs. 32.24 ± 13.84 mmHg, p = 0.238) nor histological analysis (data not shown).
3.6. TWO-WAY ANOVA analysis
Finally, the two-way ANOVA performed to investigate the impact of COVID-19 symptoms, pharmacological treatment and histological placental alterations on cord pO 2 at birth did not show any significant interaction effect (data not shown).
4. Discussion
Herein we report no relevant differences in placental histopathologic patterns between SARS-CoV-2 infected pregnant women and non-infected controls with similar maternal characteristics. Previous studies have also suggested no specific histopathologic differences in placentas from infected patients [[14], [15], [16], [17], [18], [20], [23], [24], [25], [26]], but these reports were lacking appropriately selected controls group.
Differently, Shanes et al. have recently reported higher rates of maternal (MVM) and fetal vascular malperfusion (FVM) lesions and intramural fibrin deposition in placentas of women infected with SARS-CoV-2 [1].
However, Shanes and colleagues performed a retrospective analysis and compared data with historical controls and with a group being examined due to maternal history of melanoma [1], while in the current study data were prospectively collected and compared to a control group selected from consecutively enrolled women, according to precise demographic features which made the study groups comparable. This is important since pregnancies associated with COVID-19 have been reported more often in women with risk factors, such as increased BMI [27].
To date conflicting data have been reported from the analysis of placental and fetal tissues positive to SARS-CoV-2. This was summarized in a recent systematic review [28] with some Authors reporting increased rates of maternal (MVM) and fetal vascular malperfusion (FVM) lesions and intramural fibrin deposition in placentas of women infected with SARS-CoV-2, while others reported rates of MVM and FVM comparable to controls.
In our study population we did not observe increased frequencies of the aforementioned histologic lesions. Of note, we studied a population affected by SARS-CoV-2 during the second half of pregnancy, mostly during the third trimester. Moreover, the majority of our enrolled women were asymptomatic or developed only a mild infection. We could therefore hypothesize a smaller impact upon placental histology on almost completely developed placentas.
Theoretically, infection with SARS-CoV-2 during pregnancy might influence placental development either directly via viral infection, or indirectly by modifying the maternal environment, i.e. with increased inflammation, or by changes in uterine oxygenation. In our series, seven placentas were positive to SARS-CoV-2 PCR evaluation. However, these placentas did not show any specific histological and morphological features different from non-positive placentas or from controls. Indeed, these patients developed a late and mild infection, similar to the rest of the analyzed cases.
Several authors suggest that COVID-19 may trigger a severe systemic inflammatory response, with consequent MVM caused by the SARS-CoV-2 infection derived-hypoxia [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [20], [19], [21], [22]]. We also recently reported [29] evidence of an increased immune activation profile in SARS-CoV-2 positive subjects, with higher levels of cytokines and chemokines in placental tissue, maternal and funicular plasma. However, this acute inflammatory response seems not to have a significant effect on the histological placental pattern in late gestation.
In our study population, the analyzed placentas showed an increase in delayed villous maturation in the pharmacologically treated group. As delayed villous maturation is considered a sign of metabolic alterations, its meaning in relation to SARS-CoV-2 infection still has to be investigated.
We did not show differences in placental histopathological and biometric parameters between cases and controls, apart from heavier but not more efficient placentas in the treated women's group. We adjusted our statical analysis to the confounding variables as maternal body mass index (BMI), since overweight and obese pregnant women have heavier and less efficient placentas [30], but we did not find any significant difference, ascribing the evidence as a casual result.
Interestingly, umbilical cord pO2 levels were significantly higher in symptomatic compared to not-symptomatic patients. Actually, oxygen therapy was administered to the symptomatic subjects of our study, in relation to the increasing clinical severity (moderate and severe groups). Therefore we may hypothesize that placental oxygen exchange was preserved in these placentas.
4.1. Strengths and limitations
This prospective study has made benefit of a specialized team of pathologists that made all the histopathological, morphological and biometric analyses thus minimizing the possible bias derived from a multiple interpretation of the diagnoses. The study data derived from comparisons of the cases to a control group chronologically selected upon specific chosen demographical features that was evaluated by the same pathologists in the previous year [31].
To the best of our knowledge, this is the first study on placentas from pregnant women infected with SARS-Cov-2 comparing treated versus not treated and symptomatic versus asymptomatic patients, analyzing them for the presence of the viral strain (with PCR analysis) in placenta.
However, the small size of our study population, to date one of the biggest to our knowledge, could have contributed to the lack of significance in some of our results, thus not allowing to drive definitive conclusions. The sample size problem is further complicated by the complexity of the issue of SARS-CoV-2 infection timing, symptoms and treatment which further reduces the number of patients in any one group.
In conclusion, the presented evidence suggests that SARS-CoV-2 infection during the third trimester does not affect placental histology and morphology, nor causes obstetrical or fetal adverse outcomes.
COVID-19 severity and its pharmacological treatment have not affected placental status and development in this study. Nevertheless, the oxygen therapy administered to our patients seems to ameliorate the neonatal oxygenation at birth (thus increasing cord pO2), independently from SARS-CoV2 severity of infection.
Declaration of competing interest
Irene Cetin
I declare that I participated in the conception and planning of the study and in the writing and revision of the article and that I have seen and approved the final version. I have no conflicts of interest.
Chiara Tasca
I declare that I participated in the clinical enrollment of the patients, in the analysis of data and in writing the first draft of the paper and that I have seen and approved the final version. I have no conflicts of interest.
Silvia Corti
I declare that I participated in the clinical enrollment of the patients, in the analysis of data and in writing the first draft of the paper and that I have seen and approved the final version. I have no conflicts of interest.
Roberta Simona Rossi
I declare that I participated in the macroscopical and microscopical analysis of placentas and in the revision of the article and that I have seen and approved the final version. I have no conflicts of interest.
Gaia Maria Anelli
I declare that I participated in the analysis of data and in the revision of the file and that I have seen and approved the final version. I have no conflicts of interest.
Valeria Savasi
I declare that I participated in the clinical enrollment of the patients and in the revision of the article and that I have seen and approved the final version. I have no conflicts of interest.
Federica Brunetti
I declare that I participated in the clinical enrollment of the patients and their placentas and that I have seen and approved the final version. I have no conflicts of interest.
Emilio Caselli
I declare that I participated in the macroscopical and microscopical analysis of placentas and that I have seen and approved the final version. I have no conflicts of interest.
Cristina Tonello
I declare that I participated in the PCR analysis of placentas and that I have seen and approved the final version. I have no conflicts of interest.
Prof. Patrizia Vergani
I declare that I participated in the design of the study and in the revision of the article and that I have seen and approved the final version. I have no conflicts of interest.
Prof. Manuela Nebuloni
I declare that I participated in the design of the study and in the revision of the article and that I have seen and approved the final version. I have no conflicts of interest.
Manuela Cardellicchio
I declare that I participated in the clinical enrollment of the patients and their placentas and that I have seen and approved the final version. I have no conflicts of interest.
Acknowledgements
We are particularly grateful to all the pregnant women that contributed to the study with their clinical and biological data. We thank the ethical committee for their contribution and rapid evaluation of the protocol of the study. Lastly, we thank all the Italian doctors and midwives that with no fear have provided an incredible effort during the first wave of this terrible pandemic.
References
- 1.Shanes E.D., Mithal L.B., Otero S., et al. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020 May;2(2):100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020;99(7):823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi J., Gong W., Gao Q. Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: a systematic review. Arch. Gynecol. Obstet. 2020:1–9. doi: 10.1007/s00404-020-05889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baud D., Gilbert G., Guillaume F., et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penfield C.A., Brubaker S.G., Limaye M.A., et al. Detection of SARS-COV-2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020:100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosier H., Farhadian S.F., Morotti R.A., et al. SARS–CoV-2 infection of the placenta. J. Clin. Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Assaf N, Khan R. Significance of placental swab in diagnosing vertical transmission in SARS-COV-2 positive mothers. Res. Square. doi: 10.21203/rs.3.rs-62590/v1. [PubMed]
- 9.Fenizia C., Biasin M., Cetin I., et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020 doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotlyar A.M., Grechukhina O., Chen A., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021 Jan;224(1):35–53. doi: 10.1016/j.ajog.2020.07.049. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamouroux A., Attie-Bitach T., Martinovic J., et al. Evidence for and against vertical transmission for SARS-CoV-2 (COVID-19) Am. J. Obstet. Gynecol. July 01, 2020;223(1):91.E1–91.E4. doi: 10.1016/j.ajog.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z., Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am. J. Perinatol. 2020 Aug;37(10):1055–1060. doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patanè L., Morotti D., Giunta M.R., et al. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am. J. Obstetrics Gynecol. MFM. 2020 doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hecht J.L., Quade B., Deshpande V., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz D., Beilin Y. Disorders of coagulation in pregnancy. Br. J. Anaesth. 2015;115 doi: 10.1093/bja/aev374. [DOI] [PubMed] [Google Scholar]
- 16.Baergen R., Heller D. Placental pathology in covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020;23(3):177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvey J., Magrob C.A., Mab L., et al. A mechanistic analysis placental intravascular thrombus formation in COVID-19 patients. Ann. Diagn. Pathol. 2020;46:151529. doi: 10.1016/j.anndiagpath.2020.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosier H., Farhadian S.F., Morotti R.A., et al. SARS‐CoV‐2 infection of the placenta. J. Clin. Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patberg E.T., Adams T., Rekawek P., et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2020;S0002–9378(20):31194–31197. doi: 10.1016/j.ajog.2020.10.020. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M., Skaria P., Kreutz K., et al. Histopathology of third trimester placenta from SARS-CoV-2-positive women. Fetal Pediatr. Pathol. 2020 doi: 10.1080/15513815.2020.1828517. [DOI] [PubMed] [Google Scholar]
- 21.Mando C., Calabrese S., Mazzocco M.I., et al. Sex specific adaptations in placental biometry of overweight and obese women. Placenta. 2016;38:1–7. doi: 10.1016/j.placenta.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Khong T.Y., Mooney E.E., Ariel I., et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch. Pathol. Lab Med. 2016;140(7):698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 23.Menter T., Mertz K.D., Jiang S., et al. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiology. 2020 Sep 18:1–9. doi: 10.1159/000511324. Epub ahead of print. PMID: 32950981; PMCID: PMC7573905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prabhu M., Cagino K., Matthews K.C., et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020 Nov;127(12):1548–1556. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P., Salafia C., Heyman T., et al. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am. J. Obstet. Gynecol. MFM. 2020 Nov;2(4) doi: 10.1016/j.ajogmf.2020.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulersen M., Prasannan L., Tam Tam H., et al. Histopathologic evaluation of placentas after diagnosis of maternal severe acute respiratory syndrome coronavirus 2 infection. Am. J. Obstet. Gynecol. MFM. 2020 Nov;2(4):100211. doi: 10.1016/j.ajogmf.2020.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savasi V.M., Parisi F., Patanè L., et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstet. Gynecol. 2020 Aug;136(2):252–258. doi: 10.1097/AOG.0000000000003979. PMID: 32433453. [DOI] [PubMed] [Google Scholar]
- 28.Sharps M.C., Hayes D.J.L., Lee S., et al. Vol. 101. 2020 Nov. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection Placenta; pp. 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenizia C., Biasin M., Cetin I., et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020 Oct 12;11(1):5128. doi: 10.1038/s41467-020-18933-4. PMCID: PMC7552412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandò C., Anelli G.M., Novielli C., et al. Impact of obesity and hyperglycemia on placental mitochondria. Oxid. Med. Cell Longev. 2018 Aug 14;2018 doi: 10.1155/2018/2378189. PMCID: PMC6112210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zambon M., Tasca C., Bonato S., et al. Reproductive Sciences; 2021. Methodology for Biometrical Analysis of the Placenta: Feasibility and Reproducibility. [DOI] [PubMed] [Google Scholar]